Abstract
Congenital myasthenic syndromes (CMS) are clinically and genetically heterogeneous diseases caused by mutations affecting neuromuscular transmission. Even if the first symptoms mainly occur during childhood, adult neurologists must confront this challenging diagnosis and manage these patients throughout their adulthood. However, long-term follow-up data from large cohorts of CMS patients are lacking, and the long-term prognosis of these patients is largely unknown.
We report the clinical features, diagnostic difficulties, and long-term prognosis of a French nationwide cohort of 235 adult patients with genetically confirmed CMS followed in 23 specialized neuromuscular centres. Data were retrospectively analysed.
Of the 235 patients, 123 were female (52.3%). The diagnosis was made in adulthood in 139 patients, 110 of whom presented their first symptoms before the age of 18. Mean follow-up time between first symptoms and last visit was 34 years [standard deviation (SD) = 15.1]. Pathogenic variants were found in 19 disease-related genes. CHRNE-low expressor variants were the most common (23.8%), followed by variants in DOK7 (18.7%) and RAPSN (14%). Genotypes were clustered into four groups according to the initial presentation: ocular group (CHRNE-LE, CHRND, FCCMS), distal group (SCCMS), limb-girdle group (RAPSN, COLQ, DOK7, GMPPB, GFPT1), and a variable-phenotype group (MUSK, AGRN). The phenotypical features of CMS did not change throughout life. Only four genotypes had a proportion of patients requiring intensive care unit admission that exceeded 20%: RAPSN (54.8%), MUSK (50%), DOK7 (38.6%) and AGRN (25.0%). In RAPSN and MUSK patients most ICU admissions occurred before age 18 years and in DOK7 and AGRN patients at or after 18 years of age. Different patterns of disease course (stability, improvement and progressive worsening) may succeed one another in the same patient throughout life, particularly in AGRN, DOK7 and COLQ. At the last visit, 55% of SCCMS and 36.3% of DOK7 patients required ventilation; 36.3% of DOK7 patients, 25% of GMPPB patients and 20% of GFPT1 patients were wheelchair-bound; most of the patients who were both wheelchair-bound and ventilated were DOK7 patients. Six patients died in this cohort. The positive impact of therapy was striking, even in severely affected patients.
In conclusion, even if motor and/or respiratory deterioration could occur in patients with initially moderate disease, particularly in DOK7, SCCMS and GFPT1 patients, the long-term prognosis for most CMS patients was favourable, with neither ventilation nor wheelchair needed at last visit. CHRNE-LE patients did not worsen during adulthood and RAPSN patients, often severely affected in early childhood, subsequently improved.
Keywords: genetic, neuromuscular junction, myasthenia, CMS
Theuriet et al. present long-term clinical data from adults with genetically defined congenital myasthenic syndromes. Most patients had a favourable long-term prognosis; however, motor and/or respiratory deterioration occurred in subgroups of patients, especially those with DOK7 mutations and slow-channel congenital myasthenic syndromes.
Introduction
Congenital myasthenic syndromes (CMS) are clinically and genetically heterogeneous diseases characterized by a neuromuscular transmission defect caused by mutations affecting the synaptic structure or function.1 Over recent decades, the molecular bases of CMS have expanded, and more than 35 genes have been associated with the disease.2-4 CMS is usually present at birth or during early childhood.5-8 However, the first symptoms can occur in adulthood.9 The reported prevalence of CMS, around 2.8–14.8/1 000 000, is likely underestimated, given the complexity of the diagnostic process, especially for mild or late-onset forms and those presenting with atypical or complex phenotypes.10-14 Indeed, the diagnosis can be particularly challenging in adult patients, in whom autoimmune myasthenia gravis (MG) is frequently considered in the first place due to its higher prevalence.9 Moreover, some CMS patients presenting with marked proximal weakness can easily be misdiagnosed with limb-girdle dystrophy or congenital myopathy.9 Clinical symptoms and severity range from mild ocular or bulbar symptoms, such as ptosis, diplopia or swallowing disturbances, to severe limb weakness leading to loss of ambulation.5 Decrement or increment evidenced on repetitive nerve stimulation (RNS) supports the neuromuscular transmission defect in these diseases. Moreover, electrophysiological features can help to orient the genetic diagnosis and validate the pathogenicity of variants.15 Like the phenotype, the response to treatment is also heterogeneous and depends on the underlying molecular mechanism and thus the precise genetic defect.1 For example, acetylcholinesterase (AChE) inhibitors can worsen the symptoms of patients with COLQ or slow-channel variants.16,17 Although the clinical spectrum of the different CMS subtypes keeps expanding,5-8 large cohorts of adult CMS patients with long-term follow-up and detailed clinical characteristics are lacking. In previous studies, the median follow-up time was frequently short (maximum 12.8 years) and/or the cohort size was small, without precise evaluation criteria.9,16,18,19 Yet these data are of the utmost importance to better define the long-term prognosis of CMS according to the genetic background and help neurologists to improve their management of adult patients.
By retrospectively analysing the clinical data of 235 adult patients with genetically confirmed CMS followed in 23 French specialized neuromuscular centres, we aimed to better define the long-term prognosis of these patients. We also aimed to determine the most common misdiagnoses to help clinicians better recognize this condition and to give straightforward treatment recommendations.
Materials and methods
Study design and population
This retrospective, observational, multicentre study included all adult patients followed for genetically confirmed CMS until July 2023 in the specialized neuromuscular centres of 23 University Hospitals in France (Amiens, Angers, Bordeaux, Brest, Caen, Clermont, Créteil, Grenoble, Limoges, Lille, Lyon, Marseille, Montpellier, Nancy, Nantes, Nice, Nîmes, Paris, Rennes, Rouen, Saint-Etienne, Strasbourg and Toulouse) within the French neuromuscular network FILNEMUS. All clinical data were collected anonymously from the study units’ medical files. All patients provided written informed consent for genetic tests and the use of their data for research purposes. All procedures involving patients performed in this study were carried out in accordance with the ethical standards of Assistance Publique des Hôpitaux de Paris (APHP ethics approval #20230524134437) and with the 1964 Helsinki declaration.
Clinical, laboratory and electrophysiological data
The demographic data collected included sex, ethnic origin, family history of CMS, mode of inheritance, consanguinity, age at first symptoms, age at clinical and molecular diagnoses and age at last follow-up visit. The patients were further classified according to age at onset of their symptoms in six subgroups: neonatal period, infancy (1–3 years), childhood (4–9 years), teenage (10–17 years), adulthood (18–40 years) and late onset (>40 years). Clinical data of interest included the presence of limb weakness, either proximal or distal, axial muscle deficit, facial weakness, fatigability, bulbar symptoms (including dysphonia and swallowing disturbances), ptosis, oculomotor disturbances, arthrogryposis, intellectual disability, delayed motor milestones, scoliosis, dyspnoea, need for ventilation, need for tube feeding, need for a wheelchair and need for admission to an intensive care unit (ICU). The Myasthenia Gravis Foundation of America (MGFA) score was collected when available. All these data were collected at disease onset and at the last follow-up visit. Electroneuromyography (ENMG) examinations were performed in each specialized neuromuscular centre by trained neurologists. We collected the presence of an RNS decrement or increment, post-effort increment and repetitive compound muscle action potential (R-CMAP). Creatine kinase (CK) values were also recorded and were considered elevated if above 200 UI/l. Lastly, we collected the type of treatment [AChE inhibitors, 3,4-diaminopyridine (3,4-DAP) quinidine, fluoxetine, ephedrine and salbutamol] and its efficacy according to the clinician in charge of the patients, based on patients’ feedback and clinical examination.
Genetic analyses
Until 2016, PCR and Sanger sequencing of CMS genes was used in a gene-after-gene approach. All exons and flanking intronic sequences of genes were PCR-amplified using patients’ genomic DNA and sequenced using the BigDye® Terminator v3.1 Cycle Sequencing kit (Applied Biosystems®, Life Technologies™). From 2016, next-generation sequencing (NGS) of CMS gene panels was used. Three panels (v2 2016–2017, v3 2017–2021 and v4 since 2021) were designed and successively used. NGS panel v2 targeted 25 CMS genes (AGRN, ALG14, ALG2, CHAT, CHRNA1, CHRNB1, CHRND, CHRNE, CHRNG, COLQ, DOK7, DPAGT1, GFPT1, LAMB2, LRP4, MUSK, PLEC, PREPL, RAPSN, SCN4A, SLC18A3, SLC25A1, SLC5A7, SNAP25, SYT2). NGS panel v3 targeted five additional genes (COL13A1, GMPPB, LAMA5, MYO9A, UNC13A, VAMP1) and v4 one additional gene (TOR1AIP1). NGS-based screening of CMS panel genes was performed using a SeqCapEZ capture design (Nimblegen) and a MiSeq sequencer (Illumina). Variants were identified through a bioinformatics pipeline (Genodiag, Paris, France). Copy number variations (CNVs) in targeted regions were searched for by a dedicated algorithm based on comparison of normalized number of reads of each region among the 12 samples of the sequence run.
Statistical analyses
All data were analysed with R 4.0. To visualize the relationship between neuromuscular symptoms and implicated genes, a heat map was generated using the library ComplexHeatmap.20 Only the genotypes with at least four patients were included in the heat map. The remaining genotypes, with fewer than four patients, were described separately. The hierarchical clustering of rows was conducted using the Ward.D2 method and the distance between rows was computed using the maximum method on the percentage-based data matrix. The heat map was colour-coded to represent the range of proportions of symptoms, and an accompanying metadata panel was incorporated to display the mean age at onset of the first symptom. To identify symptoms that exhibited significant patterns in the heat map, Z-scores were calculated to facilitate the interpretation of symptoms in relation to the involved gene, normalizing the data around a mean of zero and a standard deviation of one. Associations between prognostic outcomes and implicated genes were evaluated using Chi-squared tests. Bonferroni correction was applied to adjust P-values for multiple comparisons, employing a significance threshold of 0.05.
Results
Demographic, genetic and diagnostic characteristics
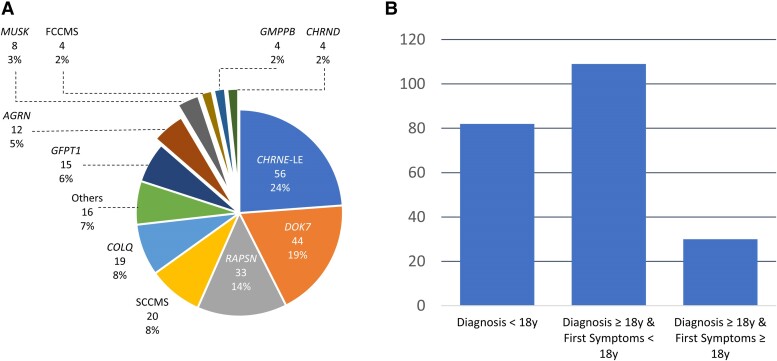
A total of 235 patients belonging to 195 unrelated families were included in the study; 123 were female (52.3%). A positive family history was reported in 107 patients (45.6%), and consanguinity was found in 55 cases (23.4%). In terms of ethnicity, 177 patients were Caucasian (75.3%), 32 originated from North Africa (13.6%), 10 from the Middle East (4.2%), seven from Sub-Saharan Africa (3.0%) and five came from Romani families (2.1%); the remaining four (1.7%) had diverse other origins (Asia and South America). Causative variants (Supplementary Table 1) were found in 19 disease-related genes (AGRN, CHAT, CHRNA1, CHRNB1, CHRND, CHRNE, COL13A1, COLQ, DOK7, DPAGT1, GFPT1, GMPPB, LRP4, MUSK, RAPSN, SCNA4, SLC5A7, SLC18A3, TOR1AIP1). These 180 variants were either described as likely pathogenic or pathogenic in the literature or were novel and retained as probably or certainly disease-causing. All patients had pathogenic mutations linked to CMS: 56 in CHRNE (23.8%), characterized as low-expressor (CHRNE-LE) variants responsible for a low expression of the encoded protein, 44 in DOK7 (18.7%), 33 in RAPSN (14.0%), 20 slow-channel congenital myasthenic syndromes (SCCMS, 8.5%) due to variants in CHRNA1 for 14 and in CHRNE for six, 19 in COLQ (8.1%), 15 in GFPT1 (6.4%), 12 in AGRN (5.1%), eight in MUSK (3.4%), four in CHRND and four in GMPPB (1.7% for each), four fast-channel congenital myasthenic syndromes (FCCMS) due to already described CHRNE variants (1.7%),21,22 and 16 (6.8%) in other genes (CHAT, CHRNA1 low-expressor, CHRNB1, COL13A1, LRP4, SCNA4, SLC5A7, SLC18A3, TOR1AIP1) (Fig. 1A). Inheritance was recessive in 215 patients (91.5%), dominant in 16 patients (6.8%) and de novo in four patients (1.7%). Only patients with SCCMS had dominant or de novo inheritance. Symptom onset occurred in the neonatal period in 81 patients (34.4%), in infancy in 55 patients (23.4%), in childhood in 44 patients (18.7%) and in the teenage years in 18 patients (7.7%). Twenty-five patients had their first symptoms between ages 18 and 40 (10.6%). These patients belonged to the DOK7 (6/25), AGRN (4/25), SCCMS (4/25), RAPSN (3/25), COLQ (2/25), GMPPB (2/25), CHRND (1/25), GFPT1 (1/25), LRP4 (1/25) and TOR1AIP1 (1/25) groups. Only five patients had their first symptoms after age 40 (2.1%). In these five patients, the genetic analysis disclosed SCCMS, DOK7, MUSK genes for one patient each and RAPSN gene for two. The age at first symptoms could not be clearly determined in seven patients (3.0%). A total of 138 patients were previously misdiagnosed (58.7%). Among them, the main misdiagnoses were congenital myopathy (50%), autoimmune MG (29.0%), muscular dystrophy (15.9%) and mitochondrial myopathy (8.7%) (Table 1 and Supplementary Fig. 1). Some patients were misdiagnosed with several different pathologies during their disease course. The mean delay between first symptoms and clinical diagnosis was 17.2 years (SD = 15.3), while the mean delay until molecular diagnosis was 22.0 years (SD = 15.2). The clinical diagnosis was made before 18 years in 82 patients (35.0%). Among the 139 patients in whom the diagnosis was made in adulthood (59.1%), 110 presented symptoms before the age of 18 (46.8%) and 29 had their first symptoms at or after the age of 18 (12.3%; Fig. 1B). This categorization was not possible in 14 patients (5.9%) as age at first symptoms was not available in seven patients and age at clinical diagnosis could not be determined in seven others. The mean follow-up time between first symptoms and last visit was 34 years (SD = 15.1). The mean age at last visit was 40.5 years (SD = 15.1). There was no significant difference in age at last visit according to the genotype (P = 0.11).
Figure 1.
Genetic and diagnostic characteristics of the cohort. (A) Proportion of genotypes present in the cohort. (B) Diagnostic categories according to age at first symptoms and age at diagnosis. CMS = congenital myasthenic syndromes; FCCMS = fast-channel congenital myasthenic syndrome; SCCMS = slow-channel congenital myasthenic syndrome; y = years; LE = low-expressor.
Table 1.
Misdiagnoses in the adult congenital myasthenic syndrome cohort
| Misdiagnosis | Patients, n (% patients with misdiagnosis/% of all patients)a |
|---|---|
| Congenital myopathy | 69 (50/29.4) (DOK7/RAPSN/CHRNE) |
| Myasthenia gravis | 40 (29.0/17.0) (DOK7/RAPSN/CHRNE) |
| Muscular dystrophy | 22 (15.9/9.4) (DOK7/RAPSN/GMPPB) |
| Mitochondrial myopathy | 12 (8.7/5.1) (CHRNE/MUSK/COLQ) |
| Distal myopathy | 4 (2.9/1.7) |
| Spinal muscular atrophy | 4 (2.9/1.7) |
| Metabolic myopathy | 3 (2.2/1.3) |
| Channelopathies and periodic paralysis | 3 (2.2/1.3) |
| Myositis | 2 (1.5/0.9) |
| Amyotrophic lateral sclerosis | 1 (0.7/0.4) |
| Moebius syndrome | 1 (0.7/0.4) |
| Lambert-Eaton syndrome | 1 (0.7/0.4) |
| Fibromyalgia | 1 (0.7/0.4) |
| Lyme disease | 1 (0.7/0.4) |
aFor the four main misdiagnoses, the three genes that are mostly involved in terms of the number of patients are shown in parentheses.
Genotype-phenotype correlations
Initial and final phenotype
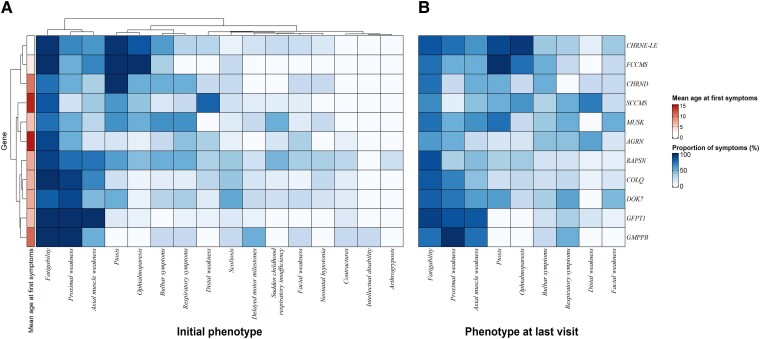
The proportion of symptoms per genotype at initial presentation is shown in Fig. 2A. Genes were clustered using the Ward.D2 method according to the initial clinical presentation in different groups. The first one included CHRNE-LE, CHRND and FCCMS. In this group, ptosis was found in 53/54 CHRNE patients (98.1%), 4/4 CHRND patients (100%) and 4/4 FCCMS patients (100%). Moreover, ophthalmoparesis was reported in 46/54 CHRNE patients (85.2%), in 4/4 FCCMS patients (100%) and in 2/4 CHRND patients (50%). The second group was composed of SCCMS patients, with a high proportion having upper distal weakness at initial presentation (16/20 patients, 80%). The third group was composed of AGRN- and MUSK-mutated patients. In this group, the phenotype was variable. Some AGRN patients could also have a distal weakness (4/12, 33%), while others had a proximal weakness (6/12, 50%). These patients could also have facial weakness (4/12, 33.3%), respiratory symptoms (5/12, 41.7%) or bulbar symptoms (25%). MUSK patients also presented with variable symptoms, such as proximal weakness (4/8, 50%), respiratory symptoms (5/8, 62.5%), bulbar symptoms (5/8, 62.5%) and ophthalmoparesis (4/8, 50%). The last group included RAPSN, COLQ, DOK7, GMPPB and GFPT1. Proximal weakness was the hallmark of this last group, as observed in the following mutated patients: GMPPB (4/4, 100%), GFPT1 (15/15, 100%), COLQ (18/19, 94.7%), DOK7 (40/44, 90.9%) and RAPSN (25/33, 75.8%). Interestingly, axial muscle weakness was always found in all GFPT1 patients (10/10, 100%) and GMPPB patients could have intellectual disability (1/4, 25.0%). Then, 23/44 DOK7 patients (52.2%) had scoliosis, while 7/32 (21.9%) and 9/32 (28.2%) RAPSN patients had arthrogryposis and contractures, respectively. Only four genotypes had a mean age at first symptoms of over 10 years: AGRN (14.75, SD = 12.8), SCCMS (14.4, SD = 14.7), GMPPB (11, SD = 10.5) and CHRND (10.25, SD = 11.8). The proportion of symptoms per genotype at last follow-up is shown in Fig. 2B. There were no significant differences in any proportion of symptoms per genotype between the initial presentation and the last follow-up visit. The genotypes that were associated with symptoms at initial presentation with a Z-score > 0.85 are shown in Fig. 3.
Figure 2.
Genotype-phenotype correlations heat map and clustering of genotypes according to symptoms. Symptoms at initial presentation (A) and at last follow-up (B), and age at first symptoms. LE = low-expressor.
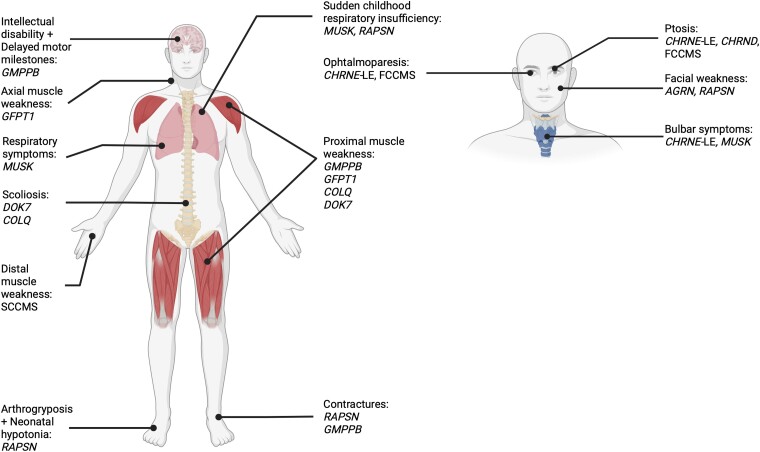
Figure 3.
Characteristic symptoms at diagnosis, by genotype. Illustration of the human body showing genotypes with high prevalence of symptoms (Z-score > 0.85) in specific categories of symptoms. Created with BioRender.com. LE = low-expressor.
Adult onset
Regarding the 30 patients with onset of symptoms in adulthood, 5/7 DOK7 (71%) patients presented with upper limb proximal weakness as the first symptom. The remaining two patients developed acute respiratory insufficiency as the initial manifestation of the disease. Interestingly, two adult-onset AGRN patients initially manifested respiratory symptoms and the remaining two had fatigability and proximal muscle weakness. All five SCCMS patients with an onset of symptoms in adulthood presented finger extension weakness as the first manifestation of the disease. The two COLQ patients, two GMPPB patients and one GFPT1 patient who had their first symptoms in adulthood had proximal muscle weakness as the initial manifestation of the disease. The first symptoms of the five RAPSN patients with disease onset at or after 18 years were not specific and included ptosis, proximal muscle weakness and bulbar symptoms. The only CHRND patient with disease onset after 18 years presented with ptosis, ophthalmoparesis and bulbar symptoms. Finally, the MUSK patient who developed her first symptoms in adulthood presented with fatigability, bulbar symptoms, proximal muscle weakness and respiratory symptoms. The TOR1AIP1 and LRP4 adult-onset patients have previously been reported.23,24
Paraclinical investigations
Regarding electrophysiological features, 213/220 patients (96.8%) with available ENMG data had a decrement superior to 10% on RNS at 3 Hz in at least one nerve-muscle pair. Four patients had an increment on post-exercise CMAP: three AGRN patients and one TOR1AIP1 patient. An R-CMAP was found in 15/19 COLQ patients (78.9%) and in 15/20 SCCMS patients (75.0%) and was significantly more frequent in these genotypes compared to the others (P < 0.01).
CK levels were available in 134 patients and were elevated (>200 UI/l) in 34 of them (25.4%). The proportion of patients with raised CK was significantly increased in the GMPPB group (4/4 patients, P < 0.01) compared to the others. In this group, the mean CK level was 2035.3 UI/l (SD = 1291.8). The second genotype associated with elevated CK was the GFPT1 genotype, with 7/12 patients with available CK having raised levels (mean of 311.5 UI/l, SD = 238.0). All three MUSK patients with available CK data had elevated values (339, 57 and 4558 UI/l, respectively). Regarding the two most frequently represented genes, 3/30 CHRNE patients (10%) and 7/28 DOK7 patients (25%) had elevated CK values.
Muscle biopsy was performed in 117 patients (49.8%). The results were available in 104 patients. The biopsy was considered normal in 16 patients (15.4%). The main abnormality was type 1 fibre predominance (n = 44, 42.3%), type 2 fibre atrophy (n = 30, 28.9%) and fibre size disproportion (n = 21, 20.2%). Other features included: lipid surcharge (n = 14, 13.4%), nuclear internalizations (n = 10, 9.6%), mitochondrial abnormalities (n = 5, 4.8%) and core-like lesions (n = 4, 3.8%). Tubular aggregates were seen in eight patients (7.7%), including seven GFPT1 patients and one DGAPGT1 patient. Necrotic/regenerating fibres were observed in six patients (5.8%), including the four GMPPB patients. Abnormal neuromuscular junctions on electron microscopy were reported in 11 patients (10.6%). All clinical and paraclinical findings are detailed according to genotype in Supplementary Tables 2–12.
Long-term prognosis
Disease course
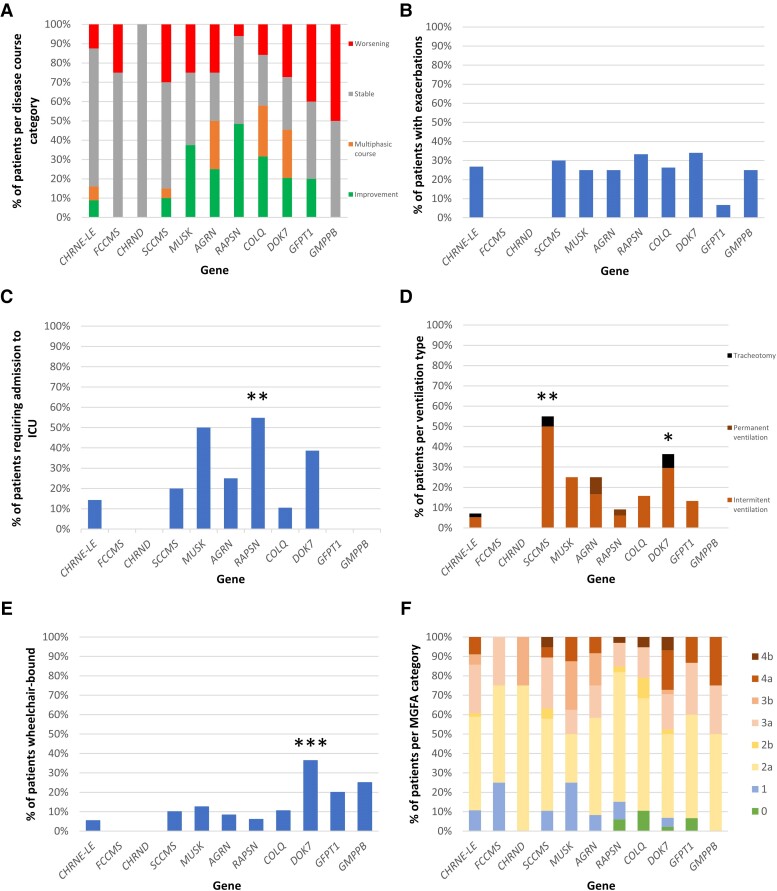
The type of disease course according to the genotype is shown in Fig. 4A. Most CHRNE-LE (40/56, 71.4%), CHRND (4/4, 100%) and FCCMS (3/4, 75.0%) patients had a stable disease course. A progressive improvement was reported in 16/33 RAPSN (48.5%) and in 3/8 MUSK patients (37.5%). Conversely, 6/20 SCCMS (30%), 12/44 DOK7 (27%), 2/4 GMPPB (50.0%) and 6/15 GFPT1 (40.0%) patients had a progressively worsening course. A proportion of DOK7 (11/44, 25.0%), COLQ (5/19, 26.3%) and AGRN (3/12, 25.0%) patients had a multiphasic disease course, combining successive periods of improvement, stability and progressive worsening. An example of multiphasic disease, with late-onset deterioration, is given in Supplementary Fig. 2. Intrafamilial variability was also a hallmark in some CHRNE-LE and SCCMS families.
Figure 4.
Long-term data of congenital myasthenic syndrome patients, by genotype. (A) Disease course category. (B) Proportion of patients with exacerbations. Proportion of patients requiring (C) intensive care unit (ICU) admission during their disease course, (D) ventilation at last follow-up, (E) wheelchair at last follow-up. (F) Myasthenia Gravis Foundation of America (MGFA) category at last follow-up. CMS = congenital myasthenic syndrome; LE = low-expressor. *P < 0.05, **P < 0.01, ***P < 0.001.
Exacerbations and ICU admissions
The proportion of patients having acute disease exacerbations reached 20% in most of the genotypes (Fig. 4B). The duration of exacerbations could be very long in some cases, as noted in a COLQ patient who lost her ambulation within a week for 3 years before regaining it completely in 1 month. RAPSN patients required significantly more ICU admissions compared to the others (17/31, 54.8%, P < 0.01); three of them required non-invasive ventilation, 11 were intubated and three required tracheostomies. Moreover, four RAPSN patients required two ICU admissions in their disease course. Three other genotypes had a proportion of patients requiring ICU admission that exceeded 20% (Fig. 4C): MUSK (4/8, 50%), DOK7 (17/44, 38.6%) and AGRN (3/12, 25.0%). Four DOK7 patients and one AGRN patient required two ICU admissions in their disease course. In RAPSN and MUSK patients, 19/21 (90.4%) and 4/4 (100%) ICU admissions, respectively, occurred before age 18; in DOK7 and AGRN patients, 11/21 (52.3%) and 4/4 (100%) ICU admissions, respectively, occurred at or after age 18.
Pregnancy and other triggers
Of the 74 female patients who had a pregnancy, 24 (32.4%) reported a worsening of symptoms during pregnancy; 20/123 female patients (16.2%) reported a worsening of symptoms during menstruation. In the cohort of 235 patients, other triggers for symptom worsening were infection in 35 patients (14.9%), warm temperatures in 19 patients (8%), cold temperatures in 15 patients (6.3%), anaesthesia in nine patients (3.8%) and psychological stress in five patients (2.1%).
Disability and ventilation
Among the different genotypes, the proportion of patients requiring ventilation at the last follow-up was significantly elevated in SCCMS (11/20, 55%, P < 0.01) and DOK7 patients (16/44, 36.3%, P = 0.04) (Fig. 4D). This proportion did not exceed 25% in the other genotypes (Fig. 4D). Six patients were tracheotomized, with invasive ventilation, at last follow-up: one SCCMS patient, one CHRNE patient, three DOK7 patients and one SLC5A7 patient. Only two patients (one CHRND and one SCCMS) required a feeding tube at last visit. Regarding the motor long-term prognosis, the proportion of DOK7 patients who were wheelchair-bound was significantly higher compared to the other genotypes (16/44, 36.3%, P < 0.001). One GMPPB patient (25.0%) and 3/15 GFPT1 patients (20%) were wheelchair-bound at last visit (Fig. 4E). The proportion of patients per MGFA category according to the genotype is shown in Fig. 4F. The highest proportion of MGFA category 4 patients was found in the DOK7 patients (12/44, 27.2%) (Fig. 4F). The highest proportion of patients both wheelchair-bound and ventilated was in the DOK7 patients (9/44, 20.5%). This proportion did not exceed 10% in the other genotypes (SCCMS: 2/20, 10%, AGRN: 1/12, 8.3%, GFPT1: 1/15, 6.7%, RAPSN: 2/33, 6.1%, COLQ: 1/19, 5.2%, CHRNE: 1/56, 1.8%).
Death
Only six patients died in our cohort (2.6%). An AGRN patient died at 50 years of age from respiratory insufficiency. She was tetraplegic with severe bulbar involvement after three decades of progressive worsening (Patient 1 in Jacquier et al.25). One COLQ patient died at 52 years of age from cancer. Two patients with DOK7 variants died. The first one was misdiagnosed with seronegative autoimmune MG and died from acute vocal cord palsy at 41 years of age, possibly favoured by AChE inhibitors. The second died at 56 years of age after an accidental fall in the stairs (Supplementary Fig. 2). A DPAGT1 patient died at 36 years from aspiration pneumonia secondary to swallowing disorders. Finally, a patient with RAPSN variants died at 86 years, but the cause of death was not available. Moreover, a family history of early death during infancy was reported in 15 patients (three RAPSN, three COLQ, two DOK7 and one patient each for CHRNE-LE, MUSK, GMPPB, GFPT1, COL13A1, SCNA4, SCL5A7).
Treatment
Twenty-five patients (10.6% of the cohort), misdiagnosed as seronegative autoimmune MG, received immunomodulatory treatments before the diagnosis of CMS. These treatments included corticosteroids, intravenous immunoglobulin, plasma exchange and immunosuppressive treatments (azathioprine, mycophenolate mofetil). Moreover, eight of them had a thymectomy. None of these 25 patients reported a long-term improvement with these therapies.
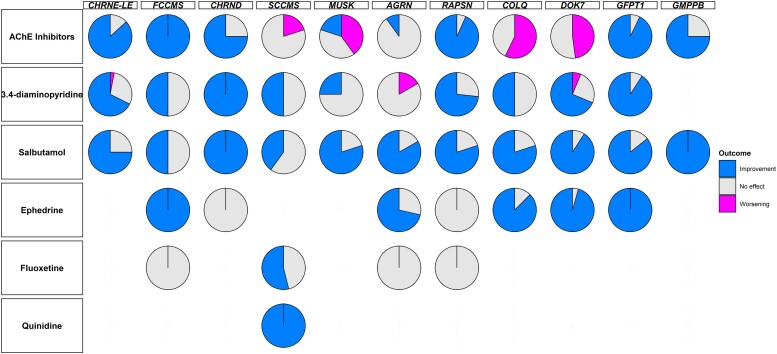
A total of 224 patients received non-immunomodulatory CMS treatments (95.3%). These treatments included AChE inhibitors, 3,4-DAP, salbutamol, ephedrine, fluoxetine and quinidine; 138 patients received more than one of these treatments (58.8%). Responses to non-immunomodulatory therapies are summarized in Fig. 5.
Figure 5.
Treatment efficacy according to the genotype. AChE = acetylcholinesterase; LE = low-expressor.
All SCCMS (5/5), COLQ (7/7) and DOK7 (23/23) patients reported either no effect or worsening with AChE inhibitors. Only 1/5 MUSK patients (20.0%) and 1/10 AGRN patients (10.0%) claimed symptom improvement with these treatments, the remaining patients reporting no effect or symptom worsening. AChE inhibitors were effective in 75% or more of the patients in the other genotypes: CHRNE-LE (46/53), FCCMS (4/4), CHRND (3/4), RAPSN (28/30), GFPT1 (13/14) and GMPPB (3/4). 3,4-DAP was reported as effective in more than half of the CHRNE-LE (23/33), CHRND (3/3), RAPSN (11/15), DOK7 (11/16) and GFPT1 (10/11) patients. Half of FCCMS (1/2), SCCMS (1/2) and COLQ (3/6) patients had their symptoms improved with 3,4-DAP, while this treatment was ineffective in 3/4 MUSK patients (75.0%) and 6/7 AGRN patients (85.7%) and even led to symptom worsening in 1/7 AGRN patients (14.3%). Salbutamol improved the symptoms of 75% or more of CHRNE-LE (12/16), CHRND (1/1), MUSK (4/5), AGRN (5/6), RAPSN (4/5), COLQ (8/10), DOK7 (20/22), GFPT1 (6/7) and GMPPB (1/1) patients. One of the two FCCMS patients (50.0%) treated with salbutamol reported symptom improvement, while the other reported no effect. Two of the five SCCMS patients (40.0%) treated with salbutamol reported treatment efficacy, while the three others reported no effect. Ephedrine was reported to improve symptoms in the only FCCMS patient treated, and in 5/7 AGRN patients (71.4%), 7/8 COLQ patients (87.5%), 22/23 DOK7 patients (95.7%) and 3/3 GFPT1 patients (100%). No effect was reported with this therapy in the one CHRND patient and one RAPSN patient treated, and in 2/7 AGRN (28.6%), 1/8 COLQ (12.5%) and 1/23 DOK7 (4.3%) patients. Four previously wheelchair-bound DOK7 patients were able to walk unaided, three after being treated with both salbutamol and ephedrine and one after ephedrine alone. One previously wheelchair-bound COLQ patient became ambulant after being treated with salbutamol, as did another COLQ patient thanks to ephedrine. Fluoxetine was found to be effective in 7/13 SCCMS patients (53.8%), the remaining 6/13 patients (46.1%) reporting no benefit. In the other genotypes, one FCCMS patient, one AGRN patient and three RAPSN patients were treated with fluoxetine but reported no effect. Finally, all five SCCMS patients treated with quinidine reported an improvement of motor weakness.
Clinical summary for genes in a small number of patients
TOR1AIP1 gene
Three patients with TOR1AIP1 variants were included in our cohort (Table 2). One of them (Patient 1) had previously been published (main proband of Malfatti et al.23). We report herein two other patients, who are brothers, harbouring the same c.63dupC (p.Arg22Glnfs*88) found in Patient 1, and the c.72dupC (p.Ile25Hisfs*85) heterozygous variants (Patients 2 and 3). They were born to Algerian healthy parents. The first variant was inherited from the mother and the second variant from the father. Their motor milestones were normal, but they had difficulties in sports activities during their teenage years. Patient 2 developed progressive walking and respiratory difficulties at age 35 years, leading to his needing a banister to climb stairs at age 40. At age 47, the patient underwent a coronary angiography for an acute coronary syndrome. Because he did not tolerate the supine position during the examination, pulmonary investigations including blood-gas analysis and pulmonary functional tests were performed and showed an alveolar hypoventilation requiring non-invasive ventilation. Neurological examination found proximal muscle and finger extensor muscle weakness in the upper limbs, associated with distal muscle weakness in the lower limbs, and cervical spine, finger, wrist and Achilles tendon contractures. Repetitive nerve stimulation at 3 Hz performed at age 48 showed a 48% decrement in tibialis anterior and anconeus muscles and a 39% decrement in trapezius muscle. Serum CK was mildly elevated at 227 UI/l (n < 200 U/l). He was mildly improved by AChE inhibitors with an increased walking distance. At last follow-up (age 51), he was still ambulant without walking aid but required nocturnal non-invasive ventilation. Patient 3 was admitted to the ICU at age 48 for an acute respiratory insufficiency revealing an alveolar hypoventilation and requiring intubation. At discharge, he walked unaided and had non-invasive ventilation. His neurological examination showed elbow and finger contractures and mild deltoid muscle weakness. His CK levels were normal. RNS at 3 Hz showed a 19% decrement on tibialis anterior and 15% on trapezius muscle.
Table 2.
Clinical characteristics and long-term prognosis of patients with rare genotypes (n ≤ 3)
| Patient | Gene | Sex | Age at first symptoms | First symptoms | Disease course | ICU admission (age in years) | Wheelchair at last visit | Respiratory assistance at last visit (type) | Treatment response | Other features |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | TOR1AIP1 | F | 25 | Gowers’ sign, axial muscle weakness, fatigability | Stable | No | No | No | AChE inhibitors (+) | Small stature |
| 2 | TOR1AIP1 | M | 10 | Fatigability, difficulties in sports activities | Worsening | Yes (47) | No | Yes (NIV) | AChE inhibitors (+) | Contractures |
| 3 | TOR1AIP1 | M | 10 | Fatigability, difficulties in sports activities | Stable | Yes (48) | No | Yes (NIV) | NA | Contractures |
| 4 | DPAGT1 | F | 0 | Neonatal hypotonia and respiratory insufficiency | Stable | No | No | No | AChE inhibitors (−) | Contractures |
| 5 | DPAGT1 | F | 0 | Neonatal hypotonia | Worsening | No | Yes | No | AChE inhibitors (+) | Contractures, delayed motor milestones, intellectual disability, optic disk atrophy, epilepsy, deafness, cerebellar ataxia |
| 6 | DPAGT1 | F | 0 | Neonatal hypotonia | Worsening | Yes (18) | Yes | No | AChE inhibitors (+) | Contractures, delayed motor milestones, intellectual disability, optic disk atrophy, epilepsy |
| 7 | SLC5A7 | M | 0 | Neonatal hypotonia respiratory insufficiency, sudden apnoea | Improvement | Yes (0) | No | No | AChE inhibitors (+) | / |
| 8 | SLC5A7 | M | 0 | Neonatal hypotonia and respiratory insufficiency | Worsening | Yes (0) | Yes | Yes (tracheotomy) | AChE inhibitors (+) | Arthrogryposis (equinovarus), epilepsy |
Treatment response: (+) = improvement; (/) = no effect; (−) = worsening. F = female; M = male; ICU = intensive care unit; AChE = acetylcholinesterase; 3,4-DAP = 3, 4-diaminopyridine; NIV = non-invasive ventilation; NA = not available.
DPGAT1 gene
Three patients in our cohort had DPAGT1 variants (Table 2; Patients 4, 5 and 6). They all presented at birth with hypotonia and contractures. Two (Patients 5 and 6) had associated CNS signs characterized by delayed motor milestones, intellectual disability, optic disk atrophy and epilepsy, associated with deafness and cerebellar ataxia in one of them. AChE inhibitors were reported to improve their symptoms in two of the three patients and were considered ineffective in the other. At last follow-up, Patient 4 (age 49) was still ambulant but required a banister to climb stairs, while Patients 5 and 6 were wheelchair-bound (age 34 and 21, respectively). None required respiratory assistance. Patient 5 died at age 36 from pneumonia secondary to swallowing disorders.
SLC5A7 gene
Two patients had biallelic variants in the SLC5A7 gene (Table 2; Patients 7 and 8). They both presented with hypotonia and respiratory insufficiency at birth, requiring ICU admission. One of them (Patient 8) had arthrogryposis (equinovarus) and developed epileptic seizures. Mild efficacy was reported for both with AChE inhibitors. However, their final prognosis was different. While Patient 7 improved and was asymptomatic at age 20, Patient 8 progressively became wheelchair-bound and required nocturnal non-invasive ventilation at last visit (age 22).
The remaining patients are described in Supplementary Table 13. The paraclinical findings associated with genes with a small number of mutated patients (n ≤ 3) are available in Supplementary Table 14. Of note, the LRP4 and SCN4A patients have previously been reported.24,26
Discussion
This study of a French nationwide multicentre cohort of 235 adult patients has enabled us to better describe CMS patients’ phenotype and long-term prognosis, according to their genotype. CHRNE-LE variants were the most common and are considered as the main cause of CMS worldwide.6,27 As described in an Austrian cohort, DOK7 was herein the second most commonly involved gene.28RAPSN variants were also frequent, as in previously published cohorts.6,8,29COLQ was only the fifth most frequently involved gene in our cohort, whereas it was one of the three main genes in several previous studies in different populations.7,8,16
Adult neurologists can encounter CMS patients in three different situations. In the first and most straightforward scenario, the diagnosis has already been made by a paediatric neurologist, and the adult neurologist assumes responsibility for the patient’s follow-up (35% of patients in our cohort). In the second situation, symptoms have already been present in childhood or infancy, but the diagnosis has not been reached due to mild and/or short-duration symptoms insufficient to initiate a diagnostic investigation or due to misdiagnosis (mainly congenital myopathy). This was the most common situation in our study (46.8% of patients), as previously observed.9 In the third, more rarely encountered situation, symptom onset occurs in adulthood (12.3% of patients in our cohort). These last two situations are particularly challenging, especially for adult neurologists non-specialized in the neuromuscular field. In these cases, finger extension weakness or proximal muscle weakness, even if non-specific, are suggestive of the diagnosis. Clinicians should also keep in mind that acute respiratory insufficiency can be the first manifestation of the disease in adult patients.
Misdiagnoses were frequent in our cohort (58.7%) and the diagnostic delay was long, in line with previously published cohorts.9,27 Congenital myopathy was the most common misdiagnosis as there are overlapping clinical and histological features. Autoimmune MG can easily be suspected in late-onset cases, and this was the second most frequent misdiagnosis in our cohort, leading to an immunosuppressive treatment in 25 patients. With the development of new immunosuppressive treatments in the past decade, we recommend considering the diagnosis of CMS in patients with seronegative MG before starting such treatments which could cause serious adverse events.30
We clustered patients’ genotypes according to their initial phenotypes. The clustering method we applied led to the formation of four groups of phenotypes. The first group was composed of CHRNE, CHRND and FCCMS patients and was characterized by predominantly ocular symptoms, such as ptosis and ophthalmoparesis. Acetylcholine receptor endplate deficiencies are known to cause predominantly ocular symptoms, and FCCMS patients have essentially the same phenotype.4,8,29 The second group was represented solely by SCCMS patients, who presented a particular phenotype with predominantly upper limb distal weakness, especially affecting finger extensors, frequently associated with neck extensor muscle weakness, as previously found.31 The third group was composed of AGRN and MUSK patients. They developed variable symptoms such as ocular symptoms, bulbar symptoms, respiratory involvement and muscle weakness, which led us to consider this group as a variable-phenotype group. These genes have already been associated with such diverse symptoms.32,33 Interestingly, AGRN patients frequently had distal weakness but, contrary to SCCMS patients, they rarely had axial muscle weakness. The fourth group was composed of GMPPB, GFPT1, DOK7, COLQ and RAPSN patients, and could be categorized as having a limb-girdle muscle dystrophy (LGMD)-like phenotype, associated with some additional characteristic features for some of these genes. GMPPB and GFPT1 are essential for N- and O-glycosylation and N- and O-mannosylation.4 As previously described, GMPPB and GFPT1 patients presented with relatively pure proximal weakness.34,35 However, GMPPB patients could also have CNS involvement with delayed motor milestones and intellectual disability. DOK7 and COLQ patients also presented with a proximal weakness but were more prone to have associated symptoms, such as bulbar or ocular symptoms.36,37DOK7 was associated with a high rate of scoliosis, as previously observed.36RAPSN was included by the clustering method in this group due to frequent proximal and axial muscle weakness. However, RAPSN patients developed more ocular, bulbar and respiratory symptoms than the other patients of this group. RAPSN has already been associated with such clinically diverse symptoms.8 In our cohort, RAPSN patients had more arthrogryposis, hypotonia at birth and sudden respiratory insufficiency during childhood than other patients. Taken together, these hallmarks are evocative of RAPSN-related CMS.38 Regarding the electrophysiological data, we confirmed that an R-CMAP is a hallmark of COLQ and SCCMS patients, present in around three-quarters of these cases, resulting from a neuromuscular junction gain-of-function.15 Highly elevated CK levels are suggestive of GMPPB gene mutation, as this gene has also been reported in LGMD or overlapping LGMD-CMS phenotype.39 We confirmed that tubular aggregates point towards the diagnosis of GFPT1− and DPAGT1-associated CMS, and that features of muscular dystrophies can be observed in GMPPB patients.35,40,41
Our main objective was to describe the long-term prognosis of adult CMS patients. Firstly, we noticed that CMS patients did not switch from one phenotype group to another along their disease course. We acknowledge that this finding may have been influenced by the different treatments that patients received throughout their lives. CHRNE, CHRND and FCCMS patients were prone to have a stable disease course. Moreover, they remained mainly ambulant at the end of the follow-up and did not require ventilation. Even if they could experience symptom exacerbations, these later exacerbations were relatively moderate since patients rarely required ICU admission. This relatively good prognosis is supported by the findings reported in previous cohorts.16,18 SCCMS patients were frequently stable regarding their disease course but could worsen in approximately one-third of cases. Although they remained ambulant, more than half of them required respiratory support at the end of follow-up. This proportion was higher than in previous cohorts, leading us to recommend monitoring the respiratory functions of these patients through regular pulmonary functional tests.31RAPSN, DOK7, MUSK, COLQ and AGRN patients had various disease courses, represented either by stability, worsening or improvement. Moreover, DOK7, COLQ and AGRN patients frequently presented several types of disease course during their lives. Regarding the latter finding, clinicians should be aware that phases of worsening and improvement can succeed one other, and caution is needed when informing a particular patient about the long-term prognosis. Patients with RAPSN, DOK7, MUSK and AGRN mutations were more prone to have severe exacerbations requiring ICU admissions. While most ICU admissions in RAPSN and MUSK patients occurred in childhood, most of them in DOK7 and AGRN patients occurred in adulthood. Thus, adult neurologists should be aware that severe exacerbations can be expected in their DOK7 and AGRN patients. DOK7 had the more severe motor prognosis among these genes. Indeed, while most RAPSN, MUSK, COLQ and AGRN patients were ambulant at last follow-up, DOK7 patients were wheelchair-bound in approximately one-third of cases. This proportion was higher than in a previously published cohort of adult CMS patients.9 Regarding respiratory functions, ventilation was also more frequent in DOK7 patients.19 Our previous study on COLQ patients, which included some of the patients reported here, had already shown that most of these patients remained ambulant without respiratory assistance at last follow-up.20 It is interesting to note that, despite severe initial phenotypes characterized by hypotonia and respiratory distress during childhood requiring ICU, the overall final phenotype of RAPSN patients was quite favourable. Finally, GMPPB and GFPT1 patients were prone to have worsening disease courses and around 20% of them were wheelchair-bound at last follow-up. However, they remained ventilation free. In these patients with glycosylation defects, myopathic changes can be observed in muscle biopsies and MRI, which could partly explain the worsening course.35 Pregnancy seems to be a risk period for symptom exacerbations. Indeed, 32.4% of our female patients with at least one pregnancy experienced a symptom exacerbation during pregnancy. This frequency was lower than that previously reported.9,42 This apparent discrepancy could be explained by the retrospective nature of our study, which was not specifically designed to address this question. Only six patients died in our adult cohort. Thus, the overall vital prognosis of adult CMS patients appears quite favourable. However, we found a family history of early death in infancy in 15 patients, with most of them bearing RAPSN and COLQ gene mutations, suggesting a possible life-threatening condition for these genes, in some cases during childhood. Importantly, future clinical trials aiming to evaluate the efficacy of treatments already available or in development will need to consider the different clinical courses. Outcomes and effect sizes need to be conceived and chosen according to the disease course of each genotype. For example, investigators will have to consider the improving course of RAPSN patients, the progressive worsening of GMPPB and GFPT1 patients and the multiphasic course of DOK7, COLQ and AGRN patients. Moreover, further large-scale prospective studies will help to better define the natural history of CMS according to the genotype.
The small number of patients per gene prevented us from drawing conclusions regarding the long-term prognosis of patients with rare CMS genes. However, patients with mutations in pre-synaptic genes implicated in acetylcholine production and transport (CHAT, SLC5A7, SLC18A3) seem to have a favourable long-term motor and respiratory prognosis despite severe symptoms in infancy, such as hypotonia, feeding difficulties and episodic apnoea, even if one SLC5A7 patient was wheelchair-bound and ventilated at last visit. This favourable prognosis contrasting with a severe onset was suggested in a previous study in SLC5A7 patients.43DPAGT1 patients were prone to develop CNS signs such as intellectual disability, as previously reported;41 they seem to have a poor motor prognosis with the need for a wheelchair.
This cohort also provides important and valuable information regarding CMS treatment. We confirm that AChE inhibitors should be avoided in SCCMS, COLQ and DOK7 patients, in whom their use could lead to symptoms worsening.16,17,44 This treatment was often ineffective in AGRN patients.45 Furthermore, 3,4-DAP was frequently ineffective in AGRN and MUSK patients, raising the question of early treatment with salbutamol. SCCMS patients’ symptoms were difficult to improve because 3,4-DAP and salbutamol were not effective in about half of treated patients. Fluoxetine, a selective serotonin reuptake inhibitor that acts as a channel blocker therapy, can be useful in some patients, and a previous study suggests that treatment is more effective the sooner it is started after the onset of symptoms.46 Finally, quinidine could be an interesting option in these patients. Apart from these cases, most patients responded favourably to AChE inhibitors and other treatments regularly administrated as second-line therapies. Salbutamol and ephedrine were particularly effective in DOK7 and COLQ patients, with a high proportion of patients reporting a durable and significant improvement. Interestingly, the few patients in whom the treatment was considered ineffective had particularly severe disease with marked muscle weakness.
We also report two new cases of TOR1AIP1-related CMS. To our knowledge, this is only the third published family for this phenotype, with one of the variants (c.63dupC; p.Arg22Glnfs*88) having already been published.23,47 This frameshift variant is localized between the first two alternative start codons for LAP1B and LAPC isoforms and was associated with a selectively decreased level of LAP1B isoform in patients’ fibroblasts, when present in a homozygous state. The second frameshift variant (c.72dupC; p.Ile25Hisfs*85), not previously reported, is also present between the two first start codons and, like the first variant, is predicted to selectively impact LAP1B. It was absent from the gnomAD database. Each variant was heterozygous in the parents, confirming the familial segregation. These patients shared common features with the previously published patients: normal developmental milestones, a late-onset disease, contractures and a predominant proximal muscle weakness associated with mild distal weakness, such as finger extensors.23,48 However, contrary to previous cases, our patients developed severe acute respiratory insufficiency requiring admission to ICU, and they required non-invasive ventilation at discharge. Thus, our data indicate that respiratory involvement can be a major feature of TOR1AIP1-related CMS. Nevertheless, the motor prognosis seems favourable because all published patients were still ambulant at last visit.23,48
Our study has several limitations. Due to its retrospective design some clinical data on the initial phenotype could have been missed. The number of patients per genotype was not equal between genes, due to the variable prevalence of the different CMS genotypes, and this could lead to difficulties in comparing them. Spirometry data were not available, but a recent study reported a progressive worsening of forced vital capacity in DOK7 and COLQ patients.19 Finally, treatment efficacy was determined retrospectively, based on clinicians’ reports in the medical file and not on objective and repeated validated scales. However, the large size of this cohort and the mean follow-up of 34 years allowed us to obtain reliable data regarding prognosis and follow-up.
In conclusion, even if the phenotypical features of CMS do not change during the patient’s life, the long-term prognosis is more complex and difficult to foresee due to various patterns of evolution from worsening to improvement, which can be multiphasic in some patients. However, knowing which gene is involved is informative: no long-term worsening was observed in CHRNE-LE patients. RAPSN patients, even if severely affected in infancy, improved later. The situation is more critical for SCCMS, DOK7 and GFPT1 genotypes, with a significant proportion of patients requiring, at last visit, ventilation (SCCMS and DOK7), a wheelchair (DOK7 and GFPT1), or both (DOK7). The positive impact of therapy was striking even in severely affected patients, some of them regaining walking capacity. Most patients of this cohort did not require ventilation and/or a wheelchair at last follow-up. Our results inform clinical practice, hopefully improving the diagnosis and management of these rare conditions.
Supplementary Material
Acknowledgements
The authors thank Fatou Diop, Floriane Llorens, Marina Dupre, Karine Ferraud, Mathilde Ferey, Maud Pirotte and Emilie Laheranne-Martinez for their help in data acquisition. They thank Prof. Michel Fardeau, Prof. Jeanine Koenig, Dr Daniel Hantaï and Dr Arnaud Isapof for their key role in the initiation and development of the CMS project in France. The thumbnail was generated using BioRender.com and an illustration (https://commons.wikimedia.org/wiki/File:CPAP.png) licensed under the Creative Commons Attribution 3.0 unported license.
Contributor Information
Julian Theuriet, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, Institut de Myologie, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France; Service d’ENMG et de pathologies neuromusculaires, centre de référence des maladies neuromusculaires PACA-Réunion-Rhône-Alpes, Hôpital Neurologique Pierre Wertheimer, Hospices Civils de Lyon, Groupement Est, 69500 Bron, France; Pathophysiology and Genetics of Neuron and Muscle, CNRS UMR 5261, INSERM U1315, Université Lyon 1, Faculté de Médecine Lyon Est, 69008 Lyon, France.
Marion Masingue, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, Institut de Myologie, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France; Centre de Recherche en Myologie, GH Pitié-Salpêtrière, Sorbonne Université-Inserm UMRS974, 75013 Paris, France.
Anthony Behin, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, Institut de Myologie, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France; Centre de Recherche en Myologie, GH Pitié-Salpêtrière, Sorbonne Université-Inserm UMRS974, 75013 Paris, France.
Ana Ferreiro, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, Institut de Myologie, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France; Centre de Recherche en Myologie, GH Pitié-Salpêtrière, Sorbonne Université-Inserm UMRS974, 75013 Paris, France; Basic and Translational Myology laboratory, Université Paris Cité, BFA, UMR 8251, CNRS, 75013 Paris, France.
Guillaume Bassez, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, Institut de Myologie, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France; Centre de Recherche en Myologie, GH Pitié-Salpêtrière, Sorbonne Université-Inserm UMRS974, 75013 Paris, France.
Pauline Jaubert, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, Institut de Myologie, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France.
Oriana Tarabay, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, Institut de Myologie, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France.
Frédéric Fer, Centre de Recherche en Myologie, GH Pitié-Salpêtrière, Sorbonne Université-Inserm UMRS974, 75013 Paris, France.
Antoine Pegat, Service d’ENMG et de pathologies neuromusculaires, centre de référence des maladies neuromusculaires PACA-Réunion-Rhône-Alpes, Hôpital Neurologique Pierre Wertheimer, Hospices Civils de Lyon, Groupement Est, 69500 Bron, France; Pathophysiology and Genetics of Neuron and Muscle, CNRS UMR 5261, INSERM U1315, Université Lyon 1, Faculté de Médecine Lyon Est, 69008 Lyon, France.
Françoise Bouhour, Service d’ENMG et de pathologies neuromusculaires, centre de référence des maladies neuromusculaires PACA-Réunion-Rhône-Alpes, Hôpital Neurologique Pierre Wertheimer, Hospices Civils de Lyon, Groupement Est, 69500 Bron, France; Pathophysiology and Genetics of Neuron and Muscle, CNRS UMR 5261, INSERM U1315, Université Lyon 1, Faculté de Médecine Lyon Est, 69008 Lyon, France.
Juliette Svahn, Pathophysiology and Genetics of Neuron and Muscle, CNRS UMR 5261, INSERM U1315, Université Lyon 1, Faculté de Médecine Lyon Est, 69008 Lyon, France; Service de Neurologie, troubles du mouvement et pathologies neuromusculaires, Hôpital Neurologique Pierre-Wertheimer, Hospices Civils de Lyon, Groupement Est, 69500 Bron, France.
Philippe Petiot, Service d’ENMG et de pathologies neuromusculaires, centre de référence des maladies neuromusculaires PACA-Réunion-Rhône-Alpes, Hôpital Neurologique Pierre Wertheimer, Hospices Civils de Lyon, Groupement Est, 69500 Bron, France.
Laurentiu Jomir, Service d’ENMG et de pathologies neuromusculaires, centre de référence des maladies neuromusculaires PACA-Réunion-Rhône-Alpes, Hôpital Neurologique Pierre Wertheimer, Hospices Civils de Lyon, Groupement Est, 69500 Bron, France.
Guy Chauplannaz, Service d’ENMG et de pathologies neuromusculaires, centre de référence des maladies neuromusculaires PACA-Réunion-Rhône-Alpes, Hôpital Neurologique Pierre Wertheimer, Hospices Civils de Lyon, Groupement Est, 69500 Bron, France.
Catherine Cornut-Chauvinc, Service de Neurologie clinique et fonctionnelle, Centre Hospitalier Lyon Sud, Hospices Civils de Lyon, 69310 Pierre-Bénite, France.
Véronique Manel, Service de Médecine Physique et Réadaptation Pédiatrique, L’Escale, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon, Groupement Est, 69500 Bron, France.
Emmanuelle Salort-Campana, Service de pathologies neuromusculaires, Hôpital de la Timone, Assistance Publique des Hôpitaux de Marseille, 13005 Marseille, France.
Shahram Attarian, Service de pathologies neuromusculaires, Hôpital de la Timone, Assistance Publique des Hôpitaux de Marseille, 13005 Marseille, France.
Etienne Fortanier, Service de pathologies neuromusculaires, Hôpital de la Timone, Assistance Publique des Hôpitaux de Marseille, 13005 Marseille, France.
Annie Verschueren, Service de pathologies neuromusculaires, Hôpital de la Timone, Assistance Publique des Hôpitaux de Marseille, 13005 Marseille, France.
Ludivine Kouton, Service de pathologies neuromusculaires, Hôpital de la Timone, Assistance Publique des Hôpitaux de Marseille, 13005 Marseille, France.
Jean-Philippe Camdessanché, Service de neurologie, centre référent pour les maladies neuromusculaires, Hôpital Nord, CHU de Saint Etienne, 42270 Saint-Etienne, France.
Céline Tard, Service de Neurologie, U1172, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, CHU de Lille, 59000 Lille, France.
Armelle Magot, Centre de référence des Maladies Neuromusculaires AOC, Euro-NMD, Filnemus, Hôtel-Dieu, CHU de Nantes, 44000 Nantes, France.
Yann Péréon, Centre de référence des Maladies Neuromusculaires AOC, Euro-NMD, Filnemus, Hôtel-Dieu, CHU de Nantes, 44000 Nantes, France.
Jean-Baptiste Noury, Inserm, LBAI, UMR1227, Centre de référence des Maladies Neuromusculaires AOC, CHRU de Brest, 29200 Brest, France.
Marie-Christine Minot-Myhie, Service de Neurologie, CHU de Rennes, 35000 Rennes, France.
Maud Perie, Service de Neurologie, CHU Gabriel Montpied, 63000 Clermont-Ferrand, France.
Frederic Taithe, Service de Neurologie, CHU Gabriel Montpied, 63000 Clermont-Ferrand, France.
Yacine Farhat, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, Institut de Myologie, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France.
Anne-Laure Millet, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, CHU Charles Nicolle, 76000 Rouen, France.
Pascal Cintas, Service de Neurologie, Centre de référence des Maladies Neuromusculaires, CHU de Toulouse Purpan, 31300 Toulouse, France.
Guilhem Solé, Service de Neurologie et des Maladies Neuromusculaires, Centre de référence des Maladies Neuromusculaires AOC, FILNEMUS, EURO-NMD, Hôpital Pellegrin, CHU de Bordeaux, 33000 Bordeaux, France.
Marco Spinazzi, Service de Neurologie, Centre de référence des Maladies Neuromusculaires, CHU d’Angers, 49100 Angers, France.
Florence Esselin, Service de Neurologie, CHU Gui de Chauliac, 34295 Montpellier, France.
Dimitri Renard, Service de Neurologie, Hôpital Caremeau, CHU de Nîmes, 30900 Nîmes, France.
Sabrina Sacconi, Service de Neurologie: Système nerveux périphérique, Muscle et SLA, Hôpital Pasteur 2, CHU de Nice, 06000 Nice, France.
Andra Ezaru, Service de Neurologie: Système nerveux périphérique, Muscle et SLA, Hôpital Pasteur 2, CHU de Nice, 06000 Nice, France.
Edoardo Malfatti, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, Hôpital Henry Mondor, Assistance Publique des Hôpitaux de Paris, Université Paris Est Créteil, INSERM, U955, IMRB, 94000 Créteil, France.
Martial Mallaret, Service de Neurologie, CHU de Grenoble, 38700 La Tronche, France.
Laurent Magy, Service de Neurologie, Centre de référence des Maladies Neuromusculaires, Hôpital Dupuytren, CHU de Limoges, 87000 Limoges, France.
Eva Diab, Service de Neurophysiologie Clinique, CHU Amiens Picardie, 80000, Amiens, France; Unité de Recherche Chimère UR 7516, Université Picardie Jules Verne, 80000 Amiens, France.
Philippe Merle, Service de Neurophysiologie Clinique, CHU Amiens Picardie, 80000, Amiens, France.
Maud Michaud, Service de Neurologie, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile-de-France, CHU de Nancy, 54000 Nancy, France.
Maxime Fournier, Service de Neurologie, CHU de Caen, 14000 Caen, France.
Aleksandra Nadaj Pakleza, Service de Neurologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, CHU de Strasbourg, 67000 Strasbourg, France; European Reference Network—Neuromuscular Diseases (ERN EURO-NMD), 75013 Paris, France.
Jean-Baptiste Chanson, Service de Neurologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, CHU de Strasbourg, 67000 Strasbourg, France; European Reference Network—Neuromuscular Diseases (ERN EURO-NMD), 75013 Paris, France.
Claire Lefeuvre, Service de Neurologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, Hôpital Raymond-Poincaré, Assistance Publique des Hôpitaux de Paris, 92380 Garches, France.
Pascal Laforet, Service de Neurologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, Hôpital Raymond-Poincaré, Assistance Publique des Hôpitaux de Paris, 92380 Garches, France; FHU PHENIX, Université Versailles, Université Paris-Saclay, 78000 Saint-Quentin-en-Yvelines, France.
Pascale Richard, Service de Biochimie Métabolique et Centre de Génétique, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France; Unité Fonctionnelle de Cardiogénétique et Myogénétique Moléculaire et cellulaire, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France.
Damien Sternberg, Service de Biochimie Métabolique et Centre de Génétique, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France.
Rocio-Nur Villar-Quiles, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, Institut de Myologie, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France; Centre de Recherche en Myologie, GH Pitié-Salpêtrière, Sorbonne Université-Inserm UMRS974, 75013 Paris, France.
Tanya Stojkovic, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, Institut de Myologie, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France; Centre de Recherche en Myologie, GH Pitié-Salpêtrière, Sorbonne Université-Inserm UMRS974, 75013 Paris, France.
Bruno Eymard, Centre de référence des Maladies Neuromusculaires Nord/Est/Ile de France, Institut de Myologie, Hôpital Pitié-Salpêtrière, Assistance Publique des Hôpitaux de Paris, 75013 Paris, France; Centre de Recherche en Myologie, GH Pitié-Salpêtrière, Sorbonne Université-Inserm UMRS974, 75013 Paris, France.
Data availability
The anonymized data that support the findings of this study are available from the corresponding author, upon reasonable request.
Funding
No financial assistance was received in support of the study.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Engel AG, Shen XM, Selcen D, Sine SM. Congenital myasthenic syndromes: Pathogenesis, diagnosis, and treatment. Lancet Neurol. 2015;14:420–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vanhaesebrouck AE, Beeson D. The congenital myasthenic syndromes: Expanding genetic and phenotypic spectrums and refining treatment strategies. Curr Opin Neurol. 2019;32:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramdas S, Beeson D. Congenital myasthenic syndromes: Where do we go from here? Neuromuscul Disord. 2021;31:943–954. [DOI] [PubMed] [Google Scholar]
- 4. Ohno K, Ohkawara B, Shen XM, Selcen D, Engel AG. Clinical and pathologic features of congenital myasthenic syndromes caused by 35 genes—A comprehensive review. Int J Mol Sci. 2023;24:3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Engel AG. Congenital myasthenic syndromes in 2018. Curr Neurol Neurosci Rep. 2018;18:46. [DOI] [PubMed] [Google Scholar]
- 6. Abicht A, Dusl M, Gallenmüller C, et al. . Congenital myasthenic syndromes: Achievements and limitations of phenotype-guided gene-after-gene sequencing in diagnostic practice: A study of 680 patients. Hum Mutat. 2012;33:1474–1484. [DOI] [PubMed] [Google Scholar]
- 7. Aharoni S, Sadeh M, Shapira Y, et al. . Congenital myasthenic syndrome in Israel: Genetic and clinical characterization. Neuromuscul Disord. 2017;27:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Natera-de Benito D, Töpf A, Vilchez JJ, et al. . Molecular characterization of congenital myasthenic syndromes in Spain. Neuromuscul Disord. 2017;27:1087–1098. [DOI] [PubMed] [Google Scholar]
- 9. Kao JC, Milone M, Selcen D, Shen XM, Engel AG, Liewluck T. Congenital myasthenic syndromes in adult neurology clinic: A long road to diagnosis and therapy. Neurology. 2018;91:e1770–e1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parr JR, Andrew MJ, Finnis M, Beeson D, Vincent A, Jayawant S. How common is childhood myasthenia? The UK incidence and prevalence of autoimmune and congenital myasthenia. Arch Dis Child. 2014;99:539–542. [DOI] [PubMed] [Google Scholar]
- 11. Troha Gergeli A, Neubauer D, Golli T, et al. . Prevalence and genetic subtypes of congenital myasthenic syndromes in the pediatric population of Slovenia. Eur J Paediatr Neurol. 2020;26:34–38. [DOI] [PubMed] [Google Scholar]
- 12. Finsterer J. Congenital myasthenic syndromes. Orphanet J Rare Dis. 2019;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iyadurai SJP. Congenital myasthenic syndromes. Neurol Clin. 2020;38:541–552. [DOI] [PubMed] [Google Scholar]
- 14. Garg N, Yiannikas C, Hardy TA, et al. . Late presentations of congenital myasthenic syndromes: How many do we miss?. Muscle Nerve. 2016;54:721–727. [DOI] [PubMed] [Google Scholar]
- 15. Stojkovic T, Masingue M, Turmel H, et al. . Diagnostic yield of a practical electrodiagnostic protocol discriminating between different congenital myasthenic syndromes. Neuromuscul Disord. 2022;32(11–12):870–878. [DOI] [PubMed] [Google Scholar]
- 16. Durmus H, Shen XM, Serdaroglu-Oflazer P, et al. . Congenital myasthenic syndromes in Turkey: Clinical clues and prognosis with long term follow-up. Neuromuscul Disord. 2018;28:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colomer J, Müller JS, Vernet A, et al. . Long-term improvement of slow-channel congenital myasthenic syndrome with fluoxetine. Neuromuscul Disord. 2006;16:329–333. [DOI] [PubMed] [Google Scholar]
- 18. Della Marina A, Wibbeler E, Abicht A, et al. . Long term follow-up on pediatric cases with congenital myasthenic syndromes—A retrospective single centre cohort study. Front Hum Neurosci. 2020;14:560860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poulos J, Samuels M, Palace J, et al. . Congenital myasthenic syndromes: A retrospective natural history study of respiratory outcomes in a single centre. Brain Commun. 2023;5:fcad299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wargon I, Richard P, Kuntzer T, et al. . Long-term follow-up of patients with congenital myasthenic syndrome caused by COLQ mutations. Neuromuscul Disord. 2012;22:318–324. [DOI] [PubMed] [Google Scholar]
- 21. Sine SM, Shen XM, Wang HL, et al. . Naturally occurring mutations at the acetylcholine receptor binding site independently alter ACh binding and channel gating. J Gen Physiol. 2002;120:483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang HL, Ohno K, Milone M, et al. . Fundamental gating mechanism of nicotinic receptor channel revealed by mutation causing a congenital myasthenic syndrome. J Gen Physiol. 2000;116:449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malfatti E, Catchpool T, Nouioua S, et al. . A TOR1AIP1 variant segregating with an early onset limb girdle myasthenia—Support for the role of LAP1 in NMJ function and disease. Neuropathol Appl Neurobiol. 2022;48:e12743. [DOI] [PubMed] [Google Scholar]
- 24. Masingue M, Cattaneo O, Wolff N, et al. . New mutation in the β1 propeller domain of LRP4 responsible for congenital myasthenic syndrome associated with cenani–lenz syndrome. Sci Rep. 2023;13:14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacquier A, Risson V, Simonet T, et al. . Severe congenital myasthenic syndromes caused by agrin mutations affecting secretion by motoneurons. Acta Neuropathol. 2022;10:707–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Echaniz-Laguna A, Biancalana V, Nadaj-Pakleza A, et al. . Homozygous C-terminal loss-of-function na V 1.4 variant in a patient with congenital myasthenic syndrome. J Neurol Neurosurg Psychiatry. 2020;91:898–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polavarapu K, Sunitha B, Töpf A, et al. . Clinical and genetic characterisation of a large Indian congenital myasthenic syndrome cohort. Brain. 2024;147:281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krenn M, Sener M, Rath J, et al. . The clinical and molecular landscape of congenital myasthenic syndromes in Austria: A nationwide study. J Neurol. 2023;270:909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Estephan EP, Zambon AA, Thompson R, et al. . Congenital myasthenic syndrome: Correlation between clinical features and molecular diagnosis. Eur J Neurol. 2022;29:833–842. [DOI] [PubMed] [Google Scholar]
- 30. Verschuuren JJ, Palace J, Murai H, Tannemaat MR, Kaminski HJ, Bril V. Advances and ongoing research in the treatment of autoimmune neuromuscular junction disorders. Lancet Neurol. 2022;21:189–202. [DOI] [PubMed] [Google Scholar]
- 31. Chaouch A, Müller JS, Guergueltcheva V, et al. . A retrospective clinical study of the treatment of slow-channel congenital myasthenic syndrome. J Neurol. 2012;259:474–481. [DOI] [PubMed] [Google Scholar]
- 32. Luan X, Tian W, Cao L. Limb-girdle congenital myasthenic syndrome in a Chinese family with novel mutations in MUSK gene and literature review. Clin Neurol Neurosurg. 2016;150:41–45. [DOI] [PubMed] [Google Scholar]
- 33. Nicole S, Chaouch A, Torbergsen T, et al. . Agrin mutations lead to a congenital myasthenic syndrome with distal muscle weakness and atrophy. Brain. 2014;137(Pt ):2429–2443. [DOI] [PubMed] [Google Scholar]
- 34. Senderek J, Müller JS, Dusl M, et al. . Hexosamine biosynthetic pathway mutations cause neuromuscular transmission defect. Am J Hum Genet. 2011;88:162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Belaya K, Cruz R, Liu PM, et al. . Mutations in GMPPB cause congenital myasthenic syndrome and bridge myasthenic disorders with dystroglycanopathies. Brain. 2015;138:2493–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ben Ammar A, Petit F, Alexandri N, et al. . Phenotype genotype analysis in 15 patients presenting a congenital myasthenic syndrome due to mutations in DOK7. J Neurol. 2010;257:754–766. [DOI] [PubMed] [Google Scholar]
- 37. Mihaylova V, Müller JS, Vilchez JJ, et al. . Clinical and molecular genetic findings in COLQ-mutant congenital myasthenic syndromes. Brain. 2008;131:747–759. [DOI] [PubMed] [Google Scholar]
- 38. Burke G, Cossins J, Maxwell S, et al. . Rapsyn mutations in hereditary myasthenia: Distinct early- and late-onset phenotypes. Neurology. 2003;61:826–828. [DOI] [PubMed] [Google Scholar]
- 39. Rodríguez Cruz PM, Belaya K, Basiri K, et al. . Clinical features of the myasthenic syndrome arising from mutations in GMPPB. J Neurol Neurosurg Psychiatry. 2016;87:802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bauché S, Vellieux G, Sternberg D, et al. . Mutations in GFPT1-related congenital myasthenic syndromes are associated with synaptic morphological defects and underlie a tubular aggregate myopathy with synaptopathy. J Neurol. 2017;264:1791–1803. [DOI] [PubMed] [Google Scholar]
- 41. Selcen D, Shen XM, Brengman J, et al. . DPAGT1 myasthenia and myopathy: Genetic, phenotypic, and expression studies. Neurology. 2014;82:1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O’Connell K, Rooney T, Alabaf S, Ramdas S, Beeson D, Palace J. Pregnancy outcomes in patients with congenital myasthenic syndromes. Muscle Nerve. 2022;66:345–348. [DOI] [PubMed] [Google Scholar]
- 43. Bauché S, O’Regan S, Azuma Y, et al. . Impaired presynaptic high-affinity choline transporter causes a congenital myasthenic syndrome with episodic apnea. Am J Hum Genet. 2016;99:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lozowska D, Ringel SP, Winder TL, Liu J, Liewluck T. Anticholinesterase therapy worsening head drop and limb weakness due to a novel DOK7 mutation. J Clin Neuromuscul Dis. 2015;17:72–77. [DOI] [PubMed] [Google Scholar]
- 45. Huang K, Duan H, Li Q, Luo Y, Bi F, Yang H. Clinicopathological-genetic features of congenital myasthenic syndrome from a Chinese neuromuscular centre. J Cell Mol Med. 2022;26:3828–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Di L, Chen H, Lu Y, et al. . Determinants of the repetitive-CMAP occurrence and therapy efficacy in slow-channel myasthenia. Neurology. 2020;95:e2781–e2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lornage X, Mallaret M, Silva-Rojas R, et al. . Selective loss of a LAP1 isoform causes a muscle-specific nuclear envelopathy. Neurogenetics. 2021;22:33–41. [DOI] [PubMed] [Google Scholar]
- 48. Cossins J, Webster R, Maxwell S, et al. . Congenital myasthenic syndrome due to a TOR1AIP1 mutation: A new disease pathway for impaired synaptic transmission. Brain Commun. 2020;2:fcaa174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The anonymized data that support the findings of this study are available from the corresponding author, upon reasonable request.