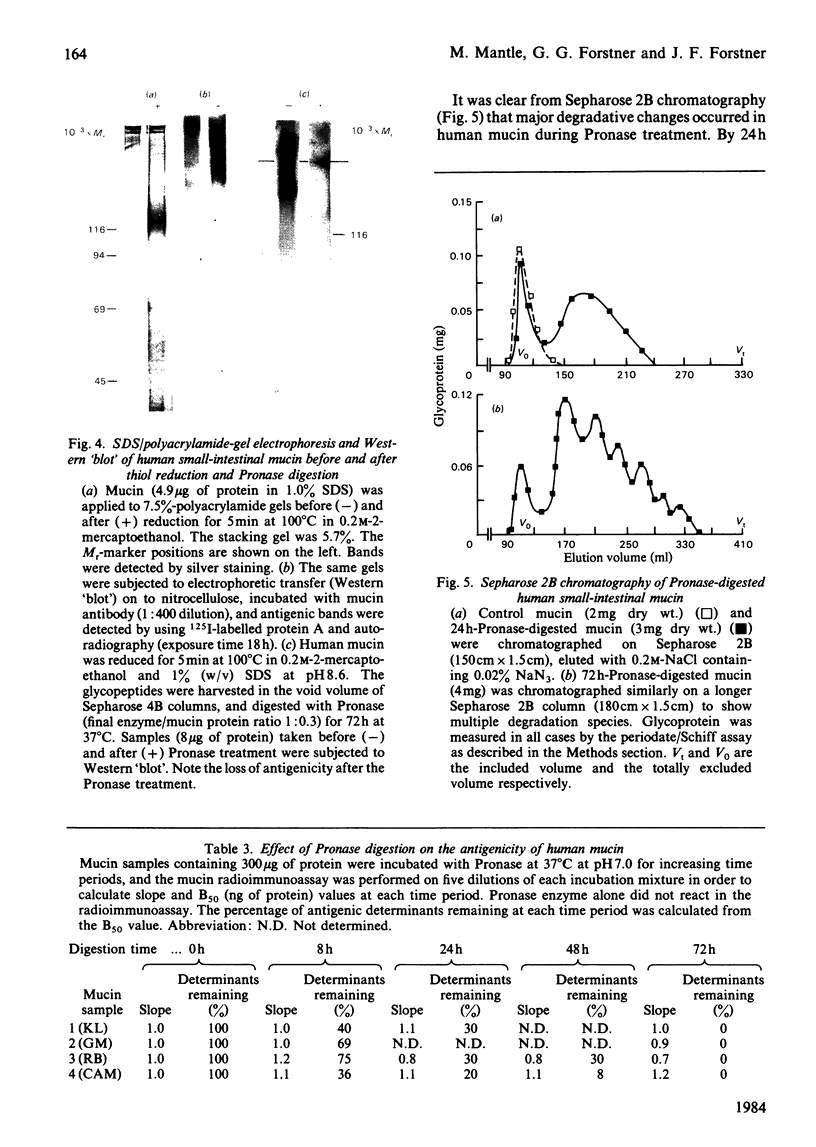
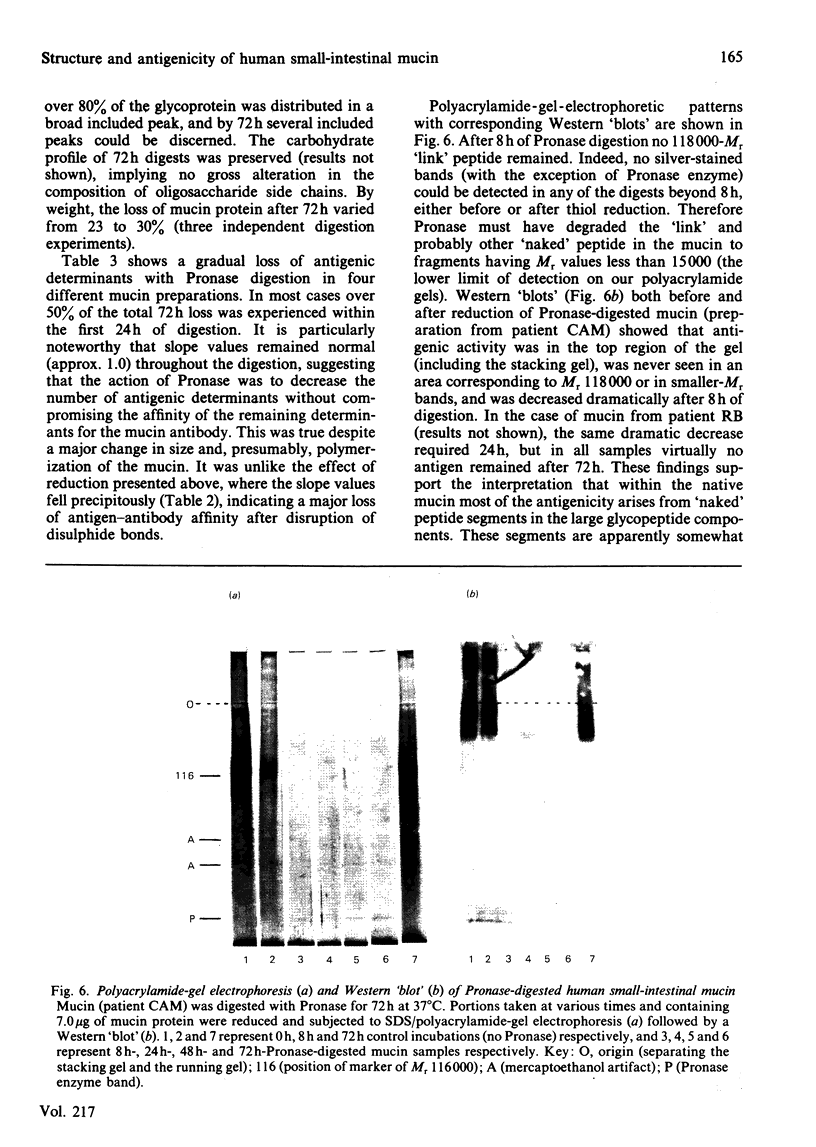
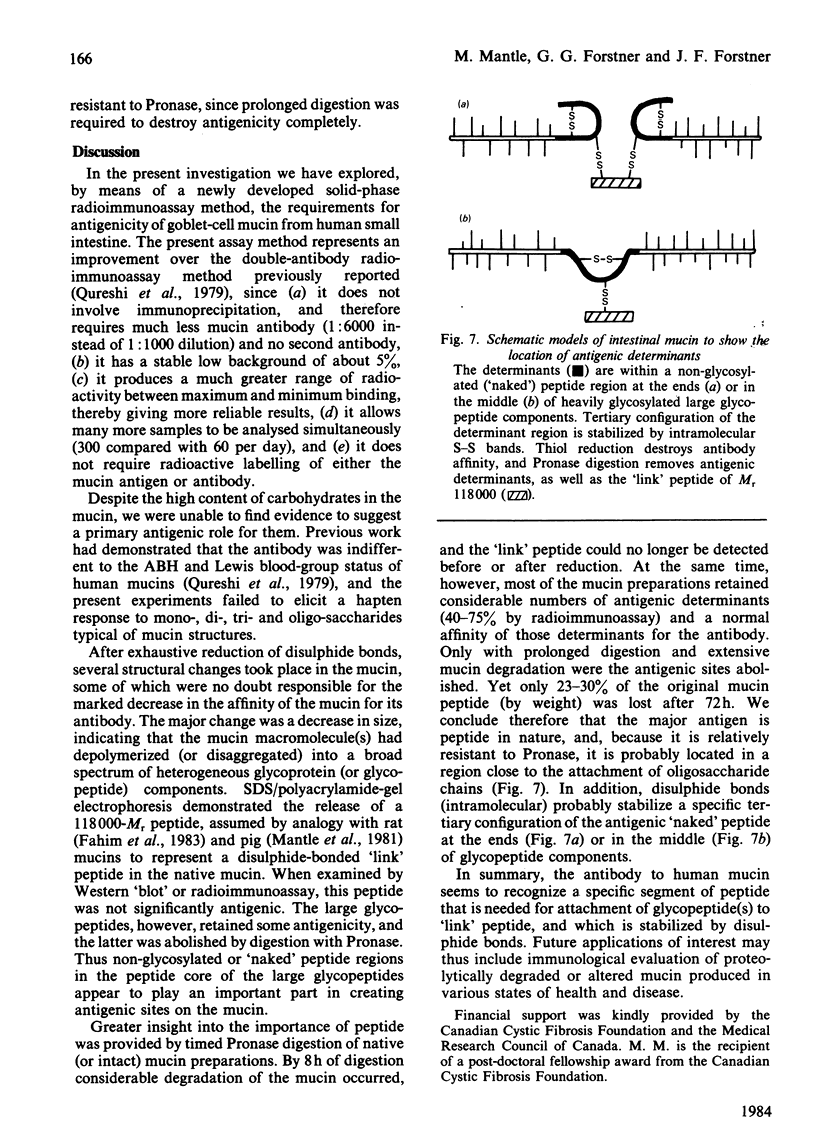
Abstract
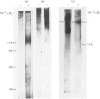
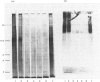
With the use of a newly developed solid-phase radioimmunoassay method, the major antigenic determinants of human small-intestinal goblet-cell mucin were investigated and related to the overall tertiary structure of the mucin. Preliminary hapten inhibition studies with various oligosaccharides of known sequence and structure suggested that the determinants did not reside in carbohydrate. Exhaustive thiol reduction, however, almost abolished antigenicity, caused breakdown of the mucin into small heterogeneous glycopeptides, and liberated a 'link' peptide of Mr 118000. Western 'blots' of reduced mucin from polyacrylamide gels on to nitrocellulose sheets showed that a small amount of residual antigenicity remained in large-Mr glycopeptides (Mr greater than 200000). The 'link' peptide was not antigenic. Timed Pronase digestion of native mucin resulted in a progressive loss of antigenic determinants. Gel electrophoresis revealed that after 8h of digestion the 118000-Mr peptide had disappeared, whereas antigenicity, which was confined to large-Mr glycopeptides, was destroyed much more slowly with time (70% by 24h, 100% by 72h). Despite the loss of antigenicity, 72h-Pronase-digested glycopeptides retained all of the carbohydrate of the native mucin. Therefore the antibody to human small-intestinal mucin appears to recognize a 'naked' (non-glycosylated and Pronase-susceptible) peptide region(s) of mucin glycopeptides. For full antigenicity, however, disulphide bonds are required to stabilize a specific three-dimensional configuration of the 'naked' region.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boland C. R., Montgomery C. K., Kim Y. S. A cancer-associated mucin alteration in benign colonic polyps. Gastroenterology. 1982 Apr;82(4):664–672. [PubMed] [Google Scholar]
- Boland C. R., Montgomery C. K., Kim Y. S. Alterations in human colonic mucin occurring with cellular differentiation and malignant transformation. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2051–2055. doi: 10.1073/pnas.79.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp J. R., Fraser G., Read A. E. Study of the carbohydrate content of mucus glycoproteins from normal and diseased colons. Clin Sci (Lond) 1981 Aug;61(2):229–234. doi: 10.1042/cs0610229. [DOI] [PubMed] [Google Scholar]
- Ehsanullah M., Filipe M. I., Gazzard B. Morphological and mucus secretion criteria for differential diagnosis of solitary ulcer syndrome and non-specific proctitis. J Clin Pathol. 1982 Jan;35(1):26–30. doi: 10.1136/jcp.35.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanullah M., Filipe M. I., Gazzard B. Mucin secretion in inflammatory bowel disease: correlation with disease activity and dysplasia. Gut. 1982 Jun;23(6):485–489. doi: 10.1136/gut.23.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim R. E., Forstner G. G., Forstner J. F. Heterogeneity of rat goblet-cell mucin before and after reduction. Biochem J. 1983 Jan 1;209(1):117–124. doi: 10.1042/bj2090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M. I., Fenger C. Histochemical characteristics of mucins in the small intestine. A comparative study of normal mucosa, benign epithelial tumours and carcinoma. Histochem J. 1979 May;11(3):277–287. doi: 10.1007/BF01005027. [DOI] [PubMed] [Google Scholar]
- Forstner J. F., Jabbal I., Qureshi R., Kells D. I., Forstner G. G. The role of disulphide bonds in human intestinal mucin. Biochem J. 1979 Sep 1;181(3):725–732. doi: 10.1042/bj1810725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner J. F., Roomi N. W., Fahim R. E., Forstner G. G. Cholera toxin stimulates secretion of immunoreactive intestinal mucin. Am J Physiol. 1981 Jan;240(1):G10–G16. doi: 10.1152/ajpgi.1981.240.1.G10. [DOI] [PubMed] [Google Scholar]
- Gold D. V., Miller F. Comparison of human colonic mucoprotein antigen from normal and neoplastic mucosa. Cancer Res. 1978 Oct;38(10):3204–3211. [PubMed] [Google Scholar]
- Henderson L. E., Oroszlan S., Konigsberg W. A micromethod for complete removal of dodecyl sulfate from proteins by ion-pair extraction. Anal Biochem. 1979 Feb;93(1):153–157. [PubMed] [Google Scholar]
- Jabbal I., Kells D. I., Forstner G., Forstner J. Human intestinal goblet cell mucin. Can J Biochem. 1976 Aug;54(8):707–716. doi: 10.1139/o76-102. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. P. Hypersecretion of mucus glycoprotein by the gallbladder epithelium in experimental cholelithiasis. J Pathol. 1981 Jul;134(3):199–207. doi: 10.1002/path.1711340304. [DOI] [PubMed] [Google Scholar]
- Lee S. P., LaMont J. T., Carey M. C. Role of gallbladder mucus hypersecretion in the evolution of cholesterol gallstones. J Clin Invest. 1981 Jun;67(6):1712–1723. doi: 10.1172/JCI110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings]. Biochem Soc Trans. 1978;6(3):607–609. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- Mantle M., Allen A. Isolation and characterization of the native glycoprotein from pig small-intestinal mucus. Biochem J. 1981 Apr 1;195(1):267–275. doi: 10.1042/bj1950267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Mantle D., Allen A. Polymeric structure of pig small-intestinal mucus glycoprotein. Dissociation by proteolysis or by reduction of disulphide bridges. Biochem J. 1981 Apr 1;195(1):277–285. doi: 10.1042/bj1950277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Nawa Y. Nippostrongylus brasiliensis: intestinal goblet-cell response in adoptively immunized rats. Exp Parasitol. 1979 Feb;47(1):81–90. doi: 10.1016/0014-4894(79)90010-9. [DOI] [PubMed] [Google Scholar]
- Qureshi R., Forstner G. G., Forstner J. F. Radioimmunoassay of human intestinal goblet cell mucin. Investigation of mucus from different organs and species. J Clin Invest. 1979 Nov;64(5):1149–1156. doi: 10.1172/JCI109568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr H. P., Mertens R. B. Cholera toxin-induced glycoprotein secretion in rabbit small intestine. Gastroenterology. 1979 Jul;77(1):18–25. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley A., Forstner J., Qureshi R., Mantle M., Forstner G. Human intestinal mucin in cystic fibrosis. Pediatr Res. 1983 Jan;17(1):65–69. doi: 10.1203/00006450-198301000-00013. [DOI] [PubMed] [Google Scholar]
- Younan F., Pearson J., Allen A., Venables C. Changes in the structure of the mucous gel on the mucosal surface of the stomach in association with peptic ulcer disease. Gastroenterology. 1982 May;82(5 Pt 1):827–831. [PubMed] [Google Scholar]