Abstract
Rates of muscle protein synthesis and degradation measured in the perfused hindquarter were compared with those in incubated epitrochlearis muscles. With fed or starved mature rats, results without insulin treatment were identical. With insulin treatment, protein synthesis in perfused hindquarters was greater, though protein degradation was the same. Thus rates of muscle protein degradation estimated by these two methods in vitro correspond closely.
Full text
PDF

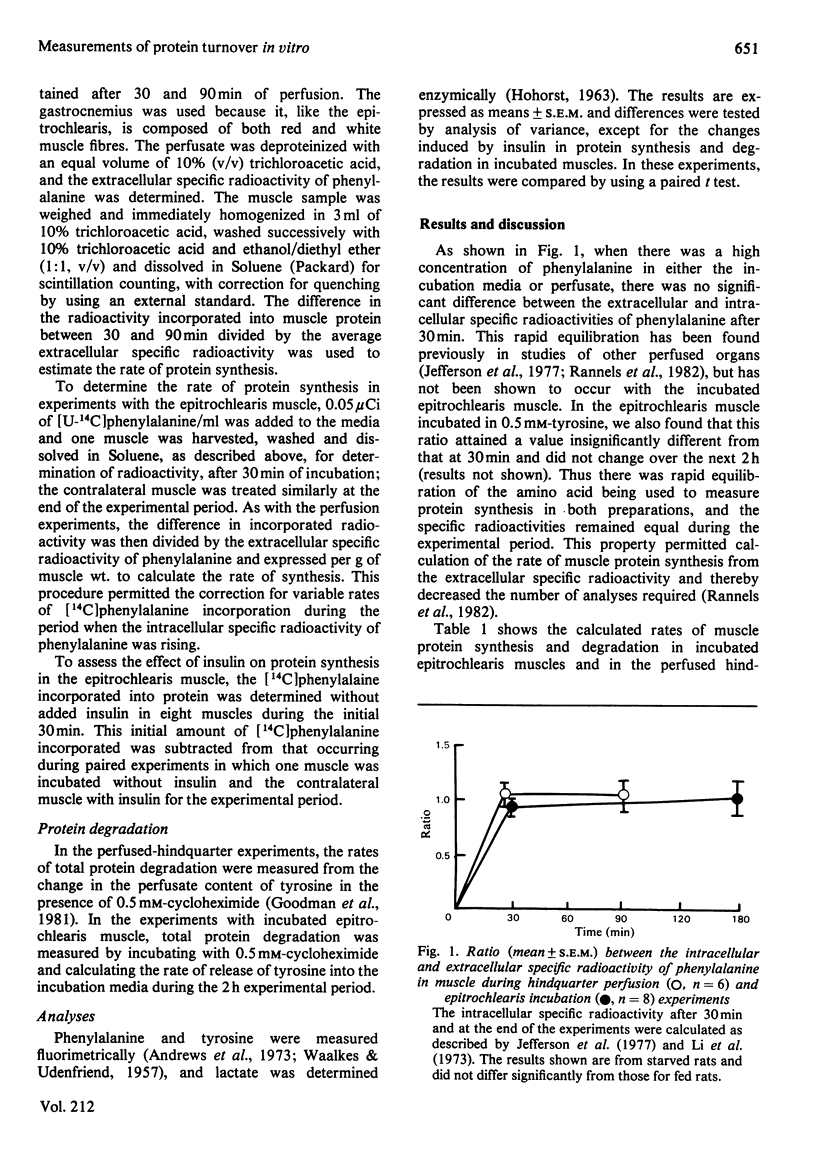
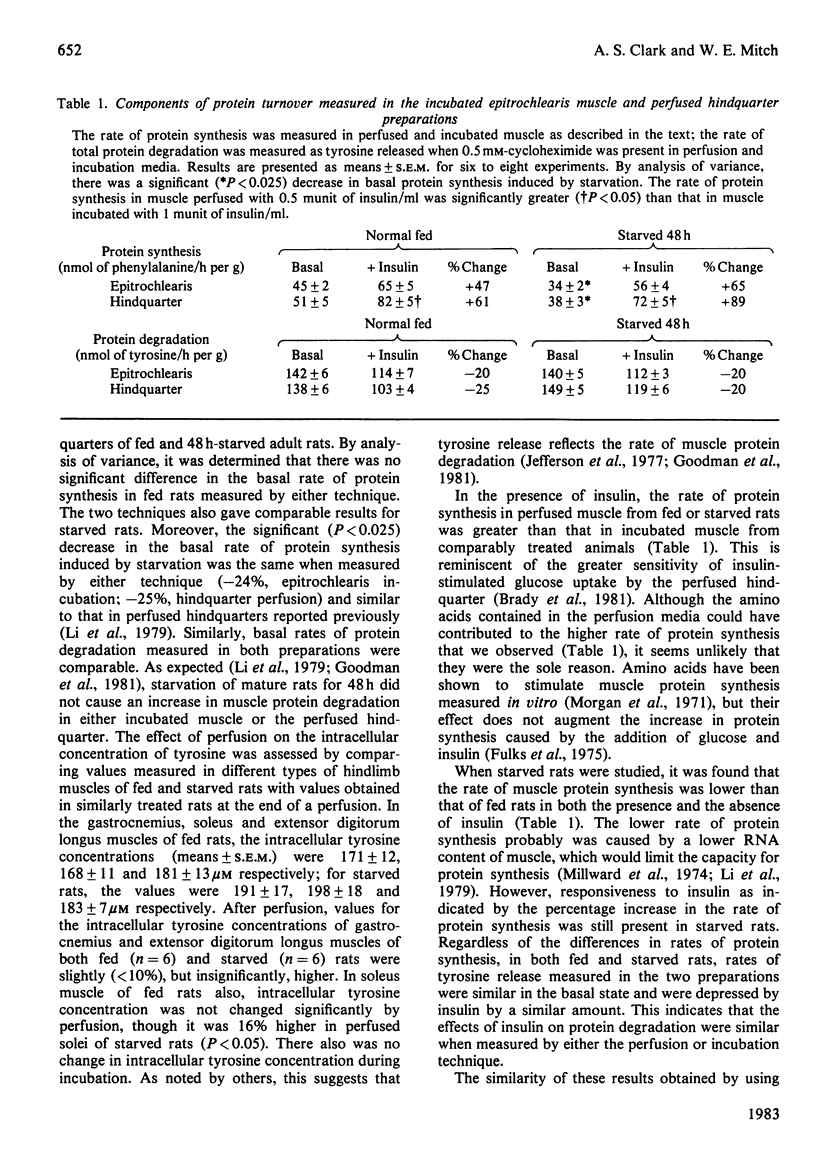

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. M., Goldthorp R., Watts R. W. Fluorimetric measurement of the phenylalanine content of human granulocytes. Clin Chim Acta. 1973 Feb 12;43(3):379–387. doi: 10.1016/0009-8981(73)90477-4. [DOI] [PubMed] [Google Scholar]
- Berger M., Hagg S. A., Goodman M. N., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Effects of starvation, diabetes, fatty acids, acetoacetate, insulin and exercise on glucose uptake and disposition. Biochem J. 1976 Aug 15;158(2):191–202. doi: 10.1042/bj1580191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady L. J., Goodman M. N., Kalish F. N., Ruderman N. B. Insulin binding and sensitivity in rat skeletal muscle: effect of starvation. Am J Physiol. 1981 Feb;240(2):E184–E190. doi: 10.1152/ajpendo.1981.240.2.E184. [DOI] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Goldberg A. L., Martel S. B., Kushmerick M. J. In vitro preparations of the diaphragm and other skeletal muscles. Methods Enzymol. 1975;39:82–94. doi: 10.1016/s0076-6879(75)39012-5. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., McElaney M. A., Ruderman N. B. Adaptation to prolonged starvation in the rat: curtailment of skeletal muscle proteolysis. Am J Physiol. 1981 Oct;241(4):E321–E327. doi: 10.1152/ajpendo.1981.241.4.E321. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S. A technique for perfusion of an isolated preparation of rat hemicorpus. Methods Enzymol. 1975;39:73–82. doi: 10.1016/s0076-6879(75)39011-3. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Li J. B., Fulks R. M., Goldberg A. L. Evidence that the intracellular pool of tyrosine serves as precursor for protein synthesis in muscle. J Biol Chem. 1973 Oct 25;248(20):7272–7275. [PubMed] [Google Scholar]
- Li J. B., Higgins J. E., Jefferson L. S. Changes in protein turnover in skeletal muscle in response to fasting. Am J Physiol. 1979 Mar;236(3):E222–E228. doi: 10.1152/ajpendo.1979.236.3.E222. [DOI] [PubMed] [Google Scholar]
- Millward D. J., Nnanyelugo D. O., James W. P., Garlick P. J. Protein metabolism in skeletal muscle: the effect of feeding and fasting on muscle RNA, free amino acids and plasma insulin concentrations. Br J Nutr. 1974 Jul;32(1):127–142. doi: 10.1079/bjn19740063. [DOI] [PubMed] [Google Scholar]
- Mitch W. E. Amino acid release from the hindquarter and urea appearance in acute uremia. Am J Physiol. 1981 Dec;241(6):E415–E419. doi: 10.1152/ajpendo.1981.241.6.E415. [DOI] [PubMed] [Google Scholar]
- Mitch W. E., Chan W. alpha-Ketoisocaproate stimulates branched-chain amino acid transaminase in kidney and muscle. Am J Physiol. 1979 May;236(5):E514–E518. doi: 10.1152/ajpendo.1979.236.5.E514. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Earl D. C., Broadus A., Wolpert E. B., Giger K. E., Jefferson L. S. Regulation of protein synthesis in heart muscle. I. Effect of amino acid levels on protein synthesis. J Biol Chem. 1971 Apr 10;246(7):2152–2162. [PubMed] [Google Scholar]
- Nesher R., Karl I. E., Kaiser K. E., Kipnis D. M. Epitrochlearis muscle. I. Mechanical performance, energetics, and fiber composition. Am J Physiol. 1980 Dec;239(6):E454–E460. doi: 10.1152/ajpendo.1980.239.6.E454. [DOI] [PubMed] [Google Scholar]
- Preedy V. R., Garlick P. J. Rates of protein synthesis in skin and bone, and their importance in the assessment of protein degradation in the perfused rat hemicorpus. Biochem J. 1981 Jan 15;194(1):373–376. doi: 10.1042/bj1940373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannels D. E., Wartell S. A., Watkins C. A. The measurement of protein synthesis in biological systems. Life Sci. 1982 May 17;30(20):1679–1690. doi: 10.1016/0024-3205(82)90300-9. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Garetto L. P., Goodman M. N., Ruderman N. B. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982 Apr;69(4):785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]


