Abstract
Background
Darunavir is a potent HIV protease inhibitor with a high barrier to resistance. We conducted a nested pharmacokinetic sub-study within CHAPAS-4 to evaluate darunavir exposure in African children with HIV, taking once-daily darunavir/ritonavir for second-line treatment.
Methods
We used data from the CHAPAS-4 pharmacokinetic sub-study treating children with once-daily darunavir/ritonavir (600/100 mg if 14–24.9 kg and 800/100 mg if ≥25 kg) with either tenofovir alafenamide fumarate (TAF)/emtricitabine (FTC), abacavir/lamivudine or zidovudine/lamivudine. Steady-state pharmacokinetic sampling was done at 0, 1, 2, 4, 6, 8, 12 and 24 hours after observed darunavir/ritonavir intake. Non-compartmental and population pharmacokinetic analyses were used to describe the data and identify significant covariates. Reference adult pharmacokinetic data were used for comparison. We simulated the World Health Organization (WHO) recommended 600/100 mg darunavir/ritonavir dose for the 25–34.9 kg weight band.
Results
Data from 59 children with median age and weight 10.9 (range 3.8–14.7) years and 26.0 (14.5–47.0) kg, respectively, were available. A two-compartment disposition model with transit absorption compartments and weight-based allometric scaling of clearance and volume best described darunavir data. Our population achieved geometric mean (%CV) darunavir AUC0–24h, 94.3(50) mg·h/L and Cmax, 9.1(35) mg/L, above adult reference values and Ctrough, 1.5(111) mg/L, like adult values. The nucleoside reverse-transcriptase inhibitor backbone was not found to affect darunavir concentrations. Simulated WHO-recommended darunavir/ritonavir doses showed exposures equivalent to adults. Higher alpha-1-acid glycoprotein increased binding to darunavir and decreased apparent clearance of darunavir.
Conclusions
Darunavir exposures achieved in our trial are within safe range. Darunavir/ritonavir can safely be co-administered with TAF/FTC. Both WHO-recommended 600/100 mg and CHAPAS-4 800/100 mg darunavir/ritonavir doses for the 25–34.9 kg weight band offer favourable exposures. The choice between them can depend on tablet availability.
Introduction
In 2022, only 57% of children with HIV globally were on antiretroviral therapy (ART), which was lower than in adults (77%).1 Among children on ART, treatment outcomes are worse than in adults, which is partly a result of suboptimal ART treatment (limited and complex drug regimens) and the challenge to retain children living with HIV in care.2,3 To improve therapy in children, the World Health Organization (WHO) recommended dolutegravir with abacavir and lamivudine as first-line ART for children.4 In the case of failing on a dolutegravir regimen, a boosted protease inhibitor (bPI) with an optimized nucleoside reverse-transcriptase inhibitor (NRTI) backbone is recommended as second line.4
BPIs are highly effective second-line therapy when combined with two NRTIs, even after prolonged failure on first-line NNRTI-based ART in low-income countries.5 Currently, the only co-formulated bPI available for children is lopinavir/ritonavir, offered in tablet and syrup forms. Challenges with these formulations include unpleasant taste, large uncrushable tablets, high alcohol concentration (42.4% v/v) in the syrup requiring refrigeration and the need for twice-daily dosing, which may hinder adherence.6–8
An alternative bPI is darunavir boosted with ritonavir (darunavir/ritonavir). Darunavir is potent and retains high efficacy in children who failed on other bPIs. It has a high genetic barrier to resistance, a favourable lipid profile, fewer gastrointestinal side effects than lopinavir/ritonavir and does not require refrigeration.9 It is metabolized by CYP3A4 and is 95% bound to plasma proteins, mainly alpha-1-acid glycoprotein (AAG).10,11
In children >3 years and weighing at least 14 kg who have no previous exposure to PIs, once-daily darunavir/ritonavir can be used.4 However, paediatric dosing recommendations for darunavir/ritonavir vary among the European Medicines Agency, Food and Drug Administration and WHO, particularly regarding weight-based dosing exceeding 600 mg darunavir in combination with ritonavir [refer to Table S2 (available as Supplementary data at JAC Online)]. In our study, we implemented a simplified once-daily dosing regimen similar to the WHO recommendation. However, we initiated treatment with the higher (adult) dose of 800/100 mg darunavir/ritonavir for children weighing 25 kg or more, rather than the WHO-recommended 35 kg threshold.
In adults, darunavir/ritonavir is often given once daily with tenofovir disoproxil fumarate and emtricitabine, a highly efficacious NRTI backbone.12 In children, however, with tenofovir disoproxil fumarate is not recommended due to concerns of bone and renal toxicity, and the recommended standard of care. NRTI backbones are abacavir/lamivudine and zidovudine/lamivudine, which are bulky, and zidovudine/lamivudine is administered twice daily. Children can benefit from tenofovir alafenamide fumarate (TAF), a safer alternative to with tenofovir disoproxil fumarate. Data on the combined use of darunavir/ritonavir with TAF/emtricitabine and once-daily darunavir/ritonavir in African children are lacking. Therefore, there is a need to investigate the pharmacokinetics of once-daily darunavir/ritonavir with TAF/emtricitabine or other NRTI backbones, following WHO weight-band dosing guidelines, in African paediatric populations.
In this work, we present results of an intensive once-daily darunavir/ritonavir PK sub-study nested within the CHAPAS-4 trial. We compared exposures achieved in this study to previously reported adult exposures and investigated the effect of NRTI backbones and other covariates on the pharmacokinetics of darunavir. Finally, we simulated and compared darunavir exposures achieved with the 2018 WHO-recommended doses with those achieved with doses used in the CHAPAS-4 trial.
Methods
Study design and participants
CHAPAS-4 (#ISRCTN22964075) was an open-label, multicentre, 4 × 2 randomized trial evaluating the virological efficacy and pharmacokinetics of bPIs (lopinavir/ritonavir, darunavir/ritonavir and atazanavir/ritonavir) and dolutegravir, in combination with either TAF or non-TAF-based NRTI backbone. The NRTI backbone contained TAF plus emtricitabine or abacavir or zidovudine, both with lamivudine.
Children with HIV aged 3–15 years weighing at least 14 kg from Zambia, Uganda and Zimbabwe, who were receiving abacavir- or zidovudine- containing NRTI backbone and failing according to WHO criteria (confirmed viral load >1000 copies/mL after adherence counselling or CD4 criteria for failure or clinical criteria for failure), were enrolled in this sub-study. Children with illness(es) that could affect pharmacokinetic results, and those using medication with known drug–drug interactions with trial drugs, were not eligible for inclusion. Written informed consent was given by parents/care givers and local and national ethics committees approved main trial and nested pharmacokinetic sub-studies. Consent documents were translated into local languages.
Procedures
We report pharmacokinetic results of the darunavir/ritonavir arm of the CHAPAS-4 intensive pharmacokinetic sub-study. Darunavir/ritonavir was dosed according to WHO weight bands (14–19.9 kg, 20–24.9 kg, 25–34.9 kg, ≥ 35 kg) using tablets of 400 or 150 mg darunavir alone and 100 mg ritonavir alone. Children in weight bands 14–19.9 and 20–24.9 kg were treated with once-daily 600/100 mg, and those in weight bands 25–34.9 and ≥35 kg with once-daily 800/100 mg darunavir/ritonavir. Doses were derived from previous pharmacokinetic studies in non-African children and selected before the 2018 WHO-recommended darunavir doses were released.13–15 NRTI backbones were also dosed according to weight (see Table S4).
Children were followed up for 96 weeks and intensive pharmacokinetic blood samples collected at week 6 at pre-dose, 1, 2, 4, 6, 8, 12 and 24 hours post-dose. The children in the TAF-based arm had another blood sample drawn 0.5 hours post-dose. On the day of pharmacokinetic blood sampling, ART was administered under direct observation, with food (breakfast with 5% fat, ∼250 kCal). Any co-medication was taken at least 2 hours after the study drugs.
Darunavir and ritonavir plasma concentrations were measured at the Department of Pharmacy, Radboud University Medical Center, Nijmegen, the Netherlands using a validated and externally validated high-performance liquid chromatography quantification method with a lower limit of quantification (LLOQ) of 0.105 mg/L for darunavir and 0.045 mg/L for ritonavir.16,17 AAG plasma concentrations were measured at the department of Clinical Chemistry and Laboratory Medicine, Leiden University Medical Center, Leiden, the Netherlands, using a validated immunoturbidimetric method with a LLOQ of 0.10 g/L. Pharmacokinetic results of TAF and tenofovir are reported elsewhere.18 Results of other nested pharmacokinetic sub-studies within the CHAPAS-4 trial and main study outcome data will be reported in various papers elsewhere.19,20
Non-compartmental pharmacokinetic analysis
We considered haemolysed blood samples as non-evaluable for non-compartmental analysis (NCA) and excluded pharmacokinetic profiles of children if therapy non-adherence was likely. Therapy non-adherence was defined as when a pre-dose plasma concentration (C0) of darunavir was >15 times lower than plasma concentration 24 hours after observed drug administration [trough concentration (Ctrough)]. Samples were also considered non-evaluable for NCA if there were protocol violations, or if concomitant medications were taken that could affect the pharmacokinetics of darunavir.
Phoenix WinNonlin v.8.4 (Pharsight Corporation, Mountain View, CA, USA) was used to calculate pharmacokinetic parameters for darunavir, namely Ctrough, C0, area under the concentration–time curve from 0–24 hours (AUC0–24h), maximum plasma concentration (Cmax), and apparent elimination half-life (T1/2). T1/2 was used to estimate Ctrough if a sample was not taken at 24 h. The pharmacokinetic parameters were reported as geometric means (GM) with coefficients of variation (CV%) except for Tmax, which was reported as median with interquartile range.
We compared the observed pharmacokinetic parameters (AUC0–24h, Ctrough, Cmax) with values observed in adults in the ARTEMIS III trial, after 4 weeks of treatment with once-daily darunavir/ritonavir 800/100 mg.14 Adequate exposure was defined as a GM AUC0–24h in children like the median adult AUC0–24h
Population pharmacokinetic analysis
Darunavir concentrations were also interpreted with population pharmacokinetics modelling using NONMEM v.7.5.1 (ICON Development Solutions, Ellicott City, MD, USA) with the first-order conditional estimation with interaction (FOCE-I) algorithm. Data were visualized and processed using R v.4.2.2, Perl Speaks NONMEM v.5.3.0 and Pirana v.2.9.9.21
We tested one- and two-compartment disposition models with first-order absorption (with or without a delay in absorption or transit compartments22), first-order elimination, as well as first-pass and saturable elimination through a liver compartment. We included between-subject variability (BSV) on disposition parameters and between-occasion variability on absorption parameters assuming a lognormal distribution. Combined additive and proportional error models to describe residual unexplained variability were evaluated.
To account for differences in body size, we tested allometric scaling of clearance and volume of distribution parameters with a fixed exponent of 0.75 and 1, respectively, scaled by either total body weight, fat-free mass or fat mass.23 After accounting for allometry, we tested the effect of age, ritonavir exposure, NRTI backbone and AAG concentration on pharmacokinetic parameters of darunavir. We retained covariates reaching statistical significance of P < 0.05 on forward inclusion and P < 0.01 on backward elimination. We assessed performance of intermediate and final models with goodness of fit plots, visual predictive checks and evaluated parameter precision using sampling importance resampling procedure.24,25
Simulations
We used our final model to simulate darunavir exposures in a representative virtual population of 100 000 children.26 We simulated darunavir AUC0–24h, Ctrough and Cmax achieved when the children are dosed according to once-daily darunavir doses recommended by WHO in 2018 (Table S1). These exposures were compared with simulated exposures achieved with doses used in the CHAPAS-4 trial, all the while using historic adult darunavir exposures from the ARTEMIS III trial14 as the reference.
Results
Study population
Between January 2019 and March 2021, 59 children were enrolled into the darunavir/ritonavir arm. Median age and weight were 10.9 (range 3.8–14.7) years and 26.0 (14.5–47.0) kg, respectively, 56% were female. One out of 491 darunavir concentrations was below LLOQ and this participant was excluded from NCA due to non-adherence. This participant was retained in population pharmacokinetic analysis, only C0 concentration was ignored. Table 1 summarizes baseline characteristics for all children in the darunavir/ritonavir arm.
Table 1.
Baseline characteristics of children who received darunavir in the CHAPAS-4 trial, stratified by NRTI backbone
| Total (N = 59) |
TAF/FTC (N = 34) |
ABC/3TC (N = 12) |
ZDV/3TC (N = 13) |
||
|---|---|---|---|---|---|
| Age (years) | Median [min–max] | 10.9 [3.8–14.7] | 10.9 [3.8–14.7] | 12.5 [6.9–14.3] | 9.5 [3.9–12.9] |
| Sex: n (%) | |||||
| Female | 33 (55.9%) | 18 (52.9%) | 7 (58.3%) | 8 (61.5%) | |
| Male | 26 (44.1%) | 16 (47.1%) | 5 (41.7%) | 5 (38.5%) | |
| Weight (kg) | Median [min–max] | 26.0 [14.5–47.0] | 24.0 [14.5–47.0] | 31.7 [19.2–45.8] | 22.4 [16.5–44.0] |
| Weight band (kg): n (%) | |||||
| 14–19.9 | 12 (20.3%) | 7 (20.6%) | 1 (8.3%) | 4 (30.8%) | |
| 20–24.9 | 17 (28.8%) | 11 (32.4%) | 1 (8.3%) | 5 (38.5%) | |
| 25–34.9 | 17 (28.8%) | 9 (26.5%) | 6 (50.0%) | 2 (15.4%) | |
| ≥35 | 13 (22.0%) | 7 (20.6%) | 4 (33.3%) | 2 (15.4%) | |
| Height (cm) | Median [min–max] | 132 [97.8–161] | 133 [97.8–161] | 142 [113–156] | 126 [101–144] |
| Darunavir dose (mg/kg) | Median [min–max] | 26.7 [17.0–41.4] | 26.5 [17.0–41.4] | 25.2 [17.5–31.3] | 28.6 [18.2–36.4] |
| Ritonavir dose (mg/kg) | Median [min–max] | 3.85 [2.13–6.90] | 4.17 [2.13–6.90] | 3.16 [2.18–5.21] | 4.46 [2.27–6.06] |
| AAG (g/L) | Median [min–max] | 0.7 [0.3–1.9] | 0.7 [0.3–1.5] | 0.6 [0.3–1.1] | 0.7 [0.4–1.9] |
TAF/FTC, tenofovir alafenamide/emtricitabine; ABC/3TC, abacavir/lamivudine, ZDV/3TC, zidovudine/lamivudine.
Non-compartmental analysis
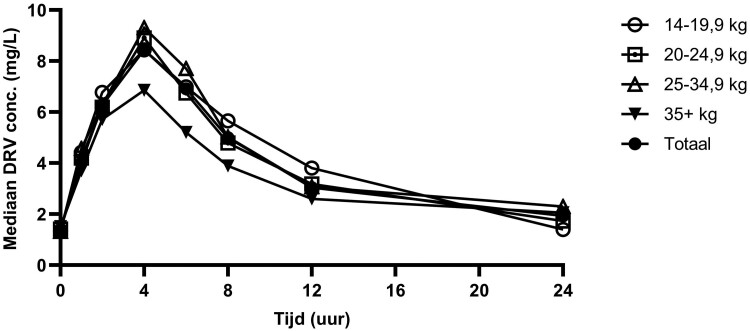
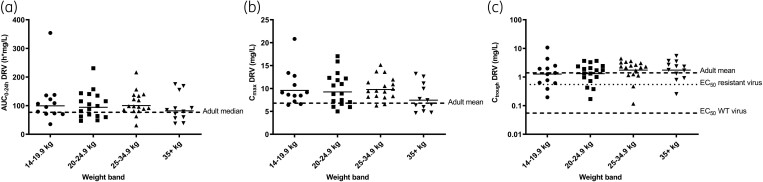
We observed a GM (CV%) AUC0–24h of 94.3 (50%) mg·h/L, and Cmax of 9.1 (35%) mg/L in this population, which are all slightly above observed median(range) adult AUC0–24h of 69.4 (33.0–88.4) mg·h/L, and Cmax of 5.5 (1.3) mg/L {mean [standard deviation (SD)]}. Our observed Ctrough of 1.5 (111%) mg/L GM (CV%) was similar to the adult Ctrough of 1.4 (0.5) mg/L [mean (SD)].14 The 50% effective concentration (EC50) of darunavir for wild-type virus is 0.055 mg/L.14 All children in CHAPAS-4 achieved a Ctrough above 0.055 mg/L and 86% had Ctrough above EC90 of 0.495 mg/L. Table 2 summarizes NCA pharmacokinetic parameters of darunavir/ritonavir stratified by NRTI backbone. Median darunavir pharmacokinetic profiles and observed AUC0–24h, Cmax and Ctrough stratified by weight band are shown in Figures 1 and 2. Table S3 summarizes NCA pharmacokinetic parameters of darunavir and ritonavir stratified by weight band. Figure S3 shows median ritonavir pharmacokinetic profiles per weight band and Figure S4 shows median darunavir and ritonavir pharmacokinetic profiles for the whole group.
Table 2.
Summary of darunavir/ritonavir pharmacokinetic parameters in children within CHAPAS-4 and reference study
| Total | TAF/FTC | ABC/3TC | ZDV/3TC | Adultsa | |
|---|---|---|---|---|---|
| Darunavir | |||||
| AUC0–24h (h mg/L) | 94.3 (50) | 90.0 (49) | 79.5 (52) | 122.4 (45) | Median (range); 69.4 (33.0–88.4) |
| Ctrough (mg/L) | 1.5 (111) | 1.4 (94) | 1.1 (191) | 2.5 (70) | Mean (SD); 1.4 (0.5) |
| Cmax (mg/L) | 9.1 (35) | 9.0 (35) | 7.8 (26) | 10.4 (37) | Mean (SD); 5.5 (1.3) |
| Tmax, h | 4 (3.9–4.0) | 4 (3.9–4.03) | 4 (2.0–4.0) | 4.0 (4.0–5.02) | Median (range); 3.0 (1.0–4.1) |
| T1/2, h | 9.1 (54) | 8.5 (50.0) | 8.4 (68) | 11.8 (48) | Mean (SD); 16.8 (7.2) |
| Cl/F (L/h) | 7.4 (54) | 7.7 (52) | 9.6 (50) | 5.4 (45) | — |
| Vd/F | 97.0 (67) | 90.5 (70) | 131.2 (69) | 88.8 (54) | — |
| Ritonavir | |||||
| AUC0–24h (h mg/L) | 9.1 (47) | 9.1 (40) | 7.0 (38) | 11.8 (58) | — |
| Cmax (mg/L) | 1.1 (67) | 1.2 (46) | 0.8 (33) | 1.3 (129) | — |
Data are presented as GM (coefficient of variation), except for Tmax, which is presented as median (interquartile range).
AUC0–24h/Ctrough: n = 57, Cmax/Tmax: n = 58, T1/2: n = 45.
TAF/FTC, tenofovir alafenamide/emtricitabine; ABC/3TC, abacavir/lamivudine; ZDV/3TC, zidovudine/lamivudine.
aWeek 4 data from ARTEMIS trial, adults on 800/100 mg darunavir/ritonavir QD.14
Figure 1.
Median darunavir plasma concentrations observed in the CHAPAS-4 trial, stratified by weight band and for all children together (total). DRV; darunavir.
Figure 2.
Individual pharmacokinetic parameter of darunavir stratified by weight-band groups. The solid horizontal lines indicate the geometric mean for each group. (a) Individual darunavir (DRV) area under the concentration–time curve (AUC0–24h); the dashed line indicates the AUC0–24h adult median (69.4 h mg/L).14 (b) Individual DRV maximum concentrations (Cmax); the dashed line indicates the Cmax adult mean (5.5 mg/L).14 (c) Individual DRV trough concentrations (Ctrough); the upper dashed line indicates the Ctrough adult mean (1.4 mg/L),14 the middle dotted line shows the concentration at 50% (EC50) for resistant virus inhibition27 and the lowest dotted line the EC50 of wild-type virus (0.055 mg/L).28 DRV, darunavir; WT, wild type.
Population pharmacokinetic analysis
Darunavir concentrations were best described by a two-compartment disposition model [change in objective function value (dOFV) = 40.3; P < 0.001, compared to one-compartment model] with transit absorption compartments (dOFV = 269.8; P < 0.001, compared to a model with no delay in absorption). Weight-based allometric scaling of clearance and volume parameters improved the model fit (dOFV = 27.9) and was preferred over fat-free mass (dOFV = 2.62).
An exponential relationship best described the inverse relationship between AAG and clearance, with a slope of −0.582 (95%CI −0.236 to −0.667, dOFV = 12.8; P < 0.001). In addition, we developed a semi-mechanistic model where AAG concentrations affected darunavir binding to AAG. Increasing AAG concentrations increased AAG’s maximum binding capacity for darunavir through a power relationship. Bound concentrations of darunavir to AAG followed Michaelis–Menten kinetics, assuming instantaneous binding of darunavir to AAG. The model fit significantly improved (dOFV = 11; P < 0.001) and 12.8% BSV in clearance was explained. However, considering parsimony and usability, we decided to adopt the simpler model with AAG affecting clearance directly. The details of the protein binding model are in the Supplemental Materials.
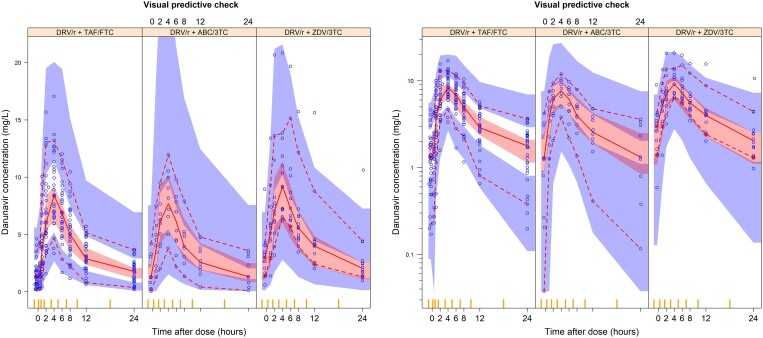
No significant effect of NRTI backbone on darunavir pharmacokinetics was found. Furthermore, neither ritonavir AUC nor a joint (direct or indirect inhibitory Emax relationship) model of ritonavir individual concentrations and darunavir clearance significantly improved the model fit (P > 0.05 for all tested relationships). Final population pharmacokinetic parameter estimates and the model fit to the data are shown in Table 3 and Figure 3, respectively.
Table 3.
Final population pharmacokinetic model parameters for darunavir
| Parameter (units) | Typical values (95% confidence-intervala) |
|---|---|
| Clearance: CL (L/h)b | 8.05 (6.85–8.81) |
| Central volume of distribution: V (L)b | 46.3 (37.2–56.0) |
| Intercompartmental clearance: Q (L/h)b | 7.79 (6.34–9.05) |
| Peripheral volume of distribution: V2 (L)b | 82.6 (65.1–101.5) |
| Bioavailability: F | 1 FIXED |
| Mean absorption transit time: MTT (h) | 0.763 (0.655–0.910) |
| Number of absorption transit compartments: NN (n) | 11.6 (5.03–23.8) |
| First-order absorption rate constant: Ka (1/h) | 0.551 (0.437–0.696) |
| Slope of AAG concentration effect on CLc | −0.582 (−0.236 to −0.667) |
| BSV (%) | |
| Clearance | 32.4 (25.5–40.8) |
| BOV (%) | |
| Bioavailability | 44.4 (31.7–55.5) |
| Mean absorption transit time | 60.8 (48.8–79.7) |
| First-order absorption rate constant | 43.4 (31.8–58.5) |
| Residual unexplained error | |
| Proportional error (%) | 12.5 (10.6–15.0) |
| Additive error (mg/L) | 0.291 (0.202–0.382) |
BOV, between-occasion variability.
aBased on sampling importance resampling.
bAll clearances and volumes of distribution were allometrically scaled, and the typical values reported here refer to a child weighing 26 kg.
cThe relationship between CL and AAG is as follows: , where Weighti, CLi and AAGi are the values of weight, CL and AAG for patient i.
Figure 3.
Visual predictive check of darunavir concentration versus time after dose, stratified by non-NRTI backbone. The solid and dashed lines represent the fifth, 50th and 95th percentiles of the observed data, while the shaded areas represent the model-predicted 95% confidence intervals for the same percentiles. The dots are the observed concentrations. DRV/r, darunavir/ritonavir; TAF/FTC, tenofovir alafenamide/emtricitabine; ABC/3TC, abacavir/lamivudine; ZDV/3TC, zidovudine/lamivudine.
Simulation results
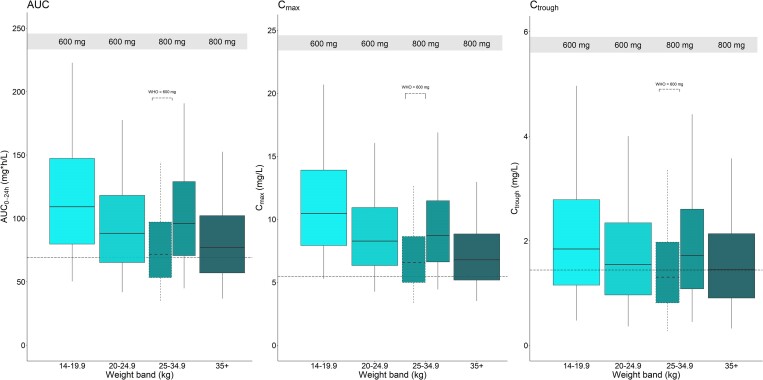
Results of exposure simulations are in Figure 4. Across all weight bands, median AUC0–24h, Cmax and Ctrough values achieved using CHAPAS-4 dosing are similar or exceed medians observed in adults. The WHO-recommended dosing differs from that used in CHAPAS-4 in the 25–34.9 kg weight band, i.e. 600/100 mg darunavir/ritonavir once daily according to WHO guidelines and 800/100 mg administered in CHAPAS-4. This lower WHO-recommended dose showed simulated exposures in line with adult values and, while Ctrough is slightly lower than the mean previously observed in adults, >99% of patients are expected to remain above twice the EC50 in all weight bands.
Figure 4.
Simulated steady-state darunavir area under the curve from 0 to 24 h (AUC0–24h), maximum concentrations (Cmax) and trough concentrations (Ctrough) versus weight band, with concentrations achieved after administration of the weight-based dosing used in CHAPAS-4. The box with dashed boundaries shows the WHO-recommended dosing for the weight band where it differs from CHAPAS-4. The black dashed horizontal lines (from left to right) represent the reported adult median AUC0–24h (69.4 h mg/L), mean Cmax (5.5 mg/L) and mean Ctrough (1.4 mg/L), respectively.14 The boxes indicate the interquartile range and the whiskers the 95% range.
Discussion
We conducted pharmacokinetic analysis of once-daily ritonavir-boosted darunavir used as second-line ART and co-administered with TAF/emtricitabine, abacavir/lamivudine or zidovudine/lamivudine in African children aged 3.8–14.7 years and developed a population pharmacokinetic model accounting for the effect of body weight and binding of darunavir to AAG. In all weight bands, we observed GM AUC0–24h and Cmax slightly above observed median AUC0–24h and mean Cmax in adults taking once-daily 800/100 mg darunavir/ritonavir.14 The observed GM Ctrough in our population was similar to adult reference values and >99% of the children reached a Ctrough about twice the EC50% and 86% were above the EC90 for wild-type virus. As the dose in children weighing 25–34.9 kg in CHAPAS-4 differed from the 2018 WHO-recommended dose, the model was used to simulate the WHO dose in this weight band as well. The simulated 2018 WHO-recommended dose also led to exposures similar to adult reported values. There was no difference in darunavir exposure for the different NRTI backbones.
This last finding is reassuring and important. TAF is a prodrug of tenofovir and is considered safer than with tenofovir disoproxil fumarate, the other prodrug of tenofovir.29 Moreover, it can be administered once daily, has a higher barrier to resistance and is more efficacious than abacavir or zidovudine.12,19,30 It is therefore encouraging to note that a TAF-based NRTI backbone does not interfere with darunavir exposures. Additionally, darunavir/ritonavir has also been shown to not significantly alter exposures of TAF18 and this leads us to conclude that this ART combination is viable and safe for children failing on first-line ART. If adopted in the WHO guidelines, this once-daily combination could transform second-line ART treatment for African children.
Across all weight bands, we observed median AUC0–24h and Cmax above adult reported values (Figure 4). Safety of these higher exposures can be ascertained from safety results of the CHAPAS-4 main trial, which found no difference in the safety profile of children on darunavir versus other PIs.19 The median Ctrough in this study was comparable to reported adult mean Ctrough. The WHO-recommended 600/100 mg darunavir/ritonavir dose in the 24.9–35 kg weight band (which is a lower dose than 800/100 mg used in CHAPAS-4 in this weight band) in our simulations still led to median AUC0–24h and Cmax comparable with adults taking 800/100 mg darunavir/ritonavir. While the Ctrough for this dosing is slightly lower than the mean previously observed in adults, >99% of patients are expected to remain above twice the EC50 in all weight bands. CHAPAS-4 trial doses simulated for this weight band achieved median AUC0–24h higher than both WHO-recommended doses and reported adult median AUC. Despite the high darunavir exposures, there were no safety concerns reported in the 24.9–35 kg weight band in the CHAPAS-4 trial.19 Given that both 600/100 mg and 800/100 mg doses within this weight band achieve desirable exposures, there exists flexibility in selecting the appropriate dose based on considerations of convenience and tablet availability.
Our population pharmacokinetic model describing darunavir concentration–time data was similar to a previous model in children, except for a few differences.13 Although both models had two disposition compartments, an effect of weight on volume and clearance parameters, the effect of AAG was different. The model by Brochot et al. included the effect of AAG on the binding affinity of darunavir, assuming linear binding within the range of observed darunavir concentrations. Although this model gave satisfactory results, we think it is more appropriate to have AAG affect both clearance and distribution, through affecting the fraction of unbound (FU) drug (as opposed to affecting only clearance). In our model, we fixed the value of the FU to a literature value but soon realized that the model was unidentifiable as changing the value of FU did not affect the model. We then tried a more mechanistic approach as reported by Schalkwijk et al., which assumes non-linear binding of darunavir to AAG. However, due to the unavailability of unbound darunavir concentrations in our dataset we had to use priors from Schalkwijk et al. to inform our model and estimate the parameters.31 Details of this model are shown in the Supplemental Materials. Although this model fitted the data well, the model improvements were not sufficient for taking parsimony into account. In addition, the simpler model not only provides a more straightforward interpretation of the data but also enhances practical applicability without compromising the integrity and reliability of our findings.
The effect of ritonavir on darunavir pharmacokinetics was evaluated to explore possibility of reducing ritonavir doses, but no significant effect was found with the observed range of ritonavir exposures. This is not to say that ritonavir does not adequately inhibit activity of CYP3A4 to boost darunavir exposure but rather, it suggests that both 800/100 and 600/100 mg darunavir/ritonavir provide adequate ritonavir for maximum boosting, making it difficult to establish a relationship. To quantify the effect of ritonavir, one would need to compare children on darunavir/ritonavir with children on darunavir without ritonavir or with much lower doses of ritonavir, and neither of these scenarios was available within CHAPAS-4. This is a limitation of our study since we are unable to advise whether 100 mg ritonavir is higher than necessary and could be reduced. Second, while our non-linear darunavir-AAG binding model characterized the pharmacokinetics of darunavir making assumptions about the equilibrium between total and unbound concentrations, the last were not directly observed in our study. Our findings should be confirmed in studies measuring unbound concentrations, and possibly including participants with extreme AAG concentrations e.g. malnourished children.32
In conclusion, compared to reported adult exposures, the CHAPAS-4 trial had higher median exposures of darunavir and these exposures are within the safe range. We found no difference in darunavir exposures among children on TAF/emtricitabine versus abacavir/lamivudine or zidovudine/lamivudine. This finding paves the way to considering darunavir/ritonavir with TAF/emtricitabine as an attractive treatment option for second-line ART in children. The WHO-recommended 600/100 mg darunavir/ritonavir dose in the 24.9–35 kg weight band led to exposures comparable with adults and may be maintained. We found that 100 mg ritonavir provides sufficient boosting to 600 or 800 mg darunavir and, if possible, lower ritonavir doses could be investigated. We recommend that effect of AAG on darunavir exposure should be considered in therapeutic drug monitoring settings, and the clinical relevance of this effect be further investigated.
Supplementary Material
Acknowledgements
We are thankful to the study participants and their families. We also thank the ICTS High Performance Computing team at the University of Cape Town (http://hpc.uct.ac.za) for providing us with the resources to perform the calculations in this study. We also thank the department of Clinical Chemistry and Laboratory Medicine of Leiden University Medical Center for measuring AAG concentration and providing information about the measurement.
Contributor Information
Lufina Tsirizani, Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, Cape Town, South Africa; Training and Research Unit of Excellence, Kamuzu University of Health Sciences, Blantyre, Malawi.
Shaghayegh Mohsenian Naghani, Department of Pharmacy, Radboudumc Research Institute for Medical Innovation (RIMI), Radboud University Medical Center, Nijmegen, The Netherlands.
Hylke Waalewijn, Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, Cape Town, South Africa; Department of Pharmacy, Radboudumc Research Institute for Medical Innovation (RIMI), Radboud University Medical Center, Nijmegen, The Netherlands.
Alexander Szubert, Medical Research Council Clinical Trials Unit at University College London, London, UK.
Veronica Mulenga, Department University Teaching Hospital, University of Lusaka, Lusaka, Zambia.
Chishala Chabala, Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, Cape Town, South Africa; Department University Teaching Hospital, University of Lusaka, Lusaka, Zambia.
Mutsa Bwakura-Dangarembizi, Department of Paediatrics and Child Health, Clinical Research Centre, University of Zimbabwe, Faculty of Medicine and Health Sciences, Harare, Zimbabwe; Department of Child, Adolescent and Women’s Health, University of Zimbabwe Faculty of Medicine and Health Sciences, Harare, Zimbabwe.
Moses Chitsamatanga, Department of Paediatrics and Child Health, Clinical Research Centre, University of Zimbabwe, Faculty of Medicine and Health Sciences, Harare, Zimbabwe.
Diana A Rutebarika, Department of Paediatrics, Joint Clinical Research Centre, Kampala, Uganda.
Victor Musiime, Department of Paediatrics, Joint Clinical Research Centre, Kampala, Uganda; Department of Paediatrics and Child Health, Makerere University, College of Health Sciences, School of Medicine, Kampala, Uganda.
Mariam Kasozi, Department of HIV Reasearch, Joint Clinical Research Centre, Mbarara, Uganda.
Abbas Lugemwa, Department of HIV Reasearch, Joint Clinical Research Centre, Mbarara, Uganda.
Lara N Monkiewicz, Medical Research Council Clinical Trials Unit at University College London, London, UK.
Helen M McIlleron, Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, Cape Town, South Africa; Wellcome Centre for Infectious Diseases Research in Africa (CIDRI-Africa), Institute of Infectious Disease and Molecular Medicine, University of Cape Town, Cape Town, South Africa.
David M Burger, Department of Pharmacy, Radboudumc Research Institute for Medical Innovation (RIMI), Radboud University Medical Center, Nijmegen, The Netherlands.
Diana M Gibb, Medical Research Council Clinical Trials Unit at University College London, London, UK.
Paolo Denti, Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, Cape Town, South Africa.
Roeland E Wasmann, Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, Cape Town, South Africa.
Angela Colbers, Department of Pharmacy, Radboudumc Research Institute for Medical Innovation (RIMI), Radboud University Medical Center, Nijmegen, The Netherlands.
Funding
The CHAPAS-4 trial is sponsored by University College London (UCL), with central management by the Medical Research Council (MRC) Clinical Trials Unit at UCL, supported by Medical Research Council United Kingdom core funding (Blood Borne Viruses program number: MC_UU_00004/03). The main funding for this study was provided by the European and Developing Countries Clinical Trials Partnership (EDCTP; TRIA2015-1078). Additional funding was received from Janssen Pharmaceuticals and Gilead Sciences. The TAF and TFV assay was developed and performed at the Division of Clinical Pharmacology, University of Cape Town, which is supported by the South African National Institute of Allergy and Infectious Diseases of the South African National Institutes of Health (award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701). L.T. was partly supported by the Research council of Norway through the Global Health and Vaccination Programme (GLOBVAC), project number 285284, which is part of the EDCTP2 programme supported by the European Union.
Transparency declarations
D.M.B. has received research grants from ViiV Healthcare, Merck and Gilead Sciences; payments from ViiV Healthcare and Gilead Sciences for serving on advisory boards; payment from ViiV Healthcare for speaking at symposia; payment or honoraria for lectures from Pfizer and Gilead Sciences and for advisory board for Merck; and is the co-founder of Global DDI Solutions. A.C. has received honoraria from Merck Sharp & Dohme and Gilead (fees paid to institution) and has received study grants from MSD, Gilead Sciences and ViiV Healthcare. The rest of the authors declare no potential conflicts of interest.
Authors’ contributions
D.M.G. conceived the study, the CHAPAS-4 trial team conducted the clinical trial, A.S. managed the trial data, L.N.M. managed the trial. H.M.M., D.M.B., A.C. and H.W. led the pharmacokinetic sub-study. S.M.N., A.C. and D.M.B. conducted the NCA. L.T., R.E.W. and P.D. performed the population pharmacokinetic analysis. C.C., V.M., M.B., M.C., D.A.R., V.M., M.K. and A.L. carried out the trial activities. All authors read and approved the final manuscript.
Supplementary data
Figures S1 to S4 and Tables S1 to S4 are available as Supplementary data at JAC Online.
References
- 1. UNAIDS . Global HIV statistics. Global HIV & AIDS statistics — Fact sheet. Fact Sheet 2022; (June):1–3. UNAIDS.
- 2. Ubesie AC. Pediatric HIV/AIDS in sub-Saharan Africa: emerging issues and way forward. Afr Health Sci 2012; 12: 297–304. 10.4314/ahs.v12i3.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. UNAIDS . UNAIDS Global AIDS Update — Confronting inequalities — Lessons for pandemic responses from 40 years of AIDS. Global AIDS Update. 2021; 3862021.
- 4. World Health Organization . Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. WHO, 2021; 548. [PubMed] [Google Scholar]
- 5. Paton NI, Kityo C, Hoppe Aet al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med 2014; 371: 234–47. 10.1056/NEJMoa1311274 [DOI] [PubMed] [Google Scholar]
- 6. Musiime V, Fillekes Q, Kekitiinwa Aet al. The pharmacokinetics and acceptability of lopinavir/ritonavir minitab sprinkles, tablets, and syrups in African HIV-infected children. J Acquir Immune Defic Syndr 2014; 66: 148–54. 10.1097/QAI.0000000000000135 [DOI] [PubMed] [Google Scholar]
- 7. AbbVie Pharmaceutical Research & Development . Kaletra 80 mg/20 mg oral solution. 2022[Product Monograph Template - Standard] (abbvie.ca).
- 8. Bamford A, Turkova A, Lyall Het al. Paediatric European Network for Treatment of AIDS (PENTA) guidelines for treatment of paediatric HIV-1 infection 2015: optimizing health in preparation for adult life. HIV Med 2018; 19: e1–42. 10.1111/hiv.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keating GM. Darunavir: a review in pediatric HIV-1 infection. Pediatr Drugs 2015; 17: 411–21. 10.1007/s40272-015-0146-0 [DOI] [PubMed] [Google Scholar]
- 10. Rittweger M, Arasteh K. Clinical pharmacokinetics of darunavir. Clin Pharmacokinet 2007; 46: 739–56. 10.2165/00003088-200746090-00002 [DOI] [PubMed] [Google Scholar]
- 11. Huisman M, Smit J, Schinkel A. Significance of P-glycoprotein for the pharmacology and clinical use of HIV protease inhibitors. AIDS 2000; 14: 237–42. 10.1097/00002030-200002180-00005 [DOI] [PubMed] [Google Scholar]
- 12. Sax PE, Tierney C, Collier ACet al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis 2011; 204: 1191–201. 10.1093/infdis/jir505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brochot A, Kakuda T, Van De Casteele Tet al. Model-based once-daily darunavir/ritonavir dosing recommendations in pediatric HIV-1-infected patients aged ≥3 to <12 years. CPT Pharmacometrics Syst Pharmacol 2015; 4: 406–14. 10.1002/psp4.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kakuda TN, Brochot A, Tomaka FLet al. Pharmacokinetics and pharmacodynamics of boosted once-daily darunavir. J Antimicrob Chemother 2014; 69: 2591–605. 10.1093/jac/dku193 [DOI] [PubMed] [Google Scholar]
- 15. Bastiaans DET, Geelen SPM, Visser EGet al. Pharmacokinetics, short-term safety and efficacy of the approved once-daily darunavir/ritonavir dosing regimen in HIV-infected children. Pediatr Infect Dis J 2018; 37: 1008–10. 10.1097/INF.0000000000001964 [DOI] [PubMed] [Google Scholar]
- 16. Holland DT, DiFrancesco R, Connor JDet al. Quality assurance program for pharmacokinetic assay of antiretrovirals: ACTG proficiency testing for pediatric and adult pharmacology support laboratories, 2003 to 2004. Ther Drug Monit 2006; 28: 367–74. 10.1097/01.ftd.0000211817.58052.b8 [DOI] [PubMed] [Google Scholar]
- 17. Burger D, Teulen M, Eerland Jet al. The international interlaboratory quality control program for measurement of antiretroviral drugs in plasma: a global proficiency testing program. Ther Drug Monit 2011; 33: 239–43. 10.1097/FTD.0b013e31820fa528 [DOI] [PubMed] [Google Scholar]
- 18. Waalewijn H, Szubert AJ, Wasmann REet al. First pharmacokinetic data of tenofovir alafenamide fumarate and tenofovir with dolutegravir or boosted protease inhibitors in African children: a substudy of the CHAPAS-4 trial. Clin Infect Dis 2023; 77:875–82. 10.1093/cid/ciad267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bwakura-Dangarembizi M, Szubert AJ, Mumbiro Vet al. CHAPAS-4 trial: second-line anchor drugs for children with HIV in Africa. medRxiv 2024; 2024.04.12.24305333. 10.1101/2024.04.12.24305333 [DOI] [Google Scholar]
- 20. Bevers LAH, Waalewijn H, Szubert AJet al. Pharmacokinetic data of dolutegravir in second-line treatment of children with human immunodeficiency virus: results from the CHAPAS4 trial. Clin Infect Dis 2023; 77: 1312–7. 10.1093/cid/ciad346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keizer R, Karlsson M, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol 2013; 2: e50. 10.1038/psp.2013.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savic RM, Jonker DM, Kerbusch Tet al. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 2007; 34: 711–26. 10.1007/s10928-007-9066-0 [DOI] [PubMed] [Google Scholar]
- 23. Holford NHG, Anderson BJ. Allometric size: the scientific theory and extension to normal fat mass. Eur J Pharm Sci 2017; 109(May): S59–64. 10.1016/j.ejps.2017.05.056 [DOI] [PubMed] [Google Scholar]
- 24. Dosne A-G, Bergstrand M, Karlsson MO. An automated sampling importance resampling procedure for estimating parameter uncertainty. J Pharmacokinet Pharmacodyn 2017; 44: 509–20. 10.1007/s10928-017-9542-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergstrand M, Hooker AC, Wallin JEet al. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 2011; 13: 143–51. 10.1208/s12248-011-9255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wasmann RE, Svensson EM, Walker ASet al. Constructing a representative in-silico population for paediatric simulations: application to HIV-positive African children. Br J Clin Pharmacol 2021; 87: 2847–54. 10.1111/bcp.14694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tibotec Inc . PREZISTATM* (Tibotec, Inc.) (darunavir). 2006. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021976s001lbl.pdf
- 28. Boffito M, Miralles D, Hill A. Pharmacokinetics, efficacy, and safety of darunavir/ritonavir 800/100 mg once-daily in treatment-naïve and -experienced patients. HIV Clin Trials 2008; 9: 418–27. 10.1310/hct0906-418 [DOI] [PubMed] [Google Scholar]
- 29. Hill A, Hughes SL, Gotham Det al. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety? J Virus Erad 2018; 4: 72–9. 10.1016/S2055-6640(20)30248-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruane PJ, DeJesus E, Berger Det al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1–positive adults. J Acquir Immune Defic Syndr 2013; 63: 449–55. 10.1097/QAI.0b013e3182965d45 [DOI] [PubMed] [Google Scholar]
- 31. Schalkwijk S, ter Heine R, Colbers Aet al. Evaluating darunavir/ritonavir dosing regimens for HIV-positive pregnant women using semi-mechanistic pharmacokinetic modelling. J Antimicrob Chemother 2019; 74: 1348–56. 10.1093/jac/dky567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramli RM, Sinrang AW, Aminuddin. Levels of alpha-1 acid glycoprotein (AGP) in stunting and non stunting tolls age 36–60 months. Int J Health Med Sci 2021; 4: 145–9. 10.31295/ijhms.v4n1.1666 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.