Abstract
Adjuvant oxaliplatin plus capecitabine (XELOX) therapy is recommended for patients with curatively resected colon cancer. However, prospective data on its practical application in Japanese patients are limited. Therefore, we aimed to conduct a long-term clinical evaluation of the efficacy and safety of adjuvant XELOX in patients with curatively resected stage III colon cancer (MCSCO-1024). This prospective, multi-center, open-label, single-arm, phase II study enrolled patients with curatively resected stage III colon cancer. The treatment protocol consisted of a 120-minute intravenous infusion of oxaliplatin (130 mg/m2) on day 1 and two divided doses oral capecitabine (2000 mg/m2/day) for 14 days in a 3-week cycle, totaling eight cycles (24 weeks). The primary endpoint was 3-year disease-free survival (DFS), and the secondary endpoints were 5-year overall survival and long-term prognosis of peripheral sensory neuropathy. A total of 196 patients were enrolled between November 2011 and August 2014 (34 months). The 3-year DFS rate was 73%, and the 5-year overall survival rate was 87%. The overall incidence of peripheral sensory neuropathy was 17%, with a 1% rate of grade 3 neuropathy after 5 years. Adjuvant XELOX demonstrated utility and safety in the clinical management of Japanese patients with stage III colon cancer.
Keywords: XELOX, Adjuvant chemotherapy, Japanese, Peripheral sensory neuropathy
Subject terms: Cancer, Cancer therapy, Chemotherapy
Introduction
Although studies have assessed the safety and tolerability of adjuvant XELOX therapy in Asian patients with curatively resected colon cancer1,2, there are limited reports on long-term outcomes, including peripheral neuropathy, in Japanese individuals. We previously published interim safety data from this study3. The aim of the present study was to present the long-term efficacy of adjuvant XELOX therapy and peripheral sensory neuropathy (PSN) associated with it for Japanese patients who underwent curative resection for stage III colon cancer (MCSCO-1024). In this study, the primary endpoint was 5-year Disease-Free Survival (DFS), whereas the secondary endpoints included degree of peripheral neuropathy after 5-years of treatment and 5-year overall survival (OS). Andre et al. reported that peripheral neuropathy at four years occurred in 15.4% of cases4. Hence, in the present study, we assumed that peripheral neuropathy is about 12% after five years.
Patients and methods
Eligibility criteria
Eligible patients were 20 years or older with a histologically confirmed diagnosis of colon adenocarcinoma, specifically stage III colon cancer, which included cancers of the rectosigmoid region. They underwent colectomy with D2 or D3 lymph node dissection and achieved curative resection (curative level A) without macroscopic or microscopic residual tumors. Other eligibility criteria included an Eastern Clinical Oncology Group (ECOG) performance status of 0 or 1, no prior chemotherapy or radiotherapy, adequate oral food intake, and proper functioning of vital organs (e.g., white blood cell count ≥ 3000/mm3, neutrophil count ≥ 1500/mm3, platelet count ≥ 100,000/mm3, serum creatinine ≥ 1.5 × the institutional upper limit of normal (ULN), serum total bilirubin ≥ 2.5 × ULN, and serum aspartate aminotransferase and alanine aminotransferase ≥ 2.5 × ULN). Written informed consent was obtained from all patients before enrollment. Patients were excluded if they had watery diarrhea, a synchronous or metachronous (within 5 years) malignancy other than carcinoma in situ, Common Terminology Criteria for Adverse Events (CTCAE) v 4.0, grade > 1 of PSN, regularly used insulin, uncontrollable congestive heart failure, angina, hypertension, arrhythmia, severe mental disorders, active infections, pregnancy or lactation, or sexually active patients unwilling to use contraception. Inadequate physical conditions diagnosed by primary physicians also led to exclusion. These criteria are consistent with that in our previous study3.
Study design and treatment
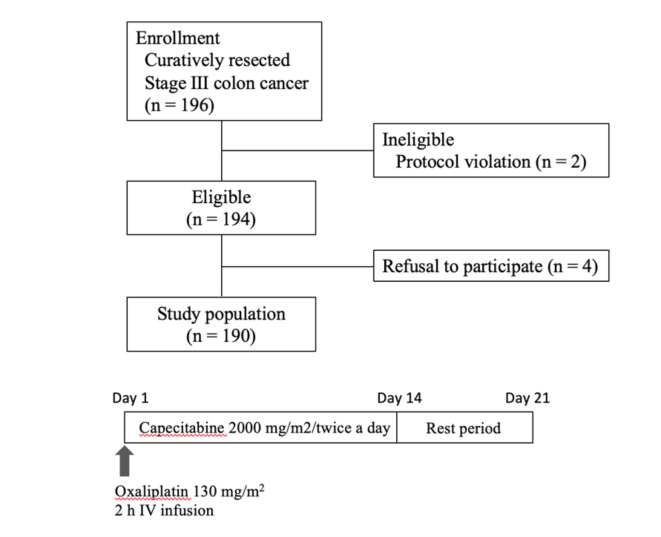
The study protocol for this multicenter, open-label, single-arm, phase II study was approved by the review board of each participating institution and registered in the University Hospital Medical Information Network (UMIN) registry system (UMIN ID: 000006742). All the methods employed in this study were performed in accordance with the guidelines stipulated in the Declaration of Helsinki. Patients were enrolled, and XELOX therapy was initiated within 8 weeks of surgery. The treatment protocol consisted of a 120-minute intravenous infusion of oxaliplatin (130 mg/m2) on day 1 and oral capecitabine (2000 mg/m2/day) in two divided doses for 14 days in a 3-week cycle, for a total of eight cycles (24 weeks), or until unacceptable toxicity occurred. The treatment protocol mirrors that in our previous study3.
Dose modification
If the following criteria for the initiation of treatment were not met on the day of or the day before the start of each course, treatment was postponed for a maximum of 42 days: neutrophil count ≥ 1500/mm3, platelet count ≥ 75,000/mm3, PSN persistent grade ≥ 1, grade ≥ 1 hand-foot syndrome (HFS), and other parameters at the attending physician’s discretion. Dose modifications were based on the most severe adverse events observed during the previous treatment cycle. If any of the following adverse events occurred (except PSN and HFS), oxaliplatin and capecitabine doses were reduced to 100 or 85 mg/m2 and 1500 or 1000 mg/m2, respectively: grade ≥ 2 leukopenia, neutropenia, or thrombocytopenia, and any other grade ≥ 3 hematological or grade ≥ 2 non-hematological adverse events. Oxaliplatin dose was reduced to 100 mg/m2 or 85 mg/m2 if the patient developed persistent grade 2 PSN or grade 3 PSN, respectively. Oxaliplatin was discontinued if the patient developed a second occurrence of grade 2/3 or 4 PSN and if grade ≥ 3 adverse events occurred. Capecitabine monotherapy was permitted in patients who refused or discontinued oxaliplatin due to drug-induced PSN. Capecitabine dose was adjusted for adverse events of grade ≥ 2 HFS. At the first occurrence of grade ≥ 2 HFS, treatment was interrupted and resumed at a reduced dose of 1500 mg/m2 after resolution to grade 1 or better. For second occurrences of grade ≥ 2 HFS, treatment was interrupted and resumed at a reduced dose of 1000 mg/m2 after resolution to grade 1 or better. These modifications are consistent with that our previous study3.
Staging criteria
The Japanese Classification of Colorectal Carcinoma, 7th edition (Second English edition)5, and the Union against Cancer (UICC) TNM Classification of Malignant Tumors 7th edition6 were used for tumor staging.
Statistical analysis
The primary endpoint was the 3-year DFS. The 3-year DFS rate was 70.9% in the XELOXA/NO16968 trial7. A total of 165 patients were required to test the null hypothesis versus the alternative hypothesis with a one-sided α level of 0.05 and β level of 0.1 when the critical value of the 3-year DFS was 60% and the expected value of 3-year DFS was 71%. In total, 195 patients were recruited for this study. The safety population was defined as patients who received at least one dose of the treatment protocol. PN was assessed using a patient-reported treatment diary and dictation and evaluated by a doctor according to CTCAE v. 4.0. Adherence to capecitabine was verified using a patient-reported treatment diary. Tumor assessments with abdominal computed tomography, ultrasonography, and chest radiography were performed at baseline and every 6 months for 5 years after the primary surgery. Tests for carcinoembryonic antigen and carbohydrate antigen 19–9 were conducted every 3 months for the first 3 years and every 6 months thereafter. The Kaplan–Meier method was used to evaluate the 3-year DFS and 5-year overall survival. The JMP® 10 software (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Patient characteristics
196 patients were enrolled at 25 institutions belonging to the Multi-center Clinical Study Group of Osaka, Colorectal Cancer Treatment Group (MCSGO). Among the 196 patients, one did not start the study protocol and one was in violation of protocol. Of the remaining 194 patients, four were excluded from the safety analysis of the per protocol set (PPS) (Fig. 1). Thus, a total of 190 patients were included in the safety analysis. The median patient age was 64 years (range: 29–78). There were 90 males and 100 females. The tumor invasion depth was T1 in six patients, T2 in 16 patients, T3 in 98 patients, and T4 in 70 patients. Lymph node status was N1 in 99 patients and N2 in 90 patients (Table 1). This result has already been reported in an earlier paper, 3) but is included again because it is necessary to understand the contents of this paper.
Fig. 1.
Flow chart.
Table 1.
Baseline clinical characteristics.
| n = 190 | |
|---|---|
| Age, years | |
| Median | 64 |
| Range | 29–78 |
| Sex | |
| Female | 100 |
| Male | 90 |
| ECOG PS | |
| 0 | 173 |
| 1 | 17 |
| Tumor location | |
| Accending | 54 |
| Transverse | 22 |
| Descending | 17 |
| Sigmoid/Rrectosigmoid | 97 |
| Depth of invasion | |
| T1 | 6 |
| T2 | 16 |
| T3 | 98 |
| T4a | 50 |
| T4b | 20 |
| Lymph node classification | |
| N1 | 99 |
| N2 | 91 |
| TNM Stagea | |
| IIIA | 16 |
| IIIB | 104 |
| IIIC | 70 |
| Japanese Stageb | |
| IIIa | 92 |
| IIIb | 98 |
ECOG PS: Eastern Clinical Oncology Group Performance Status.
aUICC TNM Classification of Malignant Tumors, 7th edition.
bJapanese Classification of Colorectal Carcinoma, 7th edition (Second English edition).
Efficacy
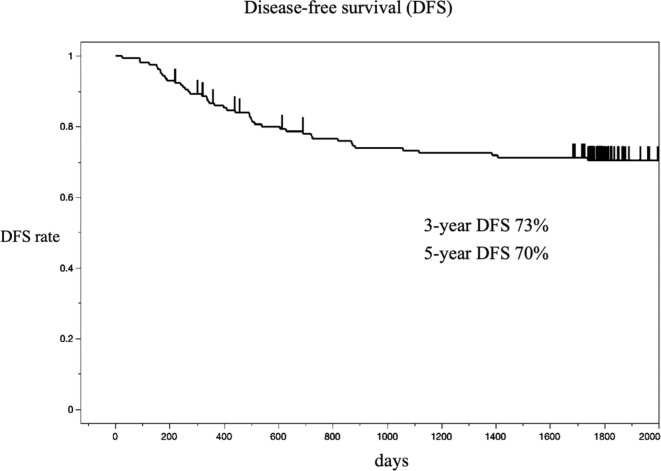
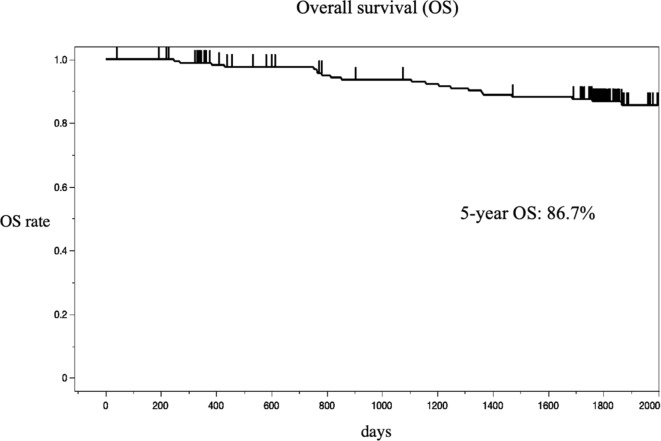
The 3-year and the 5-year DFS were 73% and 70%, respectively (Fig. 2). The 5-year overall survival (OS) was 87% (Fig. 3).
Fig. 2.
Kaplan-Meier estimates of the probability of disease-free survival (DFS).
Fig. 3.
Kaplan-Meier estimates of the probability of overall survival (OS).
Safety
Short-term safety has already been reported3. So, in this study, we primarily focused on the long-term prognosis of peripheral neuropathy. However, data were available for only 141 patients (72%) after 5 years. After one year, the overall incidence of PSN was 33%, with grade 3 cases accounting for 2% of all cases. The overall incidence of PSN was17%, with grade 3 cases accounting for 1% after 5 years. We investigated the grade and timing of PSN onset during XELOX treatment in 24 patients with residual PSN for up to 5 years but found no correlation with onset at 5 years (Table 2).
Table 2.
Peripheral sensory neuropathy (grades in CTCAEv4.0) after different courses of XELOX treatment.
| Patients number | 1 course | 2 course | 3 course | 4 course | 5 course | 6 course | 7 course | 8 course | 5 years |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 | 1 | 1 | 1 | 1 | Stop | 3 | |
| 2 | 0 | Stop | 2 | ||||||
| 3 | 1 | Stop | 1 | ||||||
| 4 | 1 | 2 | 2 | Stop | 1 | ||||
| 5 | 0 | 0 | 0 | 0 | Stop | 1 | |||
| 6 | 1 | 1 | 1 | 1 | Stop | 1 | |||
| 7 | 1 | 1 | 1 | 1 | 1 | 1 | Stop | 1 | |
| 8 | 0 | 2 | 2 | 1 | 1 | 1 | 1 | Stop | 1 |
| 9 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | Stop | 1 |
| 10 | 0 | 0 | 1 | 1 | 1 | 1 | 3 | Stop | 1 |
| 11 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 1 |
| 12 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| 13 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 |
| 14 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 15 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| 16 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 17 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| 18 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 19 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 20 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 21 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 22 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| 23 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Discussion
In our previous study, we discussed the short-term outcomes of adjuvant XELOX therapy in Japanese patients with curatively resected colon cancer3. Therefore, the primary focus of this study was to determine the long-term safety of this regimen in Japanese patients. We assessed the primary endpoint of 3-year DFS, and the long-term outcomes of 5-year DFS and OS.
The ACHIEVE Trial was a prospective investigation of the prognosis of Japanese patients8 to assess the safety and efficacy of different dosing periods. Their results showed 5-year DFS rates of 75.2% and 74.2% for the 3-month and 6-month doses, respectively. In our study, this value was slightly lower (70%). Conversely, our 5-year OS was 87%, consistent with the 87% for the 3-month treatment and 86% for the 6-month treatment in their study. Compared to the results of the IDEA study, which included data from other countries, our 3-year DFS rates were 72.9% and 74.1% for the 3-month and 6-month doses, respectively9. These results are consistent with previously reported data.
The MOSAIC Trial by Andre T et al. reported a 5-year DFS rate of 73.3% in patients with Stage III FOLFOX4 disease4. They found no significant difference in efficacy between FOLFOX and XELOX therapies, and the 5-year DFS rate for oxaliplatin-based adjuvant chemotherapy was 70–75%.
In European countries, Andre et al. reported that peripheral neuropathy at four years occurred in 15.4% of cases overall, with 0.7% experiencing grade 3 and 2.8% experiencing grade 24. In our study, after 5 years, the overall incidence was 17%, and grade 3 was observed in 1% of the patients. Although there were no significant differences in the values, the frequency of residual peripheral neuropathy was the same or slightly higher in Japanese patients than in Western individuals. Although the reasons for this discrepancy are unknown, it may be due to racial differences.
We evaluated 24 cases in which peripheral neuropathy persisted after 5 years of treatment and examined whether there was a correlation between the timing and severity of peripheral nerve symptoms during treatment. However, no clear correlation was observed. One reason for this could be that the assessment of numbness is subjective, as it relies on self-evaluation and varies in resistance among individuals, making it difficult to assign a specific grade. Therefore, it is possible that the results may have been different if there were assessed using objective criteria for peripheral nerve symptoms.
Nonetheless, this study had some limitations. First, data on peripheral neuropathy were available for only 72% of patients. Second, regarding the 5-year OS rate, chemotherapy treatment affected the survival rate in recurrent cases; however, there were no data on which chemotherapeutic agents were used or when they were administered in this study. Although the number of cases was small, and the results cannot be considered definitive, the number of Japanese patients with long-term grade 3 persistence was slightly higher than that of Western patients, possibly reflecting racial differences10.
In conclusion, in real clinical practice, the 5-year DFS and 5-year OS after XELOX adjuvant chemotherapy in Japan were similar to those in previous clinical trials. Thus, adjuvant XELOX is useful and safe for the treatment of patients with Stage III colon cancer in Japan. To the best of our knowledge, this is the first report on long-term peripheral neuropathy in Asian patients.
Acknowledgements
This study was supported by the Multicenter Clinical Study Group of the Osaka Colorectal Cancer Treatment Group.
Author contributions
K.D. designed this study, T.H. drafted the manuscript. All authors have read and approved the final manuscript.
Data availability
The supporting data are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Taishi Hata and Mamoru Uemura have contributed equally to this work.
References
- 1.Osawa, H., Handa, N. & Minakata, K. Efficacy and safety of capecitabine and oxaliplatin (CapOX) as an adjuvant therapy in Japanese for stage II/III colon cancer in a group at high risk of recurrence in retrospective study. Oncol. Res.22, 325 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu, J. et al. Efficacy and tolerability of adjuvant oral capecitabine plus intravenous oxaliplatin (XELOX) in Asian patients with colorectal cancer: 4-year analysis. Asian Pac. J. Cancer Prev.14, 6585–6590 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Danno, K. et al. Interim analysis of a phase II trial evaluating the safety and efficacy of capecitabine plus oxaliplatin (XELOX) as adjuvant therapy in Japanese patients with operated stage III colon cancer. Cancer Chemother. Pharmacol.80, 777–785 (2017). [DOI] [PubMed] [Google Scholar]
- 4.André, T. et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol.27, 3109–3116 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal Carcinoma 2nd edn (English) (Kanehara Shuppan, 2009).
- 6.Sobin, L. H. et al. Union against cancer (UICC) TNM Classification of Malignant Tumours 7th edn (Wiley, 2009).
- 7.Haller, D. G. et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J. Clin. Oncol.29, 1465–1471 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Yoshino, T. et al. Final analysis of 3 versus 6 months of adjuvant oxaliplatin and fluoropyrimidine-based therapy in patients with stage III colon cancer: the randomized phase III ACHIEVE trial. J. Clin. Oncol.40, 3419–3429 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Souglakos, J. et al. Three-versus six-month adjuvant FOLFOX or CAPOX for high-risk stage II and stage III colon cancer patients: the efficacy results of Hellenic Oncology Research Group (HORG) participation to the International Duration evaluation of adjuvant chemotherapy (IDEA) project. Ann. Oncol.30, 1304–1310 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Kamei, K. et al. A new monitoring tool CLIP test for progression of oxaliplatin-induced peripheral neuropathy: a multicenter prospective study. Asia Pac. J. Clin. Oncol.16, e257–e262 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The supporting data are available from the corresponding author on reasonable request.