Abstract
Data evaluating effectiveness of XBB.1.5-adapted vaccines against JN.1-related endpoints are scarce. This nationwide test-negative case-control study within the US Veterans Affairs Healthcare System aims to estimate vaccine effectiveness (VE) of BNT162b2 XBB.1.5-adapted vaccine compared to not receiving an XBB vaccine of any kind against COVID-19 hospitalization, emergency department or urgent care visits (ED/UC), and outpatient visits. Between September 25, 2023 and January 31, 2024, effectiveness was 24–35% during a period of JN.1 predominance and 50–61% during XBB predominance across all outcomes. VE within 60 days of vaccination during the likely JN.1 period was 32% (95% confidence interval 3–52%) against hospitalization, 41% (23–54%) against ED/UC visits, and 31% (1–52%) against outpatient visits. Corresponding VE during the likely XBB period was 62% (44–74%), 52% (37–63%), and 50% (25–66%) by setting, respectively. Here, we show the importance of strain match to maximize the public health impact of COVID-19 vaccination.
Subject terms: Outcomes research, Epidemiology, SARS-CoV-2, Vaccines
This study evaluates the effectiveness of the BNT162b2 XBB.1.5-adapted vaccine against COVID-19 outcomes within the US Veterans Affairs Healthcare System, finding 50–61% during XBB predominance and 24–35% effectiveness during JN.1 predominance, emphasizing the importance of strain match.
Introduction
Coronavirus disease 2019 (COVID-19), caused by the SARS-CoV-2 virus, continues to pose significant global health challenges more than four years after its emergence. COVID-19 still causes hundreds of thousands of deaths annually worldwide, and millions of survivors have long-term sequalae following acute COVID-191,2. Highly effective vaccines targeting SARS-CoV-2 have been key in blunting the public health impact of COVID-19 over the past several years3–8. Variant-adapted versions of the vaccines have been developed to maintain protection against COVID-19 as SARS-CoV-2 continues to evolve9–13. On September 11th, 2023, the United States Food and Drug Administration authorized and approved the use of the 2023–2024 updated COVID-19 mRNA vaccines of Moderna and Pfizer-BioNTech for all individuals ≥6 months of age14. On October 3rd, 2023, the FDA authorized and approved the use of the updated 2023–2024 Novavax COVID-19 vaccine for all individuals ≥12 years of age14. The 2023–2024 updated COVID-19 vaccines (hereafter referred to as XBB vaccines) were adapted to include a monovalent mRNA component to target the SARS-CoV-2 XBB.1.5 sublineage14. As of September 2, 2023, XBB.1.5 sublineage variants accounted for over 99% of the sequenced SARS-CoV-2 specimens in the United States15. Data from the early portion of the 2023–2024 respiratory season indicated that XBB vaccines have been effective at preventing COVID-19, including severe disease16–20. In the US, individuals aged 12 years and older are considered up to date with their COVID-19 vaccinations once they have received a single dose of an updated XBB vaccine, regardless of their prior vaccination history, according to Centers for Disease Control and Prevention (CDC) recommendations21.
JN.1, which is antigenically and phylogenetically distinct from XBB sub-lineages, was first detected in late August of 2023 and became the predominant circulating variant in the United States and elsewhere by mid-to-late December 202315. JN.1 has shown some signs of immune-escape22–24, which in turn could reduce the effectiveness of current XBB vaccines. Data evaluating the effectiveness of XBB vaccines against JN.1 are both scarce and urgently needed to help guide regulators and public health policy about the need for COVID-19 vaccine strain updates prior to the 2024–2025 respiratory virus season18. We evaluated the effectiveness of the Pfizer-BioNTech 2023–2024 formulation, which was a monovalent XBB.1.5-containing vaccine (hereafter referred to as BNT162b2 XBB vaccine), against COVID-19 hospitalization, emergency department or urgent care visits, and outpatient visits across time periods of XBB and JN.1 sub-lineage predominance among adults in a large US nationwide integrated healthcare system. Here, we show the importance of strain match to maximize the public health impact of COVID-19 vaccination.
Results
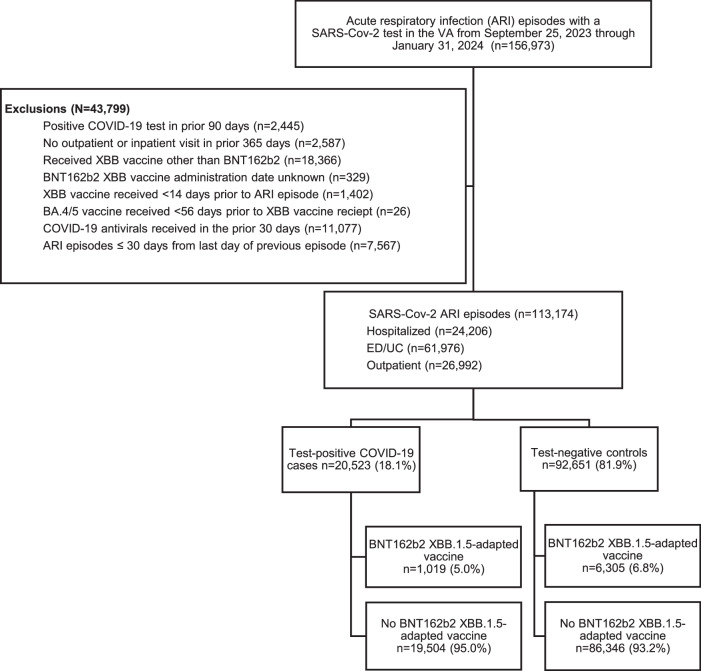
This study included 113,174 ARI episodes with corresponding SARS-CoV-2 test results (Fig. 1), of which 24,206 (21.4%) were hospital admissions, 61,976 (54.8%) were ED/UC visits, and 26,992 (23.9%) were outpatient visits. The cumulative frequency of ARI episodes and vaccination over time are shown in Supplemental Fig. 2. Median age was 65 years (interquartile range [IQR]: 52–75; Table 1). There were 9 cases and 4372 controls with multiple events. Most ARI episodes occurred among males (98,172; 86.7%) and those who were White (71,345; 63%); 29,699 (26.2%) were Black or African American, 29,083 (25.7%) had a Charlson Comorbidity Index ≥4, and 40,309 (35.6%) were immunocompromised. Overall, 18.1% (20,523/113,174) were SARS-CoV-2 test-positive cases and 81.9% (92,651/113,174) were test-negative controls. A total of 6.5% (7,324/113,174) received the BNT162b2 XBB vaccine with a median time since receipt of 54 days (range: 15–133; IQR: 35–76). Of cases and controls, 1,019 (5.0%) and 6,305 (6.8%), respectively, had ever received BNT162b2 XBB vaccine. Most (105,850/113,174 [93.5%]) had never received an XBB vaccine of any kind, and 24,747 (21.9%) had never received a COVID-19 vaccine of any kind. Among those who received the BNT162b2 XBB vaccine, the median time since receipt of their most recent previous (non-XBB) dose of COVID-19 vaccine was 433 days (IQR: 385–477).
Fig. 1. Study selection criteria.
ED/UC= emergency department/urgent care; VA = Veterans Affairs. Patients could contribute more than one ARI episode to the study if the episodes were more than 30 days apart. There were 9 cases and 4372 controls with multiple events.
Table 1.
Demographics and clinical characteristics of acute respiratory infection episodes (hospitalization, ED/UC visits, outpatient visits) with SARS-CoV-2 testing by COVID-19 case-control status
| Total (n = 113,174) | Test-positive COVID-19 cases (n = 20,523) | Test-negative controls (n = 92,651) | P value | |
|---|---|---|---|---|
| COVID vaccine status* | ||||
| ≥1 dose of BNT162b2 XBB vaccine | 7324 (6.5) | 1019 (5.0) | 6305 (6.8) | <0.001 |
| ≥1 dose of BA.4/5-adapted bivalent vaccine | 29,907 (26.4) | 5316 (25.9) | 24,591 (26.5) | 0.060 |
| ≥3 doses of original wild-type mRNA vaccine but no bivalent-adapted vaccines | 29,463 (26.0) | 5512 (26.9) | 23,951 (25.9) | 0.003 |
| ≥2 doses of original wild-type mRNA vaccine but no bivalent-adapted vaccines | 51,546 (45.6) | 9467 (46.1) | 42,079 (45.4) | 0.064 |
| No original wild-type mRNA or bivalent-adapted or non-mRNA vaccines | 24,747 (21.9) | 4454 (21.7) | 20,293 (21.9) | 0.530 |
| Time since last non-XBB adapted vaccine, median days (IQR) | 670 (413–820) | 679 (421–825) | 668 (412–819) | <0.001 |
| Age group | 0.002 | |||
| 18–64 years | 54,563 (48.2) | 10,099 (49.2) | 44,464 (48.0) | |
| 65–74 years | 27,821 (24.6) | 4820 (23.5) | 23,001 (24.8) | |
| ≥75 years | 30,790 (27.2) | 5604 (27.3) | 25,186 (27.2) | |
| Sex | 0.099 | |||
| Male | 98,172 (86.7) | 17,730 (86.4) | 80,442 (86.8) | |
| Female | 15,002 (13.3) | 2793 (13.6) | 12,209 (13.2) | |
| Body mass index category | <0.001 | |||
| Underweight ( < 18.5 kg/m2) | 1399 (1.2) | 183 (0.9) | 1216 (1.3) | |
| Healthy weight (18.5–24.9 kg/m2) | 34,083 (30.1) | 6223 (30.3) | 27,860 (30.1) | |
| Overweight (25–29.9 kg/m2) | 22,780 (20.2) | 4387 (21.4) | 18,393 (19.9) | |
| Obese ( ≥ 30 kg/m2) | 54,398 (48.3) | 9620 (46.9) | 44,778 (48.3) | |
| Missing | 514 (0.4) | 110 (0.5) | 404 (0.4) | |
| Region | <0.001 | |||
| Midwest | 22,167 (19.6) | 4599 (22.4) | 17,568 (19.0) | |
| Northeast | 15,063 (13.3) | 2940 (14.3) | 12,123 (13.1) | |
| West | 21,933 (19.4) | 3787 (18.5) | 18,146 (19.6) | |
| South | 54,011 (47.7) | 9197 (44.8) | 44,814 (48.4) | |
| Race | 0.397 | |||
| Black or African American | 29,699 (26.2) | 5364 (26.1) | 24,335 (26.3) | |
| White | 71,345 (63.0) | 12,905 (62.9) | 58,440 (63.1) | |
| Other race | 12,130 (10.7) | 2254 (11.0) | 9876 (10.7) | |
| Ethnicity | 0.831 | |||
| Hispanic or Latino | 10,527 (9.3) | 1917 (9.3) | 8610 (9.3) | |
| Not Hispanic or Latino | 102,647 (90.7) | 18,606 (90.7) | 84,041 (90.7) | |
| Smoking | <0.001 | |||
| Current or former | 58,062 (51.3) | 9987 (48.7) | 48,075 (51.9) | |
| Never | 36,570 (32.3) | 7106 (34.6) | 29,464 (31.8) | |
| Unknown | 18,542 (16.4) | 3430 (16.7) | 15,112 (16.3) | |
| Area deprivation index (ADI)** Quintile | 0.013 | |||
| 1 (Least Deprived) | 22,140 (19.6) | 4041 (19.7) | 18,099 (19.5) | |
| 2 | 22,134 (19.6) | 4127 (20.1) | 18,007 (19.4) | |
| 3 | 22,143 (19.6) | 4084 (19.9) | 18,059 (19.5) | |
| 4 | 22,126 (19.6) | 3981 (19.4) | 18,145 (19.6) | |
| 5 (Most Deprived) | 22,136 (19.6) | 3852 (18.8) | 18,284 (19.7) | |
| Unknown | 2495 (2.2) | 438 (2.1) | 2057 (2.2) | |
| VA Frailty index (VA-FI)*** | <0.001 | |||
| Non-frail (VA-FI ≤ 0.1) | 40,182 (35.5) | 7832 (38.2) | 32,350 (34.9) | |
| Pre-frail (VA-FI > 0.1-0.2) | 27,422 (24.2) | 5195 (25.3) | 22,227 (24.0) | |
| Mildly frail (VA-FI > 0.2-0.3) | 18,818 (16.6) | 3438 (16.8) | 15,380 (16.6) | |
| Moderately frail (VA-FI > 0.3-0.4) | 12,559 (11.1) | 1998 (9.7) | 10,561 (11.4) | |
| Severely frail (VA-FI ≥ 0.5) | 14,193 (12.5) | 2060 (10.0) | 12,133 (13.1) | |
| Healthcare exposures, 1 year prior | <0.001 | |||
| Hospital admission | 32,067 (28.3) | 4762 (23.2) | 27,305 (29.5) | |
| Nursing home admission | 3924 (3.5) | 947 (4.6) | 2977 (3.2) | <0.001 |
| Intensive care unit admission | 8825 (7.8) | 1172 (5.7) | 7653 (8.3) | <0.001 |
| Emergency department visit | 69,978 (61.8) | 11,733 (57.2) | 58,245 (62.9) | <0.001 |
| Primary care visit | 106,691 (94.3) | 19,349 (94.3) | 87,342 (94.3) | 0.957 |
| Charlson Comorbidity Index | <0.001 | |||
| 0 | 38,598 (34.1) | 7769 (37.9) | 30,829 (33.3) | |
| 1 | 21,698 (19.2) | 4057 (19.8) | 17,641 (19.0) | |
| 2 | 12,962 (11.5) | 2273 (11.1) | 10,689 (11.5) | |
| 3 | 10,833 (9.6) | 1912 (9.3) | 8921 (9.6) | |
| ≥ 4 | 29,083 (25.7) | 4512 (22.0) | 24,571 (26.5) | |
| Immunocompromised**** | 40,309 (35.6) | 5720 (27.9) | 34,589 (37.3) | <0.001 |
| Week of infection | <0.001 | |||
| Sep 25–Sep 30,2023 | 5503 (4.9) | 876 (4.3) | 4627 (5.0) | |
| Oct 01–Oct 07, 2023 | 4834 (4.3) | 731 (3.6) | 4103 (4.4) | |
| Oct 08–Oct 14, 2023 | 4358 (3.9) | 665 (3.2) | 3693 (4.0) | |
| Oct 15–Oct 21, 2023 | 4707 (4.2) | 779 (3.8) | 3928 (4.2) | |
| Oct 22–Oct 28, 2023 | 4624 (4.1) | 691 (3.4) | 3933 (4.2) | |
| Oct 29–Nov 04, 2023 | 4453 (3.9) | 663 (3.2) | 3790 (4.1) | |
| Nov 05–Nov 11, 2023 | 4741 (4.2) | 731 (3.6) | 4010 (4.3) | |
| Nov 12–Nov 18, 2023 | 5622 (5.0) | 972 (4.7) | 4650 (5.0) | |
| Nov 19–Nov 25, 2023 | 5155 (4.6) | 952 (4.6) | 4203 (4.5) | |
| Nov 26–Dec 02, 2023 | 6746 (6.0) | 1162 (5.7) | 5584 (6.0) | |
| Dec 03–Dec 09, 2023 | 6855 (6.1) | 1285 (6.3) | 5570 (6.0) | |
| Dec 10–Dec 16, 2023 | 6959 (6.1) | 1315 (6.4) | 5644 (6.1) | |
| Dec 17–Dec 23, 2023 | 8160 (7.2) | 1597 (7.8) | 6563 (7.1) | |
| Dec 24–Dec 30, 2023 | 8366 (7.4) | 1752 (8.5) | 6614 (7.1) | |
| Dec 31, 2023–Jan 06, 2024 | 8814 (7.8) | 1774 (8.6) | 7040 (7.6) | |
| Jan 07–Jan 13, 2024 | 7624 (6.7) | 1537 (7.5) | 6087 (6.6) | |
| Jan 14–Jan 20, 2024 | 5663 (5.0) | 1153 (5.6) | 4510 (4.9) | |
| Jan 21–Jan 27, 2024 | 6316 (5.6) | 1237 (6.0) | 5079 (5.5) | |
| Jan 28–Jan 31, 2024 | 3674 (3.2) | 651 (3.2) | 3023 (3.3) | |
| Prior COVID-19 infection**** | 31,195 (27.6) | 4878 (23.8) | 26,317 (28.4) | <0.001 |
| Virtual visit (outpatient only)***** | 2773 (10.3) | 1129 (23.9) | 1644 (7.4) | <0.001 |
| ICU admission (hospitalized only)****** | 4955 (20.5) | 624 (13.7) | 4331 (22.0) | <0.001 |
| Current influenza vaccine | 39,077 (34.5) | 7300 (35.6) | 31,777 (34.3) | <0.001 |
| Pneumococcal vaccine in last 5 years | 41,032 (36.3) | 7116 (34.7) | 33,916 (36.6) | <0.001 |
ARI acute respiratory infection, ED/UC emergency department/urgent care, VA Veterans Affairs.
Data are n (%) unless otherwise specified.
The data source did not indicate whether sex was self-reported or assigned.
All ARI encounters within a 30-day window were considered a single ARI episode. If multiple encounter types occurred during the 30-day window, the highest level of care was used (hospitalization > ED/UC > outpatient).
*The categories under “COVID vaccine status” were categorized as present or absent for each category.
**Area deprivation index (ADI) is a measure of socioeconomic disadvantage and was grouped into quintiles from least to most deprived neighborhoods (based on zip code)51.
***Frailty was defined using the ICD-10 updated Veterans Affairs Frailty Index (VA-FI) and categorized as non-frail (VA-FI ≤ 0.1), prefrail ( > 0.1–0.2), mildly frail ( > 0.2–0.3), moderately frail ( > 0.3–0.4), and severely frail ( > 0.4)52.
****Immunocompromised status was based on immunocompromising conditions in the year prior and immunosuppressive medications in the 90 days prior to the ARI episode based on a slightly modified algorithm that has been previously described49. Unlike the previously described algorithm, we used diagnosis codes to identify solid organ or hematopoietic stem cell transplantation and HIV/AIDs versus patient registries. Consistent with the previously described algorithm, we required one inpatient or two outpatient diagnosis code for an immunocompromising condition (leukemia, lymphoma, congenital immunodeficiencies, asplenia/hyposplenia, HIV/AIDS, and organ transplant) in the year prior and any immunosuppressive medication (alkylating agents, antibiotics, antimetbolites, antimitotics, monoclonal antibodies, other, immune-modulating agents, TNF Alpha antagonist, and steroids) with an outpatient days supply or inpatient administration in the 90 days prior.
****Prior COVID-19 infection was defined as any previous documented SARS-CoV-2 infection or no prior documented infection (yes or no).
*****Virtual visit was only assessed among those with an outpatient visit and defined as a virtual visit or not.
*****ICU admission was only assessed among those with a hospital admission and defined as admission to an ICU or not.
A higher proportion of those who tested negative for SARS-CoV-2 (compared to test-positive cases) had a Charlson Comorbidity Index of ≥4 (26.5% vs. 22.0%; P < 0.001), were immunocompromised (37.3% vs 27.9%; P < 0.001), or were hospitalized in the past year (29.5% vs. 23.2%; P < 0.001). These notable differences and other differences by case-control status are described in Table 1 and Supplemental Table 2. Table 1 presents aggregate SARS-CoV-2 positive cases and SARS-CoV-2 negative controls. Demographics and clinical characteristics for cases and controls for each VE outcome are presented in Supplemental Tables 3–5.
Among those with an ARI episode and corresponding SARS-CoV-2 test result, a higher proportion of those who received the XBB vaccine (compared to those who did not) were ≥65 years of age (75.3% vs 50.2%; P < 0.001), were Black or African American (31.4% vs 25.9%; P < 0.001), had a Charlson Comorbidity Index of ≥4 (38.4% vs 24.8%; P < 0.001), or had previously received a BA.4/5-adapted bivalent vaccine (76.8% vs 22.9%; P < 0.001). These notable differences and other differences by XBB vaccination status in those with an ARI episode and corresponding SARS-CoV-2 test result are described in Supplemental Table 6.
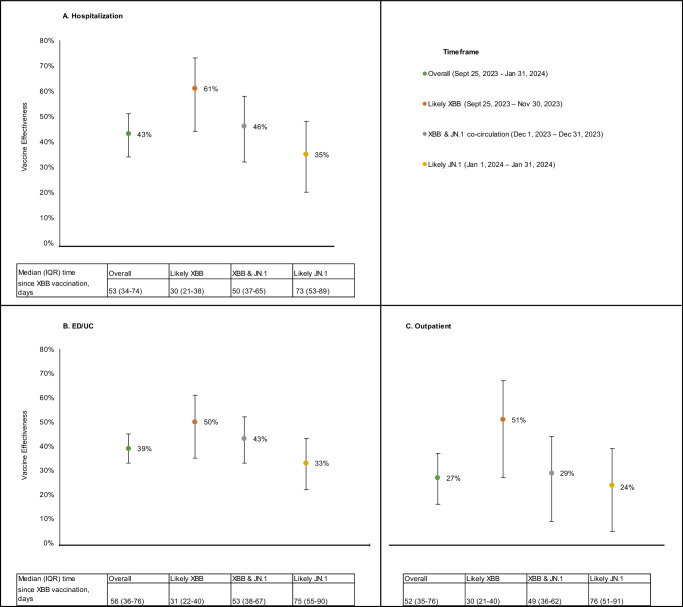
Overall adjusted VE of BNT162b2 XBB vaccine was 43% (95% CI: 34–51%) against hospitalization (Fig. 2A), 39% (33–45%) against ED/UC visits (Fig. 2B), and 27% (16–37%) against outpatient visits (Fig. 2C) compared to not receiving an XBB vaccine of any kind. Across all three outcomes VE declined after the likely XBB period during the JN.1 co-circulation and likely JN.1 periods, although CIs overlapped: 61% (44–73%), 46% (32–58%), and 35% (20–48%), respectively, against hospitalization (Fig. 2A); 50% (35–61%), 43% (33–52%), and 33% (22–43%), respectively, against ED/UC visits (Fig. 2B); and 51% (27–67%), 29% (9–44%), and 24% (5–39%), respectively, against outpatient visits (Fig. 2C).
Fig. 2. Adjusted vaccine effectiveness of the BNT162b2 XBB vaccine by time period.
A Hospitalization, B ED/UC visits, C Outpatient visits. ED/UC= emergency department/urgent care; IQR= interquartile range; VA= Veterans Affairs Compared the odds of receiving a BNT162b2 XBB vaccine between SARS-CoV-2 positive cases and SARS-CoV-2 negative controls. Adjusted for week of ARI episode, age, sex, race, ethnicity, BMI category, Charlson Comorbidity Index, receipt of 2023–2024 influenza vaccine, receipt of pneumococcal vaccine in the past 5 years, interactions with healthcare systems in the year prior, previous SARS-CoV-2 infection, smoking status, immunocompromised status, and Census region.
Median time since receipt of BNT162b2 XBB vaccine was ≤76 days for all variant periods, but longer in the XBB and JN.1 co-circulation (51 days, IQR: 37–65) and likely JN.1 (75 days, IQR: 54–90) periods, than the likely XBB time period (30 days, IQR: 21–40). Thus, to help tease apart the impact of waning effectiveness and increasing prevalence of JN.1 over time, Table 2 presents VE of the BNT162b2 XBB vaccine by both days since receipt of the vaccine and variant period. VE within 60 days of BNT162b2 XBB vaccination during the likely XBB period was 62% (95% CI 44–74%) against hospitalization, 52% (37–63%) against ED/UC visits, and 50% (25–66%) against outpatient visits. VE could not be calculated beyond 60 days of BNT162b2 XBB vaccination during the likely XBB period due to the small number of ARI episodes with a time since vaccination longer than 60 days during this time period. During the likely JN.1 period, VE within 60 days of vaccination was 32% (3–52%) against hospitalization, 41% (23–54%) against ED/UC visits, and 31% (1–52%) against outpatient visits, while VE 61–133 days since vaccination was 30% (16–41%) against ED/UC episodes and 20% (−4–38%) against outpatient visits.
Table 2.
Adjusted vaccine effectiveness of the BNT162b2 XBB vaccine for hospitalization, ED/UC visits, and outpatient visits by variant period and time since vaccination
| Outcome | Likely XBB (Sep 25 – Nov 30, 2023) | Likely JN.1 (Jan 1 – Jan 31, 2024) | ||
|---|---|---|---|---|
| VE (95% CI) by days since receipt of XBB dose | ||||
| ≤ 60 | 61–133 | ≤ 60 | 61–133 | |
| Hospitalization | 62 (44–74) | –* | 32 (3–52) | 37 (19–51) |
| ED/UC visit | 52 (37–63) | –* | 41 (23–54) | 30 (16–41) |
| Outpatient visit | 50 (25–66) | –* | 31 (1–52) | 20 (-4–38) |
CI= confidence interval; ED/UC= emergency department/urgent care; VE= vaccine effectiveness
Compared the odds of receiving a BNT162b2 XBB vaccine between SARS-CoV-2 positive cases and SARS-CoV-2 negative controls by days since vaccination. Adjusted for the week of ARI episode, age, sex, race, ethnicity, BMI category, Charlson Comorbidity Index, receipt of 2023-2024 influenza vaccine, receipt of pneumococcal vaccine in the past 5 years, interactions with healthcare systems in the year prior, previous SARS-CoV-2 infection, smoking status, immunocompromised status, and Census region.
*VE estimates based on 2 × 2 comparison tables with a cell size <5 were not reported due to imprecision.
Supplemental Tables 7, 8, 9, and 10 present VE results stratified by age group, immunocompromised status, obesity status, and smoking status, respectively. Although CIs overlapped across all stratified estimates, effectiveness estimates were 24–41% in those ≥65 years of age and 34–58% in those <65 years of age across all outcomes. VE was 33% against hospitalization and 34% against ED/UC visits in those who were immunocompromised and 49% and 42%, respectively, in those who were not. Finally, VE estimates were 34–50% across all outcomes in those who were obese and 21–39% in those who were not, and VE was 51% against hospitalization in non-smokers and 38% among current or former smokers.
Supplemental Tables 11 and 12 present VE results stratified by previous COVID-19 vaccination history. VE was 45% (34–54%) and 56% (36–69%) against hospitalization, 44% (37–51%) and 41% (25–53%) against ED/UC visits, and 26% (11–39%) and 38% (11–57%) against outpatient visits among those with 1 or more doses of BA.4/5-adapted bivalent vaccine and among those with 3 or more doses of original wild-type mRNA but no bivalent-adapted vaccines, respectively.
Discussion
In this test-negative case-control study conducted among a large national US Veteran population between September 25, 2023 and January 31, 2024, overall effectiveness of the BNT162b2 XBB vaccine compared to not receiving an XBB vaccine of any kind was 43% (95% CI: 34–51%) against COVID-19-associated hospitalization, 39% (33–45%) against ED/UC visits, and 27% (16–37%) against outpatient visits. These data add to a growing body of evidence that BNT162b2 XBB vaccine was effective at preventing a range of COVID-19 outcomes during the 2023–2024 respiratory virus season16–20,25. Notably, however, VE point estimates were lower for all three COVID-19 outcomes, including hospitalization, during the time period when COVID-19 was likely caused by JN.1 sub-lineages (24–35%) than when caused by XBB sub-lineages (50–61%), although CIs overlapped.
The observed reduction in VE during the likely JN.1 period did not appear to be driven by waning effectiveness over time. First, all VE estimates in our study had a median time since receipt of a BNT162b2 XBB dose of ≤76 days. Previous work has demonstrated that COVID-19 VE is generally stable, across all outcomes, for at least 10 to 12 weeks, with waning beginning thereafter, most often against less severe endpoints first26–28. Thus, it seems unlikely that the waning of protection would meaningfully impact our estimates given our relatively short follow-up period. Additionally, during the likely JN.1 period (when durability could be assessed), there appeared to be modest waning of effectiveness against JN.1 for less severe outcomes (i.e., ED/UC and outpatient visits) through a maximum of 133 days since BNT162b2 XBB vaccination. Finally, VE still appeared lower during the likely JN.1 period than the likely XBB period even when analyses were restricted to within 60 days of BNT162b2 XBB vaccination (32% [3–52%] and 62% [44–74%], respectively, against hospitalization; 41% [23–54%] and 52% [37–63%] against ED/UC visits; and 31% [1–52%] and 50% [25–66%] against outpatient visits).
These results suggest that XBB vaccines likely have reduced effectiveness against COVID-19 caused by JN.1 and its sub-lineages, which have now become predominant globally. Thus, like the last two years, a strain change for the upcoming 2024–2025 season also appears warranted to combat not only waning immunity over time, but reduced effectiveness stemming from continued SARS-CoV-2 evolution and vaccine strain mismatch. Reassuringly, annual updates to mRNA COVID-19 vaccines have restored protection against COVID-19 that has eroded over time due to a combination of waning of vaccine protection and the emergence of antigenically distinct strains9–13,16,18,25. Thus, analogous to influenza, although prior versions of COVID-19 vaccines once provided high levels of protection, the combination of waning vaccine-induced immunity and continuous SARS-CoV-2 strain evolution eventually renders prior versions of vaccines less effective over time, even against severe clinical outcomes like hospitalization. Vaccine effectiveness in the full study population was similar to VE among those who previously received a BA.4/5 vaccine, which is consistent with other published data suggesting that residual protection from prior COVID-19 vaccinations is limited29. This, in turn, warrants routine updates to COVID-19 vaccines, as with influenza, so long as SARS-CoV-2 continues to circulate and cause disease.
Other preliminary data also support our findings that XBB vaccines may have reduced effectiveness against JN.1. A recent test-negative design study conducted by CDC that evaluated the effectiveness of XBB vaccines against symptomatic COVID-19 detected in the pharmacy setting between September 21, 2023, and January 14, 2024, suggested that VE against JN.1 sub-lineages may be lower than XBB sub-lineages (49% [19–68%] and 60% [35–75%], respectively), although variant-specific CIs overlapped in this analysis as well18. In addition, when looking at the totality of XBB VE data published to date globally, there appears to be a general decline in VE over time—corresponding to increases in the prevalence of JN.1 over the same time period. For example, early reports showed >70% effectiveness for XBB vaccines against COVID-19 hospitalization during time periods when JN.1 was not circulating or was present only at low levels17,20. However, subsequent reports with longer study periods that included more JN.1 co-circulation have shown sequentially lower VE. A report from Kaiser Permanente Southern California that included data through mid-December showed a slightly lower overall VE of roughly 60% against COVID-19 hospitalization16, and a recent CDC report that included data through the end of January 2024 showed roughly 40–50% VE against COVID-19 hospitalization25. This latter CDC estimate25 is similar to the 40% overall VE against hospitalization we observed in this study which also included data through the end of January 2024. Finally, these VE data are consistent with data showing that JN.1 is phylogenetically and antigenically distant from XBB sub-lineages22,23, and that neutralization activity of XBB vaccines is lower against JN.1 compared to compared to XBB strains24.
It is possible that the lower overall VE against all COVID-19 measured outcomes observed in our study may also be explained by the national VA population, which is generally older, predominantly male, and with a high prevalence of multiple comorbid conditions30. Reductions in vaccine immunogenicity and effectiveness with increasing age have been demonstrated previously31, and we observed slightly lower effectiveness among individuals ≥65 years of age in our study. Male sex and comorbidities are also well-established risk factors for severe COVID-1932,33. Further, our study differed from previous VE studies by including both NAAT and RAT to identify SARS-CoV-2 rather than NAAT alone. The sensitivity of RAT is lower than molecular testing, particularly as population immunity against SARS-CoV-2 has increased over time34,35. Although the inclusion of RAT allowed a more robust outpatient sample to be included in the study and represents real-world testing behaviors, an under-detection of cases (due to potential reduced RAT sensitivity) could bias VE estimates towards the null presuming non-differential misclassification across vaccination status. Despite these study population and design differences that could partially explain the slightly lower overall VE against COVID-19 outcomes observed in our study compared with previous reports, these differences would not explain the marked differences in point estimates for VE against XBB-related and JN.1-related COVID-19 we saw in our study population across multiple outcomes. Finally, ours and other recent XBB VE estimates against COVID-19 outcomes, were derived from study populations where nearly all participants had some level of pre-existing COVID-19 immunity stemming from prior vaccination and infection36, which may result in lower apparent effectiveness compared to the initial COVID-19 vaccine rollout when levels of baseline immunity were lower37.
VE estimates against hospitalization, ED/UC visits, and outpatient visits were all somewhat lower in individuals ≥65 years of age than those 18‒64 years of age. However, in subgroup analyses evaluating VE by immunocompromised status, obesity, and smoking, results were somewhat mixed and with mostly overlapping 95% CIs12,38,39. More studies are needed to evaluate the effectiveness of COVID-19 by various sociodemographic characteristics and by underlying risk conditions.
There are several important limitations to consider when interpreting these data. The test-negative case-control study, while considered a reliable design for evaluating real-world VE due to its ability to mitigate both healthcare-seeking and testing behavior, is still susceptible to selection bias40–42. Namely, although we employed statistical adjustments to control for potentially confounding patient clinical and sociodemographic characteristics, there may still have been residual confounding by unmeasured factors. For instance, variations in health-seeking behavior and adherence to public health recommendations could influence both vaccination status and COVID-19 outcomes. We accounted for SARS-CoV-2 activity and circulating strain by adjusting for calendar week and performing analyses that were stratified by variant time period, however, this may not have fully accounted for increased SARS-CoV-2 activity in December 2023 and January 202443. It is also important to note that our VE estimates against XBB or JN.1 sub-lineages relied on time periods rather than sequencing data. However, our variant time period estimates are likely still a valid approximation of strain-specific VE because XBB and JN.1 sub-lineages accounted for >90% and >80% of sequenced SARS-CoV-2 strains in the United States during our likely XBB and likely JN.1 periods, respectively44. Another limitation is that we were unable to evaluate VE against COVID-19 outcomes beyond roughly 4 months since receipt of a BNT162b2 XBB dose given our study period. Thus, additional analyses of longer-term durability are needed. Similarly, our analysis, like others before it, was unable to evaluate the durability of VE against XBB sub-lineages given they were overtaken by JN.1 fewer than 90 days after vaccine rollout16–20,25.
We were also unable to assess whether VE estimates against COVID-19 differed between those tested by NAAT versus RAT, as the type of laboratory test is not uniformly captured in the VA database. Additionally, it is possible that some of the SARS-CoV-2-positive ARI episodes were incidental infections (i.e., sought care “with COVID-19” rather than “for COVID-19”), which could also lead to underestimation of VE. The accuracy of XBB vaccination status depended on the completeness and reliability of the vaccination records within the VA system. However, VA data capture most vaccines given outside of the VA, and any rare instances of uncaptured COVID-19 vaccine administrations would likely result in an underestimation of VE. Due to heterogeneity in both the number of previous COVID-19 vaccinations received and the vaccine manufacturer of previous vaccinations, we did not conduct stratified analyses by specific vaccination history. The impact of specific COVID-19 vaccination history on our results is likely limited given that nearly all individuals in the US have some level of pre-existing immunity36 and it is now well accepted that there is waning of both infection- and vaccine-induced immunity over time45. Finally, our findings may not be generalizable to the general US population or globally, due to the unique characteristics of the VA population that include a higher proportion of patients that are older, male, and have underlying chronic medical conditions. Veterans may also exhibit unique healthcare-seeking behavior given their access to the VA Healthcare System. Further, we excluded individuals who received COVID-19 antiviral treatment prior to their ARI episode, which may affect the representativeness of our study population, as those who received antivirals are likely to have different health profiles compared to those who did not. This could influence the generalizability of our findings, however, previous reports have shown that including these individuals had little impact on study results16.
BNT162b2 XBB vaccine was effective at preventing a range of COVID-19 outcomes, including against COVID-19 hospitalization, ED/UC visits, and outpatient visits, during the 2023–2024 respiratory virus season. However, VE appeared lower during a period when most COVID-19 cases were caused by JN.1 even after accounting for time since receipt of a BNT162b2 XBB dose. These data underscore the importance of strain match to maximize the public health impact of COVID-19 vaccination.
Methods
Ethics approval
Our study complies with all relevant ethical regulations and was determined to be exempt by the VA Providence Healthcare System (VAPHS) Institutional Review Board (IRB) and approved by the VAPHS Research and Development Committee. As this was a retrospective study of existing health records and exempt from IRB review, informed consent requirements are not applicable.
Setting and Participants
We conducted a nationwide test-negative case-control study using clinical data from patients of the US Veterans Affairs (VA) Healthcare System. The VA Healthcare system is the largest integrated healthcare system in the US, with over 9 million enrolled Veterans and over 1,300 healthcare facilities nationwide, including 172 VA Medical Centers (hospitals) and 1138 outpatient clinics46.
We assessed the effectiveness of the BNT162b2 XBB vaccine among adult patients ≥18 years of age diagnosed with an acute respiratory infection (ARI; see Supplemental Table 1) in the hospital, emergency department (ED), urgent care (UC), or outpatient setting (in-person or virtual) between September 25, 2023 and January 31, 2024. To be included, patients had to be tested for SARS-CoV-2 via nucleic acid amplification test (NAAT) or rapid antigen test (RAT) within 14 days prior through 3 days after the ARI encounter. All ARI encounters within a 30-day window were considered a single ARI episode, and the encounter at the highest level of care (i.e., hospitalization > ED/UC visit > outpatient visit) was selected for inclusion (Supplemental Fig. 1). Patients were excluded if they (1) did not have at least one visit to the VA Healthcare System in the previous 12 months, (2) had another prior positive SARS-CoV-2 test in the 90 days prior to their ARI episode, (3) received an XBB vaccine other than BNT162b2, (4) received BNT162b2 XBB vaccine within 8 weeks of a prior COVID-19 vaccine dose, (5) received BNT162b2 XBB vaccine within 14 days prior to their ARI episode, (6) received BNT162b2 XBB vaccine but the date of administration was unknown, or (7) received a COVID-19 antiviral (nirmatrelvir/ritonavir, remdesivir, or molnupiravir) within 30 days prior to their ARI episode. Patients could contribute more than one ARI episode to the study if the episodes were more than 30 days apart (Supplemental Fig. 1).
Outcomes
Three mutually exclusive ARI episode outcome categories were assessed: (1) hospital admission, (2) ED or UC visit (without subsequent hospital admission), and (3) outpatient visits (without a subsequent ED/UC visit or hospital admission). Within each ARI outcome category, cases were those with a positive SARS-CoV-2 NAAT or RAT result, and controls were those who tested negative.
Exposure
The exposure of interest was the receipt of the BNT162b2 XBB vaccine at least 14 days before the ARI episode. Those who had not received an XBB vaccine of any kind were considered unexposed, regardless of prior COVID-19 vaccination history, and included those who were not up to date with COVID-19 vaccination and those who were not previously vaccinated. Vaccine exposure was evaluated using the VA integrated electronic health record, which captures data across all healthcare settings, including all vaccines administered47. COVID-19 vaccines were offered free of charge to all Veterans enrolled in VA healthcare based on Centers for Disease Control and Prevention (CDC) recommendations during the study period48.
Statistical Analyses
The primary analysis estimated the vaccine effectiveness (VE) of the BNT162b2 XBB vaccine against hospitalization, ED/UC visits, and outpatient visits separately compared to not receiving an XBB vaccine of any kind. Effectiveness was estimated overall (September 25, 2023 through January 31, 2024) and during three time periods based on the predominantly circulating SARS-CoV-2 strains, which were determined using GISAID variant tracking data for the United States44: (1) likely XBB period defined as September 25, 2023, through November 30, 2023; (2) XBB and JN.1 co-circulation period defined as December 1, 2023, through December 31, 2023; and (3) likely JN.1 period defined as January 1, 2024 through January 31, 2024. XBB and JN.1 sub-lineages accounted for >90% and >80% of sequenced SARS-CoV-2 strains in the United States during our likely XBB and likely JN.1 periods, respectively44.
Separate multivariable logistic regression models were used to compare the odds of receiving a BNT162b2 XBB vaccine between SARS-CoV-2 positive cases and test-negative controls within each ARI outcome category (i.e., hospitalization, ED/UC visit, outpatient visit) and variant period while adjusting for potential confounding variables. Adjusted odds ratios (OR) and corresponding 95% Wald confidence intervals (CI) were constructed. VE was calculated as 1 minus the corresponding adjusted OR (and 95% CI), multiplied by 100%. The following variables were selected a priori based on previous literature, and controlled for in each multivariable logistic regression model: calendar week of ARI episode, age (18–64, 65–74, ≥75 years), sex (male or female), race (Black, White, or other race), ethnicity (Hispanic or non-Hispanic), body mass index (BMI) categories (underweight, healthy weight, overweight, obese, missing), Charlson Comorbidity Index (0, 1, 2, 3, ≥ 4), receipt of influenza vaccine during the 2023–2024 season (yes or no), receipt of pneumococcal vaccine in the past 5 years (yes or no), encounters with the VA healthcare system in the year prior (intensive care unit admission, hospital admission, nursing home admission, ED visit, primary care visit; 0 or ≥1 for each), smoking status (current or former smoker or never smoker), immunocompromised (yes or no), Census region (Northeast, Midwest, South, or West)12, and prior documented SARS-CoV-2 infection.
In secondary analyses, we evaluated adjusted VE by time since BNT162b2 XBB vaccination within both the likely XBB and likely JN.1 time periods to simultaneously assess the impact of the potential waning of protection and variant predominance. We calculated adjusted VE estimates within 60 and 61–133 days since vaccination for the likely XBB and JN.1 time periods18.
Additional stratified VE analyses were also conducted by age group ( < 65 and ≥65 years), immunocompromised status (yes or no)49, obesity status (BMI ≥ 30 kg/m2 and <30 kg/m2)50, smoking status (current or former smoker and never smoker), and previous COVID-19 vaccination history (one or more doses of BA.4/5-adapted bivalent vaccine, 3 or more doses of original wild-type mRNA but no bivalent-adapted vaccines).
Chi-square tests were used to compare differences in proportions between SARS-CoV-2 positive cases and test-negative controls. For continuous variables, comparisons of medians were performed using the Wilcoxon Rank Sum test. Statistical significance was set at a two-sided p value of <0.05. All logistic regression models were carefully checked for assumptions and model fit. All analyses were conducted using SAS (Version 9.2, SAS Institute Inc., Cary, NC, USA).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
Views expressed are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs. The research study would not have been possible without the health information from patients under the care of the Veterans Health Administration. We express our gratitude to the VA patients for their invaluable contributions to medical and scientific progress. This study was conducted as a collaboration between VA Providence Healthcare System and Pfizer. Pfizer is the study sponsor. HJA, ARC, VVL and KLL are employees of the VA Providence Healthcare System, which received funding from Pfizer in connection with the development of this manuscript and for data analysis.
Author contributions
Conception and design of the study: A.R.C., H.J.A., V.V.L., L.P., E.J.Z., J.M.M. Data generation: A.R.C., H.J.A., V.V.L. Analysis and/or interpretation of the data: A.R.C., H.J.A., V.V.L., L.P., E.J.Z., L.J., K.L.L., J.M.M. Preparation or critical revision of the manuscript: A.R.C., H.J.A., V.V.L., L.P., E.J.Z., L.J., K.L.L., J.M.M.
Peer review
Peer review information
Nature Communications thanks Brechje de Gier, who co-reviewed with Bente Smagge, Weibing Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data supporting the findings of this study are not publicly available due to the inclusion of identifiable protected health information from the Veterans Health Administration. Privacy regulations prevent the open sharing of the individual-level data used in this study and any data covered under these regulations cannot be shared. The Veterans Health Administration may approve the sharing of some study data after verifying de-identification, though this may not include all final study data. Each request is subject to approval by the ethics board, privacy office, and information systems and security office. For such requests, please contact the corresponding author.
Competing interests
Haley J. Appaneal has received research funding from Pfizer. Aisling R. Caffrey has received research funding from AbbVie, Merck, and Pfizer. Vrishali V. Lopes has no competing interest to declare. Kerry L. LaPlante has received research funding from AbbVie, Merck, and Pfizer and has been an advisor for Ferring Pharmaceuticals, AbbVie, and Seres Therapeutics. Laura Puzniak, Evan J. Zasowski, Luis Jodar, and John M. McLaughlin are employees and shareholders of Pfizer Inc.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-53842-w.
References
- 1.World Health Organization (WHO). Coronavirus (COVID-19) Dashboard. Accessed 21 Feb 2024, https://covid19.who.int/.
- 2.Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol21, 133–146 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack, F. P. et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden, L. R. et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med384, 403–416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey, M. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet397, 99–111 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadoff, J. et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med384, 2187–2201 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marra, A. R. et al. The effectiveness of COVID-19 vaccine in the prevention of post-COVID conditions: a systematic literature review and meta-analysis of the latest research. Antimicrob. Steward Health. Epidemiol.3, e168 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson, O. J. et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect. Dis.22, 1293–1302 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin, D. Y. et al. Effectiveness of Bivalent Boosters against Severe Omicron Infection. N. Engl. J. Med388, 764–766 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Link-Gelles, R. et al. Effectiveness of Bivalent mRNA Vaccines in Preventing Symptomatic SARS-CoV-2 Infection - Increasing Community Access to Testing Program, United States, September-November 2022. MMWR Morb. Mortal. Wkly Rep.71, 1526–1530 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbel, R. et al. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: a retrospective cohort study. Lancet Infect. Dis.23, 914–921 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tartof, S. Y. et al. Effectiveness of BNT162b2 BA.4/5 bivalent mRNA vaccine against a range of COVID-19 outcomes in a large health system in the USA: a test-negative case-control study. Lancet Respir. Med11, 1089–1100 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Tenforde, M. W. et al. Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults - VISION Network, Nine States, September-November 2022. MMWR Morb. Mortal. Wkly Rep.71, 1637–1646 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Food and Drug Administration. COVID-19 Vaccines for 2023-2024. Accessed 13 Dec 2023, https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines-2023-2024 (2023).
- 15.Centers for Disease Control and Prevention (CDC). COVID Data Tracker: Monitoring Variant Proportions. Accessed 30 Jan 2024, https://covid.cdc.gov/covid-data-tracker/#variant-proportions.
- 16.Tartof, S. Y. et al. BNT162b2 XBB1.5-adapted Vaccine and COVID-19 Hospital Admissions and Ambulatory Visits in US Adults. medRxiv, 2023.2012.2024.23300512 (2023).
- 17.Hansen, C. H. et al. Short-term effectiveness of the XBB.1.5 updated COVID-19 vaccine against hospitalisation in Denmark: a national cohort study. Lancet Infect. Dis.24, e73–e74 (2024). [DOI] [PubMed] [Google Scholar]
- 18.Link-Gelles, R. et al. Early Estimates of Updated 2023-2024 (Monovalent XBB.1.5) COVID-19 Vaccine Effectiveness Against Symptomatic SARS-CoV-2 Infection Attributable to Co-Circulating Omicron Variants Among Immunocompetent Adults - Increasing Community Access to Testing Program, United States, September 2023-January 2024. MMWR Morb. Mortal. Wkly Rep.73, 77–83 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skowronski, D. M. et al. 2023/24 mid-season influenza and Omicron XBB.1.5 vaccine effectiveness estimates from the Canadian Sentinel Practitioner Surveillance Network (SPSN). Euro Surveill29, 10.2807/1560-7917.Es.2024.29.7.2400076 (2024). [DOI] [PMC free article] [PubMed]
- 20.van Werkhoven, C. H. et al. Early COVID-19 vaccine effectiveness of XBB.1.5 vaccine against hospitalisation and admission to intensive care, the Netherlands, 9 October to 5 December 2023. Euro Surveill29, 10.2807/1560-7917.ES.2024.29.1.2300703 (2024). [DOI] [PMC free article] [PubMed]
- 21.Centers for Disease Control and Prevention (CDC). Stay Up to Date with COVID-19 Vaccines. Accessed 17 May 2024, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html (Updated May 14, 2024).
- 22.World Health Organization (WHO). Initial risk evaluation of JN.1, 19 December 2023https://www.who.int/docs/default-source/coronaviruse/18122023_jn.1_ire_clean.pdf?sfvrsn=6103754a_3 (2024). Accessed 21 Feb.
- 23.Yang, S. et al. Fast evolution of SARS-CoV-2 BA.2.86 to JN.1 under heavy immune pressure. Lancet Infect. Dis.24, e70–e72 (2024). [DOI] [PubMed] [Google Scholar]
- 24.Wang, Q. et al. XBB.1.5 monovalent mRNA vaccine booster elicits robust neutralizing antibodies against XBB subvariants and JN.1. Cell Host Microbe10.1016/j.chom.2024.01.014 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeCuir, J. et al. Interim Effectiveness of Updated 2023-2024 (Monovalent XBB.1.5) COVID-19 Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalization Among Immunocompetent Adults Aged >/=18 Years - VISION and IVY Networks, September 2023-January 2024. MMWR Morb. Mortal. Wkly Rep.73, 180–188 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng, H. F. et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med28, 1063–1071 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gazit, S. et al. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. BMJ377, e071113 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, Z. et al. Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant infection, severe illness, and death. BMC Med20, 400 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tartof, S. Y. et al. Estimated Effectiveness of the BNT162b2 XBB Vaccine Against COVID-19. JAMA Intern Med10.1001/jamainternmed.2024.1640 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberger, D. M. et al. Excess Mortality Among Patients in the Veterans Affairs Health System Compared With the Overall US Population During the First Year of the COVID-19 Pandemic. JAMA Netw. Open6, e2312140 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palacios-Pedrero, M. et al. Signs of immunosenescence correlate with poor outcome of mRNA COVID-19 vaccination in older adults. Nat. Aging2, 896–905 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Twitchell, D. K. et al. Examining Male Predominance of Severe COVID-19 Outcomes: A Systematic Review. Androg. Clin. Res Ther.3, 41–53 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention (CDC). Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals, https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. [PubMed]
- 34.Chu, V. T. et al. Comparison of Home Antigen Testing With RT-PCR and Viral Culture During the Course of SARS-CoV-2 Infection. JAMA Intern Med182, 701–709 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frediani, J. K. et al. The New Normal: Delayed Peak SARS-CoV-2 Viral Loads Relative to Symptom Onset and Implications for COVID-19 Testing Programs. Clin. Infect. Dis.78, 301–307 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC). 2022-2023 Nationwide COVID-19 Infection- and Vaccination-Induced Antibody Seroprevalence (Blood donations). Accessed 25 Mar 2024, https://covid.cdc.gov/covid-data-tracker/#nationwide-blood-donor-seroprevalence-2022.
- 37.Centers for Disease Control and Prevention (CDC). Nationwide COVID-19 Infection-Induced Antibody Seroprevalence (Commercial laboratories). Accessed 25 Mar 2024, https://covid.cdc.gov/covid-data-tracker/#national-lab.
- 38.Ferrara, P., Gianfredi, V., Tomaselli, V. & Polosa, R. The Effect of Smoking on Humoral Response to COVID-19 Vaccines: A Systematic Review of Epidemiological Studies. Vaccines (Basel)10, 10.3390/vaccines10020303 (2022). [DOI] [PMC free article] [PubMed]
- 39.Faizo, A. A. et al. A potential association between obesity and reduced effectiveness of COVID-19 vaccine-induced neutralizing humoral immunity. J. Med Virol.95, e28130 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foppa, I. M., Haber, M., Ferdinands, J. M. & Shay, D. K. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine31, 3104–3109 (2013). [DOI] [PubMed] [Google Scholar]
- 41.De Serres, G., Skowronski, D. M., Wu, X. W. & Ambrose, C. S. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill18, 10.2807/1560-7917.es2013.18.37.20585 (2013). [DOI] [PubMed]
- 42.Jackson, M. L. et al. The impact of selection bias on vaccine effectiveness estimates from test-negative studies. Vaccine36, 751–757 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention (CDC). Trends in United States COVID-19 Hospitalizations, Deaths, Emergency Department (ED) Visits, and Test Positivity by Geographic Area. Accessed 22 Mar 2023, https://covid.cdc.gov/covid-data-tracker/#trends_weeklyhospitaladmissions_select_00.
- 44.GISAID. Tracking of hCoV-19 Variants. Accessed 1 Mar 2024, https://gisaid.org/hcov19-variants/.
- 45.Ciesla, A. A. et al. Effectiveness of Booster Doses of Monovalent mRNA COVID-19 Vaccine Against Symptomatic Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Children, Adolescents, and Adults During Omicron Subvariant BA.2/BA.2.12.1 and BA.4/BA.5 Predominant Periods. Open Forum Infect. Dis.10, ofad187 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veterans Health Administration. About VHA. Accessed 16 May 2023, https://www.va.gov/health/aboutvha.asp (August 15, 2022).
- 47.U.S. Department of Veterans Affairs. Department of Veterans Affairs COVID-19 National Summary. Accessed 31 Jan 2024, https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary.
- 48.U.S. Department of Veterans Affairs. COVID-19 vaccines at VA, https://www.va.gov/health-care/covid-19-vaccine/.
- 49.Tartof, S. Y. et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: A retrospective cohort study. Lancet Reg. Health Am.9, 100198 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention (CDC). Defining Adult Overweight & Obesity. Accessed 31 Jan 2024, https://www.cdc.gov/obesity/basics/adult-defining.html (June 3, 2022).
- 51.Kitchen, C., Hatef, E., Chang, H. Y., Weiner, J. P. & Kharrazi, H. Assessing the association between area deprivation index on COVID-19 prevalence: a contrast between rural and urban U.S. jurisdictions. AIMS Public Health8, 519–530 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng, D. et al. Updating and Validating the U.S. Veterans Affairs Frailty Index: Transitioning From ICD-9 to ICD-10. J. Gerontol. A Biol. Sci. Med Sci.76, 1318–1325 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are not publicly available due to the inclusion of identifiable protected health information from the Veterans Health Administration. Privacy regulations prevent the open sharing of the individual-level data used in this study and any data covered under these regulations cannot be shared. The Veterans Health Administration may approve the sharing of some study data after verifying de-identification, though this may not include all final study data. Each request is subject to approval by the ethics board, privacy office, and information systems and security office. For such requests, please contact the corresponding author.