Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a fatal malignancy due to the difficulty in diagnosis and poor prognosis because of the high recurrence rate, necessitating reliable biomarkers to improve the diagnosis and prognosis. However, the existing markers have limitations. We previously identified extracellular vesicles (EVs) recognized by O‐glycan‐binding lectins (Amaranthus caudatus agglutinin [ACA]) as a novel diagnostic biomarker for PDAC using an EV‐counting system (ExoCounter). This retrospective study analyzed changes in ACA‐positive EVs in perioperative PDAC serum and its association with prognosis using ExoCounter. Absolute EV levels in the pre‐ and postoperative sera of 44 patients who underwent curative pancreatectomy for PDAC were quantified using ExoCounter. The carbohydrate antigen 19‐9 levels declined in most samples postoperatively, and presented no correlation with poor prognosis. In contrast, ACA‐positive EVs increased in serum at 7 days postoperatively in 27 of 44 patients (61.4%). We therefore divided participants with ACA‐positive EVs before and after surgery into elevation and decline groups. The overall survival (OS) and recurrence‐free survival (RFS) of patients with higher ACA‐positive EVs were significantly shorter than those with lower ACA‐positive EVs (26.1 months vs. not reached, P = 0.018; 11.9 vs. 38.6 months, P = 0.013). Multivariable analysis revealed that ACA‐positive EV elevation in postoperative serum was an independent prognostic factor for poor OS (hazard ratio [HR] = 3.891, P = 0.023) and RFS (HR = 2.650, P = 0.024). The detection of ACA‐positive EVs in perioperative serum may be used to predict the prognosis of PDAC in the early postoperative period.
Keywords: extracellular vesicles, lectin, liquid biopsy, O‐glycan, pancreatic cancer
This retrospective, observational study developed a system to predict poor prognosis of pancreatic ductal adenocarcinoma after surgery based on the change in Amaranthus caudatus agglutinin‐positive extracellular vesicles in patients' sera before surgery and after surgical resection.
1. INTRODUCTION
Despite progress in medical and health sciences, the prognosis of pancreatic ductal adenocarcinoma (PDAC) remains poor. The 5‐year survival rate of patients with PDAC is approximately 10%, which is mainly attributable to aggressive tumor behavior and delay in clinical detection. 1 Although surgical resection is the only curative treatment for PDAC, only 10%–20% of patients have resectable disease at initial presentation. 1 , 2 Furthermore, 80% of patients experience disease recurrence postoperatively, 3 and the 5‐year survival rate after resection for PDAC is approximately 20%. 4 In recent years, multidisciplinary treatment, including preoperative treatment and surgery, has become a focal point for improving the diagnosis and prognosis of PDAC. 5 , 6 , 7 Identifying a reliable biomarker is crucial for determining surgical indications, evaluating treatment efficacy, and predicting prognosis.
The most commonly used serum biomarker for detecting pancreatic cancer and predicting its prognosis is carbohydrate antigen 19‐9 (CA19‐9). The CA19‐9 levels are elevated in 69%–92% of patients with PDAC, 8 and preoperative elevation in CA19‐9 levels can predict the long‐term prognosis. 9 , 10 In addition, several liquid biopsy biomarkers have been evaluated to facilitate prognosis prediction, 11 but the prognosis for PDAC has not improved. Overall, there is dearth of reliable biological markers to guide adjuvant therapy and determine the prognosis of PDAC.
In PDAC, liquid biopsy analysis of cell‐free DNA, circulating tumor cells, and extracellular vesicles (EVs) from patients' blood samples, ascites fluid, saliva, or urine has been widely used for the detection of cancer‐related mutations, disease diagnosis at an early stage, and monitoring the treatment response. 12 , 13 , 14 , 15 , 16 , 17 EVs, measuring 40–160 nm in diameter, are membranous vesicles with a lipid bilayer that are actively released by most cells and circulate stably in body fluids. 18 EVs contain genetic biomaterials, such as nucleic acids and proteins, and contribute to intercellular communication. 18 , 19 , 20 , 21 EVs via are superior to other biomarkers, such as circulating tumor cells and DNA, for liquid biopsy assessment because of the abundance of EVs in biological fluids, inherent copious biological information from living cells, and innate stability conferred by the lipid bilayer. 21 , 22 , 23 Several studies have suggested that quantitative determination of EVs in blood samples would be clinically helpful for the diagnosis and prognostication of cancer. 24 , 25
Despite the importance of EV analysis, accurate quantification of EVs is encumbered by their small size and abundance of contaminants such as lipids and protein aggregates in body fluids. We developed an EV counting system, ExoCounter (JVC), by combining the properties of nanobeads with optical disc technology. 26 This system facilitated detection of the absolute number of specific EVs in serum, and we successfully detected a specific elevation in human epidermal growth factor receptor 2‐positive EVs in sera obtained from patients with ovarian and breast cancer. 26 Recently, we showed that O‐glycan altered EVs, which are recognized by Agaricus bisporus aggulutinin or Amaranthus caudatus agglutinin (ACA) lectins, were significantly elevated in PDAC sera in the early stage. 15 In that study, the O‐glycan altered EVs were reduced in the postoperative sera compared with those in the preoperative sera; however, the relationship between the change in their perioperative levels and prognosis, such as recurrence or survival after surgery, remains unclear.
In this study, we aimed to develop a system to predict the poor postoperative prognosis of PDAC by analyzing changes in ACA‐positive EVs in pre‐ and postoperative sera.
2. MATERIALS AND METHODS
2.1. Patients
The data of patients who underwent curative pancreatectomy for PDAC at Keio University Hospital between January 2016 and March 2020 were retrospectively analyzed. Invasive ductal adenocarcinoma was confirmed histologically in all patients. Patients who underwent R2 resection and those who did not undergo pre‐ and postoperative serum sampling were excluded. This study is a retrospective study and lacks randomization and blinding.
Written informed consent was obtained from all patients. This study was approved by the Ethics Committee of Keio University School of Medicine (No. 20170086) and conducted in accordance with the Helsinki Declaration of 1975.
2.2. Clinicopathological characteristics
This study analyzed preoperative clinical variables such as sex, age, presence of diabetes mellitus, treatment with neoadjuvant therapy, and surgical procedure. The postoperative variables analyzed were operative time, blood loss, and complications graded according to the Clavien‐Dindo classification.
The pathological stage was determined according to the eighth edition of the Union for international cancer control (UICC) TNM classification. R0 resection was characterized by the absence of residual tumor, R1 resection by microscopically positive margins, and R2 resection showed some remnant gross tumor. The histologically evaluated prognostic pathological parameters included tumor size, lymph node metastasis, lymphatic infiltration, venous infiltration, intrapancreatic nerve infiltration, distal bile duct invasion, duodenal invasion, serosal invasion, retropancreatic tissue invasion, portal vein invasion, arterial invasion, extrapancreatic nerve plexus invasion, and invasion of other organs. 27
2.3. Serum sample collection
Preoperative serum samples (12 mL) were collected 1 day before or on the day of the operation. Postoperative serum samples (12 mL) were obtained 7 days after the operation or on the day before hospital discharge if patients showed no signs of inflammation, such as high fever and elevated levels of inflammatory indicators in blood tests. ACA‐positive EVs were measured using pre‐ and postoperative serum samples. The CA19‐9 serum levels were measured in the first postoperative outpatient blood sample as well as in other general blood tests. The first postoperative outpatient visit was usually held approximately 2 weeks after discharge from the hospital.
Blood samples were collected in Venoject II tubes (Terumo) and processed on the same day as sample collection. To separate serum from peripheral blood cells, the samples were centrifuged at 1900g at 4°C for 10 min, and the sera were stored at −80°C.
2.4. Antibody and lectin
The anti‐cluster of differentiation 9 (anti‐CD9) antibody (clone 12A12, Cosmo Bio Co.) and the ACA lectin (EY Laboratories) were used for analysis.
2.5. Preparation of antibody‐conjugated nanobeads
Carboxylated affinity magnetic nanobeads (Formyl Glycine beads) were purchased from Tamagawa Seiki (Nagano). The beads were incubated with 200 mM 1‐ethyl‐3‐(3‐dimethylaminopropyl) carbodiimide hydrochloride and N‐hydroxysuccinimide in phosphate‐buffered saline (PBS) (pH = 7.4) for 4 h at room temperature. The beads were washed with 50 mM acetate buffer (pH = 5.2), followed by incubation with 1.0 g/L anti‐CD9 antibody in acetate buffer overnight at 4°C. The beads were then incubated with 1 M ethanolamine in PBS for 5 h at 4°C. The antibody‐conjugated beads were washed and stored in 4‐(2‐hydroxyethyl)‐1‐piperazinnethanesulfonic acid (HEPES) buffer (10 mM HEPES [pH = 7.9]), 50 mM KCl, 1 mM Ethylenediaminetetraacetic acid (EDTA), and 0.1% Tween 20 at 4°C.
2.6. Isolation of EVs from serum
The EVs were isolated using size‐exclusion chromatography (SEC) using qEV single 70‐nm columns (IZON). The qEV column was equilibrated with 4 mL of PBS. Thereafter, serum samples (100 μL) centrifuged at 2000g at 4°C for 5 min were applied to the column. The column was loaded with 1.1 mL PBS (discarded) and the EV fraction was eluted with 600 μL of PBS.
2.7. Quantification of EVs with ExoCounter
The EV fractions from pre‐ and postoperative sera were analyzed with ExoCounter using the following protocol. An optical disc was attached to a removal plate containing 16 wells for injecting samples. Each well was coated with 5 μg/mL of lectin (ACA) or antibody (anti‐CD9 antibody) in carbonate buffer (pH = 9.6) for 30 min at 37°C. After washing with PBS with 0.05% Tween 20 (PBS‐T), the disc was incubated with a blocking solution in PBS‐T (ACA:1% Carbo‐free blocking solution; Vector Laboratories, Inc.) or anti‐CD9 antibody:0.1% skim milk for 30 min at 37°C.
The EV fraction (50 μL sample) was incubated for 2 h at 37°C, followed by washing three times with PBS‐T. Subsequently, approximately 1.6 × 108 anti‐CD9‐antibody conjugated beads in blocking solution (0.1% casein in PBS‐T for ACA or 0.1% skim milk in PBS‐T for anti‐CD9 antibody) were incubated for 2 min under a magnetic field. Each well was first washed with PBS‐T and then with deionized water. The disc was dried in a thermostatic oven at 37°C for 15 min, and the ACA positive‐ or CD9 positive‐EVs were quantified with ExoCounter. The counts of ACA‐positive EVs from the pre‐ and postoperative samples were normalized to the counts of the CD9‐positive EVs from the same samples.
2.8. Statistical analysis
For the patient characteristics, summary statistics were constructed using frequencies and proportions for categorical data, and means and standard deviations (SDs) or medians and ranges for continuous variables. We compared patient characteristics using chi‐squared or Fisher's exact test for categorical outcomes and t‐tests for continuous variables, as appropriate.
For time‐to‐event outcomes, the lengths of time to a first event were compared using the log‐rank test, while the Kaplan–Meier method was used to estimate the absolute risk of each event for each group. Moreover, hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated by the Cox proportional hazards model. To identify pre‐ and postoperative variables associated with overall survival (OS), multivariable analysis was performed using the Cox proportional hazard model with a stepwise selection procedure. The stepwise procedure was set to a threshold of 0.05 for inclusion and 0.05 for exclusion.
A P value <0.01 was considered to be statistically significant. All statistical analyses were initially performed by using the IBM SPSS statistics version 27.0 (IBM Japan).
3. RESULTS
3.1. Patient characteristics
A total of 128 patients underwent curative resection at our institution between January 2016 and March 2020. Eighty‐four patients were excluded because of incomplete pre‐ and postoperative serum samples or lack of informed consent; the remaining 44 patients were enrolled in this study.
The patients' background characteristics are summarized in Table 1. The median age of all patients was 72 years and 28 patients (63%) were men. Fourteen patients (32%) received neoadjuvant treatment and 38 patients (86%) underwent adjuvant chemotherapy after resection. The median hospitalization duration was 22 days and 12 patients (27%) experienced postoperative morbidity. The serum levels of CA19‐9 were >100 (U/mL) in 18 (41%) and three (7%) patients pre‐ and postoperatively, respectively.
TABLE 1.
Clinicopathological characteristics of patients in the two groups.
| Parameter | Total (n = 44) | Group 1 (n = 27) | Group 2 (n = 17) | P value |
|---|---|---|---|---|
| Male gender, n (%) | 28 (63) | 19 (70) | 9 (53) | 0.088 |
| Age, years (median (range)) | 72 (49–94) | 72 (49–82) | 72 (55–94) | 0.453 |
| Diabetes mellitus, n (%) | 18 (41) | 11 (41) | 7 (41) | 0.956 |
| Procedure, n (PD/DP/TP) | 25/16/3 | 15/10/2 | 10/6/1 | 0.238 |
| Neoadjuvant treatment, n (%) | 14 (32) | 10 (37) | 4 (24) | 0.055 |
| Resection status, n (%) | 24 (55) | 15 (56) | 9 (53) | 0.931 |
| Lymph node metastasis, n (%) | 33 (75) | 20 (74) | 13 (76) | 0.726 |
| Stage, n (IA/IB/IIA/IIB/III/IV) | 1/0/9/32/2/0 | 1/0/5/19/2/0 | 0/0/4/13/0/0 | 0.523 |
| Adjuvant chemotherapy, n (%) | 38 (86) | 24 (89) | 14 (82) | 0.538 |
| First recurrence region, n (liver/lung/peritoneal/local/others) | 6/5/7/7/6 | 4/2/6/3/6 | 2/3/1/4/0 | 0.381 |
| Hospital stay, days (median (range)) | 22 (13–92) | 21 (13–39) | 24 (13–92) | 0.204 |
| Morbidity (Clavien–Dindo ≧grade3), n (%) | 12 (27) | 7 (26) | 5 (29) | 0.630 |
| Preoperative CA19‐9 >100 | 18 (41) | 9 (33) | 9 (53) | 0.198 |
| Postoperative CA19‐9 >100 | 3 (7) | 1 (4) | 2 (12) | 0.556 |
| Change ratio of ACA‐positive EVs | 1.11 | 1.55 | 0.68 | 0.013 |
Abbreviations: ACA, Amaranthus caudatus agglutinin; CA19‐9, carbohydrate antigen 19‐9; DP, distal pancreatectomy; EV, extracellular vesicle; PD, pancreatoduodenectomy; TP, total pancreatectomy.
3.2. Analysis of changes in ACA‐positive EVs between pre‐ and postoperative PDAC sera using ExoCounter
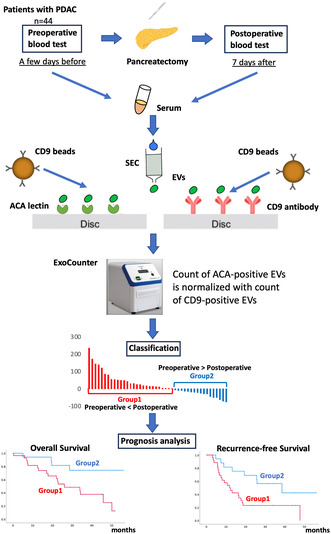
To evaluate ACA‐positive EVs, an analysis was performed using the experimental method schematically depicted in Figure 1. The EV fraction of PDAC sera from pre‐ and postoperative patients was obtained using SEC purification. The EVs isolated with SEC were validated using nanoparticle tracking analysis and scanning transmission electron microscopy (Figure 2A,B). The purified EVs were quantified using the exosome‐counting system, ExoCounter, which can measure the absolute number of EVs by detecting the FG bead–EV complexes on the optical disc using an optical disc drive. The ACA‐positive EVs were counted using ACA‐coated discs and anti‐CD9 antibody conjugated FG beads. We also analyzed CD9/CD9 double‐positive EVs, and the counts of ACA‐positive EVs were normalized to the counts of CD9/CD9 double‐positive EVs. Based on this protocol, we evaluated the changes in ACA‐positive EVs and CA19‐9 levels between pre‐ and postoperative PDAC serum samples (Figure 3A,B). While the CA19‐9 levels were significantly lower in the postoperative sera compared to the preoperative samples (P = 0.031), no significant difference was detected between the preoperative ACA‐positive EVs (P = 0.961). Examination of the changes in ACA‐positive EVs in each serum sample led to stratification into two groups: the postoperative ACA‐positive EV elevation group (Group 1) and the ACA‐positive EV decline group (Group 2). The waterfall plot of the change in the ratio of ACA‐positive EVs during operation identified 27 patients (27/44, 61.4%) in Group 1 and 17 patients (17/44, 38.6%) in Group 2 (Figure 3C).
FIGURE 1.
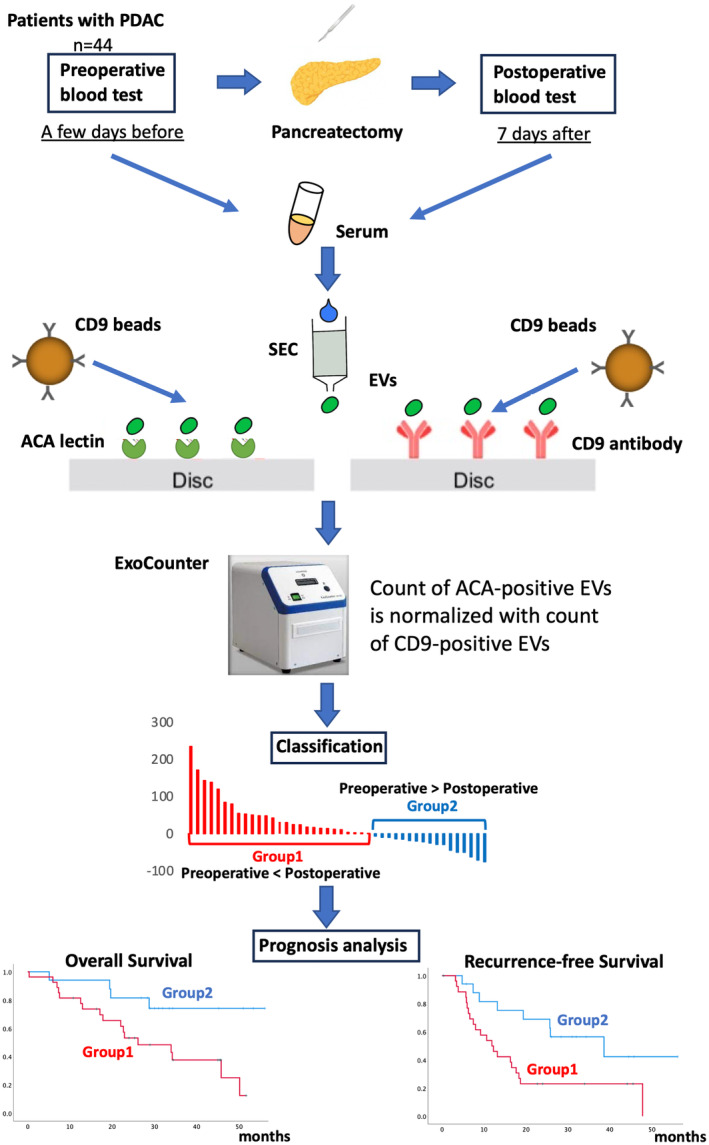
Experimental scheme of this study. The EV fraction from pre‐ and postoperative sera was purified using SEC in 44 patients with PDAC. ACA‐positive and CD9‐positive EVs were then measured using the ExoCounter. The ACA‐positive EV counts from pre‐ and postoperative samples were normalized to the CD9‐positive EV counts from the same sample. We evaluated the changes in ACA‐positive EVs between pre‐ and postoperative PDAC sera, classifying them into the postoperative ACA‐positive EV elevation group (Group 1) and the decline group (Group 2). Prognostic analyses, including overall survival and recurrence‐free survival, were conducted for these two groups. ACA, Amaranthus caudatus agglutinin; CD9, cluster of differentiation 9; EV, extracellular vesicle; PDAC, pancreatic ductal adenocarcinoma; SEC, size exclusion chromatography.
FIGURE 2.
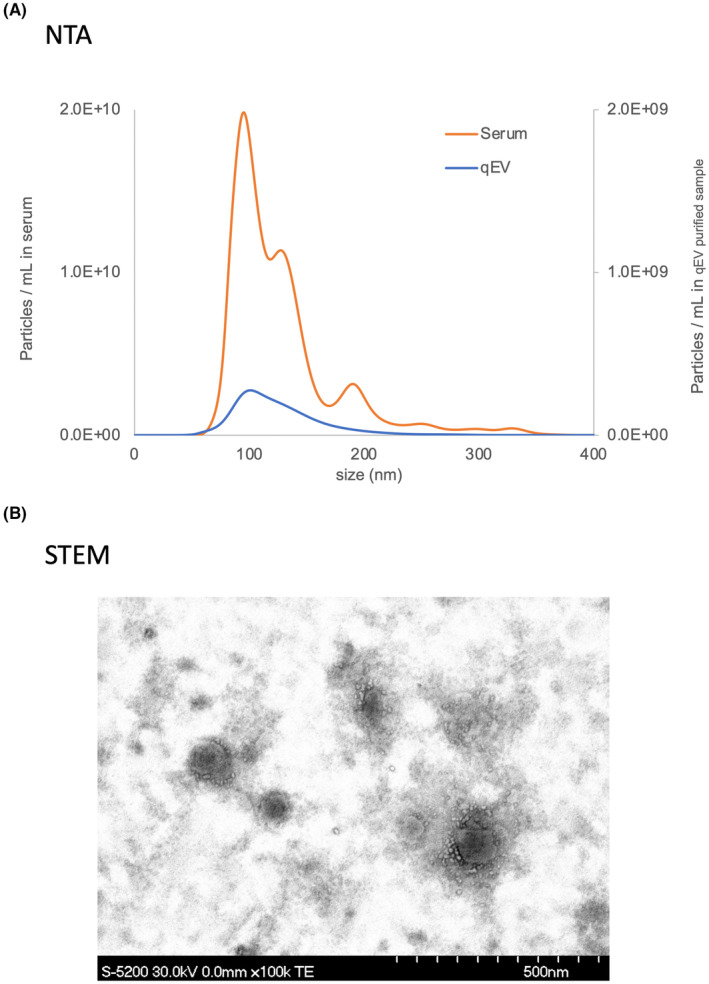
(A) The EVs in PDAC serum (orange line) and purified EVs in PDAC serum by size exclusion chromatography (qEV column) (blue line) were analyzed using NTA. (B) The purified EVs were observed using STEM. EV, extracellular vesicle; NTA, nanoparticle tracking analysis; PDAC, pancreatic ductal adenocarcinoma; qEV, ; STEM, scanning transmission electron microscopy.
FIGURE 3.
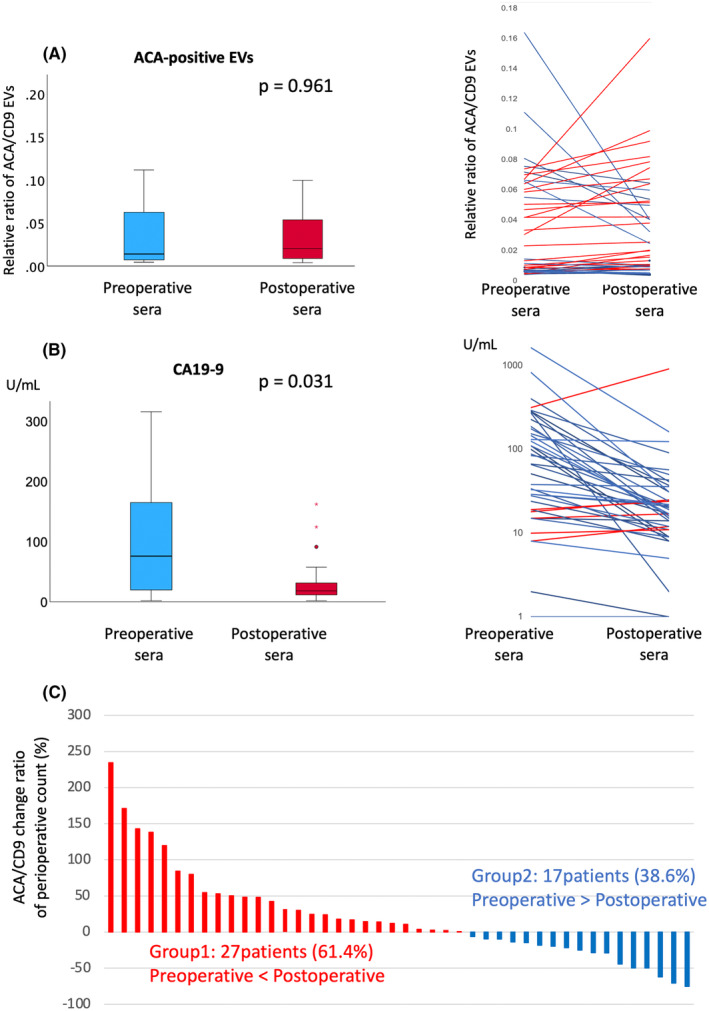
Comparison of ACA‐positive EVs (A) and CA19‐9 levels (B) between pre‐ and postoperative PDAC sera. ACA‐positive EV counts were normalized to CD9‐positive EV counts. The box plot indicates the 25th to 75th percentiles, with medians represented by colored bars. P values were calculated using the Mann–Whitney U test. Raw data for the comparison of ACA‐positive EVs (A) and CA19‐9 levels (B) between pre‐ and postoperative PDAC sera are shown. The blue line represents cases with decreased postoperative levels compared to preoperative samples, while the red line represents cases with increased levels. (C) Waterfall plot showing the change ratio of ACA‐positive EVs between pre‐ and postoperative sera. Samples were classified into postoperative ACA‐positive EV elevation (Group 1) and decline groups (Group 2). ACA, Amaranthus caudatus agglutinin; CA19‐9, carbohydrate antigen 19–9; CD9, cluster of diffentiation 9; EV, extracellular vesicle; PDAC, pancreatic ductal adenocarcinoma.
3.3. Increased ACA‐positive EVs in postoperative sera were highly correlated with poor prognosis
The demographic and clinical characteristics of both groups are presented in Table 1. All factors, including resection status, lymph node metastasis, and pathological stage, did not differ significantly between Groups 1 and 2. We performed a prognostic follow‐up of the two groups. The mean time duration of follow‐up was 27.6 (range 0.4–56.1) months. The OS was significantly shorter in Group 1 than that in Group 2 according to the log‐rank test (HR 3.452, 95% CI 1.155–10.321, P = 0.018; Figure 4A). The median OS was 26.1 months in Group 1 and not reached in Group 2. The 1‐ and 3‐year OS rates were 81.5% and 37.6% in Group 1 and 94.1% and 74.2% in Group 2, respectively. Recurrence‐free survival (RFS) was also significantly shorter in Group 1 than in Group 2 according to the log‐rank test (HR 2.735, 95% CI 1.195–2.626, P = 0.013; Figure 4B). The median RFS was 11.9 months in Group 1 and 38.6 months in Group 2. The 1‐ and 3‐year RFS rates were 50.0% and 23.1% in Group 1, and 81.6% and 56.5% in Group 2, respectively. In contrast, the CA19‐9 levels did not differ significantly concerning the OS and RFS between Groups 1 and 2 (Figure 4C,D).
FIGURE 4.
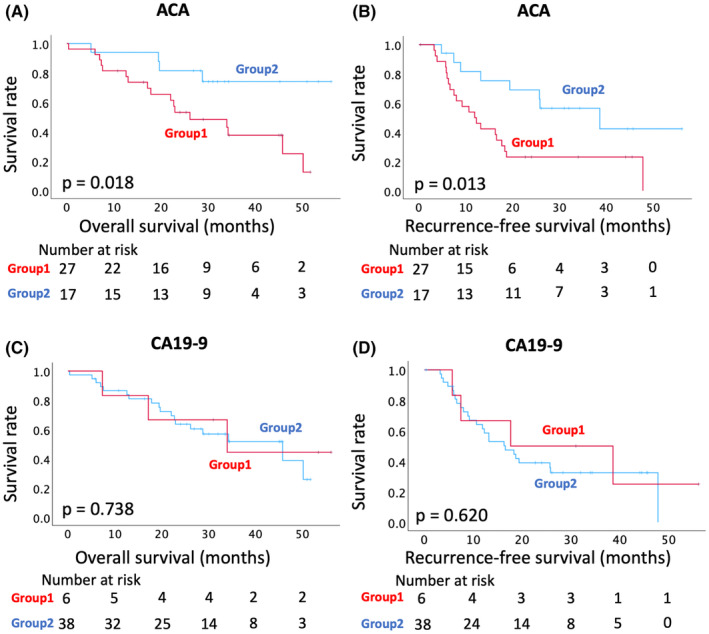
Kaplan–Meier survival analyses with the change in the ACA‐positive EVs in PDAC sera (A) Comparison of the overall survival (OS) between Groups 1 and 2. (B) Comparison of the recurrence‐free survival (RFS) between Groups 1 and 2. (C, D) Kaplan–Meier survival analyses with the change in CA19‐9 levels in PDAC sera. (C) Comparison of the OS between Groups 1 and 2. (D) Comparison of the RFS between Group 1 and 2. Survival curves and P values were calculated using the log‐rank test. ACA, Amaranthus caudatus agglutinin; CA19‐9, carbohydrate antigen 19‐9; EV, extracellular vesicle; PDAC, pancreatic ductal adenocarcinoma.
Univariable analysis of various factors, including pathological features, identified only ACA‐positive EV elevation in postoperative sera as a significant prognostic factor for poor OS and RFS (Table 2).
TABLE 2.
Univariable analysis of clinicopathological variables in relation to recurrence‐free and overall survival.
| Factor | Overall survival | Recurrence‐free survival | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| Increase of ACA‐positive EVs | 3.452 | 1.155–10.321 | 0.027 | 2.735 | 1.195–2.626 | 0.017 |
| Preoperative CA19‐9 >100 | 1.850 | 0.571–5.997 | 0.305 | 1.372 | 0.484–3.885 | 0.552 |
| Postoperative CA19‐9 >100 | 0.769 | 0.102–5.789 | 0.799 | 0.437 | 0.059–3.232 | 0.417 |
| Increase in CA19‐9 | 0.810 | 0.234–2.800 | 0.739 | 1.228 | 0.459–3.281 | 0.682 |
| Age | 0.584 | 0.225–1.514 | 0.269 | 0.989 | 0.951–1.029 | 0.583 |
| Sex (male) | 0.964 | 0.921–1.010 | 0.125 | 1.412 | 0.667–2.988 | 0.368 |
| Diabetes mellitus (+) | 0.687 | 0.277–1.702 | 0.417 | 0.666 | 0.308–1.439 | 0.301 |
| Neoadjuvant therapy | 1.986 | 0.832–4.739 | 0.122 | 1.809 | 0.845–3.874 | 0.127 |
| Operation time >450 min | 3.121 | 0.897–8.917 | 0.107 | 1.329 | 0.491–1.932 | 0.677 |
| Amount of bleeding >300 g | 1.340 | 0.319–3.971 | 0.539 | 1.109 | 0.490–2.239 | 0.449 |
| Complication (CD ≧ IIIa) (+) | 0.706 | 0.256–1.945 | 0.501 | 1.179 | 0.514–2.705 | 0.697 |
| Lymphatic infiltration (0 or 1) | 4.466 | 0.598–33.321 | 0.144 | 3.611 | 0.853–15.282 | 0.081 |
| Venous infiltration (0 or 1) | 22.282 | 0.006–79,274 | 0.457 | 23.121 | 0.051–10444.77 | 0.314 |
| Neural infiltration (0 or 1) | 1.983 | 0.264–14.913 | 0.506 | 3.028 | 0.411–22.326 | 0.277 |
| Serosal invasion (+) | 0.769 | 0.324–1.828 | 0.553 | 1.445 | 0.685–3.046 | 0.333 |
| Retropancreatic tissue invasion (+) | 1.022 | 0.236–4.428 | 0.977 | 1.117 | 0.345–4.019 | 0.794 |
| Distal bile duct invasion (+) | 2.246 | 0.849–5.942 | 0.103 | 1.114 | 0.490–2.532 | 0.797 |
| Duodenal invasion (+) | 0.613 | 0.223–1.690 | 0.345 | 0.483 | 0.195–1.200 | 0.117 |
| Extrapancreatic nerve plexus invasion (+) | 1.468 | 0.615–3.504 | 0.387 | 1.594 | 0.752–3.376 | 0.224 |
| Lymph node metastasis | 3.717 | 0.864–15.991 | 0.078 | 1.560 | 0.631–3.860 | 0.336 |
| Resection margin (R0) | 2.171 | 0.913–5.164 | 0.079 | 1.181 | 0.549–2.539 | 0.671 |
Abbreviations: ACA, Amaranthus caudatus agglutinin; CA19‐9, carbohydrate antigen 19‐9; CD, Clavien–Dindo; CI, confidence interval.
Multivariable analysis of ACA‐positive EV elevation and perioperative CA19‐9 decline revealed that ACA‐positive EV elevation in postoperative sera was an independent prognostic factor for poor OS and RFS (Table 3) (HR 3.891, 95% CI 1.209–12.519, P = 0.023; HR 2.650, 95% CI 1.134–6.192, P = 0.024). Thus, ACA‐positive EV elevation in postoperative sera could be useful for predicting poor prognosis in the early postoperative period.
TABLE 3.
Multivariable analysis of clinicopathological variables in relation to recurrence‐free and overall survival.
| Factor | Overall survival | Recurrence‐free survival | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| Increase of ACA‐positive EVs | 3.891 | 1.209–12.519 | 0.023 | 2.650 | 1.134–6.192 | 0.024 |
| Preoperative CA19‐9>100 | 1.810 | 0.697–4.701 | 0.223 | 1.447 | 0.660–3.171 | 0.356 |
| Postoperative CA19‐9>100 | 1.159 | 0.126–10.645 | 0.896 | 0.550 | 0.067–4.544 | 0.579 |
Abbreviations: ACA, Amaranthus caudatus agglutinin; CA19‐9, carbohydrate antigen 19‐9; CI, confidence interval; EV, extracellular vesicle.
4. DISCUSSION
The present study demonstrates that the change in the ratio of ACA‐positive EVs in the total EVs from sera sampled before and after surgery is a prognostic factor in patients with PDAC. The increase in ACA‐positive EVs after resection is an independent biomarker for poor OS and RFS. To the best of our knowledge, this study is the first to focus on changes in specific EVs among the total EVs obtained from pre‐ and postoperative PDAC sera.
CA19‐9 ranks among the most frequently used diagnostic markers for PDAC. Furthermore, CA19‐9 is often used as a marker for determining chemotherapeutic efficacy and predicting prognosis, including the evaluation of recurrence after PDAC treatment. 28 , 29 , 30 , 31 , 32 Several studies have reported that the CA19‐9 level in preoperative sera serves as a useful predictive marker of poor survival. 28 , 29 Other studies have shown that CA19‐9 in postoperative sera is also a predictive marker of recurrence. 30 , 31 However, the use of CA19‐9 as a predictive prognostic marker is beset by limitations. CA19‐9 cannot be detected in patients with Lewis‐antigen negative pancreatic cancer, who comprise approximately 10% of the total population. 33 , 34 , 35 , 36 , 37 , 38 We previously showed that ACA‐positive EVs could be detected in the sera of Lewis‐negative patients. 15 Furthermore, in many cases, the serum CA19‐9 levels immediately drop to normal values following curative resection, indicating that CA19‐9 cannot be used for prognosis in the early post‐surgical period. 39 , 40 , 41 In our study, almost all CA19‐9 levels decreased immediately postoperatively, while elevation of ACA‐positive EVs was observed in some patients, which was highly corelated with poor prognosis, indicating that the change in ACA‐positive EVs might be more sensitive to recurrence than CA19‐9 levels in the immediate postoperative period.
The reason underlying the persistence of ACA‐positive EVs in some patients and immediate decline in the CA19‐9 levels in serum postoperatively remains unclear. One possibility is that the EVs are released from residual or potentially metastatic tumors due to tumor manipulation during surgery. Badovinac et al. reported that the total EV concentration in plasma was elevated postoperatively for PDAC, 42 supporting this hypothesis. However, other studies have reported that the release of circulating tumor cells was higher due to the surgical procedures involved in pancreatoduodenectomy than those in distal pancreatectomy. 43 , 44 In our study, there was no significant difference in the surgical procedure between Groups 1 and 2. Another possibility may be the release of cancer‐specific EVs from the remnant tumor cells. In our study, there was no significant difference in the change in ACA‐positive EVs between patients who underwent R0 and R1 resection. Minimal residual disease (MRD) is defined as a small number of residual tumor cancer cells in the body after curative treatment, which cannot be detected by clinical examination, imaging, or pathological examination. 45 , 46 , 47 Although it is difficult to evaluate MRD, ACA‐positive EV may reflect MRD perioperatively. In summary, although the cause underlying the elevation of ACA‐positive EVs immediately after curative resection is unclear, ACA‐positive EVs may be elevated by the surgical manipulation of PDAC lesions with poor prognosis.
The prediction of poor prognosis using ACA‐positive EVs may support the decision to institute individualized follow‐up regimens. For example, in patients with elevated ACA‐positive EVs, blood tests and imaging are performed more frequently to confirm the diagnosis of recurrence more quickly.
Additionally, our data may contribute to the selection of adjuvant treatment strategies following surgery. 6 Generally, S‐1 adjuvant treatment for 1 year is strongly recommended in Japan, based on the results of the JASPAC 01 trial. 48 A recent study showed that S‐1 treatment did not prevent recurrence in patients with non‐normalized postoperative CA19‐9 levels. 40 For patients with unresectable, recurrent, or metastatic PDAC, more aggressive treatments such as gemcitabine plus nab‐paclitaxel or folinic acid + fluorouracil + irinotecan hydrochloride + oxaliplatin (FOLFIRINOX) are recommended, 49 , 50 which may improve prevention of recurrence in high‐risk patients, including those with elevated ACA‐positive EVs.
Several studies have suggested that alterations in O‐glycosylation are closely associated with multiple steps in carcinogenesis and tumor progression, including in breast cancer. 51 , 52 The O‐glycosylation enzyme, N‐acetylgalactosaminyltransferase (GALNT) 6 reportedly contributes to disease pathogenesis and affects prognosis in breast cancer. 53 , 54 In PDAC, several aberrations in O‐glycosylation have been shown to influence cancer regulation. 55 , 56 Zhang et al. reported that GALNT3 plays a crucial role in distinct phenotypes and metastatic behavior in PDAC cell lines. 57 Our previous study revealed that glycans stained with Agaricus bisporus Agglutinin (ABA) or ACA were present in PDAC, pancreatic intraepithelial neoplasia, and acinar‐to‐ductal metaplasia lesions. 15 These findings suggest that changes in O‐glycans contribute to the malignant progression of PDAC, although the correlation between these changes and PDAC prognosis requires further investigation.
This study had several limitations. First, this was a single‐center, retrospective analysis, therefore the possibility of selection bias cannot be excluded. Moreover, the sample size of this study was small, which could have attenuated the statistical accuracy in some aspects. Future studies should incorporate a large population of patients with PDAC. Second, in this study, blood tests were performed only at two time points, that is, pre‐ and postoperatively. Performing blood tests at several postoperative points may help confirm the effectiveness of pharmacotherapy and the tumor extent.
In conclusion, this study showed that the change in O‐glycan altered EVs between the pre‐ and postoperative sera could have potential clinical utility as a biomarker for predicting the survival and recurrence of PDAC postoperatively.
AUTHOR CONTRIBUTIONS
Sho Uemura: Conceptualization; formal analysis; investigation; software; validation; writing – original draft; writing – review and editing. Yasuaki Kabe: Conceptualization; methodology; project administration; software; writing – review and editing. Minoru Kitago: Conceptualization; formal analysis; funding acquisition; investigation; writing – review and editing. Sachiko Matsuda: Conceptualization; project administration. Yuta Abe: Data curation. Yasushi Hasegawa: Data curation. Shutaro Hori: Data curation. Masayuki Tanaka: Data curation. Yutaka Nakano: Funding acquisition; investigation. Yasunori Sato: Formal analysis. Makoto Itonaga: Methodology; validation. Masayuki Ono: Methodology; validation. Tatsuya Kawakami: Methodology; validation. Makoto Suematsu: Supervision; visualization. Yuko Kitagawa: Supervision; visualization.
FUNDING INFORMATION
This research was funded by AMED‐CREST (to Y.K., Grant No: JPgm0710010) and JSPS KAKENHI (to Y.K., Grant No: 18K06921). This study was funded partly by the JST ERATO Suematsu Gas Biology Project (until March 2015). This work was also funded by JST [Moonshot R&D, Grant Number JPMJPS2022]. This research was supported by JSPS KAKENHI (to Y.N., Grant No: 19K18132 and to M.K., Grant No: 24K11897). This research was supported by a joint research agreement with JVCKENWOOD Corporation (approval no. 2021122).
CONFLICT OF INTEREST STATEMENT
Yuko Kitagawa is an editorial board member of Cancer Science. The other authors have no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: This study was approved by Keio University Hospital (approval no. 20170086) and was registered in the University Hospital Medical Information Network (http://www.umin.ac.jp; registration number ID 000017751).
Informed Consent: This retrospective, observational study was conducted after obtaining informed content from patients.
Registry and the Registration No. of the study/trial: This study was approved by Keio University Hospital (approval no. 20170086).
Animal Studies: This study did not constitute animal investigations.
ACKNOWLEDGMENTS
We would like to thank Editage (http://www.editage.com/) for the English language editing.
Uemura S, Kabe Y, Kitago M, et al. Prognosis prediction of PDAC via detection of O‐glycan altered extracellular vesicles in perioperative sera. Cancer Sci. 2024;115:3718‐3728. doi: 10.1111/cas.16341
Contributor Information
Yasuaki Kabe, Email: ykabe@z3.keio.jp.
Minoru Kitago, Email: dragonpegasus@keio.jp.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7‐30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607‐620. doi: 10.1016/S0140-6736(10)62307-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19‐9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987;47(20):5501‐5503. [PubMed] [Google Scholar]
- 4. Matsumoto I, Murakami Y, Shinzeki M, et al. Proposed preoperative risk factors for early recurrence in patients with resectable pancreatic ductal adenocarcinoma after surgical resection: a multi‐center retrospective study. Pancreatology. 2015;15(6):674‐680. doi: 10.1016/j.pan.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 5. Endo Y, Kitago M, Kitagawa Y. Evidence and future perspectives for neoadjuvant therapy for resectable and borderline resectable pancreatic cancer: a scoping review. Cancers (Basel). 2024;16(9):1632. doi: 10.3390/cancers16091632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kitago M, Endo Y, Aiura K, et al. Adjuvant 5‐fluorouracil and portal vein infusion chemotherapy followed by gemcitabine for pancreatic cancer. Cancer Med. 2024;13(14):e7459. doi: 10.1002/cam4.7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Endo Y, Kitago M, Aiura K, et al. Efficacy and safety of preoperative 5‐fluorouracil, cisplatin, and mitomycin C in combination with radiotherapy in patients with resectable and borderline resectable pancreatic cancer: a long‐term follow‐up study. World J Surg Oncol. 2019;17(1):145. doi: 10.1186/s12957-019-1663-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Brien DP, Sandanayake NS, Jenkinson C, et al. Serum CA19‐9 is significantly upregulated up to 2 years before diagnosis with pancreatic cancer: implications for early disease detection. Clin Cancer Res. 2015;21(3):622‐631. doi: 10.1158/1078-0432.CCR-14-1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imaoka H, Shimizu Y, Senda Y, et al. Post‐adjuvant chemotherapy CA19‐9 levels predict prognosis in patients with pancreatic ductal adenocarcinoma: a retrospective cohort study. Pancreatology. 2016;16(4):658‐664. doi: 10.1016/j.pan.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 10. Dong Q, Yang XH, Zhang Y, et al. Elevated serum CA19‐9 level is a promising predictor for poor prognosis in patients with resectable pancreatic ductal adenocarcinoma: a pilot study. World J Surg Oncol. 2014;12:171. doi: 10.1186/1477-7819-12-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakano Y, Kitago M, Matsuda S, et al. KRAS mutations in cell‐free DNA from preoperative and postoperative sera as a pancreatic cancer marker: a retrospective study. Br J Cancer. 2018;118(5):662‐669. doi: 10.1038/bjc.2017.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kitago M, Koyanagi K, Nakamura T, et al. mRNA expression and BRAF mutation in circulating melanoma cells isolated from peripheral blood with high molecular weight melanoma‐associated antigen‐specific monoclonal antibody beads. Clin Chem. 2009;55(4):757‐764. doi: 10.1373/clinchem.2008.113225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takai E, Totoki Y, Nakamura H, et al. Clinical utility of circulating tumor DNA for molecular assessment in pancreatic cancer. Sci Rep. 2015;5:18425. doi: 10.1038/srep18425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao Y, Zhu Y, Yuan Z. Circulating tumor cells and circulating tumor DNA provide new insights into pancreatic cancer. Int J Med Sci. 2016;13(12):902‐913. doi: 10.7150/ijms.16556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yokose T, Kabe Y, Matsuda A, et al. O‐glycan‐altered extracellular vesicles: a specific serum marker elevated in pancreatic cancer. Cancers (Basel). 2020;12(9):2469. doi: 10.3390/cancers12092469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Street JM, Barran PE, Mackay CL, et al. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med. 2012;10:5. doi: 10.1186/1479-5876-10-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shinozaki M, O'Day SJ, Kitago M, et al. Utility of circulating B‐RAF DNA mutation in serum for monitoring melanoma patients receiving biochemotherapy. Clin Cancer Res. 2007;13(7):2068‐2074. doi: 10.1158/1078-0432.CCR-06-2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17(2):170. doi: 10.3390/ijms17010170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255‐289. doi: 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- 20. Cai X, Janku F, Zhan Q, Fan JB. Accessing genetic information with liquid biopsies. Trends Genet. 2015;31(10):564‐575. doi: 10.1016/j.tig.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 21. Yu W, Hurley J, Roberts D, et al. Exosome‐based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32(4):466‐477. doi: 10.1016/j.annonc.2020.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Théry C. Cancer: diagnosis by extracellular vesicles. Nature. 2015;523(7559):161‐162. doi: 10.1038/nature14569 [DOI] [PubMed] [Google Scholar]
- 23. An T, Qin S, Xu Y, et al. Exosomes serve as tumour markers for personalized diagnostics owing to their important role in cancer metastasis. J Extracell Vesicles. 2015;4:27522. doi: 10.3402/jev.v4.27522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Badovinac D, Goričar K, Zavrtanik H, et al. Plasma extracellular vesicle characteristics correlate with tumor differentiation and predict overall survival in patients with pancreatic ductal adenocarcinoma undergoing surgery with curative intent. J Pers Med. 2021;11(2):77. doi: 10.3390/jpm11010077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan BD, Wong WY, Lee MM, et al. Exosomes in inflammation and inflammatory disease. Proteomics. 2019;19(8):e1800149. doi: 10.1002/pmic.201800149 [DOI] [PubMed] [Google Scholar]
- 26. Kabe Y, Suematsu M, Sakamoto S, et al. Development of a highly sensitive device for counting the number of disease‐specific exosomes in human sera. Clin Chem. 2018;64(10):1463‐1473. doi: 10.1373/clinchem.2018.288084 [DOI] [PubMed] [Google Scholar]
- 27. Ishida M, Fujii T, Kishiwada M, et al. Japanese classification of pancreatic carcinoma by the Japan pancreas society. J Hepatobiliary Pancreat Sci. 2024. doi: 10.1002/jhbp.12056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez‐delCastillo C, Warshaw AL. Perioperative CA19‐9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24(18):2897‐2902. doi: 10.1200/JCO.2005.05.4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kondo N, Murakami Y, Uemura K, et al. Prognostic impact of perioperative serum CA 19‐9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2010;17(9):2321‐2329. doi: 10.1245/s10434-010-1031-6 [DOI] [PubMed] [Google Scholar]
- 30. Motoi F, Rikiyama T, Katayose Y, Egawa S, Unno M. Retrospective evaluation of the influence of postoperative tumor marker status on survival and patterns of recurrence after surgery for pancreatic cancer based on RECIST guidelines. Ann Surg Oncol. 2011;18(2):371‐379. doi: 10.1245/s10434-010-1323-x [DOI] [PubMed] [Google Scholar]
- 31. Berger AC, Garcia M Jr, Hoffman JP, et al. Postresection CA 19‐9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008;26(36):5918‐5922. doi: 10.1200/JCO.2008.17.7188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoshimasu T, Maebeya S, Suzuma T, et al. Disappearance curves for tumor markers after resection of intrathoracic malignancies. Int J Biol Markers. 1999;14(2):99‐105. [DOI] [PubMed] [Google Scholar]
- 33. Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP. Preoperative neutrophil‐to‐lymphocyte ratio (NLR) is associated with reduced disease‐free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011;35(4):868‐872. doi: 10.1007/s00268-010-0919-z [DOI] [PubMed] [Google Scholar]
- 34. Tempero MA, Malafa MP, Chiorean EG, et al. Pancreatic Adenocarcinoma, Version 1.2019. J Natl Compr Cancer Netw. 2019;17(3):202‐210. [DOI] [PubMed] [Google Scholar]
- 35. Proctor MJ, Morrison DS, Talwar D, et al. An inflammation‐based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow inflammation outcome study. Br J Cancer. 2011;104(4):726‐734. doi: 10.1038/bjc.2011.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267(5):936‐945. doi: 10.1097/SLA.0000000000002253 [DOI] [PubMed] [Google Scholar]
- 37. Luo G, Liu C, Guo M, et al. Potential biomarkers in Lewis negative patients with pancreatic cancer. Ann Surg. 2017;265(4):800‐805. doi: 10.1097/SLA.0000000000001766 [DOI] [PubMed] [Google Scholar]
- 38. Kawa S, Oguchi H, Kobayashi T, et al. Elevated serum levels of Dupan‐2 in pancreatic cancer patients negative for Lewis blood group phenotype. Br J Cancer. 1991;64(5):899‐902. doi: 10.1038/bjc.1991.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azizian A, Rühlmann F, Krause T, et al. CA19‐9 for detecting recurrence of pancreatic cancer. Sci Rep. 2020;10(1):1332. doi: 10.1038/s41598-020-58337-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ariake K, Okada T, Tsuchiya H, et al. Postoperative carbohydrate antigen 19‐9 level as a good indicator of ineffective response to the currently recommended S‐1 adjuvant chemotherapy for pancreatic ductal adenocarcinoma: a single‐center. Retrospective Study Ann Surg Oncol. 2024;31(1):525‐534. doi: 10.1245/s10434-023-13693-1 [DOI] [PubMed] [Google Scholar]
- 41. Rieser CJ, Zenati M, Hamad A, et al. CA19‐9 on postoperative surveillance in pancreatic ductal adenocarcinoma: predicting recurrence and changing prognosis over time. Ann Surg Oncol. 2018;25(12):3483‐3491. doi: 10.1245/s10434-018-6701-6 [DOI] [PubMed] [Google Scholar]
- 42. Badovinac D, Goričar K, Lavrin T, et al. Plasma extracellular vesicle characteristics as biomarkers of resectability and radicality of surgical resection in pancreatic cancer‐a prospective cohort study. Cancers (Basel). 2023;15(3):605. doi: 10.3390/cancers15030605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hirota M, Shimada S, Yamamoto K, et al. Pancreatectomy using the no‐touch isolation technique followed by extensive intraoperative peritoneal lavage to prevent cancer cell dissemination: a pilot study. JOP. 2005;6(2):143‐151. [PubMed] [Google Scholar]
- 44. Hirota M, Kanemitsu K, Takamori H, et al. Pancreatoduodenectomy using a no‐touch isolation technique. Am J Surg. 2010;199(5):e65‐e68. doi: 10.1016/j.amjsurg.2009.08.021 [DOI] [PubMed] [Google Scholar]
- 45. Honoré N, Galot R, van Marcke C, Limaye N, Machiels JP. Liquid biopsy to detect minimal residual disease: methodology and impact. Cancers (Basel). 2021;13(21):5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early‐stage lung cancer evolution. Nature. 2017;545(7655):446‐451. doi: 10.1038/nature22364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Valcz G, Buzás EI, Gatenby RA, Újvári B, Molnár B. Small extracellular vesicles from surviving cancer cells as multiparametric monitoring tools of measurable residual disease and therapeutic efficiency. Biochim Biophys Acta Rev Cancer. 2024;1879(2):189088. doi: 10.1016/j.bbcan.2023.189088 [DOI] [PubMed] [Google Scholar]
- 48. Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S‐1 versus gemcitabine for resected pancreatic cancer: a phase 3, open‐label, randomised, non‐inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248‐257. doi: 10.1016/S0140-6736(16)30583-9 [DOI] [PubMed] [Google Scholar]
- 49. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817‐1825. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 50. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691‐1703. doi: 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540‐555. doi: 10.1038/nrc3982 [DOI] [PubMed] [Google Scholar]
- 52. Liu C, Li Z, Xu L, et al. GALNT6 promotes breast cancer metastasis by increasing mucin‐type O‐glycosylation of α2M. Aging (Albany NY). 2020;12(12):11794‐11811. doi: 10.18632/aging.103332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Park JH, Nishidate T, Kijima K, et al. Critical roles of mucin 1 glycosylation by transactivated polypeptide N‐acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 2010;70(7):2759‐2769. doi: 10.1158/0008-5472.CAN-09-3792 [DOI] [PubMed] [Google Scholar]
- 54. Kimura R, Yoshimaru T, Matsushita Y, et al. The GALNT6‐LGALS3BP axis promotes breast cancer cell growth. Int J Oncol. 2020;56(2):581‐595. doi: 10.3892/ijo.2020.4974 [DOI] [PubMed] [Google Scholar]
- 55. Taniuchi K, Cerny RL, Tanouchi A, et al. Overexpression of GalNAc‐transferase GalNAc‐T3 promotes pancreatic cancer cell growth. Oncogene. 2011;30(49):4843‐4854. doi: 10.1038/onc.2011.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Munkley J. The glycosylation landscape of pancreatic cancer. Oncol Lett. 2019;17(3):2569‐2575. doi: 10.3892/ol.2019.9885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang T, van Die I, Tefsen B, et al. Differential O‐ and glycosphingolipid glycosylation in human pancreatic adenocarcinoma cells with opposite morphology and metastatic behavior. Front Oncol. 2020;10:732. doi: 10.3389/fonc.2020.00732 [DOI] [PMC free article] [PubMed] [Google Scholar]


