Abstract
Linezolid has gained increased use for the treatment of infections caused by multidrug-resistant Gram-positive bacteria in recent years. It can cause rare but potentially life-threatening lactic acidosis. Here, we presented a case report of linezolid-induced lactic acidosis (LILA), along with a systematic review of current literature.
The patient was a 55-year-old male who presented with the symptoms of acute cholecystitis. He had been treated for sepsis due to acute cholecystitis with broad-spectrum antibiotics and intravenous fluids as per protocol. Still, his lactate level was getting elevated. After excluding other causes of lactic acidosis, LILA was diagnosed, and linezolid was discontinued. His lactic acid level, as well as his physical condition, improved after that.
Studies related to LILA were searched in Medline via PubMed. After screening titles, abstracts, and full texts, data were extracted, tabulated, and presented in this article. The risk of bias was also assessed. We found 78 relevant articles in the primary search, and 26 articles, including 496 patients, were included in the study. From 23 studies of 129 patients, 28 patients (21.7%) died in the setting of LILA. The peak lactate level in which the patient developed LILA was 38.1 mmol/L after four weeks of therapy. The most common health conditions associated with LILA were end-stage renal failure (ESRD), diabetes mellitus (DM), hypertension, chronic obstructive pulmonary disease (COPD), etc. Eighteen studies with a total of 30 patients discontinued it after the development of LILA. Twenty-four patients (80%) out of 30 survived after the discontinuation.
We recommend including LILA in the differential diagnoses when treating patients with lactic acidosis since LILA is associated with a relatively elevated mortality rate.
Keywords: drug reaction, lactic acidosis, linezolid, oxazolidinones, zyvox
Introduction
Linezolid is a drug of the oxazolidinone class, approved by the US Food and Drug Administration in 2000. It is mainly used for treating multidrug-resistant Gram-positive bacteria such as vancomycin-resistant Enterococcus faecium, vancomycin-resistant Staphylococcus aureus, multidrug-resistant tuberculosis, and methicillin-resistant S. aureus. Its use is increasing due to emerging orthopedic implant infections in the elderly and bone and joint infections [1,2].
Lactic acidosis can be classified into two types. Type A occurs due to hypoxia or decreased perfusion secondary to sepsis, hypovolemia, etc. Type B occurs due to deterioration of cellular metabolism secondary to other causes, such as drug toxicity, diabetes mellitus, alcoholism, malignancy, etc. [3]. Though the most common adverse effect of linezolid is reversible myelosuppression (anemia, thrombocytopenia, leukopenia), the first case of linezolid-induced lactic acidosis (LILA) was reported in 2003 [4,5]. It results in serious adverse effects such as multiorgan failure, which can be life-threatening. Little is known about the pathophysiology, risk factors, and characteristics of patients who develop LILA.
Here, we presented a case of a 55-year-old male with LILA whose symptoms mimicked acute cholecystitis, along with a review of current literature.
Case presentation
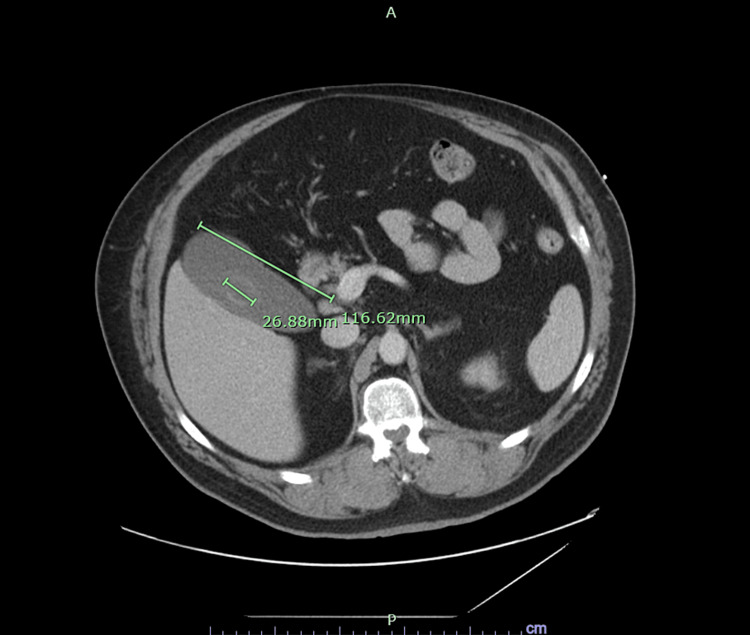
A 55-year-old male with a history of obesity, tobacco use, and uncontrolled diabetes mellitus complicated by osteomyelitis of the left foot presented to the emergency department with complaints of epigastric and left upper quadrant pain for one day associated with nausea and vomiting. Vitals signs were as follows: temperature of 35.5 degrees Celsius, heart rate of 123 bpm, blood pressure of 174/105 mmHg, and oxygen saturation of 97% on room air. Relevant labs were a WBC count of 14.3 k/cumm, lactate level of 7.3 mmol/L, pH 7.22, anion gap of 18 mmol/L, and creatinine of 1.39 mg/dL. No other abnormalities were noted on the rest of the complete metabolic panel. On a CT scan, the patient had an enlarged gall bladder (Figure 1).
Figure 1. Enlarged Gallbladder (116.62 mm × 26.88 mm) in a Patient With Linezolid-Induced Lactic Acidosis.
Initially, the patient was diagnosed and treated for sepsis due to acute cholecystitis with broad-spectrum antibiotics and intravenous fluids per protocol. Despite an additional two-liter fluid bolus, the lactic acid level continued to be elevated.
On review of his medication history, the patient had a previous history of chronic diabetic left foot osteomyelitis and methicillin-sensitive S. aureus (MSSA) septicemia, requiring hospitalization for three days. He was treated with oral linezolid due to his inability to tolerate any adhesives required for a peripherally inserted central catheter (PICC) line. After six weeks of treatment with linezolid, the patient was presented to the emergency room.
After investigating alternative differentials such as hypoperfusion, tissue necrosis, or liver failure, LILA was suspected. Linezolid was discontinued and transitioned to doxycycline. After 12 hours of discontinuation, his lactate level decreased to 1.8 mmol/L, and his pH improved to 7.32. The laboratory findings during linezolid therapy and 12 hours after discontinuation are shown in Table 1.
Table 1. Time Course of Laboratory Test Results.
ALT: alanine transaminase; AST: aspartate aminotransferase; BUN: blood urea nitrogen; PO2: partial pressure of oxygen; PCO2: partial pressure of carbon dioxide; WBC: white blood cell
| Pertinent Laboratory Values | Reference Range | Initial Findings | After 12 Hours of Discontinuation |
| pH Arterial | 7.35-7.45 | 7.22 | 7.32 |
| PCO2 Arterial (mmHg) | 35-45 | 35 | 37 |
| PO2 (mmHg) | 80-110 | 77 | 68 |
| Venous Lactate (mmol/L) | 0.5-2.2 | 7.3 | 1.8 |
| Anion Gap (mmol/L) | 3-11 | 18 | 9 |
| Glucose (mg/dL) | 70-99 | 128 | 339 |
| Alkaline Phos (Units/L) | 25-125 | 47 | - |
| ALT (Units/L) | 7-52 | 44 | - |
| AST (Units/L) | 13-39 | 27 | - |
| Bilirubin Total (mg/dL) | 0.0-1.0 | 0.4 | - |
| BUN (mg/dL) | 5-20 | 27 | 18 |
| Creatinine (mg/dL) | 0.8-1.40 | 1.39 | 1.17 |
| Sodium (mmol/L) | 135-145 | 139 | 132 |
| Potassium (mmol/L) | 3.5-5.5 | 2.9 | 4.6 |
| Chloride (mmol/L) | 98-108 | 101 | 100 |
| Bicarb (mmol/L) | 22-29 | 15 | 18.9 |
| WBC (k/cumm) | 3.6-10.6 | 14.3 | - |
Discussion
Methodology
We have done this systematic review, maintaining the standard of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, 2020 [6].
Eligibility Criteria
The inclusion criteria for this study encompassed all articles about LILA, written in English, and published studies, covering people of all ages, genders, ethnicities, and geographic locations. In contrast, the exclusion criteria ruled out editorials, short comments, letters to the editor, viewpoints, opinion papers, and commentary. Animal studies and articles without available full text were also excluded from the review.
Search Methodology
Following a comprehensive search criterion, we searched Medline through PubMed for studies of LILA until April 2023. The search strategy was developed following PICO criteria (Population, Intervention, Comparison, Outcome). Advanced searching was done following the keywords ("linezolid" [All Fields]) OR ("linezolid" [MeSH Terms]) OR ("zyvox" [All Fields]), ("lactic acidosis" [All Fields]) OR (lactic acidosis [MeSH Terms]) in combination.
Screening Process
Two reviewers individually screened the title and abstract of all eligible articles following the inclusion and exclusion criteria. After the primary title and abstract screening, relevant articles were selected for the full-text screening. Both reviewers independently reviewed all the selected articles. There were no duplicates. Any conflict of opinion that appeared in any screening steps is resolved by discussion of themselves and by the expert advice of the senior authors.
Data Extraction
All the articles have been read thoroughly for relevant information regarding LILA and were documented precisely. Data points including author name, study design, age and gender of the patients, comorbid conditions, duration, doses and route of linezolid, reasons for using this medication, if any concurrent medications used that can have an impact on lactate level, peak lactate levels, pH, therapeutic approaches, resultant effects, disease prevalence, and associated risk factors were reviewed and summarized.
Assessment of Risk of Bias
The risk of bias was assessed using the "JBI Checklist for Case Reports" [7], for case reports and case series and the RoBANS tool (Risk of Bias Assessment tool for Non-randomized Studies) for other observational studies [8]. Graphical representations of the risk of bias for case reports, case series, and observational studies are shown in two stacked bar charts.
Data Synthesis
A narrative data synthesis was performed to bring together the findings, examine the similarities and differences among the studies, and assess the strength of the evidence. Meta-analysis was not done because of the heterogeneity of including articles regarding interventions, outcomes, and scenarios to provide a meaningful summary.
Results
Characteristics of Included Studies
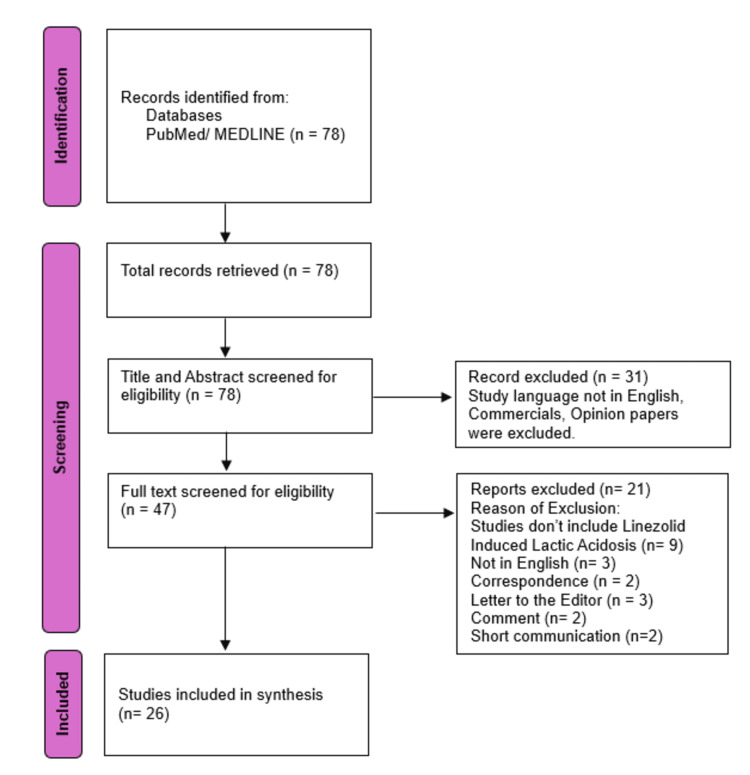
The study search and inclusion process are shown in the PRISMA flow diagram, 2020 (Figure 2).
Figure 2. Flow Diagram of Identification of Studies.
In the initial search, 78 relevant articles were found. Forty-seven articles were selected for full-text screening after title-abstract screening. After full-text screening, 21 articles were excluded for not fulfilling our strict inclusion and exclusion criteria. Twenty-six articles with 496 patients were included in this study and selected for data extraction. Among 26 studies, 17 were case reports, two case series (including five cases total), two retrospective cohort studies, two cross-sectional studies, two reviews, and one case-control study. The characteristics and outcomes of the included studies are shown in Table 2.
Table 2. Characteristics of Included Studies and Treatment Outcomes.
AF: atrial fibrillation; AIDS: acquired immunodeficiency syndrome; AKI: acute kidney disease; ALL: acute lymphoblastic leukemia; AML: acute myeloblastic leukemia; AV: aortic valve; BMT: bone marrow transplant; CAD: coronary artery disease; CKD: chronic kidney disease; CLD: chronic liver disease; COPD: chronic obstructive pulmonary disease; CRRT: continuous renal replacement therapy; CVVH: continuous veno-venous hemofiltration; DM: diabetes mellitus; DVT: deep venous thrombosis; D50W: dextrose 50% in water; ESRD: end-stage renal disease; F: female; HD: hemodialysis; HF: heart failure; HTN: hypertension; IV: intravenous; M: male; MRSA: methicillin-resistant Staphylococcus aureus; MS: mitral stenosis; MV: mitral valve; NE: nor-epinephrine; NR: not-reported; PH: portal hypertension; PVD: peripheral vascular disease; qm: every month; qn: every night; q/12h: every 12 hours; SC: subcutaneous; UTI: urinary tract infection; VRE: vancomycin-resistant Enterococcus faecium
| S. no. | Author name | Study design | Age (years) | Sex | Comorbid conditions | Reason of therapy | Concurrent medications | Duration of therapy | Dose and route | Peak lactate (mmol/L) | pH | Treatment | Outcome |
| 1 | Narita et al. [9] | Minireview | 64.8 | F=4; M=2 | AML, BMT Biliary cirrhosis, HTN, Cutaneous T-cell lymphoma | Prosthetic joint infection, Osteomyelitis, Disseminated Nocardiosis | NR | 1-16 weeks | NR | 10-24.5 | NR | Discontinued | Survived (n=5); Died (n=2) |
| 2 | Ozkaya-Parlakay et al. [10] | Cross-sectional study | Mean= 6.1 years | F=20 M=30 | NR | Bacteremia, Pneumonia, UTI, Sepsis, | Amikacin, Ciprofloxacin, Caspofungin, Ceftriaxone, Imipenem, Clarithromycin, Fluconazole, Ornidazole, Ampicillin/sulbactam, Vancomycin, Trimethoprim/sulfamethoxazole, Clindamycin, Amphotericin B | 18.1 (3-93 days) | 10 mg/kg, q/8h in age 0-11 years, 10 mg/kg q/12h in older children. | NR | NR | NR | Nine children died |
| 3 | Smolka et al. [11] | Case report | 9y | F | B-cell-precursor ALL, Allogeneic hematopoietic stem cell transplant | Multifocal pulmonary abscesses by mycobacterium | Amikacin, Meropenem, Posaconazole, Valaciclovir, Prednisone, Budesonide | 51 days | 810 mg/day | 19 | 7.06 | Discontinued, Sodium bicarbonate | Survived |
| 4 | Su et al. [12] | Case series (Case-1) | 6 months | M | Prematurity, Necrotizing enterocolitis, Liver disease with coagulopathy, Bronchopulmonary dysplasia, | Surgical site MRSA following repair of mucocutaneous fistulas, VRE bacteremia | NR | 39 days | NR | 24 | NR | Discontinued | Died |
| Su et al. [12] | Case-2 | 6 months | F | Protein-losing enteropathy, Hepatic insufficiency | Prior VRE infection, Suspected sepsis | NR | 4 weeks | NR | 38.1 | NR | NR | Died | |
| Su et al. [12] | Case-3 | 16 Y | M | Cryptogenic cirrhosis, Bilateral hepatic vein occlusion, PH | VRE in urine | NR | 7 days | NR | 28 | NR | CRRT | Died | |
| 5 | Liu et al. [13] | Case-control study | 94 | M+F=35 | CKD (n=24), HTN (n=29), AF (n=16), CAD (n=29), DM (n=19), COPD (n=27) | Pulmonary, skin, and soft tissue infections | NR | 10 days | IV 600 mg qm, Oral 600 mg qn | 4.6 | NR | NR | 30-day mortality rate = 48.6% |
| 6 | Mori et al. [14] | Retrospective cohort study | >20 yrs | M=65 F=29 | DM (n=19), COPD (n=10), CKD (n=13), HF (n=11), CLD (n=5), malignancy (n=29), | Respiratory Tract Infection | NR | 8-10 days | NR | 17.1% developed lactate >5 | <7.35 | NR | 30 days mortality 28.8% |
| 7 | Im et al. [15] | Retrospective Cohort study | 61.4± 17 | F=2 M=3 | DM (n=4), CKD (n=2), AKI (n=2) | Skin and soft tissue infection, Bacteremia, Pneumonia, Intra-abdominal infection | NR | 19.7± 18 days. | NR | >4 | <7.25 | HD (n=2), Bivone (n=1), none (n=1) | Survived (n=3), Died (n=2) |
| 8 | Velez and Janech [16] | Case Report | 36 y | Male | ESRD | VRE bacteremia | Meropenem, Metronidazole Gabapentin, Metoprolol, Clonidine, Pantoprazole, Hydrocodone, Darbepoetin, Metoclopramide | 6 weeks | 600 mg q/12h | 12.5 | 7.31 | Discontinued | Survived |
| 9 | Del Pozo et al. [17] | Case Series (Case-1) | 72y | F | DM, Liver transplant due to primary biliary cirrhosis, | Disseminated nocardiosis (i.e., bone and central nervous system) | Ceftriaxone | 9 weeks | 600 mg q/12h | 4.8 | 7.25 | Discontinued, Thiamine | Survived |
| Del Pozo et al. [17] | Case-2 | 43y | M | Liver transplant, Acute renal failure | Lung tuberculosis | Ethambutol, Isoniazid | 8 weeks | 600 mg q/12h | 7.20 | 7.29 | Discontinued, Bicarbonate, Thiamine | Survived | |
| 10 | Nightingale et al. [18] | Case report | 67 y | M | COPD, CAD, HTN, CKD | Post-operative intra-abdominal sepsis, Pneumonia, bacteremia after elective abdominal aortic aneurysm repair | NR | 7 days | 600mg q/12h | 7.7 | NR | Discontinued | Survived |
| 11 | Kopterides et al. [19] | Case Report | 70y | M | cutaneous T-cell lymphoma, | MRSA bacteremia | NR | 7 days | NR | 12.5 | NR | Discontinued, thiamine 100 mg per day | Survived |
| 12 | Santini et al. [20] | Review | 63±17 | F=22 M=26 | Renal Failure (n=13) | NR | NR | 35±29 days | 600mg, q/12h | 13±7 | 7.14± 0.16 | NR | Survived (n=39), Died(n=9) |
| 13 | Mao et al. [21] | Case Report | 57 | M | T-2 DM, Poor renal function | Severe Pneumonia | IV Imipenem, Cilastatin sodium, Vancomycin | NR | 600 mg, IV q/12h, 3 doses | 10 | NR | Discontinued | Died |
| 14 | Dai et al. [22] | Cross-sectional study | 18-60 | M =125 F=111 | HTN, DM | Infectious diseases, | Meropenem, Warfarin, Ertapenem | 1-2 weeks | IV, Oral | NR | NR | NR | NR |
| 15 | Xiao et al. [23] | Case Report | 50 | F | AV, MV replacement, Rheumatic heart disease | Endocarditis | Warfarin | 25 days | 600 mg IV q/12h | 19 | 6.94 | HD, NE | died |
| 16 | Wiener et al. [24] | Case Report | 80 | F | MS | Post-operative bacteremia after MV replacement | Amiodarone, Metoprolol, Pantoprazole, Coumadin. | 19 days | IV, Oral | 19 | 7.026 | Discontinued | Survived |
| 17 | Tobias et al. [25] | Case report | 52 y | F | HTN, ulcerative colitis, osteoarthritis | Diverticulitis complicated by abscess | NR | 6 weeks | 600 mg IV piggyback (IVPB) q/12 h | 16.1 | 7.187 | Thiamine 100 mg IV push daily, HD | Survived |
| 18 | Zuccarini et al. [26] | Case report | 67y | F | HTN, dyslipidemia, bilateral avascular necrosis of the femoral head | Post-operative MRSA infection after left total hip replacement | NR | 6 weeks | NR | 9.4 | NR | Discontinued | Survived |
| 19 | Chen et al. [27] | Case report | 54y | M | Lupus nephritis, Uremic state, Cadaveric kidney transplant | Pulmonary non-tuberculosis mycobacterial infection | Azithromycin, Prednisolone, Mycophenolic acid, Sirolimus | NR | 600 mg q/12h | 18.4 | 7.122 | HD for 4 hours | Survived |
| 20 | Protti et al. [28] | Case Report | 64 y | M | Single lung transplant, Pulmonary fibrosis, | Acute respiratory failure due to ground glass opacities at the right lung (graft) | Meropenem, Amphotericin-B, Methylprednisolone | NR | 1200 mg/day IV continuous infusion | 22 | NR | Discontinued | Died |
| 21 | Johnson et al. [29] | Case Report | 34 y | M | Sickle cell disease, renal disease, Secondary hemochromatosis, Stroke, seizure | VRE bacteremia | Amphotericin B, Micafungin, Piperacillin/Tazobactam | 11 days | 600 mg IV q/12h | 26 | 7.07 | Discontinued, several amps. of D50W | Survived |
| 22 | Belani et al. [30] | Case Report | 81y | F | PVD, CAD | Deep heel abscess by VRE | IV Meropenem | 12 days | NR | 14.2 | 6.89 | Discontinued | Survived |
| 23 | Hsu et al. [31] | Case Report | 38y | F | HTN, alcoholic cirrhosis, stage 3 CKD | VRE UTI | NR | 12 days | NR | 24 | 6.918 | Discontinued, Continuous HD, IV Thiamine | Survived |
| 24 | Carson et al. [32] | Case Report | 35 y | F | AIDS | Disseminated infection with Mycobacterium avium-intracellular complex | Moxifloxacin, Tenofovir, Emtricitabine, Efavirenz, Pyridoxine, Ethambutol, Clarithromycin, Atovaquone, Valacyclovir, Erythropoietin, Sargramostim, Leucovorin | 35 days | 600 mg q/12h | 11.38 | 7.16 | Discontinued, CVVH, Riboflavin, Thiamine, Folic acid, Coenzyme Q10, L-carnitine | Survived |
| 25 | Scotton et al. [33] | Case Report | 81y | F | NR | Bacterial spondylodiscitis, Disseminated disease with involvement of the meninges, Mediastinum lymph nodes, Bilateral pleural effusion, Paravertebral abscess | Isoniazid Rifampin, Ethambutol | 12 days | 600 mg q/12h | 18.6 | 7.25 | Discontinued, Bicarbonate infusion | Survived |
| 26 | Lee et al. [34] | Case report | 56y | Caucasian F | Renal transplant, Gastric bypass for morbid obesity, | Urosepsis | Doxycycline Liposomal amphotericin- B, Meropenem | 3 days | 600 mg q/12h | 4.3 | 7.19 | Discontinued, | Survived |
Evidence of LILA
The lowest duration of linezolid therapy that caused lactic acidosis was three days for a 56-year-old Caucasian female due to urosepsis with a peak lactate level of 4.3 mmol/L. Her condition improved after discontinuation of the therapy [35]. One mini-review including six patients revealed that the highest duration of therapy was 112 days (16 weeks) with a peak lactate level of 24.5 mmol/L [9].
Ninety-six patients from 14 studies who developed lactic acidosis received a standard dose of linezolid (600 mg twice a day). One cross-sectional study included 50 patients receiving linezolid 10 mg/kg three times daily for 0-11 months old and 10 mg/kg twice daily for older children [10]. One nine-year-old female patient received 810 mg/day for 51 days before emerging LILA [11]. Ten studies did not mention the dosing of linezolid.
Among these 26 studies, the peak lactate level associated with LILA was 38.1 mmol/L, developed in a six-month-old child after four weeks of linezolid therapy [12]. Three studies did not specifically mention peak lactate levels.
From 23 studies, including 129 patients, 28 (21.7%) patients died in the setting of LILA. Three studies were not included for lack of mortality data. One case-control study in people older than 85 years showed that 30-day mortality was 48.6%, even after a relatively shorter duration of linezolid therapy. The risk factors associated with lactic acidosis development after linezolid use in patients older than 85 are the duration of treatment longer than nine days (odds ratio (OR): 3.541; 95% confidence interval (CI): 1.161-10.793; p = 0.026), an arterial blood glucose level >8 mmol/L (OR: 4.548; 95% CI: 1.507-13.725; p = 0.007), and a high sequential organ failure assessment score (OR: 1.429; 95% CI: 1.213-1.685; p < 0.0001) [13]. Another study showed that they found 28.8% mortality after 8-10 days of administration of linezolid in 94 patients older than 20 years [14].
Out of 496 individuals, 261 were male and 200 were female. The gender of 35 patients was not specified. Regarding age, 369 individuals were under 65, while 102 were over 65. The age of 25 participants was not provided.
Risk of Bias Assessment of the Included Studies
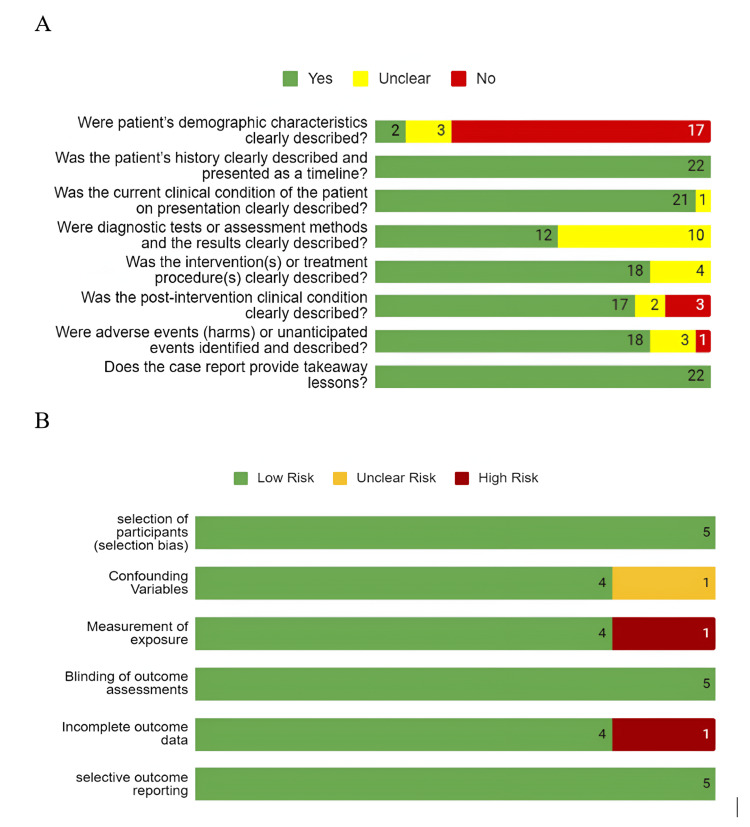
We assessed the risk of bias for 24 articles, as two out of 26 articles were reviews. The detailed findings are shown in Figure 3.
Figure 3. Risk of Bias of the Included Studies (n = number of articles).
(A) Risk of Bias of the Case Reports and Case Series; (B) Risk of Bias of the Non-randomized Studies
"JBI Checklist for Case Reports" was used to analyze the bias of all case reports and case series. Eight domains of this tool, including demographic characteristics, history, current clinical condition, diagnostics tests, interventions and treatment procedure, post-intervention clinical condition, adverse events, and takeaway lessons, were checked precisely and noted.
For observational studies, we checked six domains of the RoBANS tool, including participant selection, confounding variables, measurement of exposure, blinding of the outcome assessments, incomplete outcome data, and selective outcome reporting.
Among the 22 case reports, 17 didn't describe patients' demographic location clearly. Most case reports described patients' history (n=22) and clinical condition on presentation (n=21). Ten studies didn't mention assessment methods and results, i.e., the dose of linezolid administration, lactic acid level after discontinuation of linezolid, didn't exclude the differential diagnosis of lactic acidosis like sepsis, etc. Seventeen case reports described properly the post-intervention clinical condition of the patients.
For observational studies, we assessed the six domains of the RoBANS tool. Among the six studies, none had selection bias, blinding of the outcome assessments bias, or selective outcome reporting bias. One study didn't mention if they assessed the patient for potential confounding bias. One study had a high risk of measurement of exposure bias as the data was self-reported by the patient, which can cause recall bias. One study mentioned that they had missing data. Hence, the incomplete outcome bias risk is high.
Discussion
Lactic acidosis is a condition with serum lactate level >4 mmol/L and metabolic acidosis (pH <7.35) in arterial blood gas analysis [13]. Common risk factors for LILA are pregnancy, renal impairment, obesity, lipid dystrophy, female sex, etc. [15].
Although in our study we found a larger number of males (260) were affected compared to females (200), the US Food and Drug Administration reported 90 LILA cases from August 2011 to August 2016 [36]. Since 2003, it has been thought that prolonged use of linezolid leads to lactic acidosis due to mitochondrial toxicity [4]. Though a standard dose of 600 mg twice a day is thought to be non-toxic, evidence suggests that advanced age, impaired renal function, prolonged therapy, and co-administration of some drugs, e.g., omeprazole, amiodarone or amlodipine increase the risk of lactic acidosis due to mitochondrial toxicity [1,37-39].
Linezolid most likely binds to the A-site of the peptidyl transferase center in the 23rRNA of the bacterial ribosome and disrupts the correct positioning of aminoacyl-tRNAs. Similarly, they can bind to the human mitochondrial ribosomes at the same location in the 16S rRNA and interfere with protein synthesis. It explains some of the effects of lactic acidosis associated with linezolid [40-42].
De Vriese et al. demonstrated that linezolid can inhibit protein synthesis in human and animal tissue after prolonged use. Though mitochondrial morphology didn't change, biochemical analysis revealed severe inhibition of respiratory chain complex I in the muscle and kidney and inhibition of complex IV in the muscle, kidney, and liver in humans. Those complexes are encoded by mtRNA. No change was noticed in complex II, which is encoded by nuclear DNA. Those findings were also detected in rats. mtRNA-encoded respiratory complexes were severely reduced after high doses and prolonged administration of linezolid [43].
Garrabou et al. also found decreased complex 4 activity and cytochrome c oxidase subunit 2 level in peripheral blood mononuclear cells of patients with LILA, which resolved with drug withdrawal [44].
In our study, we have found 496 cases of LILA. Patients mainly received this treatment due to pneumonia, MRSA bacteremia, urinary tract infection (UTI), sepsis, skin and soft tissue infection, pulmonary mycobacterial infection, disseminated Mycobacterium avium complex (MAC) infection, disseminated nocardiosis, diverticulitis, endocarditis, etc.
Previous studies denoted that LILA develops after prolonged use of linezolid [4,16,45-46]. A case series of two patients, 72- and 43-years old, male and female, received linezolid for nine and eight weeks, respectively, before developing lactic acidosis [17]. But LILA is also reported to occur even after a short duration of therapy of three to seven days. Their age ranged from 16 to 70 years, which supports that LILA can develop in patients of any age with an even shorter duration of medication use [12,18,19,35].
The majority of patients had underlying health conditions. These included prevalent conditions such as end-stage renal failure (ESRD), diabetes mellitus, hypertension, COPD, coronary artery disease, chronic liver disease, as well as a history of kidney and liver transplantation.
Linezolid is primarily metabolized by the liver, with the remaining 30% metabolized by the kidney. Kidney excretion occurs when the lactic acid level is above 6-10 mmol/L [47]. Therefore, severe renal impairment can be a risk factor for lactic acidosis [16]. In our study, a total number of 57 patients had renal impairment. Three separate studies of 24, 13, and another 13 patients with renal failure and CKD developed lactic acidosis after linezolid use. Mori et al. showed that renal insufficiency (estimated glomerular filtration rate (eGFR)) <30 mL/min (OR: 7.4; 95% CI: 1.0-84.4, p = 0.02) increases the risk of LILA [14].
Seventeen patients either had chronic liver disease, liver transplants, or biliary cirrhosis. Five out of 12 patients didn't survive even after discontinuation of the drug or continuous renal replacement therapy (CRRT). One study of 94 patients in which five of them had chronic liver disease denoted that their overall 30-day mortality was 28.8% [11]. A six-month-old female patient with hepatic insufficiency and protein-losing enteropathy developed LILA with a peak lactate level of 38.1 mmol/L after receiving four weeks of linezolid. Although the study did not mention the dosing and treatment, the patient unfortunately did not survive [12].
According to Jae et al., diabetes is familiar to be a cause of lactic acidosis, but no statistically significant differences between diabetes and LILA were found [16]. Forty-five patients (9.07%) out of 496 had diabetes in this study. Further studies on this subject need to be carried out to know if diabetes is the definitive risk factor for LILA.
The treatment is discontinuation of the medication. Twenty-three studies from 21 articles discussed the treatment modalities used for LILA. Seventeen articles, including 18 studies with 30 patients, denoted that they discontinued linezolid after developing lactic acidosis. Twenty-four patients out of those 30 survived after the discontinuation. The rest of the studies did not mention the treatment modality or the survival status.
Five patients required hemodialysis. The only death was a 16-year-old male with vancomycin-resistant Enterococcus faecium UTI requiring CRRT [13]. Some patients also received thiamine and sodium bicarbonate with unclear efficacy.
Our study has several limitations. Due to the relatively small sample size, a definitive conclusion cannot be made. Therefore, more relevant studies are necessary for more substantial evidence. Due to data heterogenicity, meta-analysis was not possible. We could not account for all the confounding factors, such as disease severity, use of other unreported medication, etc. All 26 studies were either observational studies or reviews. Among them, 17 were case reports, three cross-sectional studies, two case series, two reviews, one case-control, and one retrospective cohort study.
Conclusions
Linezolid use is associated with lactic acidosis with a relatively elevated mortality rate. We recommend including LILA in the differential diagnoses when treating patients with lactic acidosis.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Fahima Akter, Hannah Bozell, Tyson Neumann, Cheng Chung
Acquisition, analysis, or interpretation of data: Fahima Akter, Hannah Bozell
Drafting of the manuscript: Fahima Akter, Hannah Bozell, Tyson Neumann, Cheng Chung
Critical review of the manuscript for important intellectual content: Tyson Neumann, Cheng Chung
Supervision: Tyson Neumann, Cheng Chung
References
- 1.Risk factors associated with high linezolid trough plasma concentrations. Morata L, De la Calle C, Gómez-Cerquera JM, et al. Expert Opin Pharmacother. 2016;17:1183–1187. doi: 10.1080/14656566.2016.1182154. [DOI] [PubMed] [Google Scholar]
- 2.Linezolid for the treatment of adults with bone and joint infections. Falagas ME, Siempos II, Papagelopoulos PJ, Vardakas KZ. Int J Antimicrob Agents. 2007;29:233–239. doi: 10.1016/j.ijantimicag.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Cohen RD, Woods HF, Krebs HA. Boston, MA: Blackwell Scientific Publications. London: Blackwell Scientific Publications; 1976. Clinical and Biochemical Aspects of Lactic Acidosis. [Google Scholar]
- 4.Linezolid-induced lactic acidosis. Apodaca AA, Rakita RM. N Engl J Med. 2003;348:86–87. doi: 10.1056/NEJM200301023480123. [DOI] [PubMed] [Google Scholar]
- 5.Linezolid: a review of safety and tolerability. Vinh DC, Rubinstein E. J Infect. 2009;59:59–74. doi: 10.1016/S0163-4453(09)60009-8. [DOI] [PubMed] [Google Scholar]
- 6.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. BMJ. 2020;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The CARE guidelines: consensus-based clinical case reporting guideline development. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. BMJ Case Rep. 2013;2013:0. doi: 10.1186/1752-1947-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. Kim SY, Park JE, Lee YJ, et al. J Clin Epidemiol. 2013;66:408–414. doi: 10.1016/j.jclinepi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Linezolid-associated peripheral and optic neuropathy, lactic acidosis, and serotonin syndrome. Narita M, Tsuji BT, Yu VL. Pharmacotherapy. 2007;27:1189–1197. doi: 10.1592/phco.27.8.1189. [DOI] [PubMed] [Google Scholar]
- 10.Early lactic acidosis associated with linezolid therapy in paediatric patients. Ozkaya-Parlakay A, Kara A, Celik M, Ozsurekci Y, Karadag Oncel E, Ceyhan M, Cengiz AB. Int J Antimicrob Agents. 2014;44:334–336. doi: 10.1016/j.ijantimicag.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Severe linezolid-induced lactic acidosis in a child with acute lymphoblastic leukemia: a case report. Smolka V, Rohanova M, Ludikova B, Novak Z, Zapalka M, Pospisilova D, Volejnikova J. J Infect Chemother. 2020;26:1316–1318. doi: 10.1016/j.jiac.2020.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Linezolid and lactic acidosis: a role for lactate monitoring with long-term linezolid use in children. Su E, Crowley K, Carcillo JA, Michaels MG. Pediatr Infect Dis J. 2011;30:804–806. doi: 10.1097/INF.0b013e3182186035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Incidence and associated risk factors for lactic acidosis induced by linezolid therapy in a case-control study in patients older than 85 years. Liu T, Hu C, Wu J, et al. Front Med (Lausanne) 2021;25:604680. doi: 10.3389/fmed.2021.604680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comparative analysis of lactic acidosis induced by linezolid and vancomycin therapy using cohort and case-control studies of incidence and associated risk factors. Mori N, Kamimura Y, Kimura Y, Hirose S, Aoki Y, Bito S. Eur J Clin Pharmacol. 2018;74:405–411. doi: 10.1007/s00228-017-2377-1. [DOI] [PubMed] [Google Scholar]
- 15.Incidence and risk factors of linezolid-induced lactic acidosis. Im JH, Baek JH, Kwon HY, Lee JS. Int J Infect Dis. 2015;31:47–52. doi: 10.1016/j.ijid.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 16.A case of lactic acidosis induced by linezolid. Velez JC, Janech MG. Nat Rev Nephrol. 2010;6:236–242. doi: 10.1038/nrneph.2010.20. [DOI] [PubMed] [Google Scholar]
- 17.Linezolid-induced lactic acidosis in two liver transplant patients with the mitochondrial DNA A2706G polymorphism. Del Pozo JL, Fernández-Ros N, Sáez E, Herrero JI, Yuste JR, Banales JM. Antimicrob Agents Chemother. 2014;58:4227–4229. doi: 10.1128/AAC.02856-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linezolid induced lactic acidosis: the side effect, clinician should be aware of. Nightingale S, Austin C, Agarwal KK, Patel M, Hossain M. Cureus. 2020;16:0. doi: 10.7759/cureus.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linezolid use associated with lactic acidosis. Kopterides P, Papadomichelakis E, Armaganidis A. Scand J Infect Dis. 2005;37:153–154. doi: 10.1080/00365540410026022. [DOI] [PubMed] [Google Scholar]
- 20.Linezolid-induced lactic acidosis: the thin line between bacterial and mitochondrial ribosomes. Santini A, Ronchi D, Garbellini M, Piga D, Protti A. Expert Opin Drug Saf. 2017;16:833–843. doi: 10.1080/14740338.2017.1335305. [DOI] [PubMed] [Google Scholar]
- 21.The risk factors of linezolid-induced lactic acidosis: a case report and review. Mao Y, Dai D, Jin H, Wang Y. Medicine (Baltimore) 2018;97:0. doi: 10.1097/MD.0000000000012114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linezolid and the risk of lactic acidosis: data mining and analysis of the FDA Adverse Event Reporting System. Dai Y, Wang Y, Zeng Y, Zhang C, Zhou Z, Shi D. J Clin Pharm Ther. 2020;45:1422–1426. doi: 10.1111/jcpt.13245. [DOI] [PubMed] [Google Scholar]
- 23.Lactic acidosis and thrombocytopenia associated with linezolid therapy: a case report. Xiao B, Deng P, Jin H, Wang H, Cao Y. Am J Case Rep. 2018;19:1117–1120. doi: 10.12659/AJCR.911362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lactic acidosis after treatment with linezolid. Wiener M, Guo Y, Patel G, Fries BC. Infection. 2007;35:278–281. doi: 10.1007/s15010-007-6302-x. [DOI] [PubMed] [Google Scholar]
- 25.A case of linezolid induced toxicity. Tobias PE, Varughese CA, Hanson AP, Gurnani PK. J Pharm Pract. 2020;33:222–225. doi: 10.1177/0897190018782787. [DOI] [PubMed] [Google Scholar]
- 26.Lactic acidosis induced by linezolid mimics symptoms of an acute intracranial bleed: a case report and literature review. Zuccarini NS, Yousuf T, Wozniczka D, Rauf AA. J Clin Med Res. 2016;8:753–756. doi: 10.14740/jocmr2687w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lactic acidosis associated with standard dose linezolid in a kidney recipient with impaired renal function. Chen CC, Liu WT, Lin SH. Braz J Infect Dis. 2022;26:101701. doi: 10.1016/j.bjid.2021.101701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Changes in whole-body oxygen consumption and skeletal muscle mitochondria during linezolid-induced lactic acidosis. Protti A, Ronchi D, Bassi G, Fortunato F, Bordoni A, Rizzuti T, Fumagalli R. Crit Care Med. 2016;44:579–582. doi: 10.1097/CCM.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 29.A triad of linezolid toxicity: hypoglycemia, lactic acidosis, and acute pancreatitis. Johnson PC, Vaduganathan M, Phillips KM, O'Donnell WJ. Proc (Bayl Univ Med Cent) 2015;28:466–468. doi: 10.1080/08998280.2015.11929310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linezolid-induced lactic acidosis sets stage for surgery to rule out mesenteric ischemia: a case report. Belani K, Leibowitz A, Bose S. A A Pract. 2018;11:93–95. doi: 10.1213/XAA.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 31.Severe linezolid-induced lactic acidosis in a cirrhosis patient. Hsu SN, Shih MF, Yang CW, Wu CC, Chen CC. Nephrology (Carlton) 2015;20:47–48. doi: 10.1111/nep.12346. [DOI] [PubMed] [Google Scholar]
- 32.Severe lactic acidosis associated with linezolid use in a patient with the mitochondrial DNA A2706G polymorphism. Carson J, Cerda J, Chae JH, Hirano M, Maggiore P. Pharmacotherapy. 2007;27:771–774. doi: 10.1592/phco.27.5.771. [DOI] [PubMed] [Google Scholar]
- 33.Early linezolid-associated lactic acidosis in a patient treated for tuberculous spondylodiscitis. Scotton P, Fuser R, Torresan S, et al. Infection. 2008;36:387–388. doi: 10.1007/s15010-008-7329-3. [DOI] [PubMed] [Google Scholar]
- 34.Early development of lactic acidosis with short term linezolid treatment in a renal recipient. Lee YR, Powell N, Bonatti H, et al. J Chemother. 2008;20:766–767. doi: 10.1179/joc.2008.20.6.766. [DOI] [PubMed] [Google Scholar]
- 35.Does linezolid cause lactic acidosis by inhibiting mitochondrial protein synthesis? Palenzuela L, Hahn NM, Nelson RP Jr, et al. Clin Infect Dis. 2005;15:113–116. doi: 10.1086/430441. [DOI] [PubMed] [Google Scholar]
- 36.FDA: Reporting System (FAERS). Freedom of Information Act (FOIA). Detailed report: Linezolid. [ May; 2023 ]. 2016. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting pp. 5–2023.https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting
- 37.Hyperlactacidemia potentially due to linezolid overexposure in a liver transplant recipient. Pea F, Scudeller L, Lugano M, et al. Clin Infect Dis. 2006;42:434–435. doi: 10.1086/499533. [DOI] [PubMed] [Google Scholar]
- 38.Therapeutic drug management of linezolid: a missed opportunity for clinicians? Cattaneo D, Gervasoni C, Cozzi V, Castoldi S, Baldelli S, Clementi E. Int J Antimicrob Agents. 2016;48:728–731. doi: 10.1016/j.ijantimicag.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 39.Linezolid trough concentrations correlate with mitochondrial toxicity-related adverse events in the treatment of chronic extensively drug-resistant tuberculosis. Song T, Lee M, Jeon HS, et al. EBioMedicine. 2015;9:1627–1633. doi: 10.1016/j.ebiom.2015.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Leach KL, Swaney SM, Colca JR, et al. Mol Cell. 2007;26:393–402. doi: 10.1016/j.molcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 41.The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning. Wilson DN, Schluenzen F, Harms JM, Starosta AL, Connell SR, Fucini P. Proc Natl Acad Sci USA. 2008;9:13339–13344. doi: 10.1073/pnas.0804276105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. Ippolito JA, Kanyo ZF, Wang D, Franceschi FJ, Moore PB, Steitz TA, Duffy EM. J Med Chem. 2008;51:3353–3356. doi: 10.1021/jm800379d. [DOI] [PubMed] [Google Scholar]
- 43.Linezolid-induced inhibition of mitochondrial protein synthesis. De Vriese AS, Coster RV, Smet J, et al. Clin Infect Dis. 2006;42:1111–1117. doi: 10.1086/501356. [DOI] [PubMed] [Google Scholar]
- 44.Reversible inhibition of mitochondrial protein synthesis during linezolid-related hyperlactatemia. Garrabou G, Soriano A, López S, et al. Antimicrob Agents Chemother. 2007;51:962–967. doi: 10.1128/AAC.01190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fatal lactic acidosis after prolonged linezolid exposure for treatment of multidrug-resistant tuberculosis. Boutoille D, Grossi O, Depatureaux A, Tattevin P. Eur J Intern Med. 2009;20:0–5. doi: 10.1016/j.ejim.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Linezolid-induced lactic acidosis corrected with sustained low-efficiency dialysis: a case report. Sawyer AJ, Haley HL, Baty SR, McGuffey GE, Eiland EH 3rd. Am J Kidney Dis. 2014;64:457–459. doi: 10.1053/j.ajkd.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 47.Lactic acidosis: an update. Seheult J, Fitzpatrick G, Boran G. Clin Chem Lab Med. 2017;53:322–333. doi: 10.1515/cclm-2016-0438. [DOI] [PubMed] [Google Scholar]