Abstract
Introduction
Understanding of adverse reactions to coronavirus disease 2019 vaccines remains limited.
Case presentation
Case 1: A 52‐year‐old woman, post‐kidney transplantation, experienced sudden vision loss in her left eye after receiving a second dose of coronavirus disease 2019 vaccine. She was diagnosed with ischemic optic neuropathy.
Case 2: A 53‐year‐old woman, post‐kidney transplantation, presented with worsening diplopia and left eye pain following the second dose of coronavirus disease 2019 vaccine. She was diagnosed with left abducens nerve palsy.
Conclusion
Vigilance is essential for recognizing the potential for delayed cranial neuropathy in immunocompromised individuals after coronavirus disease 2019 vaccination.
Keywords: COVID‐19, COVID‐19 vaccine, cranial neuropathy, infection, kidney transplantation
Keynote message.
We have encountered cases of cranial nerve damage attributed to COVID‐19 vaccination in post‐renal transplant patients. Notably, in immunosuppressed individuals, COVID‐19 vaccine‐induced neuropathy may manifest with a delayed onset compared to previous reports. Clinicians caring for immunosuppressed patients should maintain vigilance and consider a range of potential scenarios.
Abbreviations & Acronyms
- AION
arteritis ION
- COVID‐19
coronavirus disease 2019
- ION
ischemic optic neuropathy
- MRI
magnetic resonance imaging
- NAION
non‐arteritis ION
- SARS‐Cov‐2
severe acute respiratory syndrome coronavirus 2
- SOT
solid organ transplantation
Introduction
COVID‐19 is a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. COVID‐19 has become a pandemic and serious health threat worldwide.
Vaccination is one of the most effective means to protect against viral infections and recommended particularly in patients with SOT because the survival rate 60 days after COVID‐19 in patients with SOT has been reported to be significantly higher in vaccinated than in unvaccinated patients (83.6% vs 97.8%, p = 0.019). 1
Efros et al. 2 reported that the third dose of the COVID‐19 vaccine in patients undergoing SOT resulted in the type and frequency of adverse reactions to the vaccine being similar to those in healthy controls.
Most adverse reactions to the COVID‐19 vaccine are mild and temporary, such as puncture site pain, fatigue, myalgia, and fever. However, serious adverse reactions associated with mRNA vaccines are rarely reported.
Case reports
Case 1
A 52‐year‐old Japanese female patient, diagnosed with kidney failure due to immunoglobulin A nephropathy, underwent an ABO‐compatible living donor kidney transplantation, with her sister as the donor. The patient received the induction immunosuppressive drugs basiliximab, tacrolimus, mycophenolate mofetil, prednisolone, and everolimus. Postoperative renal function and general condition were stable. One year and 7 months after the kidney transplantation, she received her first dose of the COVID‐19 vaccine (Pfizer/BioNTech (BNT‐162b2)). Two weeks later, the patient visited the otolaryngology clinic because of drooping of the left angle of her mouth. On examination, there was no obvious skin rash, and hearing test results were normal. Blood tests, including renal function, did not show any significant abnormalities. Based on the patient's clinical findings, the otolaryngologist diagnosed idiopathic left facial nerve palsy and initiated steroid therapy with an initial dose of 500 mg of hydrocortisone, tapering over 7 days, which quickly improved her symptoms. Five weeks after the first dose, a second dose of COVID‐19 vaccine (Pfizer/BioNTech (BNT‐162b2)) was administered. Eight weeks later, she experienced a sudden loss of vision in her left eye and was referred to the ophthalmology department. During the ophthalmology visit, no indications of facial nerve palsy were found, and visual acuity in the left eye had decreased to 0.15 (visual acuity in the right eye: 1.0). No antinuclear, anti‐Aquaporin 4, anti‐cardiolipin, or P‐ANCA antibodies were detected. Fundus examination revealed edema of the optic nerve papilla and a surrounding hemorrhage in the left eye (shown in Fig. 1). Head MRI showed no intracranial lesions that could cause sight loss. Based on these findings, the ophthalmologist diagnosed the patient with ION. The patient was administered a 3‐day treatment of 125 mg methylprednisolone. After treatment, edema of the optic papilla improved. Although visual acuity in the left eye did not improve, there was no evidence of renal allograft loss. Anti‐COVID‐19 antibody titer remained negative (shown in Fig. 2a). She has not received the COVID‐19 vaccine since the second dose.
Fig. 1.
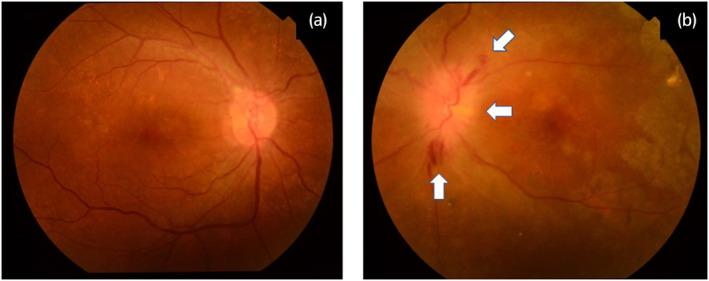
Fundoscopy: Fundoscopy shows hemorrhage and edema in the optic papilla (arrow), in the left eye (a, right eye: b, left eye).
Fig. 2.
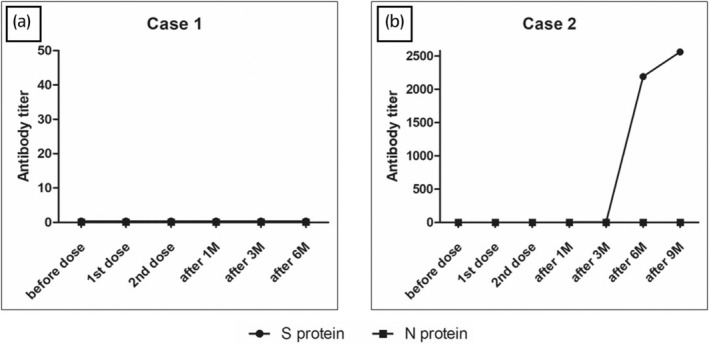
SARS‐CoV‐2 antibody titer: No increase in anti‐COVID‐19 antibody titers observed in case 1 but increased from the third dose in case 2 (a, case 1: b, case 2).
Case 2
A 53‐year‐old Japanese woman with kidney failure due to rheumatoid arthritis underwent an ABO‐compatible living‐donor kidney transplantation, with her husband as the donor. The patients received immunosuppressive therapy, similar to case 1, and their general condition was stable postoperatively.
One year and 6 months after the kidney transplantation, she received her first dose of Moderna COVID‐19 vaccine (mRNA‐1273). Two weeks after the first vaccination, she developed diplopia and pain in her left eye. The second vaccination was administered 4 weeks after the first dose. Due to worsening ocular symptoms, the patient was referred to an ophthalmologist. She was diagnosed with left upper and lower strabismus and left exotropia. Fundus examination and MRI revealed no abnormalities. There was no facial paralysis, dysarthria, or deviation of the tongue. No abnormalities were detected in the cerebrospinal fluid tests. Tests for anti‐acetylcholine receptor antibody, and anti‐aquaporin 4 antibody, and the ice‐pack test were all negative. Based on these findings, she was diagnosed with left abducens nerve palsy.
Steroid therapy (500 mg of methylprednisolone) was administered for 3 days, resulting in improvement of the patient's diplopia and eye pain from the day after starting the administration. The symptoms went into remission 5 days after treatment initiation. The anti‐COVID‐19 antibody titer remained negative until 6 months after the second vaccination but turned positive 1 month after the third vaccination (shown in Fig. 2b). She has not received the COVID‐19 vaccine since the second dose.
Discussion
Pfizer and Moderna COVID‐19 vaccines utilize mRNA technology to induce cells to trigger an immune response against the spike protein of the SARS‐CoV‐2 virus. The mRNA in these vaccines facilitates S protein synthesis and induces strong cellular and humoral immune responses. 3
ION is a condition characterized by damaged blood vessels that nourish the optic nerve and can be classified as AION or NAION. AION is often associated with giant cell arteritis, while the cause of NAION has not yet been fully elucidated. 4 Most cases of ION after COVID‐19 vaccination were NAION in several case reports and a Korean case series (Table 1). 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Only one case was diagnosed as AION, which was confirmed by a temporal artery biopsy. 6 Maleki et al. 6 also proposed that neutralizing antibodies directed against SARS‐CoV‐2 spike proteins after vaccination may cross‐react with proteins in the retinal vasculature and retinal pigment epithelial cells. Moreover, in COVID‐19 infection, excessive activation of the complement lectin pathway, triggered by the spike proteins, leads to hypercoagulability via overactivating coagulation factors. 16 It is possible that the development of NAION occurred due to microthrombotic adverse reactions of the posterior ciliary arteries supplying the optic nerve resulting from a similar reaction to the spike protein of SARS‐CoV‐2 spike proteins after vaccination.
Table 1.
Reported cases of ION following COVID‐19 vaccination
| Reported cases (n = 12) | Case series in Korea | Current study | |
|---|---|---|---|
| Age (median, range) | 64 (32–79) | 63.5 (43–77) | 52 |
| Sex (female/male, n) | 6/6 | 8/6 | Female |
| Dose (n) |
First: 7 Second: 5 |
Second | |
| Onset (day, median, range) | 6 (1–16) | 13.8 (1–41) | 63 |
| Underlying condition (n) |
DM: 5 HT: 2 |
DM: 1 HT: 4 |
Post‐kidney transplantation |
| Outcome (n) |
Improved: 7 Not improved: 2 Unknown: 3 |
No significant improvement | Not improved |
Abbreviations: DM, diabetes mellitus; HT, hypertension.
In addition, several cases of abducens nerve palsy after COVID‐19 vaccination have been reported (Table 2), 17 , 18 , 19 , 20 , 21 , 22 but, to our knowledge, these two cases are the first reports after COVID‐19 vaccination in kidney transplant recipients. This delay in the onset of cranial neuropathy might be associated with the diminished immune response observed in SOT patients, considering the reported lower antibody levels and seroconversion rates to the SARS‐CoV2 mRNA vaccines in SOT recipients compared to the general population. 23 Dey et al. 24 reported that the frequency of delayed adverse reactions was significantly higher in patients with systemic lupus erythematosus than in healthy controls 7 days after vaccination. It has also been noted that antibody titers take time to rise in immunosuppressed patients, 25 and the onset of adverse reactions may be delayed due to the slower onset of the immune response.
Table 2.
Reported cases of abducens nerve palsy following COVID‐19 vaccination
| Reported cases (n = 7) | Current study | |
|---|---|---|
| Age (median, range) | 35 (23–65) | 53 |
| Sex (female/male, n) | 1/6 | Female |
| Dose (n) |
First: 6 Second: 1 |
Second |
| Onset (day, median, range) | 6 (2–7) | 14 |
| Underlying condition (n) | Not particularly: 7 | Post‐kidney transplantation |
| Outcome (n) |
Improved: 5 Unknown: 2 |
Improved |
COVID‐19 vaccine is generally considered safe for patients with SOT. However, given that the onset of serious adverse events post‐vaccination may possibly be delayed in immunosuppressed patients, it is recommended to establish regular follow‐up procedures. Considering the nature of a case report, the applicability of this conclusion is restricted. The collection of case reports from immunosuppressed individuals, including transplant recipients and those with autoimmune conditions, is crucial for examining the side effects in this demographic.
Conclusion
We encountered two cases of cranial neuropathy in patients undergoing kidney transplantation, potentially associated with COVID‐19 vaccination. For immunosuppressed patients, such as solid organ transplant recipients, it is crucial to monitor them over an extended period to ensure the detection of any potential vaccine‐related adverse reactions.
Author contributions
Shota Fukae: Visualization; writing – original draft. Kazuaki Yamanaka: Conceptualization; validation. Soichi Matsumura: Data curation; formal analysis. Ryo Tanaka: Data curation; formal analysis. Shigeaki Nakazawa: Data curation; validation. Yoichi Kakuta: Conceptualization; methodology; project administration; supervision; validation. Norio Nonomura: Project administration; supervision.
Conflict of interest
The authors declare no conflict of interest.
Approval of the research protocol by an Institutional Reviewer Board
Not applicable.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and the accompanying images.
Registry and the Registration No. of the study/trial
Not applicable.
References
- 1. Hardgrave H, Wells A, Nigh J et al. COVID‐19 mortality in vaccinated vs. unvaccinated liver & kidney transplant recipients: a single‐center United States propensity score matching study on historical data. Vaccine 2022; 10: 1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Efros O, Anteby R, Halfon M, Meisel E, Klang E, Soffer S. Efficacy and safety of third dose of the COVID‐19 vaccine among solid organ transplant recipients: a systemic review and meta‐analysis. Vaccine 2022; 10: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holm MR, Poland GA. Critical aspects of packaging, storage, preparation, and administration of mRNA and adenovirus‐vectored COVID‐19 vaccines for optimal efficacy. Vaccine 2021; 39: 457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayreh SS. Ischemic optic neuropathy. Prog. Retin. Eye Res. 2009; 28: 34–62. [DOI] [PubMed] [Google Scholar]
- 5. Tsukii R, Kasuya Y, Makino S. Nonarteritic anterior ischemic optic neuropathy following COVID‐19 vaccination: consequence or coincidence. Case Rep. Ophthalmol. Med. 2021; 2021: 5126254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maleki A, Look‐Why S, Manhapra A, Stephen Foster C. COVID‐19 recombinant mRNA vaccines and serious ocular inflammatory side effects: real or coincidence? J. Ophthalmic Vis. Res. 2021; 16: 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elhusseiny AM, Sanders RN, Siddiqui MZ, Sallam AB. Non‐arteritic anterior ischemic optic neuropathy with macular star following COVID‐19 vaccination. Ocul. Immunol. Inflamm. 2022; 30: 1274–1277. [DOI] [PubMed] [Google Scholar]
- 8. Valsero Franco S, Fonollosa A. Ischemic optic neuropathy after administration of a SARS‐CoV‐2 vaccine: a report of 2 cases. Am. J. Case Rep. 2022; 27: e935095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin WY, Wang JJ, Lai CH. Non‐arteritic anterior ischemic optic neuropathy following COVID‐19 vaccination. Vaccine 2022; 10: 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murgova S, Balchev G. Ophthalmic manifestation after SARS‐CoV‐2 vaccination: a case series. J. Ophthalmic Inflamm. Infect. 2022; 12: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nachbor KM, Naravane AV, Adams OE, Abel AS. Nonarteritic anterior ischemic optic neuropathy associated with COVID‐19 vaccination. J. Neuroophthalmol. 2023; 43: e111–e113. [DOI] [PubMed] [Google Scholar]
- 12. Elnahry AG, Asal ZB, Shaikh N et al. Optic neuropathy after COVID‐19 vaccination: a report of two cases. Int. J. Neurosci. 2023; 133: 901–907. [DOI] [PubMed] [Google Scholar]
- 13. Haseeb A, Elhusseiny AM, Chauhan MZ, Elnahry AG. Optic neuropathy after COVID‐19 vaccination: case report and systematic review. Neuroimmunol. Rep. 2022; 2: 100121. [Google Scholar]
- 14. Girbardt C, Busch C, Al‐Sheikh M et al. Retinal vascular events after mRNA and adenoviral‐vectored COVID‐19 vaccines – a case series. Vaccine 2021; 9: 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moon Y, Jung JH, Shin HJ et al. Non‐arteritic ischemic optic neuropathy following COVID‐19 vaccination in Korea: a case series. J. Korean Med. Sci. 2023; 38: e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lesgards JF, Cerdan D, Perronne C et al. Toxicity of SARS‐CoV‐2 spike protein from the virus and produced from COVID‐19 mRNA or adenoviral DNA vaccines. Arch. Microbiol. Immunol. 2023; 7: 121–138. [Google Scholar]
- 17. Reyes‐Capo DP, Stevens SM, Cavuoto KM. Acute abducens nerve palsy following COVID‐19 vaccination. J. AAPOS 2021; 25: 302–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Veisi A, Najafi M, Hassanpour K, Bagheri A. Facial and abducens nerve palsies following COVID‐19 vaccination: report of two cases. Neuroophthalmology 2022; 46: 203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pereira A, Haslett RS. Acute abducens nerve palsy following the second dose of the AstraZeneca COVID‐19 vaccine. J. Pediatr. Ophthalmol. Strabismus 2021; 58: e49–e50. [DOI] [PubMed] [Google Scholar]
- 20. Manea MM, Dragoș D, Enache I, Sirbu AG, Tuta S. Multiple cranial nerve palsies following COVID‐19 vaccination – case report. Acta Neurol. Scand. 2022; 45: 257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pawar N, Ravindran M, Padmavathy S, Chakrabarty S. Acute abducens nerve palsy after COVID‐19 vaccination in a young adult. Indian J. Ophthalmol. 2021; 9: 3764–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalita IR, Singh HV, Sharma S. Acute abducens nerve palsy with acute disseminated encephalomyelitis‐like presentation following COVID‐19 vaccination. Indian J. Ophthalmol. 2023; 71: 2279–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Werbel WA, Boyarsky BJ, Ou MT et al. Safety and immunogenicity of a third dose of SARS‐CoV‐2 vaccine in solid organ transplant recipients: a case series. Ann. Intern. Med. 2021; 174: 1330–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dey M, Doskaliuk B, Lindblom J et al. COVID‐19 vaccination‐related delayed adverse events among patients with systemic lupus erythematosus. J. Clin. Med. 2023; 12: 7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benotmane I, Gautier G, Perrin P et al. Antibody response after a third dose of the mRNA‐1273 SARS‐CoV‐2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA 2021; 326: 1063–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]


