Abstract
The efficacy and safety of venetoclax in newly diagnosed pediatric acute myeloid leukemia (AML) are not well-established as they are in adults. Children newly diagnosed with AML were recommended for induction therapy with venetoclax and chemotherapy or hypomethylating agents (HMAs) as per for the ChiCTR1900027146 trial. Venetoclax was administered at a consistent dose of 200 mg/m2/day for 28 days, with adjustments when used concurrently with azoles. The study measured both the remission rates and the safety assessments of venetoclax. We enrolled 45 newly diagnosed pediatric patients with AML. The complete remission rates were 94.7% in the low/middle-risk group and 80.8% in the high-risk group; MRD-negative rates were 52.6% and 38.5% in the low/middle-risk group and high-risk group, respectively. Venetoclax based combination therapy was well tolerated by the majority of patients. The median duration of venetoclax dosing was 18 days (range 9–28), with hematological toxicity and infection being the most common adverse events. Venetoclax-based induction regimens demonstrated a high response rate and safety profile in newly diagnosed pediatric AML cases. This underscores the significance of venetoclax as a viable treatment option for untreated AML, extending beyond its role as salvage therapy for refractory/relapsed AML.
Subject terms: Targeted therapies, Drug development
Introduction
Leukemia is the most common malignant disease in children, with an incidence rate of approximately 4.23/100000 individuals1. Acute myeloid leukemia (AML) accounts for 25%, and the overall survival (OS) rate for AML hovers around 70%2. Standard induction and consolidation therapy in AML comprise cytarabine and anthracycline. In cases of high risk or recurrence, allogeneic hematopoietic stem cell transplantation (HSCT) assumes a pivotal role in enhancing survival3. In past decades, there were only modest improvements in AML outcomes, with enhancements primarily focused on supportive care and treatment regimens. However, a significant shift occurred during the short span from April 2017 to November 2018, marked by groundbreaking changes in AML treatment. This period witnessed the realization of the potential of small molecule inhibitors and biologic agents4,5. Currently, venetoclax has become a widely employed targeted drug with significant potential in the treatment of AML. This highly selective and potent inhibitor specifically targets the antiapoptotic protein B-cell lymphoma-2 (BCL-2), enabling the reactivation of the apoptotic pathway by binding to BCL-26. Clinical trials have demonstrated the effectiveness of venetoclax in the treatment of acute myeloid leukemia, both as monotherapy and in combination with hypomethylating agents (HMAs)7–9. Venetoclax, in conjunction with HMAs or low-dose cytarabine, has received approval for use as a first-line treatment for older adults with newly diagnosed AML, particularly when intensive regimens are unsuitable. Furthermore, ongoing investigations are exploring its potential in combination with other agents for the treatment of AML and various other hematologic malignancies10. Numerous adult studies have indicated that AML patients exhibit a significant response rate to venetoclax, and the treatment is well tolerated, with a response rate as high as 60–70%8,11–15. An increasing number of clinical trials16–18 have confirmed the favorable tolerability and response rate of venetoclax in pediatric AML patients with refractory/relapsed (R/R) cases. Presently, venetoclax usage in pediatric AML is predominantly in R/R AML, and there is a notable scarcity of reports regarding its utilization in children with untreated AML.
In our hospital, we have employed venetoclax-based combination therapy for children with R/R AML as salvage therapy, showing favorable tolerability and efficacy of venetoclax in pediatric AML. With increasing clinical experience, we are progressively utilizing venetoclax as a first-line therapy for pediatric AML at the time of the initial diagnosis. This approach to ensure that more children can access the benefit of the treatment as early as possible. In adult or pediatric AML, the combination of low-dose cytarabine or HMAs with venetoclax is a well-established and classic approach to AML treatment11–18. Homoharringtonine has been established as a standard and effective therapy for adult AML in China19. Through our multicenter study, specifically the Chinese Childhood Leukemia Group (CCLG)-AML 2015 Protocol Study, we have observed that the homoharringtonine-based induction regimen can improve the remission and survival rate in Chinese childhood AML patients20. Moreover, researchers have demonstrated a synergistic effect when combining BCL-2 inhibitors and homoharringtonine in AML cell lines and xenograft AML models21,22. As a result, we incorporated homoharringtonine into the venetoclax-based induction regimen, aiming to optimize remission rates for pediatric AML patients.
In this study, we summarized the remission rate and adverse effects associated with venetoclax-based induction regimens in newly diagnosed pediatric AML patients. Additionally, we closely monitored the blood concentration of venetoclax to assess its pharmacokinetics.
Methods
Study design and participants
Patients with newly diagnosed AML who were younger than 18 years and had not previously received any chemotherapy regimen were enrolled in this study, and induced by venetoclax-based induction regimens. Each patient’s AML diagnosis was confirmed by bone marrow examination of Morphology-Immunology-Cytogenetics-Molecular Biology (MICM) examinations. We tested 248 genetic mutations and 40 fusion genes common in myeloid blood disorders by polymerase chain reaction (PCR) and performed single-cell ribonucleic acid sequencing to clarify the diagnosis and for risk stratification.
All patients were enrolled in the CCLG-AML 2019 protocol multicenter study, duly registered with the Chinese Clinical Trial Registry (ChiCTR1900027146). The multicenter clinical study was approved on November 1, 2019. This protocol indicated the potential use of targeted drugs for patients with specific indications. Since venetoclax became available in China in 2021, we incorporated venetoclax into out treatment protocol on December 1, 2021. At the outset of the study, venetoclax was recommended for initial treatment only for patients who either displayed intolerance to intensive chemotherapy due to infection or organ function impairment, or exhibited high-risk factors associated with AML. High-risk factors included myeloid sarcoma, secondary AML (sAML), which was defined as AML induced by chemotherapy or radiotherapy, or AML transformed from myelodysplastic syndrome, and adverse genetic and molecular abnormalities known to be linked with poor prognosis, including Chromosome 5/7 monomer, 5q-, 7q-, 12p/t(2; 12)/ETV6-HOXD, MLL rearrangement except t(9; 11), t(6; 9)/DEK-NUP214 or DEK-CAN, t(7; 12)/HLXB9-ETV6, t(9; 22)/BCR-ABL1, t(16; 21)/TLS-ERG or FUS-ERG, complex karyotype (three or more genetic abnormalities, but except good karyotypes), C-KIT mutation (except CBF-AML), FLT3 mutation, RUNX1 mutation, TP53 mutation and NUP98 rearrangement, et al. As the study progressed and the favorable induction effects of venetoclax became apparent, the treatment approach was expanded to include all pediatric AML patients at their initial diagnosis. This expansion encompassed those without high-risk factors and those who could tolerate traditional intensive chemotherapy, ensuring that more children could benefit from this promising treatment. The data cutoff date for this investigation was September 30, 2023. This study primarily sought to assess the efficacy and safety of venetoclax in combination therapy for 45 pediatric patients with previously untreated AML from two participating hospitals, namely Beijing Children’s Hospital and Beijing Children’s Hospital-Baoding Hospital.
This study was approved by Medical Ethics Committee of Beijing Children’s Hospital, Capital Medical University (2019k-343). Informed consent was obtained from all patients or legal guardians in accordance with the principles of the Declaration of Helsinki.
Procedures
After the diagnosis of AML, the children received oral venetoclax daily by dose escalation to avoid tumor lysis syndrome (TLS): day 1 at 50 mg/m2/day, day 2 at 100 mg/m2/day, and days 3–28 with 200 mg/m2/day (maximum 300 mg). The combination regimens included venetoclax combined with low-dose cytarabine with or without homoharringtonine, denoted as V + LDAC (cytarabine, Days 1–10 at 10 mg/m2/day) or V + HA (cytarabine, Days 1–7 at 100 mg/m2/dose every 12 h; homoharringtonine, Days 1–7 at 3 mg/m2/day). Additionally, venetoclax was employed in combination with HMAs, including azacitidine (Days 1–7 at 75 mg/m2/day) or decitabine (Days 1–5 at 20 mg/m2/day), identified as V + AZA or V + DEC, respectively. Physicians exercised discretion in selecting the most appropriate regimen for their patients, guided by the individual indications for venetoclax application. For AML patients unable to tolerate intensive chemotherapy, V + LDAC or V + HA regimens were typically preferred. High-risk AML cases were predominantly treated with a combination of venetoclax and HMAs. As homoharringtonine is a classic drug for AML in China, and the combination of homoharringtonine and venetoclax has been shown to be effective, V + HA became the primary choice for all enrolled patients in the later stages of the study. Based on the response to venetoclax therapy, patients underwent one or more cycles of venetoclax combination therapy or continued treatment with intensive chemotherapy or transplantation, as deemed suitable.
Venetoclax is known to be a substrate of CYP3A, and its in vivo exposure can be influenced when co-administered with CYP3A inhibitors or inducers, consequently impacting both its efficacy and safety23. In this study, the antifungal drugs commonly used were azoles, which are CYP3A inhibitors; Therefore, the dose of venetoclax was adjusted when it was administered alongside with azoles. Among these azoles, voriconazole is a potent CYP3A inhibitor, necessitating a reduction of the daily venetoclax dose to 25% of the original regimen when used in combination. For fluconazole, an intermediate CYP3A inhibitor, the stable daily dose of venetoclax was reduced to 50% of the original regimen. Following a 2–3 day cessation of CYP3A inhibitors, the initial venetoclax dose was restored24.
Assessment
The primary outcome measures were the remission rate to venetoclax-based induction regimens. The response was determined by bone marrow evaluation on day 28. Complete remission (CR) was defined as bone marrow blasts <5%, CRi was defined as CR with incomplete hematologic recovery, partial remission (PR) was defined as bone marrow blasts 5–20%, and non-remission (NR) was defined as bone marrow blasts >20%. Measurable residual disease (MRD) in bone marrow was assessed using flow cytometry at a sensitivity threshold of 10−3 to 10−4 per standard of care. A negative status for MRD was defined as less than 0.1%. Overall response was calculated as the sum of CR and MRD negativity. Additionally, on the fifth day of the venetoclax at full dose, pharmacokinetic samples were collected, with measurements taken at 30 min predose to determine the venetoclax trough concentration, and the peak drug concentration was assessed at 6 h postdose24.
The secondary outcome measures in this study primarily focused on safety assessments. These safety analyzes encompassed all patients who received ≥1 dose of venetoclax. To evaluate safety, toxicity grade and its relationship to venetoclax were assessed in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03. The safety assessment included the numbers of patients with different grades of adverse events (AEs) and changes from baseline in clinical laboratory parameters. AEs were defined as events occurring on or after the day of the first dose of the study drug. Events that manifested ≥5 days after the final dose of the study drug were excluded from this analysis. Bone marrow suppression (BMS) was defined as a reduction in two or three of the lineages of white blood cells, hemoglobin, and platelets. Given the uncertainty about whether the bone marrow was in remission during the first course of induction therapy, it was challenging to definitively attribute peripheral cytopenia to either the effects of venetoclax inhibition or the tumor’s impact on bone marrow hematopoiesis. Consequently, a precise determination of the onset and duration of BMS was not feasible within the study.
Statistical Analysis
The study population was stratified based on varying levels of morphologic remission and immunologic residual status on day 28 of venetoclax treatment. Demographic and clinical characteristics of the patients were presented as the medians (ranges) or percentages, as appropriate. Differences between groups were evaluated using the Mann–Whitney U test for continuous variables and the chi-square test for categorical variables. Statistical analyzes were performed with SPSS 26.0 (IBM, Chicago, IL, USA). All tests were two-sided, and a p value of <0.05 was utilized to determine statistically significant.
Results
Patient characteristics
Between December 1, 2021, and September 30, 2023, a total of 45 untreated AML patients underwent enrollment for venetoclax-based induction treatment, including 27 boys and 18 girls. The median time of follow-up time was 6 month (range 1–21). Among them, 19 were diagnosed with low- or middle-risk AML, while the remaining 26 were diagnosed with high-risk AML at their initial diagnosis. The baseline characteristics of the patients grouped by the different levels of morphologic remission status and immunologic residual status on day 28 of venetoclax are presented in Table 1. The median age at the time of their initial diagnosis was 9.3 years (range 0.9–15.8). Of the 45 enrolled patients, 11.1% (n = 5) had white blood cell counts >100 × 109/L at the initial diagnosis, 6.7% (n = 3) had myeloid sarcoma, 4.4% (n = 2) had central nervous system leukemia, and none of the patients had testicular leukemia. The most common morphological type was M2, accounting for 40.0% (n = 18) of the cases. In terms of genetics, FLT3 mutations were the most common (n = 12, 26.7%) with 6 with FLT3-ITD mutations, and the other 6 with FLT3-TKD mutations, followed by AML1-ETO, MLL rearrangement and WT1 mutations, which accounted for 22.2% (n = 10), 20% (n = 9) and 17.8% (n = 8) of cases, respectively. However, in this study, FLT3 inhibitors were not used due to the unavailability of mutation status information during the initial two weeks of the induction phase and the occurrence of myelosuppression in the subsequent two weeks. Complex karyotypes and -7/7q- chromosomal abnormalities, associated with poor prognosis, were found in 8.9% (n = 4) and 2.2% (n = 1) of cases, respectively. The most common treatment approach was a combination therapy of V + HA, which constituted 60.0% (n = 27). The median duration of venetoclax dosing was 18 days (range 9–28).
Table 1.
Baseline characteristics grouped by bone marrow remission levels on day 28 of venetoclax
| Variables | Total (n = 45) | CR/Cri (n = 39) | PR/NR (n = 6) | P1 | MRD(−) (n = 20) | MRD(+) (n = 25) | P2 |
|---|---|---|---|---|---|---|---|
| Age (years), median (range) | 9.3 (0.9–15.8) | 9.9 (0.9–15.8) | 3.2 (0.9–14.7) | 0.129 | 9.6 (0.9–14.6) | 9.3 (0.9–15.8) | 0.681 |
| Sex, n (%) | 0.929 | 0.540 | |||||
| male | 27 (60.0) | 24 (61.5) | 3 (50.0) | 11 (55.0) | 16 (64.0) | ||
| female | 18 (40.0) | 15 (38.5) | 3 (50.0) | 9 (45.0) | 9 (36.0) | ||
| WBC count, n (%) | 0.529 | 0.791 | |||||
| <100×109/L | 40 (88.9) | 35 (89.7) | 5 (83.3) | 17 (85.0) | 23 (92.0) | ||
| >100×109/L | 5 (11.1) | 4 (10.3) | 1 (16.7) | 3 (15.0) | 2 (8.0) | ||
| Risk stratification, n (%) | 0.359 | 0.345 | |||||
| low/middle | 19 (42.2) | 18 (46.2) | 1 (16.7) | 10 (50.0) | 9 (36.0) | ||
| high | 26 (57.8) | 21 (53.8) | 5 (83.3) | 10 (50.0) | 16 (64.0) | ||
| sAML, n (%) | 7 (15.6) | 4 (10.3) | 3 (50.0) | 0.039 | 3 (15.0) | 4 (16.0) | 1.000 |
| Myeloid sarcoma, n (%) | 3 (6.7) | 3 (7.7) | 0 (0.0) | 1.000 | 1 (5.0) | 2 (8.0) | 1.000 |
| CNS leukemia, n (%) | 2 (4.4) | 1 (2.6) | 1 (16.7) | 0.252 | 1 (5.0) | 1 (4.0) | 1.000 |
| Testicular leukemia, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 0 (0.0) | - |
| Morphological typing, n (%) | 0.004 | 0.453 | |||||
| M2 | 18 (40.0) | 18 (46.2) | 0 (0.0) | 8 (40.0) | 10 (40.0) | ||
| M2/M4 | 3 (6.7) | 3 (7.7) | 0 (0.0) | 2 (10.0) | 1 (4.0) | ||
| M4 | 6 (13.3) | 6 (15.4) | 0 (0.0) | 4 (20.0) | 2 (8.0) | ||
| M5 | 5 (11.1) | 5 (12.8) | 0 (0.0) | 3 (15.0) | 2 (8.0) | ||
| M7 | 4 (8.9) | 2 (5.1) | 2 (33.3) | 1 (5.0) | 3 (12.0) | ||
| Not available | 9 (20.0) | 5 (12.8) | 4 (66.7) | 2 (10.0) | 7 (28.0) | ||
| Genetics, n (%) | |||||||
| FLT3 mutation(+) | 12 (26.7) | 11 (28.2) | 1 (16.7) | 0.921 | 6 (30.0) | 6 (24.0) | 0.651 |
| AML1-ETO(+) | 10 (22.2) | 10 (25.6) | 0 (0.0) | 0.379 | 3 (15.0) | 7 (28.0) | 0.496 |
| WT1(+) | 8 (17.8) | 7 (17.9) | 1 (16.7) | 1.000 | 5 (25.0) | 3 (12.0) | 0.459 |
| MLL rearrangement(+) (except for MLL-AF9) | 7 (15.6) | 5 (12.8) | 2 (33.3) | 0.230 | 3 (15.0) | 4 (16.0) | 1.000 |
| NRAS(+) | 5 (11.1) | 5 (12.8) | 0 (0.0) | 1.000 | 2 (10.0) | 3 (12.0) | 1.000 |
| C-KIT(+) | 5 (11.1) | 5 (12.8) | 0 (0.0) | 1.000 | 3 (15.0) | 2 (8.0) | 0.791 |
| RUNX1(+) | 4 (8.9) | 4 (10.3) | 0 (0.0) | 1.000 | 1 (5.0) | 3 (12.0) | 0.770 |
| CEBPA double mutation(+) | 4 (8.9) | 4 (10.3) | 0 (0.0) | 1.000 | 2 (10.0) | 2 (8.0) | 1.000 |
| KRAS(+) | 3 (6.7) | 2 (5.1) | 1 (16.7) | 0.356 | 1 (5.0) | 2 (8.0) | 1.000 |
| MLL-AF9(+) | 2 (4.4) | 1 (2.6) | 1 (16.7) | 0.252 | 1 (5.0) | 1 (4.0) | 1.000 |
| NUP98 rearrangement(+) | 2 (4.4) | 2 (5.1) | 0 (0.0) | 1.000 | 2 (10.0) | 0 (0.0) | 0.192 |
| TP53(+) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 0 (0.0) | - |
| Chromosomal abnormalities, n (%) | |||||||
| Complex karyotype | 4 (8.9) | 2 (5.1) | 2 (33.3) | 0.08 | 0 (0.0) | 4 (16.0) | 0.178 |
| -7/7q- | 1 (2.2) | 1 (2.6) | 0 (0.0) | 1.000 | 0 (0.0) | 1 (4.0) | 1.000 |
| Therapy protocols, n (%) | 0.309 | 0.641 | |||||
| VEN + LDAC | 6 (13.3) | 5 (12.8) | 1 (16.7) | 3 (15.0) | 3 (12.0) | ||
| VEN + HA | 27 (60.0) | 25 (64.1) | 2 (33.3) | 10 (50.0) | 17 (68.0) | ||
| VEN + AZA | 10 (22.2) | 8 (20.5) | 2 (33.3) | 6 (30.0) | 4 (16.0) | ||
| VEN + DEC | 2 (4.4) | 1 (2.6) | 1 (16.7) | 1 (5.0) | 1 (4.0) | ||
| Duration of VEN (day), median (range) | 18 (9–28) | 19 (9–28) | 14 (10–28) | 0.338 | 19 (10–28) | 18 (9–28) | 0.725 |
1grouping is based on the different levels of morphologic remission status on day 28 of venetoclax; 2grouping is based on the different levels of immunologic residual status on day 28 of venetoclax; quantitative variables are presented as medians (ranges); qualitative variables are presented as numbers (percentages).
CR complete remission, CRi CR with incomplete hematologic recovery, NR nonremission, PR partial remission, MRD measurable residual disease, VEN venetoclax, LDAC low-dose cytarabine, AZA/DEC azacitidine/decitabine, HA homoharringtonine plus cytarabine, WBC white blood cell, sAML secondary AML, CNS central nervous system.
In general, the baseline characteristics were well matched across the patient groups. However, it is noteworthy that patients who did not achieve CR after venetoclax-based induction therapy had a higher proportion of sAML (P = 0.039) and a higher proportion of M7 morphological typing or unavailable morphological typing (P = 0.004).
Efficacy of venetoclax in each treatment cycle
Of the 45 patients enrolled, 60% (n = 27) received V + HA combination therapy, 26.7% (n = 12) received V + AZA or V + DEC combination therapy, and 13.3% (n = 6) received V + LDAC combination therapy. The induction efficacy of venetoclax combination therapy in all patients is shown in Fig. 1, illustrating the distribution of patients with varying levels of morphologic remission status (Fig. 1A) and immunologic residual status (Fig. 1B) on day 28 in different treatment groups. The overall CR rate and MRD-negative rate were 86.7% (n = 39) and 44.4% (n = 20), respectively, on day 28 of venetoclax application. In the low/middle-risk group, the CR rate and MRD-negative rate were 94.7% (n = 18) and 52.6% (n = 10), respectively, on day 28 of venetoclax application, while in the high-risk group, these figures were 80.8% (n = 21) and 38.5% (n = 10), respectively. The difference in the CR rate was not statistically significant between groups with different levels of risk, and neither was the MRD-negative rate on day 28 of medication (P = 0.359 and 0.345, Table 1). The CR rates in the V+HMAs, V + LDAC and V + HA therapy groups were 75.0% (n = 9), 83.3% (n = 5) and 92.6% (n = 25), respectively, on day 28 of medication. The MRD-negative rates in the V+HMAs, V + LDAC and V + HA therapy groups were 58.3% (n = 7), 50.0% (n = 3) and 37.0% (n = 10), respectively, on day 28 of venetoclax. The differences in the CR rate and MRD-negative rate were not statistically significant between groups with different combination therapies (P = 0.309 and 0.641, Table 1).
Fig. 1. The remission status for all patients.
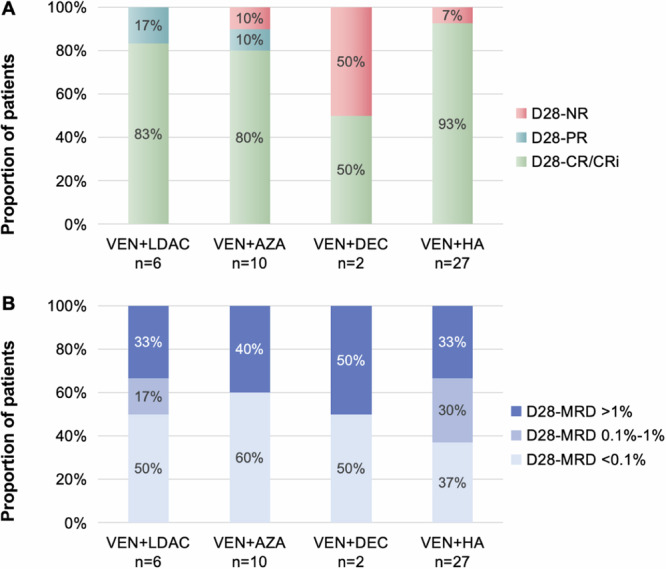
Proportion of patients with different levels of morphologic remission status (A) and immunologic residual status (B) on day 28 in different treatment groups. Note: D28: Day 28; NR: nonremission; PR: partial remission; CR: complete remission; CRi: CR with incomplete hematologic recovery; MRD: measurable residual disease; VEN: venetoclax; LDAC: low-dose cytarabine; AZA: azacitidine; DEC: decitabine; HA: homoharringtonine plus cytarabine.
Safety of venetoclax in each treatment cycle
Overall, the venetoclax-based combination therapy was well tolerated by the majority patients. Out of the total cohort, 35.6% (n = 16) of patients received venetoclax for a full 28-day cycle, while the others had to discontinue venetoclax due to drug-related hematotoxicity, with 40% (n = 18) using venetoclax for 14–28 days, and 24.4% (n = 11) for less than 14 days. The most common adverse events were related to hematology. BMS was observed in all induction courses, with hematological toxicities mainly concentrated in grades 3–4. All patients experienced grade 4 neutropenia, and febrile neutropenia was reported in 97.8% (n = 44) of cases, mostly assessed as grade 3 and not life-threatening. The second most common adverse event was infection, affecting 97.8% (n = 44) of patients. Intravenous antibiotics were administered in 93.3% (n = 42) of cases, primarily for grade 3 infections, with only two cases involving grade 4 infection. Pneumonia was the predominant type of infection (n = 11, 22.2%), with cases distributed across grades 1–4. Regarding bloodstream infection, septicemia and pyohemia were observed in 6.7% (n = 3) and 2.2% (n = 1) cases, respectively, and all bloodstream infections were grade 3 and not life-threatening. Mucositis was the third most common AE, including oral mucositis (n = 16, 35.6%) and anal mucositis (n = 10, 22.2%). Metabolic disorder was also prevalent, with tumor lysis syndrome occurring in 24.4% (n = 11) of cases, all grade as level 3, and hyperglycemia in 17.8% (n = 8), predominantly at grade 1. The next most common AEs were gastrointestinal adverse reactions, including nausea (n = 5, 11.1%), vomiting (n = 7, 15.6%) and diarrhea (n = 5, 11.1%). It’s worth noting that there were no grade 5 AEs reported in this study. Detailed information about the AEs in various systems are shown in Table 2.
Table 2.
Treatment toxicities during every treatment period of venetoclax
| Toxicities, n(%) | Cycles (n = 45) | |||||
|---|---|---|---|---|---|---|
| None | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Hematology | ||||||
| Leukopenia | 0 (0.0) | 2 (4.4) | 2 (4.4) | 12 (26.7) | 29 (64.4) | - |
| Neutropenia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 45 (100.0) | - |
| Febrile neutropenia | 1 (2.2) | - | - | 44 (97.8) | 0 (0.0) | 0 (0.0) |
| Anemia | 0 (0.0) | 0 (0.0) | 3 (6.7) | 42 (93.3) | 0 (0.0) | 0 (0.0) |
| Thrombocytopenia | 1 (2.2) | 0 (0.0) | 3 (6.7) | 12 (26.7) | 29 (64.4) | - |
| Respiratory | ||||||
| Pneumonia | 35 (77.8) | 2 (4.4) | 5 (11.1) | 1 (2.2) | 2 (4.4) | 0 (0.0) |
| Pleural effusion | 43 (95.6) | 2 (4.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pulmonary atelectasis | 45 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cardiology | ||||||
| Reduced ejection fraction | 43 (95.6) | - | 1 (2.2) | 1 (2.2) | 0 (0.0) | - |
| Sinus bradycardia | 44 (97.8) | 1 (2.2) | 0 (0.0) | 0 (0.0) | - | - |
| Sinus tachycardia | 42 (93.3) | 3 (6.7) | 0 (0.0) | 0 (0.0) | - | - |
| Abnormal T-waves on ECG | 43 (95.6) | 2 (4.4) | 0 (0.0) | - | - | - |
| hypotension | 45 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| hypertension | 44 (97.8) | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gastroenterology | ||||||
| Nausea | 40 (88.9) | 5 (11.1) | 0 (0.0) | 0 (0.0) | - | - |
| Vomiting | 38 (84.4) | 5 (11.1) | 2 (4.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 40 (88.9) | 5 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Constipation | 44 (97.8) | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Increased AST | 37 (82.2) | 3 (6.7) | 5 (11.1) | 0 (0.0) | 0 (0.0) | - |
| Increased ALT | 37 (82.2) | 4 (8.9) | 4 (8.9) | 0 (0.0) | 0 (0.0) | - |
| Increased total bilirubin | 44 (97.8) | 0 (0.0) | 0 (0.0) | 1 (2.2) | 0 (0.0) | - |
| Hepatic failure | 45 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lipase | 42 (93.3) | 0 (0.0) | 0 (0.0) | 2 (4.4) | 1 (2.2) | - |
| Amylase | 44 (97.8) | 0 (0.0) | 0 (0.0) | 1 (2.2) | 0 (0.0) | - |
| Pancreatitis | 42 (93.3) | - | 0 (0.0) | 3 (6.7) | 0 (0.0) | 0 (0.0) |
| Nephrology | ||||||
| Renal damage | 42 (93.3) | - | - | 3 (6.7) | 0 (0.0) | 0 (0.0) |
| Increased creatinine | 40 (88.9) | 4 (8.9) | 1 (2.2) | 0 (0.0) | 0 (0.0) | - |
| Metabolism and nutrition | ||||||
| Tumor lysis syndrome | 34 (75.6) | - | - | 11 (24.4) | 0 (0.0) | 0 (0.0) |
| Hypernatremia | 45 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hyponatremia | 45 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hyperkalemia | 45 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypokalemia | 41 (91.1) | 1 (2.2) | 3 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypercalcemia | 45 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypocalcemia | 41 (91.1) | 3 (6.7) | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hyperglycemia | 37 (82.2) | 7 (15.6) | 0 (0.0) | 1 (2.2) | 0 (0.0) | 0 (0.0) |
| Skin/Mucosa | ||||||
| Oral mucositis | 29 (64.4) | 7 (15.6) | 9 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anal mucositis | 35 (77.8) | 5 (11.1) | 5 (11.1) | 0 (0.0) | - | - |
| Papule | 43 (95.6) | 1 (2.2) | 0 (0.0) | 1 (2.2) | - | - |
| Others | ||||||
| Infections | 1 (2.2) | - | 0 (0.0) | 42 (93.3) | 2 (4.4) | 0 (0.0) |
| Septicemia | 42 (93.3) | - | - | 3 (6.7) | 0 (0.0) | 0 (0.0) |
| Pyohemia | 44 (97.8) | - | - | 1 (2.2) | 0 (0.0) | 0 (0.0) |
| Multiorgan failure | 43 (95.6) | - | - | 1 (2.2) | 1 (2.2) | 0 (0.0) |
| DIC | 37 (82.8) | - | 3 (6.7) | 4 (8.9) | 1 (2.2) | 0 (0.0) |
| Allergic reaction | 44 (97.8) | - | - | 1 (2.2) | 0 (0.0) | 0 (0.0) |
ECG electrocardiogram, AST aspartate aminotransferase, ALT alanine aminotransferase, DIC disseminated intravascular coagulation. Hyperkalemia and hypocalcemia were not considered if patients were diagnosed with tumor lysis syndrome.
Venetoclax concentration
Plasma concentrations of venetoclax were measured in 37 patients on day 5 of full-dose venetoclax treatment. Pharmacokinetic samples were obtained at 30 min pre-dose to determine the venetoclax trough concentration, while the peak drug concentration was measured at 6 h post-dose. In cases where venetoclax was administered alongside a potent CYP3A inhibitor, the daily venetoclax dose was reduced to 25% of the original regimen. When combined with an intermediate CYP3A inhibitor, the stable daily dose of venetoclax was reduced to 50%. Among the 37 patients for whom the venetoclax concentration was measured, 43.2% (n = 16) had received azoles during their venetoclax treatment, while 56.8% (n = 21) had not. For all patients, the trough and peak concentrations of venetoclax averaged 1007.2 ng/ml (standard deviation 1258.2) and 2004.6 ng/ml (standard deviation 1785.0), respectively. The Mann–Whitney U test was used to compare concentrations between patients using or not using azoles. Trough concentrations were significantly higher in patients who used azoles during venetoclax treatment than in those who did not (p = 0.000, Fig. 2), with mean concentrations were 1711.1 ng/ml (standard deviation 432.3) and 504.4 ng/ml (standard deviation 90.3), respectively. However, peak concentrations did not exhibit a significantly different between the two groups (p = 0.986, Fig. 2), with mean concentrations of 1934.5 ng/ml (standard deviation 483.1) for patients using azoles and 2063.1 ng/ml (standard deviation 415.7) for those who did not. To explore the relationship between the coadministration of CYP3A inhibitors and the induction response, a chi-square test was employed. The analysis revealed no significant association between achieving CR (p = 1.000) or MRD negativity (p = 0.092) and the application of CYP3A inhibitors.
Fig. 2. Venetoclax concentration monitoring.
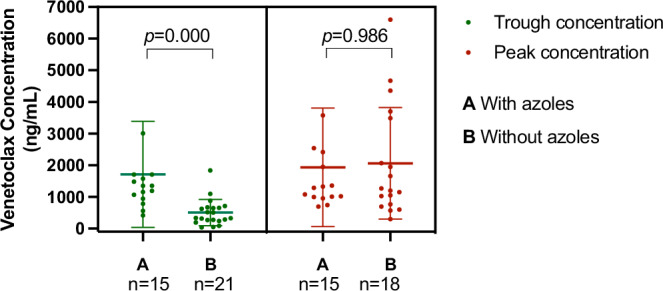
Comparation of venetoclax concentrations between patients using or not using CYP3A inhibitors.
Discussion
Venetoclax has been widely used in combination with low-dose cytarabine and HMAs in adults with R/R and untreated AML, and its safety and efficacy have been verified, with a CR rate of 70%8,9,11,12,25. Furthermore, in the realm of pediatric care, venetoclax has shown promise when employed in cases of relapsed or refractory AML, yielding a high response rate and exhibiting good tolerance17,26. Nevertheless, the application of venetoclax in the pediatric population remains less explored compared to its utilization in adults. The studies that do exist on this topic often suffer from limited sample sizes27, and, notably, none have reported on the induction efficacy of venetoclax in children with newly diagnosed AML. In this study, we embarked on the treatment of 45 newly diagnosed pediatric patients with venetoclax. Bone marrow assessments were performed on day 28 of dosing, and AEs and plasma concentrations of venetoclax were monitored closely during venetoclax treatment. To our knowledge, this study stands as the first to offer insights into the remission rate, toxicity, and pharmacokinetics of venetoclax-based induction regimens in newly diagnosed pediatric AML cases.
In this study, the combination therapy involving venetoclax demonstrated a robust response in previously untreated pediatric patients with AML, with CR rates as high as 94.7% in the low/middle-risk group and 80.8% in the high-risk group, while MRD-negative rates were 52.6% and 38.5%, respectively. The high-risk group had a lower CR rate and lower MRD-negative rates than the low/middle-risk group, but the difference was not statistically significant, which could be attributed to the relatively small sample size. Our previous research revealed that the CR rate after the conventional two-course chemotherapeutic drug induction treatment in children with AML was 76.7%20. In this study, the overall CR rate following a single course of venetoclax-based induction therapy stood at 86.7%, surpassing the CR rate attained after two courses of traditional chemotherapy induction. In another study28, the MRD-negative rate after induction 1 (daunorubicin, etoposide and cytarabine) was 30%, with 19% patients with high-risk AML. The MRD-negative rates of venetoclax-based regimens in our study was higher despite a higher proportion of high-risk patients. This finding underscores the potential of venetoclax in improving the response rates in pediatric AML patients.
Previous investigations on the use of venetoclax in pediatric leukemia predominantly focused on relapsed or refractory ALL or AML, with six of nine children with R/R AML treated with venetoclax (360 mg/2 per day) achieved CR17,26,29,30. Notably, our study revealed that high-risk group patients achieved a substantially higher CR rate even when administered a lower stable dose of venetoclax than the relapsed/refractory AML children in prior studies17. This finding suggests that early-stage administration of venetoclax might mitigate drug resistance, a promising development for high-risk AML patients. In light of the results from this study, the CR rate of venetoclax in children with previously untreated AML is indeed promising. However, it’s important to note that the follow-up period was relatively short, and the study primarily focused on short-term induction efficacy rather than long-term prognosis. Longer-term follow-up is warranted to monitor leukemia relapse and assess the treatment’s durability.
Venetoclax exhibited excellent tolerance among the majority patients in our study. The incidence rates of febrile neutropenia and infection in our regimens were almost 100%, which was consistent with the standard induction regimens in previous research31. Therefore, regardless of the induction regimen, the patient’s temperature and blood count should be closely monitored during the induction period, and symptomatic support and anti-infective treatment should be provided in a timely manner. Importantly, TLS, a serious complication in neoplastic disease, was a rare occurrence, affecting only 11 courses of venetoclax treatment in this study. It’s worth noting that the cases of TLS observed were mild, which can be attributed to the carefully implemented dose-escalating regimen32. In this research, the most prevalent AEs were hematological toxicity, infection, mucositis, metabolic disorder and digestive issues, which is consistent with previous reports except for metabolic disorder17. All the patients in our study were initially diagnosed with a heavy tumor disease burden, likely contributing to the elevated incidence rate of TLS. Notably, there is a dearth of studies reporting on the impact of venetoclax on blood glucose levels, emphasizing the need for monitoring patient’s blood glucose during the subsequent use of venetoclax.
In a previous study29 of pediatric patients with ALL, oral daily venetoclax was adjusted by weight to match the exposure of the adult-equivalent target doses of 400 mg. In a study17 of pediatric AML patients, venetoclax was administered at 240 mg/m² or 360 mg/m² (max 600 mg) once per day. The dose of venetoclax in our study was 200 mg/m2/day (maximum 300 mg), which was notably lower than the doses used in the aforementioned studies. Therefore, we closely monitored venetoclax plasma concentrations, taking into account the lower administered dose. In our study, we observed that venetoclax peak concentrations were slightly lower compared to pediatric patients receiving a dose of 360 mg/m² (max 600 mg) per day, with an average concentration of 2004.6 ng/ml. Comparing the efficacy of the two dosages, the concentrations in our study appeared to be within an acceptable range. Given that CYP3A inhibitors can lead to increased venetoclax accumulation in the body, the dose reduction when used concomitantly is crucial for ensuring safety33. In our study, we found a significant difference in the trough concentration of venetoclax when used with or without the CYP3A inhibitor, confirming that azoles can indeed contribute to the accumulation of venetoclax in the body. However, there was no significant difference in the peak concentration of venetoclax with or without the CYP3A inhibitor. This suggests that the accumulation of venetoclax due to concurrent use of azoles did not pose a safety risk in our study, and the dose adjustment of venetoclax was deemed reasonable. Interestingly, we observed a statistically significant difference in elevated trough concentrations with azoles, hinting that co-administration of azoles and venetoclax might enhance drug efficacy while maintaining tolerability. In our research, a chi-square test was conducted, revealing no significant relationship between achieving CR (p = 1.000) or MRD negativity (p = 0.092) and the application of CYP3A inhibitors. This finding might be attributed to our specific focus on monitoring the effect of azoles on venetoclax metabolism. In our study, the application of azoles was defined as their use prior to collecting the venetoclax concentration sample. Therefore, if a patient began using azoles later in the treatment course, it did not impact the already measured blood concentration of venetoclax.
This study provides preliminary validation of venetoclax’s efficacy and safety in the treatment of pediatric AML, particularly in previously untreated cases. It emphasizes the potential of venetoclax as a vital component in the management of newly diagnosed AML, extending its relevance beyond its role as salvage therapy for refractory or relapsed AML. The insights gained from this study provides valuable experience to guide future therapeutic approaches for AML patients. However, it is important to acknowledge that the absence of randomized control groups represents a limitation of this study, and extended follow-up periods are essential for assessing long-term outcomes.
Acknowledgements
This work was supported by Capital’s Funds for Health Improvement and Research (CFH) (No. 2022-1-2091), National Natural Science Foundation of China (NSFC) (No. 82070154), Beijing Natural Science Foundation (No. 7222056). And we extend our heartfelt gratitude to the patients who participated in this study, their families who actively cooperated, and the healthcare professionals who treated and cared for the patients in the clinical setting.
Author contributions
Xiaojia Wen was responsible for data extraction, analysis, result interpretation, and manuscript drafting. Xiaojia Wen, Yu Lu and Yanming Li collected clinical data. Huyong Zheng designed the treatment protocol and revised the draft. Huyong Zheng and Yan Liu provided study guidance. Yanming Li, Ning Sun and Yanni Zhang performed laboratory testing of venetoclax drug concentrations. Other authors participated in treating the patients. All authors read and approved the final manuscript.
Data availability
The data and material of this study are available from the corresponding author on reasonable request. Permission to reproduce material from other sources. I permit to reproduce material from other sources. Clinical trial registration. The clinical trial registration number is ChiCTR1900027146.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiaojia Wen, Yu Lu.
Contributor Information
Yan Liu, Email: 1516420424@qq.com.
Huyong Zheng, Email: zhenghuyong@bch.com.cn.
References
- 1.Ni, X. et al. Socioeconomic inequalities in cancer incidence and access to health services among children and adolescents in China: a cross-sectional study. Lancet400, 1020–1032 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Shiba, N. et al. Transcriptome analysis offers a comprehensive illustration of the genetic background of pediatric acute myeloid leukemia. Blood Adv.3, 3157–3169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu, H. Emerging agents and regimens for AML. J. Hematol. Oncol.14, 49 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knight, T., Edwards, H., Taub, J. W. & Ge, Y. Evaluating venetoclax and its potential in treatment-naïve acute myeloid leukemia. Cancer Manag Res11, 3197–3213 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhansali, R. S., Pratz, K. W. & Lai, C. Recent advances in targeted therapies in acute myeloid leukemia. J. Hematol. Oncol.16, 29 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonas, B. A. & Pollyea, D. A. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia33, 2795–2804 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Konopleva, M. et al. Efficacy and biological correlates of response in a phase II study of Venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov.6, 1106–1117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei, A. H. et al. Venetoclax combined with low-dose Cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J. Clin. Oncol.37, 1277–1284 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiNardo C. D. et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133 [DOI] [PMC free article] [PubMed]
- 10.Guerra, V. A., DiNardo, C. & Konopleva, M. Venetoclax-based therapies for acute myeloid leukemia. Best. Pr. Res Clin. Haematol.32, 145–153 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiNardo, C. D. et al. Azacitidine and Venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med.383, 617–629 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Wei, A. H. et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood135, 2137–2145 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiNardo, C. D. et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol.19, 216–228 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Chua, C. C. et al. Chemotherapy and Venetoclax in Elderly Acute Myeloid Leukemia Trial (CAVEAT): a Phase Ib dose-escalation study of Venetoclax combined with modified intensive chemotherapy. J. Clin. Oncol.38, 3506–3517 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Jin, H. et al. Venetoclax combined with azacitidine and homoharringtonine in relapsed/refractory AML: a multicenter, phase 2 Trial. J. Hematol. Oncol.16, 42 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trabal A. et al. Venetoclax for Acute Myeloid Leukemia in Pediatric Patients: A Texas Medical Center Experience. Cancers (Basel). 2023;15 [DOI] [PMC free article] [PubMed]
- 17.Karol, S. E. et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: a phase 1, dose-escalation study. Lancet Oncol.21, 551–560 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masetti, R. et al. Venetoclax-based therapies in pediatric advanced MDS and relapsed/refractory AML: a multicenter retrospective analysis. Blood Adv. 7, 4366–4370 (2023). [DOI] [PMC free article] [PubMed]
- 19.Jin, J. et al. Homoharringtonine-based induction regimens for patients with de-novo acute myeloid leukaemia: a multicentre, open-label, randomised, controlled phase 3 trial. Lancet Oncol.14, 599–608 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Li, J. et al. Homoharringtonine-Based Induction Regimen Improved the Remission Rate and Survival Rate in Chinese Childhood AML: A Report From the CCLG-AML 2015 Protocol Study. J Clin Oncol. 41, 4881–4892 (2023). [DOI] [PMC free article] [PubMed]
- 21.Wei, W. et al. Homoharringtonine is synergistically lethal with BCL-2 inhibitor APG-2575 in acute myeloid leukemia. J. Transl. Med.20, 299 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi, Y. et al. The basic research of the combinatorial therapy of ABT-199 and homoharringtonine on acute myeloid leukemia. Front. Oncol.11, 692497 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, A. K. et al. Clinical predictors of venetoclax pharmacokinetics in chronic lymphocytic leukemia and Non-Hodgkin’s lymphoma patients: a pooled population pharmacokinetic analysis. AAPS J.18, 1192–1202 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Agarwal, S. K. et al. Management of venetoclax-Posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin. Ther.39, 359–367 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Wei, A. H. et al. 6-month follow-up of VIALE-C demonstrates improved and durable efficacy in patients with untreated AML ineligible for intensive chemotherapy (141/150). Blood Cancer J.11, 163 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winters, A. C., Maloney, K. W., Treece, A. L., Gore, L. & Franklin, A. K. Single-center pediatric experience with venetoclax and azacitidine as treatment for myelodysplastic syndrome and acute myeloid leukemia. Pediatr. Blood Cancer67, e28398 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Xu, H. et al. Refractory pediatric acute myeloid leukemia expressing NUP98-NSD1 fusion gene responsive to chemotherapy combined with venetoclax and decitabine. Pediatr. Blood Cancer70, e30021 (2023). [DOI] [PubMed] [Google Scholar]
- 28.Tierens, A. et al. Mitoxantrone Versus Liposomal Daunorubicin in Induction of Pediatric AML With Risk Stratification Based on Flow Cytometry Measurement of Residual Disease. J Clin Oncol. 42, 2174–2185 (2024). [DOI] [PubMed]
- 29.Pullarkat, V. A. et al. Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov.11, 1440–1453 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibson, A. et al. Venetoclax for Children and Adolescents with Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma. Cancers (Basel). 14, 150 (2024). [DOI] [PMC free article] [PubMed]
- 31.Almatrafi, M. A., Dassner, A. M., Aquino, V., Slone, T. & Sebert, M. Retrospective observational assessment of the impact of cefepime prophylaxis in neutropenic pediatric patients with acute myelogenous leukemia. J. Pediatr. Infect. Dis. Soc.12, 471–476 (2023). [DOI] [PubMed] [Google Scholar]
- 32.Davids, M. S. et al. Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin. Cancer Res.24, 4371–4379 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Eisenmann, E. D. et al. Interaction of Antifungal Drugs with CYP3A- and OATP1B-Mediated Venetoclax Elimination. Pharmaceutics. 14, 694 (2022). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and material of this study are available from the corresponding author on reasonable request. Permission to reproduce material from other sources. I permit to reproduce material from other sources. Clinical trial registration. The clinical trial registration number is ChiCTR1900027146.


