Abstract
Inhaler therapy and physical activity (PA) are important methods of chronic obstructive pulmonary disease (COPD) management. This study aimed to investigate the additional benefit of moderate-to-vigorous PA (MVPA) in patients with COPD using a long-acting beta-agonists (LABA)/long-acting muscarinic antagonist (LAMA) combination. We emulated a target trial to estimate the benefit of MVPA in patients with COPD using a dual ultra-long-acting bronchodilators. We enrolled patients aged ≥ 40 who were diagnosed with COPD between 2014 and 2018, initiated a LABA/LAMA combination, and had not undergone regular MVPA. The main exposure was the initiation of MVPA, defined as vigorous aerobic exercise > 20 min per day on ≥ 3 days/week or moderate aerobic exercise > 30 min per day on ≥ 5 days/week. The main outcomes were the future usage of inhaled corticosteroids (ICS) and severe exacerbation. We identified 1,526 patients who initiated MVPA and 4,516 who did not. The median follow-up period was 3.0 years. The hazard ratio (HR) for future ICS usage in the MVPA initiation group was 0.83 (95% confidence intervals (CI): 0.72, 0.97) compared to the control group. The HR for severe exacerbation in the MVPA initiation group was 0.81 (95% CI: 0.68, 0.96) compared to the control group. Subgroup analyses by age, sex, body mass index, residence area, smoking and drinking status showed consistent benefits in these outcomes. Initiation of MVPA may offer an additional benefit for even COPD patients who use a dual ultra-long-acting bronchodilators.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75702-9.
Keywords: COPD, LABA/LAMA, Physical activity, ICS
Subject terms: Chronic obstructive pulmonary disease, Patient education
Introduction
Inhaled bronchodilators are the mainstay of treatment in patients with chronic obstructive pulmonary disease (COPD)1. Particularly, dual bronchodilator therapy with long-acting beta-agonists (LABA) and long-acting muscarinic antagonists (LAMA) is superior to monotherapy for improving lung function and quality of life and lowering the risk of exacerbation, and hospital admission2–4. A LABA/LAMA combination also improves PA levels and exercise capacity better than either LAMA or LABA5,6. This LABA/LAMA combination is now recommended for all patients with symptomatic COPD or a history of exacerbation1. However, nearly 30% of patients using a LABA/LAMA combination experiences an COPD exacerbation7, and inhaled corticosteroid (ICS) could be added for them to lower future events1. Triple therapy of ICS/LABA/LAMA showed a reduction in future exacerbation events in frequent or severe exacerbators8,9 at the expense of raised a risk of pneumonia and other infections10.
Regular physical activity (PA) is a key non-pharmacological therapy for COPD patients1, as it significantly improves symptoms such as dyspnea, enhances overall health status, and increases exercise tolerance11. While any amount of PA is beneficial in reducing functional impairments, moderate-to-vigorous physical activity (MVPA) can make more advantages in enhancing physical capacity12 and reducing symptoms13. Both the World Health Organization (2020) and PA guidelines for Americans (2nd edition, 2018) emphasize the importance of MVPA not only for healthy individuals but also for those with chronic conditions14,15. A recent study from South Korea further supports this recommendation, showing that MVPA in COPD patients significantly reduces the frequency of severe exacerbations and improves clinical outcomes16.
However, additional information is still required to recommend MVPA for patients with COPD who are using a LABA/LAMA combination. Studies in this field have largely focused on the physiological effects of the LABA/LAMA combination on the improvement of PA and exercise capacity5,6. Those imply that LABA/LAMA combination might enhance PA, thereby leading to better clinical outcomes in patients with COPD. However, LABA/LAMA therapy alone does not always lead to the engagement in MVPA, and the impact of initiating MVPA in patients who prescribed the LABA/LAMA therapy has not been thoroughly examined. Although our previous report showed that MVPA could be beneficial in patients with COPD regardless of their pharmacologic treatment, further research is needed to determine whether initiating MVPA could provide an additional benefit for patients receiving LABA/LAMA combination therapy following COPD diagnosis. As it is well established that LABA/LAMA combination is associated with reduction in the moderate-to-severe exacerbations17, our study focused on whether regular MVPA initiation provides additional benefits in terms of exacerbation-related outcomes when combined with a LABA/LAMA therapy compared with COPD patients receiving LABA/LAMA alone. Therefore, our study aimed to investigate the impact of initiating MVPA on clinical outcomes, the addition of ICS and severe exacerbation, in patients who did not regularly engage in MVPA but initiated a dual ultra-long-acting bronchodilators after COPD diagnosis. We utilized a large, nationally representative cohort for this study employing a target trial emulation framework18–21 which could generate an effect estimate similar to that of a randomized clinical trial.
Results
The mean age (standard deviation, SD) of the control and MVPA group was 68.4 (8.7) and 68.5 (8.1) years. The proportion of males in the control and MVPA groups were 88.4% and 88.2%, respectively (Table 1). All the standardized mean differences (SMDs) of the differences between the control and MVPA initiation groups were < 0.1 (Table 1).
Table 1.
Characteristics of study participants at the time of COPD diagnosis.
| Control | MVPA Initiation | SMD | |
|---|---|---|---|
| (N = 4,516) | (N = 1,526) | ||
| Age, years | 68.4 (8.7) | 68.5 (8.1) | 0.013 |
| Sex | -0.005 | ||
| Male | 3,991 (88.4) | 1,346 (88.2) | |
| Female | 525 (11.6) | 180 (11.8) | |
| Body mass index | |||
| Underweight | 302 (6.7) | 97 (6.4) | -0.013 |
| Normal | 1,713 (37.9) | 573 (37.5) | -0.008 |
| Overweight | 1,121 (24.8) | 386 (25.3) | 0.011 |
| Obesity | 1,380 (30.6) | 470 (30.8) | 0.005 |
| Residential area, metropolitan | 2,522 (55.8) | 888 (58.2) | 0.047 |
| Income | |||
| Medical Aid | 186 (4.1) | 63 (4.1) | 0.001 |
| ≤ 30th | 1,050 (23.3) | 359 (23.5) | 0.007 |
| 31st to 70th | 1,442 (31.9) | 474 (31.1) | -0.019 |
| > 70th | 1,765 (39.1) | 604 (39.6) | 0.010 |
| Unknown | 73 (1.6) | 26 (1.7) | 0.007 |
| Smoking status | |||
| Never | 1,339 (29.7) | 453 (29.7) | 0.001 |
| Past | 1,947 (43.1) | 674 (44.2) | 0.021 |
| Current | 1,230 (27.2) | 399 (26.1) | -0.025 |
| Smoking pack years | 30.6 (18.3) | 29.5 (18.1) | -0.062 |
| Current drinking status | 0.021 | ||
| No | 2,445 (54.1) | 808 (54.3) | |
| Yes | 2,070 (45.8) | 697 (45.7) | |
| Charlson comorbidity index | 1.7 (1.6) | 1.7 (1.7) | 0.014 |
| 0–1 | 2,615 (57.9) | 885 (58.0) | 0.008 |
| 2 | 867 (19.2) | 280 (18.3) | -0.022 |
| 3+ | 1,034 (22.9) | 361 (23.7) | 0.018 |
| History of severe exacerbation | 180 (4.0) | 47 (3.1) | 0.049 |
Values were presented as n (%) or mean (SD).
MVPA, moderate-to-vigorous physical activity; SMD, standardized mean difference.
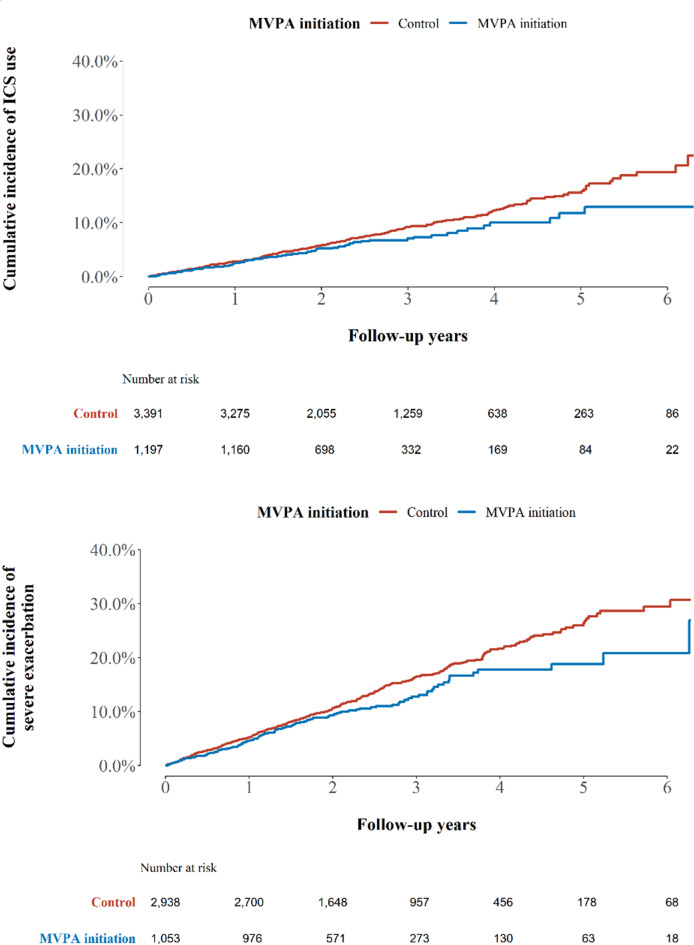
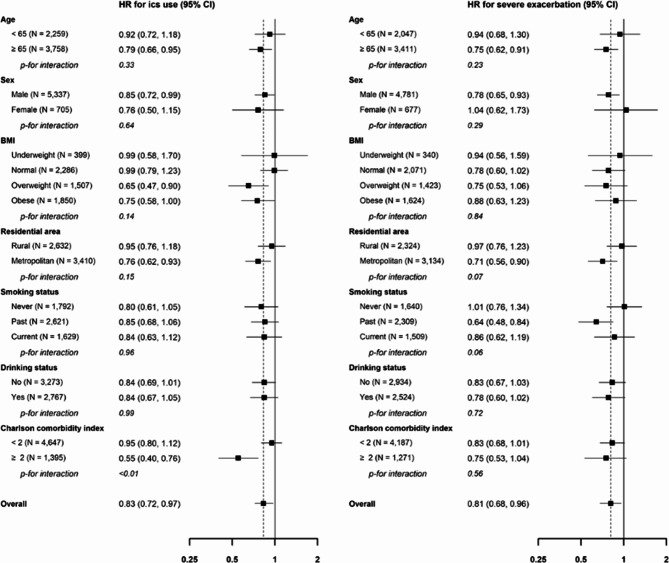
During the follow-up period (median [interquartile range], 3.0 [0.5–7.0] years), 1,060 added ICS as a combination therapy. There were 5.7 and 4.7 cases per 100-person years in the control and MVPA initiation groups, respectively (Fig. 1, log-rank test P-value < 0.01). The HR for the risk of adding ICS (MVPA initiation group vs. control) was 0.83 (95% CI: 0.72, 0.97, Table 2). Furthermore, the beneficial effects of beginning MVPA on the reduction of ICS addition remained consistent in subgroups (Fig. 2). Patients with a higher CCI score (≥ 2) showed a stronger effect of MVPA initiation on reduction of ICS addition than those with a lower CCI score (P < .05).
Fig. 1.
Kaplan-Meier curve for ICS use and severe exacerbation. MVPA, moderate-to-vigorous physical activity; ICS, inhaled corticosteroids.
Table 2.
Hazard ratio (HR, 95% confidence intervals (CIs)) for ICS use and severe exacerbation associated with MVPA initiation among COPD patients.
| Number of events | Incidence rate per 100 person-year | HR (95% CI) | |
|---|---|---|---|
| ICS use | |||
| Control | 834 | 5.7 | Reference |
| MVPA initiation | 226 | 4.7 | 0.83 (0.72, 0.97) |
| Severe exacerbation (N = 5,458) | |||
| Control | 640 | 4.9 | Reference |
| MVPA initiation | 172 | 3.9 | 0.81 (0.68, 0.96) |
MVPA, moderate-to-vigorous physical activity; COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroids.
Fig. 2.
Subgroup analysis in ICS use and severe exacerbation.
Among severe exacerbation-naïve patients (N = 5,458), 812 experienced severe exacerbations. The incidence of severe exacerbation per 100-person years was 4.9 in the control group and 3.9 in the MVPA initiation group (Fig. 1, log-rank test P-value < 0.01). The hazard ratio (HR) for the risk of severe exacerbation (MVPA initiation group vs. control) was 0.81 (95% CI: 0.68, 0.96, Table 2). In addition, the beneficial effects of initiating MVPA in reducing severe exacerbations remained consistent in all subgroups (Fig. 2). When categorizing patients with MVPA into individuals who met the criteria of MVPA but not performed daily MVPA, and those who engaged in daily MVPA, defined as daily at least 30 min of moderate aerobic exercise and over 20 min of vigorous aerobic exercise each day for 7 days a week, there also exist a dose-dependent relationship (Supplementary Table S1).
Discussion
Using a large national cohort, our study matched sets of MVPA initiation and control groups and employed a target trial emulation framework that mimicked randomized controlled trials. The current study is a follow-up analysis of our previous report16 which showed that regular MVPA initiation following COPD diagnosis was associated with a reduced risk of death. This study specifically focused on COPD patients receiving a dual ultra-long-acting bronchodilators after COPD diagnosis. In these patients, we also found that the initiation of regular MVPA significantly reduced the future addition of ICS, and the severe exacerbations compared to those who did not initiate regular MVPA. This advantage of MVPA initiation was consistent across various subgroups, including age, sex, body mass index (BMI), residence area, smoking and drinking status.
This study expands on previous findings regarding the relationship between bronchodilator therapy and PA in patients with COPD. Most studies have focused on examining the effect of the LABA/LAMA combination on the improvement of PA and exercise capacity5,6. A recent randomized controlled trial in patients with COPD with long-acting bronchodilator, tiotropium/olodaterol, PHYSACTO, showed a further increase in exercise endurance time (EET) and 6-minute walk distance after 12 weeks from baseline when they received a LABA/LAMA combination with or without exercise training22. Although exercise training provided an incremental improvement in EET when added to the LABA/LAMA combination, the improvement did not reach statistical significance between the active treatment arms. The small number of patients in this study might not have been sufficient to detect differences due to insufficient statistical power. Rather than enhancement of exercise capacity, our study focused on the impact of regular MVPA on reduced the future addition of ICS and the severe exacerbation in patients using a LABA/LAMA combination and showed that initiation of regular MVPA provides significantly better clinical outcomes compared to those who did not engage in regular MVPA even in patients who received a LABA/LAMA combination following COPD diagnosis.
It has been well demonstrated that a LABA/LAMA combination is associated with better outcomes in patients with COPD. A LABA/LAMA combination therapy was superior to the monotherapy of either LABA or LAMA in terms of hospitalization and acute exacerbation in a meta-analysis of 24 randomized trials17. A large cohort study of patients with COPD who were newly prescribed inhaler therapy found that dual bronchodilator therapy was also better than ICS/LABA in reducing the risk of exacerbation, and hospitalization due to first pneumonia23, and improving lung function, quality of life, respiratory symptoms24,25, PA, and exercise capacity5,6. These benefits are attributed by reducing systemic inflammation and improving gas exchange26–28. MVPA also enhances muscular strength29 and overall cardiorespiratory fitness12. The recent report showed that MVPA initiation in patients newly diagnosed with COPD decreased the risk of severe exacerbation by 10% and the risk of death by 16%16. MVPA might enhance the impact of the LAMA/LABA combination, as our study showed an additional benefit of MVPA initiation in patients with COPD who received the LAMA/LABA combination. In other words, pharmacological treatment alone, without the initiation of MVPA, may miss the chance to capture the additional benefits of MVPA in reducing future ICS addition and severe exacerbation. Before matching, the percentage of patients with COPD who did not undergo regular MVPA in the national cohort was 19.2%. In real-world clinical practice, although clinicians emphasize the importance of both proper inhaler use and physical activity (PA), a substantial proportion of COPD patients have low PA levels compared to healthy controls30, and have a low referral rate to pulmonary rehabilitation31,32. Furthermore, while inhaler technique is often routinely assessed, the quality and frequency of physical activity are rarely monitored. Thus, there is still a need for further investigation into how MVPA can be best integrated into the treatment plan, even in those who received optimal pharmacological therapy.
The methodology employed in this study–targeted trial emulation has gained attention for using big data when a randomized trial is not feasible. Given the limited number of clinical trials comparing COPD patients received a LABA/LAMA combination with or without regular engagement in MVPA, this study design has several strengths18,20. First, the time-varying covariables were addressed using the g-method. This approach minimizes biased effect estimates. Second, the immortal-time bias related to the delay between the diagnosis of COPD and the initiation of MVPA could be minimized. Finally, in situations where performing a randomized controlled trial is not feasible due to ethical issues, given the known benefits of regular MVPA, targeted trial emulation can be implemented to derive causal inference from observational data33.
Despite the strengths of this study, it has several limitations. First, the confirmation of COPD relied on health insurance claims data, which utilized a combination of disease codes and prescriptions for COPD medications. This approach can introduce misclassification bias. Second, PA was assessed using self-administered questionnaires34. Numerical estimation of energy expenditure, such as metabolic equivalents, was not feasible because the database did not provide the total duration of exercise. Third, patients were censored as having outcome of ICS addition when they prescribed ICS at one-time prescription. This might overestimate the ICS addition, as there could be patients who were briefly prescribed ICS but subsequently returned to LABA/LAMA. Fourth, the K-NHIS does not include data on lung function testing. Since lung function can influence an individual’s ability to perform daily physical activity, further studies based on spirometry are necessary. An individualized approach to recommending regular PA would also be important. Finally, our study did not employ biochemical methods for subgroups of patients with COPD. For instance, given blood eosinophil count is one of crucial factors for exacerbation, future studies could provide more insights by examining the effects of initiating MVPA according to eosinophil levels.
In conclusion, initiation of MVPA in patients with COPD using a dual ultra-long-acting bronchodilators was associated with a lower use of ICS and a reduced risk of severe exacerbation. With the use of large national insurance data and a target trial emulation, our study suggests there are additional effects of this lifestyle modification, and clinicians in real-world practice could recommend their patients to engage in physically active to improve their outcomes.
Methods
Study design and methods
This retrospective population-based cohort study used data from the K-NHIS database. The K-NHIS is a single national insurance system that covers approximately all the population (the NHIS and beneficiaries of the Medical Aid Program cover 97% and 3%, respectively). K-NHIS database contained information on demographics, medical treatments, procedures, prescription drugs, diagnostic codes, hospital use and National Health-Screening Examination (NHSE). The NHSE is a standardized program provided to all insured persons every 2 years35. The NHSE participation rate target population was approximately 76%35, with higher rates observed among those over 40 years of age.
Study population
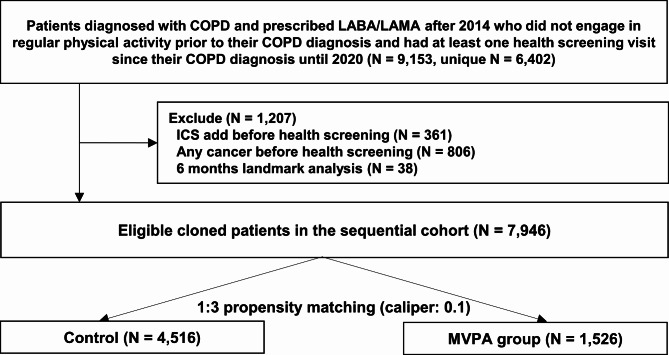
Since dual ultra-long-acting bronchodilators (LABA/LAMA combinations) were introduced to South Korea in 2014, we considered all patients ≥ 40 years who had COPD and had at least one health-screening visit before and after their COPD diagnosis between January 1, 2014, and December 31, 2018. Among them, we selected patients with COPD who received a dual ultra-long-acting bronchodilators (LABA/LAMA combinations) (LABA: vilanterol, olodaterol, and indacaterol, and LAMA: umeclidinium bromide, tiotropium, glycopyrronium bromide) as the initial treatment (N = 6,402). Of note, formoterol and aclidinium combination was not included as LABA/LAMA combination since LABA/LAMA in this study was confined to ultra-long-acting agents.
Among the eligible participants, we mimicked the sequential emulation of the target trial21. In this study, health screening was considered for each trial. According to the trial protocol, patients were considered eligible for multiple follow-up periods if they were alive and had not participated in MVPA at previous time points19 (N = 9,153). Patients who met the eligibility criteria in the previous trial were excluded from subsequent trials if they had a cancer diagnosis (N = 808), received ICS as triple therapy (N = 361) prior to the start of each trial, or died within 6 months of the trial (N = 38). These steps were repeated until June 1, 2020 (N = 7,946). The selection process was depicted in Fig. 3.
Fig. 3.
Selection process of study population.
Measurements
Diagnoses in the K-NHIS database were based on the ICD-10 codes. The K-NHIS regularly audits ICD-10 codes, procedures, and prescription records to avoid unnecessary medical expenses. NHSE data included a self-administered questionnaire on medical history, habits, anthropometric measurements, and laboratory tests35.
COPD was defined as the presence of the J43-J44 code (except J43.0) (ICD-10) and prescription of COPD medication at least twice within a year between January 1, 2014, and December 31, 201816,36. The K-NHIS routinely audits claims; the data are considered highly reliable and have been used in numerous peer-reviewed publications37–39.
Data on PA were collected using self-reported structured questionnaires that employed a 7-day recall method during national health screening. The questionnaire was similar to the International Physical Activity Questionnaire-Short Form but was simplified by the National Health Examination Committee (Supplementary Fig. 1). This questionnaire was widely used in previous studies16,34.
The study defined the main exposure as the initiation of MVPA, which is vigorous aerobic exercise for over 20 min per day on at least three days per week or moderate aerobic exercise for more than 30 min per day on at least five days per week34. Since none of the study participants engaged in regular MVPA before diagnosis, those who reported engaging in MVPA after diagnosis were considered to have initiated MVPA.
The outcomes of the current study include the addition of ICS and the severe exacerbation following the diagnosis of COPD. Given that the current Korean guidelines and the GOLD 2024 report suggest a LABA/LAMA combination as the initial therapy for COPD patients with respiratory symptoms or a history of exacerbation1,40 and considering that the escalation to triple therapy in patients already using a LABA/LAMA indicates a worsening clinical course, we chose to include ICS usage as a study outcome. As the overuse of inhaled corticosteroids in real world setting is well-known41, the rate of severe exacerbations was also used as outcome. An addition of ICS was defined as the regular prescription of ICS during follow-up. Severe COPD exacerbation was defined as hospitalization or emergency room attendance, with one of the following ICD-10 codes as the principal or secondary diagnosis: COPD (J43.X [excluding J43.0] or J44.X) or diseases related to COPD, such as pneumonia (J12.X-J17.X), pulmonary thromboembolism (I26, I26.0, or I26.9), dyspnea (R06.0), or acute respiratory distress syndrome (J80), and a prescription for systemic steroids or antibiotics may be given during the same visit.
For covariates, we included baseline age, sex, BMI, and behaviors, including smoking and alcohol intake. Information on smoking and alcohol consumption was obtained using standardized self-administered questionnaires. Smoking status was defined as never, past (former), and current smokers. Current alcohol intake was categorized as ‘no’ or ‘yes’. We also included comorbidities, defined as the presence of a disease code within a year before each baseline, and summarized them using the Charlson’s Comorbidity Index (CCI)42.
Statistical analysis
The propensity score (PS) was estimated in each emulated cohort to minimize the systematic differences in the baseline characteristics between the two groups. All covariates were included in the logistic regression model to estimate the probability of receiving treatment, conditional on their covariates. To minimize this bias, we implemented a 1:3 PS nearest-neighbor matching with a caliper of 0.1 on the PS scale43. Differences in baseline covariates between the MVPA and control groups were evaluated before and after the PS matching using an SMD with a value of > 0.1 indicating a significant difference43. Covariates with an SMD greater than 0.1, indicating possible group imbalances, were adjusted for in our survival analysis. Intention-to-treat analysis was used as the primary method. We determined the cumulative incidence of each outcome using the Kaplan-Meier method and evaluated the differences between groups using the log-rank test. We calculated HR and 95% confidence intervals (CI) for ICS addition and severe exacerbation using a Cox regression model. As the patients were matched using propensity score and all the variables were found to be well-balanced, no further adjustments were necessary. To improve the precision of the variance estimates, we used a sandwich variance estimator to control for subject correlations because some patients appeared multiple times in the model. We confirmed the proportional hazard assumption by examining log-log survival function plots and Schoenfeld residuals.
Stratified analyses were performed to examine the correlation between MVPA initiation and outcomes in the subgroups: these subgroups were determined based on factors such as age, sex, BMI, residential area, smoking status, drinking status and CCI. The selection of these subgroups was guided by previous research suggesting a potential effect of MVPA in reducing mortality rates within these cohorts.
We considered P-values below 0.05 as being statistically significant in our two-sided tests. The SAS® Visual Analytics and R software version 4.1.2 was used in performing the analyses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
TK: Writing the original draft. HK: formal analysis and investigation. HYP: Writing, review, editing, supervision, and project administration. DK: Methodology, formal analysis, investigation, writing, review, and editing. SK, SHS, and JC: Validation. All the authors discussed the results and approved the final version of the manuscript.
Funding
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) and was funded by the Korean government (MSIT) [No. 2022M3A9G8017220]. This work was supported by grants from the National Research Foundation of Korea, and was funded by the Korean government (Ministry of Science and ICT) (NRF-2021R1A2C2093987).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The Institutional Review Board of the Samsung Medical Center approved the study (approval no.: 2022-09-022) and waived the requirement for informed consent because the K-NHIS data were de-identified. This study was conducted in accordance with the Declaration of Helsinki. All procedures were performed in accordance with the relevant guidelines and regulations.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Danbee Kang and Hye Yun Park contributed equally to this work.
Contributor Information
Danbee Kang, Email: dbee.kang@skku.edu.
Hye Yun Park, Email: hyeyunpark@skku.edu.
References
- 1.Agustí, A. et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur. Respir. J.61, (2023). [DOI] [PMC free article] [PubMed]
- 2.Mammen, M. J. et al. Dual LABA/LAMA Therapy versus LABA or LAMA Monotherapy for Chronic Obstructive Pulmonary Disease. A Systematic Review and Meta-analysis in Support of the American Thoracic Society Clinical Practice Guideline. Ann. Am. Thorac. Soc 17, 1133–1143 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Rodrigo, G. J. et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis.12, 907–922 (2017). [DOI] [PMC free article] [PubMed]
- 4.Calzetta, L., Rogliani, P., Matera, M. G. & Cazzola, M. A Systematic Review With Meta-Analysis of Dual Bronchodilation With LAMA/LABA for the Treatment of Stable COPD. Chest149, 1181–1196 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Calzetta, L. et al. Impact of LABA/LAMA combination on exercise endurance and lung hyperinflation in COPD: A pair-wise and network meta-analysis. Respir. Med.129, 189–198 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Miravitlles, M. et al. Exercise capacity and physical activity in COPD patients treated with a LAMA/LABA combination: A systematic review and meta-analysis. Respir Res.23, 347 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, C. Y. et al. LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: A systematic review and meta-analysis. Ther. Adv. Respir. Dis.14, 1753466620937194. 10.1177/1753466620937194 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipson, D. A. et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N. Engl. J. Med.378, 1671–1680 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Rabe, K. F. et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N. Engl. J. Med.383, 35–48 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Agusti, A. et al. Inhaled corticosteroids in COPD: Friend or foe?. Eur. Respir. J.52, (2018). [DOI] [PubMed]
- 11.Xiang, X., Huang, L., Fang, Y., Cai, S. & Zhang, M. Physical activity and chronic obstructive pulmonary disease: A scoping review. BMC Pulm. Med.22, 301. 10.1186/s12890-022-02099-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nayor, M. et al. Physical activity and fitness in the community: The Framingham heart study. Eur. Heart J.42, 4565–4575 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oostrik, L. et al. Physical activity and symptom burden in COPD: The Canadian obstructive lung disease study. Chronic Obstr. Pulm. Dis.10, 89–101. 10.15326/jcopdf.2022.0349 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bull, F. C. et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med.54, 1451–1462 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piercy, K. L. et al. The physical activity guidelines for americans. JAMA320, 2020–2028 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, T. Association between regular moderate to vigorous physical activity initiation following COPD diagnosis and mortality: An emulated target trial using Nationwide Cohort Data. Chest (2023). [DOI] [PubMed]
- 17.Mammen, M. J. et al. Dual LABA/LAMA therapy versus LABA or LAMA monotherapy for chronic obstructive pulmonary disease. A systematic review and meta-analysis in support of the American Thoracic Society Clinical Practice Guideline. Ann. Am. Thorac. Soc.17, 1133–1143 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Hernán, M. A. & Robins, J. M. Using big data to emulate a target trial when a randomized trial is not available. Am. J. Epidemiol.183, 758–764 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernán, M. A., Sauer, B. C., Hernández-Díaz, S., Platt, R. & Shrier, I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J. Clin. Epidemiol.79, 70–75 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernán, M. A., Wang, W. & Leaf, D. E. Target trial emulation: A Framework for causal inference from observational data. JAMA328, 2446–2447 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Keogh, R. H., Gran, J. M., Seaman, S. R., Davies, G. & Vansteelandt, S. Causal inference in survival analysis using longitudinal observational data: Sequential trials and marginal structural models. Stat. Med.42, 2191–2225 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Troosters, T. et al. Effect of bronchodilation, exercise training, and behavior modification on symptoms and physical activity in Chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med.198, 1021–1032 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Feldman, W. B., Avorn, J., Kesselheim, A. S. & Gagne, J. J. Chronic obstructive pulmonary disease exacerbations and pneumonia hospitalizations among new users of combination maintenance inhalers. JAMA Intern. Med.183, 685–695 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calzetta, L., Rogliani, P., Matera, M. G. & Cazzola, M. A. Systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest149, 1181–1196 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Sion, K. Y., Huisman, E. L., Punekar, Y. S., Naya, I. & Ismaila, A. A network meta-analysis of long-acting muscarinic antagonist (LAMA) and long-acting β 2-agonist (LABA) combinations in COPD. Pulm. Ther.3, 297–316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sin, D. D. & Man, S. F. Skeletal muscle weakness, reduced exercise tolerance, and COPD: Is systemic inflammation the missing link?. Thorax61, 1–3 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elbehairy, A. F. et al. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am. J. Respir. Crit. Care Med.191, 1384–1394 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Mummy, D. G., Coleman, E. M., Wang, Z. & Bier, E. A. Regional Gas Exchange Measured by 129 Xe Magnetic Resonance Imaging Before and After Combination Bronchodilators Treatment in Chronic Obstructive Pulmonary Disease. J. Magn. Reson. Imaging. 54, 964–974 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper, A., Lamb, M., Sharp, S. J., Simmons, R. K. & Griffin, S. J. Bidirectional association between physical activity and muscular strength in older adults: Results from the UK Biobank study. Int. J. Epidemiol.46, 141–148 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitta, F. et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med.171, 972–977 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Milner, S. C., Boruff, J. T., Beaurepaire, C., Ahmed, S. & Janaudis-Ferreira, T. Rate of, and barriers and enablers to, pulmonary rehabilitation referral in COPD: A systematic scoping review. Respir. Med.137, 103–114 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Choi, J. Y. et al. Pulmonary rehabilitation is associated with decreased exacerbation and mortality in patients with COPD: A nationwide Korean study. Chest165, 313–322. 10.1016/j.chest.2023.09.026 (2024). [DOI] [PubMed] [Google Scholar]
- 33.Zuo, H., Yu, L., Campbell, S. M., Yamamoto, S. S. & Yuan, Y. The implementation of target trial emulation for causal inference: A scoping review. J. Clin. Epidemiol.162, 29–37 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Nelson, M. E. et al. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc.39, 1435–1445 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Cheol Seong, S. et al. Data resource profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol.46, 799–800 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park, H. Y. et al. Chronic obstructive pulmonary disease and lung cancer incidence in never smokers: A cohort study. Thorax75, 506–509 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin, D. W., Cho, B. & Guallar, E. Korean National Health Insurance Database. JAMA Intern. Med.176, 138 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol.46, e15 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Hwangbo, Y. et al. Incidence of diabetes after cancer development: A Korean National Cohort Study. JAMA Oncol.4, 1099–1105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park, Y. B. et al. Revised COPD clinical practice guideline of the Korean Academy of tuberculosis and respiratory disease: A summary. Tuberc. Respir. Dis.81, 261–273. 10.4046/trd.2018.0029 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffith, M. F. et al. Overuse and misuse of inhaled corticosteroids among Veterans with COPD: A cross-sectional study evaluating targets for de-implementation. J. Gen. Intern. Med.35, 679–686. 10.1007/s11606-019-05461-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis.40, 373–383 (1987). [DOI] [PubMed] [Google Scholar]
- 43.Austin, P. C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat.10, 150–161. 10.1002/pst.433 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.