Abstract
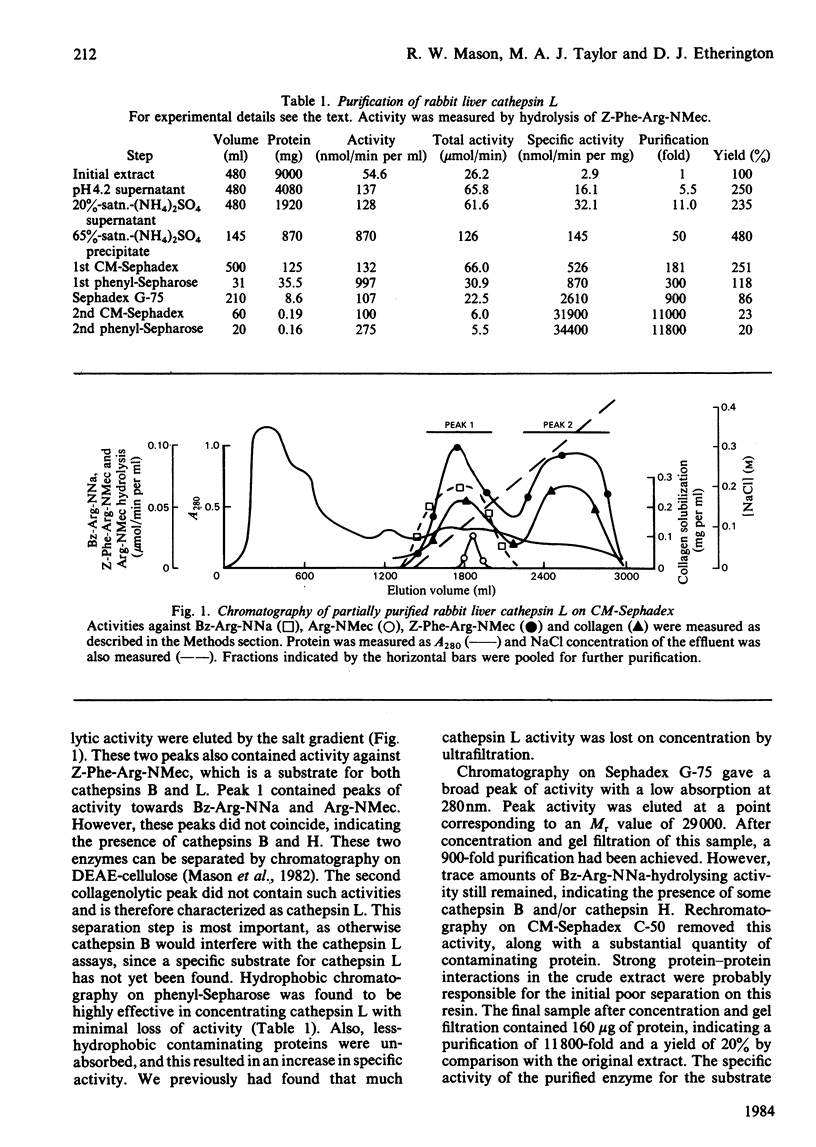
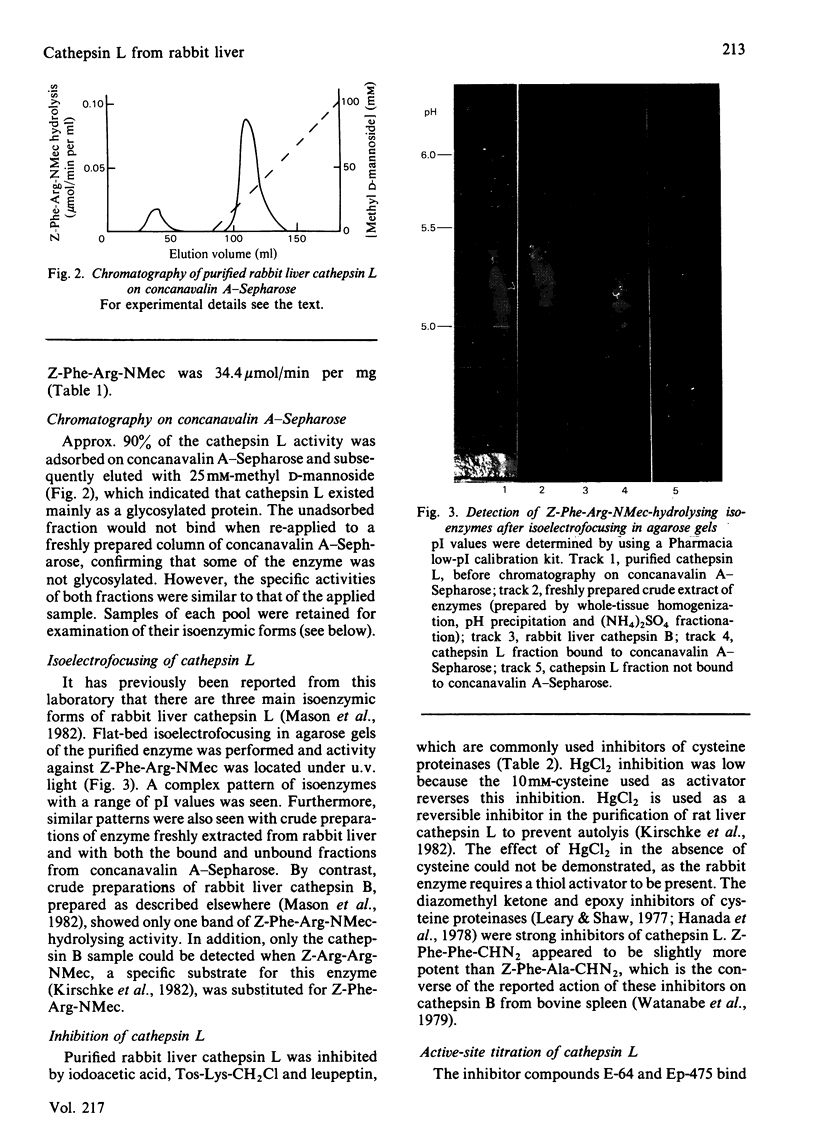
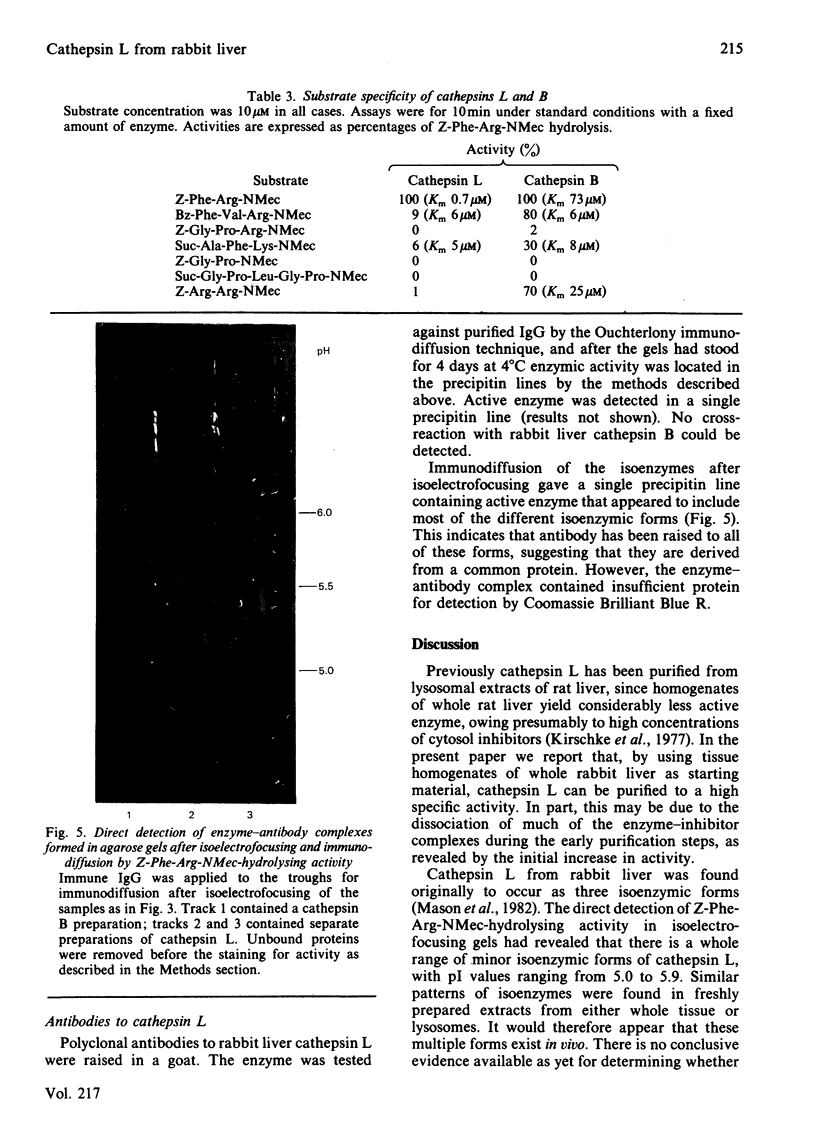
Cathepsin L was purified from rabbit liver by a method involving whole-tissue homogenization, pH precipitation, ammonium sulphate fractionation and chromatography on CM-Sephadex C-50, phenyl-Sepharose and Sephadex G-75. Pure enzyme was obtained without the necessity of laborious subcellular fractionation techniques. The Mr of the enzyme was determined to be 29 000 by gel filtration, and affinity for concanavalin A-Sepharose indicated that it was a glycoprotein. A novel technique for detection of enzyme activity in agarose isoelectrofocusing gels showed that the enzyme existed in multiple isoenzymic forms with pI values ranging from 5.0 to 5.9. The enzyme catalysed the hydrolysis of azocasein, collagen and Z-Phe-Arg-NMec (where Z and NMec indicate benzyloxycarbonyl and N-methylcoumarin derivative respectively) optimally at pH 5.2, 3.3 and 6.0 respectively. In addition, cathepsin L was found to degrade benzoyl-Phe-Val-Arg-NMec and 3-carboxypropionyl-Ala-Phe-Lys-NMec. However, cathepsin B also cleaved all of these substrates. One major difference between these two enzymes was in their Michaelis constants for Z-Phe-Arg-NMec; cathepsin B had Km 75 microM whereas that of cathepsin L was 0.7 microM. Cathepsin L was inhibited by all of the usual chemical inhibitors of thiol proteinases as well as the more specific inhibitors Z-Phe-Phe-CHN2, Z-Phe-Ala-CHN2, compound E-64 and compound Ep-475. Active-site titration with compound E-64 showed that the purified sample contained 80% active protein, which had kcat. 20s-1 for the substrate Z-Phe-Arg-NMec. Antibodies were raised to active cathepsin L, and these did not cross-react with cathepsin B, thus demonstrating that these two enzymes are immunologically distinct.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. An improved color reagent for use in Barrett's assay of Cathepsin B. Anal Biochem. 1976 Nov;76(50):374–376. doi: 10.1016/0003-2697(76)90298-0. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J. 1980 Jun 1;187(3):909–912. doi: 10.1042/bj1870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Etherington D. J. The nature of the collagenolytic cathepsin of rat liver and its distribution in other rat tissues. Biochem J. 1972 May;127(4):685–692. doi: 10.1042/bj1270685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherington D. J. The purification of bovine cathepsin B1 and its mode of action on bovine collagens. Biochem J. 1974 Mar;137(3):547–557. doi: 10.1042/bj1370547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katunuma N., Towatari T., Tamai M., Hanada K. Use of new synthetic substrates for assays of cathepsin L and cathepsin B. J Biochem. 1983 Apr;93(4):1129–1135. doi: 10.1093/oxfordjournals.jbchem.a134238. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Kembhavi A. A., Bohley P., Barrett A. J. Action of rat liver cathepsin L on collagen and other substrates. Biochem J. 1982 Feb 1;201(2):367–372. doi: 10.1042/bj2010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P. Cathepsin L. A new proteinase from rat-liver lysosomes. Eur J Biochem. 1977 Apr 1;74(2):293–301. doi: 10.1111/j.1432-1033.1977.tb11393.x. [DOI] [PubMed] [Google Scholar]
- Kärgel H. J., Dettmer R., Etzold G., Kirschke H., Bohley P., Langner J. Action of cathepsin L on the oxidized B-chain of bovine insulin. FEBS Lett. 1980 Jun 2;114(2):257–260. doi: 10.1016/0014-5793(80)81128-8. [DOI] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- Leary R., Shaw E. Inactivation of cathepsin B1 by diazomethyl ketones. Biochem Biophys Res Commun. 1977 Dec 7;79(3):926–931. doi: 10.1016/0006-291x(77)91199-8. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Taylor M. A., Etherington D. J. Purification and characterisation of collagenolytic cathepsins from rabbit liver. FEBS Lett. 1982 Sep 6;146(1):33–36. doi: 10.1016/0014-5793(82)80699-6. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- Pierart-Gallois M., Trouet A., Tulkens P. Production of rabbit antibodies against active rat cathepsin B. Acta Biol Med Ger. 1977;36(11-12):1887–1891. [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Tamai M., Hanada K., Adachi T., Oguma K., Kashiwagi K., Omura S., Ohzeki M. Papain inhibitions by optically active E-64 analogs. J Biochem. 1981 Jul;90(1):255–257. doi: 10.1093/oxfordjournals.jbchem.a133458. [DOI] [PubMed] [Google Scholar]
- Towatari T., Tanaka K., Yoshikawa D., Katunuma N. Separation of a new protease from cathepsin B1 of rat liver lysosomes. FEBS Lett. 1976 Sep 1;67(3):284–288. doi: 10.1016/0014-5793(76)80548-0. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Green G. D., Shaw E. A comparison of the behavior of chymotrypsin and cathepsin B towards peptidyl diazomethyl ketones. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1354–1360. doi: 10.1016/0006-291x(79)92158-2. [DOI] [PubMed] [Google Scholar]