Abstract
Thymic epithelial tumors (TETs) are rare tumors arising from the mediastinum. Among TETs, thymoma type B2, B3 and thymic carcinoma are highly malignant and often present invasion and dissemination. However, the biological characteristics of TETs have not been thoroughly studied, and their mechanisms of invasion and dissemination are largely unknown. α‐Actinin 4 (ACTN4) is a member of actin‐binding proteins and reportedly plays important roles in the progression of several cancers. In this study, we investigated the relationship between ACTN4 and characteristics of the malignant potential of TETs, such as invasion and dissemination. In vitro experiments using Ty‐82 thymic carcinoma cells revealed that overexpression of ACTN4 enhanced the proliferative and invasive ability of Ty‐82 cells; conversely, knockdown of ACTN4 attenuated the proliferative and invasive potential of Ty‐82 cells. In western blotting (WB) experiments, ACTN4 induced the phosphorylation of extracellular signal–regulated kinase and glycogen synthase kinase 3β to regulate the β‐catenin/Slug pathway. Furthermore, WB analysis of cancer tissue–origin spheroids from patients with TETs showed results similar to those for Ty‐82 cells. In vivo experiments showed that the knockdown of ACTN4 significantly suppressed the dissemination of Ty‐82 cells. A WB and immunohistochemistry staining comparison of primary and disseminated lesions of TETs using surgical specimens showed upregulated expression of ACTN4, β‐catenin, and Slug proteins in disseminated lesions. In summary, our study suggests ACTN4 is associated with malignant potential characteristics such as invasion and dissemination in TETs via the β‐catenin/Slug pathway.
Keywords: ACTN4, dissemination, Slug, thymic carcinoma, thymoma
ACTN4 is associated with poor prognosis in several cancers. ACTN4 enhances the aggressiveness of thymic carcinoma cells. In addition, ACTN4 is involved in the dissemination of thymic epithelial tumors.
Abbreviations
- ACTN4
α‐Actinin 4
- CTOS
cancer tissue–origin spheroid
- EMT
epithelial–mesenchymal transition
- IHC
immunohistochemistry
- KD
knockdown
- OE
overexpression
- TETs
thymic epithelial tumors
- WB
western blotting
1. INTRODUCTION
Thymic epithelial tumors are the most common malignancy arising in the mediastinum. 1 TETs are rare, accounting for only 0.2%–1.5% of malignant tumors. 2 TETs include thymoma and thymic carcinoma. The majority of TETs are thymomas, whereas thymic carcinoma accounts for 10%–20% of TETs. 3 Thymoma is further divided into types A, AB, B1, B2, and B3 according to the World Health Organization classification. 4 TETs are broadly classified into two categories according to malignancy: low grade and high grade. Low‐grade TETs include types A, AB, and B1 thymoma, and high‐grade TETs include types B2 and B3 thymoma, as well as thymic carcinoma. 1 , 5
Surgery, chemotherapy, and radiation therapy are the standard treatments for TETs. 6 The complete surgical resection is a favorable prognostic factor for TETs. 7 , 8 Even in the cases of patients with type B3 thymoma or thymic carcinoma, which are highly malignant, surgical complete resection is considered a favorable prognostic factor. 9 , 10 However, some patients with invasion to adjacent organs and with pleural dissemination have an incomplete resection, resulting in high recurrence rates. 11 In such cases of advanced TETs, chemotherapy and radiotherapy are performed preoperatively to kill tumor cells in areas of invasion and dissemination, thus increasing the rate of complete resection. As effective chemotherapies for TETs are limited, new therapeutic options are needed for advanced TETs. The development of new therapeutic options for TETs necessitates elucidation of the mechanisms of invasion and dissemination. However, few studies have examined the malignant behavior of TETs, and one of the major reasons is that there are few useful cell lines and mouse tumor models available.
ACTN4 is a member of actin‐binding proteins and plays roles in several processes, including cellular motility and cell adhesion. 12 , 13 Previous reports demonstrated ACTN4 is a poor prognostic factor in several cancers, including oral tongue cancer, upper urinary tract urothelial carcinoma, and ovarian cancer. 14 , 15 , 16 ACTN4 also plays important roles in the proliferation, invasion, and dissemination of several cancer cells, including prostate cancer and osteosarcoma. 12 , 13 , 17 Furthermore, ACTN4 is reportedly involved in the Wnt/β‐catenin signaling pathway and in EMT, which involves the metastasis, invasion, and dissemination of various cancers, including non‐small cell lung cancer and pancreatic cancer. 18 , 19 However, the function of ACTN4 and β‐catenin in TETs has not been reported.
Slug is a transcription factor in EMT, and the β‐catenin/Slug pathway contributes to tumor invasion in malignant tumors such as head and neck squamous cell carcinoma. 20 Slug is also a poor prognostic factor in colorectal carcinoma, 21 and Slug is associated with poor prognosis in thymoma. 22
In this study, we evaluated the correlation between ACTN4 and clinical malignant behavior in high‐grade TETs, including prognosis, invasion and dissemination. We also investigated the function of ACTN4 in TETs using primary cultured cells established from human samples and a mouse TETs model of pleural dissemination.
2. MATERIALS AND METHODS
2.1. Material
RPMI 1640 medium was purchased from Thermo Fisher Scientific (Waltham, MA, USA), and FBS was obtained from HyClone Laboratories (Logan, UT, USA). Penicillin and streptomycin were purchased from Invitrogen (Carlsbad, CA, USA). Antibodies against ACTN4 for WB and IHC staining were purchased from Abcam (Cambridge, UK). Anti–β‐actin, extracellular signal–regulated kinase (ERK), phosphorylated ERK (pERK), phospho‐glycogen synthase kinase 3β (pGSK3β) (ser9), phospho‐β‐Catenin (Ser33/37/Thr41) and non‐phospho (Active) β‐Catenin (Ser33/37/Thr41) antibodies for WB were obtained from Cell Signaling Technology (Beverly, MA, USA). Anti–β‐catenin and anti‐Slug antibody for IHC were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Cell line
Ty‐82 human thymic carcinoma cells were obtained from the Japanese Collection of Research Bioresources (Osaka, Japan). Ty‐82 cells were maintained in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in a CO2 incubator.
2.3. Tissue samples and clinicopathologic data of patients with TETs
Surgical specimens were obtained from patients with TETs who underwent surgery at Osaka University Hospital. Between 2015 and 2021, 141 surgeries for TETs were performed at our institution. From these, high‐grade TETs cases were selected and immunostaining for ACTN4 was performed on 49 cases for which tissue specimens were available. Formalin‐fixed, paraffin‐embedded sections of tissues were subjected to immunohistochemistry (IHC) staining analysis (see Supplementary Information Data S1). The experimental methods for PCR and WB analyses using surgical specimens are also described in Supplementary Information Data S1.
2.4. Animal studies
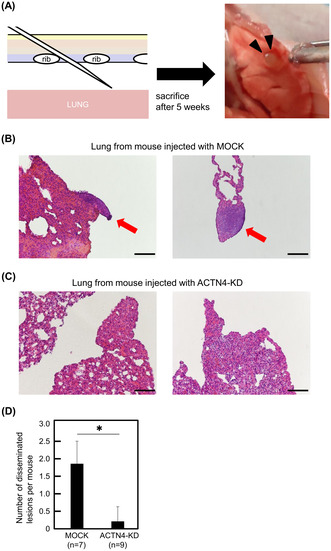
Ty‐82 cells with ACTN4 KD and control Ty‐82 cells were injected into the thoracic cavity of 4‐ to 5‐week‐old male BALB/cAJcl‐nu/nu mice (CLEA Japan, Tokyo, Japan). The mice were sacrificed 5 weeks after injection, and the thoracic cavity was observed, and lung and diaphragmatic tissues were collected. The collected tissues were formalin fixed, embedded in paraffin, and stained with hematoxylin–eosin (HE) to evaluate dissemination.
Detailed methods for each assay and statistical analysis are available in the Supplementary Information (Data S1).
3. RESULTS
3.1. ACTN4 expression in thymoma
First, we investigated ACTN4 gene expression levels in TETs using The Cancer Genome Atlas (TCGA) and GTEx databases via the GEPIA2 portal. ACTN4 gene expression in thymoma was significantly higher than in normal areas of the thymus (p < 0.01) (Figure 1A, B). Next, we investigated whether ACTN4 gene expression affected the prognosis of TETs patients using TCGA via the R2 Genomics Analysis and Visualization Platform. Thymoma patients exhibiting high ACTN4 expression had a significantly worse prognosis (p = 0.014) (Figure 1C). These data suggest that ACTN4 would be a useful TETs prognostic marker.
FIGURE 1.
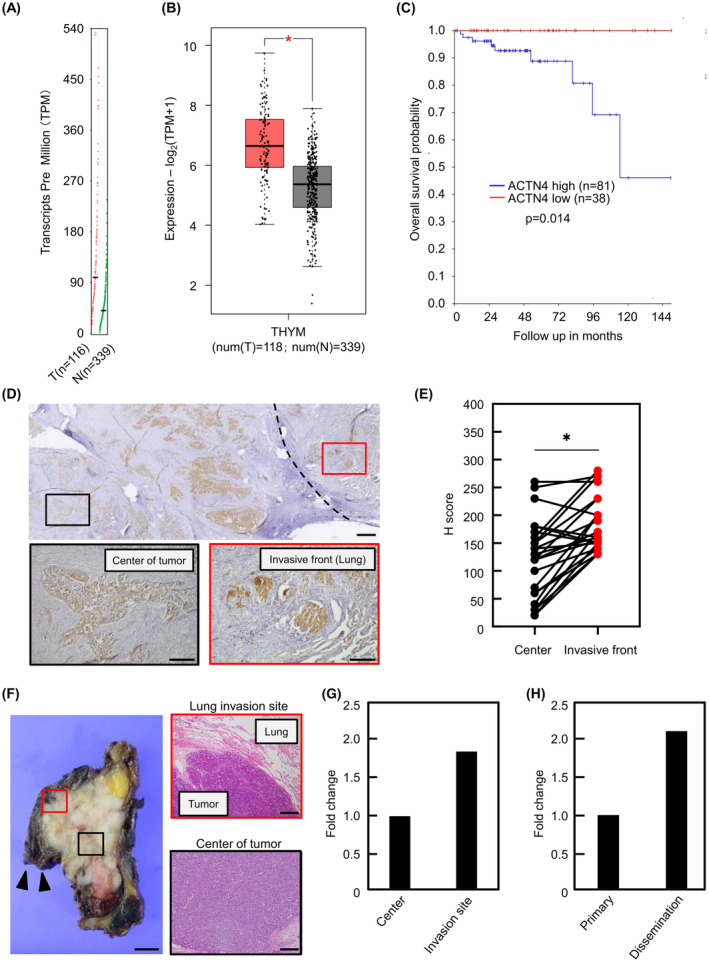
Expression and significance of α‐actinin4 (ACTN4) in TETs. (A, B) ACTN4 mRNA expression in thymoma was evaluated using The Cancer Genome Atlas (TCGA) and GTEx databases via the GEPIA2 portal. T indicates tumor tissue; N, normal tissue. (C) The overall survival rates associated with low and high levels of ACTN4 expression were analyzed using TCGA via the R2 Genomics Analysis and Visualization Platform. (D) Immunohistochemical staining of ACTN4 using surgical specimens from TETs patients. Black square indicates the center of the tumor, whereas red square indicates the site of lung invasion by the tumor. Scale bars indicate 1 mm and 200 μm (magnified image), respectively. (E) The intensity of immunohistochemical staining of ACTN4 in surgical specimens from patients with high‐grade TETs (n = 25) was compared between the frontier and invasion areas of the tumor and the center of the tumor. (F, G) ACTN4 mRNA levels at the invasion site and center of the tumor were compared by qPCR using surgical specimens of thymoma type B3. Black triangles indicate lung tissue. The red square indicates the lung invasion site, and the black square indicates the center of the tumor. Scale bars indicate 1 cm (macro image) and 200 μm (magnified image). (H) Differences in ACTN4 mRNA levels in primary and disseminated lesions were evaluated by qPCR using surgical specimens of thymic carcinoma. *p < 0.01.
Subsequently, in total, 49 surgical specimens from patients with high‐grade TETs were evaluated by IHC staining of ACTN4. Patients were divided into two groups based on ACTN4 staining intensity. The characteristics of these eligible patients are shown in Table 1. Table 2 shows the characteristics of patients classified by ACTN4‐high and ACTN4‐low or ACTN4‐negative. Although there was no difference in Masaoka stage between the two groups, the percentage of type B3 thymoma and thymic carcinoma, which is highly malignant in TETs, was significantly higher in the ACTN4 high group (84.0% vs. 54.2%, p < 0.05). No deaths were observed in ACTN4‐low or ACTN4‐negative group, and there was no significant difference in overall survival between the two groups (Figure S1).
TABLE 1.
Patients' characteristics.
| Factor | Number of patients (%) |
|---|---|
| Age (years) | |
| <60 | 21 (42.9) |
| ≥60 | 28 (57.1) |
| Sex | |
| Male | 25 (51.0) |
| Female | 24 (49.0) |
| Histology | |
| B2 | 15 (30.6) |
| B3 | 23 (46.9) |
| Sq | 11 (22.5) |
| Masaoka stage | |
| I | 17 (34.7) |
| II | 16 (32.6) |
| III | 9 (18.4) |
| IVa | 4 (8.2) |
| IVb | 3 (6.1) |
| ACTN4 | |
| High | 25 (51.0) |
| Low | 24 (49.0) |
Abbreviations: B2, thymoma type B2; B3, thymoma type B3; Sq, thymic squamous cell carcinoma.
TABLE 2.
Patients' characteristics of ACTN4 high and low groups.
| Factor | ACTN4‐high (n = 25) | ACTN4‐low or ACTN4‐negative (n = 24) | p value |
|---|---|---|---|
| Age (years) | |||
| Mean | 60.8 | 60.3 | 0.90 |
| Range | 28–84 | 43–81 | |
| Sex | |||
| Male | 14 (56.0) | 11 (45.8) | 0.48 |
| Female | 11 (44.0) | 13 (54.2) | |
| Histology | |||
| B2 | 4 (16.0) | 11 (45.8) | 0.02 |
| B3 or Sq | 21 (84.0) | 13 (54.2) | |
| Masaoka stage | |||
| I–II | 17 (68.0) | 16 (66.7) | 0.92 |
| III–IV | 8 (32.0) | 8 (33.3) | |
Abbreviations: B2, thymoma type B2; B3, thymoma type B3; Sq, thymic squamous cell carcinoma.
3.2. ACTN4 expression in sites of invasion and dissemination in high‐grade TETs
The intensity of ACTN4 staining was compared between the center and invasive front of the tumor in the 25 ACTN4‐high patients. A representative pathological image of ACTN4 IHC staining is shown in Figure 1D. ACTN4 staining intensity was evaluated using H‐scores. ACTN4 staining was more intense in the frontier edge and invasion areas of the tumor than in the center of the tumor (p < 0.01) (Figure 1E). qPCR analysis was performed to compare ACTN4 mRNA levels in the invasion and non‐invasion areas of a surgical specimen of type B3 thymoma that invaded the lung. The expression of ACTN4 mRNA was higher in the lung invasion site than that in the center of the tumor (Figure 1F,G). ACTN4 mRNA expression was also evaluated using a surgical specimen of a thymic carcinoma exhibiting pleural dissemination. Compared with the primary lesion, ACTN4 mRNA expression was higher in the disseminated lesion (Figure 1H). The background of these patients is shown in Table S1. These results indicate ACTN4 could be associated with invasion and dissemination in high‐grade TETs.
3.3. Effect of ACTN4 on the proliferative capacity of Ty‐82 cells
The effect of ACTN4 on proliferation was examined using Ty‐82 cells overexpressing ACTN4 (ACTN4 OE) and Ty‐82 cells knocked down ACTN4 (ACTN4 KD). The ACTN4 mRNA level in each cell line was confirmed using qPCR compared to respective controls (Figure 2A,B). In WST‐1 assays, the proliferation of ACTN4 OE cells was significantly enhanced compared with control cells. Conversely, the proliferation of ACTN4 KD cells was significantly suppressed compared to control cells (Figure 2C,D).
FIGURE 2.
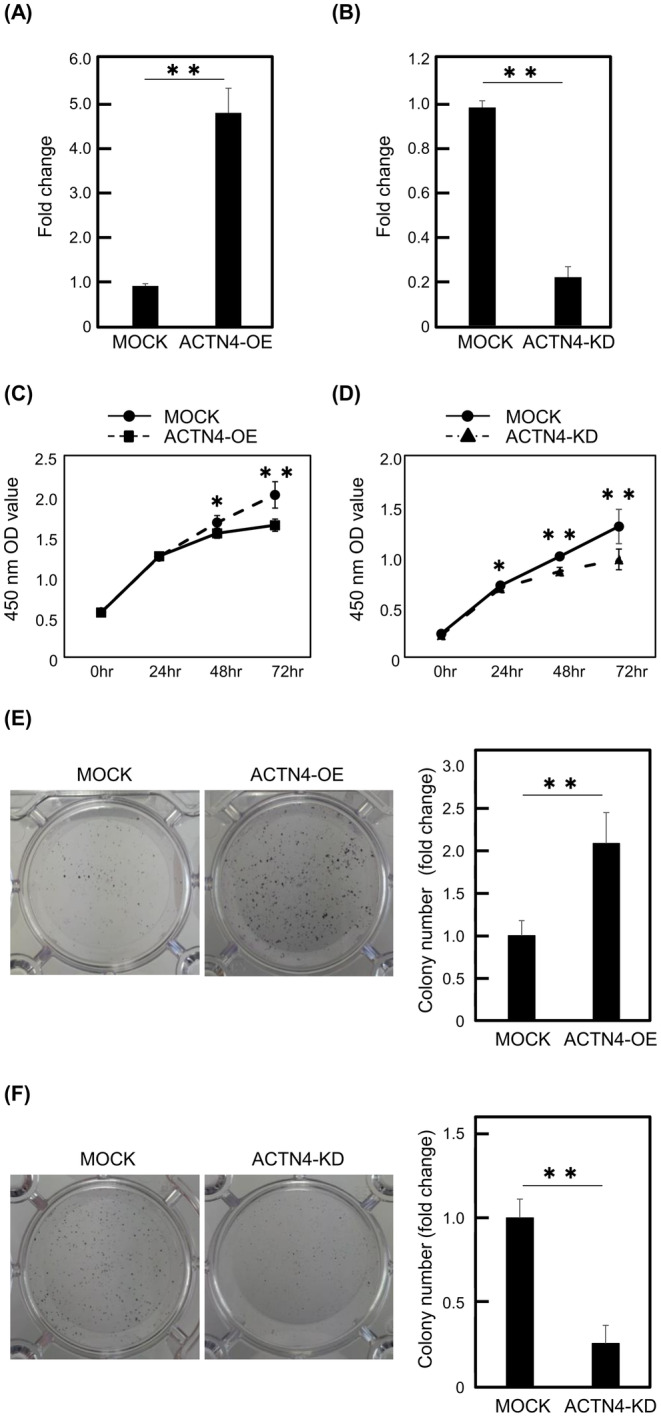
ACTN4 promotes the proliferation of Ty‐82 cells. (A, B) ACTN4 mRNA levels in ACTN4‐overexpressing and ACTN4‐knockdown Ty‐82 cells were compared with the respective controls. (C, D) The effect of ACTN4 on the proliferative capacity of Ty‐82 cells was evaluated using the WST‐1 assay. (E, F) The effect of ACTN4 on proliferative capacity was assessed using the colony formation assay. *p < 0.05, **p < 0.01.
Next, we performed colony formation assays. The number of colonies formed was significantly increased in ACTN4 OE Ty‐82 cells but decreased in ACTN4 KD Ty‐82 cells (p < 0.01) (Figure 2E,F). These results indicated that ACTN4 enhances the proliferation of Ty‐82 cells.
3.4. Effect of ACTN4 on the migration and invasive capacity of Ty‐82 cells
Transwell migration assays were performed to determine the effect of ACTN4 on the migration capability of Ty‐82. The number of migrating ACTN4 OE cells was significantly higher compared with the control cells (p < 0.05) (Figure 3A). Conversely, the number of migrating ACTN4 KD cells was reduced compared with control cells (p < 0.05) (Figure 3B). The effect of ACTN4 on the invasive capacity of Ty‐82 was consistent with the results of migration assays (Figure 3C,D). These results indicate ACTN4 enhances Ty‐82 cell migration and invasion.
FIGURE 3.
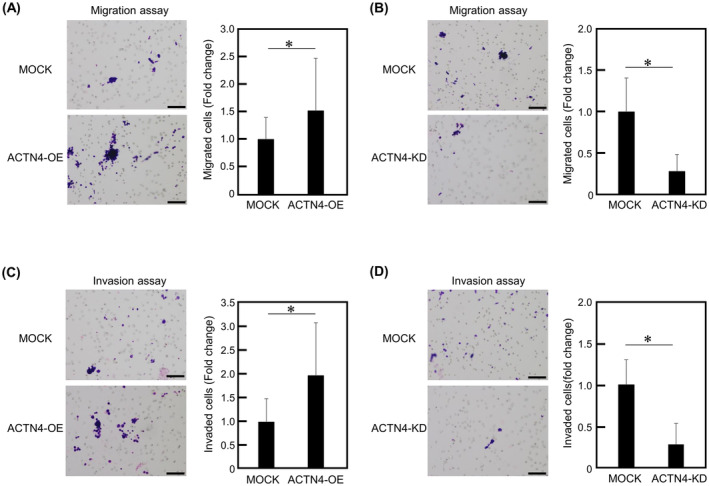
ACTN4 enhances the migration and invasion of Ty‐82 cells. (A, B) Effect of differences in ACTN4 expression on the migration of Ty‐82 cells, as examined using the Transwell migration assay. (C, D) Invasiveness of Ty‐82 cells expressing different levels of ACTN4, as examined using the invasion assay with Matrigel‐coated Transwells. Scale bars indicate 100 μm. *p < 0.05.
3.5. Regulation of the ERK/GSK‐3β/β‐catenin/Slug signaling pathway by ACTN4
As ACTN4 has been implicated in cancer invasiveness via the β‐catenin signaling pathway, 17 , 26 action of related molecules in the β‐catenin pathway was evaluated using WB. The ratio of activated β‐catenin and Slug expression was upregulated in ACTN4 OE Ty‐82 cells. Furthermore, phosphorylation of ERK and GSK‐3β (ser9) was enhanced in ACTN4 OE Ty‐82 cells compared with control cells. In addition, in ACTN4 OE Ty‐82 cells treated with ERK inhibitor PD98059, GSK‐3β phosphorylation was suppressed, the percentage of active β‐catenin was reduced, and Slug expression was suppressed (Figure 4A). This result indicated that ACTN4 activates the ERK/GSK‐3β/β‐catenin/Slug signaling pathway in Ty‐82. Conversely, activation of the ERK/GSK‐3β/β‐catenin/Slug pathway was suppressed in ACTN4 KD Ty‐82 compared with control cells (Figure 4B).
FIGURE 4.
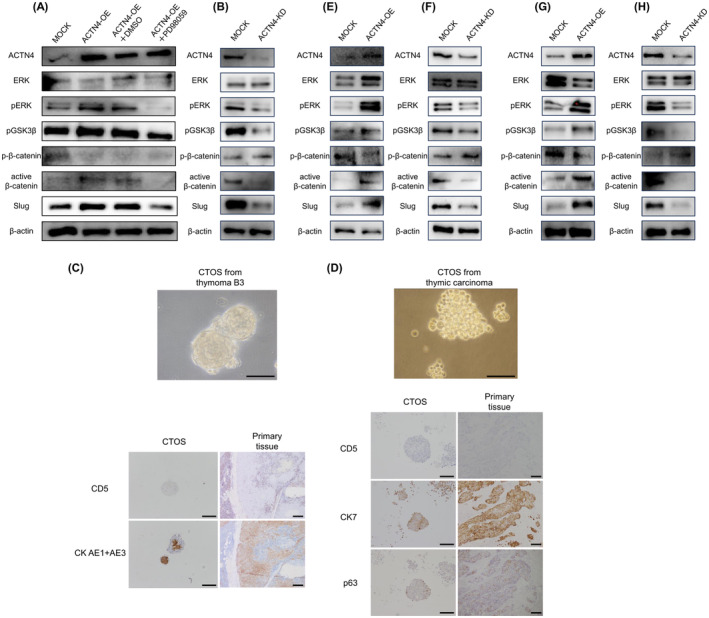
ACTN4 affects the expression of Slug via the ERK/GSK‐3β/β‐catenin signaling pathway. (A, B) Western blotting was performed to evaluate the effect of ACTN4 on the expression of ERK, pGSK3‐β, β‐catenin, and Slug in Ty‐82 cells. (C, D) Cancer tissue–origin spheroid (CTOS) cells established from surgical specimens from patients with thymoma type B3 and thymic carcinoma. A comparison of immunohistochemical staining of CTOS and primary tissue of the patient is presented. (E, F) Effect of ACTN4 on ERK, pGSK3‐β, β‐catenin, and Slug in CTOS cells of thymoma type B3 was evaluated by western blotting. (G, H) Effect of ACTN4 in CTOS cells of thymic carcinoma, as determined by western blotting.
Using the CTOS method, primary cancer cells were established using samples from a patient with thymoma type B3 and with thymic carcinoma. The success rate of CTOS establishment was 12.5% (1 of 8 cases) for B3 thymoma and 25% (one of four cases) for thymic carcinoma. The results of IHC staining of CTOS cells and primary tissues of the patients were identical (Figure 4C,D). ACTN4 OE and KD were performed in these cells using the same method used for Ty‐82 cells. In thymoma B3 CTOS cells, OE of ACTN4 activated the ERK/GSK‐3β/β‐catenin/Slug pathway, and KD of ACTN4 suppressed this pathway (Figure 4E,F). The same results were observed in thymic carcinoma CTOS cells (Figure 4G,H). WB analysis using CTOS cells established from surgical specimens showed the same results as Ty‐82, suggesting that ACTN4 regulated the ERK/GSK‐3β/β‐catenin/Slug pathway in high‐grade TETs.
3.6. Effect of ERK inhibition on the abilities of proliferation, migration and invasion in ACTN4 OE Ty‐82 cells
The effect of ERK inhibitor on the proliferative ability and migration, invasive ability of ACTN4 OE Ty‐82 cells were also examined with WST‐1 assay and migration assay. ACTN4 OE Ty‐82 cells treated with ERK inhibitor showed decreased proliferation compared with those treated with DMSO alone (Figure 5A). In addition, ACTN4 OE Ty‐82 cells treated with ERK inhibitor had reduced migratory and invasive ability (Figure 5B,C).
FIGURE 5.
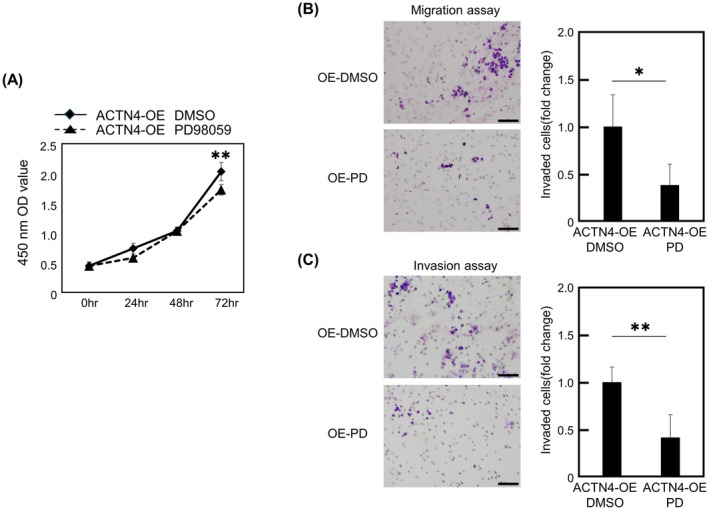
Effects of ERK inhibition on the proliferation, migration and invasion ability of ACTN4 OE Ty‐82 cells. (A) Effect of the ERK inhibitor on the proliferative ability of ACTN4 OE Ty‐82 cells was evaluated using a WST‐1 assay. (B, C) ACTN4 OE Ty‐82 cells were treated with ERK inhibitor and changes in migration and invasion ability were assessed by Transwell assay. PD indicates ERK inhibitor PD98059. Scale bars indicate 100 μm. *p < 0.05, **p < 0.01.
3.7. Effect of ACTN4 KD on dissemination of Ty‐82 cells in vivo
As KD of ACTN4 suppressed the β‐catenin/Slug pathway in Ty‐82, thymoma B3, and thymic cancer CTOS cells, we investigated the effect of ACTN4 KD in Ty‐82 cells in vivo using a dissemination model. Ty‐82 cells with ACTN4 KD and control Ty‐82 cells were injected into the thoracic cavity of BALB/cAJcl‐nu/nu mice (Figure 6A). All mice (100%, 7/7) injected with control Ty‐82 cells exhibited dissemination. H&E staining images of Ty‐82 cells disseminated on the lung surface are shown in Figure 6B. By contrast, only 22% (2/9) of mice injected with Ty‐82 cells with KD of ACTN4 showed dissemination, a significantly lower rate compared with control (p < 0.01). A representative H&E image of lungs from mice injected with ACTN4 KD Ty‐82 cells is shown in Figure 6C. Mice injected with ACTN4 KD Ty‐82 cells also exhibited significantly fewer disseminated lesions per mouse than mice injected with control Ty‐82 cells (p < 0.01) (Figure 6D). This result suggests that the inhibition of ACTN4 expression suppresses the dissemination of TETs cells. We also performed the same experiment using ACTN4 OE Ty‐82 cells and their control cells, which showed no significant difference in the number of disseminated lesions between the two groups (Figure S2).
FIGURE 6.
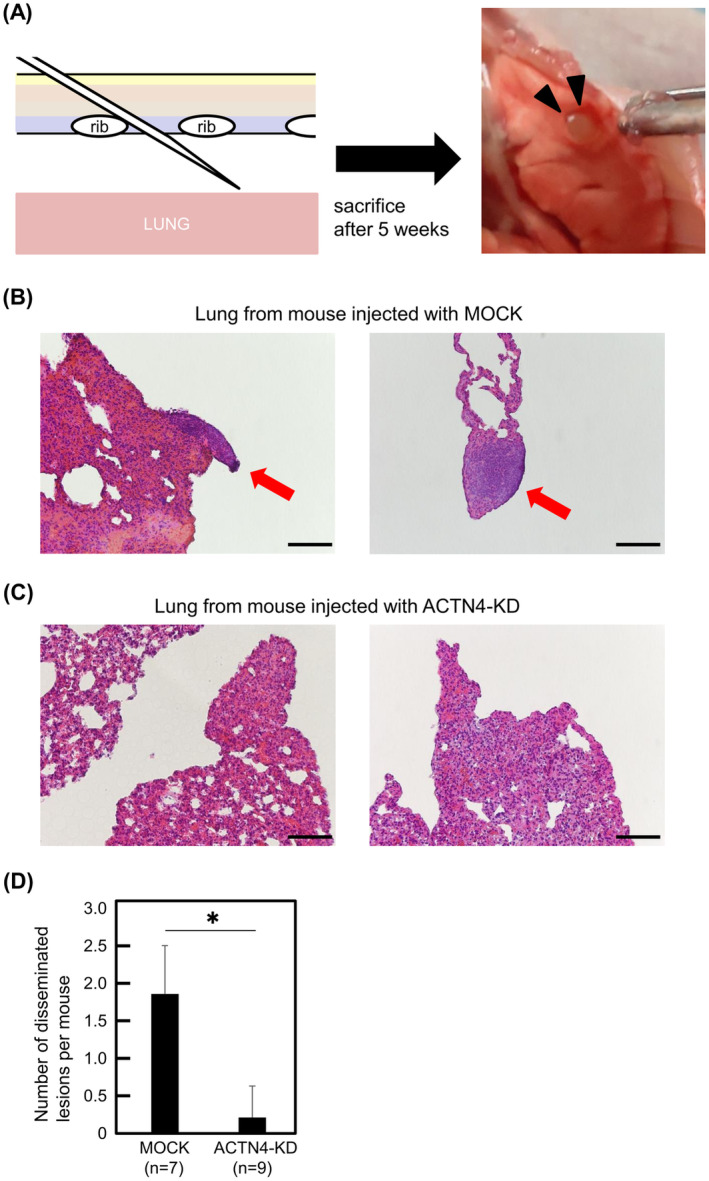
In vivo experiments to confirm the effect of ACTN4 on the dissemination of Ty‐82 cells. (A) Schematic illustration of the injection of Ty‐82 cells into the thoracic cavity of a mouse to induce dissemination. Black triangles indicate the disseminated lesion on the lung surface. (B, C) Representative hematoxylin–eosin (H&E) findings of lungs from sacrificed mice. Disseminated lesions were observed on the surface of the lungs of mice injected with control Ty‐82 cells (red arrows). Scale bars indicate 100 μm. (D) Comparison of the number of disseminated lesions per mouse between control Ty‐82 and ACTN4 KD. *p < 0.01.
3.8. Comparison of ACTN4, β‐catenin, and Slug expression between primary lesions and disseminated lesions using surgical specimens
As the in vitro and in vivo experiments suggested ACTN4 is involved in TETs invasion and dissemination via the β‐catenin/Slug pathway, we evaluated the protein expression of ACTN4, β‐catenin, and Slug using surgical specimens. First, by WB, we compared the expression of ACTN4, β‐catenin, and Slug in the central lesion of a tumor and the pericardial invasion lesion of a thymoma B3 patient with the pericardial invasion of tumor cells. We similarly compared ACTN4, β‐catenin, and Slug expression in primary and disseminated lesions in thymoma type B2 and B3 cases with pleural dissemination. The backgrounds of the patients in these three cases are shown in Table S2. The WB analysis revealed the expression of ACTN4, active β‐catenin, and Slug were all upregulated in the invasion lesion compared with the tumor center in the thymoma B3 case (Figure 7A). Similarly, expression of ACTN4, active β‐catenin, and Slug was higher in the disseminated lesion than in the primary lesion in both thymoma B2 and B3 cases (Figure 7B). We then compared the expression of ACTN4, β‐catenin, and Slug between primary and disseminated lesions using IHC staining of surgical specimens from high‐grade TETs patients for which both surgical specimens of primary lesions and disseminated lesions were available. In total, 11 specimens were available, and the backgrounds of these patients are shown in Table 3. The intensity of IHC staining for ACTN4, β‐catenin, and Slug was compared using the H‐score. Staining for ACTN4, β‐catenin, and Slug was significantly more intensein the disseminated lesion than in the primary lesion (p < 0.05) (Figure 7C,D). The results of these experiments using surgical samples indicate that ACTN4 and the β‐catenin/Slug pathway play an important role in the dissemination of TETs.
FIGURE 7.
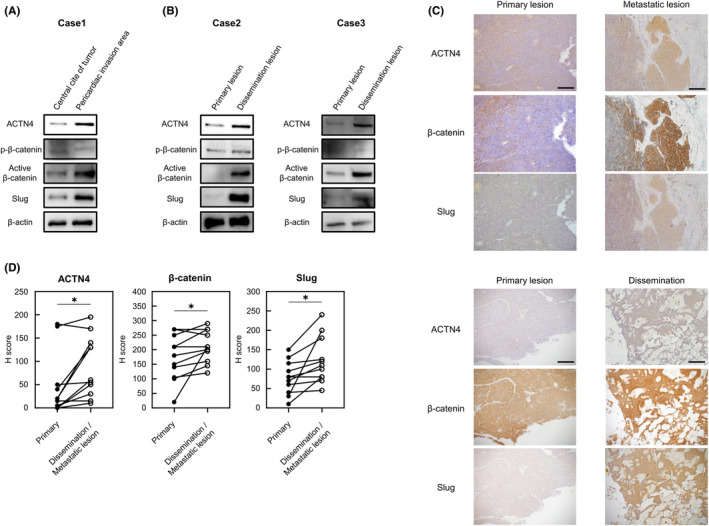
Evaluation of ACTN4, β‐catenin, and Slug expression levels using surgical specimens. (A) Western blotting analysis of ACTN4, β‐catenin, and Slug expression between the pericardial invasion site and the non‐invasion site of a tumor from a thymoma type B3 patient. (B) Differential expression of ACTN4, β‐catenin, and Slug as indicated by western blotting between disseminated and primary lesions of a tumor from a thymoma type B2 patient. (C, D) Comparison of ACTN4, β‐catenin, and Slug expression by immunohistochemical staining between primary lesions and disseminated or distant metastatic lesions using surgical specimens (n = 11). Staining intensity was evaluated. Scale bars indicate 200 μm. *p < 0.05.
TABLE 3.
Patients' characteristics.
| Case | Age | Sex | Histology | Masaoka Stage |
|---|---|---|---|---|
| 1 | 49 | M | Sq | I |
| 2 | 74 | M | Sq | IVa |
| 3 | 70 | M | B3 | I |
| 4 | 62 | F | B2 | IVa |
| 5 | 54 | M | B3 | IVa |
| 6 | 72 | F | B3 | IVb |
| 7 | 64 | M | Sq | IVb |
| 8 | 52 | F | B3 | III |
| 9 | 56 | F | B2 | I |
| 10 | 65 | F | B2 | II |
| 11 | 54 | F | B3 | IVa |
Abbreviations: Age and Stage, at surgery for primary lesion; B2, thymoma type B2; B3, thymoma type B3; F, female; M, male; Sq, thymic squamous cell carcinoma.
4. DISCUSSION
In this study, we elucidated the relationship between ACTN4 and high‐grade TETs. Our in vitro experimental results suggested that ACTN4 enhanced the ability of high‐grade TETs cells to proliferate and invade other tissues. In addition, the results of our in vivo experiments suggest that inhibition of ACTN4 can result in the suppression of high‐grade TETs dissemination. Experiments using human surgical specimens also suggested that ACTN4 was associated with the ability of invasion and disseminated in high‐grade TETs. In addition, we found the β‐catenin/Slug signaling pathway was involved in these malignant potentials of high‐grade TETs.
Details regarding the biological properties of TETs remain unclear, including the mechanisms of invasion and dissemination. One of the major reasons for this lack of information is that few TETs cell lines are commercially available, and no cell lines of type B2 or B3 thymoma are available. In addition, no animal models of TETs have been established. As a result, basic research on TETs has not progressed, and effective therapeutic targets remain largely unknown. To overcome this situation, experimental materials such as primary cultured cells derived from human samples are often used. CTOS cells are primary cultures established from human samples, and they have been used for basic research in a variety of cancers. 23 , 24 , 25 We used CTOS cells derived from thymoma type B3 and thymic carcinoma patients for WB. In addition, surgical specimens from patients with high‐grade TETs were analyzed by qPCR, WB, and IHC. The results of our experiments with CTOS cells and surgical specimens of high‐grade TETs indicated that ACTN4 was involved in the malignant potential of TETs.
Previous reports have shown that ACTN4 is a poor prognostic factor in several cancers. 14 , 15 , 16 TCGA database indicates that ACTN4 is also a poor prognostic factor in thymoma (Figure 1C). Although the prognosis did not differ significantly according to ACTN4 expression in our patients (Figure S1), this discrepancy may be because our eligible patients are smaller than those in TCGA datasets (49 vs. 119). In addition, it should be noted that our eligible patients are only high‐grade TETs, whereas patients in TCGA dataset could include low‐grade and high‐grade TETs. Conversely, the ACTN4‐high group had a higher proportion of type B3 thymoma and thymic carcinoma, which are more malignant, compared with the ACTN4‐low group (Table 2). Based on these results, we speculated that ACTN4 was involved in the malignancy of TETs.
Our in vitro experiments showed that ACTN4 enhances the invasive and proliferative capacity of Ty‐82 cells (Figures 2 and 3). In other malignancies such as prostate cancer and osteosarcoma, ACTN4 reportedly affects both tumor invasion and proliferation. 12 , 17 We speculate that ACTN4 enhances the proliferative and invasive capacity of TETs, which can lead to a poor prognosis. Notably, our experiments with surgical specimens indicated that ACTN4 is highly expressed in invasive lesions and disseminated lesions (Figures 1D–H and 7). Our report is thus important because it is the first study to demonstrate that ACTN4 regulates the invasiveness of TETs, as only a few basic studies have thus far examined this issue.
Each assay with ACTN4 OE Ty‐82 cells using ERK inhibitor and WB analyses of Ty‐82 cells, CTOS cells, and surgical specimens suggest that the enhancement of the malignant potential of high‐grade TETs by ACTN4 is mediated by the ERK/GSK‐3β/β‐catenin/Slug pathway (Figures 4 and 5). Figure 8 includes an illustration of the putative pathway. An association between ACTN4 and the β‐catenin pathway has been reported in various other cancers. 17 , 26 Activation of the ERK/GSK‐3β/β‐catenin pathway is associated with poor outcomes in non‐small cell lung cancer and plays a role in tumor proliferation and migration in ovarian cancer. 27 , 28 Slug is a transcription factor associated with EMT via β‐catenin signaling. 29 , 30 The β‐catenin/Slug pathway contributes to tumor invasion and lymph node metastasis in head and neck squamous cell carcinoma 20 and is involved in colon cancer progression and EMT. 31 EMT reportedly affects disease‐free survival in thymic carcinoma, 32 and Slug is a poor prognostic factor in thymoma. 22 Our study revealed an association between ACTN4 and Slug, and we hypothesize that ACTN4 affects the malignant potential of high‐grade TETs via the β‐catenin/Slug pathway. While KD of ACTN4 suppressed the dissemination of Ty‐82 cells in our vivo experiments (Figure 6), there was no significant difference in the number of dissemination lesions between ACTN4 OE Ty‐82 and control Ty‐82 cells (Figure S2). In pleural dissemination, multiple processes such as adhesion, in addition to proliferation and infiltrative metastasis, are important, and it may not be sufficient to explain it solely by high expression of ACTN4. Notably, the results of our WB and IHC analyses of surgical specimens from patients with high‐grade TETs also revealed that ACTN4 and Slug are involved in the dissemination of TETs (Figure 7). Patients with Masaoka Classification Stage IV TETs—patients with pleural dissemination or distant metastasis—reportedly have a poor prognosis, and dissemination is considered a poor prognostic factor for TETs. 8 Based on these results, we conclude that ACTN4 is a potential therapeutic target in patients with advanced high‐grade TETs.
FIGURE 8.
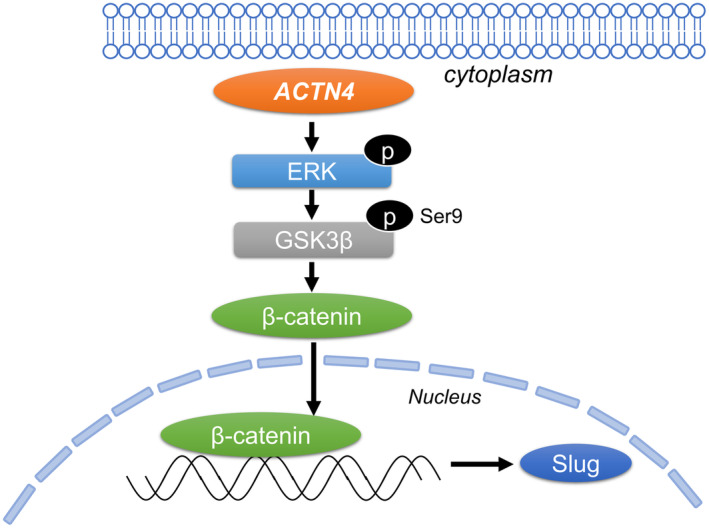
Diagram of the hypothetical pathway by which ACTN4 promotes the invasion and dissemination of TETs via Slug.
Recent studies have investigated both ACTN4 and related molecules. These studies revealed that Na+/H+ exchanger regulatory factor 1 (NHERF1) regulates ACTN4 expression, 33 and Wang et al. reported that NHERF1 attenuates β‐catenin expression in cervical cancer by suppressing the expression of ACTN4. 34 Liu et al. showed that thyroid hormone receptor‐interacting protein 13 plays a cancer‐promoting role in cervical cancer through the ACTN4/β‐catenin signaling axis. 26 Furthermore, Liao et al. reported that LIM domain kinase 1 promotes cell motility and proliferation in colorectal cancer via interaction with ACTN4. 35 We expect that further studies of ACTN4 and these related molecules could facilitate the development of new molecularly targeted therapies for high‐grade TETs.
Our study has several limitations. First, as no thymoma type B2 or B3 cell lines exist, assays of proliferation, migration, and invasion were only conducted using thymic carcinoma cell lines. It should be emphasized, however, that WB analysis showed the same results with CTOS cells derived from patients with type B3 thymoma and thymic carcinoma as with Ty‐82 cells. Second, as both thymoma and thymic carcinoma are rare diseases, surgical specimens from both primary and disseminated lesions were available for only a few cases.
In summary, our findings suggested that ACTN4 promoted the proliferation, migration, and invasion of high‐grade TETs and played a role in TETs dissemination through the β‐catenin/Slug pathway. ACTN4 is a potential therapeutic target for high‐grade TETs, and our study may provide new insights for the development of treatments for advanced TETs.
AUTHOR CONTRIBUTIONS
Hideki Nagata: Formal analysis; methodology; writing – original draft. Soichiro Funaki: Supervision; writing – review and editing. Kenji Kimura: Conceptualization; methodology. Eriko Fukui: Investigation. Toru Kimura: Investigation. Takashi Kanou: Investigation. Naoko Ose: Investigation. Eiichi Morii: Supervision. Yasushi Shintani: Project administration; supervision; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
Eiichi Morii is an editorial board member of Cancer Science and other authors declare no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: The use of human specimens was approved by the Medicine Ethics Committee of Osaka University Medical Hospital (approval number: 18518, 10026).
Informed consent: Written informed patient consent was obtained prior to the establishment of CTOS cells.
Registry and the Registration No. of the study: N/A.
Animal Studies: This animal study protocol was approved by the Osaka University Animal Experiment Committee (approval no. 05‐061‐000).
Supporting information
Figure S1.
Figure S2.
Table S1.
Data S1.
ACKNOWLEDGMENTS
We thank Miho Shimada, Reiko Inoue and Tsuyoshi Takashima for their great technical assistance.
Nagata H, Funaki S, Kimura K, et al. ACTN4 is associated with the malignant potential of thymic epithelial tumors through the β‐catenin/Slug pathway. Cancer Sci. 2024;115:3636‐3647. doi: 10.1111/cas.16313
REFERENCES
- 1. Ozono K, Onishi H, Iwamoto N, et al. Tropomyosin‐related kinase B is potentially a biomarker of prognosis and therapeutic target for malignant thymic epithelial tumors. Anticancer Res. 2022;42(8):3779‐3787. [DOI] [PubMed] [Google Scholar]
- 2. Ohtaki Y, Shimizu K, Kawabata‐Iwakawa R, et al. Carbonic anhydrase 9 expression is associated with poor prognosis, tumor proliferation, and radiosensitivity of thymic carcinomas. Oncotarget. 2019;10(13):1306‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Owen D, Chu B, Lehman AM, et al. Expression patterns, prognostic value, and intratumoral heterogeneity of PD‐L1 and PD‐1 in thymoma and thymic carcinoma. J Thorac Oncol. 2018;13(8):1204‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuhn E, Pescia C, Mendogni P, Nosotti M, Ferrero S. Thymic epithelial tumors: an evolving field. Life (Basel). 2023;13(2):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tateo V, Manuzzi L, De Giglio A, et al. Immunobiology of thymic epithelial tumors: implications for immunotherapy with immune checkpoint inhibitors. Int J Mol Sci. 2020;21(23):9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shintani Y, Funaki S, Ose N, et al. Surgical management of thymic epithelial tumors. Surg Today. 2021;51(3):331‐339. [DOI] [PubMed] [Google Scholar]
- 7. Guan S, Long W, Liu Y, Cai B, Luo J. Prognosis of concurrent versus sequential chemo‐radiotherapy induction followed by surgical resection in patients with advanced thymic epithelial tumors: a retrospective study. Ann Surg Oncol. 2023;30(11):6739‐6747. [DOI] [PubMed] [Google Scholar]
- 8. Knetki‐Wróblewska M, Kowalski DM, Olszyna‐Serementa M, Krzakowski M, Szołkowska M. Thymic epithelial tumors: do we know all the prognostic factors? Thorac Cancer. 2021;12(3):339‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okereke IC, Kesler KA, Freeman RK, et al. Thymic carcinoma: outcomes after surgical resection. Ann Thorac Surg. 2012;93(5):1668‐1673. [DOI] [PubMed] [Google Scholar]
- 10. Karube Y, Kobayashi S, Maeda S, Sado T, Ishihama H, Chida M. Fu. Tumor‐related gene expression levels in thymic carcinoma and Type B3 thymoma. J Cardiothorac Surg. 2016;11(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Funaki S, Shintani Y, Fukui E, et al. Surgical treatment strategies for invasive thymoma. J Thorac Dis. 2020;12(12):7619‐7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang Q, Li X, Huang Z, et al. ACTN4 promotes the proliferation, migration, metastasis of osteosarcoma and enhances its invasive ability through the NF‐κB pathway. Pathol Oncol Res. 2020;26(2):893‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tentler D, Lomert E, Novitskaya K, Barlev NA. Role of ACTN4 in tumorigenesis, metastasis, and EMT. Cells. 2019;8(11):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kakuya T, Mori T, Yoshimoto S, et al. Prognostic significance of gene amplification of ACTN4 in stage I and II oral tongue cancer. Int J Oral Maxillofac Surg. 2017;46(8):968‐976. [DOI] [PubMed] [Google Scholar]
- 15. Kawamura K, Miyai K, Sato K, et al. Copy number gain of ACTN4 is associated with poor prognosis in patients with upper urinary tract urothelial carcinoma. Cancer Sci. 2023;114(8):3411‐3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamamoto S, Tsuda H, Honda K, et al. Actinin‐4 gene amplification in ovarian cancer: a candidate oncogene associated with poor patient prognosis and tumor chemoresistance. Mod Pathol. 2009;22(4):499‐507. [DOI] [PubMed] [Google Scholar]
- 17. Park S, Kang M, Kim S, An HT, Gettemans J, Ko J. α‐Actinin‐4 promotes the progression of prostate cancer through the Akt/GSK‐3β/β‐catenin signaling pathway. Front Cell Dev Biol. 2020;8:588544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1–2):349‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yousefi M, Bahrami T, Salmaninejad A, Nosrati R, Ghaffari P, Ghaffari SH. Lung cancer‐associated brain metastasis: molecular mechanisms and therapeutic options. Cell Oncol (Dordr). 2017;40(5):419‐441. [DOI] [PubMed] [Google Scholar]
- 20. Moon JH, Lee SH, Lim YC. Wnt/β‐catenin/Slug pathway contributes to tumor invasion and lymph node metastasis in head and neck squamous cell carcinoma. Clin Exp Metastasis. 2021;38(2):163‐174. [DOI] [PubMed] [Google Scholar]
- 21. Shioiri M, Shida T, Koda K, et al. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br J Cancer. 2006;94(12):1816‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang T, Chen XU, Chu X, et al. Slug overexpression is associated with poor prognosis in thymoma patients. Oncol Lett. 2016;11(1):306‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoshida T, Okuyama H, Endo H, Inoue M. Spheroid cultures of primary urothelial cancer cells: cancer tissue‐originated spheroid (CTOS) method. Methods Mol Biol. 2018;1655:145‐153. [DOI] [PubMed] [Google Scholar]
- 24. Ideo H, Kondo J, Nomura T, Nonomura N, Inoue M, Amano J. Study of glycosylation of prostate‐specific antigen secreted by cancer tissue‐originated spheroids reveals new candidates for prostate cancer detection. Sci Rep. 2020;10(1):2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Endo H, Okami J, Okuyama H, et al. Spheroid culture of primary lung cancer cells with neuregulin 1/HER3 pathway activation. J Thorac Oncol. 2013;8(2):131‐139. [DOI] [PubMed] [Google Scholar]
- 26. Liu X, Shen X, Zhang J. TRIP13 exerts a cancer‐promoting role in cervical cancer by enhancing Wnt/β‐catenin signaling via ACTN4. Environ Toxicol. 2021;36(9):1829‐1840. [DOI] [PubMed] [Google Scholar]
- 27. Chen MJ, Wu DW, Wang YC, Chen CY, Lee H. PAK1 confers chemoresistance and poor outcome in non‐small cell lung cancer via β‐catenin‐mediated stemness. Sci Rep. 2016;6:34933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hao P, Li H, Wu A, et al. Lipocalin2 promotes cell proliferation and migration in ovarian cancer through activation of the ERK/GSK3β/β‐catenin signaling pathway. Life Sci. 2020;262:118492. [DOI] [PubMed] [Google Scholar]
- 29. Prasad CP, Rath G, Mathur S, Bhatnagar D, Parshad R, Ralhan R. Expression analysis of E‐cadherin, Slug and GSK3beta in invasive ductal carcinoma of breast. BMC Cancer. 2009;9:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grzegrzolka J, Biala M, Wojtyra P, et al. Expression of EMT markers SLUG and TWIST in breast cancer. Anticancer Res. 2015;35(7):3961‐3968. [PubMed] [Google Scholar]
- 31. Chen K, Liu Q, Tsang LL, et al. Human MSCs promotes colorectal cancer epithelial‐mesenchymal transition and progression via CCL5/β‐catenin/Slug pathway. Cell Death Dis. 2017;8(5):e2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Funaki S, Shintani Y, Fukui E, et al. The prognostic impact of programmed cell death 1 and its ligand and the correlation with epithelial‐mesenchymal transition in thymic carcinoma. Cancer Med. 2019;8(1):216‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun L, Zheng J, Wang Q, et al. NHERF1 regulates actin cytoskeleton organization through modulation of α‐actinin‐4 stability. FASEB J. 2016;30(2):578‐589. [DOI] [PubMed] [Google Scholar]
- 34. Wang Q, Qin Q, Song R, et al. NHERF1 inhibits beta‐catenin‐mediated proliferation of cervical cancer cells through suppression of alpha‐actinin‐4 expression. Cell Death Dis. 2018;9(6):668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liao Q, Li R, Zhou R, et al. LIM kinase 1 interacts with myosin‐9 and alpha‐actinin‐4 and promotes colorectal cancer progression. Br J Cancer. 2017;117(4):563‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.
Table S1.
Data S1.


