Abstract
Cerebral small vessel disease (SVD) is known to contribute to cognitive impairment, apathy and gait dysfunction. Although associations between cognitive impairment and either apathy or gait dysfunction have been shown in SVD, the inter-relations among these three clinical features and their potential common neural basis remain unexplored. The dopaminergic meso-cortical and meso-limbic pathways have been known as the important brain circuits for both cognitive control, emotion regulation and motor function. Here, we investigated the potential inter-relations between cognitive impairment, apathy and gait dysfunction, with a specific focus on determining whether these clinical features are associated with damage to the meso-cortical and meso-limbic pathways in SVD.
In this cross-sectional study, we included 213 participants with SVD for whom MRI and comprehensive neurobehavioural assessments were performed. These assessments comprised six clinical measures: processing speed, executive function, memory, apathy (based on the Apathy Evaluation Scale) and gait function (based on the time and steps in the Timed Up and Go Test). We reconstructed five tracts connecting the ventral tegmental area (VTA) and dorsolateral prefrontal cortex (PFC), ventral lateral PFC, medial orbitofrontal cortex, anterior cingulate cortex (ACC) and nucleus accumbens within meso-cortical and meso-limbic pathways using diffusion weighted imaging. The damage along the five tracts was quantified using the free water (FW) and FW-corrected mean diffusivity indices. Furthermore, we explored the inter-correlations among the six clinical measures and identified their common components using principal component analysis (PCA).
Linear regression analyses showed that higher FW values of tracts within meso-cortical pathways were related to these clinical measures in cognition, apathy, and gait (all P-corrected values < 0.05). The PCA showed strong inter-associations among these clinical measures and identified a common component wherein all six clinical measures loaded on. Higher FW values of tracts within meso-cortical pathways were related to the PCA-derived common component (all P-corrected values < 0.05). Moreover, FW values of the VTA-ACC tract showed the strongest contribution to the PCA-derived common component over all other neuroimaging features.
In conclusion, our study showed that the three clinical features (cognitive impairment, apathy, and gait dysfunction) of SVD are strongly inter-related and that the damage in meso-cortical pathway could be the common neural basis underlying the three features in SVD. These findings advance our understanding of the mechanisms behind these clinical features of SVD and have the potential to inform novel management and intervention strategies for SVD.
Keywords: meso-cortico-limbic system, cognitive impairment, apathy, gait dysfunction, cerebral small vessel disease
Li et al. show that the three main clinical features of small vessel disease—cognitive impairment, apathy, and gait dysfunction—are strongly interrelated and that damage to the dopaminergic meso-cortical pathway could be a common factor underlying all three symptoms.
Introduction
Cerebral small vessel disease (SVD) is a complex brain disease that encompasses a diverse range of pathological changes to small vessels in the brain.1,2 These changes manifest as various abnormalities on MRI, including white matter hyperintensities (WMH), lacunes and cerebral microbleeds (CMBs).1 The primary neurobehavioural symptoms associated with SVD are cognitive impairment, apathy, and gait dysfunction.2-4 However, these symptoms are often studied in isolation, without considering their interrelations. A recent study in older adults tentatively identified a vascular triad that includes apathy, executive dysfunction and gait disorder, highlighting the inter-connected nature of these conditions.5 In SVD, the association between cognitive impairment and apathy has been established.6-8 Furthermore, gait dysfunction was related to cognitive impairment in individuals with SVD, as well as apathy in those suffering from subcortical vascular dementia.9-11 These findings indicated that the three neurobehavioural symptoms in SVD may share some common components, potentially arising from a single neurological basis. Identifying the common component and shared neural basis of cognitive impairment, apathy and gait dysfunction may contribute to novel approaches for intervention and treatment in SVD. However, the inter-associations among the three symptoms and their potentially shared neural basis remain largely unknown in SVD.
The meso-cortical and meso-limbic pathways, wherein dopaminergic neurons from the ventral tegmental area (VTA) project to the prefrontal cortex (PFC), anterior cingulate cortex (ACC) and nucleus accumbens (NAc), respectively,12 have been regarded as the important brain circuits for cognitive control, emotion regulation and motor function.13 Multiple studies have reported that apathy might be associated with structural disconnections within the cerebral dopaminergic reward network in SVD.14-16 In addition, our previous study found an association between striatal atrophy and apathy, which was also independently associated with cognitive impairment in SVD.6 Moreover, another study on cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), a rare monogenetic form of SVD, further demonstrated that dysfunctional effort-based decision-making might be a cognitive basis for apathy and that the reduced white matter integrity of orbitofrontal-anterior cingulate white matter is associated with both apathy and this specific cognitive dysfunction.17 Regarding gait performance, as far as we are aware, no studies have specifically explored its association with dopaminergic pathway damage in SVD. However, evidence from Parkinson’s disease has demonstrated that abnormalities in the meso-cortical and meso-limbic pathways, in addition to the nigrostriatal pathway, are related to gait dysfunction.18-20 Additionally, the dopaminergic white matter (WM) pathways, with long-range fibre connections, may be vulnerable to SVD-related pathology.21,22 Hence, it can be hypothesized that the disruption of meso-cortical and meso-limbic WM pathways may contribute to cognitive impairment, apathy and gait dysfunction. However, to the best of our knowledge, no study has specifically assessed the disruption of the meso-cortical and meso-limbic WM pathways and their relations to composite performances in cognitive function, apathy and gait in SVD.
The goal of this study was (i) to reconstruct the meso-cortical and meso-limbic WM pathways in vivo and assess the damage in these pathways in patients with sporadic SVD using advanced neuroimaging approaches; (ii) to determine the inter-relations between the three clinical features (i.e. cognitive impairment, apathy and gait dysfunction); and (iii) to relate the extent of damage of the meso-cortical and meso-limbic WM pathways to these clinical features in SVD. We hypothesized that cognitive impairment, apathy and gait dysfunction are inter-related and that their dysfunction might be due to disruption in the meso-cortical and meso-limbic WM pathways.
Materials and methods
Study population
Data were derived from the Radboud University Nijmegen Diffusion tensor and Magnetic resonance imaging Cohort (RUN DMC) study, an ongoing cohort study investigating the causes and clinical consequences of sporadic SVD. Detailed information has been described previously.23 In brief, participants meeting the following criteria were included: (i) aged between 50 and 85 years; (ii) presence of SVD MRI markers (identified as WMH or lacunes); and (iii) cognitive or motor symptoms that could be attributed to SVD. After the baseline data collection in 2006, participants underwent three follow-up assessments in 2011, 2015 and 2020, respectively. However, due to a change of MRI scanners, we only used the data from the third follow-up assessment in 2020 (Supplementary Fig. 1). Additionally, all participants underwent a thorough and detailed clinical work-up, during which extensive standardized motor and neuropsychological screening tests were performed to identify participants with concomitant dementia or parkinsonism. A detailed description of the diagnostic work-up has been provided previously.24,25 Participants diagnosed with non-vascular dementia or Parkinson’s disease were excluded (n = 14) from the present study, resulting in a final sample of 213 participants (Supplementary Fig. 1).
Neuropsychological and gait assessment
Three cognitive domains (processing speed, executive function and memory) were assessed using a standardized cognitive assessment battery, including the Trail Making Test, Symbol-Digit Modalities Test, Verbal Fluency Test, Digit Span Test, Rey Auditory Verbal Learning Test and Rey-Osterrieth Complex Figure Test. The raw scores from each cognitive test were standardized to z-scores, which were subsequently used to compute a compound score for each cognitive domain (Supplementary Table 1).
Apathy was measured by the clinician-rated version of the Apathy Evaluation Scale (AES-C),26 an instrument validated across various neurological conditions, including SVD.15 The AES-C encompasses 18 items, each scored from 1 to 4 points. Higher scores indicate more severe apathy. We consider participants with an AES score ≥34 as having apathy.15 Depression was measured using the modified 18-item Centre for Epidemiologic Studies Depression Scale (CESD), excluding two items related to motivation to ensure a more pure measure of depressive symptoms (termed as modified CSED score), as described in the previous study.7 The internal consistency (α = 0.87) of the modified 18-item CESD is comparable with the original 20-item CESD (α = 0.88).7 The inclusion of depression assessment was justified by its significant overlap with apathy symptoms and its potential impact on cognitive functions and gait performance.27,28 Owing to the lack of a validated cut-off score specifically for modified 18-item CESD, we considered participants with an original 20-item CESD ≥16 as having depression.15
Gait performance was assessed by employing the Timed Up and Go (TUG) test,29 whereby both the mean time (in seconds) and the number of steps derived from three repetitive TUG tests were used in the analyses.9
MRI acquisition
All participants underwent scanning on a 3 T MRI scanner (MAGNETOM Prisma; Siemens Healthineers) with a 32-channel head coil. The following sequences were collected: magnetization prepared 2 rapid acquisition gradient echoes (MP2RAGE) sequence with sparse sampling for creating T1-weighted images, a 3D fluid-attenuated inversion recovery (FLAIR) sequence, a 3D multi-echo gradient echo (GRE) sequence for susceptibility weighted image (SWI) and multi-shell multi-directional diffusion-weighted imaging (DWI). Full acquisition details are provided in the Supplementary material and have been described previously.30
Cerebral small vessel disease MRI markers
MRI markers of SVD (i.e. WMH, lacunes, CMBs) and brain atrophy were assessed according to version 2 of the Standards for Reporting Vascular Changes on Neuroimaging criteria (STRIVE-2).1 WMH was semi-automatically segmented based on FLAIR and T1 images using a validated 3D U-net deep learning algorithm.31 All segmented WMH masks were visually inspected and manually corrected when necessary. The lacunes were identified on T1 and FLAIR images, while the CMBs were identified on SWI images; both were manually counted by two well-trained raters who were blind to the clinical data, with subsequent consensus meetings. Grey matter (GM), WM and CSF were segmented on T1 images using SPM12 software. To avoid misclassification of WMH as GM, these segmented images of GM, WM and CSF were corrected using the WMH mask. Brain volumes were determined by the ratio between the sum of GM and WM volumes and the sum of GM, WM and CSF volumes [i.e. intracranial volumes (ICV)] and served as a proxy of brain atrophy. WMH volumes were normalized to the ICV.
Diffusion MRI processing and free water mapping
Diffusion MRI data were pre-processed for denoising and removal of Gibbs artefacts, correction of head motion, eddy currents-induced distortion, susceptibility-induced distortion (top-up) and intensity bias.32-36 Next, multi-fibre directions within each voxel of the brain were estimated based on the multi-shell ball-and-two-sticks model.37 Subsequently, with the processed images (only b = 0 and b = 1000 s/mm2), fractional anisotropy (FA) and mean diffusivity (MD) images for each participant were obtained by using the ‘dtifit’ function within FSL. Additionally, free water (FW) and FW-corrected MD (MD-t) were acquired using a non-linear regularized minimization process implemented in MATLAB.38 The FW image model is a two-compartment model that enhances traditional diffusion tensor imaging (DTI) models by modelling both a FW component and a FW-corrected tissue compartment (Supplementary Fig. 2). The FW component signifies the unrestricted and undirected extracellular water fraction and was associated with multiple pathological processes, including the disruption of the blood–brain barrier, neuroinflammation and vasogenic oedema in SVD.39 The tissue compartment is estimated from the remaining signal after removing FW contribution, thus allowing for a more precise assessment of white matter fibre organization.
Regions of interest and probabilistic tractography
Probabilistic tractography was performed to capture two WM pathways originating from the VTA, with one projecting to the PFC and ACC (meso-cortical pathway) and the other projecting to the NAc (meso-limbic pathway).40,41 Additionally, the nigrostriatal WM pathway originating from the substantia nigra (SN) and projection to the striatum was identified.42 Note that our analyses focused on the meso-cortical and meso-limbic pathways, with the nigrostriatal WM pathway serving as a control in the sensitivity analyses.
First, a list of region of interest (ROI) masks was extracted. VTA and SN were identified based on a previously validated human subcortical atlas.43 NAc, putamen and globus pallidus were extracted from the structural segmentation of each participant’s T1-weighted image using the ‘samseg’ function within Freesurfer (version 7.3.2).44 In addition, considering the substantial size of the prefrontal cortex, this region was subdivided into three discrete regions: dorsolateral prefrontal cortex (dlPFC), ventral lateral PFC (vlPFC) and medial orbitofrontal cortex (mOFC) to enhance the accuracy and specificity of tractography. Three subregions of the PFC and ACC were defined based on previous studies using the Anatomical Automatic Labelling (AAL) atlas45: dlPFC (3, 4, 7 and 8), mOFC (27, 28, 5, 6, 25 and 26), vlPFC (9, 10, 15 and 16) and ACC (35 and 36).46,47 These numbers correspond to the brain parcellation in the AAL atlas. Next, a cortex mask for each participant was obtained from ‘Sequence Adaptive Multimodal SEGmentation (SAMSEG)’-based structural segmentation.44 Only the cortical part of each participant’s AAL-derived dlPFC, mOFC, vlPFC and ACC regions were retained through the cortical mask and then employed as the ROIs in subsequent analyses. According to previous studies, prior anatomical knowledge and visual inspection, multiple tailored ROIs were extracted as the exclusion masks for the WM pathway reconstruction based on the AAL atlas,45 Johns Hopkins University White Matter Atlas,48 Harvard-Oxford Cortical Atlas and structural segmentation of T1-weighted images (Supplementary Table 2).49,40 Subsequently, these ROIs in their corresponding native spaces were registered to diffusion space using the non-linear SyN registration algorithm in Advanced Normalization Tools (ANTs, http://stnava.github.io/ANTs/).50 All segmented and transformed ROIs were visually inspected.
Afterwards, probabilistic tractography was performed by repeating 5000 random samples from each voxel within the seed ROI, generating streamlines using ‘probtractx2’ function from FSL.51 For the meso-cortical pathway, VTA was employed as the seed mask, each of the three PFC subregions and the ACC were respectively employed as target mask, resulting in four different bundles of meso-cortical pathway: VTA-dlPFC, VTA-mOFC, VTA- vlPFC and VTA-ACC.40 For the meso-limbic pathway, the NAc was selected as the seeded mask, while the VTA was set as the target masks, aligning with a previous study.41 Regarding the nigrostriatal WM pathway, the SN was employed as the seeded mask, with the combination mask of putamen and globus pallidus designated as the target mask, consistent with a prior study.42
Common pathway and features extraction
To minimize the effects of individual anatomical variations, we formulated a common representative template for each tract, following similar methodology in previous studies.52,53 Briefly, an unbiased template was created based on FA images from all participants using the ANTs (http://stnava.github.io/ANTs/). Next, the individually tracked pathway was brought to this unbiased group template space using SyN registration algorithm within ANTs.50 Subsequently, the binary masks of common representative tracts were created by considering all individually warped tracts and retaining only the voxels present in a minimum of 60% of all participants.52 The selection of a 60% group threshold was guided by visual inspection, ensuring both the extent and specificity of the resultant group common pathways. Afterwards, six group-template tracts (i.e. VTA-dlPFC, VTA-mOFC, VTA-vlPFC, VTA-ACC, VTA-NAc and SN-putamen) were transformed back to each participant’s diffusion space. An ROI of the residual WM, designated as the WM reference region, was created by excluding the six group common tracts from the total WM mask in individual’s diffusion space. Finally, the mean values of MD-t and FW of six tracts were calculated. Additionally, we extracted the mean values of MD-t and FW of the residual WM mask as a reference. We prioritized the MD-t and FW indices over other diffusion indices since the MD index is less affected by crossing fibres, and FW determines diffusion alterations in SVD, as reported in previous studies.39,54
Statistical analysis
Continuous variables were presented by either mean (standard deviation, SD) or median (interquartile ranges, IQRs), based on their distribution.
To examine the effects of SVD burden on the disruption of five specified tracts (i.e. VTA-dlPFC, VTA-mOFC, VTA-vlPFC, VTA-ACC and VTA-NAc) within meso-cortical and meso-limbic pathways, the inter-correlations between mean MD-t or FW values of these tracts and WM reference regions, and SVD MRI markers, in addition to normalized brain volumes, were assessed across the total sample using Kendall rank correlation coefficient.
To test the associations between the disruption of five specified tracts and the three clinical features (i.e. cognitive function, apathy and gait performance), we performed linear regression analyses. Six clinical measures (processing speed, executive function, memory, apathy, TUG time and TUG steps) for three features were used as dependent variables in separate models, with mean MD-t or FW values across the five tracts serving as independent variables. Adjustments were made for age, sex, education, modified CESD score, the corresponding diffusion index from the WM reference mask, SVD MRI markers and normalized brain volumes. The diffusion index of WM reference mask was included as a control representing the global white matter damages, which is essential to test the specificity of any effects for the meso-cortical and meso-limbic pathways.52
To determine the potential common factors underlying cognitive function, apathy, and gait performance, we performed principal component analysis (PCA) on all clinical measures using ‘psych’ package in R.55,56 First, Pearson’s correlation analyses were performed among all pairs of the six clinical measures to explore their potential inter-correlations. Next, PCA was applied to the six clinical measures without rotation. The Kaiser–Meyer–Olkin (KMO) test examined the sampling adequacy, and Bartlett’s test of sphericity assessed whether the correlations among the six measures were sufficiently large in the PCA.57 KMO values >0.6 indicate the sampling is adequate for PCA.57 Kaiser’s criterion (>1) was used to define the number of components that were retained in the subsequent analysis.58 The clinical measures with a factor loading ≥0.30 on a given component were considered as relevant.59 Finally, each participant’s factor values of preserved PCA components were computed and used as dependent variables in the linear regression model. The independent variables and adjustments were the same as in the above linear regression models.
To identify the relative importance of meso-cortical and meso-limbic tracts, SVD burden and brain atrophy on clinical outcomes, we included mean MD-t and FW of the five tracts into separate random forest analysis models. SVD MRI markers, normalized brain volumes, the corresponding diffusion measure from WM reference mask were also included as predictors in these random forest models. The preserved PCA components and six clinical outcome measures were respectively assigned as outcome variables in these models. Subsequently, relative importance of each predictor was assessed using the percentage increase in mean squared error (%IncMSE) index through the ‘randomForest’ package in R,60,61 as described in previous studies.52 This %IncMSE index was determined through a perturbation approach, where the predictors’ values are permuted to assess their impact on model accuracy.61 The selection of random forest approach over other regression models was justified by its ability to handle multicollinearity and complex non-linear relations within these models.60
For the linear regression models, multicollinearity was assessed using variance inflation factors (VIFs) and the correlation matrix of independent variables, with a VIF value ≥5 or a correlation coefficient between any pair of independent variables ≥0.7, indicating strong multicollinearity.62 When non-normally distributed data were used as response variables, the Yeo–Johnson approach was employed to approximate a normal distribution in R.63
All statistical analyses were performed using R software (version 4.1.1) and alpha was set at 0.05 (two-tailed). The Bonferroni method was employed to correct for multiple comparisons.
Sensitivity analyses
First, to confirm the specificity of these associations between tracts within meso-cortical and meso-limbic pathways and the three clinical features, additional analyses were conducted. We assessed the associations between the diffusion features values of SN-putamen tract within nigrostriatal pathway and the six clinical measures, as well as the association with the diffusion features values of SN-putamen tract and the PCA-derived common component (i.e. PC1). The SN-putamen tract was selected as a control due to its primary association with motor function, as opposed to cognitive or mood function, and its anatomical proximity to the caudal part of meso-cortical pathways.
Furthermore, given the established effects of frontal WM on cognitive function, apathy and gait performance,15,64,65 we employed another more specific frontal WM reference mask as the reference region. This frontal WM reference region was obtained by segregating the frontal area (derived from AAL atlas) from the whole white matter mask after omitting the five meso-cortical and meso-limbic tracts. Next, we calculated the mean MD-t and FW values within this delineated region. Subsequently, we re-assessed the associations between diffusion measures of the five tracts within meso-cortical and meso-limbic pathways and the six clinical measures, as well as PC1 (i.e. the PCA-derived first principal component), while adjusting for the corresponding diffusion measures of the frontal white matter reference region, along with other covariates in the primary analysis.
Second, to ensure comprehensive exploration beyond PC1 and reduce the risk of overlooking potentially interesting components in our PCA methodology, we employed an alternative principal component selection approach. That is, selecting the number of components that generate a cumulative sum of explained variance over 80%.66 We further assessed the associations between the preserved PCA components and the diffusion measures of five specified tracts within meso-cortical and meso-limbic pathways.
Results
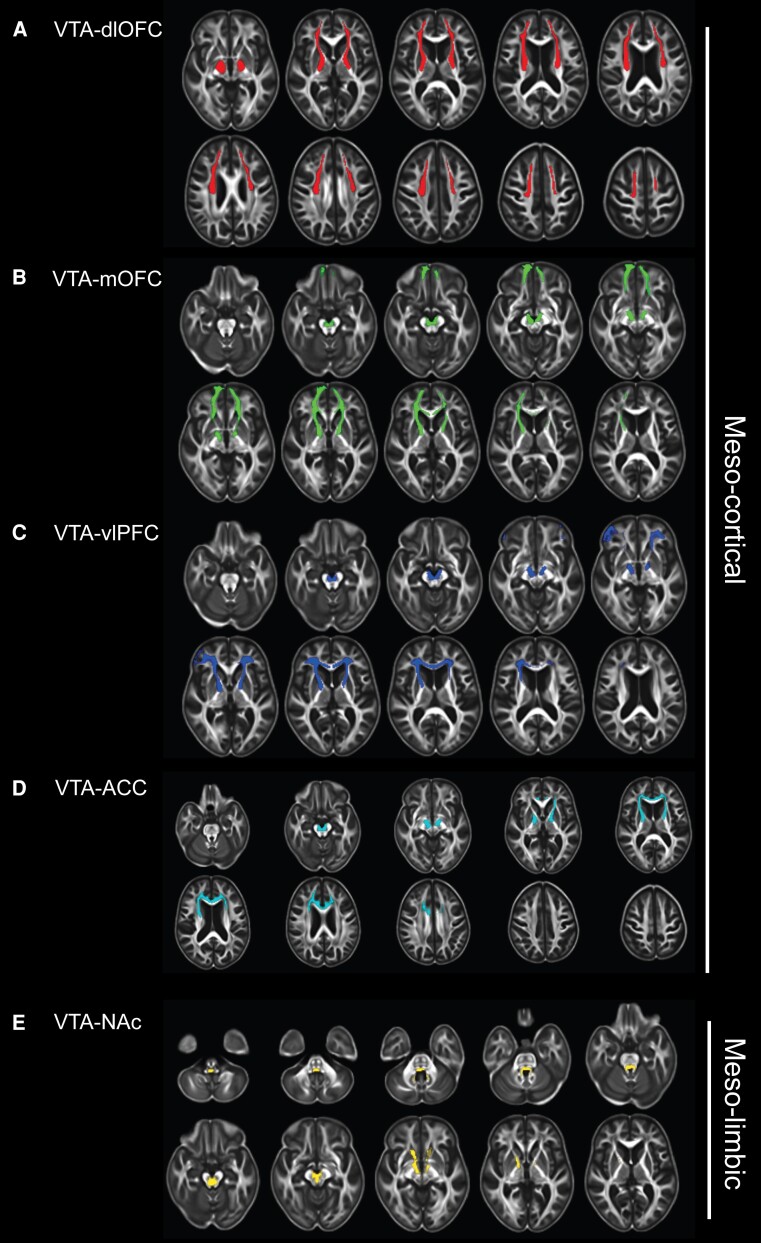
A total of 213 participants were included in the present study, with a median age of 73.0 years (IQR 69.0–79.0); 96 were female (45.1%). Detailed information on demographic data, cognitive function and neuroimaging characteristics is provided in Table 1 and Supplementary Table 3. The five common representative tracts of meso-cortical and meso-limbic white matter pathways, along with their mean MD-t and FW values, are shown in Fig. 1 and Supplementary Table 4.
Table 1.
Demographic, clinical and imaging characteristics of the study cohort
| N = 213 | |
|---|---|
| Demographic | |
| Age, years, median (IQR) | 73.0 (69.0–79.0) |
| Female, n (%) | 96 (45.1%) |
| Education, years, median (IQR) | 10.0 (10.0–15.0) |
| Vascular risk factors | |
| Hypertension, n (%) | 141 (66.2%) |
| Diabetes, n (%) | 31 (14.6%) |
| Hypercholesterolaemia, n (%) | 116 (54.5%) |
| Smoking history, n (%) | 136 (63.8%) |
| Clinical diagnosis | |
| Vascular dementia, n (%) | 4 (1.9%) |
| Vascular parkinsonism, n (%) | 9 (4.2%) |
| Neuropsychological and gait assessment | |
| MMSE, median (IQR) | 29 (27–30) |
| Processing speeda, median (IQR) | 0.2 (−0.5–0.7) |
| Executive functiona, median (IQR) | 0.1 (−0.4–0.6) |
| Memorya, median (IQR) | 0.0 (−0.7–0.6) |
| AES-score, median (IQR) | 31.0 (26–35) |
| Apathyb, n (%) | 65 (30.5%) |
| Modified CESD score, 18-item, median (IQR) | 5.0 (1.0–11.0) |
| CESD score, 20-item, median (IQR) | 7.0 (2.0–12.0) |
| Depressionc, n (%) | 31 (14.6%) |
| TUG-time, seconds, median (IQR) | 10.2 (9.3–13.1) |
| TUG-step, median (IQR) | 13.7 (12.3–15.0) |
| SVD markers | |
| WMH volumes, ml, median (IQR) | 4.3(1.8–12.1) |
| Normalized WMH, %, median (IQR) | 0.3 (0.1–0.8) |
| Lacunes, n (%) | 58 (27.2%) |
| CMBs, n (%) | 77 (36.2%) |
| Brain volume, ml, mean (SD) | 1084.1 (116.8) |
Modified Centre for Epidemiologic Studies Depression Scale (CSED) score = original 20-item CESD excluding two items related to motivation. AES = Apathy Evaluation Scale; CMBs = cerebral microbleeds; IQR = interquartile range; MMSE = Mini-Mental State Examination; SD = standard deviation; SVD = small vessel disease; TUG = Timed Up and Go test; WMH = white matter hyperintensities.
aScores were reported as z-score.
bParticipants with an AES score ≥34 considered as having apathy.
cParticipants with a 20-item CESD ≥16 considered as having depression.
Figure 1.
Meso-cortical and meso-limbic white matter pathways. (A)The ventral tegmental area (VTA)-dorsolateral prefrontal cortex (dlPFC) tract; (B) The VTA–medial orbitofrontal cortex (mOFC) tract; (C) The VTA-ventral lateral PFC (vlPFC) tract; (D) The VTA-anterior cingulate cortex (ACC) tract; and (E) The VTA-nucleus accumbens (NAc) tract. Meso-cortical pathways include the VTA-dlPFC, VTA-mOFC and VTA-vlPFC tracts; the meso-limbic pathway is referred to as the VTA-NAc tract.
Cerebral small vessel disease burden and meso-cortical and meso-limbic pathways
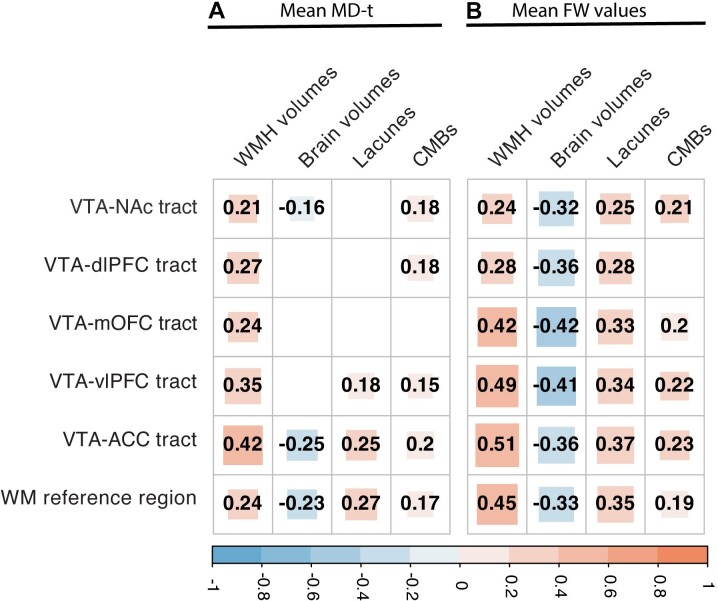
In the correlation analysis, SVD MRI markers (i.e. WMH volumes, the presence of lacunes and CMBs) and normalized brain volumes showed significant correlations with FW values across five specific tracts within the meso-cortical and meso-limbic pathways (i.e. VTA-dlPFC, VTA-mOFC, VTA-vlPFC, VTA-ACC and VTA-NAc tracts) and the WM reference regions. The only exception was the lack of correlation between the presence of CMBs and FW values of the VTA-dlPFC tract. In contrast, the correlations between SVD burden and MD-t values of these tracts and WM reference regions were markedly weaker (Fig. 2).
Figure 2.
Inter-correlation analyses among mean free water (FW) and FW-corrected mean diffusivity values of five tracts of interest and white matter reference regions, small vessel disease MRI markers and brain atrophy. (A) Correlation coefficients of FW-corrected mean diffusivity (MD-t) values. (B) Correlation coefficients of FW values. White matter hyperintensities (WMH) and brain volumes were normalized to intracranial volumes; all numbers within squares represent correlation coefficients (P-corrected <0.05), blank means no statistically significant results (P-corrected >0.05). CMBs = cerebral microbleeds.
Relation between disruption of meso-cortical, meso-limbic pathways and clinical outcomes
MD-t values of these tracts showed no associations with clinical measures. By contrast, higher FW values of the VTA-dlPFC tract were related to worse memory performance, higher AES scores and higher step number of TUG test; higher FW values of the VTA-mOFC tract were related to worse executive function and worse memory performance; higher FW values of the VTA-vlPFC and VTA-ACC tract were both related to slower processing speed, worse executive function and worse memory performance (all P-corrected values < 0.05; Table 2).
Table 2.
Relation between free water-corrected mean diffusivity and free water values of meso-cortical and meso-limbic pathways and cognitive function, apathy and gait performances
| VTA-dlPFC | VTA-mOFC | VTA-vlPFC | VTA-ACC | VTA-NAc | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (SE) | P-corrected | β (SE) | P-corrected | β (SE) | P-corrected | β (SE) | P-corrected | β (SE) | P-corrected | |
| Models for free water-corrected mean diffusivity | ||||||||||
| Processing speed | −0.02 (0.06) | 1.000 | −0.04 (0.08) | 1.000 | −0.14 (0.07) | 0.342 | −0.10 (0.08) | 1.000 | −0.05 (0.06) | 1.000 |
| Executive function | −0.02 (0.06) | 1.000 | −0.03 (0.07) | 1.000 | −0.03 (0.08) | 1.000 | 0.00 (0.07) | 1.000 | −0.04 (0.07) | 1.000 |
| Memory | 0.05 (0.06) | 1.000 | −0.04 (0.06) | 1.000 | −0.03 (0.06) | 1.000 | −0.06 (0.07) | 1.000 | −0.05 (0.05) | 1.000 |
| AES-score | −0.09 (0.06) | 0.833 | 0.00 (0.07) | 1.000 | 0.05 (0.06) | 1.000 | 0.17 (0.11) | 0.774 | 0.14 (0.07) | 0.305 |
| TUG-step | −0.13 (0.06) | 0.225 | −0.15 (0.07) | 0.197 | −0.05 (0.08) | 1.000 | 0.02 (0.06) | 1.000 | 0.10 (0.06) | 0.422 |
| TUG-time | −0.08 (0.06) | 1.000 | −0.03 (0.05) | 1.000 | −0.01 (0.06) | 1.000 | −0.01 (0.06) | 1.000 | 0.03 (0.06) | 1.000 |
| Models for free water | ||||||||||
| Processing speed | −0.15 (0.07) | 0.140 | −0.25 (0.10) | 0.070 | −0.31 (0.10) | 0.016 | −0.39 (0.11) | 0.003 | −0.13 (0.06) | 0.291 |
| Executive function | −0.11 (0.07) | 0.629 | −0.33 (0.10) | 0.011 | −0.42 (0.11) | 0.001 | −0.32 (0.10) | 0.010 | −0.16 (0.08) | 0.234 |
| Memory | −0.19 (0.07) | 0.028 | −0.29 (0.10) | 0.028 | −0.42 (0.10) | <0.001 | −0.46 (0.10) | <0.001 | −0.10 (0.06) | 0.787 |
| AES-score | 0.24 (0.07) | 0.005 | 0.16 (0.09) | 0.361 | 0.19 (0.10) | 0.327 | 0.23 (0.10) | 0.180 | −0.07 (0.12) | 1.000 |
| TUG-step | 0.20 (0.07) | 0.036 | 0.10 (0.12) | 1.000 | 0.00 (0.11) | 1.000 | 0.00 (0.11) | 1.000 | −0.05 (0.06) | 1.000 |
| TUG-time | 0.14 (0.07) | 0.260 | 0.07 (0.08) | 1.000 | 0.10 (0.09) | 1.000 | 0.13 (0.08) | 0.739 | 0.03 (0.05) | 1.000 |
Meso-cortical pathways included the ventral tegmental area (VTA)-dorsolateral prefrontal cortex (dlPFC), VTA-medial orbitofrontal cortex (mOFC), VTA-ventral lateral PFC (vlPFC) and VTA–anterior cingulate cortex (ACC) tracts; the meso-limbic pathway is referred to as the VTA-nucleus accumbens (NAc) tract. All models were adjusted for age, sex, education years, modified Centre for Epidemiologic Studies Depression Scale (CESD) score, the corresponding diffusion measures of white matter reference regions, small vessel disease (SVD) MRI markers and normalized brain volumes. β-Values are reported as the standardized β-values and standard error (SE). P-values were corrected for multiple comparisons (n = 6) using the Bonferroni method; P-corrected values in bold represent < 0.05. AES = Apathy Evaluation Scale; TUG = Timed Up and Go test.
Common factors underlying cognition, apathy and gait
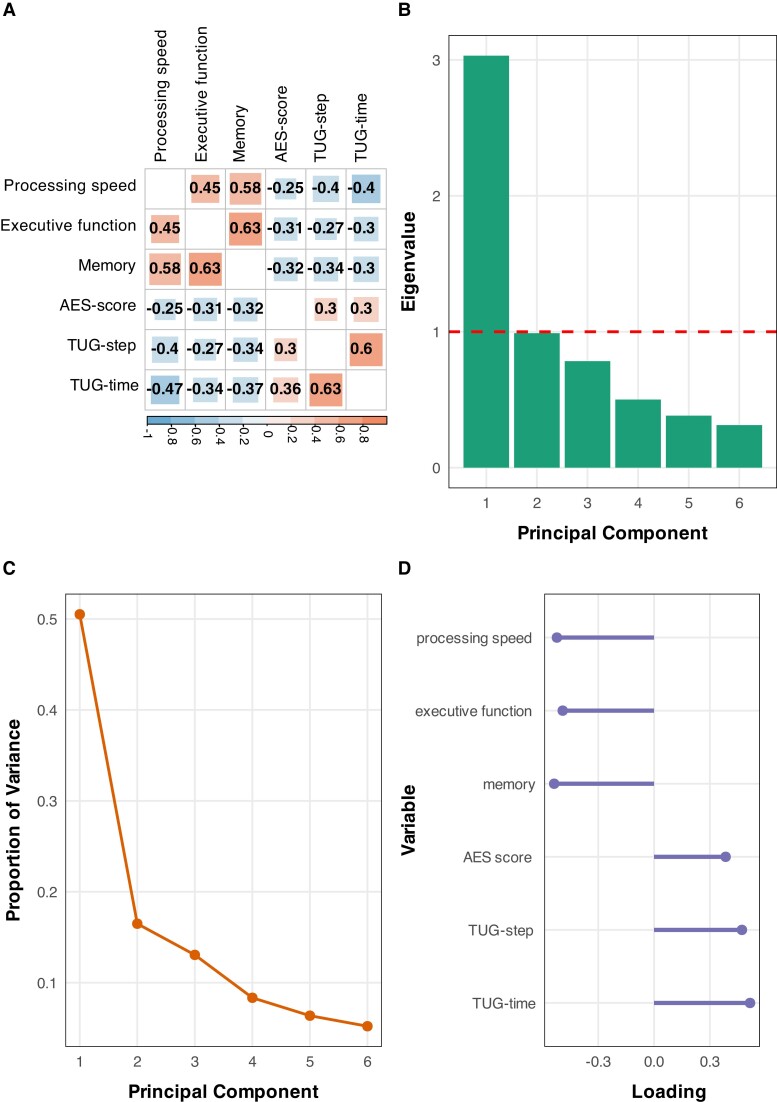
Pearson’s correlation analyses showed significant associations between each pair among the six clinical outcome measures (all P-corrected values < 0.05; Fig. 3A). This result suggests the existence of shared factors underlying the three clinical features (i.e. cognition, apathy, and gait), thus justifying subsequent PCA to identify the common components.
Figure 3.
Principal component analysis for the six clinical measures of cognition, apathy and gait. (A) Inter-correlation analyses among six clinical measures. Numbers within squares represent correlation coefficients (P-corrected <0.05); P-values were corrected using the Bonferroni method. (B) Eigenvalue of each principal component analysis (PCA)-derived PC. The PC with an eigenvalue value >1 (red line) was preserved. (C) The proportion of variance explained by any given PC. (D) The factor loading of each original clinical measure to the preserved PC (i.e. the first PC, PC1). AES = Apathy Evaluation Scale; TUG = Timed Up and Go test.
The KMO test showed a value of 0.772 and Bartlett’s test of sphericity was significant (P < 0.001). This result confirmed the appropriateness of conducting a factor analysis. Therefore, PCA was performed and identified a total of six components in the data. The first component (PC1) had eigenvalues above Kaiser’s criterion of 1 and explained 50.52% of the total variance (Fig. 3B and C). All six clinical outcome measures showed meaningful loadings on the first component (all factor loadings > 0.3; Fig. 3D). The direction of associations between each clinical outcome measure and PC1 provided excellent clinical interpretability, that is, higher PC1 values suggesting worse performances in the common factors of cognition, apathy and gait.
Linear regression analyses showed that higher FW values of VTA-dlPFC, VTA-mOFC, VTA-vlPFC and VTA-ACC tracts were related to higher PC1 values (all P-corrected values <0.05; Table 3).
Table 3.
Relation between free water-corrected mean diffusivity and free water values of meso-cortical and meso-limbic pathways and preserved PCA components
| PC1 | ||
|---|---|---|
| β (SE) | P-corrected | |
| Free water-corrected mean diffusivity | ||
| VTA-dlPFC | −0.13 (0.09) | 0.993 |
| VTA-mOFC | −0.04 (0.09) | 1.000 |
| VTA-vlPFC | 0.04 (0.10) | 1.000 |
| VTA-ACC | 0.04 (0.09) | 1.000 |
| VTA-NAc | 0.12 (0.09) | 1.000 |
| Free water | ||
| VTA-dlPFC | 0.37 (0.09) | 0.001 |
| VTA-mOFC | 0.40 (0.14) | 0.034 |
| VTA-vlPFC | 0.64 (0.14) | <0.001 |
| VTA-ACC | 0.51 (0.13) | <0.001 |
| VTA-NAc | 0.13 (0.10) | 1.000 |
Meso-cortical pathways include the ventral tegmental area (VTA)-dorsolateral prefrontal cortex (dlPFC), VTA-medial orbitofrontal cortex (mOFC) and VTA-ventral lateral PFC (vlPFC) tracts; the meso-limbic pathway is referred to as the VTA-nucleus accumbens (NAc) tract. All models were adjusted for age, sex, education years, modified Centre for Epidemiologic Studies Depression Scale (CESD) score, the corresponding diffusion measures of white matter reference regions, cerebral small vessel disease (SVD) MRI markers and normalized brain volumes. β-Values are reported as the standardized β-values and standard error (SE). P-values were corrected using the Bonferroni method; P-corrected values in bold represent < 0.05. PCA = principal component analysis; PC1 = principal component 1.
Relative importance of each neuroimaging markers on clinical outcomes
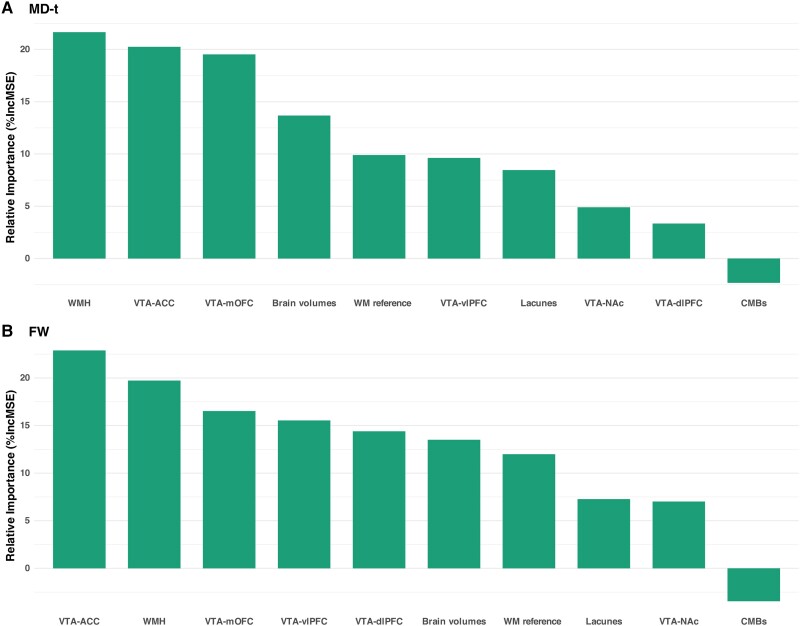
In the analyses for PC1, FW values of VTA-ACC tract showed the highest contribution compared to all other imaging features, including the FW values of VTA-dlPFC, VTA-mOFC, VTA-vlPFC, VTA-NAc tracts and WM reference regions, SVD MRI markers and normalized brain volumes (Fig. 4). Similar findings were found for each clinical measurement (i.e. processing speed, executive function, memory, AES-score, TUG-step and TUG-time; Supplementary Figs 4 and 5).
Figure 4.
Relative importance of imaging markers on the first principal component. (A) Free water (FW)-corrected mean diffusivity (MD-t) values of ventral tegmental area (VTA)-anterior cingulate cortex (ACC) tract showed a high contribution to the first principal component (PC1). (B) FW values of the VTA-ACC tract contributed most to PC1 among all imaging markers. %IncMSE is the percentage increase in mean squared error, it is calculated through a perturbation approach, where the predictors’ values are permuted to assess its impact on model accuracy. CMBs = cerebral microbleeds; dlPFC = dorsolateral prefrontal cortex; mOFC = medial orbitofrontal cortex; NAc = nucleus accumbens; vlPFC = ventral lateral prefrontal cortex; WMH = white matter hyperintensities.
Sensitivity analyses
In the analyses for nigrostriatal pathway, only an association between increased FW in the SN-putamen tract and worse executive function (β=0.26, P-corrected = 0.023) was found (Supplementary Table 5).
Associations between the diffusion features of the five tracts within the meso-cortical and meso-limbic pathways and the six clinical measures, alongside PC1, were consistent with our initial findings while adjusting for the corresponding diffusion measures of the frontal white matter reference region (Supplementary Table 6). Notably, the FW values of the VTA-ACC tract consistently showed the greatest contribution to PC1 across all examined imaging features (Supplementary Fig. 6).
Using a different approach for component selection, the combination of first three components (PC1, PC2 and PC3) explained 80.06% of the total variance (Supplementary Table 7). PC2 was mainly loaded by executive function, memory, TUG step, and TUG time; PC3 was mainly loaded by processing speed and AES score (Supplementary Table 7). Furthermore, we found no significant associations between PC2 or PC3 and the diffusion measures of the five specific tracts within the meso-cortical and meso-limbic pathways (Supplementary Table 8).
Discussion
In the present study, we found that: (i) higher MD-t and FW values of the meso-cortical and meso-limbic WM pathways were correlated with higher SVD burden, with FW showing notably stronger correlations; (ii) higher FW values of four tracts within meso-cortical pathway showed associations with clinical measures both in cognitive function, apathy and gait, whereas MD-t values of these tracts were not associated with these clinical measures; (iii) the six clinical measures (encompassing cognitive function, apathy and gait function) were strongly related to each other and collectively informed a PCA-derived common and meaningful component, which is hereafter termed as composite performances in cognition, apathy and gait; and (iv) the FW values of four tracts within meso-cortical pathway demonstrated significant associations with the composite performances in cognition, apathy and gait, with FW values of VTA-ACC tract identified as the strongest contributor. Taken together, our results suggested that cognitive impairment, apathy, and gait dysfunction shared some common pathophysiological substrates and that the white matter damage of the meso-cortical pathway in SVD, potentially serves as their common neural basis.
We found varied correlations between SVD MRI markers and the diffusion measures across meso-cortical and meso-limbic pathways, as well as the WM reference regions. Notably, the FW index showed remarkable stronger correlations compared to the MD-t index. Furthermore, the FW values of these tracts were related to clinical measures, whereas the MD-t index showed no such relations. This result is not surprising since the previous study has reported that SVD-related DTI changes (i.e. decrease in FA and increase in MD) were predominantly attributed to the increased FW, rather than the tissue compartment changes (i.e. FA-t and MD-t).39 In addition, they also reported that FW showed stronger associations with clinical deficits than MD-t.39 These findings suggested that FW measures have the potential to serve as a better marker for assessing the progression in SVD burden and clinical deficits than conventional diffusional measures.
We found significant associations between FW values of meso-cortical pathway and the clinical measures in both cognition, apathy, and gait. This finding suggested that damage to the meso-cortical pathways may serve as the common neural foundation for cognitive impairment, apathy, and gait dysfunction in SVD. So far, to the best of our knowledge, no study has investigated the role of dopaminergic meso-cortical or meso-limbic pathways in SVD. Despite the lack of pre-existing evidence in SVD, studies in other brain disorders, notably Alzheimer’s disease and Parkinson’s disease, have shown that crucial regions within the meso-cortical and meso-limbic pathways (mainly VTA, NAc, and PFC) undergo a range of alterations. These alterations include volume loss, altered inter-connectivity both functionally and structurally, changes in dopaminergic function of presynaptic terminals, and post-synaptic dopamine receptor levels.67,68 Such abnormalities have been associated, to varying degrees, with cognitive function, neuropsychiatric symptoms and motor symptoms in Alzheimer’s disease and Parkinson’s disease.67,68
Interestingly, our findings suggested that apathy was associated with deficits in the meso-cortical pathway, as opposed to the mesolimbic pathway that traditionally linked with reward and emotion regulation. A previous study demonstrated that apathy in SVD was associated with deficits in effort-based decision making,69 a process that is regarded as determined by the meso-cortical pathway.70 These findings indicated that apathy in SVD is more aligned with cognitive deficits than solely with emotional regulation dysfunction. In addition, we found that higher FW values of SN-putamen tract were related to executive function, but not to memory performance and PC1. This could be explained by the effects of motor impairment on the executive function task.
Another crucial finding in this study is the PCA-derived common component (PC1) underlying cognitive impairment, apathy, and gait dysfunction in SVD. Note that the direction of associations between each clinical measure and PC1 provided excellent clinical interpretability. In contrast, the second and third principal components derived from PCA (PC2 and PC3) presented ambiguous associations with the clinical outcome measures. Although further validation is required in another independent dataset, our findings suggested that PC1 successfully captured the predominant common components underlying cognitive impairment, apathy, and gait dysfunction in this present SVD population. The presence of this PCA-derived common component, along with the high inter-correlations among these measures for the three clinical features, strongly suggest that the three clinical features of SVD were inter-related and shared some common mechanisms. This finding is in line with previous studies that showed that cognitive impairment is associated with either apathy or gait dysfunction,7,8,71 while also offering a deeper exploration on the inter-relations of the three clinical features in SVD. Furthermore, this PCA-derived common component represents the composite performances in cognition, apathy and gait and could potentially be employed as a comprehensive metric for clinical status assessment in SVD. However, further validations in independent cohorts are required.
Furthermore, we found that deficits in meso-cortical pathways were associated with composite impairments in cognition, apathy and gait. Moreover, damage to VTA-ACC tract showed the highest contribution to the composite performance in cognitive impairment, apathy, and gait, exceeding the effects of other neuroimaging features. This result supported that the damage in meso-cortical pathways could be the shared underlying neural foundation for cognitive impairment, apathy and gait dysfunction in SVD. In addition, this result remained consistent when we employed the frontal WM reference mask, instead of the whole WM reference mask, as the reference region. This finding reinforced the specificity of the effects of meso-cortical pathway deficits on clinical outcomes and our confidence in the interpretation of our results.
The strengths of this study lay in the comprehensive assessment of cognitive function, apathy, and gait performance in a substantial sample of SVD population (n = 213), combined with state-of-the-art neuroimaging approaches that identify five tracts of the meso-cortical and meso-limbic pathways and quantify the damage along these tracts. With the detailed clinical data of cognitive function, apathy and gait performances, we were able to capture the common component underlying the three clinical features of SVD through a bottom-up analysis approach (i.e. PCA).
Several limitations should be acknowledged. First, due to the change of MRI scanners, we could only use the data from the third follow-up of our cohort. While this provided access to the advanced and high-quality MRI scans, it limited us to performing only cross-sectional analyses. The temporal and causal relations between the observed meso-cortical and meso-limbic damages and clinical outcomes need further examination in longitudinal studies. Second, we lacked the age-matched healthy controls as a reference, making it difficult to distinguish our findings from the effects of normal ageing and the concurrent neurodegenerative pathologies. Although excluding participants diagnosed with non-vascular dementia and/or parkinsonism has minimized the effects from concurrent neurodegenerative pathologies, studies including healthy controls are still essential to more accurately elucidate the effects of SVD burden on damages to meso-cortical and meso-limbic pathways and subsequently on clinical outcomes. Third, the identification of these tracts within the meso-cortical and meso-limbic pathways was based solely on diffusion imaging. While the two pathways are predominantly innervated by dopaminergic neurons, other neurotransmitters, such as glutamatergic, are also involved. Hence, caution is required to interpret the identified meso-cortical and meso-limbic pathways in this study strictly as dopaminergic. Subsequent studies employing dopamine-specific PET imaging could facilitate identifying these dopaminergic-specific tracts within meso-cortical and meso-limbic system, thereby validating our findings. Fourth, given the strong correlations among the six clinical measures and the multicollinearity observed among certain tracts, it is possible that the observed associations between FW values of these tracts within the meso-cortical pathway and clinical measures are driven by statistical correlations rather than clinically meaningful relationships. Although our results are supported by not only statistical analyses but also neurobiological evidence from prior studies, further investigation into the temporal or causal associations between changes in specific tracts and clinical outcomes is necessary to validate our findings. Fifth, the specificity of the observed effects of the meso-cortical pathway was mainly examined by adjusting for diffusion index of (frontal) WM reference region. It is still possible that other tracts, such as the cholinergic pathways or thalamus-cortex projections, might also constitute a shared neural basis for the three clinical features of SVD. Notably, disruption in the cholinergic system has been associated with cognitive impairment, apathy, and gait dysfunction in Parkinson’s disease.72-74 Further research is needed to investigate the impact of cholinergic pathway disconnections on these symptoms in SVD. In addition, studies employing a non-hypothesis-driven approach, such as lesion-symptom mapping, could also provide further insights into this question.
Conclusion
In conclusion, these findings of our study suggested that the three neurobehavioural symptoms (cognitive impairment, apathy and gait dysfunction) of SVD are strongly related to each other and that the damage in the meso-cortical pathway could be the common neural basis underlying these three clinical features. These findings advance our understanding of the mechanisms of the primary clinical features in SVD and have the potential to inform novel management and intervention strategies for SVD.
Supplementary Material
Contributor Information
Hao Li, Department of Neurology, Radboud Institute for Medical research and Innovation and Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, 6525 GA Nijmegen, The Netherlands.
Mina A Jacob, Department of Neurology, Radboud Institute for Medical research and Innovation and Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, 6525 GA Nijmegen, The Netherlands.
Mengfei Cai, Department of Neurology, Radboud Institute for Medical research and Innovation and Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, 6525 GA Nijmegen, The Netherlands; Department of Neurology, Guangdong Neuroscience Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Southern Medical University, 510000 Guangzhou, China.
Roy P C Kessels, Radboud University, Donders Institute for Brain, Cognition and Behaviour, Centre for Cognition, Radboud University, 6525 GD Nijmegen, The Netherlands; Department of Medical Psychology and Radboudumc Alzheimer Center, Radboud University Medical Center, 6525 GA Nijmegen, The Netherlands; Centre of Excellence for Korsakoff and Alcohol-Related Cognitive Disorders, Vincent van Gogh Institute for Psychiatry, 5804 AV Venray, The Netherlands.
David G Norris, Donders Institute for Brain, Cognition and Behaviour, Centre for Cognitive Neuroimaging, Radboud University, 6525 GD Nijmegen, The Netherlands.
Marco Duering, Medical Image Analysis Center (MIAC AG) and Department of Biomedical Engineering, University of Basel, 4051 Basel, Switzerland; Institute for Stroke and Dementia Research (ISD), LMU University Hospital, 81377 LMU Munich, Germany.
Frank-Erik de Leeuw, Department of Neurology, Radboud Institute for Medical research and Innovation and Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, 6525 GA Nijmegen, The Netherlands.
Anil M Tuladhar, Department of Neurology, Radboud Institute for Medical research and Innovation and Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, 6525 GA Nijmegen, The Netherlands.
Data availability
The data that support the findings of this study are available from the corresponding author, depending on reasonable request from qualified investigators after permission of the appropriate regulatory bodies.
Funding
This work was supported by the China Scholarship Council (No 202106380078 to H.L.), Dutch Brain Foundation personal fellowship (H04-12; F2009(1)-16 to F.-E.d.L.), VIDI innovational grant from The Netherlands Organization for Health Research and Development (ZonMw grant 016.126.351 to F.-E.d.L.) and the Netherlands Cardiovascular Research Initiative: The Dutch Heart Foundation (CVON 2018-28 and 2012-06 Heart Brain Connection to A.M.T.). A.M.T. is a junior staff member of the Dutch Heart Foundation (grant number 2016T044).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Duering M, Biessels GJ, Brodtmann A, et al. Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet Neurol. 2023;22:602–618. [DOI] [PubMed] [Google Scholar]
- 2. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019;18:684–696. [DOI] [PubMed] [Google Scholar]
- 3. Clancy U, Gilmartin D, Jochems ACC, Knox L, Doubal FN, Wardlaw JM. Neuropsychiatric symptoms associated with cerebral small vessel disease: A systematic review and meta-analysis. Lancet Psychiatry. 2021;8:225–236. [DOI] [PubMed] [Google Scholar]
- 4. Sharma B, Wang M, McCreary CR, Camicioli R, Smith EE. Gait and falls in cerebral small vessel disease: A systematic review and meta-analysis. Age Ageing. 2023;52:afad011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hachinski V, Finger E, Pieruccini-Faria F, Montero-Odasso M. The apathy, gait impairment, and executive dysfunction (AGED) triad vascular variant. Alzheimers Dement. 2022;18:1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li H, Cui L, Wang M, et al. Apathy is associated with striatal atrophy and cognitive impairment in cerebral small vessel disease. J Affect Disord. 2023;328:39–46. [DOI] [PubMed] [Google Scholar]
- 7. Tay J, Morris RG, Tuladhar AM, Husain M, de Leeuw FE, Markus HS. Apathy, but not depression, predicts all-cause dementia in cerebral small vessel disease. J Neurol Neurosurg Psychiatry. 2020;91:953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lohner V, Brookes RL, Hollocks MJ, Morris RG, Markus HS. Apathy, but not depression, is associated with executive dysfunction in cerebral small vessel disease. PLoS One. 2017;12:e0176943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai M, Jacob MA, Norris DG, de Leeuw FE, Tuladhar AM. Longitudinal relation between structural network efficiency, cognition, and gait in cerebral small vessel disease. J Gerontol A Biol Sci Med Sci. 2022;77:554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cai M, Jacob MA, Norris DG, Duering M, de Leeuw FE, Tuladhar AM. Cognition mediates the relation between structural network efficiency and gait in small vessel disease. Neuroimage Clin. 2021;30:102667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moretti R, Cavressi M, Tomietto P. Gait and apathy as relevant symptoms of subcortical vascular dementia. Am J Alzheimers Dis Other Demen. 2015;30:390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinstein AM. Reward, motivation and brain imaging in human healthy participants—A narrative review. Front Behav Neurosci. 2023;17:1123733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lisiecka-Ford DM, Tozer DJ, Morris RG, Lawrence AJ, Barrick TR, Markus HS. Involvement of the reward network is associated with apathy in cerebral small vessel disease. J Affect Disord. 2018;232:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tay J, Tuladhar AM, Hollocks MJ, et al. Apathy is associated with large-scale white matter network disruption in small vessel disease. Neurology. 2019;92:e1157–e1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chokesuwattanaskul A, Zanon Zotin MC, Schoemaker D, et al. Apathy in patients with cerebral amyloid angiopathy: A multimodal neuroimaging study. Neurology. 2023;100:e2007–e2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Le Heron C, Manohar S, Plant O, et al. Dysfunctional effort-based decision-making underlies apathy in genetic cerebral small vessel disease. Brain. 2018;141:3193–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Z, Xie Y, Dou K, Yang L, Xie A. Associations of striatal dopamine transporter binding with motor and non-motor symptoms in early Parkinson’s disease. Clin Transl Sci. 2023;16:1021–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung SJ, Yoo HS, Oh JS, et al. Effect of striatal dopamine depletion on cognition in de novo Parkinson’s disease. Parkinsonism Relat Disord. 2018;51:43–48. [DOI] [PubMed] [Google Scholar]
- 20. Costello H, Yamamori Y, Reeves S, Schrag AE, Howard R, Roiser JP. Longitudinal decline in striatal dopamine transporter binding in Parkinson’s disease: Associations with apathy and anhedonia. J Neurol Neurosurg Psychiatry. 2023;94:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilmskoetter J, Marebwa B, Basilakos A, et al. Long-range fibre damage in small vessel brain disease affects aphasia severity. Brain. 2019;142:3190–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lambert C, Benjamin P, Zeestraten E, Lawrence AJ, Barrick TR, Markus HS. Longitudinal patterns of leukoaraiosis and brain atrophy in symptomatic small vessel disease. Brain. 2016;139(Pt 4):1136–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Norden AG, de Laat KF, Gons RA, et al. Causes and consequences of cerebral small vessel disease. The RUN DMC study: A prospective cohort study. Study rationale and protocol. BMC Neurol. 2011;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacob MA, Cai M, Bergkamp M, et al. Cerebral small vessel disease progression increases risk of incident parkinsonism. Ann Neurol. 2023;93:1130–1141. [DOI] [PubMed] [Google Scholar]
- 25. Jacob MA, Cai M, van de Donk V, et al. Cerebral small vessel disease progression and the risk of dementia: A 14-year follow-up study. Am J Psychiatry. 2023;180:508–518. [DOI] [PubMed] [Google Scholar]
- 26. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res. 1991;38:143–162. [DOI] [PubMed] [Google Scholar]
- 27. Lanctôt KL, Ismail Z, Bawa KK, et al. Distinguishing apathy from depression: A review differentiating the behavioral, neuroanatomic, and treatment-related aspects of apathy from depression in neurocognitive disorders. Int J Geriatr Psychiatry. 2023;38:e5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodakowski J. Relationship among depression, gait disturbance, disability, and neurobiological abnormalities. Am J Geriatr Psychiatry. 2018;26:87–88. [DOI] [PubMed] [Google Scholar]
- 29. Podsiadlo D, Richardson S. The timed “up & go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 30. Li H, Jacob MA, Cai M, et al. Regional cortical thinning, demyelination, and iron loss in cerebral small vessel disease. Brain. 2023;146(11):4659–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shelhamer E, Long J, Darrell T. Fully convolutional networks for semantic segmentation. IEEE Trans Pattern Anal Mach Intell. 2017;39:640–651. [DOI] [PubMed] [Google Scholar]
- 32. Kellner E, Dhital B, Kiselev VG, Reisert M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med. 2016;76:1574–1581. [DOI] [PubMed] [Google Scholar]
- 33. Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. [DOI] [PubMed] [Google Scholar]
- 36. Tustison NJ, Avants BB, Cook PA, et al. N4ITK: Improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jbabdi S, Sotiropoulos SN, Savio AM, Graña M, Behrens TE. Model-based analysis of multishell diffusion MR data for tractography: How to get over fitting problems. Magn Reson Med. 2012;68:1846–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62:717–730. [DOI] [PubMed] [Google Scholar]
- 39. Duering M, Finsterwalder S, Baykara E, et al. Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimers Dement. 2018;14:764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carandini T, Mancini M, Bogdan I, et al. Disruption of brainstem monoaminergic fibre tracts in multiple sclerosis as a putative mechanism for cognitive fatigue: A fixel-based analysis. Neuroimage Clin. 2021;30:102587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Supekar K, Kochalka J, Schaer M, et al. Deficits in mesolimbic reward pathway underlie social interaction impairments in children with autism. Brain. 2018;141:2795–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Y, Wu IW, Buckley S, et al. Diffusion tensor imaging of the nigrostriatal fibers in Parkinson’s disease. Mov Disord. 2015;30:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pauli WM, Nili AN, Tyszka JM. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci Data. 2018;5:180063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Puonti O, Iglesias JE, Van Leemput K. Fast and sequence-adaptive whole-brain segmentation using parametric Bayesian modeling. Neuroimage. 2016;143:235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 46. van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM. Adolescent impatience decreases with increased frontostriatal connectivity. Proc Natl Acad Sci U S A. 2015;112:E3765–E3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yuan K, Zhao M, Yu D, et al. Striato-cortical tracts predict 12-h abstinence-induced lapse in smokers. Neuropsychopharmacology. 2018;43:2452–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 50. Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nemy M, Dyrba M, Brosseron F, et al. Cholinergic white matter pathways along the Alzheimer’s disease continuum. Brain. 2023;146:2075–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nemy M, Cedres N, Grothe MJ, et al. Cholinergic white matter pathways make a stronger contribution to attention and memory in normal aging than cerebrovascular health and nucleus basalis of Meynert. Neuroimage. 2020;211:116607. [DOI] [PubMed] [Google Scholar]
- 54. Dauguet J, Peled S, Berezovskii V, et al. Comparison of fiber tracts derived from in-vivo DTI tractography with 3D histological neural tract tracer reconstruction on a macaque brain. Neuroimage. 2007;37:530–538. [DOI] [PubMed] [Google Scholar]
- 55. Revelle WR. psych: Procedures for personality and psychological research. Software.2017.
- 56. Kaufmann BC, Cazzoli D, Pastore-Wapp M, et al. Joint impact on attention, alertness and inhibition of lesions at a frontal white matter crossroad. Brain. 2023;146:1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dziuban CD, Shirkey EC. When is a correlation matrix appropriate for factor analysis? Some decision rules. Psychol Bull. 1974;81:358. [Google Scholar]
- 58. Braeken J, Van Assen MA. An empirical Kaiser criterion. Psychol Methods. 2017;22:450–466. [DOI] [PubMed] [Google Scholar]
- 59. Hair J Jr, Anderson R, Tatham R, Black W. Multivariate data analysis. 5th ed. Prentice Hall; 1998. [Google Scholar]
- 60. Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 61. Strobl C, Boulesteix AL, Zeileis A, Hothorn T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinformatics. 2007;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Witten D, James G. An introduction to statistical learning with applications in R. Springer; 2013. [Google Scholar]
- 63. Yeo IK, Johnson RA. A new family of power transformations to improve normality or symmetry. Biometrika. 2000;87:954–959. [Google Scholar]
- 64. Brugulat-Serrat A, Salvadó G, Sudre CH, et al. Patterns of white matter hyperintensities associated with cognition in middle-aged cognitively healthy individuals. Brain Imaging Behav. 2020;14:2012–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134(Pt 1):73–83. [DOI] [PubMed] [Google Scholar]
- 66. Jolliffe I. Principal component analysis. In: Lovric M, ed. International encyclopedia of statistical science. Springer; 2011:1094–1096. [Google Scholar]
- 67. Krashia P, Spoleti E, D’Amelio M. The VTA dopaminergic system as diagnostic and therapeutical target for Alzheimer’s disease. Front Psychiatry. 2022;13:1039725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hirano S. Clinical implications for dopaminergic and functional neuroimage research in cognitive symptoms of Parkinson’s disease. Mol Med. 2021;27:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Saleh Y, Le Heron C, Petitet P, et al. Apathy in small vessel cerebrovascular disease is associated with deficits in effort-based decision making. Brain. 2021; 144:1247–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hauser TU, Eldar E, Dolan RJ. Separate mesocortical and mesolimbic pathways encode effort and reward learning signals. Proc Natl Acad Sci U S A. 2017;114:E7395–E7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Blumen HM, Jayakody O, Verghese J. Gait in cerebral small vessel disease, pre-dementia, and dementia: A systematic review. Int J Stroke. 2023;18:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ray NJ, Lawson RA, Martin SL, et al. Free-water imaging of the cholinergic basal forebrain and pedunculopontine nucleus in Parkinson’s disease. Brain. 2023;146:1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sperling SA, Druzgal J, Blair JC, Flanigan JL, Stohlman SL, Barrett MJ. Cholinergic nucleus 4 grey matter density is associated with apathy in Parkinson’s disease. Clin Neuropsychol. 2023;37:676–694. [DOI] [PubMed] [Google Scholar]
- 74. Morris R, Martini DN, Madhyastha T, et al. Overview of the cholinergic contribution to gait, balance and falls in Parkinson’s disease. Parkinsonism Relat Disord. 2019;63:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, depending on reasonable request from qualified investigators after permission of the appropriate regulatory bodies.