Abstract
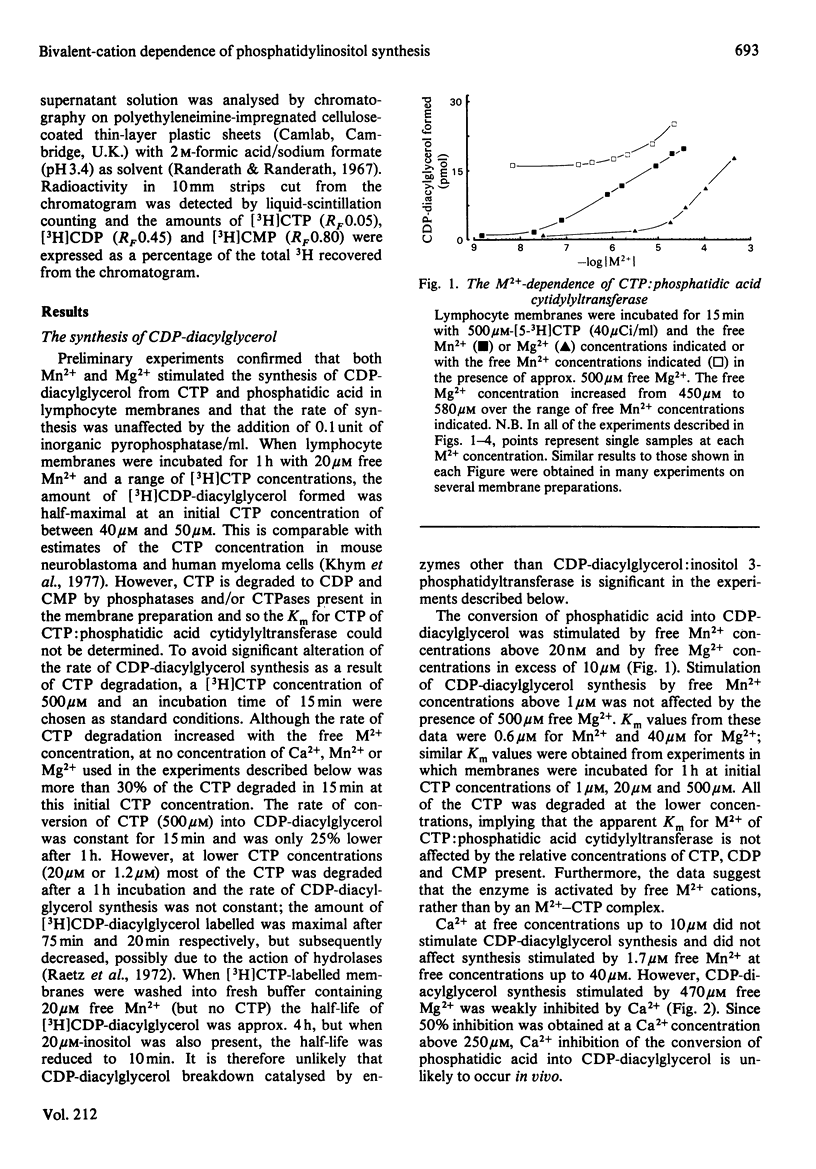
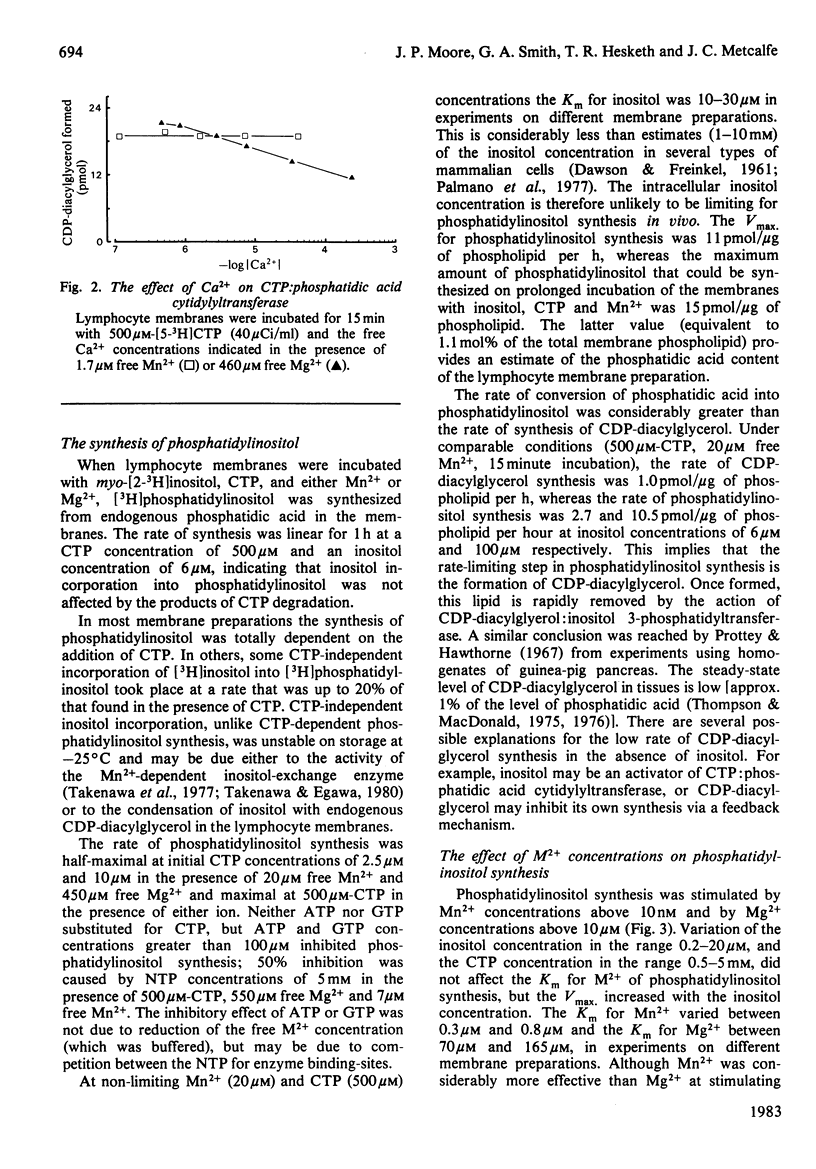
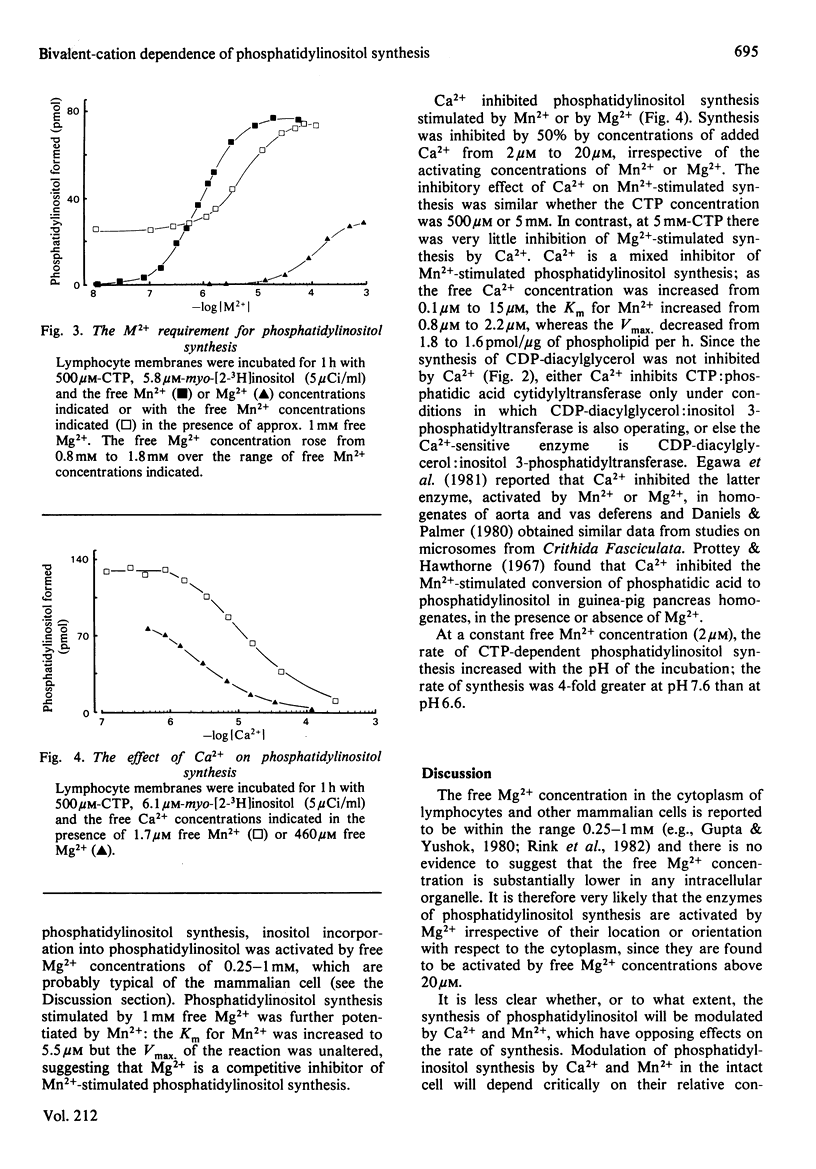
The bivalent-cation requirements of two enzymes involved in phosphatidylinositol synthesis were defined for pig lymphocyte membranes using a citric acid buffer. CTP:phosphatidic acid cytidylyltransferase (EC 2.7.7.41) is activated by free Mn2+ concentrations above 20nM and by free Mg2+ concentrations above 10 microM. When activated by Mg2+, the enzyme is weakly inhibited by Ca2+ (Ki greater than 250 microM), but Ca2+ has no effect when Mn2+ is used to stimulate CDP-diacylglycerol synthesis. The synthesis of phosphatidylinositol from phosphatidic acid is also stimulated by Mn2+ and Mg2+ concentrations similar to those above and is inhibited by free Ca2+ concentrations above 500nM, probably by its action on CDP-diacylglycerol:inositol 3-phosphatidyltransferase (EC 2.7.8.11). Taken together, these studies suggest that under physiological conditions phosphatidylinositol synthesis is activated by Mg2+ and it is possible that it is further regulated by the free concentrations of Ca2+ and/or Mn2+.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Michell R. H. Phosphatidylinositol cleavage in lymphocytes. Requirement for calcium ions at a low concentration and effects of other cations. Biochem J. 1974 Sep;142(3):599–604. doi: 10.1042/bj1420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash D. E., Schramm V. L. Determination of free and bound manganese(II) in hepatocytes from fed and fasted rats. J Biol Chem. 1982 Aug 25;257(16):9261–9264. [PubMed] [Google Scholar]
- Benjamins J. A., Agranoff B. W. Distribution and properties of CDP-diglyceride:inositol transferase from brain. J Neurochem. 1969 Apr;16(4):513–527. doi: 10.1111/j.1471-4159.1969.tb06850.x. [DOI] [PubMed] [Google Scholar]
- Butler M., Morell P. Sidedness of phospholipid synthesis on brain membranes. J Neurochem. 1982 Jul;39(1):155–164. doi: 10.1111/j.1471-4159.1982.tb04714.x. [DOI] [PubMed] [Google Scholar]
- Carter J. R., Kennedy E. P. Enzymatic synthesis of cytidine diphosphate diglyceride. J Lipid Res. 1966 Sep;7(5):678–683. [PubMed] [Google Scholar]
- DAWSON R. M., FREINKEL N. The distribution of free mesoinositol in mammalian tissues, including some observations on the lactating rat. Biochem J. 1961 Mar;78:606–610. doi: 10.1042/bj0780606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. J., Palmer F. B. Biosynthesis of phosphatidylinositol in Crithidia fasciculata. Biochim Biophys Acta. 1980 May 28;618(2):263–281. doi: 10.1016/0005-2760(80)90032-6. [DOI] [PubMed] [Google Scholar]
- Egawa K., Takenawa T., Sacktor B. Inhibition by Ca2+ of the incorporation of myo-inositol into phosphatidylinositol. Mol Cell Endocrinol. 1981 Jan;21(1):29–35. doi: 10.1016/0303-7207(81)90027-7. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Felber S. M., Brand M. D. Factors determining the plasma-membrane potential of lymphocytes. Biochem J. 1982 May 15;204(2):577–585. doi: 10.1042/bj2040577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Yushok W. D. Noninvasive 31P NMR probes of free Mg2+, MgATP, and MgADP in intact Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2487–2491. doi: 10.1073/pnas.77.5.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa K., Irvine R. F., Dawson R. M. Proteolytic activation can produce a phosphatidylinositol phosphodiesterase highly sensitive to Ca2+. Biochem J. 1982 Sep 15;206(3):675–678. doi: 10.1042/bj2060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler K. Y., Zenner B. D., Morris H. P. Increased mitochondrial CTP: phosphatidic acid cytidyltransferase in the 7777 hepatoma. Biochem Biophys Res Commun. 1976 Sep 20;72(2):418–425. doi: 10.1016/s0006-291x(76)80059-9. [DOI] [PubMed] [Google Scholar]
- Khym J. X., Bynum J. W., Volkin E. The co-use of retention time and bandwidth measurements in evaluations of nucleotide pools by ion-exchange chromatography. Anal Biochem. 1977 Feb;77(2):446–463. doi: 10.1016/0003-2697(77)90258-5. [DOI] [PubMed] [Google Scholar]
- Kimura N., Nakane K., Nagata N. Activation by GTP of liver adenylate cyclase in the presence of high concentrations of ATP. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1250–1256. doi: 10.1016/0006-291x(76)91036-6. [DOI] [PubMed] [Google Scholar]
- Lichtman M. A., Jackson A. H., Peck W. A. Lymphocyte monovalent cation metabolism: cell volume, cation content and cation transport. J Cell Physiol. 1972 Dec;80(3):383–396. doi: 10.1002/jcp.1040800309. [DOI] [PubMed] [Google Scholar]
- Liteplo R. G., Sribney M. The stimulation of rat liver microsomal CTP: phosphatidate cytidylyltransferase activity by guanosine triphosphate. Biochim Biophys Acta. 1980 Sep 8;619(3):660–668. doi: 10.1016/0005-2760(80)90115-0. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Is phosphatidylinositol really out of the calcium gate? Nature. 1982 Apr 8;296(5857):492–493. doi: 10.1038/296492a0. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. Early increases in phospholipid methylation are not necessary for the mitogenic stimulation of lymphocytes. J Biol Chem. 1982 Jul 25;257(14):8183–8189. [PubMed] [Google Scholar]
- PAULUS H., KENNEDY E. P. The enzymatic synthesis of inositol monophosphatide. J Biol Chem. 1960 May;235:1303–1311. [PubMed] [Google Scholar]
- Palmano K. P., Whiting P. H., Hawthorne J. N. Free and lipid myo-inositol in tissues from rats with acute and less severe streptozotocin-induced diabetes. Biochem J. 1977 Oct 1;167(1):229–235. doi: 10.1042/bj1670229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prottey C., Hawthorne J. N. The biosynthesis of phosphatidic acid and phosphatidylinositol in mammalian pancreas. Biochem J. 1967 Oct;105(1):379–392. doi: 10.1042/bj1050379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Hirschberg C. B., Dowhan W., Wickner W. T., Kennedy E. P. A membrane-bound pyrophosphatase in Escherichia coli catalyzing the hydrolysis of cytidine diphosphate-diglyceride. J Biol Chem. 1972 Apr 10;247(7):2245–2247. [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Sribney M., Hegadorn C. A. Biosynthesis of CDP-diacylglycerol in hog mesenteric lymph node lymphocytes. Can J Biochem. 1982 Jun;60(6):668–674. doi: 10.1139/o82-082. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Egawa K. CDP-diglyceride:inositol transferase from rat liver. Purification and properties. J Biol Chem. 1977 Aug 10;252(15):5419–5423. [PubMed] [Google Scholar]
- Takenawa T., Egawa K. Phosphatidyl inositol: myo-inositol exchange enzyme from rat liver: partial purification and characterization. Arch Biochem Biophys. 1980 Jul;202(2):601–607. doi: 10.1016/0003-9861(80)90467-1. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Saito M., Nagai Y., Egawa K. Solubilization of the enzyme catalyzing CDP-diglyceride-independent incorporation of myo-inositol into phosphatidyl inositol and its comparison to CDP-diglyceride:inositol transferase. Arch Biochem Biophys. 1977 Jul;182(1):244–250. doi: 10.1016/0003-9861(77)90304-6. [DOI] [PubMed] [Google Scholar]
- Thompson W., MacDonald G. Cytidine diphosphate diglyceride of bovine brain. Positional distribution of fatty acids and analysis of major molecular species. Eur J Biochem. 1976 May 17;65(1):107–111. doi: 10.1111/j.1432-1033.1976.tb10394.x. [DOI] [PubMed] [Google Scholar]
- Thompson W., MacDonald G. Isolation and characterization of cytidine diphosphate diglyceride from beef liver. J Biol Chem. 1975 Sep 10;250(17):6779–6785. [PubMed] [Google Scholar]
- Van Kessel W. S., Hax W. M., Demel R. A., De Gier J. High performance liquid chromatographic separation and direct ultraviolet detection of phospholipids. Biochim Biophys Acta. 1977 Mar 25;486(3):524–530. doi: 10.1016/0005-2760(77)90102-3. [DOI] [PubMed] [Google Scholar]
- Williams R. J. Free manganese (II) and iron (II) cations can act as intracellular cell controls. FEBS Lett. 1982 Apr 5;140(1):3–10. doi: 10.1016/0014-5793(82)80508-5. [DOI] [PubMed] [Google Scholar]


