Abstract
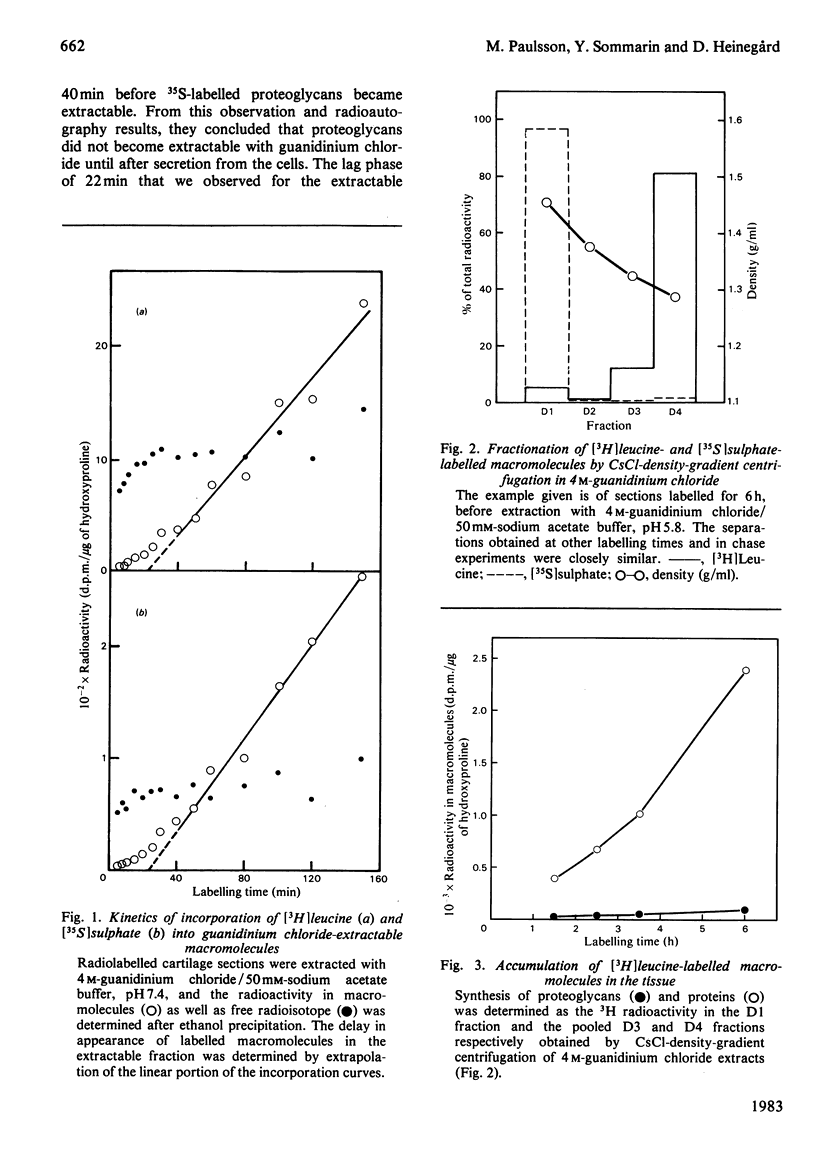
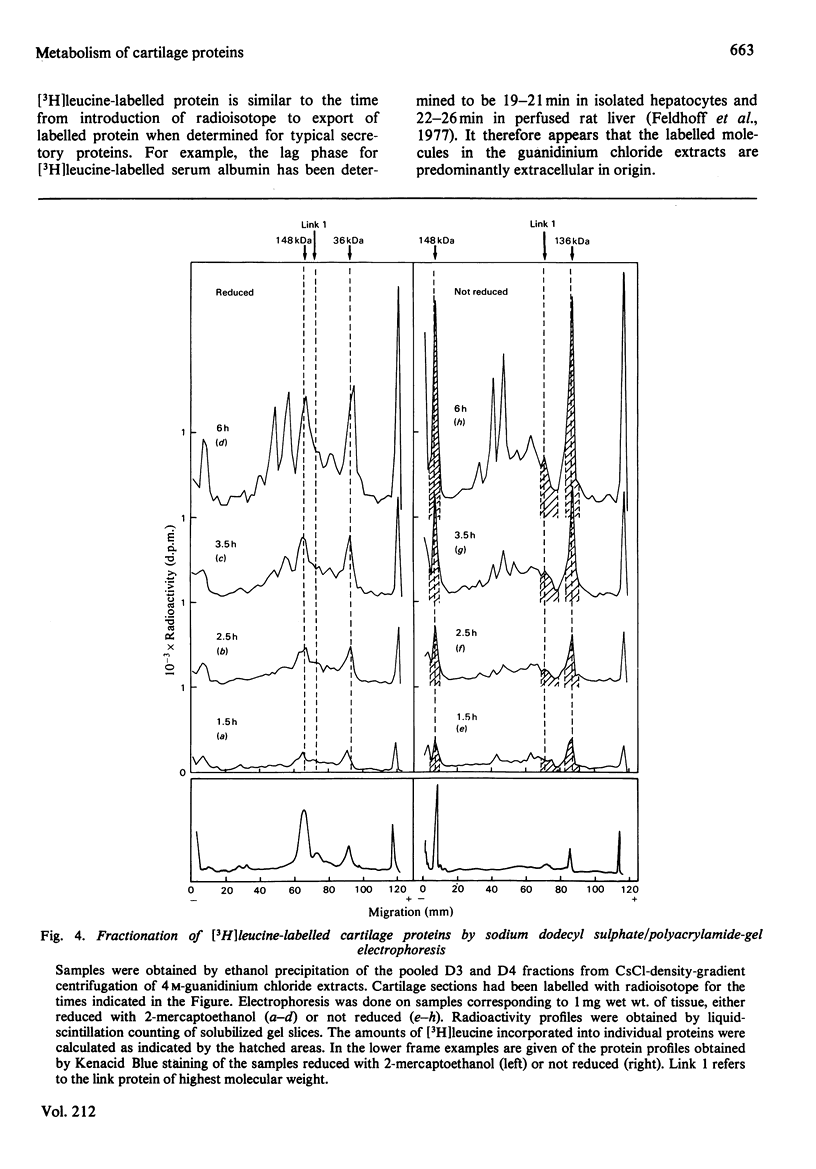
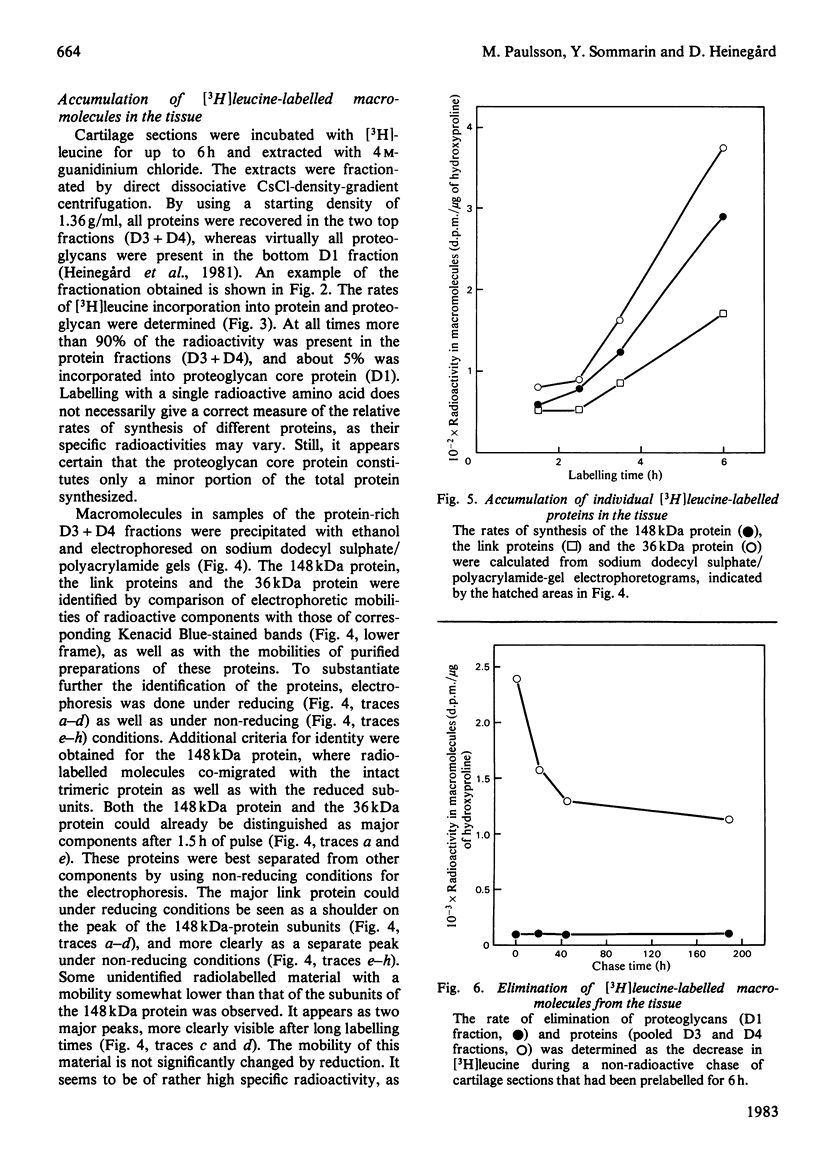
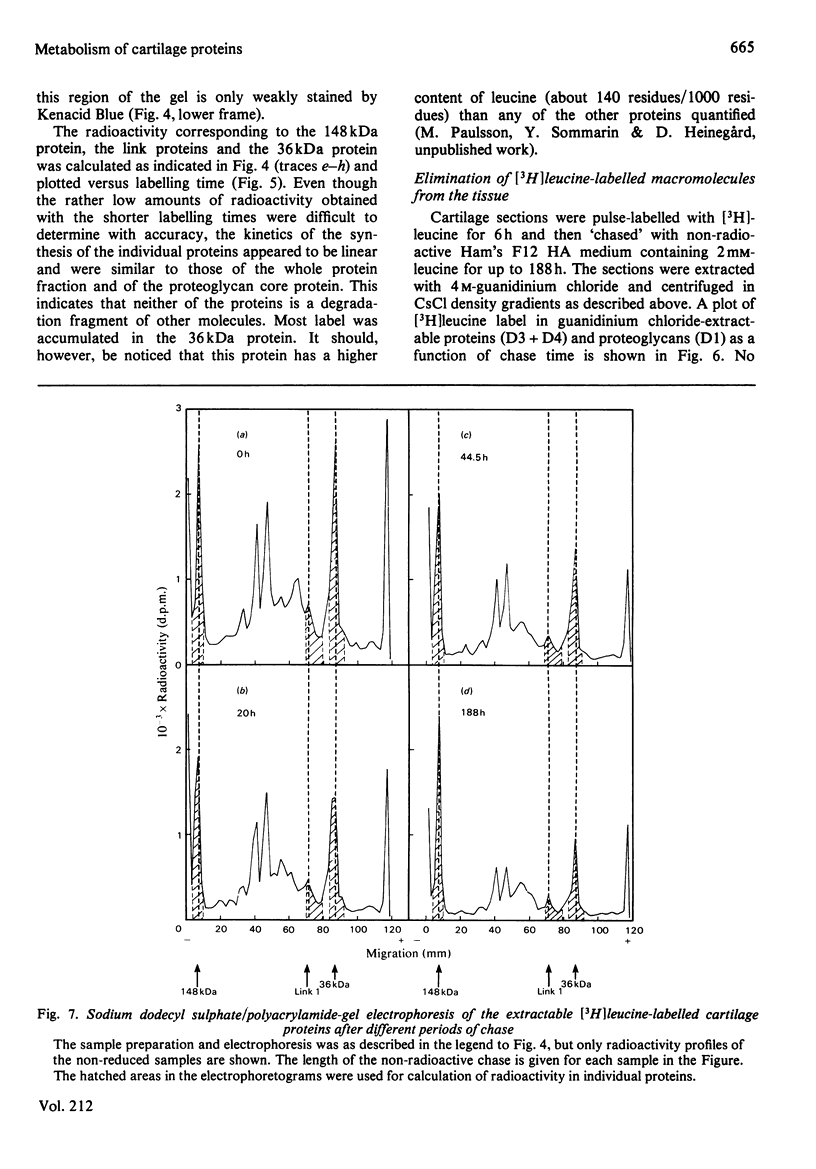
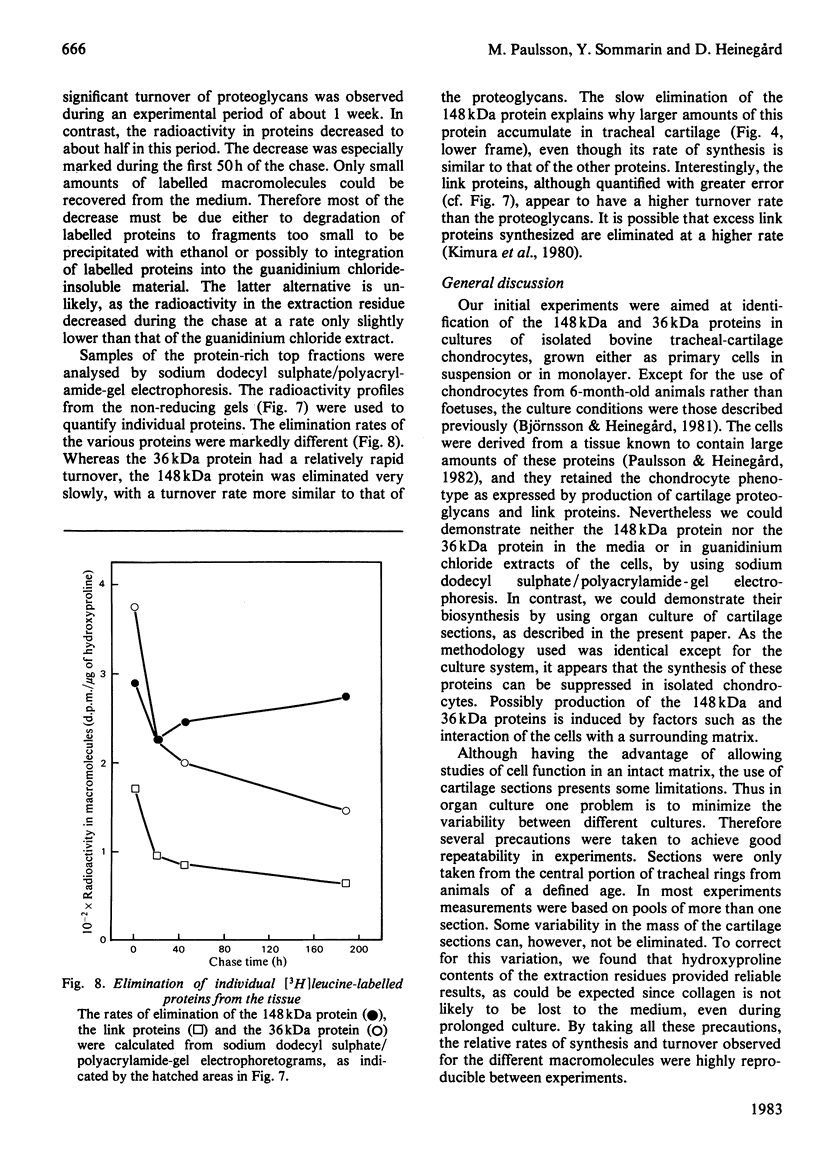
The biosynthesis and turnover of cartilage proteins was studied in organ cultures of bovine tracheal-cartilage sections. In cultures labelled with [3H]leucine, more than 99% of the labelled macromolecules were retained in the sections. About half of the [3H]leucine-labelled protein was extracted with 4M-guanidinium chloride. The incorporation of [3H]leucine into protein extractable with guanidinium chloride was linear with time, after an initial delay of 20-25 min. The 148kDa and 36kDa cartilage proteins were major labelled components in this extract. The elimination of the proteins was studied by using a pulse-chase protocol. The 148kDa protein was found to have a very slow turnover, similar to that of proteoglycans, whereas the 36kDa protein was eliminated more rapidly.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björnsson S., Heinegård D. Isolation and culture techniques of foetal calf chondrocytes. Biochem J. 1981 Jul 15;198(1):141–148. doi: 10.1042/bj1980141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R. Collagen: molecular diversity in the body's protein scaffold. Science. 1980 Mar 21;207(4437):1315–1322. doi: 10.1126/science.7355290. [DOI] [PubMed] [Google Scholar]
- Feldhoff R. C., Taylor J. M., Jefferson L. S. Synthesis and secretion of rat albumin in vivo, in perfused liver, and in isolated hepatocytes. Effects of hypophysectomy and growth hormone treatment. J Biol Chem. 1977 Jun 10;252(11):3611–3616. [PubMed] [Google Scholar]
- Franzén A., Björnsson S., Heinegård D. Cartilage proteoglycan aggregate formation. Role of link protein. Biochem J. 1981 Sep 1;197(3):669–674. doi: 10.1042/bj1970669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Biosynthesis of proteoglycans in cartilage slices. Fractionation by gel chromatography and equilibrium density-gradient centrifugation. Biochem J. 1972 Feb;126(4):791–803. doi: 10.1042/bj1260791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D., Paulsson M., Inerot S., Carlström C. A novel low-molecular weight chondroitin sulphate proteoglycan isolated from cartilage. Biochem J. 1981 Aug 1;197(2):355–366. doi: 10.1042/bj1970355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J. H., Hardingham T. E., Hascall V. C. Assembly of newly synthesized proteoglycan and link protein into aggregates in cultures of chondrosarcoma chondrocytes. J Biol Chem. 1980 Aug 10;255(15):7134–7143. [PubMed] [Google Scholar]
- Lohmander S. Turnover of proteoglycans in guinea pig costal cartilage. Arch Biochem Biophys. 1977 Apr 15;180(1):93–101. doi: 10.1016/0003-9861(77)90012-1. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. The turnover of adult rabbit articular cartilage. J Bone Joint Surg Am. 1969 Dec;51(8):1591–1600. [PubMed] [Google Scholar]
- Maroudas A. Glycosaminoglycan turn-over in articular cartilage. Philos Trans R Soc Lond B Biol Sci. 1975 Jul 17;271(912):293–313. doi: 10.1098/rstb.1975.0054. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Paulsson M., Heinegård D. Matrix proteins bound to associatively prepared proteoglycans from bovine cartilage. Biochem J. 1979 Dec 1;183(3):539–545. doi: 10.1042/bj1830539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Heinegård D. Purification and structural characterization of a cartilage matrix protein. Biochem J. 1981 Aug 1;197(2):367–375. doi: 10.1042/bj1970367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Heinegård D. Radioimmunoassay of the 148-kilodalton cartilage protein. Distribution of the protein among bovine tissues. Biochem J. 1982 Nov 1;207(2):207–213. doi: 10.1042/bj2070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegemann H., Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967 Nov;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]


