Abstract
Background
Breast cancer predominantly affects women and poses challenges in the treatment of both local and advanced diseases. In a previous study, we reported the effectiveness of ER121, a structurally resolved small compound specifically designed to target human cancers expressing or overexpressing mutant EGFR and HER2.
Purpose
The objective of this study is to assess the efficacy and toxicity of ER121 in metastatic and triple negative breast cancer (TNBC, HER2+) cells and tumor models. The Herceptin-resistant breast cancer cell line JIMT-1 was used in an in vivo tumor model, and MMTV-erbB2 (Fo5) transgenic mice models were used to evaluate the efficacy and safety of ER121 as neoadjuvant.
Methods
ER121 treatment focusing on experimental brain metastasis in TNBC, HER2+ model, was quantified by total flux employing the In Vivo Imaging System (IVIS). We also compared the brain tissue from the treated and the controls groups. Additionally, ER121 was evaluated in JIMT-1, a Herceptin-resistant breast cancer cell line, both in vitro and in vivo tumor model. We also administered ER121 orally in neoadjuvant model with the MMTV-erbB2 (Fo5) transgenic mice, the survival rates were compared with the control group. Tumor-free survival of multiple treated groups were analyzed by Kaplan-Meier analysis employing the log-rank test with the Bonferroni correction using R Statistical Software.
Results
In this study, we present findings indicating that ER121 treatment significantly attenuated breast tumor growth using a TNBC, HER2+ model, focusing on experimental brain metastasis, as quantified by total flux employing IVIS. These observations were further corroborated by analysis of brain tissue from the treatment group compared to controls. Data is presented as Mean ± S.D. statistical significance was calculated using Student t test (*p < 0.05). Additionally, ER121 significantly inhibited JIMT-1, a Herceptin-resistant breast cancer cell line was used in vivo xenograft model. Additionally, we used a neoadjuvant model with the MMTV-erbB2 (Fo5) transgenics and the tumor-free survival rates exhibited a remarkable difference between the control and treated groups when ER121 was administered orally. We found statistically significant p values of 0.048 employing log-rank test with Bonferroni Correction for comparing ER121 high, ER121 Low, Herceptin and PBS groups. All analyses were performed using R Statistical Software.
Conclusion
ER121 is a non-toxic small-molecule erbB kinase inhibitor and holds promise as an oral and systemic therapeutic agent for treating progressive erbB-driven tumors in therapeutic settings. Moreover, ER121 shows potential as a preventive therapy in neoadjuvant settings for erbB2-associated tumors and when administered systemically can dramatically limit erbB2 brain metastases in animal models.
Keywords: breast cancer, ER121, kinase inhibitor, neoadjuvant model, TNBC, brain metastasis
Introduction
Human epidermal growth factor receptors (HERs) are part of the epidermal growth factor receptor (EGFR) family and consist of four subtypes: HER1 (EGFR, erbB1), HER2 (erbB2, HER2/neu), HER3 (erbB3), and HER4 (erbB4).1–5 The erbB2 gene encodes a growth factor receptor, which activates intracellular signaling pathways in response to homo and heterodimerization as well as extracellular signals.6–8 The family of erbB receptors was recognized in 1978 with the discovery of the first tyrosine kinase, EGFR, followed by the identification of the neu or erbB2 (HER2) gene in 1984.9,10 Demonstration that p185HER2/neu mediated transformation of cells could be dramatically reversed by ectodomain targeting monoclonals11 established targeted therapy and that HER2 amplification or overexpression was linked to extremely poor survival in breast cancer which ultimately led to the development of trastuzumab or Herceptin, a monoclonal antibody targeting p185HER2/neu.11–13 The evolving comprehension of HER2 biology has paralleled the advancement of HER2-targeting agents. HER receptors comprise extracellular ligand-binding, transmembrane, and intracellular tyrosine kinase domains. Ligand binding induces homodimerization or heterodimerization of HER proteins, activating downstream signaling for cell division, growth promotion, and apoptosis inhibition.
Although HER2 lacks a known ligand, it is a dimerization partner, particularly with HER3. HER2 overexpression/amplification leads to ligand-independent dimerization, abnormal signaling, and increased ligand-dependent heterodimerization. HER2-targeted agents exhibit maximum efficacy in “HER2-positive” tumors; however, resistance inevitably develops in metastatic HER2-positive tumors, causing disease progression. The therapeutic goal in HER2-positive breast cancer is to enhance early cure rates and prevent recurrence. For HER2-positive cancers presenting with de novo stage IV disease or recurring, novel therapies are crucial, given their continued dependence on HER2 signaling.6,7 Extensive research is underway across preclinical, translational, and clinical domains to develop original and more potent therapies for the highly sensitive HER2 target.
Tyrosine kinase inhibitors (TKIs) target the intracellular catalytic kinase domain by competitively binding with ATP, impeding phosphorylation, and thwarting downstream signaling cascades. TKIs targeting p185erbB2/neu, such as Lapatinib, neratinib, Pyrotinib, and Tucatinib, show promise, but challenges, including modest effects on brain metastases and potential toxicities, exist.6,14–16 Notably, recurrent HER2 disease often re-emerges in the central nervous system (CNS), with approximately 50% of HER2-positive breast cancer patients developing brain metastases. The blood–tumor barrier (BTB), an offshoot of the blood–brain barrier (BBB), intricately regulates drug access to brain metastases, presenting a clinical hurdle. Additionally, the substantial size of HER2 antibodies, like trastuzumab, impedes penetration of the BBB, limiting efficacy in the CNS. These observations underscore the need for advanced TKIs to address the escalating challenge of brain metastases associated with HER2-positive metastatic breast cancer.6 The use of TKIs in treating HER2-positive breast cancer is not without potential toxicities. Common toxicities associated with TKIs in breast cancer include gastrointestinal disturbances, rash, and hepatotoxicity.14–16 As research continues to refine next-generation TKIs, the goal is to develop potent dual tyrosine kinase inhibitor (DTKI) to mitigate these toxicities while optimizing the therapeutic benefits for HER2-positive breast cancer patients.
This study establishes the potential of ER121, a non-toxic small molecule ErbB kinase inhibitor, as an effective oral and systemic therapeutic for addressing progressive ErbB-driven tumors. Additionally, its capacity as a preventive intervention in neoadjuvant settings for ErbB-associated tumors is evident. These findings identify ER121 as a promising candidate for treating and preventing ErbB-driven breast cancer and brain metastasis as well as identifying its versatility in therapeutic and neoadjuvant applications.
Materials and Methods
Cell Lines
The Herceptin-resistant breast cancer cell line, JIMT-1, was obtained from Addex Bio, USA, and SKBR3 was procured from the ATCC. MDA-MB 231-BrHer2+GFP-RFP-Luc/firefly3, expressing HER2, served as the model cell line for Triple negative HER2+ (TNBC, HER2+) in brain metastasis studies, utilizing In Vivo Imaging System (IVIS) imaging. The HER2-positive breast cancer control cell line, BT474 (obtained from ATCC), was included in these studies. HuHer2-6 originated from spontaneous metastasis that occurred within 6–12 months of Hu-Her2 transgenic mice, while HuHer2-Lu2 (L2) was developed from lung metastasis that occurred at around 40 weeks of age. HuHer2-TP resulted from serial transplantations of tumors.17 Cell cultures were maintained in a 37°C humidified atmosphere with 5% CO2.
Cell Cytotoxicity Assay
The cell cytotoxicity assay was conducted as previously described using the MTT colorimetric method.18 Cells in the exponential phase were seeded into a 96-well plate and subjected to treatment with varying concentrations of both Herceptin and ER121. The plates were incubated for 72 hours at 37°C in a 5% CO2 humidified atmosphere. Subsequently, MTT (1 mg/mL) was added to all wells, followed by the addition of cell lysis buffer, and the plates were left for overnight incubation. Spectrophotometric readings at 570nm were obtained using a spectrophotometer. The obtained results were then analyzed and presented as % viability versus concentration using GraphPad software.
Experimental Metastasis Model Development
Female NSG mice aged 3-to-6 weeks, obtained from the Jackson Laboratory (catalog #05557), were used for the experiments. MDA-MB-231-HER 2+ breast carcinoma cells expressing green fluorescence protein (GFP) were acquired from Dr. Hong19 and further modified using lentivirus to express a fusion target of (RFP-Luciferase/firefly3) (GenTarget Inc., Cat#: LVP674-PBS).
For brain metastasis studies, 10 mice were intracardially injected with cells labeled with both green and red fluorescence proteins (GFP, RFP), and luciferase (MB 231-BrHer2+GFP-RFP-Luc/firefly3) under isoflurane/O2 anesthesia. Tumor growth was monitored by imaging mouse heads using the IVIS (In vivo imaging system) on Days 3, 7, 10, 15, 17, 21, or 24 until they were humanely euthanized by CO2 asphyxiation. The fluorescent intensity [total flux (p/s)] was quantified from regions of interest (ROI) for both control and experimental/treatment groups. Upon reaching a specific tumor size (as measured by total flux, ~ 8e5 p/s), mice were stratified into control and experimental groups, with the experimental group receiving intraperitoneal treatment with ER121 (10 mg/kg) twice per week.
Neoadjuvant Model of Prevention of Tumor Development
Our study assessed the prophylactic potential of the orally administered inhibitor ER121 in preventing the formation of MMTV-erbB2-driven tumors in a transgenic mouse model. We have previously described the prevention of emergence of p185HER2/neu tumors in a transgenic model using monoclonal antibodies that target the ectodomain of p185.16
In this regard, we obtained a population of Fo5 huHER2-positive transgenic mice from Johns Hopkins University. These mice harbor the human HER2 gene under the control of an MMTV promoter. The Fo5 mouse line is characterized by the development of asynchronous mammary tumors, typically with a latency period averaging 28–36 weeks, but with some instances occurring as early as 23 weeks. To facilitate our experimental design, the Fo5 huHER2-positive transgenic mice were mated with FVB mice for propagation. Subsequently, the polymerase chain reaction (PCR) was employed to identify and isolate the female offspring exhibiting the desired transgenic profile. These female mice, ranging from 17 weeks of age to exceeding 52 weeks or reaching morbidity, were randomly distributed into distinct treatment cohorts. These treatment groups received varying dosages of ER121 through oral gavage.17
Each treatment group was comprised of 15 mice. The administered dosages of ER121 consisted of high doses at 200 µg per week, delivered orally twice a week. Another MMTV-huHER2 transgenic group received ER121 oral doses of 50 µg administered twice weekly. As a control a transgenic group received Herceptin, an anti-human HER2 antibody,20 at a dose of 5 mg/kg once a week. Control transgenic group received phosphate buffered saline (PBS).
Statistical Analyses
Tumor-free survival of multiple-treatment groups were analyzed by Kaplan-Meier analysis using the log-rank test with the Bonferroni correction. All analyses were performed using R Statistical Software.21–23
In vivo Xenograft Model
Female athymic nude mice obtained from Jackson Laboratories were inoculated in their right flank with JIMT-1 (4 × 106) cells. Following the establishment of measurable tumors, the mice were randomly assigned and subjected to injections (PBS, Enhertu, ER121 or Herceptin) every alternate day over a 2-week period. Tumor volumes were assessed using Vernier calipers on alternate days, and the calculated volumes were graphically represented using GraphPad Prism software.18
Study Approval
The metastases studies were conducted in accordance with the approved Institute Animal Care and Use Committee (IACUC) protocol (AUP-0320-0023) at the Houston Methodist Research Institute, and institutional guidelines for the care and use of laboratory animals were followed. For the neoadjuvant prevention studies, all procedures were conducted in facilities accredited by AAALAC at the University of Pennsylvania. Protocol number 804008 was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania.
Results
ER121 Profoundly Diminishes the Incidence of Brain Metastasis
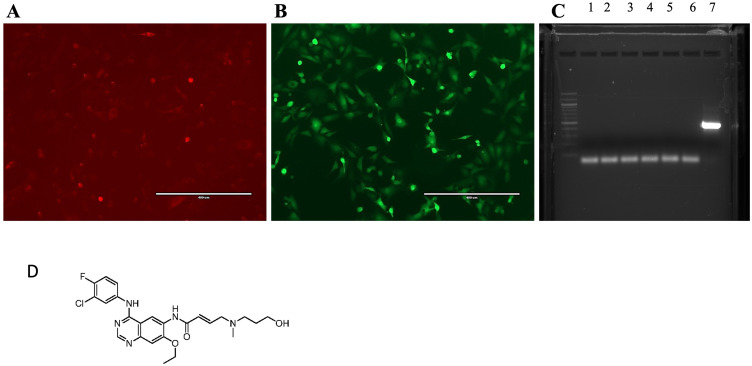
In this study, we employed MDA-MB-231-HER2+ cells, a well-established model for triple negative breast cancer (TNBC, HER2+) cells that stably expressed the fluorescent markers HER2/GFP and RFP/Luciferase to establish an experimental metastasis model, as depicted in Figure 1A and B. We tested the absence of mycoplasma as shown in Figure 1C. The chemical structure of ER121, a dual tyrosine kinase inhibitor used is shown in Figure 1D.
Figure 1.
(A) MDA MB 231-BrHer2+GFP-RFP-Luc/firefly3 cells express luciferase-RFP proteins; and (B) same cells expressing HER2+GFP proteins; (C) Mycoplasma detection by PCR, 1. HCC-NOS; 2. HCC-VC; 3. MDA MB 231-NOS; 4. MDA MB 231-VC; 5. MDA MB 231-BrHER2+GFP-Luc; 6. Negative Control; 7. Positive Control; and (D) the chemical structure of ER121.
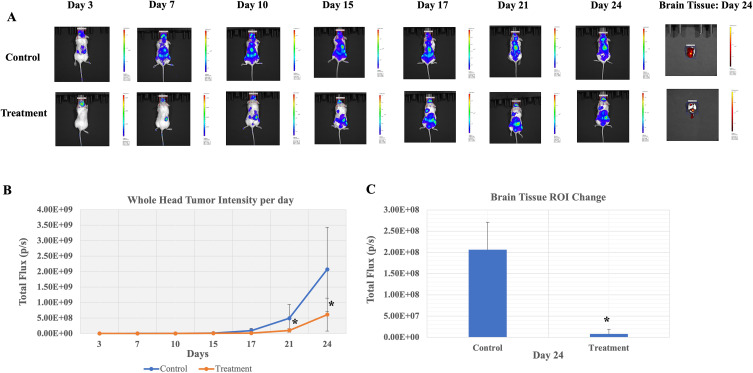
To investigate the efficacy of ER121 in preventing metastatic progression, we initiated the model by intracardially injecting HER2 tumor cells into experimental mice. Subsequently, we monitored tumor growth by employing the IVIS imaging system to capture fluorescent signals emanating from the head region of the mice at multiple time points, specifically on Days 3, 7, 10, 15, 17, 21, or 24, until a humane endpoint was reached, and the mice were euthanized via CO2 asphyxiation. The quantification of fluorescent intensity, represented as total flux (photons/second), was carried out by delineating ROI (region of interest) for both control and experimental/treatment groups. Upon attaining a predetermined tumor size threshold of approximately 8e5 photons/second, the experimental mice were meticulously stratified into distinct control and experimental groups. The experimental group received intraperitoneal treatments with ER121 at a dosage of 10 mg/kg, administered twice per week. As seen in Figure 2A and B, the administration of ER121 in the experimental group resulted in a significant reduction in tumor growth compared to the control mice. Additionally, upon euthanasia and quantification of brain tissue, as depicted in Figure 2A and C, respectively, a substantial decrease in tumor load within the brain was evident. Data is presented as Mean ± S.D., statistical significance was calculated using Student t test (*p < 0.05). These findings highlight the ability of ER121 to restrain metastatic growth and dissemination within the brain.
Figure 2.
Treatment with ER121was used in an experimental metastasis model. (A) Representative head IVIS images from a control and an experimental mouse before and after treatment with ER121. Brain tissue image was obtained on Day 24 upon sacrifice. (B) Whole head tumor fluorescent intensity per day and (C) brain tissue images after sacrifice on Day 24 showed significantly reduced tumor growth in treatment group as compared to control. Data represents mean ± S.D. * indicates statistical significance and was calculated using Student t test (*p < 0.05).
ER121 Demonstrates Efficacy in Preventing the Emergence of HER2/Neu Cancers
In this study, we used a comprehensive panel of breast cancer cell lines, each with distinct origins and characteristics, to assess the cytotoxic potential of ER121, in comparison to Herceptin. These cell lines included HuHer2-6, derived from spontaneous metastasis in Hu-Her2 transgenic mice within 6–12 months, HuHer2-Lu2 (L2), developed from lung metastasis in mice at approximately 40 weeks, and HuHer2-TP, obtained through serial transplantation of tumors.24 Additionally, BT474, expressing HER2 served as a positive control. The cell lines were subjected to treatment with both Herceptin and ER121, administered at varying doses over a 72-hour period, followed by the quantification of cell viability using the MTT assay.
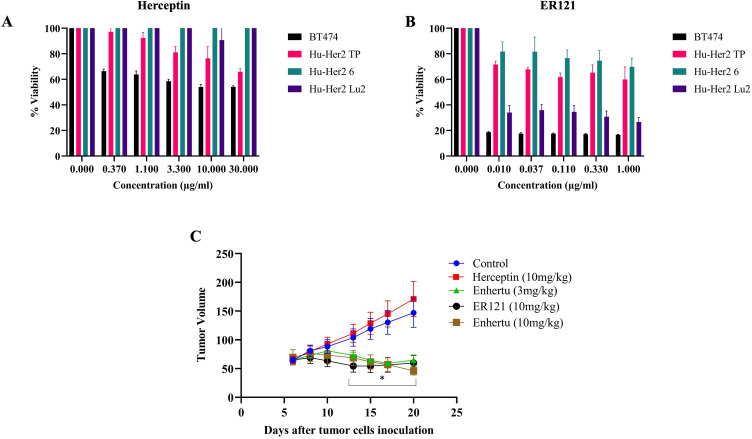
ER121 exhibited significantly higher cytotoxicity compared to Herceptin, even at lower doses, as depicted in Figure 3A and B. This observation indicates the potency and efficacy of ER121 as a therapeutic agent, particularly at lower concentrations, highlighting its potential as a promising treatment option for HER2-positive breast cancer. Furthermore, we conducted an in vivo assay employing JIMT-1 cells, which originated from a pleural metastasis in a breast cancer patient clinically resistant to trastuzumab. JIMT-1 cells are characterized by amplified HER-2 oncogene expression without identifiable mutations in the coding sequence.20 Female athymic nude mice, 15 mice in each group, were injected with JIMT-1 cells and were subsequently treated with PBS (Control), or injection of Enhertu (an FDA-approved antibody-drug conjugate consisting of trastuzumab and deruxtecan), at 3 mg/kg or 10 mg/kg or ER121 at 10 mg/kg, or Herceptin at 10 mg/kg on alternate days over a 2-week period. Tumor volumes were assessed throughout the treatment duration.
Figure 3.
In vitro comparison of activity of (A) Herceptin and (B) ER121 on breast cancer cell lines derived from Hu-HER2 transgenic mice. (C) In vivo effect of Herceptin, ER121 and Enhertu in immunocompromised mice tumors using JIMT-1 cells, a Herceptin resistant breast cancer cell line. Data was presented as Mean ± S.D., p value was calculated using Student t test (*p < 0.05).
Figure 3C demonstrates that mice treated with ER121 exhibited a substantial reduction in tumor volumes compared to the control group. It is noteworthy that both ER121 and Enhertu achieved similar reductions in tumor volumes. Data is presented as Mean ± S.D., statistical significance was calculated using Student t test (*p < 0.05). However, Enhertu, although effective, was associated with various side effects. These results emphasize the potential of ER121 in targeting HER2-positive tumors, not only in vitro but also in an in vivo setting. The data presented here indicates that ER121 is a promising therapeutic candidate for HER2-positive breast cancer, particularly in cases where conventional treatments like Herceptin or antibody-drug conjugates exhibited limitations or adverse effects.
ER121 Exhibits Promise as a Prospective Neoadjuvant Candidate for ErbB-Associated Tumors
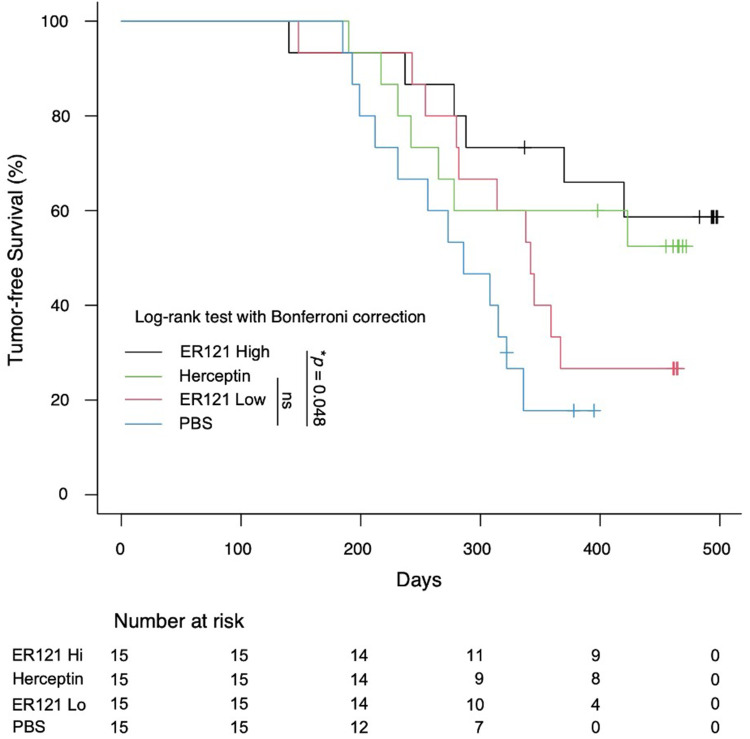
We assessed the preventive potential of orally administered ER121 in countering MMTV-erbB2-driven tumors in a transgenic mouse model that develops HER2 tumors (see Figure 4). We obtained the huHER2-positive transgenic mice (Fo5) and crossed them with wild-type FVB mice. We identified female offspring with the desired transgenic profile using PCR. These female mice were randomly divided into distinct treatment groups, each comprising 15 mice. ER121 was administered orally at varying dosages (high: 200 µg/mouse weekly; low: 100 µg/mouse weekly). A positive control group received Herceptin (100 µg/mouse weekly), an anti-human erbB2 antibody. Tumor-free survival of multiple treatment groups were analyzed by Kaplan-Meier analysis using the log-rank test with the Bonferroni correction for comparing the ER121 high, ER121 Low, Herceptin and PBS groups. Statistical analysis shows p value of 0.048.
Figure 4.
Comparison of tumor-free survival of ER121 treated groups ER121 high, ER121 low, Herceptin and PBS treated groups. Tumor free survival with multiple groups were calculated by employing Kaplan-Meier analysis using the log-rank test with the Bonferroni correction. All analyses were performed using R Statistical Software, p value was 0.048.
Discussion
The challenge of effectively targeting brain metastasis in breast cancer with TKIs is multifaceted. The blood brain barrier restricts the delivery of systemically administered TKIs to brain metastatic sites, limiting their bioavailability within the brain. Furthermore, the molecular heterogeneity of brain metastatic lesions, characterized by distinct tyrosine kinase signaling pathways, renders many conventional TKIs less effective. The development of resistance mechanisms in brain metastasis adds complexity to the issue. Potential solutions include enhancing BBB penetrability with novel drug delivery systems, designing TKIs that specifically target brain metastasis-specific pathways, exploring synergistic combination therapies, and embracing personalized medicine guided by molecular profiling. Addressing the lack of effective TKIs for brain metastasis in breast cancer requires a comprehensive and multidisciplinary approach to improve treatment outcomes in this challenging clinical scenario.
The findings from our studies reveal the potential of ER121 as a therapeutic agent in various aspects of breast cancer management. Firstly, our investigation demonstrates the ability of ER121 to profoundly reduce the incidence of brain metastasis in human breast cancer in a murine model. To assess this, we employed the well-established MDA-MB-231 cell model of TNBC expressing fluorescent markers HER2/GFP and RFP/Luciferase, creating an experimental metastasis model. Intracardial injection of these cells in experimental mice initiated the model, with rigorous monitoring of tumor growth using the IVIS imaging system. The results clearly depict the potent efficacy of ER121 in inhibiting metastatic progression, particularly within the brain, providing substantial evidence for its potential as a therapeutic agent against metastatic breast cancer. This dataset not only provides critical insights into the effectiveness of ER121 in preclinical models but also serves as a compelling rationale for further exploring its clinical utility, particularly in the context of ErbB-associated breast cancer metastasis, with a specific emphasis on the challenging landscape of triple-negative breast cancer.
Furthermore, our study extends its focus to the tumor inhibitory and cytotoxic potential of ER121 in comparison to the widely used drug Herceptin, targeting HER2-positive breast cancer. Utilizing a diverse panel of breast cancer cell lines, each with distinct origins and characteristics, ER121’s efficacy stood out prominently. Even at lower doses, ER121 demonstrated significantly higher cytotoxicity than Herceptin, indicating its potential as an effective treatment option for HER2-positive breast cancer.
Lastly, our research evaluated the prospective use of ER121 as a neoadjuvant candidate in preventing the emergence of MMTV-erbB2-driven tumors in a transgenic mouse model. By crossbreeding huHER2-positive transgenic mice with wild-type counterparts and administering ER121 orally, assessed its preventive potential. The statistical analysis using the log-rank Bonferroni correction test for comparing ER121 high, ER121 Low and Herceptin showed statistical significance.
In conclusion, this study illustrates ER121’s potential in breast cancer management, encompassing the inhibition of brain metastasis, potent cytotoxicity against HER2-positive breast cancer, and prospective use as a prevention candidate that limits the emergence of HER2 tumors. These findings provide some insights into the potential clinical utility of ER121 and encourage further studies to explore its therapeutic applications in human breast cancer.
Acknowledgment
This work was supported by grants from the National Institutes of Health (R01CA219034 and R21AI35359) and the Breast Cancer Research Foundation (BCRF-17-061) to MIG.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Professor Mark Greene reports grants from NIH, grants from BCRF, during the conduct of the study; In addition, ER121 is described in US11708335B2 ; and Dr. Greene is a director at Martell Diagnostic Laboratories, Inc., a private biotechnology company. He is also a paid consultant to 101 therapeutics, a private biotechnology company. The authors report no other conflicts of interest in this work.
References
- 1.Ullrich A, Coussens L, Hayflick JS, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309(5967):418–425. PMID: 6328312. doi: 10.1038/309418a0 [DOI] [PubMed] [Google Scholar]
- 2.Drebin JA, Stern DF, Link VC, Weinberg RA, Greene MI. Monoclonal antibodies identify a cell-surface antigen associated with an activated cellular oncogene. Nature. 1984;312(5994):545–548. PMID: 6504162. doi: 10.1038/312545a0 [DOI] [PubMed] [Google Scholar]
- 3.Coussens L, Yang-Feng TL, Liao YC, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230(4730):1132–1139. PMID: 2999974. doi: 10.1126/science.2999974 [DOI] [PubMed] [Google Scholar]
- 4.Kraus MH, Issing W, Miki T, Popescu NC, Aaronson SA. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc Natl Acad Sci U S A. 1989;86(23):9193–9197. PMID: 2687875; PMCID: PMC298460. doi: 10.1073/pnas.86.23.9193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plowman GD, Culouscou JM, Whitney GS, et al. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci U S A. 1993;90(5):1746–1750. PMID: 8383326; PMCID: PMC45956. doi: 10.1073/pnas.90.5.1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov. 2023;22(2):101–126. PMID: 36344672; PMCID: PMC9640784. doi: 10.1038/s41573-022-00579-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galogre M, Rodin D, Pyatnitskiy M, Mackelprang M, Koman I. A review of HER2 overexpression and somatic mutations in cancers. Crit Rev Oncol Hematol. 2023;186:103997. PMID: 37062337. doi: 10.1016/j.critrevonc.2023.103997 [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Berezov A, Wang Q, et al. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117(8):2051–2058. PMID: 17671639; PMCID: PMC1934579. doi: 10.1172/JCI32278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter G, King L, Cohen S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature. 1978;276(5686):409–410. PMID: 309559. doi: 10.1038/276409a0 [DOI] [PubMed] [Google Scholar]
- 10.Schechter AL, Stern DF, Vaidyanathan L, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312(5994):513–516. PMID: 6095109. doi: 10.1038/312513a0 [DOI] [PubMed] [Google Scholar]
- 11.Drebin JA, Link VC, Stern DF, Weinberg RA, Greene MI. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985;41(3):697–706. PMID: 2860972. doi: 10.1016/s0092-8674(85)80050-7 [DOI] [PubMed] [Google Scholar]
- 12.Drebin JA, Link VC, Weinberg RA, Greene MI. Inhibition of tumor growth by a monoclonal antibody reactive with an oncogene-encoded tumor antigen. Proc Natl Acad Sci U S A. 1986;83(23):9129–9133. PMID: 3466178; PMCID: PMC387088. doi: 10.1073/pnas.83.23.9129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. PMID: 11248153. doi: 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 14.Xuhong JC, Qi XW, Zhang Y, Jiang J. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am J Cancer Res. 2019;9(10):2103–2119. PMID: 31720077; PMCID: PMC6834479. [PMC free article] [PubMed] [Google Scholar]
- 15.Le Du F, Diéras V, Curigliano G. The role of tyrosine kinase inhibitors in the treatment of HER2+ metastatic breast cancer. Eur J Cancer. 2021;154:175–189. PMID: 34280871. doi: 10.1016/j.ejca.2021.06.026 [DOI] [PubMed] [Google Scholar]
- 16.Katsumata M, Okudaira T, Samanta A, et al. Prevention of breast tumour development in vivo by downregulation of the p185neu receptor. Nat Med. 1995;1(7):644–648. doi: 10.1038/nm0795-644 [DOI] [PubMed] [Google Scholar]
- 17.Park S, Nedrow JR, Josefsson A, Sgouros G. Human HER2 overexpressing mouse breast cancer cell lines derived from MMTV.f.HuHER2 mice: characterization and use in a model of metastatic breast cancer. Oncotarget. 2017;8(40):68071–68082. PMID: 28978097; PMCID: PMC5620237. doi: 10.18632/oncotarget.19174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goel PN, Zhang H, Murali R, et al. Dual kinase inhibitor for EGFR mutants and ErbB2 limit breast cancer. Biochem Biophys Res Commun. 2023;651:39–46. PMID: 36791497. doi: 10.1016/j.bbrc.2023.02.019 [DOI] [PubMed] [Google Scholar]
- 19.Zhao H, Cui K, Nie F, et al. The effect of mTOR inhibition alone or combined with MEK inhibitors on brain metastasis: an in vivo analysis in triple-negative breast cancer models. Breast Cancer Res Treat. 2012;131(2):425–436. PMID: 21394501. doi: 10.1007/s10549-011-1420-7 [DOI] [PubMed] [Google Scholar]
- 20.Finkle D, Quan ZR, Asghari V, et al. HER2-targeted therapy reduces incidence and progression of midlife mammary tumors in female murine mammary tumor virus huHER2-transgenic mice. Clin Cancer Res. 2004;10(7):2499–2511. PMID: 15073130. doi: 10.1158/1078-0432.ccr-03-0448 [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 22.Bonferroni CE. Il Calcolo Delle Assi curazioni Su Gruppi Di Test. In studi Onore Del Professore Salvatore Ortu Carboni. Rome, Italy; 1935:13–60. [Google Scholar]
- 23.R Core Team. R: a language and environment for statistical computing (v.4.4.1). Vienna, Austria: R Foundation for Statistical Computing; 2021. Available from: https://www.R-project.org/. Accessed October 24, 2024. [Google Scholar]
- 24.Tanner M, Kapanen AI, Junttila T, et al. Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Cancer Ther. 2004;3(12):1585–1592. PMID: 15634652. doi: 10.1158/1535-7163.1585.3.12 [DOI] [PubMed] [Google Scholar]