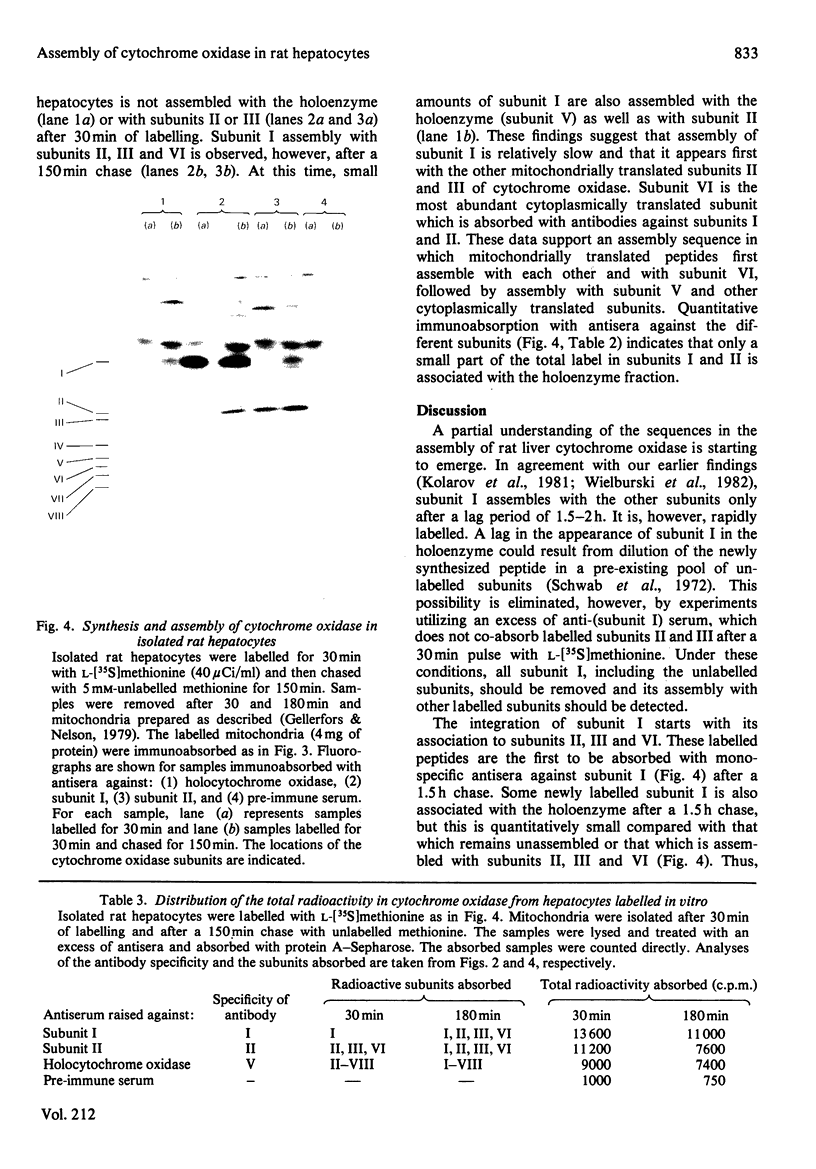
Abstract
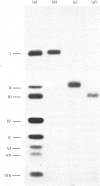
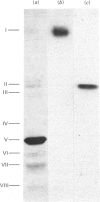
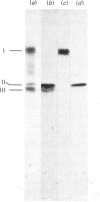
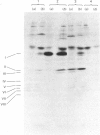
The assembly of cytochrome oxidase was studied in isolated rat liver mitochondria and isolated rat hepatocytes labelled in vitro with L-[35S]methionine. This was achieved by studying the temporal association of radioactive subunits which are immunoabsorbed with antibodies against subunits I, II and the holoenzyme. Antibodies against the holoenzyme were shown to be highly specific for subunit V. The results show that subunit I appears in the holoenzyme late in the assembly process. No radioactive subunit I is absorbed with antiserum against subunit II or the holoenzyme (subunit V) after a 30 min pulse in either isolated mitochondria or hepatocytes. However, both antisera absorb radioactive subunits I after a 150 min chase in isolated hepatocytes. This was confirmed using antibodies against subunit I, which absorbed only radioactive subunit I after a 30 min pulse but absorbed radioactive subunits I-III and VI after a 150 min chase. Thus, the late assembly of radioactive subunit I is explained by a temporal sequence in the assembly process and not by the presence of a large, non-radioactive pool of subunit I. Using the above approach and the three specific antisera, the following temporal sequence in the assembly of cytochrome oxidase was established. Subunits II and III assemble rapidly with each other or with cytoplasmically translated subunit VI. This complex of three peptides in turn assembles slowly with subunit I or with the other cytoplasmically translated subunits. The early association of subunit VI with the mitochondrially translated subunits II and III suggests a possible role of the former in integration of the holoenzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Clejan L., Beattie D. S., Gollub E. G., Liu K. P., Sprinson D. B. Synthesis of the apoprotein of cytochrome b in heme-deficient yeast cells. J Biol Chem. 1980 Feb 25;255(4):1312–1316. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gellerfors P., Nelson B. D. A rapid method for the isolation of intact mitochondria from isolated rat liver cells. Anal Biochem. 1979 Feb;93(1):200–203. [PubMed] [Google Scholar]
- Gellerfors P., Wielburski A., Nelson B. D. Synthesis of mitochondrial proteins in isolated rat hepatocytes. FEBS Lett. 1979 Dec 1;108(1):167–170. doi: 10.1016/0014-5793(79)81201-6. [DOI] [PubMed] [Google Scholar]
- Kolarov J., Wielburski A., Mendel-Hartvig I., Nelson B. D. Synthesis of cytochrome oxidase in isolated rat hepatocytes. Biochim Biophys Acta. 1981 Feb 26;652(2):334–346. doi: 10.1016/0005-2787(81)90123-4. [DOI] [PubMed] [Google Scholar]
- Kumar C. C., Padmanaban G. Role of heme in the synthesis of cytochrome c oxidase in Neurospora crassa. J Biol Chem. 1980 Dec 10;255(23):11130–11134. [PubMed] [Google Scholar]
- Kuźela S., Wielburski A., Nelson B. D. Translation of mitochondrial proteins in digitonin-treated rat hepatocytes. FEBS Lett. 1981 Nov 30;135(1):89–92. doi: 10.1016/0014-5793(81)80950-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mendel-Hartvig I. B. A simple and rapid method for the isolation of peptides from sodium dodecyl sulfate-containing polyacrylamide gels. Anal Biochem. 1982 Mar 15;121(1):215–217. doi: 10.1016/0003-2697(82)90579-6. [DOI] [PubMed] [Google Scholar]
- Merle P., Kadenbach B. The subunit composition of mammalian cytochrome c oxidase. Eur J Biochem. 1980 Apr;105(3):499–507. doi: 10.1111/j.1432-1033.1980.tb04525.x. [DOI] [PubMed] [Google Scholar]
- Michel R., Neupert W. Mitochondrial translation products before and after integration into the mitochondrial membrane in Neurospora crassa. Eur J Biochem. 1973 Jul 2;36(1):53–67. doi: 10.1111/j.1432-1033.1973.tb02884.x. [DOI] [PubMed] [Google Scholar]
- Nelson B. D., Mendel-Hartvig I. Immunological studies on beef-heart ubiquinol--cytochrome c reductase (complex III) Eur J Biochem. 1977 Oct 17;80(1):267–274. doi: 10.1111/j.1432-1033.1977.tb11879.x. [DOI] [PubMed] [Google Scholar]
- Poyton R. O., Kavanagh J. Regulation of mitochondrial protein synthesis by cytoplasmic proteins. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3947–3951. doi: 10.1073/pnas.73.11.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzgaber-Müller J., Schatz G. Heme is necessary for the accumulation and assembly of cytochrome c oxidase subunits in Saccharomyces cerevisiae. J Biol Chem. 1978 Jan 10;253(1):305–310. [PubMed] [Google Scholar]
- Schwab A. J., Sebald W., Weiss H. Different pool sizes of the precursor polypeptides of cytochrome oxidase from Neurospora crassa. Eur J Biochem. 1972 Nov 7;30(3):511–516. doi: 10.1111/j.1432-1033.1972.tb02122.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielburski A., Kuźela S., Nelson B. D. Studies on the assembly of cytochrome oxidase in isolated rat hepatocytes. Biochem J. 1982 Apr 15;204(1):239–245. doi: 10.1042/bj2040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D. B., Bruyninckx W. J., Foulke F. G., Grinich N. P., Mason H. S. Location of heme a on subunits I and II and copper on subunit II of cytochrome c oxidase. J Biol Chem. 1980 Dec 10;255(23):11408–11414. [PubMed] [Google Scholar]