Abstract
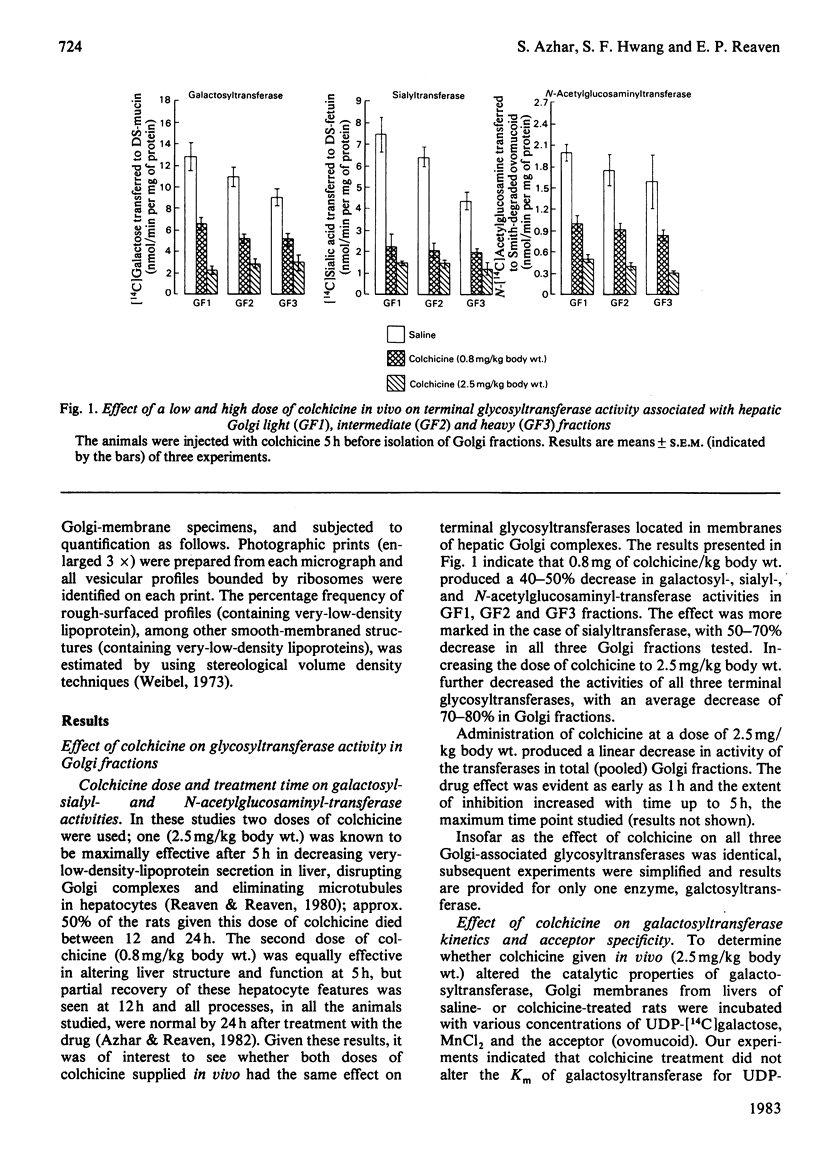
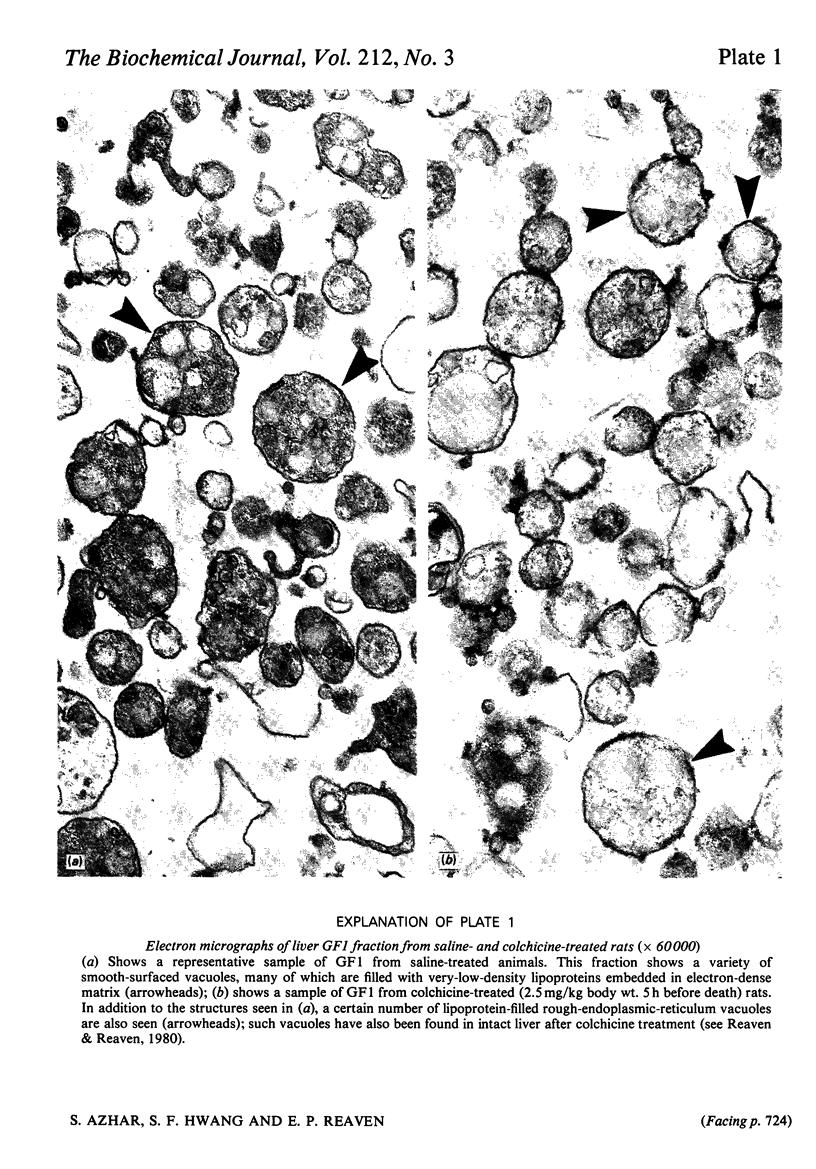
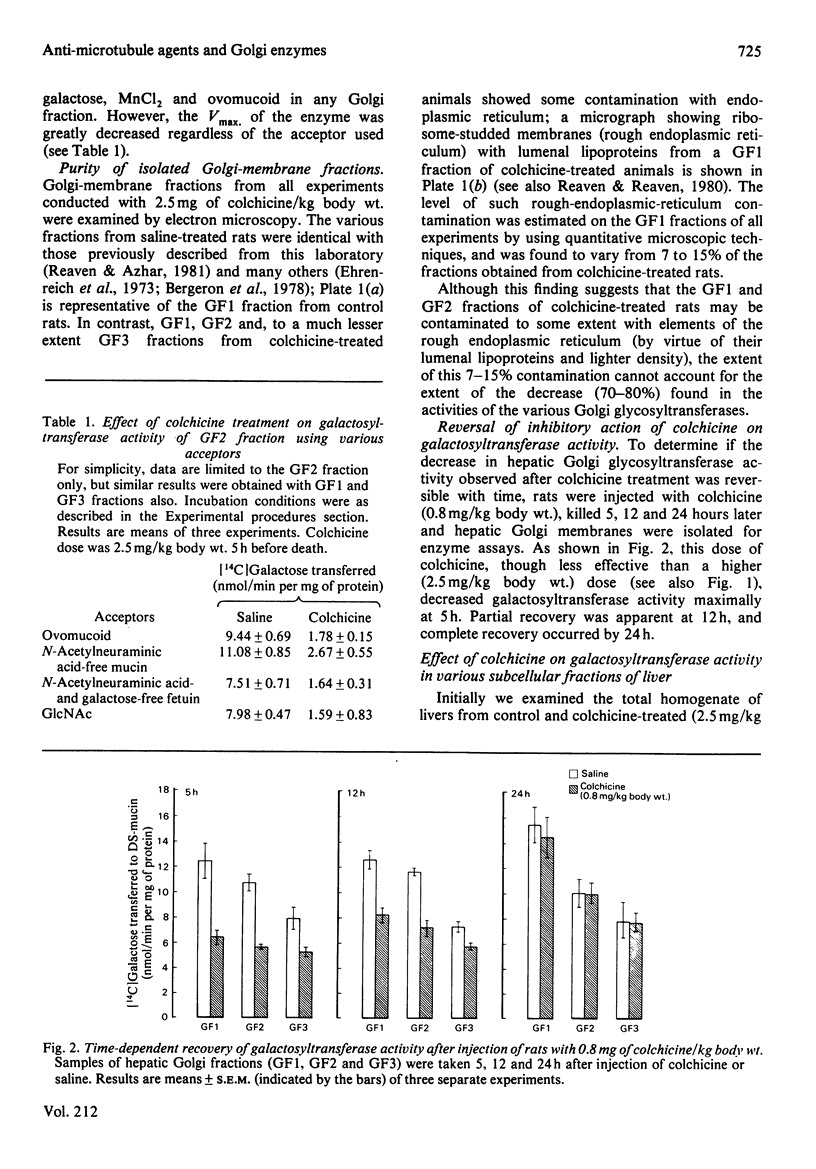
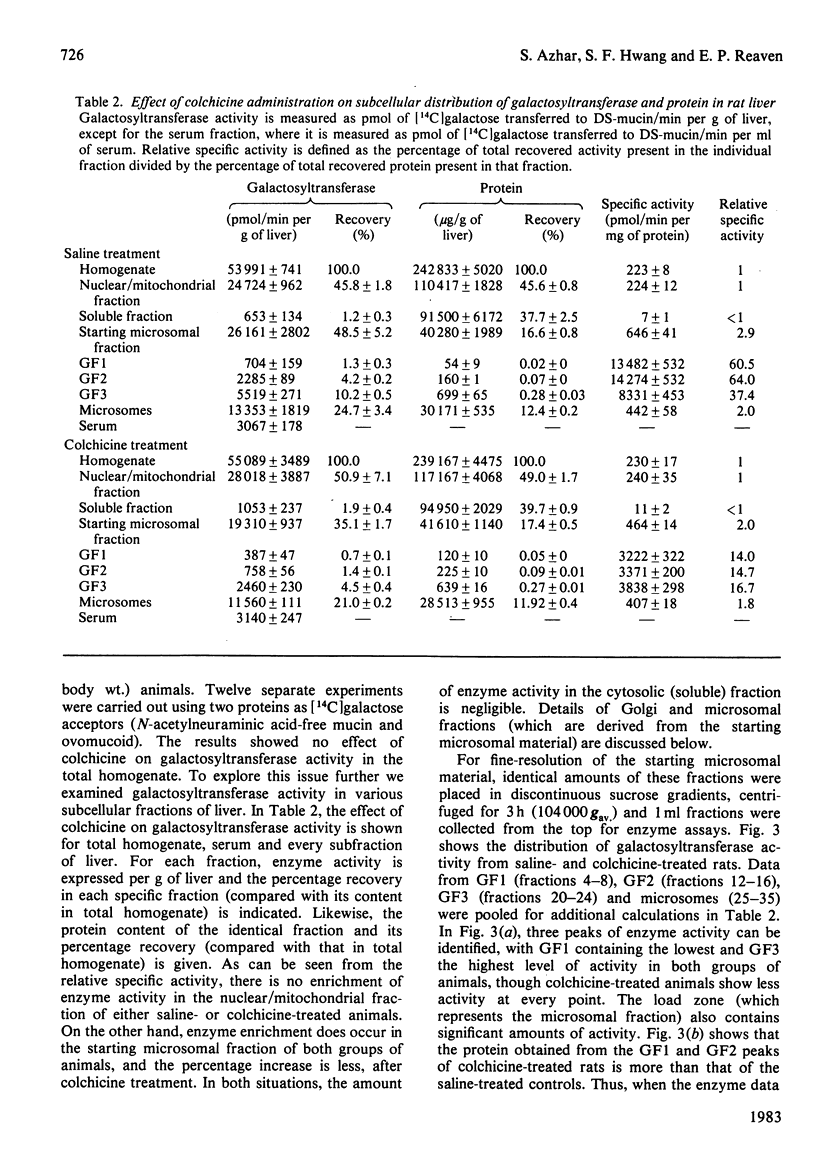
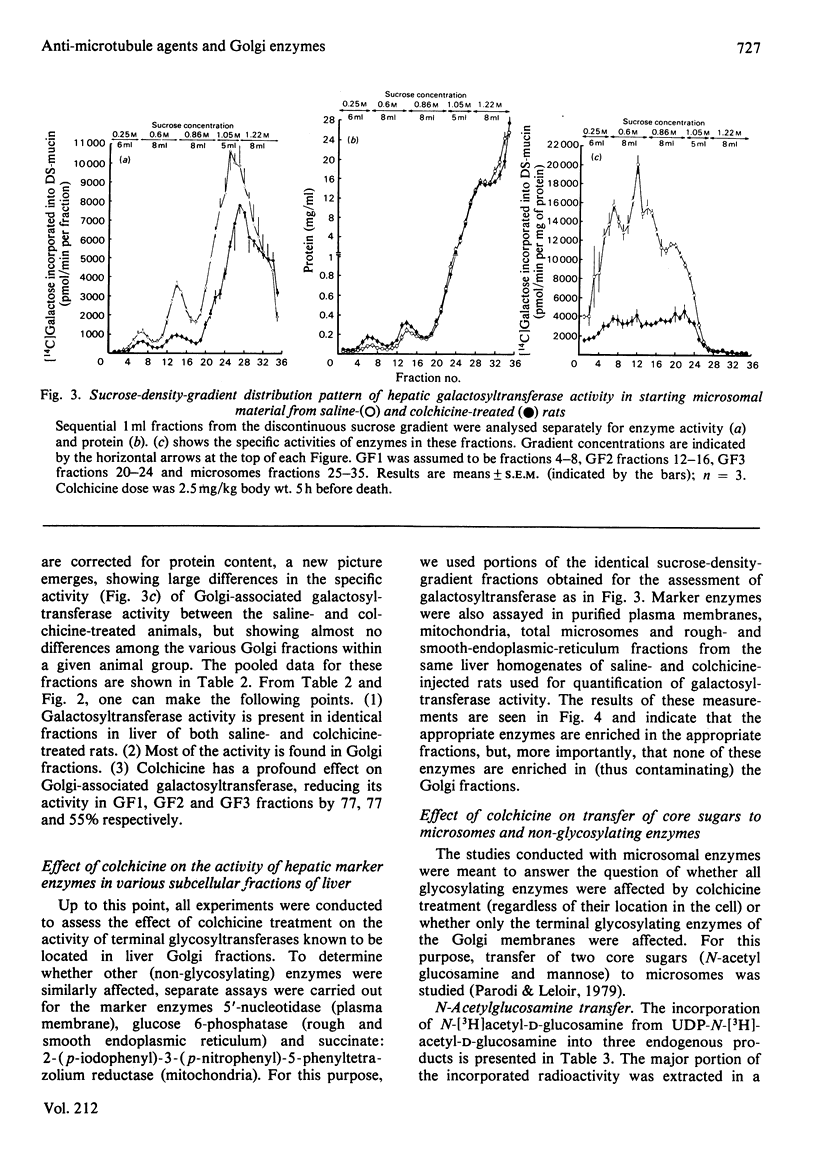
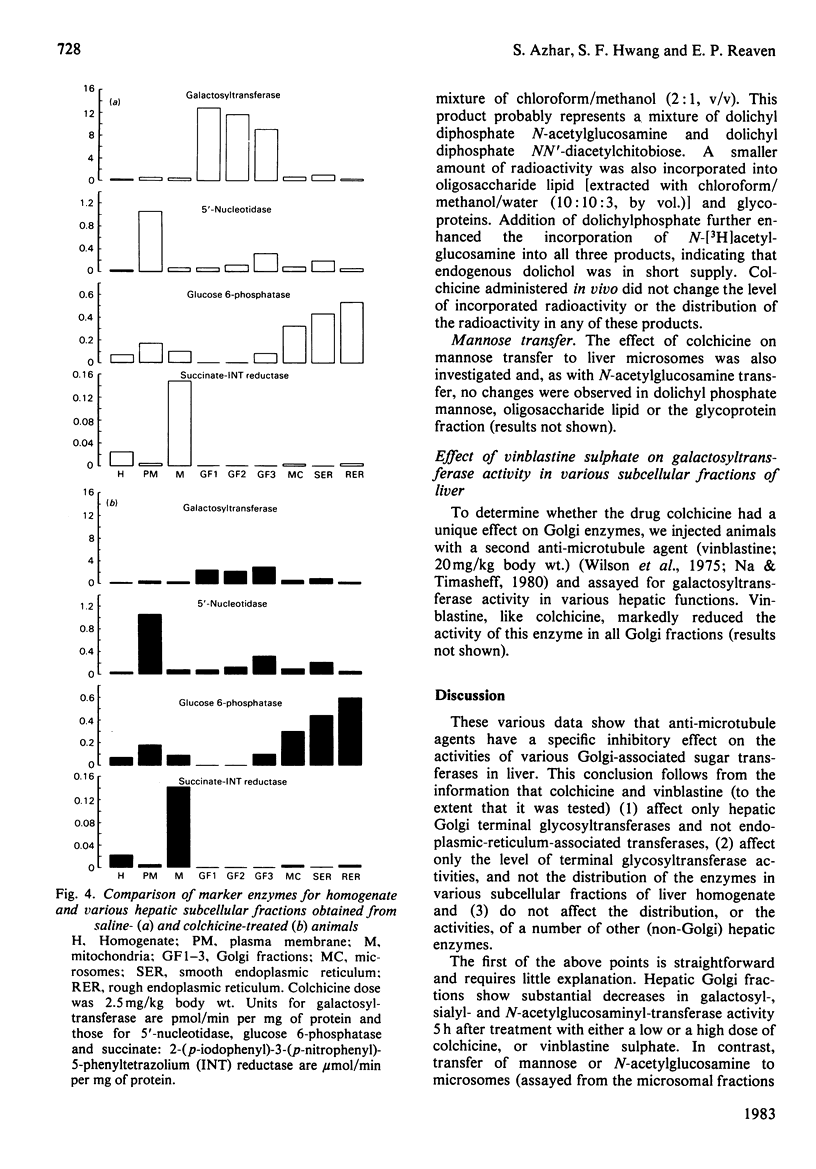
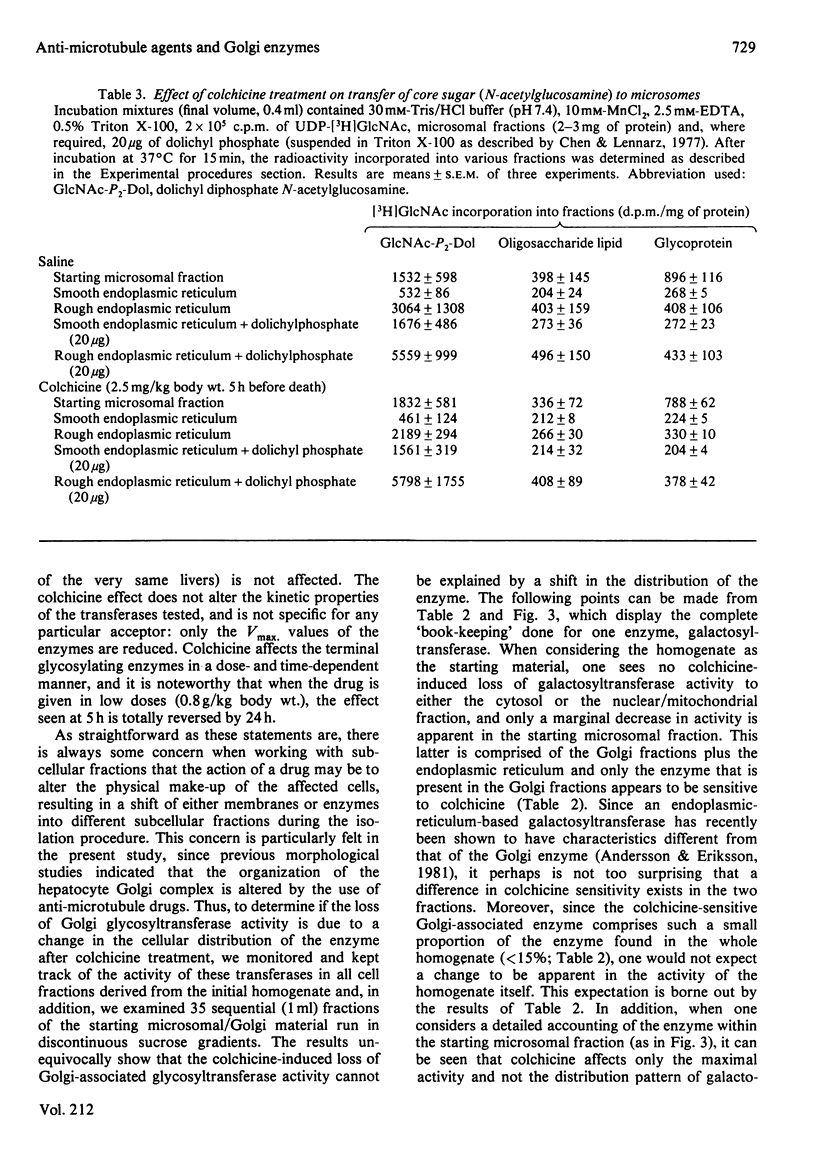
Previous studies have shown that anti microtubule agents disrupt Golgi complexes in hepatocytes and other cells, causing breakdown or vesiculation of Golgi cisternal membranes. Whether this change in the structure of the Golgi membranes is associated with changes in Golgi membrane function is not known. The present study was initiated to investigate this issue; i.e., to determine whether anti-microtubule agents that cause structural changes in Golgi membranes in vivo would, at the same time, affect characteristic enzyme functions of Golgi membranes. To this end, colchicine was given to young rats in vivo and various hepatic subcellular membranes were subsequently isolated and utilized for enzyme assays. Initially it was shown that colchicine (2.5 mg/kg body wt.) given for 5h significantly decreased the activities of the Golgi membrane associated enzymes galactosyl-, sialyl- and N-acetylglucosaminyl-transferases. More detailed experiments indicated that low doses of colchicine (0.8 mg/kg body wt.), although less effective than higher doses, decreased the activities of the terminal glycosylating enzymes maximally at 5h, with partial and complete recovery at 12 and 24h respectively. Treatment in vivo of rats with vinblastine (20 mg/kg body wt.) for 5h mimicked the action of colchicine. Two microsomal glycosylating enzymes (mannosyl and N acetylglucosaminyl transferases) were unaffected by the treatment with colchicine, as were various hepatic 'marker' enzymes such as 5' nucleotidase, glucose 6 phosphatase and succinate: 2-(p iodophenyl)-3-(p nitrophenyl)-5-phenyltetrazolium reductase (succinate dehydrogenase; EC 1.3.99.1), which were found to be enriched in plasma membrane, endoplasmic-reticulum and mitochondrial-membrane fractions respectively. These results show that anti-microtubule agents specifically suppress the activity of Golgi-associated glycosyltransferases in liver. Although it seems likely that these changes are related to the previously observed structural changes in hepatocyte Golgi complexes after colchicine treatment, to what extent the results are linked to the interaction of colchicine with microtubule protein remains to be clarified.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson G. N., Eriksson L. C. Endogenous localization of UDP-galactose:asialomucin galactosyltransferase activity in rat liver endoplasmic reticulum and Golgi apparatus. J Biol Chem. 1981 Sep 25;256(18):9633–9639. [PubMed] [Google Scholar]
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Banerjee D., Manning C. P., Redman C. M. The in vivo effect of colchicine on the addition of galactose and sialic acid to rat hepatic serum glycoproteins. J Biol Chem. 1976 Jul 10;251(13):3887–3892. [PubMed] [Google Scholar]
- Bergeron J. J., Posner B. I., Josefsberg Z., Sikstrom R. Intracellular polypeptide hormone receptors. The demonstration of specific binding sites for insulin and human growth hormone in Golgi fractions isolated from the liver of female rats. J Biol Chem. 1978 Jun 10;253(11):4058–4066. [PubMed] [Google Scholar]
- Bretz R., Bretz H., Palade G. E. Distribution of terminal glycosyltransferases in hepatic Golgi fractions. J Cell Biol. 1980 Jan;84(1):87–101. doi: 10.1083/jcb.84.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz R., Stäubli W. Detergent influence on rat-liver galactosyltransferase activities towards different acceptors. Eur J Biochem. 1977 Jul 1;77(1):181–192. doi: 10.1111/j.1432-1033.1977.tb11656.x. [DOI] [PubMed] [Google Scholar]
- Chambers J., Elbein A. D. Biosynthesis and characterization of lipid-linked sugars and glycoproteins in aorta. J Biol Chem. 1975 Sep 10;250(17):6904–6915. [PubMed] [Google Scholar]
- Chen W. W., Lennarz W. J. Metabolism of lipid-linked N-acetylglucosamine intermediates. J Biol Chem. 1977 May 25;252(10):3473–3479. [PubMed] [Google Scholar]
- Chertow B. S., Williams G. A., Baker G. R., Surbaugh R. D., Hargis G. K. The role of subcellular organelles in hormone secretion. The interactions of calcium, vitamin A, vinblastine, and cytochalasin B in PTH secretion. Exp Cell Res. 1975 Jul;93(2):388–394. doi: 10.1016/0014-4827(75)90464-4. [DOI] [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. I., Cardell R. R., Jr Alterations in the endoplasmic reticulum and Golgi complex of intestinal epithelial cells during fat absorption and after termination of this process: a morphological and morphometric study. Anat Rec. 1977 May;188(1):77–101. doi: 10.1002/ar.1091880109. [DOI] [PubMed] [Google Scholar]
- Heifetz A., Elbein A. D. Solubilization and properties of mannose and N-acetylglucosamine transferases involved in formation of polyprenyl-sugar intermediates. J Biol Chem. 1977 May 10;252(9):3057–3063. [PubMed] [Google Scholar]
- Hindelang-Gertner C., Stoeckel M. E., Porte A., Stutinsky F. Colchicine effects on neurosecretory neurons and other hypothalamic and hypophysial cells, with special reference to changes in the cytoplasmic membranes. Cell Tissue Res. 1976 Jul 20;170(1):17–41. doi: 10.1007/BF00220108. [DOI] [PubMed] [Google Scholar]
- Hoffstein S., Goldstein I. M., Weissmann G. Role of microtubule assembly in lysosomal enzyme secretion from human polymorphonuclear leukocytes. A reevaluation. J Cell Biol. 1977 Apr;73(1):242–256. doi: 10.1083/jcb.73.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Rich A., Potts J. T., Jr Microtubules and the intracellular conversion of proparathyroid hormone to parathyroid hormone. Endocrinology. 1975 Apr;96(4):903–912. doi: 10.1210/endo-96-4-903. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Nordberg J. Glycoprortein biosynthesis in small intestinal mucosa. I. A study of glycosyltransferases in microsomal subfractions. J Biol Chem. 1971 Sep 10;246(17):5466–5476. [PubMed] [Google Scholar]
- Knudson C. M., Stemberger B. H., Patton S. Effects of colchicine on ultrastructure of the lactating mammary cell: membrane involvement and stress on the Golgi apparatus. Cell Tissue Res. 1978 Dec 14;195(1):169–181. doi: 10.1007/BF00233684. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Moskalewski S., Thyberg J., Friberg U. In vitro influence of colchicine on the Golgi complex in A- and B-cells of guinea pig pancreatic islets. J Ultrastruct Res. 1976 Feb;54(2):304–317. doi: 10.1016/s0022-5320(76)80159-1. [DOI] [PubMed] [Google Scholar]
- Moskalewski S., Thyberg J., Lohmander S., Friberg U. Influence of colchicine and vinblastine on the golgi complex and matrix deposition in chondrocyte aggregates. An ultrastructural study. Exp Cell Res. 1975 Oct 15;95(2):440–454. doi: 10.1016/0014-4827(75)90569-8. [DOI] [PubMed] [Google Scholar]
- Na G. C., Timasheff S. N. Stoichiometry of the vinblastine-induced self-association of calf brain tubulin. Biochemistry. 1980 Apr 1;19(7):1347–1354. doi: 10.1021/bi00548a013. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A. J., Leloir L. F. The role of lipid intermediates in the glycosylation of proteins in the eucaryotic cell. Biochim Biophys Acta. 1979 Apr 23;559(1):1–37. doi: 10.1016/0304-4157(79)90006-6. [DOI] [PubMed] [Google Scholar]
- Patzelt C., Brown D., Jeanrenaud B. Inhibitory effect of colchicine on amylase secretion by rat parotid glands. Possible localization in the Golgi area. J Cell Biol. 1977 Jun;73(3):578–593. doi: 10.1083/jcb.73.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisner A. M., Cooke P. Microtubules and the adrenal medulla. Ann N Y Acad Sci. 1975 Jun 30;253:653–669. doi: 10.1111/j.1749-6632.1975.tb19235.x. [DOI] [PubMed] [Google Scholar]
- Reaven E. P., Reaven G. M. Distribution and content of microtubules in relation to the transport of lipid. An ultrastructural quantitative study of the absorptive cell of the small intestine. J Cell Biol. 1977 Nov;75(2 Pt 1):559–572. doi: 10.1083/jcb.75.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven E. P., Reaven G. M. Evidence that microtubules play a permissive role in hepatocyte very low density lipoprotein secretion. J Cell Biol. 1980 Jan;84(1):28–39. doi: 10.1083/jcb.84.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven E., Azhar S. Effect of various hepatic membrane fractions on microtubule assembly-with special emphasis on the role of membrane phospholipids. J Cell Biol. 1981 May;89(2):300–308. doi: 10.1083/jcb.89.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Banerjee D., Howell K., Palade G. E. Colchicine inhibition of plasma protein release from rat hepatocytes. J Cell Biol. 1975 Jul;66(1):42–59. doi: 10.1083/jcb.66.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold J., Bieger W., Kern H. F. Studies on intracellular transport of secretory proteins in the rat exocrine pancreas. II. Inhibition of antimicrotubular agents. Virchows Arch A Pathol Anat Histol. 1975 Nov 28;368(4):309–327. doi: 10.1007/BF00432309. [DOI] [PubMed] [Google Scholar]
- Stein O., Sanger L., Stein Y. Colchicine-induced inhibition of lipoprotein and protein secretion into the serum and lack of interference with secretion of biliary phospholipids and cholesterol by rat liver in vivo. J Cell Biol. 1974 Jul;62(1):90–103. doi: 10.1083/jcb.62.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock C., Launay J. F., Grenier J. F., Bauduin H. Pancreatic acinar cell changes induced by caerulein, vinblastine, deuterium oxide, and cytochalasin B in vitro. Lab Invest. 1978 Feb;38(2):157–164. [PubMed] [Google Scholar]
- Van Obberghen E., Devis G., Somers G., Ravazzola M., Malaisse-Lagae F., Malaisse W. J. Dynamics of insulin release and microtubular-microfilamentous system. IV. Effect of colchicine upon sulphonylurea-induced insulin secretion. Eur J Clin Invest. 1974 Oct;4(5):307–312. doi: 10.1111/j.1365-2362.1974.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Harford J. B. Evidence for the enzymatic transfer of N-acetylglucosamine from UDP--N-acetylglucosamine into dolichol derivative and glycoproteins by calf brain membranes. Arch Biochem Biophys. 1977 May;181(1):185–198. doi: 10.1016/0003-9861(77)90497-0. [DOI] [PubMed] [Google Scholar]
- Williams J. A. In vitro studies on the nature of vinblastine inhibition of thyroid secretion. Endocrinology. 1976 Jun;98(6):1351–1358. doi: 10.1210/endo-98-6-1351. [DOI] [PubMed] [Google Scholar]
- Wilson L., Creswell K. M., Chin D. The mechanism of action of vinblastine. Binding of [acetyl-3H]vinblastine to embryonic chick brain tubulin and tubulin from sea urchin sperm tail outer doublet microtubules. Biochemistry. 1975 Dec 30;14(26):5586–5592. doi: 10.1021/bi00697a008. [DOI] [PubMed] [Google Scholar]