Abstract
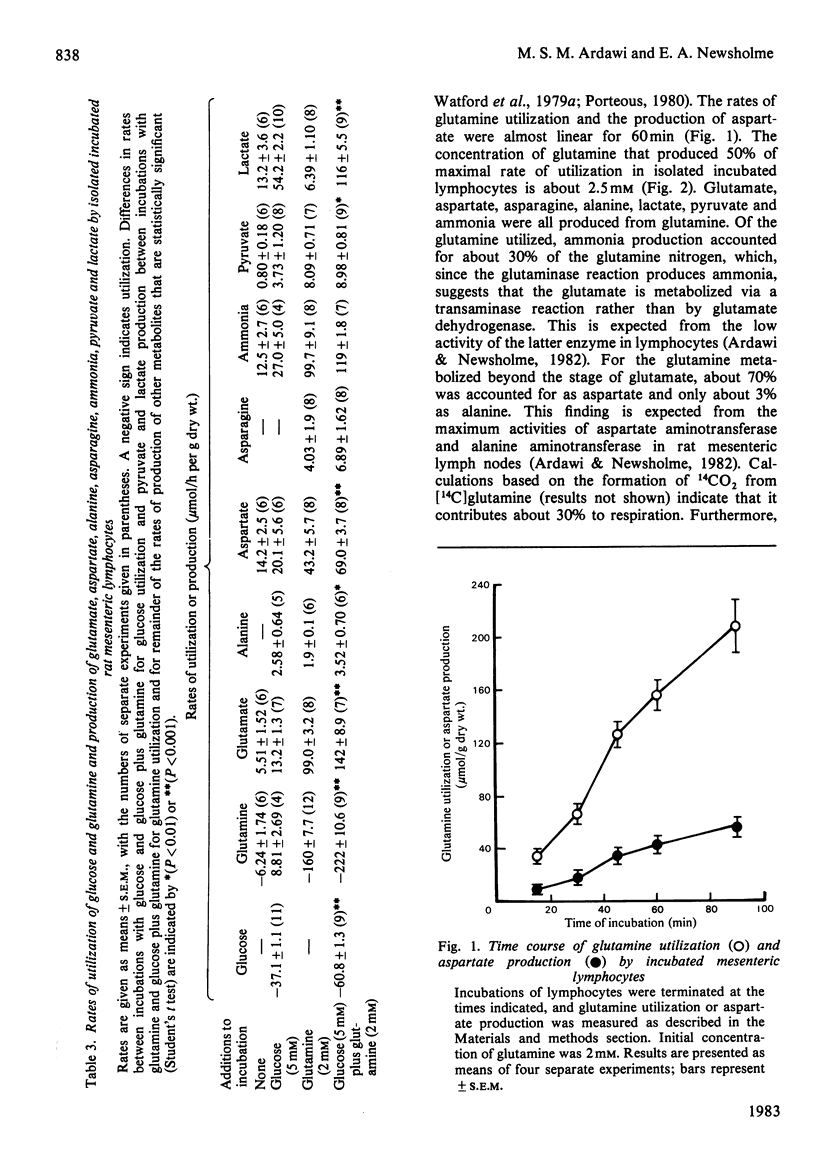
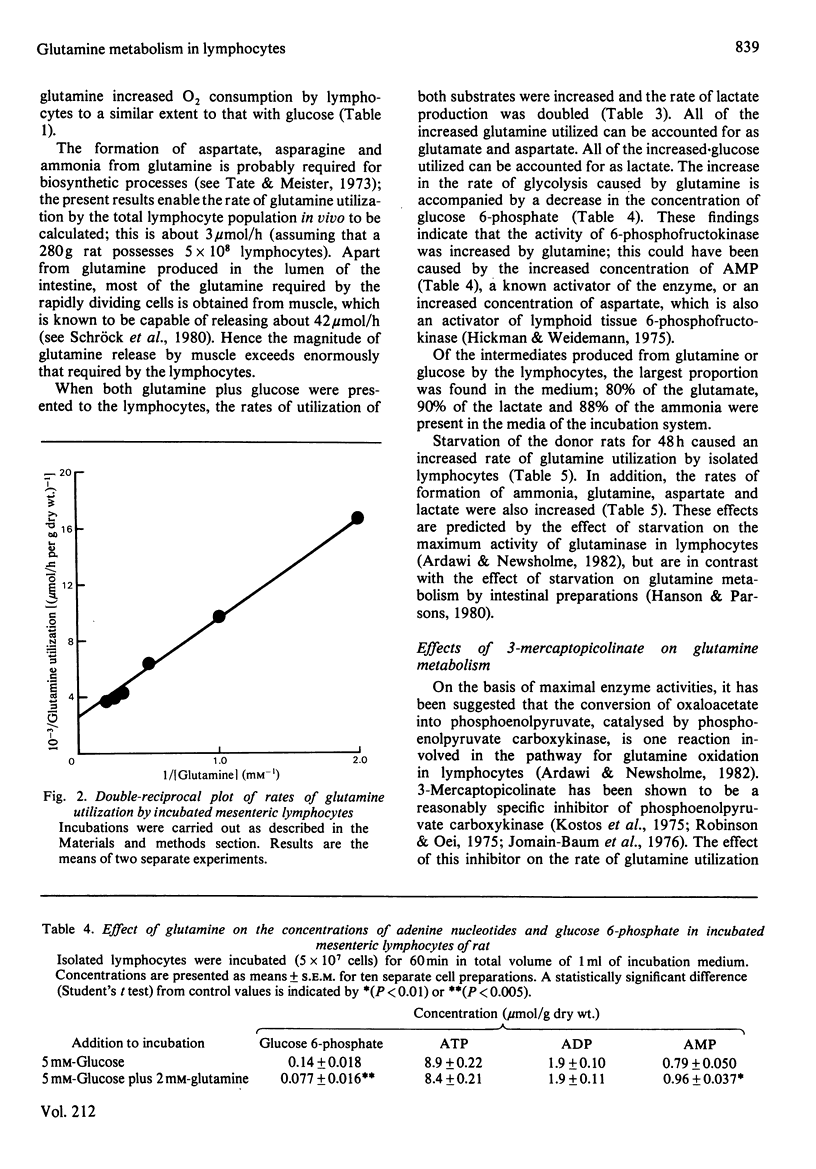
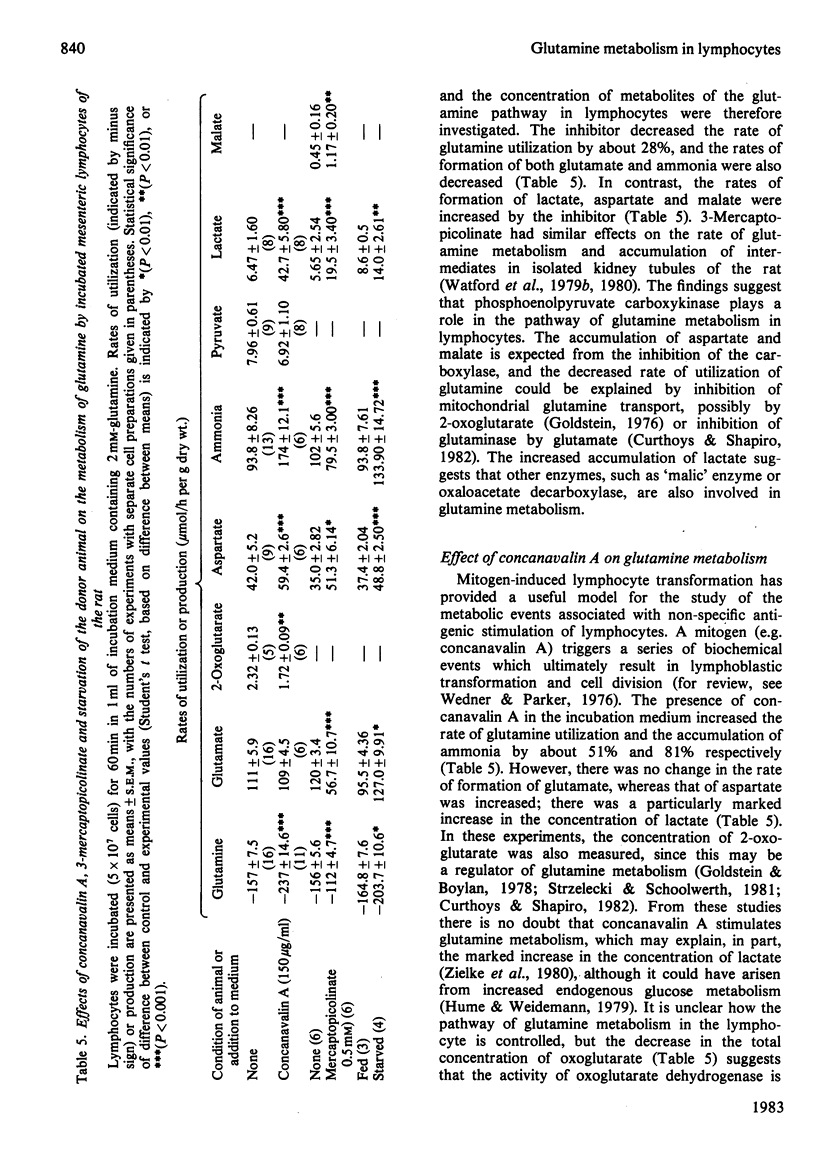
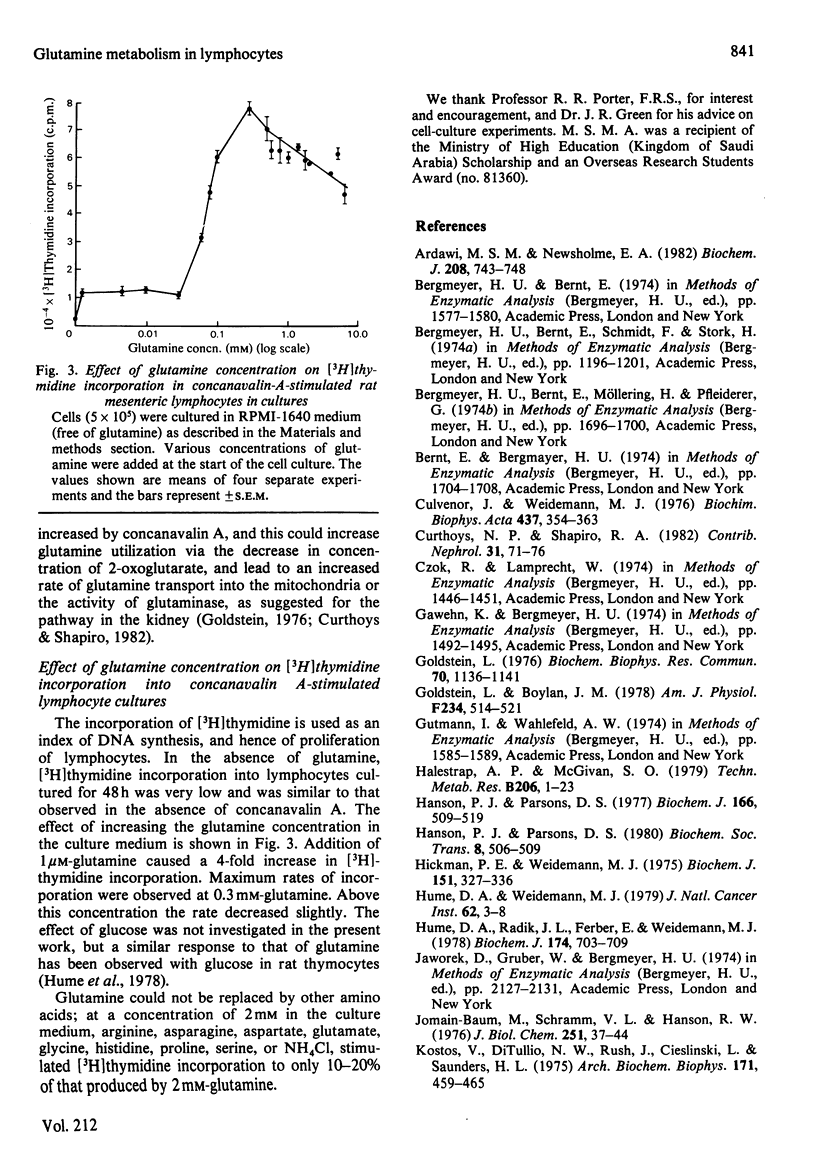
The metabolism of glutamine in resting and concanavalin-A-stimulated lymphocytes was investigated. In incubated lymphocytes isolated from rat mesenteric lymph nodes, the rates of oxygen and glutamine utilization and that of aspartate production were approximately linear with respect to time for 60 min, and the concentrations of adenine nucleotides plus the ATP/ADP or ATP/AMP concentration ratios remained approximately constant for 90 min. The major end products of glutamine metabolism were glutamate, aspartate and ammonia: the carbon from glutamine may contribute about 30% to respiration. When both glucose and glutamine were presented to the cells, the rates of utilization of both substances increased. Evidence was obtained that the stimulation of glycolysis by glutamine could be due, in part, to an activation of 6-phosphofructokinase. Starvation of the donor animal increased the rate of glutamine utilization. The phosphoenolpyruvate carboxykinase inhibitor mercaptopicolinate decreased the rate of glutamine utilization by 28%; the rates of accumulation of glutamate and ammonia were decreased, whereas those of lactate, aspartate and malate were increased. The mitogen concanavalin A increased the rate of glutamine utilization (by about 51%). The rate of [3H]thymidine incorporation into DNA caused by concanavalin A in cultured lymphocytes was very low in the absence of glutamine; it was increased about 4-fold at 1 microM-glutamine and was maximal at 0.3 mM-glutamine; neither other amino acids nor ammonia could replace glutamine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardawi M. S., Newsholme E. A. Maximum activities of some enzymes of glycolysis, the tricarboxylic acid cycle and ketone-body and glutamine utilization pathways in lymphocytes of the rat. Biochem J. 1982 Dec 15;208(3):743–748. doi: 10.1042/bj2080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culvenor J. G., Weidemann M. J. Phytohaemagglutinin stimulation of rat thymus lymphocytes glycolysis. Biochim Biophys Acta. 1976 Jul 21;437(2):354–363. doi: 10.1016/0304-4165(76)90005-2. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Shapiro R. A. Mechanism of glutamate and alpha-ketoglutarate inhibition of rat renal phosphate-dependent glutaminase. Contrib Nephrol. 1982;31:71–76. doi: 10.1159/000406618. [DOI] [PubMed] [Google Scholar]
- Goldstein L. alpha-Ketoglutarate regulation of glutamine transport and deamidation by renal mitochondria. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1136–1141. doi: 10.1016/0006-291x(76)91021-4. [DOI] [PubMed] [Google Scholar]
- Hanson P. J., Parsons D. S. The interrelationship between glutamine and alanine in the intestine. Biochem Soc Trans. 1980 Oct;8(5):506–509. doi: 10.1042/bst0080506. [DOI] [PubMed] [Google Scholar]
- Hanson P. J., Parsons S. Metabolism and transport of glutamine and glucose in vascularly perfused small intestine rat. Biochem J. 1977 Sep 15;166(3):509–519. doi: 10.1042/bj1660509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman P. E., Weidemann M. J. The purification and properties of pig spleen phosphofructokinase. Biochem J. 1975 Nov;151(2):327–336. doi: 10.1042/bj1510327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D. A., Radik J. L., Ferber E., Weidemann M. J. Aerobic glycolysis and lymphocyte transformation. Biochem J. 1978 Sep 15;174(3):703–709. doi: 10.1042/bj1740703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D. A., Weidemann M. J. Role and regulation of glucose metabolism in proliferating cells. J Natl Cancer Inst. 1979 Jan;62(1):3–8. [PubMed] [Google Scholar]
- Jomain-Baum M., Schramm V. L., Hanson R. W. Mechanism of 3-mercaptopicolinic acid inhibition of hepatic phosphoenolpyruvate carboxykinase (GTP). J Biol Chem. 1976 Jan 10;251(1):37–44. [PubMed] [Google Scholar]
- Kostos V., DiTullio N. W., Rush J., Cieslinski L., Saunders H. L. The effect of 3-mercaptopicolinic acid on phosphoenolpyruvate carboxykinase (GTP) in the rat and guinea pig. Arch Biochem Biophys. 1975 Dec;171(2):459–465. doi: 10.1016/0003-9861(75)90054-5. [DOI] [PubMed] [Google Scholar]
- Porteous J. W. Glutamate, glutamine, aspartate, asparagine, glucose and ketone-body metabolism in chick intestinal brush-border cells. Biochem J. 1980 Jun 15;188(3):619–632. doi: 10.1042/bj1880619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. H., Oei J. 3-Mercaptopicolinic acid, a preferential inhibitor of the cytosolic phosphoenolpyruvate carboxykinase. FEBS Lett. 1975 Oct 15;58(1):12–15. doi: 10.1016/0014-5793(75)80214-6. [DOI] [PubMed] [Google Scholar]
- Roediger W. E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982 Aug;83(2):424–429. [PubMed] [Google Scholar]
- Roos D., Loos J. A. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. II. Relative importance of glycolysis and oxidative phosphorylation on phytohaemagglutinin stimulation. Exp Cell Res. 1973 Mar 15;77(1):127–135. doi: 10.1016/0014-4827(73)90561-2. [DOI] [PubMed] [Google Scholar]
- Schröck H., Cha C. J., Goldstein L. Glutamine release from hindlimb and uptake by kidney in the acutely acidotic rat. Biochem J. 1980 May 15;188(2):557–560. doi: 10.1042/bj1880557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzelecki T., Schoolwerth A. C. Alpha-ketoglutarate modulation of glutamine metabolism by rat renal mitochondria. Biochem Biophys Res Commun. 1981 Sep 30;102(2):588–593. doi: 10.1016/s0006-291x(81)80172-6. [DOI] [PubMed] [Google Scholar]
- Suter D., Weidemann M. J. Regulation of carbohydrate metabolism in lymphoid tissue. Quantitative aspects of [U-14C]glucose oxidation by rat spleen slices. Biochem J. 1975 Jun;148(3):583–594. doi: 10.1042/bj1480583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay P., Lemieux G., Gougoux A., Lemieux C. Response of the rat and dog kidney to H+ concentration in vitro--a comparative study with slices and tubules. Int J Biochem. 1980;12(1-2):89–98. doi: 10.1016/0020-711x(80)90048-8. [DOI] [PubMed] [Google Scholar]
- Watford M., Lund P., Krebs H. A. Isolation and metabolic characteristics of rat and chicken enterocytes. Biochem J. 1979 Mar 15;178(3):589–596. doi: 10.1042/bj1780589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watford M., Vinay P., Lemieux G., Gougoux A. The formation of pyruvate from citric acid-cycle intermediates in kidney cortex [proceedings]. Biochem Soc Trans. 1979 Aug;7(4):753–755. doi: 10.1042/bst0070753. [DOI] [PubMed] [Google Scholar]
- Watford M., Vinay P., Lemieux G., Gougoux A. The regulation of glucose and pyruvate formation from glutamine and citric-acid-cycle intermediates in the kidney cortex of rats, dogs, rabbits and guinea pigs. Biochem J. 1980 Jun 15;188(3):741–748. doi: 10.1042/bj1880741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedner H. J., Parker C. W. Lymphocyte activation. Prog Allergy. 1976;20:195–300. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem. 1974 Aug 25;249(16):5070–5079. [PubMed] [Google Scholar]
- Yasmeen D., Laird A. J., Hume D. A., Weidemann M. J. Activation of 3-O-methyl-glucose transport in rat thymus lymphocytes by concanavalin A. Temperature and calcium ion dependence and sensitivity to puromycin but to cycloheximide. Biochim Biophys Acta. 1977 Nov 7;500(1):89–102. doi: 10.1016/0304-4165(77)90049-6. [DOI] [PubMed] [Google Scholar]
- Zielke H. R., Sumbilla C. M., Sevdalian D. A., Hawkins R. L., Ozand P. T. Lactate: a major product of glutamine metabolism by human diploid fibroblasts. J Cell Physiol. 1980 Sep;104(3):433–441. doi: 10.1002/jcp.1041040316. [DOI] [PubMed] [Google Scholar]


