Abstract
Objective
To improve the understanding of the natural disease progression of retinitis pigmentosa GTPaseregulator (RPGR)-associated X-linked retinitis pigmentosa (XLRP).
Design
A multicenter, prospective, observational natural history study over 24 months.
Participants
Male participants aged ≥7 years with a pathogenic variant in the RPGR gene, a best-corrected visual acuity (BCVA) score of ≥34 ETDRS letters, and a mean 68-loci retinal sensitivity (assessed by microperimetry) of 0.1 to 20 decibels (dB).
Methods
Participants were divided into subgroups based on their BCVA score at baseline: 34 to 73 (lower BCVA) or ≥74 (higher BCVA) ETDRS letters. There were 7 visits over 24 months.
Main Outcome Measures
Change from baseline in BCVA, retinal sensitivity, low luminance visual acuity (LLVA), fixation stability, contrast sensitivity, visual field, anatomical measures, 25-item Visual Function Questionnaire (VFQ-25), intraocular pressure, and adverse events (AEs).
Results
Overall, 201 participants were included. The mean (standard deviation [SD]) age was 30.3 (11.9) years in the lower BCVA subgroup (n = 170) and 27.7 (10.1) years in the higher BCVA subgroup (n = 31). The study eye baseline mean (SD) BCVA scores were 59.4 (10.30) and 77.3 (3.95) in the lower and higher BCVA subgroups, respectively; the lower BCVA subgroup had lower retinal sensitivity in the study eye at baseline than the higher BCVA subgroup. Over 24 months, there were small observed changes in BCVA, retinal sensitivity, LLVA, fixation, contrast sensitivity, and fundus photography findings. There were observed mean (SD) changes at 24 months in the lower and higher BCVA subgroups of −1.01 (4.67) and 0.03 (5.83) dB-steradians in the volume of full-field hill of vision, −330.6 (869.51) and −122.7 (22.01) μm in distance from foveal center to the nearest border of preserved fundus autofluorescence, −104.3 (277.80) and −207.1 (171.01) μm in central ellipsoid width, and −2.8 (9.7) and −0.6 (7.6) in VFQ-25 composite score, respectively. There was 1 death from completed suicide. There were no ocular serious adverse events, and most AEs were mild/moderate.
Conclusions
This study provides evidence of the slow natural progression of XLRP over 24 months in both subgroups and provides important functional, anatomical, and safety data.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Natural history observational study, X-linked retinitis pigmentosa, XOLARIS
X-linked retinitis pigmentosa (XLRP) is one of the most common and severe forms of retinitis pigmentosa (RP), a rare inherited disease distinguished by severe degeneration of rod and cone photoreceptors, leading to night blindness in childhood or adolescence that develops into central vision loss and legal blindness by the fourth decade.1, 2, 3, 4, 5 X-linked retinitis pigmentosa predominantly affects males with a prevalence of 3.4 to 4.4 per 100 000 males; however, female carriers can present with a wide phenotypic spectrum of XLRP owing to X chromosome inactivation.6, 7, 8 Over 70% of XLRP cases are caused by mutations in the retinitis pigmentosa GTPase regulator (RPGR) gene.6 The RPGR gene (OMIM.org 312610) is located on the short arm of the X chromosome and is alternatively spliced into 19 different exons.6,9 RPGRORF15 is a major RPGR isoform expressed only in the connecting cilia of rod and cone cells.6 Glutamylation of RPGRORF15 has been shown to be important in cargo trafficking to maintain the high metabolic rate of photoreceptors, and pathogenic variants leading to a lack of glutamylation may compromise the function of RPGRORF15.6,10 As the RPGRORF15 transcript is purine-rich and highly repetitive, it is highly susceptible to pathogenic variants and aberrant translation, which can lead to either a rod–cone phenotype or a cone/cone‒rod phenotype of retinal degeneration.6,10,11
At present, there is no approved treatment for XLRP,12,13 and there is a substantial unmet need for new and effective therapies designed to halt or reduce disease progression. Gene therapies are newly emerging approaches to XLRP treatment and aim to prevent progression by targeting the primary genetic defect in the retina using minimally invasive procedures.3,6 To date, there are 4 investigational gene therapies for XLRP in clinical development, 1 of which (BIIB112) has published results.12 The XIRIUS study (NCT03116113) assessed the efficacy and safety of a subretinal injection of BIIB112 gene therapy after vitrectomy in participants with XLRP.12,14 At 6-month follow-up, there were early improvements in visual field for 6 of 18 patients, with no significant safety concerns.12,15 Despite these findings, the primary end point of a ≥7-decibel (dB) improvement from baseline at ≥5 of the 16 central loci assessed by microperimetry (MP) was not met, although a statistically significant improvement in mean MP of treated eyes was seen.14 The suitability of MP as an outcome measure in clinical trials has been questioned given the high rate of variability between patients, the skewing of data,16 and because the current thresholds are based on glaucoma studies and not validated in inherited retinal diseases.13 Furthermore, perimetric techniques with white adapting backgrounds may not be suitable for patients with shifts in the threshold versus intensity function, so-called d1 mechanism sensitivity loss, at fixation.17,18 These findings highlight the need to further understand the natural history of XLRP and investigate better outcome measures that are likely to worsen significantly over typical clinical trial durations.
XOLARIS was a prospective, observational, 24-month study of participants with genetically confirmed RPGR-associated XLRP. The study aimed to gain a better understanding of disease progression over time to inform future interventional study design, including patient population, duration, and appropriate end points for determining efficacy.
Methods
Study Design
This was a multicenter, prospective, observational natural history study (NCT04926129) conducted between September 29, 2017, and December 16, 2022.19 The study consisted of a prescreening period, a baseline screening/enrollment period (visit 1), and 6 follow-up visits over 24 months: visit 2 (month 3 ± 14 days), visit 3 (month 6 ± 14 days), visit 4 (month 9 ± 14 days), visit 5 (month 12 ± 14 days), visit 6 (month 18 ± 14 days), and visit 7 (month 24 ± 14 days) (Fig S1; available at https://www.ophthalmologyscience.org/). In the event of participant withdrawal before visit 7, an early termination visit was conducted.
Participant Eligibility
Approximately 300 participants were planned to be enrolled in the study, including up to 20 female participants. Recruitment into the study was limited by participant availability, owing to the rarity of the disease and the impact of the coronavirus disease 2019 (COVID-19) pandemic. Participants enrolled in this study were able to withdraw to participate in any interventional study, including those sponsored by Biogen/NightstaRx, at any time during the 24-month observational period. In the final version of the protocol (Version 5), participants eligible for inclusion in the study were male or female and aged ≥7 years, with documentation of a pathogenic variant in the RPGR gene (including exon 1–14 and ORF15 mutations), a best-corrected visual acuity (BCVA) score in at least 1 eye of ≥34 ETDRS letters (equivalent to Snellen 6/60 or 20/200), and a mean retinal sensitivity in the eligible eye, as assessed by 68-loci MP, ranging from ≥0.1 dB to ≤20 dB in males, or ≥0.1 dB to ≤25 dB in females. Version 1 of the protocol did not include the mean retinal sensitivity inclusion criterion, which was added in Versions 2–4. Participants enrolled under Version 1 of the protocol remained eligible for continuation in the study, irrespective of their baseline total retinal sensitivity in the study eye. Furthermore, Protocol Version 5 added the ability to enroll up to 20 female participants who had symptomatic disease with impairment of visual function of the typical male phenotype.
For the BCVA examination, if both eyes met the eligibility criteria for the study, the eye with the worse BCVA score was the study eye, and the other was considered the fellow eye. If both eyes had the same BCVA score, the right eye was designated the study eye. No interventional treatment was administered as part of the study; however, participants may have been prescribed any concomitant medications, procedures, or treatments deemed necessary by their treating physician. Key exclusion criteria for the study included a history of amblyopia or inflammatory disorder in the eligible eye, or any other significant ocular or nonocular condition that could put the participant at risk in the study. Participants must not have taken part in a research study involving an investigational product in the past 12 weeks or received a gene- or cell-based therapy previously. Eligible participants were categorized into 2 subgroups based on the highest ETDRS BCVA score of the study eye at screening/baseline: lower BCVA (BCVA 34–73 ETDRS letters; equivalent to Snellen 6/12–6/60 or 20/40–20/200) or higher BCVA (BCVA ≥74 ETDRS letters; equivalent to Snellen 6/9 or 20/32) (Fig S1; available at https://www.ophthalmologyscience.org/).
This study was conducted in line with the appropriate laws, regulations, Good Epidemiological Practice guidelines, and the relevant articles of the Declaration of Helsinki. The protocol, informed consent and assent forms, participant information sheet, and any proposed advertising materials were submitted to the appropriate independent ethics committee/institutional review board and host institutions for written approval. All participants, or legal guardians for participants aged 7–13 years, provided written informed consent to take part in this study.
Primary and Secondary End Points
The primary end points analyzed during the XOLARIS observational study were the change from baseline in BCVA (ETDRS) over time and the change from baseline in retinal sensitivity assessed by MP over time. The functional secondary end points were low luminance visual acuity (LLVA), MP readings (other than the change in retinal sensitivity), contrast sensitivity (Pelli–Robson contrast sensitivity chart) over time, visual field, and the multiluminance mobility test. Multiluminance mobility test assessments were added to the study in Version 4 of the protocol. Anatomical secondary and exploratory outcomes included fundus autofluorescence (FAF) readings, fundus photography readings, spectral domain OCT (SD-OCT) based outcome measures, and, at a single site (University of California, San Francisco), adaptive optics-scanning laser ophthalmoscopy. Microperimetry readings, visual field, FAF readings, fundus photography readings, and SD-OCT were assessed by the central reading center. Functional questionnaires used were the 25-item Visual Function Questionnaire (VFQ-25), the RP-specific patient-reported outcome (PRO), the EuroQol 5-dimension 5-level (EQ-5D-5L) instrument, and Health Utilities Index Mark 3 (HUI3). The RP-specific PRO, EQ-5D-5L, and HUI3 questionnaires were added to the study at Version 5 of the protocol, and the EQ-5D-5L and HUI3 questionnaires were age-appropriate.
Safety analyses included change from baseline in intraocular pressure, slit lamp examination, dilated ophthalmoscopy, lens opacity grades (Lens Opacities Classification System III), and adverse events (AEs). Serious adverse events (SAEs) were defined as any medical occurrence that resulted in death, threat to life, hospitalization or prolonging of existing hospitalization, persistent or significant disability/incapacity, a congenital anomaly/birth defect, vision loss/threat to vision, or other medical events deemed to be important by the investigator.
Statistical Methods
Analyses were performed on all participants enrolled (participants who signed the informed consent form, met the inclusion criteria, and attended at least 1 study visit with at least 1 reported assessment). Participants who completed the study were defined as those who completed the month 24 assessments. Data were stored in an encrypted electronic data capture system and analyzed using SAS version 9.4 (SAS Institute Inc.). No formal hypothesis testing, statistical comparison, or sample size calculations were performed, given the noninterventional nature of the study. Continuous variables were summarized with descriptive statistics. Categorical variables, including binary variables, were summarized by counts and percentages. All analyses were performed using the ‘observed case’ method, and baseline values were defined as those recorded at visit 1, unless specified otherwise. Microperimetry was assessed in triplicate at baseline, and the final value was used. All analyses were based on the study eye, unless stated otherwise. Interocular symmetry for continuous end points was evaluated at selected visits by fitting an orthogonal (Deming) regression model on both eyes (with the fellow eye as the independent variable and the study eye as the dependent variable), and by calculating the intraclass correlation coefficient (ICC) using a 2-way mixed-effects model with measure of absolute agreement. Intraclass correlation coefficient values of <0.5 indicate poor reliability, 0.5 to 0.75 suggest moderate reliability, >0.75 to 0.9 show good reliability, and >0.9 demonstrate excellent reliability.20
Results
Participant Disposition and Baseline Characteristics
A total of 221 participants were screened, of whom 201 were enrolled across 21 centers globally (Fig S2; available at https://www.ophthalmologyscience.org/). Owing to changes in the clinical development strategy, study enrollment was halted on November 9, 2021, and follow-up continued through the remaining visits for all enrolled participants. Consequently, the study did not meet the planned sample size of 300 participants, and no female participants were enrolled between the Version 5 protocol update on February 5, 2021, and the end of the study. A total of 145 (72%) participants completed the study to month 24 (Fig S2; available at https://www.ophthalmologyscience.org/). Of participants who withdrew from the study (56/201; 28%), reasons for discontinuation were death (1/56; 2%), withdrawal by participant (13/56; 23%), loss to follow-up (13/56; 23%), physician decision (1/56; 2%), significant noncompliance (1/56; 2%), and other reasons (27/56; 48%) including 22 participants who exited XOLARIS early to join a different study, 14 of whom entered XIRIUS, the NightstaRx interventional study. The mean age of participants was balanced across the 2 subgroups, and most participants identified as ‘not Hispanic or Latino’ and White (Table 1). At baseline, ocular characteristics, including the BCVA ETDRS score, were balanced across the study and fellow eye in each subgroup and the overall population. At baseline, the mean sensitivity score in the center grid (16 loci) and whole grid (68 loci) assessed by MP and the mean SD-OCT readings for the central horizontal ellipsoid zone (EZ) width (μm) and area (mm2) were higher in participants in the higher BCVA subgroup compared with the lower BCVA subgroup (Table 1).
Table 1.
Demographics and Participant Baseline Characteristics in the Study and Fellow Eye
| Demographics and Characteristics | Lower BCVA Subgroup (n = 170) | Higher BCVA Subgroup (n = 31) | All (N = 201) | |||
|---|---|---|---|---|---|---|
| Age (yrs), mean (SD) | 30.3 (11.9) | 27.7 (10.1) | 29.9 (11.7) | |||
| Sex: male, n (%) | 170 (100) | 31 (100) | 201 (100) | |||
| Ethnicity: n (%) | ||||||
| Hispanic or Latino | 19 (11.2) | 0 | 19 (9.5) | |||
| Not Hispanic or Latino | 144 (84.7) | 30 (96.8) | 174 (86.6) | |||
| Not reported | 3 (1.8) | 1 (3.2) | 4 (2.0) | |||
| Unknown | 4 (2.4) | 0 | 4 (2.0) | |||
| Race, n (%)∗ | ||||||
| Asian | 6 (3.5) | 2 (6.5) | 8 (4.0) | |||
| Black or African American | 2 (1.2) | 0 | 2 (1.0) | |||
| Multiracial | 0 | 1 (3.2) | 1 (0.5) | |||
| Native Hawaiian or Pacific Islander | 2 (1.2) | 0 | 2 (1.0) | |||
| White |
160 (94.1) |
28 (90.3) |
188 (93.5) |
|||
| Study eye |
Fellow eye |
Study eye |
Fellow eye |
Study eye |
Fellow eye |
|
| BCVA ETDRS letters, mean (SD) | 59.4 (10.30) | 61.0 (17.14) | 77.3 (3.95) | 79.5 (4.80) | 62.2 (11.58) | 63.9 (17.21) |
| MP | ||||||
| Mean sensitivity in center grid (16 loci) (dB), mean (SD) | 8.0 (6.33) | 8.5 (6.43) | 16.2 (6.32) | 15.9 (5.95) | 9.3 (7.00) | 9.6 (6.91) |
| Mean sensitivity in whole grid (68 loci) (dB), mean (SD) | 4.3 (5.00) | 4.7 (5.21) | 8.4 (5.67) | 8.4 (5.69) | 4.9 (5.31) | 5.3 (5.45) |
| LLVA ETDRS letters, mean (SD) | 35.4 (20.75) | 39.2 (21.04) | 63.9 (7.69) | 66.5 (8.79) | 39.8 (21.88) | 43.4 (22.00) |
| LLD ETDRS letters, mean (SD) | 23.9 (14.85) | 21.9 (14.54) | 13.5 (5.40) | 12.8 (5.65) | 22.3 (14.31) | 20.5 (13.94) |
| Pelli–Robson contrast sensitivity score, mean (SD) | 0.923 (0.3987) | 0.969 (0.4144) | 1.427 (0.2408) | 1.458 (0.2826) | 1.001 (0.4199) | 1.045 (0.4342) |
| IOP, mean (SD), mmHg | 13.9 (2.87) | 13.9 (2.89) | 12.1 (2.14) | 12.1 (2.24) | 13.6 (2.84) | 13.6 (2.87) |
| SD-OCT | ||||||
| Central horizontal EZ width (μm), mean (SD) | 832.1 (1098.7) | 877.8 (1003.2) | 2338.2 (1620.8) | 2236.0 (1591.7) | 1062.9 (1304.5) | 1091.0 (1213.8) |
| Central EZ area (mm2), mean (SD) | 0.960 (1.8113) | 1.251 (2.8654) | 5.041 (6.3537) | 4.972 (6.5390) | 1.601 (3.3225) | 1.845 (3.9095) |
| FAF | ||||||
| n | 1 | 3 | 0 | 0 | 1 | 3 |
| Total area of preserved FAF (mm2), mean (SD) | 12.430 (N/E) | 12.480 (7.9701) | N/A | N/A | 12.430 (N/E) | 12.480 (7.9701) |
Lower BCVA subgroup = 34 to 73 ETDRS letters.
Higher BCVA subgroup = ≥74 ETDRS letters.
BCVA = best-corrected visual acuity; dB = decibels; EZ = ellipsoid zone; FAF = fundus autofluorescence; IOP = intraocular pressure; LLD = low-luminance deficit; LLVA = low luminance visual acuity; MP = microperimetry; N/A = not applicable; N/E = not estimable; SD = standard deviation; SD-OCT = spectral domain OCT.
No participants were reported in the ‘American Indian or Alaska Native,’ ‘Other,’ ‘Not reported,’ or ‘Unknown’ categories.
Primary Outcomes
Change in BCVA Score from Baseline
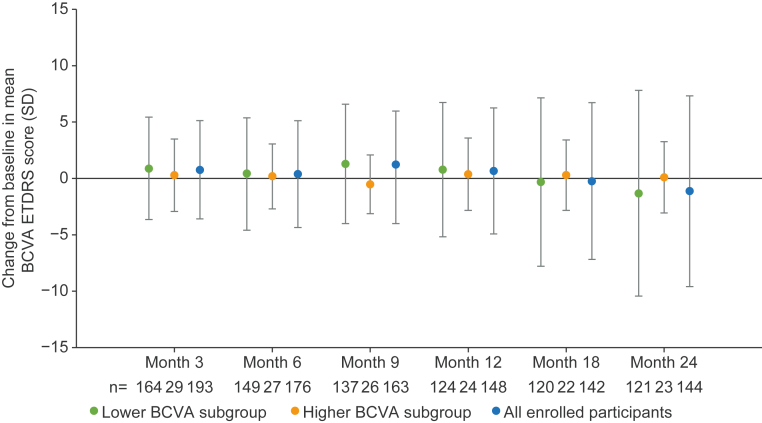
Over 24 months, the overall mean change in BCVA was consistent across subgroups, with more variability in the change from baseline for all visits for the lower BCVA subgroup compared with the higher BCVA subgroup (Fig 3). At month 24, the mean (standard deviation [SD]) change from baseline in BCVA score was −1.3 (9.14) in the lower BCVA subgroup and +0.1 (3.16) in the higher BCVA subgroup. The ICC from baseline in BCVA ETDRS scores showed poor correlation between eyes across subgroups. At months 12 and 24, the ICC reliability was poor for both the lower BCVA subgroup (0.28 and 0.29, respectively) and the higher BCVA subgroup (0.09 and 0.44, respectively).
Fig 3.
Change from baseline in mean BCVA score in study eye over time. Error bars show SD. Lower BCVA subgroup = 34 to 73 ETDRS letters. Higher BCVA subgroup = ≥74 ETDRS letters. BCVA = best-corrected visual acuity; SD = standard deviation.
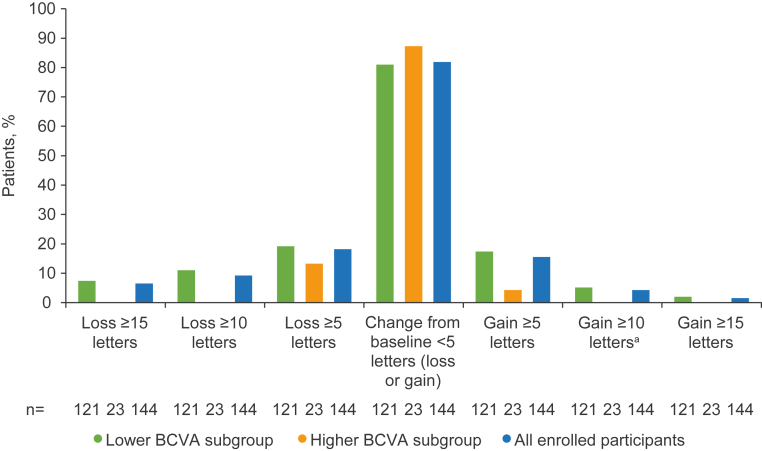
Over the 24-month period, most participants did not have a substantial change (>5 letters gained or lost) in their BCVA score (Fig 4). In the lower BCVA subgroup, 2 participants (1.7%) gained ≥15 BCVA ETDRS letters and 9 participants (7.4%) lost ≥15 BCVA ETDRS letters at month 24. No participants in the higher BCVA subgroup experienced a substantial change (gain or loss of ≥15 letters) in BCVA score at month 24.
Fig 4.
Percentage of participants achieving categorical threshold changes in study eye from baseline in BCVA score at month 24. Lower BCVA subgroup = 34 to 73 ETDRS letters. Higher BCVA subgroup = ≥74 ETDRS letters. aOf the 6 patients with a gain of ≥10 letters in BCVA score, 2 had concomitant medication for cataract surgery listed for the study eye, and 1 patient had medications listed for uveitis, macular edema, and inflammation. BCVA = best-corrected visual acuity.
Change in Retinal Sensitivity from Baseline
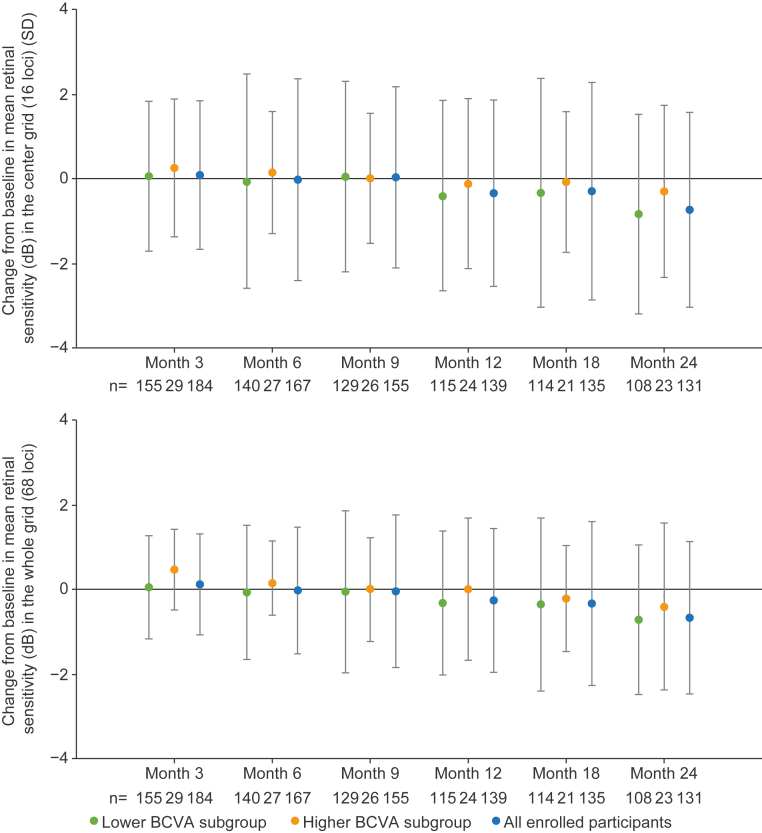
Across subgroups, most participants showed a small mean change in MP retinal sensitivity in the center grid or the whole grid at month 24 (Fig 5). At month 24, the mean (SD) change from baseline in mean retinal sensitivity in the center and whole grid was −0.8 (2.36) dB and −0.7 (1.77) dB in the lower BCVA subgroup and −0.3 (2.03) dB and −0.4 (1.97) dB in the higher BCVA subgroup, respectively. At month 24, the small decrease in the mean change from baseline in retinal sensitivity across subgroups was considered not clinically significant and indicative of natural disease progression.
Fig 5.
Change from baseline in retinal sensitivity in the center grid (16 loci) and whole grid (68 loci) in the study eye over time. Error bars show SD. N values displayed inside bars. Lower BCVA subgroup = 34 to 73 ETDRS letters. Higher BCVA subgroup = ≥74 ETDRS letters. BCVA = best-corrected visual acuity; dB = decibels; SD = standard deviation.
Secondary and Exploratory Outcomes
Functional Outcomes
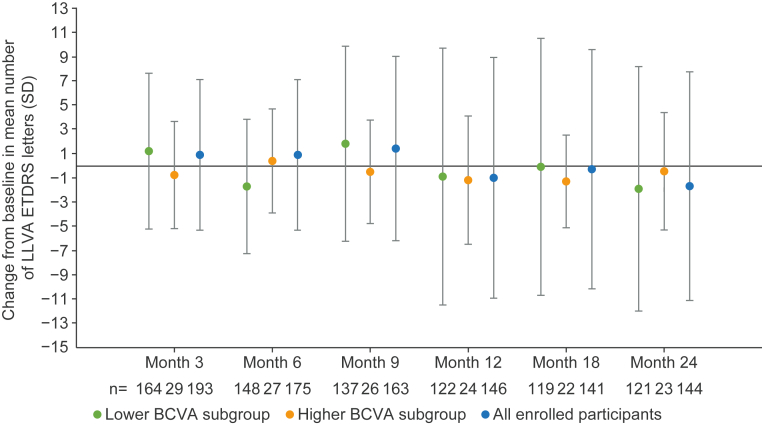
Over the 24-month period, there was a small decrease in the mean LLVA letter score across subgroups (Fig 6). At month 24, the mean (SD) change from baseline in LLVA was −1.9 (10.09) and −0.5 (4.85) for the lower and higher BCVA subgroups, respectively. The interocular symmetry for change from baseline in LLVA showed moderate correlation between eyes in both subgroups at month 12. The ICC was 0.64 and 0.63 in the lower and higher BCVA subgroups, respectively. At month 24, the reliability was moderate for the lower BCVA subgroup (ICC 0.60) and poor for the higher BCVA subgroup (ICC 0.25). Over the 24-month period, most participants did not experience a gain or loss of ≥15 LLVA letters (Fig 6). At month 24, 3 participants (2.5%) experienced a gain of ≥15 LLVA ETDRS letters and 8 participants (6.6%) experienced a loss of ≥15 LLVA ETDRS letters in the lower BCVA subgroup. No participants in the higher BCVA subgroup experienced a gain or loss of ≥15 LLVA ETDRS letters at month 24.
Fig 6.
Change from baseline in LLVA score (letters) in study eye over time. Error bars show SD. N values displayed inside bars. Lower BCVA subgroup = 34 to 73 ETDRS letters. Higher BCVA subgroup = ≥74 ETDRS letters. BCVA = best-corrected visual acuity; LLVA = low luminance visual acuity; SD = standard deviation.
For MP retinal sensitivity, in the lower BCVA subgroup, 3 participants (2.8%) showed improvement in the center grid and 16 (14.7%) in the whole grid at month 24. The higher BCVA subgroup had zero participants with improvement in the center grid and 2 (8.7%) with improvement in the whole grid at month 24.
Fixation at baseline was stable for 72% of participants in the lower BCVA subgroup and 90% of participants in the higher BCVA subgroup. At month 24, 15% of participants in the lower BCVA subgroup experienced a shift from ‘stable’ at baseline to ‘relatively unstable/unstable.’ No participants in the higher BCVA subgroup experienced a shift from ‘stable’ to ‘relatively unstable/unstable.’
There were no clinically significant changes in contrast sensitivity assessed by the Pelli–Robson contrast sensitivity chart at month 24 (Table S2; available at https://www.ophthalmologyscience.org/). Over the 24-month period, there was a decrease in the volume of full-field hill of vision for the lower BCVA subgroup of −1.01 (4.67) dB, compared with +0.03 (5.83) dB in the higher BCVA subgroup, with no appreciable decrease in volume of 30-degree hill of vision in either subgroup (Table S2; available at https://www.ophthalmologyscience.org/). Because multiluminance mobility test assessments were added to the study in Version 4 of the protocol, data were only available for 14 participants from 2 sites with the available technology; these data were not analyzed.
Anatomical Outcomes
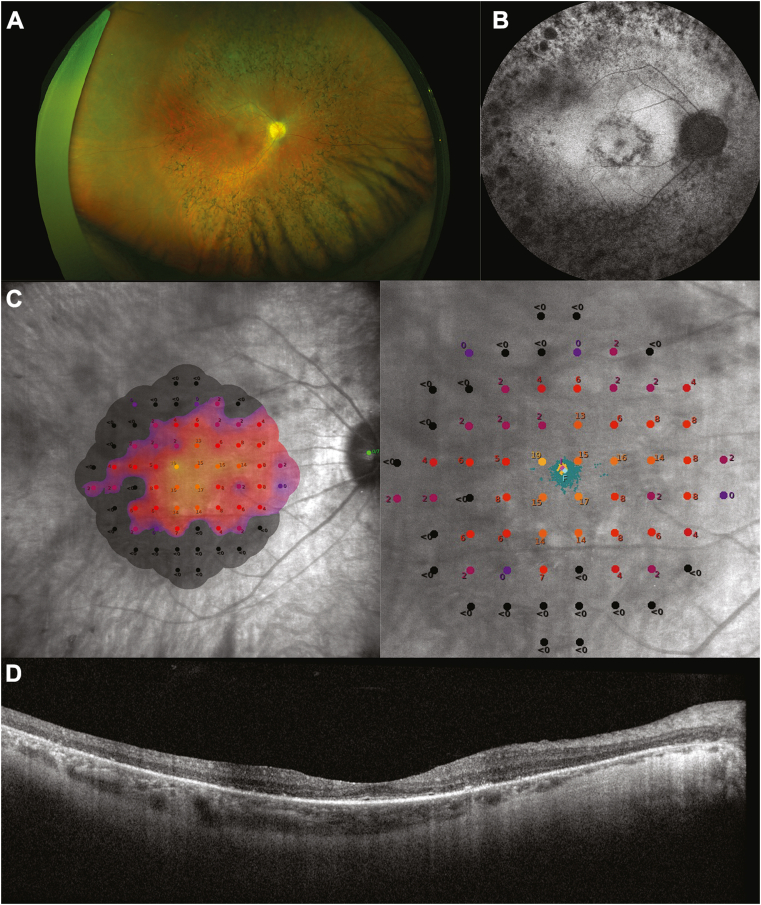
Retinal imaging is shown in Fig 7. Pseudocolor fundus imaging shows midperipheral pigment migration typical of RP, which is also evident on FAF imaging. Mesopic MP shows loss of retinal sensitivity outside of an island in the central macula; responses are reduced within the island when compared with controls. OCT imaging shows a narrow EZ band at the fovea with severe outer retinal degeneration at peripheral locations. Over 24 months, there was a gradual decrease in the mean distance from the foveal center to the nearest border of preserved autofluorescence, and the location of lesions remained stable (Fig S8; available at https://www.ophthalmologyscience.org/). The total area of preserved autofluorescence could not be graded for most participants, so no trends were determined from the data. For the fundus photography analyses over 24 months, retinal pigment epithelium hyperplasia and retinal arteriolar narrowing were present in most participants, and most abnormalities were of mild or moderate intensity. Adaptive optics-scanning laser ophthalmoscopy was available at 1 site and was performed in fewer than 10 participants; therefore, these data were not analyzed.
Fig 7.
Retinal imaging of RPGR-associated XLRP. A, Pseudocolor fundus imaging, B, FAF imaging, C, mesopic MP, and D, OCT imaging. Participants whose images are shown here signed an informed consent form giving permission for use of their photographs in publications. FAF = fundus autofluorescence; MP = microperimetry; RPGR = retinitis pigmentosa; XLRP = X-linked retinitis pigmentosa.
Spectral domain OCT measures found a large decrease in central horizontal EZ width but little change in the EZ area during the 24-month period (Table S3; available at https://www.ophthalmologyscience.org/). In line with the reduction of choroidal thickness at the foveal center and foveal subfield thickness, vitreomacular traction and macular hole were absent for most participants across both subgroups over 24 months (Table S3; available at https://www.ophthalmologyscience.org/). At month 24, there were a few shifts from ‘absent’ to ‘present’ in epiretinal membrane and from ‘none’ to ‘yes, foveal/yes, nonfoveal’ for cystoid macular edema and subretinal fluid across both subgroups (Table S3; available at https://www.ophthalmologyscience.org/).
Ocular Functional Questionnaire Outcomes
Over the 24-month period, there was an overall decrease in the mean composite VFQ-25 score. Participants in the lower BCVA subgroup had a greater decline than did those in the higher BCVA subgroup in all aspects of vision and some aspects of quality-of-life functioning (Table S4; available at https://www.ophthalmologyscience.org/). More participants in the lower BCVA subgroup experienced ≥15-point decreases in VFQ-25 composite score compared with the higher BCVA subgroup (8.6% and 0%, respectively). Analyses were not performed for RP-specific PRO, EQ-5D-5L, and HUI3 questionnaires because assessments were only available for 17, 21, and 19 participants, respectively. In total, 15 participants had available data for all 3 questionnaires, although baseline assessment and postbaseline data were sparse.
Safety
Most AEs were mild or moderate in severity in both subgroups, and incidences of ocular AEs were considered not related to study participation or assessment procedures. The most frequently reported AEs were cataract and cataract subcapsular, RP-related abnormality, blepharitis, and COVID-19 (Table S5; available at https://www.ophthalmologyscience.org/). The total number of SAEs (all nonocular) was low, with 7 events occurring in 4 participants (2%). In the lower BCVA subgroup, SAEs included nephrolithiasis (kidney stones), meniscus injury, and ankle fracture from a road traffic accident (all 1 participant each). In the higher BCVA subgroup, 1 participant experienced 3 SAEs of worsening of major depression, worsening of obsessive-compulsive disorder, and completed suicide. Ocular safety assessment results (intraocular pressure, slit lamp examinations, dilated ophthalmoscopy, and lens opacity grade) were largely similar between the 2 subgroups and were stable for each subgroup over 24 months.
Discussion
This 24-month observational study provides further useful data on the natural history of RPGR-associated XLRP, a severe, slow-progressing, retinal degenerative disorder.6 There is little understanding of how measurable outcomes progress over time in XLRP, and given the slow progression of the disease, the ability for interventional clinical trials to demonstrate efficacy over time is limited. Consequently, there is high scientific value in studies that assess the natural history of XLRP and provide valuable insights into the most suitable outcome measures for interventional clinical trials over 24 months.
It was expected that participants in XOLARIS with lower baseline BCVA scores would have a higher mean age than participants with higher BCVA scores. Interestingly, the mean age in both subgroups was similar (∼30 years); however, there were more participants in the lower BCVA subgroup (n = 170) than in the higher BCVA subgroup (n = 31), demonstrating that most patients with XLRP have severe retinal degeneration by the end of their third decade. Despite the similar mean age in both subgroups, the SD within both subgroups was large, which illustrates the different progression steps and rates among patients with RPGR-associated XLRP.4,21 Furthermore, the baseline MP and visual field values suggest that this cohort of patients may have already experienced significant visual field loss, therefore limiting the extent of further progression.
The results of this study showed few changes in most functional, anatomical, and PRO measures over 2 years. Across the 24-month observational period, there was no substantial change from baseline across subgroups in either of the primary end points: mean BCVA and MP retinal sensitivity. Most participants did not experience a BCVA gain or loss of >5 letters, which supports smaller natural history studies reporting a mean annual visual acuity reduction rate of 4% to 8%.1,21, 22, 23, 24 In addition, our retinal sensitivity data are in line with a previous interventional study that found retinal sensitivity declined by −0.9 dB in untreated fellow eyes over 12 months.15 Taken together, these findings complement previous studies that question the suitability of MP as an outcome measure in RPGR-associated XLRP clinical trials; the fluctuation between visits has been reported to impact results.13,16 Although the US Food and Drug Administration guidance states that phase 3 trials for XLRP should demonstrate a ≥7-dB change at ≥5 prespecified points on MP as the standard primary end point,13 this guidance was based on glaucoma studies and may not be feasible for clinical trials of XLRP. Furthermore, most participants in our study did not have a gain or loss of ≥15 LLVA letters, unlike a previous interventional study that reported that ∼10% of participants experienced an LLVA gain of 15 ETDRS letters in untreated eyes at 12 months.15 That study noted that the high test–retest variability impacts the use of LLVA as a clinical outcome measure.15 Over 24 months, fixation and contrast sensitivity also remained relatively stable. The small changes in these outcome measures may reflect the slow rate of natural progression of visual acuity loss in RPGR-associated XLRP and demonstrate that they may not be suitable for interventional clinical trials of this duration. However, it is important to consider that loss of fixation stability may affect comparisons of MP results over time, particularly in patients with d1 mechanism sensitivity loss.17
The outcome measures that did show a change over 24 months include the mean distance from the foveal center to the nearest border of preserved autofluorescence, the central EZ width, the loss of visual field, and the VFQ-25 composite score. In a previous study, an annual reduction in the horizontal and vertical diameter constriction of the outer border of 5.5% and 6.3%, respectively, has been reported, with a larger annual reduction in the outer and inner ring area of 10.7% and 13.1%, respectively.25 Although we did not analyze ring metrics in this study, their large annual reduction implies that they might be more appropriate outcome measures for clinical trials than diameter measurements. Furthermore, a relatively large reduction in central EZ width has also been shown previously, with a reported annual progression of 7% to 13%.26, 27, 28, 29, 30 A systematic review of XLRP natural history data found that EZ width on OCT and hyperautofluorescence ring metrics were the most sensitive measures over short periods of time owing to their high retest reliability and correlation with progression rates,24 supporting their suitability as measures in clinical trials. Moreover, an annual visual field area decline of 5% to 9%1,21 and an annual visual field mean sensitivity progression of 7% to 19%22,31 have previously been demonstrated, similar to the visual field findings in our study. Taken together, these data suggest that visual field loss might also be a suitable outcome measure in clinical trials. A 4-year clinical trial has previously shown efficacy by demonstrating the slowing of visual field progression in XLRP,28 although a floor effect has been reported given that progressive visual field loss is nearly complete in 25-year-old patients.32 It should be noted that some participants experienced an improvement in measures such as BCVA and retinal sensitivity over the 24-month observation period, potentially due to participants receiving procedures or treatments, such as cataract surgery, deemed necessary by their treating physician.
Although there were limited ocular functional questionnaire data in the present study, there was an overall decrease in the mean composite VFQ-25 score over 24 months in both subgroups. Participants in the lower BCVA subgroup experienced a greater decline than those in the higher BCVA subgroup, possibly owing to experiencing more advanced vision loss that has a greater impact on quality of life. The lack of data on PROs in XLRP has been reported in a recently published systematic review,5 and our findings reflect the importance of further additional studies of functional vision in this patient population divided into severity subgroups.
In noninterventional studies, monitoring of AEs can provide insight into the signs and symptoms experienced by patients during the course of a disease. Here, we have shown that most AEs reported were mild or moderate, the proportion of participants who reported ocular AEs was similar between subgroups, and no ocular SAEs were reported. In our study, 1 patient (0.5% of participants enrolled) reported nephrolithiasis. The annual prevalence of urolithiasis in the UK is 0.16%,33 and although the potential multiorgan effect of RPGR mutations in ciliated tissue could lead to an increase in nephrolithiasis incidence,6 the small sample size of this study limits the comparison with the overall population. The reported death from completed suicide coupled with worsening of major depression and obsessive-compulsive disorder in 1 patient is likely related to the high emotional burden of vision loss in patients with XLRP.34 Again, this event cannot be compared with the overall population owing to the small sample size of this study.
These findings provide useful insights on the most appropriate trial designs, outcome measures, and end points for future interventional studies. Given the slow rate of disease progression and the small changes in outcome measures over 2 years, therapeutic interventions should ideally demonstrate a gain in visual function rather than maintenance, and clinical trials may need to be performed over more than 3 years to demonstrate efficacy.13 In addition, clinical trials should use the most sensitive outcomes, ensure that participant numbers are powered to detect small changes, and consider the age of participants and whether they are at a more rapid stage of progression.
A key strength of the XOLARIS study is that, to our knowledge, it is the largest and most comprehensive natural history study in RPGR-associated XLRP. Nevertheless, there are several limitations that should be highlighted. First, a longer study duration, with comparison of both eyes, would have been desirable to identify larger changes in outcome measures over time. There are 2 previous studies with 13- to 16-year data on the natural history of RPGR-associated XLRP21 or XLRP in female carriers,35 although these studies focused on the variability in phenotypic expression patterns. Second, enrollment into XOLARIS was affected by the rarity of disease, the COVID-19 pandemic, early termination before the target sample size was reached, and the participant completion rate. As expected, given the noninterventional nature of this study, around 28% of enrolled participants terminated the study early. Considering this, achievement of the target sample size of 300 participants may have led to a more representative and conclusive dataset, and the limited number of participants who completed the study may have contributed to the lack of significant changes observed in many outcome measures studied. Another limitation of our study is that we did not have sufficient participant numbers to perform subgroup analyses by participant age. Given that a previous publication has described a faster progression rate in younger patients,24 it would be interesting to ascertain how changes in outcome measures differ in younger and older patients with RPGR-associated XLRP.
In this large and comprehensive natural history study of patients with RPGR-associated XLRP, our findings provide further evidence of the changes in functional, anatomical, and safety data over 2 years. These results confirm that the natural disease progression of RPGR-associated XLRP is difficult to measure over 2 years for most patients and outline the considerations and suitable outcome measures for future interventional clinical trials.
Data Sharing Statement
Individual participant data collected during the trial may be shared after anonymization and on approval of the research proposal. Biogen commits to sharing patient-level data, study-level data, clinical study reports, and protocols with qualified scientific researchers who provide a methodologically sound proposal. Biogen reviews all data requests internally based on the review criteria and in accordance with our Clinical Trial Transparency and Data Sharing Policy. Deidentified data and documents will be shared under agreements that further protect against participant reidentification. To request access to data, please visit https://vivli.org/.
Acknowledgments
The authors thank Willard Tinago, who contributed as the study lead statistician.
The authors would like to thank Laura McNamee for her contribution to the manuscript preparation and review.
Manuscript no. XOPS-D-24-00101.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosures:
All authors have completed and submitted the ICMJE disclosures form.
The authors have made the following disclosures:
R.E.M.: Grant support – Biogen; Patent on RPGR gene therapy – University of Oxford; Consultant – Biogen, Beacon Therapeutics, Applied Genetic Technologies Corporation, Novartis; Honoraria – Novartis.
J.L.D.: Grant support – Biogen/NightStaRx, National Eye Institute, Foundation Fighting Blindness, California Institute for Regenerative Medicine, PYC Therapeutics, Janssen, Acucela, SeaStar Medical, Allergan/Abbvie, Neurotech USA, Lowy Medical Research Institute, Second Sight Medical Products; Support – Funding for clinical trial expenses associated with the XOLARIS study Participation on a Data Safety Monitoring Board or Advisory Board – Biogen Advisory Board 2018–2019; Consultancy fees or honorarium for advisory boards or memberships – Applied Genetic Technologies Corporation, Biogen, California Institute for Regenerative Medicine, ConeSight Therapeutics, DTx Pharma, Editas Medicine, Eyevensys, Foundation Fighting Blindness, Gyroscope Therapeutics, Nacuit Pharmaceuticals, ProQR Therapeutics, PYC Therapeutics, Relay Therapeutics, SparingVision, Spark Therapeutics, Vedere Bio; Spousal stock – RxSight.
M.D.F.: Grant support for clinical studies – Biogen; Consultant – Adelphi Values, Advent France Biotechnology, Adverum, Alder Therapeutics, AlphaSights, Arctos Medical, Astellas, Atheneum, Atsena, Axiom Healthcare Strategies, Bayer, Biogen, Cambridge Consultants, Coave Therapeutics, Decision Resources, Dialectica, DORC, Frontera Therapeutics, Hoffmann Eitle, Janssen Research & Development, Medscape, Mogrify, Navigant, Novartis, PeerVoice, Physicians’ Education Resource, RegenxBio, Roche, Sirion Biotech, Sofinnova Partners, Sparing Vision, STZ eyetrial Therapeutics, System Analytic, Techspert, THEA, Vindico Medical Education; Honoraria – Novartis, PeerVoice, Physicians Education Rescource, Bayer, Vindico Medical Education; Participation on a Data Safety Monitoring Board or Advisory Board – RegenxBio, Alder Therapeutics, Janssen Research & Development, Novartis, Sparing Vision, SpliceBio.
B.L.L.: Grants – Applied Genetic Technologies Corporation, Biogen, Endogena, Nanoscope, Ocugen, Spark Therapeutics, Stoke Therapeutics; Consultant – Biogen, BlueRock, Janssen, Lexitas, Novartis, Stoke Therapeutics.
M.E.P.: Clinical trial expenses – Biogen; Consultant – 4D Molecular Therapeutics, Adverum Biotechnologies, Arrowhead Pharmaceuticals, Applied Genetic Technologies Corporation, Aldebaran Therapeutics, Ascidian Therapeutics, Atsena Therapeutics, Astellas Pharma, BlueRock Therapeutics – Opsis, Coave Therapeutics, ClarisBio, Dompé Farmaceutici, Editas Medicine, EdiGene, Endogena Therapeutics, Foundation Fighting Blindness, InGel Therapeutics, jCyte, Janssen, Kala Therapeutics, Kiora Pharmaceuticals, Nacuity Pharmaceuticals, Ocugen, Ora Pharmaceuticals, ProQR Therapeutics, Prime Editing, PTC Therapeutics, PYC Therapeutics, Ray Therapeutics, Rejuvitas, Restore Vision, RegenexBio, Sparing Vision, Spark Therapeutics, SpliceBio, Spotlight Therapeutics, Thea Pharmaceuticals and Theranexus; Participation on a Data Safety Monitoring Board or Advisory Board – Akouos, GenSight Biologics; Shares – Aldebaran Therapeutics, Atsena Therapeutics, Endogena Therapeutics, EnterX Biosciences, InGel Therapeutics, Kiora Pharmaceuticals, Nacuity Pharmaceuticals, Ocugen, ZipBio.
J.A.G.: Shares – Biogen; Employee – Biogen.
J.L.: Shares – Biogen; Employee – Biogen.
S.T.: Shares – Biogen; Employee – Biogen.
This study was funded by NightstaRx Ltd; NightstaRx was acquired by Biogen in 2019. NightstaRx/Biogen were involved in the research design, data analysis and interpretation, and manuscript preparation and approval. Medical writing support, including preparation of the manuscript draft according to the author’s direction, was provided by Lucy Farrow, MSci, of Selene Medical Communications, Cheshire, UK, and was funded by Biogen in accordance with Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022).
HUMAN SUBJECTS: Human subjects were included in this study. This study was conducted in line with the appropriate laws, regulations, Good Epidemiological Practice guidelines, and the relevant articles of the Declaration of Helsinki. The protocol, informed consent and assent forms, participant information sheet, and any proposed advertising materials were submitted to the appropriate independent ethics committee/institutional review board and were provided to host institutions for written approval.
No animal subjects were used in this study.
Author Contributions:
Conception and design: MacLaren, Gow, Li, Tsang
Data collection: MacLaren, Duncan, Fischer, Lam, Meunier, Pennesi, Sankila
Analysis and interpretation: Gow, Li, Tsang
Obtained funding: MacLaren, Duncan, Fischer, Lam, Meunier, Pennesi, Sankila
Overall responsibility: MacLaren, Duncan, Fischer, Lam, Meunier, Pennesi, Sankila, Gow, Li, Tsang
Contributor Information
Robert E. MacLaren, Email: enquiries@eye.ox.ac.uk.
So-Fai Tsang, Email: sofaitsang@gmail.com.
XOLARIS Study Group:
Kevin Gregory-Evans, Robert Koenekoop, Eeva-Marja K. Sankila, Henrik Bygglin, Sanna Seitsonen, Antti Riikonen, Isabelle Meunier, M. Dominik Fischer, Alex Ochakovski, Katarina Stingl, Yousof Vaheb, Paul Richter, Fabian Wozar, Felix Reichel, Caroline Gassel, Lasse Wolfram, Nora Fischer, Tobias Peters, Barbara Wilhelm, Immanuel Seitz, Frank Holz, Katharina Reinking, Amelie Clemens, Desiree Völker, Philipp Herrmann, Johannes Birtel, Pascal Schipper, Constance Weber, Louisa Bulirsch, Carel Hoyng, Caroline Klaver, T.M.L. Phan, Ramon Van Huet, Camiel Boon, X.T. Nguyen, M. Talib, Kasia Trzcionkowska, Thomas Tussenbroek, Robert E. MacLaren, Laura J. Taylor, Jasmina Cehajic-Kapetanovic, Amandeep S. Josan, Imran H. Yusuf, Kirti Jasani, Moreno Menghini, Anika Nanda, Salwah Rehman, Jasleen K. Jolly, Thomas M.W. Buckley, Andrew Lotery, Suresh Thulsidharan, Samir Khandhadia, Georgios Tsokolas, Graeme Black, Roly Megaw, Paul Bishop, Rajarshi Mukherjee, Aditi Mohla, Martin McKibbin, Raj Mukherjee, Byron L. Lam, Carlos Mendoza-Santiesteban, Jason Horowitz, Stephen Tsang, Mark E. Pennesi, Paul Yang, Andreas K. Lauer, Richard G. Weleber, David Birch, Lori Coors, Rand Spencer, Karl Csaky, Rajiv Anand, Yi-Zhong Wang, Michael Gorin, Kimberly Stepien, Jacque L. Duncan, Jay Stewart, Anthony Moore, J. Timothy Stout, Christina Weng, Ella Leung, Tahira Schlle, Benjamin Bakall, Kendra Klein, Paul Bernstein, Mary Elizabeth Hartnett, Marc Mathias, Frank Siringo, Paula Pecen, Tomas Aleman, Albert McGuire, Aaron Nagiel, Michael Larsen, Juliana Maria Ferraz Sallum, Lucas Ribeiro, and Rebeca de Azevedo Amaral
XOLARIS Study Group
The University of British Columbia–Eye Care Centre, Vancouver, Canada: Kevin Gregory-Evans; The Montreal Children’s Hospital, Montreal, Canada: Robert Koenekoop; Helsinki University Central Hospital, Helsinki, Finland: Eeva-Marja K. Sankila, Henrik Bygglin, Sanna Seitsonen, Antti Riikonen; National Reference Centre for Inherited Retinal Dystrophies University Hospital of Montpellier, University of Montpellier, France: Isabelle Meunier; Universitätsklinikum Tübingen–Institute for Ophthalmic Research, Germany: M. Dominik Fischer, Alex Ochakovski, Katarina Stingl, Yousof Vaheb, Paul Richter, Fabian Wozar, Felix Reichel, Caroline Gassel, Lasse Wolfram, Nora Fischer, Tobias Peters, Barbara Wilhelm, Immanuel Seitz; University of Bonn, Tübingen, Germany: Frank Holz, Katharina Reinking, Amelie Clemens, Desiree Völker, Philipp Herrmann, Johannes Birtel, Pascal Schipper, Constance Weber, Louisa Bulirsch; Radboud University Medical Center, Nijmegen, The Netherlands: Carel Hoyng, Caroline Klaver, T. M. L. Phan, Ramon Van Huet; Leiden Universitair Medisch Centrum, Leiden, The Netherlands: Camiel Boon, X. T. Nguyen, M. Talib, Kasia Trzcionkowska, Thomas Tussenbroek; Oxford University Hospitals NHS Trust–John Radcliffe Hospital, Oxford, UK: Robert E. MacLaren, Laura J. Taylor, Jasmina Cehajic-Kapetanovic, Amandeep S. Josan, Imran H. Yusuf, Kirti Jasani, Moreno Menghini, Anika Nanda, Salwah Rehman, Jasleen K. Jolly, Thomas M.W. Buckley; University Hospital Southampton NHS Foundation Trust–Southampton General Hospital, Southampton, UK: Andrew Lotery, Suresh Thulsidharan, Samir Khandhadia, Georgios Tsokolas; Manchester University NHS Foundation Trust–Manchester Royal Infirmary, Manchester, UK: Graeme Black, Roly Megaw, Paul Bishop; St. James’s University Hospital, Leeds, UK: Rajarshi Mukherjee, Aditi Mohla, Martin McKibbin, Raj Mukherjee; Bascom Palmer Eye Institute–University of Miami Health System, FL, USA: Byron L. Lam, Carlos Mendoza-Santiesteban; Columbia University Irving Medical Center–New York, NY, USA: Jason Horowitz, Stephen Tsang; Oregon Health & Science University–Casey Eye Institute, OR, USA: Mark E. Pennesi, Paul Yang, Andreas K. Lauer, Richard G. Weleber; Retina Foundation of the Southwest, TX, USA: David Birch, Lori Coors, Rand Spencer, Karl Csaky, Rajiv Anand, Yi-Zhong Wang; University of California, Los Angeles–Jules Stein Eye Institute, CA, USA: Michael Gorin; University of Wisconsin Health–University Station Clinic, WI, USA: Kimberly Stepien; University of California San Francisco–Koret Vision Center, CA, USA: Jacque L. Duncan, Jay Stewart, Anthony Moore; Baylor College of Medicine–The Cullen Eye Institute–Alkek Eye Center, TX, USA: J. Timothy Stout, Christina Weng, Ella Leung, Tahira Schlle; Associated Retina Consultants–Phoenix, AZ, USA: Benjamin Bakall, Kendra Klein; John A. Moran Eye Center–University of Utah Health Care, UT, USA: Paul Bernstein, Mary Elizabeth Hartnett; University of Colorado Health Sue Anschutz-Rodgers Eye Center–Anschutz Medical Campus, CO, USA: Marc Mathias, Frank Siringo, Paula Pecen; Perelman School of Medicine at the University of Pennsylvania, PA, USA: Tomas Aleman, Albert McGuire; Children's Hospital Los Angeles, CA, USA: Aaron Nagiel; Glostrup Hospital, Glostrup, Denmark: Michael Larsen; Instituto de Genetica Ocular, São Paulo, Brazil: Juliana Maria Ferraz Sallum, Lucas Ribeiro, Rebeca de Azevedo Amaral.
Supplementary Data
References
- 1.Sandberg M.A., Rosner B., Weigel-DiFranco C., et al. Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Invest Ophthalmol Vis Sci. 2007;48:1298–1304. doi: 10.1167/iovs.06-0971. [DOI] [PubMed] [Google Scholar]
- 2.Tee J.J., Smith A.J., Hardcastle A.J., Michaelides M. RPGR-associated retinopathy: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2016;100:1022–1027. doi: 10.1136/bjophthalmol-2015-307698. [DOI] [PubMed] [Google Scholar]
- 3.Cehajic Kapetanovic J., McClements M.E., Martinez-Fernandez de la Camara C., MacLaren R.E. Molecular strategies for RPGR gene therapy. Genes. 2019;10:674. doi: 10.3390/genes10090674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Iorio V., Karali M., Melillo P., et al. Spectrum of disease severity in patients with X-linked retinitis pigmentosa due to RPGR mutations. Invest Ophthalmol Vis Sci. 2020;61:36. doi: 10.1167/iovs.61.14.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam B.L., Scholl H.P., Doub D., et al. A systematic literature review of disease progression reported in RPGR-associated X-linked retinitis pigmentosa. Retina. 2024;44:1–9. doi: 10.1097/IAE.0000000000003920. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Fernandez de la Camara C., Cehajic-Kapetanovic J., MacLaren R.E. Emerging gene therapy products for RPGR-associated X-linked retinitis pigmentosa. Expert Opin Emerg Drugs. 2022;27:431–443. doi: 10.1080/14728214.2022.2152003. [DOI] [PubMed] [Google Scholar]
- 7.Vinikoor-Imler L.C., Simpson C., Narayanan D., et al. Prevalence of RPGR-mutated X-linked retinitis pigmentosa among males. Ophthalmic Genet. 2022;43:581–588. doi: 10.1080/13816810.2022.2109686. [DOI] [PubMed] [Google Scholar]
- 8.Talib M., van Schooneveld M.J., Van Cauwenbergh C., et al. The spectrum of structural and functional abnormalities in female carriers of pathogenic variants in the RPGR gene. Invest Ophthalmol Vis Sci. 2018;59:4123–4133. doi: 10.1167/iovs.17-23453. [DOI] [PubMed] [Google Scholar]
- 9.Online mendelian inheritance in man. OMIM 312610: retinitis pigmentosa GTPase regulator; RPGR. 2023. https://www.omim.org/entry/312610
- 10.Cehajic-Kapetanovic J., Martinez-Fernandez de la Camara C., Birtel J., et al. Impaired glutamylation of RPGRORF15 underlies the cone-dominated phenotype associated with truncating distal ORF15 variants. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2208707119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X., Park J.H., Gumerson J., et al. Loss of RPGR glutamylation underlies the pathogenic mechanism of retinal dystrophy caused by TTLL5 mutations. Proc Natl Acad Sci U S A. 2016;113:E2925–E2934. doi: 10.1073/pnas.1523201113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cehajic-Kapetanovic J., Xue K., Martinez-Fernandez de la Camara C., et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat Med. 2020;26:354–359. doi: 10.1038/s41591-020-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birch D.G., Cheetham J.K., Daiger S.P., et al. Overcoming the challenges to clinical development of X-linked retinitis pigmentosa therapies: proceedings of an expert panel. Transl Vis Sci Technol. 2023;12:5. doi: 10.1167/tvst.12.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ClinicalTrials.gov, NCT03116113 A clinical trial of retinal gene therapy for X-linked retinitis pigmentosa using BIIB112 (XIRIUS) 2023. https://clinicaltrials.gov/study/NCT03116113
- 15.von Krusenstiern L., Liu J., Liao E., et al. Changes in retinal sensitivity associated with cotoretigene toliparvovec in X-linked retinitis pigmentosa with RPGR gene variations. JAMA Ophthalmol. 2023;141:275–283. doi: 10.1001/jamaophthalmol.2022.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor L.J., Josan A.S., Jolly J.K., MacLaren R.E. Microperimetry as an outcome measure in RPGR-associated retinitis pigmentosa clinical trials. Transl Vis Sci Technol. 2023;12:4. doi: 10.1167/tvst.12.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simunovic M.P., Mammo Z. Mechanisms of cone sensitivity loss in retinitis pigmentosa. Ophthalmic Physiol Opt. 2024;44:605–612. doi: 10.1111/opo.13280. [DOI] [PubMed] [Google Scholar]
- 18.Hood D.C., Greenstein V. Models of the normal and abnormal rod system. Vis Res. 1990;30:51–68. doi: 10.1016/0042-6989(90)90127-7. [DOI] [PubMed] [Google Scholar]
- 19.ClinicalTrials.gov, NCT04926129 Natural history of the progression of X-linked retinitis pigmentosa (XOLARIS) https://clinicaltrials.gov/study/NCT04926129
- 20.Koo T.K., Li M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang W.C., Wright A.F., Roman A.J., et al. RPGR-associated retinal degeneration in human X-linked RP and a murine model. Invest Ophthalmol Vis Sci. 2012;53:5594–5608. doi: 10.1167/iovs.12-10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tee J.J., Yang Y., Kalitzeos A., et al. Characterization of visual function, interocular variability and progression using static perimetry-derived metrics in RPGR-associated retinopathy. Invest Ophthalmol Vis Sci. 2018;59:2422–2436. doi: 10.1167/iovs.17-23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talib M., van Schooneveld M.J., Thiadens A.A., et al. Clinical and genetic characteristics of male patients with RPGR-associated retinal dystrophies: a long-term follow-up study. Retina. 2019;39:1186–1199. doi: 10.1097/IAE.0000000000002125. [DOI] [PubMed] [Google Scholar]
- 24.Zada M., Cornish E.E., Fraser C.L., et al. Natural history and clinical biomarkers of progression in X-linked retinitis pigmentosa: a systematic review. Acta Ophthalmol. 2021;99:499–510. doi: 10.1111/aos.14662. [DOI] [PubMed] [Google Scholar]
- 25.Tee J.J., Kalitzeos A., Webster A.R., et al. Quantitative analysis of hyperautofluorescent rings to characterize the natural history and progression in RPGR-associated retinopathy. Retina. 2018;38:2401. doi: 10.1097/IAE.0000000000001871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birch D.G., Locke K.G., Wen Y., et al. Spectral-domain optical coherence tomography measures of outer segment layer progression in patients with X-linked retinitis pigmentosa. JAMA Ophthalmol. 2013;131:1143–1150. doi: 10.1001/jamaophthalmol.2013.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai C.X., Locke K.G., Ramachandran R., et al. A comparison of progressive loss of the ellipsoid zone (EZ) band in autosomal dominant and X-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2014;55:7417–7422. doi: 10.1167/iovs.14-15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman D.R., Hughbanks-Wheaton D.K., Spencer R., et al. Docosahexaenoic acid slows visual field progression in X-linked retinitis pigmentosa: ancillary outcomes of the DHAX trial. Invest Ophthalmol Vis Sci. 2015;56:6646–6653. doi: 10.1167/iovs.15-17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tee J.J., Carroll J., Webster A.R., Michaelides M. Quantitative analysis of retinal structure using spectral-domain optical coherence tomography in RPGR-associated retinopathy. Am J Ophthalmol. 2017;178:18–26. doi: 10.1016/j.ajo.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tee J.J.L., Yang Y., Kalitzeos A., et al. Natural history study of retinal structure, progression, and symmetry using ellipzoid zone metrics in RPGR-associated retinopathy. Am J Ophthalmol. 2019;198:111–123. doi: 10.1016/j.ajo.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birch D.G., Locke K.G., Felius J., et al. Rates of decline in regions of the visual field defined by frequency-domain optical coherence tomography in patients with RPGR-mediated X-linked retinitis pigmentosa. Ophthalmology. 2015;122:833–839. doi: 10.1016/j.ophtha.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellingrath J.-S., Ochakovski G.A., Seitz I.P., et al. High symmetry of visual acuity and visual fields in RPGR-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2017;58:4457–4466. doi: 10.1167/iovs.17-22077. [DOI] [PubMed] [Google Scholar]
- 33.Heers H., Turney B.W. Trends in urological stone disease: a 5-year update of hospital episode statistics. BJU Int. 2016;118:785–789. doi: 10.1111/bju.13520. [DOI] [PubMed] [Google Scholar]
- 34.Chivers M., Li N., Pan F., et al. The burden of X-linked retinitis pigmentosa on patients and society: a narrative literature review. Clinicoecon Outcomes Res. 2021;13:565–572. doi: 10.2147/CEOR.S297287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comander J., Weigel-DiFranco C., Sandberg M.A., Berson E.L. Visual function in carriers of X-linked retinitis pigmentosa. Ophthalmology. 2015;122:1899–1906. doi: 10.1016/j.ophtha.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.