Abstract
We present a genome assembly from an individual of Inga leiocalycina (Streptophyta; Magnoliopsida; Fabales; Fabaceae). The genome sequence has a total length of 948.00 megabases. Most of the assembly is scaffolded into 13 chromosomal pseudomolecules. The assembled mitochondrial genome sequences have lengths of 1,019.42 and 98.74 kilobases, and the plastid genome assembly is 175.51 kb long. Gene annotation of the nuclear genome assembly on Ensembl identified 33,457 protein-coding genes.
Keywords: Inga leiocalycina, genome sequence, chromosomal, Fabales
Species taxonomy
Eukaryota; Viridiplantae; Streptophyta; Streptophytina; Embryophyta; Tracheophyta; Euphyllophyta; Spermatophyta; Magnoliopsida; Mesangiospermae; eudicotyledons; Gunneridae; Pentapetalae; rosids; fabids; Fabales; Fabaceae; Caesalpinioideae; mimosoid clade; Ingeae; Inga; Inga leiocalycina Benth. (NCBI:txid486065).
Background
Inga Mill. (Fabaceae) is a characteristic component of the species-rich neotropical flora, and is ubiquitous in the rainforests of the tropical Americas. Inga typifies the rapid evolutionary radiations that generated most neotropical tree diversity, exhibiting the highest diversification rate of any tree genus in the Amazon ( Baker et al., 2014; Richardson et al., 2001). Inga leiocalycina Benth. is a tree species reaching 35m in height that is widespread in tropical American rainforests, both east and west of the Andes. This species ranges from Southern Mexico to Bolivia and Amazonian Brazil, occupying an elevational range of around 0-1000m above sea level ( Pennington, 1997). Inga leiocalycina tolerates a relatively wide range of rainfall conditions, ranging from the perma-wet rainforest of northwestern Colombia to the seasonally dry climate of Ecuador’s Pacific coast. Inga leiocalycina is also notable for having apparent edaphic ‘ecotypes’ in the Amazon, where populations on rich, bottomland soils differ morphologically from those on poorer, upland soils ( Dexter et al., 2010).
Trees in tropical rainforests are subject to relentless insect herbivory, and so Inga leiocalycina has evolved a range of defensive strategies to deter herbivores. Specifically, I. leiocalycina possesses extra-floral nectaries on its leaf midribs for attracting ants that defend the plant against herbivores ( Kursar et al., 2009), as well as defending itself chemically by producing a range of compounds, primarily flavan3ol_polymers, in its young leaves ( Forrister et al., 2023). Inga species are widely used for agroforestry and ecosystem restoration due to their ability to fix nitrogen, in addition to their utility as food and forage crops ( Pennington, 1997). The fruits of Inga leiocalycina are edible thanks to the sweet, white sarcotesta surrounding the seeds, and are gathered from the wild as food ( López Diago & García Castro, 2021), but are not as widely used or cultivated as other Inga species (e.g. Inga edulis or I. macrophylla). The Inga leiocalycina sample sequenced here, originally collected from Manabí in western Ecuador but grown at RBGE, was a diploid (2 n=2 x=26) as is typical for the species ( Hanson, 1995).
Here we present one of three chromosomally complete, annotated genome sequences for Inga which are the first for the genus. We believe the Inga leiocalycina genome will provide an important resource for future work, given the prominent role of Inga in studies examining the ecology and evolution of tropical rainforest floras. Potential research themes could include assessing the role of herbivory and chemical defence in driving genomic divergence and speciation in tropical trees, building on previous work (e.g. Endara et al., 2018).
Genome sequence report
The sequenced genome is of an Inga leiocalycina specimen (drIngLeio1, Figure 1). Using flow cytometry of leaf tissue, the genome size (1C-value) was estimated as 1.17 pg, equivalent to 1,150 Mb. The genome was sequenced using Pacific Biosciences single-molecule HiFi long reads, generating a total of 30.30 Gb (gigabases) from 2.90 million reads, providing approximately 29-fold coverage. Primary assembly contigs were scaffolded with chromosome conformation Hi-C data, which produced 117.73 Gb from 779.69 million reads, yielding an approximate coverage of 124-fold. Specimen and sequencing information is summarised in Table 1.
Figure 1. Photograph of the Inga leiocalycina (drIngLeio1) specimen used for genome sequencing.
Table 1. Specimen and sequencing data for Inga leiocalycina.
| Project information | |||
|---|---|---|---|
| Study title | Inga leiocalycina | ||
| Umbrella BioProject | PRJEB64758 | ||
| Species | Inga leiocalycina | ||
| BioSample | SAMEA111531407 | ||
| NCBI taxonomy ID | 486065 | ||
| Specimen information | |||
| Technology | ToLID | BioSample accession | Organism part |
| PacBio long read sequencing | drIngLeio1 | SAMEA111531416 | Leaf |
| Hi-C sequencing | drIngLeio1 | SAMEA111531409 | Leaf |
| RNA sequencing | drIngLeio2 | SAMEA113598545 | leaf |
| Sequencing information | |||
| Platform | Run accession | Read count | Base count (Gb) |
| Hi-C Illumina NovaSeq 6000 | ERR11814135 | 7.80e+08 | 117.73 |
| PacBio Sequel IIe | ERR11809161 | 2.90e+06 | 30.3 |
| RNA Illumina NovaSeq 6000 | ERR12321232 | 7.00e+07 | 10.57 |
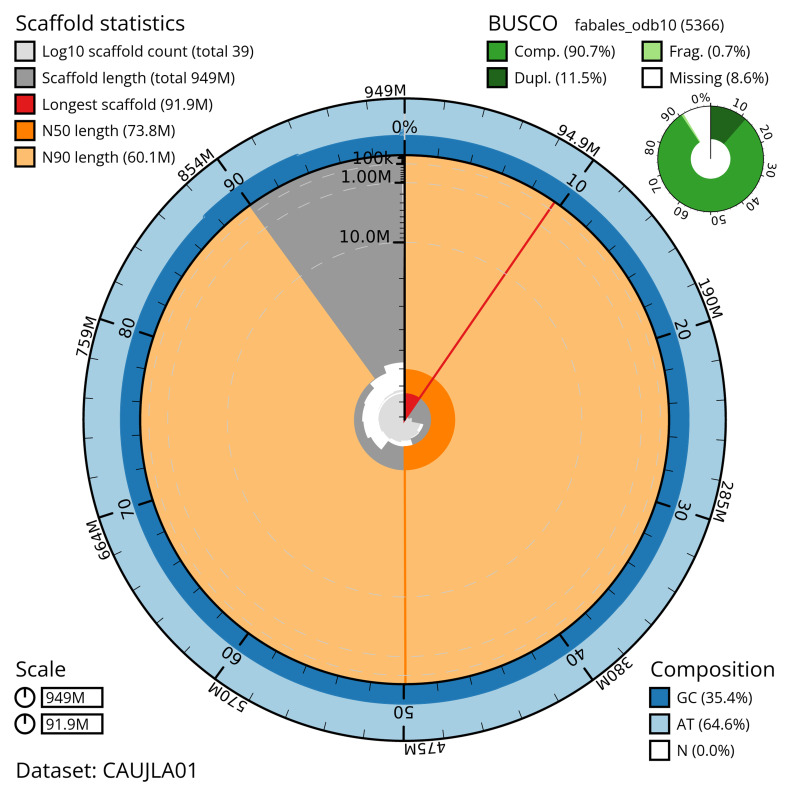
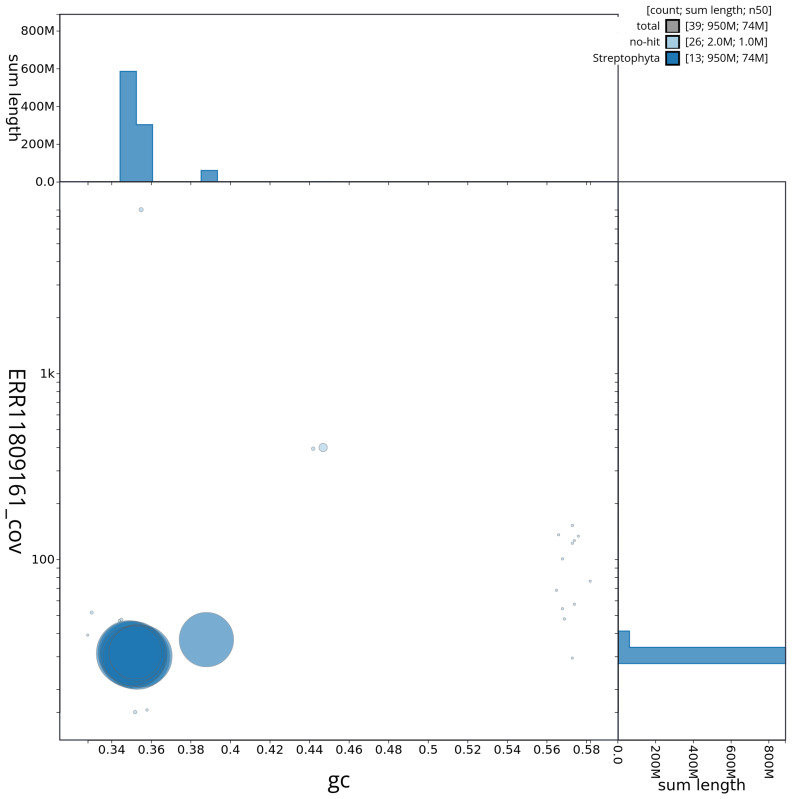
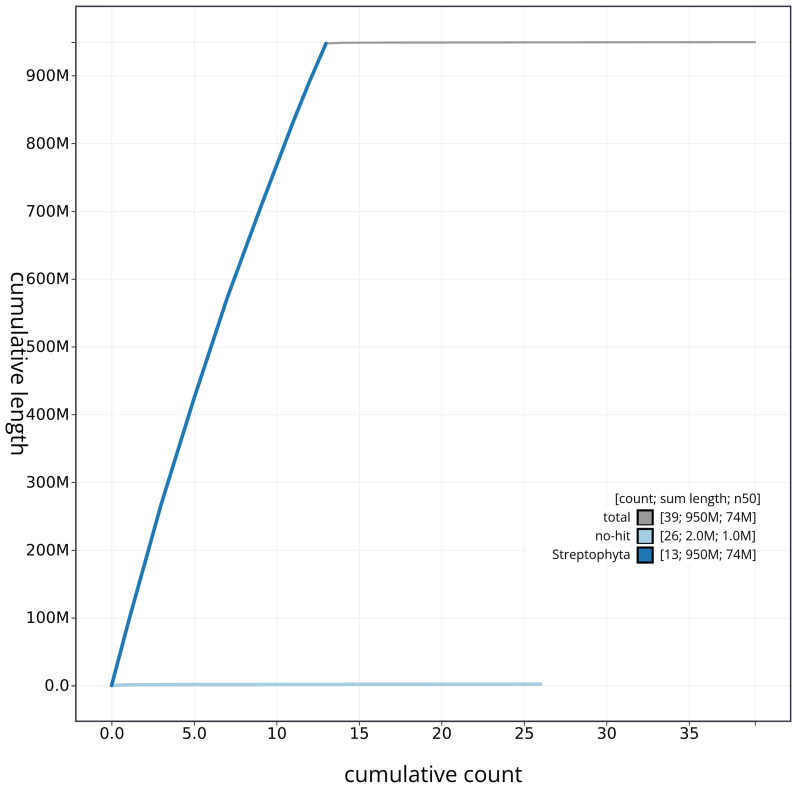
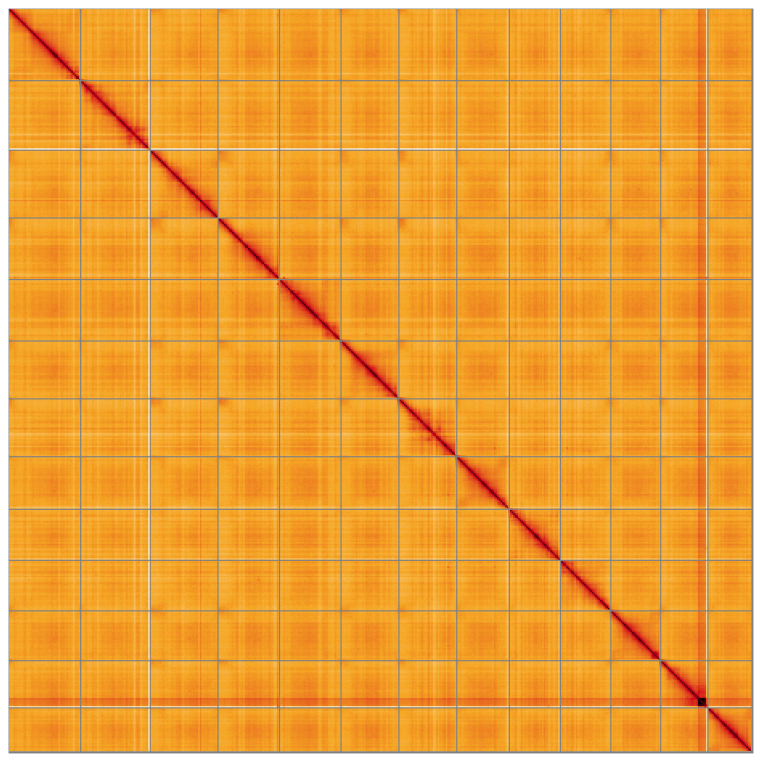
Manual assembly curation corrected 100 missing joins or mis-joins and 25 haplotypic duplications, reducing the assembly length by 2.52%, and decreasing the scaffold N50 by 18.54%. The final assembly has a total length of 948.00 Mb in 36 sequence scaffolds with a scaffold N50 of 73.8 Mb ( Table 2) with 362 gaps. The snail plot in Figure 2 provides a summary of the assembly statistics, while the distribution of assembly scaffolds on GC proportion and coverage is shown in Figure 3. The cumulative assembly plot in Figure 4 shows curves for subsets of scaffolds assigned to different phyla. Most (99.78%) of the assembly sequence was assigned to 13 chromosomal-level scaffolds. Chromosome-scale scaffolds confirmed by the Hi-C data are named in order of size ( Figure 5; Table 3). The order and orientation of contigs along Chromosome 12 between 49.5 Mb and 58.3 Mb is uncertain. While not fully phased, the assembly deposited is of one haplotype. Contigs corresponding to the second haplotype have also been deposited. The mitochondrial and plastid genomes were also assembled and can be found as contigs within the multifasta file of the genome submission.
Figure 2. Genome assembly of Inga leiocalycina, drIngLeio1.1: metrics.
The BlobToolKit snail plot shows N50 metrics and BUSCO gene completeness. The main plot is divided into 1,000 size-ordered bins around the circumference with each bin representing 0.1% of the 949,261,181 bp assembly. The distribution of scaffold lengths is shown in dark grey with the plot radius scaled to the longest scaffold present in the assembly (91,898,272 bp, shown in red). Orange and pale-orange arcs show the N50 and N90 scaffold lengths (73,756,888 and 60,100,043 bp), respectively. The pale grey spiral shows the cumulative scaffold count on a log scale with white scale lines showing successive orders of magnitude. The blue and pale-blue area around the outside of the plot shows the distribution of GC, AT and N percentages in the same bins as the inner plot. A summary of complete, fragmented, duplicated and missing BUSCO genes in the fabales_odb10 set is shown in the top right. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/CAUJLA01/dataset/CAUJLA01/snail.
Figure 3. Genome assembly of Inga leiocalycina, drIngLeio1.1: BlobToolKit GC-coverage plot.
Sequences are coloured by phylum. Circles are sized in proportion to sequence length. Histograms show the distribution of sequence length sum along each axis. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/CAUJLA01/dataset/CAUJLA01/blob.
Figure 4. Genome assembly of Inga leiocalycina drIngLeio1.1: BlobToolKit cumulative sequence plot.
The grey line shows cumulative length for all sequences. Coloured lines show cumulative lengths of sequences assigned to each phylum using the buscogenes taxrule. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/CAUJLA01/dataset/CAUJLA01/cumulative.
Figure 5. Genome assembly of Inga leiocalycina, drIngLeio1.1: Hi-C contact map of the drIngLeio1.1 assembly, visualised using HiGlass.
Chromosomes are shown in order of size from left to right and top to bottom. An interactive version of this figure may be viewed at https://genome-note-higlass.tol.sanger.ac.uk/l/?d=abMPGhQDQSOODVsPWKcBtw.
Table 2. Genome assembly data for Inga leiocalycina, drIngLeio1.1.
| Genome assembly | ||
|---|---|---|
| Assembly name | drIngLeio1.1 | |
| Assembly accession | GCA_963242795.1 | |
| Accession of alternate haplotype | GCA_963242585.1 | |
| Span (Mb) | 948.00 | |
| Number of contigs | 401 | |
| Contig N50 length (Mb) | 5.6 | |
| Number of scaffolds | 36 | |
| Scaffold N50 length (Mb) | 73.8 | |
| Longest scaffold (Mb) | 91.9 | |
| Assembly metrics * | Benchmark | |
| Consensus quality (QV) | 64.6 | ≥ 50 |
| k-mer completeness | 100.0% | ≥ 95% |
| BUSCO ** | C:90.7%[S:79.2%,D:11.5%],
F:0.7%,M:8.6%,n:5,366 |
C ≥ 95% |
| Percentage of assembly mapped
to chromosomes |
99.78% | ≥ 95% |
| Organelles | Mitochondrial genome: 1,019.42 kb and
98.74 kb; plastid genome: 175.51 kb |
complete single alleles |
| Genome annotation at Ensembl | ||
| Number of protein-coding genes | 33,457 | |
| Number of non-coding genes | 14,611 | |
| Number of gene transcripts | 69,098 | |
* Assembly metric benchmarks are adapted from column VGP-2020 of “Table 1: Proposed standards and metrics for defining genome assembly quality” from Rhie et al. (2021).
** BUSCO scores based on the fabales_odb10 BUSCO set using version 5.4.3. C = complete [S = single copy, D = duplicated], F = fragmented, M = missing, n = number of orthologues in comparison. A full set of BUSCO scores is available at https://blobtoolkit.genomehubs.org/view/CAUJLA01/dataset/CAUJLA01/busco.
Table 3. Chromosomal pseudomolecules in the genome assembly of Inga leiocalycina, drIngLeio1.
| INSDC accession | Name | Length (Mb) | GC% |
|---|---|---|---|
| OY725320.1 | 1 | 91.9 | 35.0 |
| OY725321.1 | 2 | 88.61 | 35.0 |
| OY725322.1 | 3 | 86.41 | 35.5 |
| OY725323.1 | 4 | 78.49 | 35.5 |
| OY725324.1 | 5 | 78.27 | 35.0 |
| OY725325.1 | 6 | 73.76 | 35.5 |
| OY725326.1 | 7 | 73.71 | 35.0 |
| OY725327.1 | 8 | 67.02 | 35.0 |
| OY725328.1 | 9 | 65.06 | 35.0 |
| OY725329.1 | 10 | 64.34 | 35.0 |
| OY725330.1 | 11 | 63.58 | 35.5 |
| OY725331.1 | 12 | 60.1 | 39.0 |
| OY725332.1 | 13 | 56.0 | 35.0 |
| OY725335.1 | Pltd | 0.18 | 35.5 |
| OY725333.1 | MT1 | 1.02 | 44.5 |
| OY725334.1 | MT2 | 0.1 | 44.0 |
The estimated Quality Value (QV) of the final assembly is 64.6 with k-mer completeness of 100.0%, and the assembly has a BUSCO v5.4.3 completeness of 90.7% (single = 79.2%, duplicated = 11.5%), using the fabales_odb10 reference set ( n = 5,366).
Metadata for specimens, BOLD barcode results, spectra estimates, sequencing runs, contaminants and pre-curation assembly statistics are given at https://links.tol.sanger.ac.uk/species/486065.
Genome annotation report
The Inga leiocalycina genome assembly (GCA_963242795.1) was annotated at the European Bioinformatics Institute (EBI) on Ensembl Rapid Release. The resulting annotation includes 69,098 transcribed mRNAs from 33,457 protein-coding and 14,611 non-coding genes ( Table 2; https://rapid.ensembl.org/Inga_leiocalycina_GCA_963242795.1/Info/Index). The average transcript length is 3,476.11. There are 1.44 coding transcripts per gene and 4.78 exons per transcript.
Methods
Sample acquisition and nucleic acid extraction
A specimen of Inga leiocalycina (specimen ID SAN2000548, ToLID drIngLeio1) was collected on 2021-09-09 from the wet tropics glasshouse at the Royal Botanic Garden Edinburgh, Scotland, UK. The specimen used for RNA sequencing (specimen ID SAN20001664, ToLID drIngLeio2) was collected from the same individual on 2023-05-31. The specimens were collected by Rowan Schley (University of Exeter). The original individual was collected in Manabí, Ecuador in 1993 under the collector number ‘T.D. Pennington 13822’ and identified by Terence D. Pennington (Royal Botanic Gardens Kew). The herbarium voucher associated with the sequenced plant is RBGE:BROWP2035 and is deposited in the herbarium of the Royal Botanic Garden Edinburgh (Herbarium code: E).
The workflow for high molecular weight (HMW) DNA extraction at the Wellcome Sanger Institute (WSI) Tree of Life Core Laboratory includes a sequence of core procedures: sample preparation; sample homogenisation, DNA extraction, fragmentation, and clean-up. Leaf tissue of the drIngLeio1 sample was weighed and dissected on dry ice ( Jay et al., 2023), and cryogenically disrupted using the Covaris cryoPREP ® Automated Dry Pulverizer ( Narváez-Gómez et al., 2023). HMW DNA was extracted using the Manual Plant MagAttract v4 protocol ( Jackson & Howard, 2023). HMW DNA was sheared into an average fragment size of 12–20 kb in a Megaruptor 3 system ( Bates et al., 2023). Sheared DNA was purified by solid-phase reversible immobilisation ( Oatley et al., 2023): in brief, the method employs AMPure PB beads to eliminate shorter fragments and concentrate the DNA. The concentration of the sheared and purified DNA was assessed using a Nanodrop spectrophotometer and Qubit Fluorometer and Qubit dsDNA High Sensitivity Assay kit. Fragment size distribution was evaluated by running the sample on the FemtoPulse system.
RNA was extracted from leaf tissue of drIngLeio2 in the Tree of Life Laboratory at the WSI using the RNA Extraction: Automated MagMax™ mirVana protocol ( do Amaral et al., 2023). The RNA concentration was assessed using a Nanodrop spectrophotometer and a Qubit Fluorometer using the Qubit RNA Broad-Range Assay kit. Analysis of the integrity of the RNA was done using the Agilent RNA 6000 Pico Kit and Eukaryotic Total RNA assay.
Protocols developed by the WSI Tree of Life core laboratory are publicly available on protocols.io ( Denton et al., 2023).
Sequencing
Pacific Biosciences HiFi circular consensus DNA sequencing libraries were constructed according to the manufacturers’ instructions. Poly(A) RNA-Seq libraries were constructed using the NEB Ultra II RNA Library Prep kit. DNA and RNA sequencing was performed by the Scientific Operations core at the WSI on Pacific Biosciences Sequel IIe (HiFi) and Illumina NovaSeq 6000 (RNA-Seq) instruments. Hi-C data were also generated from leaf tissue of drIngLeio1 using the Arima-HiC v2 kit. The Hi-C sequencing was performed using paired-end sequencing with a read length of 150 bp on the Illumina NovaSeq 6000 instrument.
Genome assembly, curation and evaluation
Assembly
The original assembly of HiFi reads was performed using Hifiasm ( Cheng et al., 2021) with the --primary option. Haplotypic duplications were identified and removed with purge_dups ( Guan et al., 2020). Hi-C reads were further mapped with bwa-mem2 ( Vasimuddin et al., 2019) to the primary contigs, which were further scaffolded using the provided Hi-C data ( Rao et al., 2014) in YaHS ( Zhou et al., 2023) using the --break option. Scaffolded assemblies were evaluated using Gfastats ( Formenti et al., 2022), BUSCO ( Manni et al., 2021) and MERQURY.FK ( Rhie et al., 2020). The organelle genomes were assembled using OATK ( Zhou, 2023).
Curation
The assembly was decontaminated using the Assembly Screen for Cobionts and Contaminants (ASCC) pipeline (article in preparation). Manual curation was primarily conducted using PretextView ( Harry, 2022), with additional insights provided by JBrowse2 ( Diesh et al., 2023) and HiGlass ( Kerpedjiev et al., 2018). Scaffolds were visually inspected and corrected as described by Howe et al. (2021). Any identified contamination, missed joins, and mis-joins were corrected, and duplicate sequences were tagged and removed. The process is documented at https://gitlab.com/wtsi-grit/rapid-curation (article in preparation).
Evaluation of final assembly
A Hi-C map for the final assembly was produced using bwa-mem2 ( Vasimuddin et al., 2019) in the Cooler file format ( Abdennur & Mirny, 2020). To assess the assembly metrics, the k-mer completeness and QV consensus quality values were calculated in Merqury ( Rhie et al., 2020). This work was done using the “sanger-tol/readmapping” ( Surana et al., 2023a) and “sanger-tol/genomenote” ( Surana et al., 2023b) pipelines. The genome readmapping pipelines were developed using the nf-core tooling ( Ewels et al., 2020), use MultiQC ( Ewels et al., 2016), and make extensive use of the Conda package manager, the Bioconda initiative ( Grüning et al., 2018), the Biocontainers infrastructure ( da Veiga Leprevost et al., 2017), and the Docker ( Merkel, 2014) and Singularity ( Kurtzer et al., 2017) containerisation solutions. The genome was also analysed within the BlobToolKit environment ( Challis et al., 2020) and BUSCO scores ( Manni et al., 2021) were calculated.
Table 4 contains a list of relevant software tool versions and sources.
Table 4. Software tools: versions and sources.
| Software tool | Version | Source |
|---|---|---|
| BlobToolKit | 4.2.1 | https://github.com/blobtoolkit/blobtoolkit |
| BUSCO | 5.3.2 | https://gitlab.com/ezlab/busco |
| bwa-mem2 | 2.2.1 | https://github.com/bwa-mem2/bwa-mem2 |
| Cooler | 0.8.11 | https://github.com/open2c/cooler |
| Gfastats | 1.3.6 | https://github.com/vgl-hub/gfastats |
| Hifiasm | 0.19.5-r587 | https://github.com/chhylp123/hifiasm |
| HiGlass | 1.11.6 | https://github.com/higlass/higlass |
| Merqury | MerquryFK | https://github.com/thegenemyers/MERQURY.FK |
| OATK | 0.9 | https://github.com/c-zhou/oatk |
| PretextView | 0.2 | https://github.com/wtsi-hpag/PretextView |
| purge_dups | 1.2.3 | https://github.com/dfguan/purge_dups |
| sanger-tol/genomenote | v1.0 | https://github.com/sanger-tol/genomenote |
| sanger-tol/readmapping | 1.1.0 | https://github.com/sanger-tol/readmapping/tree/1.1.0 |
| YaHS | 1.1a.2 | https://github.com/c-zhou/yahs |
Wellcome Sanger Institute – Legal and Governance
The materials that have contributed to this genome note have been supplied by a Darwin Tree of Life Partner. The submission of materials by a Darwin Tree of Life Partner is subject to the ‘Darwin Tree of Life Project Sampling Code of Practice’, which can be found in full on the Darwin Tree of Life website here. By agreeing with and signing up to the Sampling Code of Practice, the Darwin Tree of Life Partner agrees they will meet the legal and ethical requirements and standards set out within this document in respect of all samples acquired for, and supplied to, the Darwin Tree of Life Project.
Further, the Wellcome Sanger Institute employs a process whereby due diligence is carried out proportionate to the nature of the materials themselves, and the circumstances under which they have been/are to be collected and provided for use. The purpose of this is to address and mitigate any potential legal and/or ethical implications of receipt and use of the materials as part of the research project, and to ensure that in doing so we align with best practice wherever possible. The overarching areas of consideration are:
• Ethical review of provenance and sourcing of the material
• Legality of collection, transfer and use (national and international)
Each transfer of samples is further undertaken according to a Research Collaboration Agreement or Material Transfer Agreement entered into by the Darwin Tree of Life Partner, Genome Research Limited (operating as the Wellcome Sanger Institute), and in some circumstances other Darwin Tree of Life collaborators.
Acknowledgements
The authors wish to thank Sadie Barber, Peter Brownless and David Bell at Royal Botanic Garden Edinburgh for coordinating sampling of the living collections. In addition, we wish to thank María-José Endara for her extensive help with acquiring sampling permission for the sequenced accessions from the Ecuadorian Ministry of Environment. We also extend our thanks to Catherine McCarthy and the Nagoya team at Sanger, and China Williams at Royal Botanic Gardens, Kew, for their extensive help with ABS and sample permissions, as well as to the RBGE horticulture staff for their care of the living Inga collections from which we sampled.
Funding Statement
This work was supported by Wellcome through core funding to the Wellcome Sanger Institute [206194, <a href=https://doi.org/10.35802/206194>https://doi.org/10.35802/206194</a>]. The authors were also supported by a Natural Environment Research Council standard grant (grant number NE/V012258/1) held by R. T. Pennington.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
Data availability
European Nucleotide Archive: Inga leiocalycina. Accession number PRJEB64758; https://identifiers.org/ena.embl/PRJEB64758 ( Wellcome Sanger Institute, 2023). The genome sequence is released openly for reuse. The Inga leiocalycina genome sequencing initiative is part of the Darwin Tree of Life (DToL) project. All raw sequence data and the assembly have been deposited in INSDC databases. Raw data and assembly accession identifiers are reported in Table 1.
Author information
Members of the Royal Botanic Garden Edinburgh Genome Acquisition Lab are listed here: https://doi.org/10.5281/zenodo.4786682.
Members of the Plant Genome Sizing collective are listed here: https://doi.org/10.5281/zenodo.7994306.
Members of the Wellcome Sanger Institute Tree of Life Management, Samples and Laboratory team are listed here: https://doi.org/10.5281/zenodo.12162482.
Members of Wellcome Sanger Institute Scientific Operations: Sequencing Operations are listed here: https://doi.org/10.5281/zenodo.12165051.
Members of the Wellcome Sanger Institute Tree of Life Core Informatics team are listed here: https://doi.org/10.5281/zenodo.12160324.
Members of the Tree of Life Core Informatics collective are listed here: https://doi.org/10.5281/zenodo.12205391.
References
- Abdennur N, Mirny LA: Cooler: scalable storage for Hi-C data and other genomically labeled arrays. Bioinformatics. 2020;36(1):311–316. 10.1093/bioinformatics/btz540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TR, Pennington RT, Magallon S, et al. : Fast demographic traits promote high diversification rates of Amazonian trees. Ecol Lett. 2014;17(5):527–536. 10.1111/ele.12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates A, Clayton-Lucey I, Howard C: Sanger Tree of Life HMW DNA fragmentation: diagenode Megaruptor ®3 for LI PacBio. protocols.io. 2023. 10.17504/protocols.io.81wgbxzq3lpk/v1 [DOI] [Google Scholar]
- Challis R, Richards E, Rajan J, et al. : BlobToolKit – interactive quality assessment of genome assemblies. G3 (Bethesda). 2020;10(4):1361–1374. 10.1534/g3.119.400908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Concepcion GT, Feng X, et al. : Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods. 2021;18(2):170–175. 10.1038/s41592-020-01056-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Veiga Leprevost F, Grüning BA, Alves Aflitos S, et al. : BioContainers: an open-source and community-driven framework for software standardization. Bioinformatics. 2017;33(16):2580–2582. 10.1093/bioinformatics/btx192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton A, Yatsenko H, Jay J, et al. : Sanger Tree of Life wet laboratory protocol collection. protocols.io. 2023. 10.17504/protocols.io.8epv5xxy6g1b/v1 [DOI] [Google Scholar]
- Dexter KG, Pennington TD, Cunningham CW: Using DNA to assess errors in tropical tree identifications: how often are ecologists wrong and when does it matter? Ecol Monogr. 2010;80(2):267–286. 10.1890/09-0267.1 [DOI] [Google Scholar]
- Diesh C, Stevens GJ, Xie P, et al. : JBrowse 2: a modular genome browser with views of synteny and structural variation. Genome Biol. 2023;24(1): 74. 10.1186/s13059-023-02914-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Amaral RJV, Bates A, Denton A, et al. : Sanger Tree of Life RNA extraction: automated MagMax TM mirVana. protocols.io. 2023. 10.17504/protocols.io.6qpvr36n3vmk/v1 [DOI] [Google Scholar]
- Endara MJ, Coley PD, Wiggins NL, et al. : Chemocoding as an identification tool where morphological- and DNA-based methods fall short: Inga as a case study. New Phytol. 2018;218(2):847–858. 10.1111/nph.15020 [DOI] [PubMed] [Google Scholar]
- Ewels P, Magnusson M, Lundin S, et al. : MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32(19):3047–3048. 10.1093/bioinformatics/btw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewels PA, Peltzer A, Fillinger S, et al. : The nf-core framework for community-curated bioinformatics pipelines. Nat Biotechnol. 2020;38(3):276–278. 10.1038/s41587-020-0439-x [DOI] [PubMed] [Google Scholar]
- Formenti G, Abueg L, Brajuka A, et al. : Gfastats: conversion, evaluation and manipulation of genome sequences using assembly graphs. Bioinformatics. 2022;38(17):4214–4216. 10.1093/bioinformatics/btac460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrister DL, Endara MJ, Soule AJ, et al. : Diversity and divergence: evolution of secondary metabolism in the tropical tree genus Inga. New Phytol. 2023;237(2):631–642. 10.1111/nph.18554 [DOI] [PubMed] [Google Scholar]
- Grüning B, Dale R, Sjödin A, et al. : Bioconda: sustainable and comprehensive software distribution for the life sciences. Nat Methods. 2018;15(7):475–476. 10.1038/s41592-018-0046-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D, McCarthy SA, Wood J, et al. : Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics. 2020;36(9):2896–2898. 10.1093/bioinformatics/btaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson L: Some new chromosome counts in the genus Inga (Leguminosae: Mimosoideae). Kew Bull. 1995;50(4):801–804. 10.2307/4110243 [DOI] [Google Scholar]
- Harry E: PretextView (Paired REad TEXTure Viewer): a desktop application for viewing pretext contact maps.2022. Reference Source
- Howe K, Chow W, Collins J, et al. : Significantly improving the quality of genome assemblies through curation. GigaScience. 2021;10(1): giaa153. 10.1093/gigascience/giaa153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B, Howard C: Sanger Tree of Life HMW DNA extraction: manual plant MagAttract v.4. protocols.io. 2023; [Accessed 8 July 2024]. 10.17504/protocols.io.261ged5k7v47/v1 [DOI] [Google Scholar]
- Jay J, Yatsenko H, Narváez-Gómez JP, et al. : Sanger Tree of Life sample preparation: triage and dissection. protocols.io. 2023. 10.17504/protocols.io.x54v9prmqg3e/v1 [DOI] [Google Scholar]
- Kerpedjiev P, Abdennur N, Lekschas F, et al. : HiGlass: web-based visual exploration and analysis of genome interaction maps. Genome Biol. 2018;19(1): 125. 10.1186/s13059-018-1486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursar TA, Dexter KG, Lokvam J, et al. : The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga. Proc Natl Acad Sci U S A. 2009;106(43):18073–8. 10.1073/pnas.0904786106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzer GM, Sochat V, Bauer MW: Singularity: scientific containers for mobility of compute. PLoS One. 2017;12(5): e0177459. 10.1371/journal.pone.0177459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Diago D, García Castro NJ: Wild edible fruits of Colombia: diversity and use prospects. [Frutos silvestres comestibles de Colombia: diversidad y perspectivas de uso. Biota Colombiana. 2021;22(2):16–55. 10.21068/c2021.v22n02a02 [DOI] [Google Scholar]
- Manni M, Berkeley MR, Seppey M, et al. : BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol Biol Evol. 2021;38(10):4647–4654. 10.1093/molbev/msab199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel D: Docker: lightweight Linux containers for consistent development and deployment. Linux J. 2014;2014(239):2. [Accessed 2 April 2024]. Reference Source [Google Scholar]
- Narváez-Gómez JP, Mbye H, Oatley G, et al. : Sanger Tree of Life sample homogenisation: Covaris cryoPREP® automated dry pulverizer. protocols.io. 2023. 10.17504/protocols.io.eq2lyjp5qlx9/v1 [DOI] [Google Scholar]
- Oatley G, Sampaio F, Howard C: Sanger Tree of Life fragmented DNA clean up: automated SPRI. protocols.io. 2023. 10.17504/protocols.io.q26g7p1wkgwz/v1 [DOI] [Google Scholar]
- Pennington TD: The genus inga: botany. London: Royal Botanic Gardens, Kew,1997. Reference Source [Google Scholar]
- Rao SSP, Huntley MH, Durand NC, et al. : A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159(7):1665–1680. 10.1016/j.cell.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, McCarthy SA, Fedrigo O, et al. : Towards complete and error-free genome assemblies of all vertebrate species. Nature. 2021;592(7856):737–746. 10.1038/s41586-021-03451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, Walenz BP, Koren S, et al. : Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020;21(1): 245. 10.1186/s13059-020-02134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JE, Pennington RT, Pennington TD, et al. : Rapid diversification of a species-rich genus of neotropical rain forest trees. Science. 2001;293(5538):2242–2245. 10.1126/science.1061421 [DOI] [PubMed] [Google Scholar]
- Surana P, Muffato M, Qi G: sanger-tol/readmapping: sanger-tol/readmapping v1.1.0 - Hebridean Black (1.1.0). Zenodo. 2023a. 10.5281/zenodo.7755669 [DOI] [Google Scholar]
- Surana P, Muffato M, Sadasivan Baby C: sanger-tol/genomenote (v1.0.dev). Zenodo. 2023b. 10.5281/zenodo.6785935 [DOI] [Google Scholar]
- Vasimuddin Md, Misra S, Li H, et al. : Efficient architecture-aware acceleration of BWA-MEM for multicore systems.In: 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS).IEEE,2019;314–324. 10.1109/IPDPS.2019.00041 [DOI] [Google Scholar]
- Wellcome Sanger Institute: The genome sequence of Inga leiocalycina Benth. European Nucleotide Archive.[dataset], accession number PRJEB64758,2023.
- Zhou C: c-zhou/oatk: Oatk-0.1.2023. 10.5281/zenodo.7631375 [DOI] [Google Scholar]
- Zhou C, McCarthy SA, Durbin R: YaHS: yet another Hi-C scaffolding tool. Bioinformatics. 2023;39(1): btac808. 10.1093/bioinformatics/btac808 [DOI] [PMC free article] [PubMed] [Google Scholar]