Abstract
Purpose
This study evaluated the influence of different agents such as blood, artificial saliva, and normal saline on preload force of dental implants with bio-high-performance poly-ether-ether-ketone (Bio-HPP) abutments to determine its effect on screw loosening.
Methods
Forty (N = 40) Grade 5 titanium dental implant analog (GM Implant Analog; Neodent, Straumann) with Bio-HPP poly ether-ether ketone (PEEK) abutment and titanium screw was used in the study. The samples were embedded in acrylic split mold. In the control Group C, no agent was added. In the other three groups, blood (B), normal saline (N) and saliva (S) was added in the access cavity of the samples. A sequential torque of 15 Ncm, 20 Ncm, 25 Ncm, 30 Ncm up to 35 Ncm was applied with a digital torque meter (Eclatorq, model: SD-05bn, range:2.5–50 Ncm, torque accuracy: ± 2%cw). Samples were subjected to thermomechanical cyclic loading at 5–550 Celsius for 1000 cycles (Chewing simulator, CS 4.4) to simulate six months of clinical service. Preload was measured as reverse torque value (RTV). Raw data in the form of mean ± standard deviation was documented and subjected to statistical analysis. A one-way ANOVA was performed to contrast the groups. Tukey HSD test was used to determine the multiple comparison assessment (P < 0. 05).
Results
A mean reverse torque value of 35 Ncm ±0.00 was observed in both control and in groups exposed to normal saline (P >.05). Measurements of 33.4 Ncm ±2.51 and 34.8 Ncm ±0.40 were found when exposed to blood and artificial saliva in order (P < .05). When compared with control, exposure to blood showed significant variation in preload (P = .03).
Conclusion
A significant reduction in reverse torque force was observed when titanium implants and Bio-HPP abutments were exposed to blood, suggesting a potential risk of screw loosening (P < .05). In contrast, minimal decrease and no significant change in preload were noted with exposure to saliva and normal saline (P > .05).
Graphical abstract
1. Introduction
Dental implant prosthesis is proven to be one of the most predictable treatment options today. With continuous research in materials science and technology, increase in implant success rate is appreciated up to 90 % by improvement in mastication, esthetics, patient comfort and phonetics in edentulous individuals.1 Prosthetic abutments are secured to the implant fixture with a screw that acts as a fastener. Jung RE et al., evidenced abutment screw loosening to be the second commonest complication followed by lack of osseointegration. Application of right torque force to seat the components properly is needed to prevent the deleterious effects in the surrounding bone, and prosthesis.2 While tightening, energy is transmitted to the screw and internal thread portion of the implant. This act of stretching keeps the screw threads in tight contact with its counterpart, resulting in a clamping or preload force between the screw head and its seat.3
Optimizing the abutment screw preload is of pivotal importance. When the abutment screw loosens over time, it causes patient discomfort, detachment of superstructure or prosthetic crown, leading to disuse of the implant restoration.4 Factors such as screw geometry, material, abutment angulation, implant design, abutment collar dimension, abutment screw head form, and implant-abutment connection influence the abutment screw loosening.5,6 The preload force tends to fluctuate on exposure to different oral fluids and debris. Contact of blood and saliva with implant and or abutment parts always exists until completion of final restoration.7, 8, 9 Normal saline is commonly used as an irrigant and decontaminant during implant treatment. Ingress of any of these agents into the implant thread lumen, prior to abutment placement may hinder the appropriate application of torque force.9,10 Presence of these agents around the surface of implant and or abutment parts, reduces the friction and may increase the preload. The tightening torque must be proportional to the screw's elastic limit. Any amplification of screw limit, predisposes to screw fracture.3
In recent years, metal-free materials are used as abutments owing to superior properties comparable to previously used titanium alloys. Polyether ether ketone (PEEK) has comparable elastic modulus of 3.6 GPa to that of bone, 90–100 MPa of tensile strength, high resistance to thermal injury, and aesthetically pleasing to be used in anterior region.11,12 Recently, a high-performance PEEK polymer was developed for use as an alternative to polymethyl methacrylate, cobalt-chromium, and titanium alloys. PEEK when modified with nanoparticles has shown enhanced biological and mechanical properties leading to newer materials such as Bio-HPP. It is an enhanced PEEK with 20 % ceramic molecules of aluminum oxide & zirconia oxide nanoparticles.13,14 Koutouzis et al. detected no bone recession and soft tissue reaction with PEEK abutments when compared against titanium. Negligible difference between PEEK, zirconia, polymethyl methacrylate and titanium abutments with regards to oral microbial adhesion was observed. PEEK's equivalent elastic modulus with bone, decreased stress and increased bone remodeling, enabled the material to be a proven alternative to titanium abutments.15
Control of factors that cause screw loosening is critical to prevent functional impairment, pain, inconveniences, increased risk of peri-implantitis, reduced esthetics, require repeated patient visit for adjustment and psychologically impact the patients.5,6 Till date, not many studies are done to understand the screw mechanics when inserted into newer Bio-HPP PEEK abutment.3,11,12 No studies are performed to determine the variation in preload and revers torque force when implants and PEEK abutments are exposed to normal saline, blood and saliva. Understanding the clinical importance and current lacunae, in vitro research was structured to examine the impact of blood, saliva and saline over preload force of newer PEEK abutments on titanium implants. The null hypothesis stated there would be no difference in preload and revers torque force of Bio-HPP PEEK abutment before and after exposure to different agents.
2. Methods
Prior to commencement of the study, institutional review board clearance was obtained (SRMDC/IRB/2021/MDS/NO.204). A total of forty implant-abutment screws were tested in this study. Grade 5 titanium dental implant analog (GM Implant Analog; Neodent, Straumann) was used. Bio HPP poly ether-ether ketone (GM Pro PEEK abutment, Neo dent, Straumann) abutment of 4.5 × 2.5 mm dimension with titanium screw was used as test specimens in this study (Fig. 1).
Fig. 1.
Grade 5 titanium implant analog with Bio-HPP PEEK abutment.
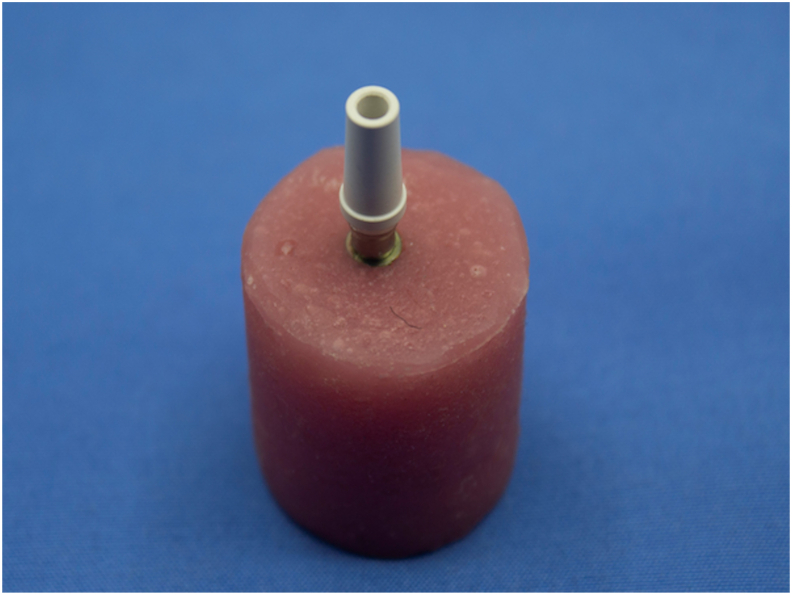
For standardization of specimen position, a mold was prepared. A stainless-steel split mold was designed in the length of 20 mm, width of 20 mm, and the thickness of 3 mm, and a block to fit into the opening of the stainless-steel form was fabricated for positioning of samples (Fig. 2). At the center of the block, a small recess was created as per the implant analog dimension to enable the fixture to be screwed vertically. The split mold was filled with self-cure polymethyl methacrylate (DPI, RR Cold Cure) until the implant top become flushed with a resin block. Resin material was mixed according to the manufacturer's recommendation and gushed into the split mold. Mold, filled with resin was undisturbed until polymerization was completed. On completion of polymerization, the acrylic block was removed from the split mold. Acrylic mold was finished and verified to be sure that PEEK abutment fitted into the analog. The abutment had an inbuilt titanium screw for attachment into the implant analog (Fig. 3).
Fig. 2.
Stainless split mold for sample preparation.
Fig. 3.
Titanium implant – PEEK abutment secured in the acrylic block for testing.
The samples were divided into four groups with ten analogs each as shown in Table 1. Group C served as control where the implant-Bio-HPP PEEK abutment unit was not exposed to oral fluids. In other three groups, blood, normal saline and artificial saliva was added into the implant lumen prior to abutment attachment and torque measurement. Whole blood served as Group B. For this, authentically stored whole blood consisting of red blood cells, white blood cells, platelets and blood plasma was used. One unit of whole blood irrespective of blood grouping was selected. It was stored at 2–4 °C under refrigeration for up to 35 days until blood transfusion. After 35 days it was considered as “expired” and was obsolete for clinical use. This expired blood was obtained from a blood bank (Blood Bank, SRM Medical College Hospital and Research Centre) and used in this study. Normal saline (0.9 % sodium chloride, NS Proline) was added in Group N and artificial saliva (Sigma Aldrich Chemical) was used in Group S.
Table 1.
Study Groups and different agents used for preload test.
| S.no | Groups | Agents | No. of samples (N) |
|---|---|---|---|
| 1 | Group C | No agents | 10 |
| 2 | Group B | Expired whole blood | 10 |
| 3 | Group N | Normal saline | 10 |
| 4 | Group S | Artificial saliva | 10 |
A sterile pipette (Salco 10 mL) was used for carrying the agents. It was filled into the inner surface of the implant fixture to simulate the oral environment. After the addition of the agents, the torque measurements were carried out. All the forty abutments were fixed to implant analogs before torque value was recorded. An electronically calibrated digital torque meter device (Eclatorq, model: SD-05bn, range: 2.5–50 Ncm with an accuracy of ± 2 %) was employed for testing. A display monitor in the device showed the applied torque values. For preload measurement, a sequential order was adhered. The screws were tightened in five steps of 15 Ncm, 20 Ncm, 25 Ncm, 30 Ncm up to 35 Ncm at an interval of 15 s after each tightening. After 10 min of completion, the abutment screw was re-tightened to final 35 Ncm. This was performed to ensure the screw settled properly. On completion of initial torque measurements in all the groups, the samples were subjected to thermomechanical cycling at 5–55 °C for 1000 cycles (Chewing simulator, CS 4.4) to simulate a clinical service of six months. Reverse torque value (RTV) was observed following the same method. All measurements were made by the same operator. The recorded torque values were documented in an Excel sheet. One-way analysis of variance (ANOVA) and Tukey HSD was used for the statistical analysis. Raw data measurement was subjected to biostatistical analysis using IBM SPSS Version 20 (IBM Corp.) software.
3. Results
The overall mean (Ncm) and the variance (SD) of the torque measurements were calculated and the raw data showed normal distribution. In all the groups, including the control where no agent was added, the same mean torque value of 35 ± 0.00 Ncm (mean ± SD), before thermomechanical cycling was observed as shown in Table 2. Reverse torque values were found to be 35 ± 0.00 Ncm in both the control group and in the group which was exposed to normal saline. Group that was contaminated with blood showed a mean reverse torque value of 33.4 ± 2. 51 Ncm. When samples were filled with artificial saliva, it was detected to be 34. 8 Ncm ±0.40. (Table 2).
Table 2.
Descriptive statistics of initial and reverse torque values in stud groups.
| S.no | Groups | Initial torque value Mean ± SD (Ncm) |
Reverse torque value Mean ± SD (Ncm) |
|---|---|---|---|
| 1 | Group C | 35 ± 0. 00 | 35 ± 0. 00 |
| 2 | Group B | 35 ± 0. 00 | 33.4 ± 2.51 |
| 3 | Group N | 35 ± 0. 00 | 35 ± 0. 00 |
| 4 | Group S | 35 ± 0. 00 | 34.8 ± 0.40 |
Group C: Control, Group B: Blood, Group N: Normal saline and Group S: Artificial saliva.
In control and normal saline groups, both the initial and reverse torque values were the same 35 Ncm ±0. 00 with a standard error mean of 0. 00. Torque values when exposed to blood showed a numerical decline from 35 Ncm to 33.4 Ncm with standard error mean of 0.79. When subjected to artificial saliva the reverse torque value reduced from 35 Ncm to 34.8 Ncm with standard error mean of 0.12. Table 3 showed one-way ANOVA tests to reveal statistical differences among the groups while comparing initial and reverse torque values as the P value was 0.02 (P < 0. 05). Table 4 furnished multiple comparison with the control specimen using Tukey HSD test. On comparison of control and blood-contaminated groups, a statistically significant difference of 1. 60 at P < 0. 03 was observed. When measured against normal saline groups, no statistical variation was detected as the difference was 0. 00 at P > .05. While equating artificial saliva with the control group, the torque values showed a negligible difference of 0. 19 (P > .05).
Table 3.
Comparison of torque value among the groups with ANOVA test.
| Sum of squares | df | Mean square | F | Significance | ||
|---|---|---|---|---|---|---|
| Initial torque | Between groups | 0.00 | 3 | 0.00 | ||
| Within groups | 0.00 | 36 | 0.00 | |||
| Total | 0.00 | 39 | ||||
| Reverse torque | Between groups | 17.95 | 3 | 5.98 | 3.68 | 0.02a |
| Within groups | 58.42 | 36 | 1.62 | |||
| Total | 76.38 | 39 |
Level of significance was set at P < .05.
Table 4.
Multiple comparisons of reverse torque measurements between groups with Tukey HSD test.
| 95 % Confidence interval | |||||||
| S.no | Control Group | Comparison Group | Mean difference | Standard Error | Sig | Lower bound | Upper bound |
| 1 | Group B | 1.60 | 0.56 | 0.03a | 0.06 | 3.13 | |
| 2 | Group N | 0.00 | 0.56 | 1.00 | −1.53 | 1.53 | |
| 3 | Group S | 0.19 | 0.56 | 0.98 | −1.34 | 1.72 | |
Level of significance was set at P < .05.
4. Discussion
Dental implant therapy involves many components like fixture, abutment, screws, healing caps, copings, prosthetic part and more. Screws are important part that secure the implant fixture and prosthetic abutment in position. Loosening of screws is a frequent implant complication that cause patient discomfort and if unattended can lead to detachment of the prosthesis.2 Many studies performed previously, analysed the factors that caused screw loosening on titanium material.2, 3, 4, 5, 6, 7, 8 During and after implant treatment procedure, the screw access holes get exposed to different oral fluids.7 Presence of patient's blood, saliva and conventional use of well-tolerated normal saline for irrigation, flushing our debris, and for maintenance of sterile zone are indispensable in implant therapeutics.9,10 Frictional coefficient between the implant parts tend to change after exposure to these agents and affect the attachment of components.7,8 Understanding the importance of screw loosening and inevitable contact of implant components to varying agents, urged to investigate screw loosening on newer non-metallic biomaterials as an in-vitro study. No studies evaluated the influence of normal saline, blood and saliva on Bio-HPP PEEK abutments. This reinforced PEEK material was recommended for use as long-term implant provisional restorations as it demonstrated enhanced properties of 180–185 MPa flexural strength, 700–1600 MPa fracture strength, 4.2–4.8 MPa elastic modulus and polished surface with Ra value less than 0.02 μm.12, 13, 14
To simulate clinical scenario, specimen from Straumann Bio-HPP PEEK abutment with grand morse implant-abutment connection, and designed for long term implant provisional restoration was opted for testing in the study. Its application in metal allergic patients was another added benefit of this material.14 The geometric design of the abutment was an internal conical connection to ensure precise fit and prevent micromovements while seated into the threads of implant fixture. To eliminate the influence of confounding factors, all the implant-abutment samples were of same size, form, design, material and subjected to uniform quantity of torque force while screw tightening was done by single investigator. Acrylic mold was prepared to stabilize the position of implant.3 To replicate 6 months of PEEK abutment service, a cyclic loading test at 5–55 °C for 1000 cycles, and 60 s of dwelling time was followed. In this study, a pre calibrated digital torque meter was used for preload measurements over hand held torque wrench to limit the manual errors and inter or intra operator variability.3 An orderly application of clinically proven torque value of 35N was adapted in the study. Implant preload was measured as it is a critical factor affirming secure implant-abutment connection and is essential for long term stability and durability of prosthesis.7, 8, 9 Reverse torque values were determined as, Bio-HPP PEEK abutments were designed for long-term provisional restoration and had to be replaced with definitive prosthesis.14
Results of the study rejected the null hypothesis as there was difference when titanium implants with Bio - HPP PEEK abutments were exposed to different agents. In control group and in samples exposed to normal saline, the initial preload and reverse torque force remained the same. This result was supported by Siamos G et al. who perceived reverse torque values indicated the preload in a functional state, thereby reflecting the ability to withstand the screw from loosening.16 This indicated there was no loss of preload over time and the implant-Bio HPP PEEK abutment connection remained stable without any screw loosening on exposure to normal saline. A significant reduction in RTV on exposure to blood (P < .05) and numerical decline in mean RTV with artificial salivary group (P > .05) was observed. Obtained results were corroborated with those of Christersson et al. and Mostafavi AS et al., who perceived reduced reverse torque values on exposure to blood and artificial saliva with titanium abutment material.17,18 In a study performed by Rathe et al., no significant effect on preload force was noticed when titanium implant, abutment and screw was exposed to chlorhexidine, saliva, blood and special sealing silicone.3 The results of the current study were further supported by the work done by Gumus HO et al., who perceived decreased reverse torque value when titanium implant-abutment assembly was subjected to blood than with chlorhexidine, fresh human saliva and dry control groups.9 In contrast, Tzenakis et al. observed higher preload with gold prosthetic retaining screw when contaminated with saliva.19. Koosha et al. proved reverse torque values were considerably greater in the chlorhexidine group than in the normal saline group.8
The difference in torque force could be explained by “sedimentation effect”. According to this phenomenon, there is energy dissipation when two surfaces encounter each other on force application. Gradual settling is impeded owing to the presence of microroughness that does not permit complete attachment of implant to abutment.20,21 Presence of third component in the form of any oral fluid between implant, abutment and screw could have exacerbated the settling process. The potential reasons for reduction in reverse torque value when exposed to blood, could be attributed to the fibrinogen and platelets that attach to the surfaces of implant-abutment screw assembly forming a biofilm. Exchange of reaction between titanium surfaces occurs instantly after platelets attachment. Gross differences in the viscosity of blood, saliva and normal saline (blood viscosity 3.33 cP, saliva with 1.9 cP and saline is 1.01 cP) influence a significant effect in decreased reverse torque values with exposure to blood.9,21 This difference could be attributed to differing uniaxial compressive strength metrics for titanium and PEEK abutment as 1.38 ± 0.17 mm and 1.06 ± 0.05 mm.22,23 Variations between fresh human and artificial saliva that differ in composition were stated by Gumus et al.9 According to Norton et al. there was no increase in reverse torque values with titanium abutment screw when subjected to saliva as the residual salivary fluid could enter micro gaps and deposit bacterial glycoproteins in the implant-abutment connection, serving as lubrication spreading pressures and reducing friction through viscous components such as polysaccharides, pathogens, and glycoproteins.24
In the current study, gross variation in torques values was not appreciated owing to the presence of an unlocking feature in the PEEK abutment-screw assembly, which was in-built design to facilitate easy removal while placing definitive crowns.25 It demonstrated higher chance of screw loosening when the implant-Bio HPP PEEK abutments were exposed to blood than over saline or saliva. It affirmed removal of oral fluids before attachment of components to limit the chances of screw loosening. The results of present study must be inferred within its limitations. The lack of clinical environment simulation is of significant concern. In the clinical scenarios, exposure to multiple agents such as blood, saliva, and normal saline occurs together in contrast to the laboratory setup adhered in the study. The tested agents were only limited to the implant lumen unlike oral cavity. The current study was performed on a specific design of implant-abutment screw assembly. It is yet unclear if the same preload force will be applicable in different designs of PEEK abutments. More studies with different implant/abutment materials and forms are needed to generalize the inference.
Patient consent.
This research work did not involve patient or guardian for getting consent.
Funding
This research work was not funded by any government or non-government organization and did not involve any promotion of commercial products or materials.
Fund was self-supported.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to express our deepest gratitude to the Department of Prosthodontics, SRM dental college, Ramapuram, for the support towards the dental laboratory in the fabrication and testing of the samples, White Lab, Saveetha dental college, towards the support for thermocycling of all samples, and Dr.Cyril, Public Health dentist, Chettinad dental college for his support in the statistical analysis.
References
- 1.Shemtov-Yona K., Rittel D. An Overview of the mechanical integrity of dental implants. BioMed Res Int. 2015;2015 doi: 10.1155/2015/547384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung R.E., Pjetursson B.E., Glauser R., Zembic A., Zwahlen M., Lang N.P. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin Oral Implants Res. 2008 Feb;19(2):119–130. doi: 10.1111/j.1600-0501.2007.01453.x. [DOI] [PubMed] [Google Scholar]
- 3.Rathe F., Ratka C., Kaesmacher C., Winter A., Brandt S., Zipprich H. Influence of different agents on the preload force of implant abutment screws. J Prosthet Dent. 2021 Oct;126(4):581–585. doi: 10.1016/j.prosdent.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Bajoghli F., Sabouhi M., Pourali M., Davoudi A. Stability of implant-abutment connection in three different systems after fatigue test. J Indian Prosthodont Soc. 2022 Oct-Dec;22(4):338–342. doi: 10.4103/jips.jips_247_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Londhe S.M., Gowda E.M., Mandlik V.B., Shashidhar M.P. Factors associated with abutment screw loosening in single implant supported crowns: a cross-sectional study. Med J Armed Forces India. 2020 Jan;76(1):37–40. doi: 10.1016/j.mjafi.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakaeen L.G., Winkler S., Neff P.A. The effect of implant diameter, restoration design, and occlusal table variations on screw loosening of posterior single-tooth implant restorations. J Oral Implantol. 2001;27(2):63–72. doi: 10.1563/1548-1336(2001)027<0063:TEOIDR>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Adawi H.A., Dewan H., Khawaji A., et al. Effects of blood contamination and decontamination protocol on reverse torque value of abutment screws in dental implants: an in vitro study. Biomimetics. 2023 April;8(2):157. doi: 10.3390/biomimetics8020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koosha S., Toraji S., Mostafavi A.S. Effect of fluid contamination on the reverse torque values of abutment screws at implant-abutment connections. J Prosthet Dent. 2020 April;123(4):618–621. doi: 10.1016/j.prosdent.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Gumus H.O., Zortuk M., Albayrak H., Dincel M., Kocaagaoglu H.H., Kilinc H.I. Effect of fluid contamination on reverse torque values in bone-level implants. Implant Dent. 2014 Oct;23(5):582–587. doi: 10.1097/ID.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 10.Tonon C.C., Panariello B.H.D., Spolidorio D.M.P., Gossweiler A.G., Duarte S. Antibiofilm effect of ozonized physiological saline solution on peri-implant-related biofilm. J Periodontol. 2021 Aug;92(8):1151–1162. doi: 10.1002/JPER.20-0333. [DOI] [PubMed] [Google Scholar]
- 11.Wang B., Huang M., Dang P., Xie J., Zhang X., Yan X. PEEK in fixed dental prostheses: application and adhesion improvement. Polymers. 2022 Jun;14(12):2323. doi: 10.3390/polym14122323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo C., Liu Y., Peng B., et al. PEEK for oral applications: recent advances in mechanical and adhesive properties. Polymers. 2023 Jan;15(2):386. doi: 10.3390/polym15020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo L., Zou Z., Smeets R., et al. Attachment and osteogenic potential of dental pulp stem cells on non-thermal plasma and UV light treated titanium, zirconia and modified PEEK surfaces. Materials. 2022 Mar;15(6):22–25. doi: 10.3390/ma15062225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo L., Smeets R., Kluwe L., et al. Cytocompatibility of titanium, zirconia and modified PEEK after surface treatment using UV light or non-thermal plasma. Int J Mol Sci. 2019 Nov;20(22):55–96. doi: 10.3390/ijms20225596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koutouzis T., Richardson J., Lundgren T. Comparative soft and hard tissue responses to titanium and polymer healing abutments. J Oral Implantol. 2011 Mar;37:174–182. doi: 10.1563/AAID-JOI-D-09-00102.1. [DOI] [PubMed] [Google Scholar]
- 16.Siamos G., Winkler S., Boberick K.G. Relationship between implant preload and screw loosening on implant-supported prostheses. J Oral Implantol. 2002;28(2):67–73. doi: 10.1563/1548-1336(2002)028<0067:TRBIPA>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Christersson C.E., Lindh L., Arnebrant T. Film-forming properties and viscosities of saliva substitutes and human whole saliva. Eur J Oral Sci. 2000 Oct;108(5):418–425. doi: 10.1034/j.1600-0722.2000.108005418.x. [DOI] [PubMed] [Google Scholar]
- 18.Mostafavi A.S., Memarian M., Seddigh M.A. Effect of fluid contamination on reverse torque values in implant-abutment connections under oral conditions. J Adv Prosthodont. 2021 Feb;13(1):65–70. doi: 10.4047/jap.2021.13.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzenakis G.K., Nagy W.W., Fournelle R.A., et al. The effect of repeated torque and salivary contamination on the preload of slotted gold implant prosthetic screws. J Prosthet Dent. 2002 Aug;88(2):183–191. doi: 10.1067/mpr.2002.127604. [DOI] [PubMed] [Google Scholar]
- 20.Bulaqi H.A., Mousavi Mashhadi M., Safari H., Samandari M.M., Geramipanah F. Dynamic nature of abutment screw retightening: finite element study of the effect of retightening on the settling effect. J Prosthet Dent. 2015 May;113(5):412–419. doi: 10.1016/j.prosdent.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Mishra S.K., Chowdhary R., Kumari S. Microleakage at the different implant abutment interface: a systematic review. J Clin Diagn Res. 2017 Jun;11(6):ZE10–ZE15. doi: 10.7860/JCDR/2017/28951.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang S., Qin Y., Guo X., Li Y. An in vitro study of fluid contaminations influences on reverse torque values of implant-abutment connections. BioMed Res Int. 2022 Mar;2022 doi: 10.1155/2022/4111710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ligtenberg A.J.M., Liem E.H.S., Brand H.S., Veerman E.C.I. The effect of exercise on salivary viscosity. Diagnostics. 2016 Nov;6(4):40. doi: 10.3390/diagnostics6040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norton M.R. Assessment of cold-welding properties of the internal conical interface of two commercially available implant systems. J Prosthet Dent. 1999 Feb;81(2):159–166. doi: 10.1016/s0022-3913(99)70243-x. [DOI] [PubMed] [Google Scholar]
- 25.Ortega-Martínez J., Delgado L.M., Ortiz-Hernández M., et al. In vitro assessment of PEEK and titanium implant abutments: screw loosening and microleakage evaluations under dynamic mechanical testing. J Prosthet Dent. 2022 Mar;127(3):470–476. doi: 10.1016/j.prosdent.2020.09.033. [DOI] [PubMed] [Google Scholar]