Abstract
Background
The genus Haplopappus Cass. [Asteraceae] comprises a large number of species distributed mainly in Chile and with various traditional medicinal uses.
Purpose
The present review addresses the botany, traditional uses, chemistry, biological and pharmacological activities of the genus, aiming to further potentiate the associated research and applications.
Study design and Methods
Literature data on the chemistry and bioactivity of the genus Haplopappus were mainly retrieved from digital databases such as SciFinder®, PubMed®, and Google Scholar®, as well as from the scientific journal publishers’ platforms linked with these databases.
Results and discussion
Although the majority of the botanical taxa of the genus Haplopappus has been understudied, available information is promising regarding its phytochemistry and bioactivity. A total of more than 400 compounds are present in different Haplopappus species, mostly terpenoids and phenolic compounds. Scientific literature supports various health promoting effects of Haplopappus extracts and isolated compounds, principally their effect against human pathogenic bacteria and their high antioxidant capacity. The existing limitations highlighted hereby are mainly associated to the lack of modern investigation regarding a wider number of Haplopappus species and chemical compounds, as well as to the absence of in vivo bioactivity results and clinical trials.
Conclusion
Scientific literature supports the ethnopharmacological, phytochemical and bioactive potential of the genus Haplopappus, however the aforementioned limitations need to be addressed in order to further promote and broaden both scientific research and future applications and uses.
Keywords: Haplopappus genus, ethnobotany, traditional uses, phytochemistry, pharmacology
1 Introduction
Haplopappus Cass. (Asteraceae (Compositae) - Astereae - Machaerantherinae), is a strictly endemic botanical genus of southern South America, distributed in Chile, with some species also present in Argentina (Klingenberg, 2007). The vernacular name ‘bailahuén’ (‘baylahuén’ or ‘vaila-huen’) has been mainly attributed to the species Haplopappus baylahuen Remy, although the other species of the genus are commonly referred to using the same name (Vogel et al., 2007).
The different species of the genus Haplopappus, although used without differentiation in terms of botanical taxa, are of high ethnopharmacological importance and form part of the longstanding traditional medicines of the Andean peoples. In Chile, where the genus is mainly distributed, its species have been widely used in all territory, from the Aymara communities in the north to Mapuche communities in the south, and in big cities by different social groups (Hoffmann et al., 1992). Bailahuén is used at the prevention and/or treatment of various human and animal pathologies, mainly -but not exclusively-associated to gastrointestinal ailments and wound healing (Muñoz et al., 1981; de Mösbach, 1992; Hoffmann et al., 1992). Alongside its traditional use, H. baylahuen is also included in the German Homeopathic Pharmacopeia as a herbal medicine against fatigue and low blood pressure, although its use is considered limited (Arzneibuch, 2006; Vogel et al., 2007).
Regarding its commercialization, it is reported that its production in Chile is exclusively based on the collection of plant material in the wild, which, in most cases, is realized by non-trained individuals (Vogel et al., 2007). Furthermore, in the same study it is highlighted that the 80% of bailahuén commercial samples correspond to Haplopappus multifolius, probably due to the fact that this species is distributed in the Metropolitan Region of Santiago, where the companies that commercialize the plant material at a national and international level are also located. The over-exploitation of H. multifolius, along with inadequate collection practices, have led to the species being recently included in The IUCN Red List of Threatened Species as Near Threatened (Plummer, 2022).
In this context, despite its high botanical diversity and the rich ethnopharmacological background of the genus Haplopappus, both scientific investigation and commercial use is often limited to a few botanical taxa, while in many cases the traditional knowledge associated with the genus is not taken into consideration, thus hindering unravelling the full phytochemical and bioactive potential of the genus.
Thus, the present article aims to present a comprehensive review of the current state of knowledge regarding the botany, traditional uses, chemistry, biological and pharmacological activities of the genus Haplopappus in an attempt to underline its phytochemical uniqueness, elucidate its bioactive potential, and highlight future research opportunities.
2 Methods
Literature data on the chemistry and bioactivity of the genus Haplopappus were mainly retrieved from digital databases such as SciFinder®, PubMed®, and Google Scholar®, as well as from the scientific journal publishers’ platforms linked with these databases. The search strategy included the scientific name of the genus, excluding the species presently classified in other genera, i.e., Ericameria Nutt., Grindelia Willd., Gundlachia A.Gray, Isocoma Nutt., Notopappus L. Klingberg (Klingenberg, 2007; POWO, 2024). All publications in peer-reviewed journals until May 2024 were considered. The chemical compounds present in the raw materials were classified according to their pathway and superclass (Supplementary Table S1; Figures 1–11) using the NPClassifier tool (Kim et al., 2021).
FIGURE 1.
Chemical structures of miscellaneous compounds identified from Haplopappus species.
FIGURE 11.
Chemical structures of cinnamic acid derivatives identified from Haplopappus species.
FIGURE 2.

(A) Chemical structures of acyclic monoterpenes identified from Haplopappus species. (B) Chemical structures of monocyclic monoterpenes identified from Haplopappus species. (C) Chemical structures of aromatic monocyclic monoterpenes identified from Haplopappus species. (D) Chemical structures of bicyclic monoterpenes identified from Haplopappus species. (E) Chemical structures of tricyclic monoterpenes identified from Haplopappus species.,
FIGURE 3.

(A) Chemical structures of acyclic sesquiterpenes identified from Haplopappus species. (B) Chemical structures of monocyclic sesquiterpenes identified from Haplopappus species. (C) Chemical structures of bicyclic sesquiterpenes identified from Haplopappus species. (D) Chemical structures of tricyclic sesquiterpenes identified from Haplopappus species.,
FIGURE 4.

(A) Chemical structures of diterpenes labdane-type1 identified from Haplopappus species. (B) Chemical structures of diterpenes labdane-type2 identified from Haplopappus species. (C) Chemical structures of diterpenes labdane-type3 identified from Haplopappus species. (D) Chemical structures of diterpenes friedolabdane-type identified from Haplopappus species. (E) Chemical structures of diterpenes clerodane-type identified from Haplopappus species. (F) Chemical structures of miscellaneous diterpenes identified from Haplopappus species., ,
FIGURE 5.
Chemical structures of triterpenes and triterpenoids identified from Haplopappus species.
FIGURE 6.

Chemical structures of meroterpenes identified from Haplopappus species.
FIGURE 7.
Chemical structures of steroids identified from Haplopappus species.
FIGURE 8.
Chemical structures and substitution patterns of flavonoids identified from Haplopappus species.
FIGURE 9.
Chemical structures and substitution patterns of coumarins identified from Haplopappus species.
FIGURE 10.
Chemical structures of benzoic acid derivatives identified from Haplopappus species.
3 Botany and distribution
The genus Haplopappus Cass. (Asteraceae - Astereae - Machaerantherinae) is a strictly endemic genus of South America and its species are mainly distributed in Chile and, to a lesser extent, Argentina (Klingenberg, 2007; Rodriguez et al., 2018; Zuloaga et al., 2019; García et al., 2024).
According to the latest taxonomic studies of the genus and after the separation of numerous, mainly North American, species that formed the genus Notopappus L. Klingenberg, the genus Haplopappus consists of 70 specific and intraspecific taxa (Table 1) and is subdivided into three subgenera (Haplopappus subgen. Haplopappus, H. subgen. Grindelioidae Klingenberg, and H. subgen. Baylahuen Klingenberg) and five sections: Haplopappus sect. Haplopappus, H. sect. Gymnocoma Nuttall, H. sect. Grindelioidae Klingenberg, H. sect. Chromochaeta Candolle, and H. sect. Leiachaenium Candolle (Klingenberg, 2007; Garcia et al., 2018; García et al., 2024). Haplopappus taxonomy is mainly based on morphological traits, due to the limited phylogenetic data available up to date (García et al., 2024). In general, Haplopappus species are shrubs or subshrubs, with aerial parts that bear glandular trichomes, usually yellow florets, and numerous pappus bristles (Klingenberg, 2007; García et al., 2024).
TABLE 1.
Scientific names and distribution of reported Haplopappus species (Klingenberg, 2007; Garcia et al., 2018; García et al., 2024).
| No. | Haplopappus species | Synonyms | Distribution a |
|---|---|---|---|
| 1 | H. angustifolius (DC.) Reiche subsp. angustifolius | Aster atenes Kuntze, Aster sternbergii Kuntze, H. durus Reiche, Pyrrocoma angustifolia DC., Pyrrocoma rigida Phil | Chile (Atacama, Coquimbo) |
| 2 | H. angustifolius (DC.) Reiche subsp. saxatilis (Remy) Klingenb | Aster saxatilis (Remy) Kuntze, Haplodiscus sphacelatus Phil., H. saxatilis (Remy) Reiche, H. sphacelatus (Phil.) Reiche, Pyrrocoma saxatilis Remy | Chile (Coquimbo, Metropolitan of Santiago, Maule) |
| 3 | H. anthylloides Meyen & Walp | Aster anthylloides (Meyen & Walp.) Kuntze, Aster radicans (Remy) Kuntze, H. radicans Remy | Chile (Valparaíso, Libertador Bernardo O’Higgins, Maule, Metropolitan of Santiago) Argentina (Mendoza) |
| 4 | H. arbutoides Remy | Aster arbutoides (Remy) Kuntze, H. obovatus Phil., H. baccharidifolius Phil., H. zanartui (Phil.) Reiche | Chile (Coquimbo, Valparaíso, Libertador Bernardo O’Higgins, Maule, Ñuble, Biobío, Araucanía) |
| 5 | H. baylahuen Remy subsp. baylahuen | Aster baylahuen (Remy) Kuntze, H. domeykoi Phil., H. lastarrianus Remy, H. medicinalis Phil | Chile (Atacama, Coquimbo) Argentina (San Juan) |
| 6 | H. baylahuen Remy subsp. fluehmannii (Phil.) Klingenb | H. fluehmannii Phil | Chile (Atacama) |
| 7 | H. bezanillanus (Remy) Reiche | Aster bezanillanus (Remy) Kuntze, Pyrrocoma bezanillana Remy | Chile (Coquimbo) |
| 8 | H. boelckei Tortosa & A. Bartoli | - | Argentina (Mendoza) |
| 9 | H. bustillosianus Remy | Aster bustillosianus (Remy) Kuntze, Aster patagoniensis Kuntze, H. australis Phil., H. glutinosus f. patagonicus (Phil.) Cabrera, H. patagonicus Phil., H. subandinus Phil | Chile (Maule, Ñuble, Biobío, Araucanía, Los Lagos) Argentina |
| 10 | H. cerberoanus (Remy) Reiche subsp. cerberoanus | Aster cerberoanus (Remy) Kuntze, Pyrrocoma cerberoana Remy | Chile (Atacama, Coquimbo) Peru |
| 11 | H. cerberoanus (Remy) Reiche subsp. elquianus Klingenb | - | Chile (Coquimbo) |
| 12 | H. chrysanthemifolius (Less.) DC. | Andromachia alternifolia Kuntze, Diplopappus chrysanthemifolius Less., Grindelia glutinosa Bertero, H. berteroi DC., H. leucanthemifolius Phil | Chile (Coquimbo, Valparaíso, Libertador Bernardo O’Higgins, Maule, Ñuble, Biobío, Metropolitan of Santiago) |
| 13 | H. coquimbensis (Hook. & Arn.) Klingenb | Aster hirtellus (Phil.) Kuntze, Diplopappus coquimbensis Hook. & Am, Haplodiscus elatus Phil., H. acanthodon Phil. Reiche, H. elatus (Phil.) Reiche, H. hirtellus Phil., (Phil.) H. hirtellus Phil. var. hirsutus, H. limarensis Phil., H. vidalii Phil | Chile (Atacama, Coquimbo, Valparaíso, Libertador Bernardo O’Higgins) |
| 14 | H. colliguayensis M.A.Villalobos, V.Morales & Nic.García | - | Chile (Valparaíso) |
| 15 | H. decurrens Remy | Aster remyanus Kuntze | Chile (Coquimbo, Valparaíso, Libertador Bernardo O’Higgins, Metropolitan of Santiago) |
| 16 | H. deserticola Phil | H. involucratus Phil., H. rengifoanus Phil | Chile (Antofagasta, Atacama, Coquimbo) |
| 17 | H. diplopappus Remy subsp. diplopappus |
Aster diplopappus (Remy) Kuntze, Diplopappus spinulosus Hook. & Arn., H. heterophysus Phil., H. pallidus Phil H. peteroanus Phil., H. reticulatus Phil |
Chile (Valparaíso, Libertador Bernardo O’Higgins, Maule, Metropolitan of Santiago) |
| 18 | H. diplopappus Remy subsp. villosus (Phil.) L. Klingenberg | Aster villiger Kuntze, Diplopappus spinulosus Hook. & Arn., H. diplopappus Remy var. struthionum (Speg.) Cabrera, H. villosus Phil | Chile (Valparaíso, Libertador Bernardo O’Higgins, Maule, Metropolitan of Santiago) Argentina (Chubut, Mendoza, Santa Cruz) |
| 19 | H. donianus (Hook. & Arn.) Sch.Bip. ex Reiche | Diplopappus donianus Hook. & Arn., Haplodiscus exserens Phil., Haplodiscus tenuifolius Phil., H. canescens var. exserens (Phil.) Reiche | Chile (Valparaíso, Libertador Bernardo O’Higgins, Maule, Biobío) |
| 20 | H. foliosus (Hook. & Arn.) Hook. & Arn. subsp. foliosus | Aster foliosus (DC.) Kuntze, Aster polyphyllus (Phil.) Kuntze, Diplopappus foliosus Hook. & Arn., Haplodiscus densifolius Phil., Haplodiscus polyphyllus Phil., H. foliosus DC., H. phyllophorus Reiche | Chile (Coquimbo, Valparaíso, Libertador Bernardo O’Higgins, Maule, Metropolitan of Santiago) |
| 21 | H. foliosus (Hook. & Arn.) Hook. & Arn. subsp. meyenii (Walp.) L. Klingenberg | Aster meyenii (Walp.) Kuntze, H. meyenii Walp | Chile (Coquimbo) |
| 22 | H. glabratus Phil | Aster glabratus (Phil.) Kuntze, H. arbutoides Remy var. glabratus (Phil.) Reiche | Chile (Valparaíso, Libertador Bernardo O’Higgins, Maule, Metropolitan of Santiago) Argentina (Chubut, Neuquén, Río Negro, Santa Cruz) |
| 23 | H. glutinosus Cass | Aster senebierifolius Kuntze, Diplopappus coronopifolius Less., H. coronopifolius DC. | Chile (Valparaíso, Libertador Bernardo O’Higgins, Maule, Biobío, Araucania, Los Ríos, Los Lagos, Aisén) Argentina |
| 24 | H. grindelioides (Less.) DC. | Aster grindelioides (Less.) Kuntze, Aster marginalis (Phil.) Kuntze, Aster reversus Kuntze, Diplopappus grindelioides Less., H. corniculatus Phil., H. heterocomus Phil., H. marginalis Phil., H. reflexus Phil | Chile (Libertador Bernardo O’Higgins, Maule, Ñuble, Biobío, Araucania, Magallanes, Metropolitan of Santiago) Argentina (Chubut, Mendoza, Neuquén, Río Negro, Santa Cruz) |
| 25 | H. humilis (Phil.) Reiche | Haplodiscus humilis Phil | Chile (Libertador Bernardo O’Higgins, Maule, Ñuble, Biobío, Metropolitan of Santiago) |
| 26 | H. integerrimus (Hook. & Arn.) H.M. Hall | Diplopappus integerrimus Hook. & Arn., Grindelia acerosa Bertero, H. acerosus Phil., H. pulchellus var. elongaus Remy, Steriphe acerosa Phil | Chile (Coquimbo, Valparaíso, Libertador Bernardo O’Higgins, Maule, Biobío, Metropolitan of Santiago) |
| 27 | H. kingii (Phil.) Reiche | Haplodiscus kingii Phil | Chile (Atacama) |
| 28 | H. linifolius (Phil.) Reiche | Aster linodes (Phil.) Kuntze, Pyrrocoma linifolia Phil | Chile (Atacama, Coquimbo) |
| 29 | H. litoralis Phil | - | Chile (Coquimbo, Valparaíso) |
| 30 | H. macrocephalus (Poepp. Ex Less.) DC. | Aster macrocephalus (Poepp. ex Less.) Kuntze, Aster spinuliger Kuntze, Diplopappus macrocephalus Poepp. ex Less., H. caespitosus Nutt., H. scaposus Remy, H. serrulatus Reiche, H. spinulosus Phil | Chile (Valparaíso, Libertador Bernardo O’Higgins, Maule, Ñuble Biobío, Araucania, Metropolitan of Santiago) |
| 31 | H. maulinus Klingenb | - | Chile (Maule, Biobío) |
| 32 | H. mendocinus Tortosa & A. Bartoli | - | Argentina (La Pampa, Mendoza) |
| 33 | H. mieresii P. Medina & Nic. García | - | Chile (Coquimbo) |
| 34 | H. mucronatus (Hook. & Arn.) Hook | Aplopappus macraenus Gray, Aster ilicifolius (Remy) Kuntze, Aster macraenus (Remy) Kuntze, Baccharis hookeriana DC., Baccharis mucronata Hook. & Arn., Diplopappus mucronatus Hook. & Arn., H. axilliflorus Phil., H. fonckii Phil., H. hookerianus DC., H. ilicifolius Remy, H. ilicifolius var. platylepis (Phil.) Reiche, H. platylepis Phil., H. macraenus (Remy) Reiche, Pyrrocoma macraena Remy | Chile (Atacama, Coquimbo, Valparaíso) |
| 35 | H. multifolius Reiche subsp. baccharidiformis Klingenb | - | Chile (Metropolitan of Santiago) |
| 36 | H. multifolius Reiche subsp. multifolius | Aster multifolius (Reiche) Kuntze, Diplopappus foliolosus Hook. & Arn., Diplopappus ilicifolius Hook. & Arn., H. rotundifolius H.M. Hall, Pyrrocoma foliosa Phil | Chile (Coquimbo, Valparaíso, Metropolitan of Santiago) |
| 37 | H. multifolius Reiche subsp. ovalifolius Klingenb | - | Chile (Valparaíso, Metropolitan of Santiago) |
| 38 | H. nahuelbutae Klingenb | - | Chile (Biobío, Araucania) |
| 39 | H. ochagavianus Phil | Aster ochayaviensis Kuntze, H. reicheanus H.M. Hall, H. tiltilensis Phil., H. vernicosus Reiche | Chile (Coquimbo, Valparaíso, Metropolitan of Santiago) |
| 40 | H. paucidentatus Phil | Aster glutinosus (Less.) Kuntze, Aster oligodontus Kuntze, Diplopappus glutinosus Less., H. glutinosus (Less.) DC., H. glutinosus f. spathulata Cabrera., H. prostratus Phil | Chile (Maule, Ñuble Biobío, Araucania, Los Lagos) |
| 41 | H. parvifolius (DC.) Gay | Aster parvifolius (DC.) Kuntze, Pyrrocoma parvifolia DC. | Chile (Atacama, Coquimbo) |
| 42 | H. pulchellus DC. | Aster valparaisanus Kuntze | Chile (Coquimbo, Valparaíso, Libertador Bernardo O’Higgins, Maule, Metropolitan of Santiago) |
| 43 | H. philippii (Kuntze) H.M. Hall | Aster philippii Kuntze, H. breviradiatus Reiche, H. paniculatus Phil | Chile (Atacama, Coquimbo, Valparaíso) |
| 44 | H. pinea (Phil.) Reiche | Aster pineus (Phil.) Kuntze, Pyrrocoma pinea Phil | Chile (Coquimbo, Valparaíso) |
| 45 | H. pinnatifidus Nutt | Aster andinus Kuntze, Aster setiger (Phil.) Kuntze, Diplopappus setiger Hook. & Arn., H. setigerus (Phil.) Meigen, Pyrrocoma nuttalli Remy, Pyrrocoma setigera Phil | Chile (Coquimbo, Valparaíso, Libertador Bernardo O’Higgins, Maule, Metropolitan of Santiago) |
| 46 | H. poeppigianus (Hook. & Arn.) A. Gray | Aster griseus Kuntze, Diplopappus poeppigianus Hook. & Arn., Grindelia canescens Bertero, Haplodiscus polycladus Phil., H. argenteus Steud., H. canescens (Phil.) Reiche, Pyrrocoma canescens Phil | Chile (Valparaíso, Libertador Bernardo O’Higgins, Metropolitan of Santiago) |
| 47 | H. punctatus (Willd.) Hall | Aster adalbertii Kuntze, Aster pedunculosus (Remy) Kuntze, Conyza punctata Willd., Diplopappus chamissonis Less., H. chamissonis (Less.) DC., H. corymbosus (Phil.) Reiche, H. pedunculosus Remy, H. rosmarinifolius Reiche, Steriphe corymbosa Phil | Chile (Maule, Biobío) |
| 48 | H. pusillus Klingenb | Aster cuneifolius (Nutt.) Kuntze, Diplopappus bellidifolius Hook. & Arn., H. cuneifolius Nutt., H. nanus Phil | Chile (Coquimbo, Valparaíso, Metropolitan of Santiago) |
| 49 | H. racemiger Klingenb | - | Chile (Atacama, Coquimbo) |
| 50 | H. reicheanus H.M. Hall | - | Chile (Coquimbo, Valparaíso, Metropolitan of Santiago) |
| 51 | H. remyanus Wedd | Aster remyanus (Wedd.) Kuntze, Haplodiscus latifolius Phil., Haplodiscus vernicosus Phil., Haplodiscus vernicosus var. geissei Phil., H. latifolius (Phil.) Reiche, H. prinophyllus Phil., Pyrrocoma ilicifolia Remy | Chile (Atacama, Coquimbo, Valparaíso, Libertador Bernardo O’Higgins, Metropolitan of Santiago) |
| 52 | H. rengifoanus Remy | Aster rengifoanus Kuntze, Haplodiscus pachyphyllus Phil., Pyrrocoma densifolia Phil | Chile (Antofagasta, Atacama, Coquimbo, Libertador Bernardo O’Higgins) |
| 53 | H. retinervius (Kuntze) Klingenb | Aster retinervius Kuntze, Haplodiscus ischnos Phil., Haplodiscus landbecki Phil., Pyrrhocoma reticulata Phil., H. ischnos (Phil.) Reiche, H. reticulatus (Phil.) Reiche | Chile (Coquimbo, Valparaíso) |
| 54 | H. rigidus Phil | Aster atacamensis Kuntze | Chile (Antofagasta, Atacama, Coquimbo) Argentina (Catamarca, Salta) Bolivia (Potosí) |
| 55 | H. rosulatus H.M. Hall | - | Chile (Antofagasta, Atacama, Coquimbo) |
| 56 | H. schumannii (Kuntze) G.K. Br. & W.D. Clark | Aster schumannii Kuntze, H. armerioides Phil., H. poeppigianus (Hook. & Arn.) A. Gray var. radiatus A. Gray, H. sericeus Phil., Steriphe navarroi Phil | Chile (Valparaíso, Metropolitan of Santiago) |
| 57 | H. scrobiculatus (Nees) DC. | Aster cuneifolius (Nutt.) Kuntze, Aster densifolius (Remy) Kuntze, Diplopappus cuneatus Hook. & Arn., Diplopappus scrobiculatus Nees, H. densifolius Remy, Perezia spathulata Phil | Chile (Coquimbo, Valparaíso, Libertador Bernardo O’Higgins, Maule, Ñuble, Biobío, Araucania, Metropolitan of Santiago) Argentina (Mendoza, San Juan) |
| 58 | H. setulosus Klingenb | - | Chile (Maule, Ñuble) |
| 59 | H. stelliger Remy | Aster denticulatus (Phil.) Kuntze, Aster stelliger (Remy) Kuntze, H. denticulatus (Phil.) Reiche, Pyrrocoma denticulata Phil | Chile (Coquimbo) |
| 60 | H. stolpii Phil | - | Chile (Maule, Ñuble, Biobío, Araucania, Metropolitan of Santiago) |
| 61 | H. taeda Reiche | Haplodiscus peteroanus Phil., Haplodiscus graveolens Phil., H. graveolens (Phil.) Reiche | Chile (Valparaíso, Libertador Bernardo O’Higgins, Maule, Metropolitan of Santiago) |
| 62 | H. teillieri A.Cádiz-Véliz, V.Morales & Nic.García | - | Chile (Coquimbo, Valparaíso) |
| 63 | H. uncinatus Phil | Aster uncinatus (Phil.) Kuntze, Diplopappus canescens Hook. & Arn., H. candolei Phil., H. uncinatus Phil. var. candolei (Phil.) Reiche | Chile (Coquimbo, Valparaíso, Libertador Bernardo O’Higgins, Maule, Metropolitan of Santiago) |
| 64 | H. undulatus Klingenb | - | Chile (Coquimbo, Valparaíso, Metropolitan of Santiago) |
| 65 | H. valparadisiacus Klingenb | Diplopappus inuloides Hook. & Arn., H. berteroi var. lanceolatus DC., H. formosus Phil | Chile (Coquimbo, Valparaíso, Libertador Bernardo O’Higgins, Metropolitan of Santiago) |
| 66 | H. velutinus Remy subsp. illinitus (Phil.) Klingenb | H. glutinosus var. illinitus (Phil.) Reiche, H. illinitus Phil | Chile (Libertador Bernardo O’Higgins, Maule) |
| 67 | H. velutinus Remy subsp. longipes (Phil.) Klingenb | Aster longipes (Phil.) Kuntze, Pyrrocoma longipes Phil | Chile (Libertador Bernardo O’Higgins, Maule) |
| 68 | H. velutinus Remy subsp. velutinus | Aster gayanus Kuntze, Aster scopiformis Kuntze, Diplopappus glutinosus Hook. & Arn., Haplodiscus fallax Phil., Haplodiscus longiscapus Phil., H. fallax (Phil.) Reiche, H. stenophyllus Phil., H. virgatus Phil., Pyrrocoma scaposa Phil | Chile (Coquimbo, Valparaíso, Libertador Bernardo O’Higgins, Maule, Metropolitan of Santiago) Argentina (Mendoza) |
| 69 | H. vicuniensis Klingenb | - | Chile (Coquimbo) |
| 70 | H. villanuevae Phil | - | Chile (Antofagasta, Atacama) |
4 Phytochemistry
Available scientific literature provides relevant information on the phytochemistry of the genus Haplopappus. However, it must be mentioned that this information refers to only 28 species and subspecies of a total of 70 taxa (Table 1), thus highlighting the largely understudied potential of the genus Haplopappus and stressing the need to further investigate its phytochemistry. Moreover, of these 28 taxa for which scientific evidence is available, for the 24 there are less than 35 compounds reported per taxa, whereas the remaining four species are associated to a higher -yet still rather diverse-number of reported compounds, i.e., H. foliosus (n = 146), H. velutinus (n = 59), H. chrysanthemifolius (n = 52), H. bustillosianus (n = 40).
Regarding the type of metabolites reported in Haplopappus species, more than 400 different molecules have been detected in various plant parts of the studied taxa. However, the number of reported compounds per chemical group is highly diverse, to an extent that it raises the question of whether this variability can be solely attributed to differences at a plant metabolic level or it can also be associated with a focus of scientific research towards certain groups of metabolites, e.g., terpenoids and phenolics. Indeed, products of the terpenoid metabolic pathway are by far the most abundant group of molecules reported in the genus Haplopappus, including more than 200 compounds, i.e. 54 monoterpenoids (abbreviated as Mon in compound codification used in the present review), 60 sesquiterpenoids (Sqt), 107 diterpenoids (Dit), five triterpenoids (Tri), one meroterpenoid (Mer) and two steroids (Str). The second most abundant group of reported compounds includes flavonoids (Flv; flavonols, n = 46; flavones, n = 20; flavanones, n = 8; flavanonols, n = 11) and other products of the metabolic pathway of shikimic acid, i.e., coumarins (Cum, n = 16), benzoic (Ben, n = 3) and cinnamic (Cin, n = 12) acid derivatives. Other compounds reported in the genus Haplopappus include alkanes (Ala, n = 29), alkenes (Ale, n = 4), alkynes (Aly, n = 1), alcohols (Alc, n = 5), ethers (Eth, n = 1), aromatic hydrocarbons and derivatives (Arh, n = 10), aldehydes (Ald, n = 8), ketones (Ket, n = 7), esters (Est, n = 8), furanones (Fur, n = 1), lactones (Ltn, n = 2) and lactams (Ltm, n = 1).
The aforementioned compounds as classified per chemical group are detailed in Figures 1–11 and Tables 2–4, and Supplementary Table S1, while their distribution among the studied Haplopappus taxa is presented as follows.
TABLE 2.
Substitution pattern of flavonols and flavones reported in species of the genus Haplopappus.
| No. | Compound | R3 | R5 | R6 | R7 | R8 | R3' | R4' | R5' |
|---|---|---|---|---|---|---|---|---|---|
| Flavonols | |||||||||
| Flv1 | quercetin | OH | OH | H | OH | H | OH | OH | H |
| Flv2 | quercetin 3-methyl ether | OMe | OH | H | OH | H | OH | OH | H |
| Flv3 | tamarixetin (quercetin 4′-methyl ether) | OH | OH | H | OH | H | OH | OMe | H |
| Flv4 | rhamnazin (quercetin 7,3′-dimethyl ether) | OH | OH | H | OMe | H | OMe | OH | H |
| Flv5 | quercetin 3,3′-dimethyl ether | OMe | OH | H | OH | H | OMe | OH | H |
| Flv6 | quercetin 3,7-dimethyl ether | OMe | OH | H | OMe | H | OH | OH | H |
| Flv7 | ayanin | OMe | OH | H | OMe | H | OH | OMe | H |
| Flv8 | retusin (5-hydroxy-3,7,3′,4′-tetramethoxyflavone) | OMe | OH | H | OMe | H | OMe | OMe | H |
| Flv9 | 3-O-acetyl-7-methylquercetin | OAc | OH | H | OMe | H | OH | OH | H |
| Flv10 | isoquercitrin (quercetin-3-β-D-glucoside) | O-Glu | OH | H | OH | H | OH | OH | H |
| Flv11 | hyperoside (quercetin-3-β-D-galactoside) | O-Gal | OH | H | OH | H | OH | OH | H |
| Flv12 | quercetagetin 3-methyl ether | OMe | OH | OH | OH | H | OH | OH | H |
| Flv13 | quercetagetin 3,7-dimethyl ether | OMe | OH | OH | OMe | H | OH | OH | H |
| Flv14 | centaureidin | OMe | OH | OMe | OH | H | OH | OMe | H |
| Flv15 | betuletol (3,5,7-trihydroxy-6,4′-dimethoxyflavone) | OH | OH | OMe | OH | H | H | OMe | H |
| Flv16 | eupatolitin | OH | OH | OMe | OMe | H | OH | OH | H |
| Flv17 | rhamnetin | OH | OH | H | OMe | H | OH | OH | H |
| Flv18 | isorhamnetin | OH | OH | H | OH | H | OMe | OH | H |
| Flv19 | isorhamnetin-3-β-D-glucoside | O-Glu | OH | H | OH | H | OMe | OH | H |
| Flv20 | isorhamnetin-3-β-D-galactoside | O-Gal | OH | H | OH | H | OMe | OH | H |
| Flv21 | kaempferol | OH | OH | H | OH | H | H | OH | H |
| Flv22 | astragalin (kaempferol 3-β-D-glucoside) | O-Glu | OH | H | OH | H | H | OH | H |
| Flv23 | isokaempferide (kaempferol 3-methyl ether) | OMe | OH | H | OH | H | H | OH | H |
| Flv24 | kaempferol 3-methyl ether 7-β-D-glucoside | OMe | OH | H | O-Glu | H | H | OH | H |
| Flv25 | rhamnocitrin (kaempferol 7-methyl ether) | OH | OH | H | OMe | H | H | OH | H |
| Flv26 | ermanin (kaempferol 3,4′-dimethyl ether) | OMe | OH | H | OH | H | H | OMe | H |
| Flv27 | kaempferol 7,4′-dimethyl ether | OH | OH | H | OMe | H | H | OMe | H |
| Flv28 | kumatakenin (kaempferol 3,7-dimethyl ether) | OMe | OH | H | OMe | H | H | OH | H |
| Flv29 | kaempferol 3,7,4′-trimethyl ether | OMe | OH | H | OMe | H | H | OMe | H |
| Flv30 | 3-O-acetyl-7,4′-dimethylkaempferol | OAc | OH | H | OMe | H | H | OMe | H |
| Flv31 | haplopappin | OMe | OH | H | OH |
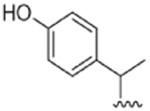
|
H | OMe | H |
| Flv32 | haplopappin A | OH | OH | H | OMe |
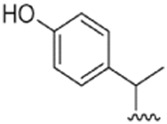
|
H | OMe | H |
| Flv33 | myricetin | OH | OH | H | OH | H | OH | OH | OH |
| Flv34 | myricetin 3′,4′-dimethyl ether | OH | OH | H | OH | H | OMe | OMe | OH |
| Flv35 | myricetin 3,3′,4′-trimethyl ether | OMe | OH | H | OH | H | OMe | OMe | OH |
| Flv36 | myricetin 3,7,4′-trimethyl ether | OMe | OH | H | OMe | H | OH | OMe | OH |
| Flv37 | 3,8-dimethylherbacetin (5,7,4′-trihydroxy-3,8-dimethoxyflavone) | OMe | OH | H | OH | OMe | H | OH | H |
| Flv38 | 3,8,4′-trimethylherbacetin (5,7-dihydroxy-3,8,4′-trimethoxyflavone) | OMe | OH | H | OH | OMe | H | OMe | H |
| Flv39 | 5,7,4′-trihydroxy-3,8,3′-trimethoxyflavone | OMe | OH | H | OH | OMe | OMe | OH | H |
| Flv40 | 3,5-dihydroxy-3′,4′,6,7-tetramethoxyflavone | OH | OH | OMe | OMe | H | OMe | OMe | H |
| Flv41 | santin | OMe | OH | OMe | OH | H | H | OMe | H |
| Flv42 | eupatorin | H | OH | OMe | OMe | H | OH | OMe | H |
| Flv43 | jaceidin | OMe | OH | OMe | OH | H | OMe | OH | H |
| Flv44 | jaceidin 7-methyl ether | OMe | OH | OMe | OMe | H | OMe | OH | H |
| Flv45 | penduletin | OMe | OH | OMe | OMe | H | H | OH | H |
| Flv46 | pachypodol | OMe | OH | H | OMe | H | OMe | OH | H |
| Flavones | |||||||||
| Flv47 | apigenin | H | OH | H | OH | H | H | OH | H |
| Flv48 | 3,6-dimethoxyapigenin | OMe | OH | OMe | OH | H | H | OH | H |
| Flv49 | vicenin-2 | H | OH | C-Glu | OH | C-Glu | H | OH | H |
| Flv50 | vitexin | H | OH | H | OH | C-Glu | H | OH | H |
| Flv51 | isovitexin | H | OH | C-Glu | OH | H | H | OH | H |
| Flv52 | isoschaftoside | H | OH | C-Ara | OH | C-Glu | H | OH | H |
| Flv53 | luteolin | H | OH | H | OH | H | OH | OH | H |
| Flv54 | luteolin 5-glucoside | H | O-Glu | H | OH | H | OH | OH | H |
| Flv55 | luteolin 7-glucoside | H | OH | H | O-Glu | H | OH | OH | H |
| Flv56 | chrysoeriol | H | OH | H | OH | H | OMe | OH | H |
| Flv57 | velutin (luteolin 7, 3′-dimethyl ether) | H | OH | H | OMe | H | OMe | OH | H |
| Flv58 | diosmetin | H | OH | H | OH | H | OH | OMe | H |
| Flv59 | eupafolin (6-methoxyluteolin) | H | OH | OMe | OH | H | OH | OH | H |
| Flv60 | 6-methoxyluteolin 4′-methyl ether | H | OH | OMe | OH | H | OH | OMe | H |
| Flv61 | cirsiliol (6-methoxyluteolin 7-methyl ether) | H | OH | OMe | OMe | H | OH | OH | H |
| Flv62 | hispidulin (scutellarein 6-methyl ether) | H | OH | OMe | OH | H | H | OH | H |
| Flv63 | pectolinaringenin | H | OH | OMe | OH | H | H | OMe | H |
| Flv64 | scutellarein 6-β-D-glucoside | H | OH | O-Glu | OH | H | H | OH | H |
| Flv65 | 3′,4′-dihydroxyflavone 5-glucoside | H | O-Glu | H | H | H | OH | OH | H |
| Flv66 | verbenacoside | H | O-Glu | H | H | H | H | OH | H |
TABLE 4.
Substitution pattern of coumarins reported in species of the genus Haplopappus.
| No. | Compound | R6 | R7 |
|---|---|---|---|
| Cum1 | esculetin | OH | H |
| Cum2 | esculin | Glu | H |
| Cum3 | prenyletin | OH |

|
| Cum4 | haplopinol | OH |
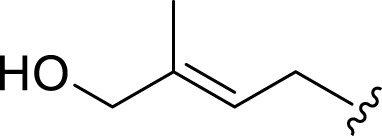
|
| Cum5 | 6-deoxyhaplopinol | H |

|
| Cum6 | 6-hydroxy-7-(5′-hydroxy-3′,7′-dimethylocta-2′,6′-dien)-oxycoumarin | OH |

|
| Cum7 | 6-hydroxy-7-(7′-hydroxy-3′,7′-dimethylocta-2′,5′-dien)-oxycoumarin | OH |

|
| Cum8 | 6-hydroxy-7-[(E,E)-3′,7′-dimethyl-2′,4′,7′-octatrienyloxy] coumarin | OH |

|
| Cum9 | scopoletin | OMe | H |
| Cum10 | 7-O-prenylscopoletin | OMe |

|
| Cum11 | 7-O-geranylscopoletin | OMe |
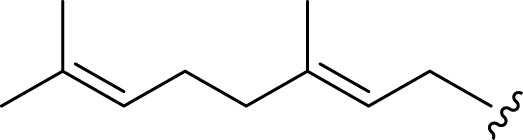
|
| Cum12 | scoparone | OMe | Me |
| Cum13 | hernianin | H | Me |
| Cum14 | umbelliferone | H | H |
| Cum15 | O-prenylumbelliferone | H |
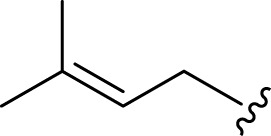
|
TABLE 3.
Substitution pattern of flavanones and flavanonols reported in species of the genus Haplopappus.
| No. | Compound | R3 | R5 | R6 | R7 | R8 | R3' | R4' | R5' |
|---|---|---|---|---|---|---|---|---|---|
| Flavanones | |||||||||
| Flv67 | sakuranetin (5,4′-dihydroxy-7-methoxyflavonone) | H | OH | H | OMe | H | H | OH | H |
| Flv68 | sakuranetin 4′-methyl ether | H | OH | H | OMe | H | H | OMe | H |
| Flv69 | persicogenin | H | OH | H | OMe | H | OH | OMe | H |
| Flv70 | sternbin | H | OH | H | OMe | H | OH | OH | H |
| Flv71 | eriodictyol | H | OH | H | OH | H | OH | OH | H |
| Flv72 | eriodictyol 7,3′-dimethyl ether | H | OH | H | OMe | H | OMe | OH | H |
| Flv73 | eriodictyol 7,3′,4′-trimethyl ether | H | OH | H | OMe | H | OMe | OMe | H |
| Flv74 | pinostrobin | H | OH | H | OMe | H | H | H | H |
| Flavanonols | |||||||||
| Flv75 | 7,4′-dimethylaromadendrin | OH | OH | H | OMe | H | H | OMe | H |
| Flv76 | 7-O-methylaromadenrin | OH | OH | H | OMe | H | H | OH | H |
| Flv77 | 3-O-acetyl-7-O-aromadendrin | OAc | OH | H | OMe | H | H | OH | H |
| Flv78 | padmatin | OH | OH | H | OMe | H | OH | OH | H |
| Flv79 | 3-O-acetylpadmatin | OAc | OH | H | OMe | H | OH | OH | H |
| Flv80 | blumeatin B | OH | OH | H | OMe | H | OH | OMe | H |
| Flv81 | 7,3′-di-O-methyltaxifolin | OH | OH | H | OMe | H | OMe | OH | H |
| Flv82 | dihydromyricetin | OH | OH | H | OH | H | OH | OH | OH |
| Flv83 | alpinone 3-acetate | OAc | OH | H | OMe | H | H | H | H |
4.1 H. angustifolius (DC.) Reiche
Information on the chemical composition of H. angustifolius is limited to reports of the presence of hentriacontane (Ala22), hexacosanol (Alc1), the diterpenes haplopappic acid (Dit96) and its methylester (Dit97) and the triterpenes friedelin (Tri1) and epi-friedelinol (Tri3) in the aerial parts of the plant (Silva and Sammes, 1973).
4.2 H. anthylloides Meyen & Walp
The ketone 4-hydroxyacetophenone (Ket2) is the only compound identified in the aerial parts of H. antylloides (Zdero et al., 1990).
4.3 H. arbutoides Remy
The majority of the compounds identified in the aerial parts and/or resin of H. arbutoides belong to the diterpenoids group, i.e. 15-oxo-labda-8(17),14E-diene-18-oic acid (Dit32), 15-oxo-labda-8(17),14Z-diene-18-oic acid (Dit33), labda-8(17),13E-dien-15,18-dioic acid 15-methyl ester (Dit34), 15-hydroxylabd-8(17)-en-18-oic acid (Dit35), labd-13(E)-ene-8α,15-diol (Dit62), 13R-labdane-8,15-diol (Dit63), 8α-hydroxy-ent-labd-13(14)Z-en-15-al (Dit64), 8α-hydroxylabdan-15-al (Dit65), epi-manoyl oxide (Dit68), 8,13-epoxy-14-labdeb-3-ol (Dit74), 8,13-epoxy-labdan-15-al (Dit75), 15-oxocleroda-3,13E-dien-18-oic acid (Dit91), and 15-oxocleroda-3,13Z-dien-18-oic acid (Dit92) (Zdero et al., 1991a; Rossomando et al., 1995). Additionally, the aerial parts are reported to contain 4-hydroxyacetophenone (Ket2), the sesquiterpene 1β-hydroxy-β-cyperone (Sqt44) and the flavonols santin (Flv41) and penduletin (Flv45) (Zdero et al., 1991a; Rossomando et al., 1995).
4.4 H. baylahuen Remy
The essential oil of the leaves of H. baylahuen is reported to contain eicosane (Ala11), benzene (Arh1), azulene (Arh2), naphthalene (Arh4), and the sesquiterpenes bergamotol (Sqt8) and α-cadinol (Sqt24) (Becerra et al., 2010). Phenolic compounds detected in this species include quercetin (Flv1), quercetin 3-methyl ether (Flv2), rhamnetin (Flv17), isorhamnetin (Flv18), kaempferol (Flv21), rhamnocitrin (Flv25), velutin (Flv57), sakuranetin (Flv67), persicogenin (Flv69), sternbin (Flv70), 7,4′-dimethylaromadendrin (Flv75), 7-O-methylaromadenrin (Flv76), 7,3′-di-O-methyltaxifolin (Flv81), dihydromyricetin (Flv82), prenyletin (Cum3), and 3,5-dicaffeoylquinic acid (Cin12) (Schwenker et al., 1967; Hörhammer et al., 1973; Nuñez-Alarcon et al., 1993; Vera et al., 2001; Schmeda-Hirschmann et al., 2015).
4.5 H. bezanillanus (Remy) Reiche
The compounds detected in the aerial parts of H. bezanillanus are the diterpenoid labd-13(E)-ene-8α,15-diol (Dit62), the steroid β-sitosterol (Str2) and the flavonol jaceidin 7-methyl ether (Flv44) (Maldonado et al., 1993).
4.6 H. bustillosianus Remy
The aerial parts of H. bustillosianus contain the alkenes C11H24 – C14H30 (Ala2 – Ala5), C16H34 – C33H68 (Ala7 – Ala24), along with 3-hydroxyacetophenone (Ket1) and the flavonoids santin (Flv41) and 3,6-dimethoxyapigenin (Flv48) (Urzúa et al., 2007a). Their phenolic profile includes α-linalool (Mon4), α-pinene (Mon37), β-pinene (Mon38), α-bisabolol (Sqt4), humulene (Sqt5), α-cadinene (Sqt18), γ-cadinene (Sqt20), δ-cadinene (Sqt21), (−)-isocaryophyllene (Sqt30), α-cubebene (Sqt48), β-cubebene (Sqt49), α-copaene (Sqt58), populifolic acid (Dit89) and its methyl ester (Dit90), and thunbergol (Dit102) (Urzúa et al., 2007a).
4.7 H. chrysanthemifolius (Less.) DC
The phytochemistry of H. chrysanthemifolius has been thoroughly investigated and various chemical groups of compounds have been identified in this species. Among them, in the flower heads there are present the alkanes C10H22 – C19H40 (Ala1 – Ala10), C21H44 – C33H68 (Ala12 – Ala24), 2-methyldecalin (Ala25), 2,4,6-trimethyloctane (Ala26), 2,6-dimethylundecane (Ala27), 4,6-dimethylundecane (Ala28), and 2,10-dimethylundecane Ala29) (Urzúa et al., 2007a). Furthermore, the terpenoid profile of the species includes β-myrcene (Mon3), limonene (Mon8), α-pinene (Mon37), β-pinene (Mon38), humulene (Sqt5), δ-cadinene (Sqt21), (−)-isocaryophyllene (Sqt30), β-bulgarene (Sqt31), γ-bulgarene (Sqt32), (−)-amorpha-4,11-diene (Sqt33), α-cubebene (Sqt48), β-cubebene (Sqt49), (−)-calarene (Sqt50), 1,3,4,5,6,7-hexahydro-2,5,5-trimethyl-2H-2,4a-ethanonaphthalene (Sqt51), α-copaene (Sqt58), 6α-hydroxy-ent-labd-8(17)-en-15-oic acid (Dit1), 3β-acetoxy-ent-labd-8(17)-en-15-oic acid (Dit2), and 18α-acetoxylabd-8(17)-en-15-oic acid (Dit3) (Faini et al., 1999; Urzua et al., 2007b). Regarding the phenolic compounds of H. chrysanthemifolius, it is reported the presence of quercetin (Flv1), tamarixetin (Flv3), ayanin (Flv7), myricetin 3,7,4′-trimethyl ether (Flv36), luteolin (Flv53), and diosmetin (Flv58) (Faini et al., 1999; Urzua et al., 2007b; Urzúa et al., 2012).
4.8 H. coquimbensis (Hook. & Arn.) Klingenb
The aerial parts of H. coquimbensis (syn. H. hirtellus Phil. (Klingenberg, 2007)) contain the terpenoids 7,13-labdadien-15,18-dioic acid 15-methyl ester (Dit44) and epi-friedelin (Tri2), as well as stigmasterol (Str1) (Maldonado et al., 1993). Regarding its flavonoid profile, the following compounds were detected in its aerial parts: kaempferol 7,4′-dimethyl ether (Flv27), kaempferol 3,7,4′-trimethyl ether (Flv29), pachypodol (Flv46), sakuranetin 4′-methyl ether (Flv68), eriodictyol 7,3′-dimethyl ether (Flv72), 7,4′-dimethylaromadendrin (Flv75), and 7,3′-di-O-methyltaxifolin (Flv81) (Maldonado et al., 1993).
4.9 H. deserticola Phil
In the aerial parts of H. deserticola there were detected the diterpenoids methyl-ent-4-epi-agath-18-oate (Dit17), dimethyl-ent-4-epi-agathoate (Dit18), copaiferolic acid (Dit19), copaiferolic acid 15-methyl ester (Dit20), methyl haplodesertoate (Dit26), 8α-hydroxyanticopalic acid (Dit60), 8α-hydroxyanticopalic acid methyl ester (Dit61), ent-19-hydroxy-cis-cleroda-3,13(E)-dien-15-oic acid (Dit98), and 18-acetoxy-cis-cleroda-3,13(E)-dien-15-oic acid (Dit99), along with the sesquiterpenoid germacrene D (Sqt7) (Zdero et al., 1990; Urzúa Moll et al., 1997; Tojo et al., 1999).
Regarding its phenolic composition, the aerial parts of this species are reported to contain the flavonoids quercetin (Flv1), quercetin 3-methyl ether (Flv2), isokaempferide (Flv23), 3,8-dimethylherbacetin (Flv37), 3,8,4′-trimethylherbacetin (Flv38), and 5,7,4′-trihydroxy-3,8,3′-trimethoxyflavone (Flv39); the coumarins 7-O-prenylscopoletin (Cum10), 7-O-geranylscopoletin (Cum11), O-prenylumbelliferone (Cum15) and the dimeric umbelliferone 3,3-dimethylallyl ether (Cum16), as well as the cinnamic acid derivatives chlorogenic acid (Cin10), 3,4-dicaffeoylquinic acid (Cin11), and 3,5-dicaffeoylquinic acid (Cin12) (Zdero et al., 1990; Tojo et al., 1999; Schmeda-Hirschmann et al., 2015).
4.10 H. diplopappus Remy
The resinous exudate of H. diplopappus is reported to contain the diterpenoid ent-manool (Dit9) and its 13-O-β-xylopyranoside (Dit8) (Urzua et al., 1995a).
4.11 H. foliosus (Hook. & Arn.) Hook. & Arn
H. foliosus is the species for which the greatest number of compounds has been reported. Among them, there are the alkanes C12H26 (Ala3), C14H30 (Ala5), C16H34 (Ala7), C18H38 (Ala9), and C23H48 – C33H68 (Ala14 – Ala24) (Silva and Sammes, 1973; Urzúa et al., 2000; Urzúa, 2004). Furthermore, the aerial parts of this species contain 11-tricosene (Ale1), hexacosanol (Alc1), ethylresorcinol (Alc2), diisopropyl ether (Eth1), α-asarone (Arh3), 1,2,3,4,5,6,7,8-octahydro-1-methylphenantrene (Arh5), eugenol (Arh6), styrene (Arh7), safrol (Arh8), elemicin (Arh9), dihydrobenzofuran (Arh10), benzaldehyde (Ald1), 2,3-dichloro-2-methylpropanal (Ald2), trans-2-hexenal (Ald3), nonanal (Ald4), decanal (Ald5), 3-ethylbenzaldehyde (Ald6), 4-vinylbenzaldehyde (Ald7), 3-hydroxyacetophenone (Ket1), 3-ethylacetophenone (Ket3), 4-ethylacetophenone (Ket4), dihydro-α-ionone (Ket6), 4,4-dimethyl-2-allylcyclohexanone (Ket7), (Z)-3-hexenyl acetate (Est8), tetrahydroactinidiolide (Ltn2), 4-phenyl-2-azetidinone (Ltm1), and stigmasterol (Str1) (Silva and Sammes, 1973; Urzúa et al., 2000; 2010; Urzúa, 2004; Villagra et al., 2021).
The terpenoid fraction of H. foliosus has been thoroughly studied and more than 70 compounds have been reported. Among them, there are the monoterpenoids cis-α-ocimene (Mon1), β-ocimene (Mon2), β-myrcene (Mon3), limonene (Mon8), α-terpinene (Mon9), γ-terpinene (Mon10), terpinen-4-ol (Mon11), terpinolene (Mon16), isoterpinolene (Mon17), α-terpineol (Mon18), p-menth-2-en-4-ol (Mon19), trans-p-menth-2-en-1-ol (Mon21), cis-p-menth-2-en-1-ol (Mon22), α-phellandrene (Mon25), m-cymene (Mon27), p-cymene (Mon28), p-cymen-8-ol (Mon29), o-cumenol (Mon30), 3-carene (Mon31), thujane (Mon32), α-thujene (Mon33), cis-(+/−)-4-thujanol (Mon34), 4-thujanol (Mon35), α-thujone (Mon36), α-pinene (Mon37), β-pinene (Mon38), pinocarveol (Mon39), borneol (Mon42), bornyl acetate (Mon43), camphor (Mon44), camphene (Mon45), fenchol (Mon46), 1,5-dimethyl-6-methylenespiro[2.4]heptane (Mon48), sabinene (Mon49), 5-(acetyloxy)-4,6,6-trimethyl-endobiciclo[2.2.1]heptan-2-one (Mon50), ascaridole (Mon52), and tricyclene (Mon54) (Urzúa et al., 2000; 2010; Urzúa, 2004; Villagra et al., 2021). The equally diverse sesquiterpenoid fraction includes germacrene D (Sqt7), (1α,7β,10β)-11-hydroxy-4-guaien-3-one (Sqt9), (1β,7β,10β)-1,11-dihydroxy-4-guaien-3-one (Sqt10), (1α,6α,7β,10β)-6,11-dihydroxy-4-guaien-3-one (Sqt11), α-selinene (Sqt13), γ-selinene (Sqt14), 5-eudesmen-11-ol (Sqt15), γ-eudesmol (Sqt16), cadalene (Sqt17), α-cadinene (Sqt18), β-cadinene (Sqt19), γ-cadinene (Sqt20), δ-cadinene (Sqt21), guaiol (Sqt22), 1(10),11-eremophiladiene (Sqt23), α-cadinol (Sqt24), ionene (Sqt25), 6-(1,1-dimethylethyl)-2,3-dihydro-1,1-dimethyl-3-methylene-1H-indene (Sqt26), δ-ambrinol (Sqt27), decahydro-3a,8-dimethyl-5-(1-methylethenyl)azulene (Sqt28), 1,2,3,4,5,6,7,8-octahydro-1,4-dimethyl-7-(1-methylethylidene)azulene (Sqt29), β-guaiene (Sqt36), (−)-caryophyllene (Sqt38), epi-bicyclosesquiphellandrene (Sqt39), α-muurolene (Sqt40), γ-muurolene (Sqt41), agarospirol (Sqt42), aromadendrene (Sqt47), α-cubebene (Sqt48), β-cubebene (Sqt49), spathulenol (Sqt52), β-bourbonene (Sqt55), α-copaene (Sqt58), β-copaene (Sqt59), and β-ylangene (Sqt60) (Labbé et al., 1998; Urzúa et al., 2000; 2010; Urzúa, 2004; Villagra et al., 2021). Much less diverse are the reported di- and triterpenoid profiles of the species, which include 2α-hydroxy-cis-clero-3,13(Z),8(17)-trien-15-oic acid (Dit87), 2α-acetoxy-cis-clero-3,13(Z),8(17)-trien-15-oic acid (Dit88), haplopappic acid (Dit96), friedelin (Tri1), and epi-friedelinol (Tri3) (Silva and Sammes, 1973; Urzúa et al., 2003).
The flavonoid profile of H. foliosus has also been thoroughly investigated and reported to include quercetin 3-methyl ether (Flv2), rhamnazin (Flv4), isoquercitrin (Flv10), hyperoside (Flv11), beturetol (Flv15), eupatolin (Flv16), isorhamnetin (Flv18), isorhamnetin 3-β-D-glucoside (Flv19), kaempferol (Flv21), astragalin (Flv22), isokaempferide (Flv23), kaempferol 3-methyl ether 7-β-D-glucoside (Flv24), ermanin (Flv26), kumatakenin (Flv28), haplopappin (Flv31), and haplopappin A (Flv32) (Ulubelen et al., 1982; Tschesche et al., 1985; Urzúa, 2004).
Furthermore, the following coumarins were detected in H. foliosus: esculetin (Cum1), prenyletin (Cum3), scopoletin (Cum9), and scoparone (Cum12) (Ulubelen et al., 1982; Urzúa, 2004), along with the benzoic and cinnamic acid derivatives methyl salicylate (Ben3), trans-cinnamic acid (Cin1), cis-cinnamic acid (Cin2), isobutyl-(E)-cinnamate (Cin3), pentyl-(E)-cinnamate (Cin4), benzyl-(E)-cinnamate (Cin5), and 2-phenylethyl-(E)-cinnamate (Cin6) (Urzúa et al., 2000; Urzúa, 2004; Villagra et al., 2021).
4.12 H. glutinosus Cass
The aerial parts of H. glutinosus are reported to contain 4-hydroxyacetophenone (Ket2), β-farnesene (Sqt2), germacrene D (Sqt7), 6,18-dihydroxy-ent-labd-7,13E-dien-15-oic acid (Dit41), 4-hydroxybenzoic acid (Ben1), syringic acid (Ben2), trans-cinnamic acid (Cin1), caffeic acid (Cin9), and chlorogenic acid (Cin10) (Jakupovic et al., 1986; Marambio and Silva, 1996). Furthermore, the flavonoid profile of the species includes isokaempferide (Flv23), ermanin (Flv26), santin (Flv41), jaceidin (Flv43), apigenin (Flv47), 3,6-dimethoxyapigenin (Flv48), luteolin 5- (Flv54) and 7- (Flv55) glucosides, hispidulin (Flv62), pectolinaringenin (Flv63), 3′,4′-dihydroxyflavone 5-glucoside (Flv65), and verbenacoside (Flv66) (Marambio and Silva, 1996; Valant-Vetschera and Wollenweber, 2007).
4.13 H. integerrimus (Hook. & Arn.) H.M. Hall
Scientific literature only contains information on the flavonoid profile of the leaves of H. integerrimus var. punctatus (Willd.) G.K.Br. & W.D.Clark, according to which the following compounds were detected: quercetin (Flv1), quercetin 3-methyl ether (Flv2), rhamnazin (Flv4), quercetin 3,3′-dimethyl ether (Flv5), quercetin 3,7-dimethyl ether (Flv6), isoquercitrin (Flv10), isorhamnetin (Flv18), myricetin 3′,4′dimethyl ether (Flv34), and myricetin 3,3′,4′-trimethyl ether (Flv35) (Ayanoglu et al., 1981).
4.14 H. litoralis Phil
The resin of H. litoralis is reported to contain the diterpenoids 18α-acetoxylabd-8(17)-en-15-oic acid (Dit3),18-hydroxylabd-8(17)-en-15-oic acid (Dit14), (+)-copalic acid (Dit16), and (−)-eperuic acid (Dit21) (Urzúa et al., 2004b). Moreover, the flavonols ayanin (Flv7) and retusin (Flv8) were identified in the resinous exudate of this species (Urzúa et al., 2012).
4.15 H. multifolius Reiche
The terpenoids 2,9-epoxy-p-menth-6-en-8-ol (Mon51), 9-cis-p-coumaroyloxy-α-terpineol (Sqt12), 18-hydroxylabda-7,13(E)-dien-15-oic acid (Dit39), and 18-hydroxylabda-7,13(Z)-dien-15-oic acid (Dit42) are present in the aerial parts of H. multifolius (Maatooq et al., 2002). However, the phenolic composition of this species has been more thoroughly investigated and the following compounds have been identified: quercetin (Flv1), quercetin 3-methyl ether (Flv2), isorhamnetin (Flv18), persicogenin (Flv69), sternbin (Flv70), 3-O-acetylpadmatin (Flv79), blumeatin B (Flv80), esculetin (Cum1), esculin (Cum2), prenyletin (Cum3), haplopinol (Cum4), 6-deoxyhaplopinol (Cum5), 6-hydroxy-7-(5′-hydroxy-3′,7′-dimethylocta-2′,6′-dien)-oxycoumarin (Cum6), 6-hydroxy-7-(7′-hydroxy-3′,7′-dimethylocta-2′,5′-dien)-oxycoumarin (Cum7), 6-hydroxy-7-[(E,E)-3′,7′-dimethyl-2′,4′,7′-octatrienyloxy] coumarin (Cum8), hernianin (Cum13), umbelliferone (Cum14), O-prenylumbelliferone (Cum15), and 3,5-dicaffeoylquinic acid (Cin12) (Chiang et al., 1982; Nuñez-Alarcón and Quiñones, 1995; Urzúa et al., 1995b; Maatooq et al., 2002; Torres et al., 2004; 2006; 2013; Schmeda-Hirschmann et al., 2015).
4.16 H. parvifolius (DC.) Gay
The group of compounds identified in the aerial parts of H. parvifolius includes mainly diterpenoids, as well as the sesquiterpenoids 2,8-dimethyl-2′-vinyl-5-[4-methyl-pent-3-enyl]-chromane (Sqt43) and aphanamol I (Sqt46) (Zdero et al., 1991b). The diterpenoids detected in this species are 13-hydroxylabda-6,8,14-triene (Dit27), 13-hydroxylabda-6,8(17),14-triene (Dit28), 9α,13-epoxy-labda-6,8(17),14-triene (Dit29), 6β-acetoxy-13-hydroxylabda-8,14-dien-7-one (Dit30), 6β-acetoxy-7β,13-dihydroxylabda-8,14-diene (Dit31), 6β-acetoxy-13-hydroxylabda-7,14-diene (Dit47), 13-hydroxy-6α-butyryloxylabda-7,14-diene (Dit48), 13-hydroxylabda-7,14-diene-6-one (Dit49), 9α,13-dihydroxylabda-7,14-dien-6-one (Dit50), 6α,13-dihydroxylabda-7,14-dien-17-al (Dit51), isomanool (Dit52), 6α-hydroxy-9α,13-epoxy-labda-7,14-diene (Dit53), 6α-acetoxy-9α,13-epoxy-labda-7,14-diene (Dit54), 6α-butyryloxy-9α,13-epoxy-labda-7,14-diene (Dit55), 5α-hydroxy-9α,13-epoxy-labda-7,14-diene-6-one (Dit56), 6α-acetoxy-9α,13-epoxy-labda-7,14-dien-17-al (Dit57), 6-oxo-14,15-nor-labda-7-ene (Dit58), 8α,13-dihydroxylabda-6,14-diene (Dit66), 8α,13-dihydroxylabda-5,14-dien-7-one (Dit67), epi-manoyl oxide (Dit68), 6,7-dehydro-13-epi-manoyl oxide (Dit69), 6,7-dehydro-8,13-bis-epi-manoyl oxide (Dit70), 13,17-epoxy-labda-5,7,14-triene (Dit71), 9α,13-epoxy-5α,8α-dihydroxylabda-6,14-diene (Dit72), 5α-hydroxy-7,8-epoxy-7,8-seco-6,7-dehydro-13-epi-manoyl oxide (Dit73), haploparvone (Dit103), 5α-hydroxyhaploparvone (Dit104), haploparviolide (Dit105), 1,1,5,6-tetramethyl-4-[3-hydroxy-3-methyl-pent-(4)-enyl]-tetralin (Dit106), and 1,1,5-trimethyl-6-(3-hydroxy-3-methyl-pent-4-enyl)-tetralin (Dit107) (Zdero et al., 1991b).
4.17 H. poeppigianus (Hook. & Arn.) A. Gray
The aerial parts of H. poeppigianus (syn. H. canescens (Phil.) Reiche (Klingenberg, 2007)) contain the flavonoid compounds centaureidin (Flv14), myricetin (Flv33), chrysoeriol (Flv56), diosmetin (Flv58), hispidulin (Flv62), and scutellarein 6-β-D-glucoside (Flv64) (Oksuz et al., 1981).
4.18 H. paucidentatus Phil
The aerial parts of H. paucidentatus contain 4-hydroxyacetophenone (Ket2) and the terpenoids germacrene D (Sqt7), caryophyllene oxide (Sqt34), 8-oxo-β-cyperone (Sqt45), 18-hydroxy-friedolabd-5-en-15-oic acid (Dit78), 18-hydroxy-cis-cleroda-3-en-15-oic acid (10βH, 16ξ, 19β, 17β, 20α form) (Dit83), 19-hydroxy-cis-cleroda-3-en-15-oic acid (10βH, 16ξ, 19β, 17β, 20α form) (Dit85), 18-hydroxy-cis-cleroda-3,13(E)-dien-15-oic acid (Dit93), and 18-acetoxy-cis-cleroda-3,13(E)-dien-15-oic acid (Dit99) (Jakupovic et al., 1986).
4.19 H. pulchellus DC
Regarding the compounds identified in the aerial parts of H. pulchellus, those include the diterpenoids 7α-hydroxylabd-8(17)-en-15,18-dioic acid (Dit4), labd-7-en-15,18-dioic acid (Dit36), 18-acetoxy-friedolabd-5-en-15-oic acid (Dit76), 18-acetoxy-friedolabd-5-en-7-one-15-oic acid (Dit77), 18-hydroxy-friedolabd-5-en-15-oic acid (Dit78), 18-hydroxy-7-oxo-friedolabd-5-en-15-oic acid (Dit79), friedolabd-5-en-15,18-dioic acid (Dit80), and 15-hydroxy-friedolabd-5-en-18-oic acid (Dit81) (Zdero et al., 1991a).
4.20 H. remyanus Wedd
The esters benzenepropanoic acid, 2-methyl-6-methylene-2,7-octadienyl ester (Est3), (±)-1-acetoxy-2-(p-tolyl)-2-propanol (Est4), 2-hydroxy-2-(4-methylphenyl)propyl benzenepropanoate (Est5), 2-hydroxy-2-(4-methyl-3-cyclohexen-1-yl)propyl benzenepropanoate (Est6), and 2-hydroxy-2-(4-methyl-3-cyclohexen-1-yl)propyl 3-phenyl-2-propenoate (Est7) have been detected in the aerial parts of H. remyanus (Zdero et al., 1991a). Regarding its terpenoid profile, the species contains uroterpenol (Mon12), 9-benzoyloxy-(1-formyl)-α-terpineol (Mon13), 9-benzoyloxy-α-terpineol (Mon14), 7-hydroxy-9-benzoyloxy-α-terpineol (Mon15), 8-hydroxy-9-acetoxy-β-phellandrene (Mon26), 18-hydroxylabda-7,13(E)-dien-15-oic acid (Dit39), 18-acetoxy-labda-7,13(E)-dien-15-oic acid (Dit40), and 18-dihydrocinnamoyloxy-labda-7,13E-dien-l5-oic acid (Dit46) (Zdero et al., 1991a; Faini et al., 2011). Morever, the following flavonoid compounds are present in H. remyanus: quercetin (Flv1), 3-O-acetyl-7-methylquercetin (Flv9), kaempferol 7,4′-dimethyl ether (Flv27), kaempferol 3,7,4′-trimethyl ether (Flv29), 3-O-acetyl-7,4′-dimethylkaempferol (Flv30), sakuranetin 4′-methyl ether (Flv68), eriodictyol (Flv71), pinostrobin (Flv74), 7,4′-dimethylaromadendrin (Flv75) and alpinone 3-acetate (Flv83) (Zdero et al., 1991a; Faini et al., 2011).
4.21 H. rengifoanus Remy
The aerial parts and/or leaves of H. rengifoanus are reported to contain the sesquiterpenoid liguloxide (Sqt57) and the flavonoids quercetagetin 3-methyl ether (Flv12), quercetagetin 3,7-dimethyl ether (Flv13), isorhamnetin (Flv18), isorhamnetin 3-β-D-glucoside (Flv19), isorhamnetin 3-β-D-galactoside (Flv20), apigenin (Flv47), luteolin (Flv53), and scutellarein 6-β-D-glucoside (Flv64) (Ulubelen et al., 1981; Zdero et al., 1991a).
4.22 H. rigidus Phil
The diterpenoids rigiduside (Dit6), 18-acetoxy-cis-clerode 3,13(Z)-dien-15 oic acid (Dit82), rigidusol (Dit100), and deacetylrigidusol (Dit101) are present in the aerial parts of H. rigidus (Morales et al., 2000a; 2000b; 2003). Furthermore, the flavonoids quercetin 3-methyl ether (Flv2), beturetol (Flv15), kaempferol (Flv21), isokaempferide (Flv23), sakuranetin (Flv67) and sternbin (Flv70) were detected in the aerial parts (Morales et al., 2000a; 2003; 2009; Schmeda-Hirschmann et al., 2015), along with 3,5-dicaffeoylquinic acid (Cin12) (Schmeda-Hirschmann et al., 2015).
4.23 H. schumannii (Kuntze) G.K. Br. & W.D. Clark
The alkanes C23H48 – C31H64 (Ala14 – Ala22) and C33H68 (Ala14) have been identified in the aerial parts of H. schumannii, along with 1-octadecyne (Aly1), dihydro-α-ionone (Ket6), and the lactone tetrahydroactinidiolide (Ltn2) (Urzúa et al., 2004a). The terpenoid profile of this species includes the sesquiterpenoids β-cadinene (Sqt19), β-bourbonene (Sqt55), and globulol (Sqt56), as well as the diterpenoids manool (Dit7), (−)-eperuic acid (Dit21), epi-manool (Dit25), 8α-hydroxylabdan-15-oic acid (Dit59), and 2-oxoclerod-3-en-15-oic acid (Dit86) (Urzúa et al., 1997; 2004a). Moreover, the flavonoids quercetin (Flv1), isoquercitrin (Flv10), vicenin-2 (Flv49), vitexin (Flv50), and isovitexin (Flv51) are present in the leaves of H. schumannii (Ates et al., 1982).
4.24 H. scrobiculatus (Nees) DC
The presence of the terpenoids α-farnesene (Sqt1), 18-hydroxymanool (Dit15), and 2-oxokolavenic acid (Dit94) has been reported in the case of the aerial parts and resinous exudates of H. scrobicultus (Rossomando et al., 1995; Urzúa et al., 2004b). However, the largest group of compounds in this species is that of phenolics, namely, quercetin (Flv1), isoquercitrin (Flv10), isorhamnetin (Flv18), isorhamnetin 3-β-D-glucoside (Flv19), rhamnocitrin (Flv25), santin (Flv41), eupatorin (Flv42), penduletin (Flv45), vicenin-2 (Flv49), vitexin (Flv50), isovitexin (Flv51), isoschaftoside (Flv52), eupafolin (Flv59), 6-methoxyluteolin 4′-methyl ether (Flv60), cirsiliol (Flv61), and esculetin (Cum1) (Ates et al., 1982; Rossomando et al., 1995; Urzúa et al., 2012).
4.25 H. taeda Reiche
The terpenoid profile of H. taeda includes taedol (Mon41), 18-hydroxylabda-7,13(E)-dien-15-oic acid (Dit39), 7,13-labdadien-15,18-dioic acid (Dit43), cleroda-3,13 (E)-dien-15,18-diol (Dit95), and 18-acetoxy-cis-cleroda-3,13(E)-dien-15-oic acid (Dit99) (Marambio and Silva, 1989; Torres et al., 2005; Faini et al., 2007; 2008). However, scientific literature provides more information on the phenolic composition of this species, with the following compounds being reported: quercetin (Flv1), quercetin 3-methyl ether (Flv2), quercetin 3,7-dimethyl ether (Flv6), kaempferol (Flv21), sakuranetin (Flv67), sternbin (Flv70), eriodictyol 7,3′-dimethyl ether (Flv72), eriodictyol 7,3′,4′-trimethyl ether (Flv73), 3-O-acetyl-7-O-aromadendrin (Flv77), padmatin (Flv78), 3-O-acetylpadmatin (Flv79), 9-trans-p-coumaroyloxy-α-terpineol (Cin7), 7-trans-p-coumaroyloxy-taedol (Cin8), chlorogenic acid (Cin10), 3,4-dicaffeoylquinic acid (Cin11), and 3,5-dicaffeoylquinic acid (Cin12) (Marambio and Silva, 1989; Faini et al., 2007; 2008; Schmeda-Hirschmann et al., 2015).
4.26 H. uncinatus Phil
The alkanes C23H48 – C31H64 (Ala14 – Ala22) and C33H68 (Ala14) have been identified in the resinous exudates and/or aerial parts of H. uncinatus (Urzúa et al., 2000; 2004a; 2006), along with 2,7-dimethyl-5-(1-methylethenyl)-1,8-nonadiene (Ale3) and 3,5-dihydroxy-3′,4′,6,7-tetramethoxyflavone (Flv40) (Urzúa et al., 2004a; 2006). Regarding its terpenoid profile, the species is reported to synthesize 3,3,7,7-tetramethyl-5-(2-methyl-1-propenyl)-tricyclo[4.1.0.0(2,4)]heptane (Mon53), the sesquiterpenoids cadalene (Sqt17), aromadendrene (Sqt47), α-cubebene (Sqt48), β-cubebene (Sqt49), spathulenol (Sqt52), cedryl acetate (Sqt53), β-bourbonene (Sqt55), globulol (Sqt56), α-copaene (Sqt58), as well as the clerodane diterpenoid 18-acetoxy-cis-cleroda-3-en-15-oic acid (10βH, 16ξ, 19β, 17β, 20α form) (Dit84) (Urzúa et al., 2000; 2004a; 2006).
4.27 H. velutinus Remy; H. velutinus Remy subsp. illinitus (Phil.) Klingenb
Several compounds are reported to be present in both H. velutinus and the subspecies H. velutinus subsp. illinitus. These are the alkanes C23H48 – C31H64 (Ala14 – Ala22) and C33H68 (Ala14), 5,5-dimethyl-2(5H)-furanone (Fur1), β-myrcene (Mon3), limonene (Mon8), α-pinene (Mon37), β-pinene (Mon38), labd-7-en-15,18-dioic acid-18α-methylester (Dit37), β-sitosterol (Str2), and quercetin (Flv1) (Latorre et al., 1990; Marambio and Silva, 1996; Faini et al., 2002; Urzúa et al., 2004a; Echeverría et al., 2019).
In contrast, compounds solely identified in H. velutinus include 3-ethyl-1,4-hexadiene (Ale2), 2-nonyn-1-ol (Alc3), 2-pentadecen-1-ol (Al4), n-dodecenyl-1-ol (Alc5), vanillin (Ald8), picein (Ket5), lavender lactone (Ltn1), linalyl anthranilate (Mon5), davanone (Mon6), davana ether (Mon7), 1,2:8,9-diepoxy-p-menthane (Mon19), cis-p-menth-2-en-1-ol (Mon22), trans-pulegone oxide (Mon23), α-campholenal (Mon24), m-cymene (Mon27), α-thujene (Mon33), pinocarveol (Mon39), trans-2-pinanol (Mon40), cis-verbenol (Mon47), α-sinensal (Sqt3), humulene epoxide II (Sqt6), caryophyllene oxide (Sqt34), α-guaiene (Sqt35), (−)-oplopanone (Sqt37), spathulenol (Sqt52), patchouli alcohol (Sqt54), dehydropinipholic acid 19-methyl ester (Dit11), 4α-hydroxy-18-norlabd-8(17)-en-15-oic acid (Dit12), 4β-hydroxy-19-norlabd-8(17)-en-15-oic acid (Dit13), 18-hydroxylabd-8(17)-en-15-oic acid (Dit14), 7,13-(E)-labdadien-15,18-dioic-acid-18-methyl ester (Dit45), friedelin (Tri1), epi-friedelinol (Tri3), taraxerol (Tri4), erythrodiol (Tri5), stigmasterol (Str1), isoquercitrin (Flv10), isokaempferide (Flv23), kumatakenin (Flv28), luteolin (Flv53), and scopoletin (Cum9) (Urzúa and Mendoza, 1989; Urzúa et al., 1991; Urzúa et al., 1995a; 2004a; Urzua and Mendoza, 1993; Marambio and Silva, 1996; Echeverría et al., 2019).
The group of compounds identified solely in the subspecies H. velutinus subsp. illinitus consists of 3,3,5,5-tetramethylcyclopentene (Ale4), methyl octanoate (Est1), 5-methyl-octanoic acid methyl ester (Est2), β-cadinene (Sqt19), procerin (Mer1), as well as the diterpenoids 7α-hydroxylabd-8(17)-en-15,18-dioic acid-15-methylester (Dit5), pinifolic acid 15-methyl ester (Dit22), pinifolic acid 18-methyl ester (Dit23), pinifolic acid dimethyl ester (Dit24), labd-7-en-15,18-dioic acid (Dit36), labd-7-en-15,18-dioic acid-15-methylester (Dit38), and 7-oxo-labd-8(9)-en-15,18-dioic acid-15-methylester (Dit10), (Faini et al., 2002; Urzúa et al., 2004a).
5 Traditional uses and evidence-based pharmacological activities related to human health
5.1 Traditional uses
The plants of the genus Haplopappus are of high medicinal value and form essential part of the traditional medicines of the Andean region (Chile, Argentina), where the genus presents high endemicity. Haplopappus species and their preparations have traditionally been associated with numerous health benefits, associated with multiple aspects of the human health and also with veterinary applications (Table 5).
TABLE 5.
Traditional uses of Haplopappus species.
| Species | Plant part(s) – preparation(s) | Traditional use(s) | References |
|---|---|---|---|
| Haplopappus spp. | whole plant (alone or combined with Satureja parvifolia or Lycopodium Saururus); aerial parts; leaf/aerial parts infusion (with or without milk); stem juice; resin (applied externally or ingested) | antidiarrheic; antiseptic; antispasmodic; antitussive; aphrodisiac; cholagogue; choleretic; cicatrizant (in particular, to treat horses); digestive; disinfectant; emmenagogue; hepatic; stimulant; sudorific; against altitude sickness, abdominal colic, dysentery, chronic dyspepsia, colds, flu and urinary diseases | Alonso, 2005; de Mösbach (1992), Hoffmann et al. (1992), Mellado Campos (1996), Ministerio de Salud (2010), Montes and Wilkomirsky (1987), Ratera and Ratera (1980), Schrickel and Bittner (2001) |
| H. baylahuen | whole plant; aerial parts; leaf/aerial parts infusion; leaf decoction; stem juice; taken with milk | aphrodisiac; antidiarrheic; antirheumatic; antiseptic; antispasmodic; antitussive; antiviral, astringent; carminative; cholagogue; choleretic; cicatrizant (in particular, to treat horses and other animals); digestive; disinfectant; emmenagogue; expectorant; hepatic; stimulant; stomachic; against altitude sickness, chronic hemorrhagic intestinal inflammation, colds, flu, flatulent dyspepsia, dysentery, gastritis, male and female hormonal disorders, pneumonia, pains provoked by air currents, genital, renal and urinary disorders | Cárdenas (1998), Del Vitto et al. (2010), Espinoza (1897), Gómez-Parra and Siarez Flores (1995), Hoffmann et al. (1992), Houghton and Manby (1985), Laval (1957), Madaleno and Delatorre-Herrera (2013), Ministerio de Salud (2010), Montes and Wilkomirsky (1987), Mostny et al. (1954), Munizaga (1963), Munizaga and Gunkel (1958), Muñoz S. et al. (1981), Murillo (1861), 1889; Remington and Woods (1918), Serracino et al. (1974), Steinmetz (1954), Vogel et al. (2005b) |
| H. multifolius | whole plant; leaf infusion | antidiarrheic; antiseptic; digestive; emmenagogue; hepatic; stomachic; against dysentery and urinary disorders | Muñoz S. et al. (1981), Vogel et al. (2005b) |
| H. remyanus | whole plant; leaf infusion | antidiarrheic; antiseptic; antispasmodic; digestive; emmenagogue; hepatic; stomachic; against dysentery and urinary disorders | Montes and Wilkomirsky (1987), Muñoz S. et al. (1981), Vogel et al. (2005b) |
| H. rigidus | whole plant; aerial parts infusion; taken with milk; decoction with fruits of Opuntia camachoi Espinosa | antirheumatic; antitussive; aphrodisiac; diuretic; febrifuge; hepatic; laxative; stomachic; against colds, flu, pains provoked by air currents, pneumonia, renal colic, cardiac pain, gastrointestinal, ovary and urinary disorders; against veterinary ailments | Aldunate et al. (1981), Gómez et al. (1997), Hoffmann et al. (1992), Mellado Campos (1996), Monterrey (1996), Montes and Wilkomirsky (1987), Muñoz S. et al. (1981), Ratera and Ratera (1980), Villagrán et al. (2003), 1998; Wickens (1993) |
| H. taeda | whole plant; resinous leaves; leaf infusion | antidiarrheic; antiseptic; digestive; emmenagogue; hepatic; stomachic; against dysentery, intestinal and urinary disorders | Faini et al. (2007), Vogel et al. (2005b) |
The main health benefits traditionally attributed to different preparations of Haplopappus plants are associated with pathologies of the human alimentary tract and metabolism. Various species and preparations have widespread use as digestives, antidiarrheic, remedies against dyspepsia, dysentery and gastrointestinal ailments, in general.
Moreover, there are reported several traditional uses associated with the human genitourinary system, with Haplopappus preparations being considered as aphrodisiacs, emmenagogues, diuretic and as remedies against urinary and renal disorders and colics or even against male and female hormonal disorders.
Other traditional uses are associated with health benefits for the human respiratory (antitussives, expectorants, cold remedies) and nervous (stimulant, antispasmodic) system, as well as with their role as disinfectants.
Finally, it is well-documented in traditional Andean medicines the use of Haplopappus preparations as cicatrizants with veterinary applications, especially to treat horses’ wounds.
It has to be mentioned that H. baylahuen Remy is recognized by the Chilean health authorities as a traditional herbal medicine against liver diseases, abdominal colics, chronic dyspepsia, kidney stones, flus and colds, as well as an aphrodisiac and wound disinfectant (Ministerio de Salud, 2010). Meanwhile, pharmaceutical products that include bailahuén, e.g., the formulations ‘Ulcenat’ and ‘Ubenat’ (Grüne Leben) and ‘Bailahuen extracto fluido’ (Knop Laboratorios S.A.) are commercialized in Chile as treatments against digestive disorders. However, there are no internationally or nationally established norms and/or protocols regarding quality, standardization, safety, and adulteration control of bailahuén preparations and commercial products.
5.2 Evidence-based pharmacological activity related to the human health
Scientific literature provides evidence related to various human health-promoting effects of extracts and isolated compounds of Haplopappus species (Table 6), with their inhibitory effect against human pathogens of bacterial origin being the most thoroughly investigated.
TABLE 6.
Biological activity attributed to the species of the genus Haplopappus.
| Biological activity | Plant species | Plant part(s) | Type of extract and/or isolated compound | Outcome | References |
|---|---|---|---|---|---|
| Antibacterial | H. anthylloides | resin | extract (CH2Cl2) | In vitro growth inhibition of Bacillus anthracis, B. pumilis, B. subtilis, Escherichia coli, Micrococcus flavus, M. luteus, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus, S. epidermidis | Urzúa et al. (1995b) |
| H. baylahuen | aerial parts | decoction, extracts (EtOH, EtOAc) | In vitro growth inhibition of Acremonium falciforme, Bacillus subtilis, Staphylococcus aureus | Lazo, (1990) | |
| leaves | extract (H2O/EtOH) | Bactericide activity against Salmonella enteritidis and inhibition of its ability to form biofilm, express adrA/hilA genes and adhere to Caco-2 cells | Elgueta et al. (2021) | ||
| H. chrysanthemifolius | resin | extract (MeOH) | In vitro growth inhibition of Bacillus cereus, B. subtilis, Enterococcus faecalis, Listeria monocytogens, Micrococcus luteus, Staphylococcus aureus | Urzúa et al., 2004a, 2012 | |
| H. deserticola | resin | 18-acetoxy-cis-cleroda-3,13(E)-dien-15-oic acid (Dit99) | Bactericidal effect against Streptococcus mutans | Urzúa Moll et al. (1997) | |
| H. diplopappus subsp. diplopappus | resin | extract (CH2Cl2) | In vitro growth inhibition of Bacillus anthracis, B. pumilis, B. subtilis Bordetella bronchiseptica, Micrococcus flavus, M. luteus, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus, S. epidermidis | Urzúa et al. (1995b) | |
| 13-O-β-xylopyranosyl-ent-manool (Dit8) | Urzúa et al. (1995a) | ||||
| H. foliosus | resin | extracts (MeOH, CH2Cl2) | In vitro growth inhibition of Bacillus anthracis, B.cereus, B. coagulans, B. pumilis, B. subtilis, Micrococcus luteus, Proteus vulgaris, Staphylococcus aureus, S. epidermidis | Urzúa et al., 1995b, 2003; Urzúa and Mendoza, 2001 | |
| 2α-hydroxy-cis-clero-3,13(Z),8(17)-trien-15-oic acid (Dit87); 2α-acetoxy-cis-clero-3,13(Z),8(17)-trien-15-oic acid (Dit88) | In vitro growth inhibition of Bacillus cereus, B. coagulans, B. subtilis, Micrococcus luteus, Staphylococcus aureus | Urzúa et al. (2003) | |||
| H. litoralis | resin | extract (MeOH) | In vitro growth inhibition of Bacillus cereus, B. subtilis, Enterococcus faecalis, Listeria monocytogens, Micrococcus luteus, Staphylococcus aureus | Urzúa et al., 2004, 2012 | |
| H. multifolius | aerial parts | esculetin (Cum1) | In vitro growth inhibition and bactericide effect against Escherichia coli, Sarcina lutea, Staphylococcus aureus | Chiang et al. (1982) | |
| prenyletin (Cum3) | In vitro growth inhibition and bactericide effect against Sarcina lutea, Staphylococcus aureus | ||||
| haplopinol (Cum4) | In vitro growth inhibition and bactericide effect against Escherichia coli, Staphylococcus aureus | ||||
| aerial parts | extracts (EtOH), infusion | In vitro growth inhibition of Bacillus cereus, B. subtilis, Staphylococcus aureus, S. epidermidis, S. pyogenes | Padilla et al. (2021) | ||
| resin | extract (CH2Cl2) | In vitro growth inhibition of Bacillus anthracis, B. pumilis, B. subtilis, Bordetella bronchiseptica, Micrococcus flavus Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus | Urzúa et al. (1995b) | ||
| H. rigidus | aerial parts | extracts (EtOH/H2O, CHCl3, EtOAc) | In vitro growth inhibition of Bacillus cereus, B. subtilis, Corynobacterium minutissimum, Enterococcus faecalis, Listeria monocytogenes, Staphylococcus aureus, S. lugdunesis | Morales et al., 2003; Ortiz et al., 2019 | |
| H. schumannii | resin | extract (CH2Cl2) | In vitro growth inhibition of Bacillus anthracis, B. pumilis, B. subtilis, Bordetella bronchiseptica, Escherichia coli, Micrococcus flavus, M. luteus, Proteus vulgaris, Staphylococcus aureus, S. epidermidis | Urzúa et al. (1995b) | |
| H. scrobiculatus | resin | extracts (MeOH, CH2Cl2) | In vitro growth inhibition of Bacillus anthracis, B. cereus, B. pumilis, B. subtilis, Enterococcus faecalis, Escherichia coli, Listeria monocytogens, Micrococcus flavus, M. luteus, Proteus vulgaris, Staphylococcus aureus, S. epidermidis | Urzúa et al., 1995b; Urzúa et al., 2004, 2012 | |
| H. taeda | aerial parts | extracts (EtOH), infusion | In vitro growth inhibition of Bacillus cereus, B. subtilis, Staphylococcus agalactiae, S. aureus, S.epidermidis, S. pyogenes | Padilla et al. (2021) | |
| H. uncinatus | resin | extract (MeOH) | In vitro growth inhibition of Bacillus cereus, B. coagulans, B. subtilis, Micrococcus luteus, Staphylococcus aureus | Urzúa and Mendoza, (2001) | |
| extract (CH2Cl2) | In vitro growth inhibition of Bacillus anthracis, B. pumilis, B. subtilis, Bordetella bronchiseptica, Escherichia coli, Micrococcus flavus, M. luteus, Proteus vulgaris, Staphylococcus aureus, S. epidermidis | Urzúa et al. (1995b) | |||
| aerial parts | resin | In vitro growth inhibition of Bacillus cereus, B. subtilis, Micrococcus luteus | Urzúa et al. (2006) | ||
| 18-acetoxy-cis-cleroda-3-en-15-oic acid (10βH, 16ξ, 19β, 17β, 20α form) (Dit84) | |||||
| H. velutinus | resin | extract (CH2Cl2) | In vitro growth inhibition of Bacillus anthracis, B. pumilis, B. subtilis, Bordetella bronchiseptica, Proteus vulgaris, Micrococcus flavus, M. luteus, Staphylococcus aureus, S. epidermidis | Urzúa et al. (1995b) | |
| H. velutinus subsp. illinitus | resin | extract (CH2Cl2) | In vitro growth inhibition of Bacillus anthracis, B. pumilis, B. subtilis, Bordetella bronchiseptica, Micrococcus flavus, Pseudomonas aeruginosa, Staphylococcus aureus | Urzúa et al. (1995b) | |
| Antidysenteric | H. baylahuen | resin | extract suspended in milk, cream or almond emulsion | Symptomatic treatment of dysentery in humans | Fingland, (1903) |
| Anti-inflammatory | H. baylahuen | aerial parts | aqueous extract | Inhibition of carrageenan-induced edema in rats | Adzet and Gene, (1991) |
| H. multifolius | leaves | esculetin (Cum1); esculin (Cum2); prenyletin (Cum3); 6-hydroxy-7-(5′-hydroxy-3′,7′-dimethylocta-2′,6′-dien)-oxycoumarin (Cum6); 6-hydroxy-7-(7′-hydroxy-3′,7′-dimethylocta-2′,5′-dien)-oxycoumarin (Cum7); umbelliferone (Cum14); O-prenylumbelliferone (Cum15) | In vitro inhibition of soybean 15-lipoxygenase (15-sLOX) | Torres et al. (2013) | |
| H. remyanus | resin | extract | Inhibition of arachidonic acid-induced ear edema in mice | Faini et al. (2011) | |
| H. taeda | - | extract (EtOH); taedol (Mon41); 18-acetoxy-cis-cleroda-3,13(E)-dien-15-oic acid (Dit99); sakuranetin (Flv67) | Inhibition of arachidonic acid-induced ear edema in mice | Faini et al. (2008) | |
| Antioxidant | H. baylahuen | commercial product (herbal tea) | infusion | Antioxidant capacity in vitro (ORAC, TEAC-ABTS, HClO quenching and ONOO−quenching assays) | Speisky et al., 2006; Alarcón et al., 2008 |
| aerial parts | infusion, extract (MeOH) | Antioxidant capacity in vitro (DPPH assay) | Schmeda-Hirschmann et al. (2015) | ||
| leaves | infusion, extract (MeOH, H2O/EtOH, EtOH), resin | Antioxidant capacity in vitro (DPPH assay) | Vogel et al., 2005a; Méttola et al., 2018; Elgueta et al., 2021 | ||
| H. deserticola | aerial parts | infusion, extract (MeOH) | Antioxidant capacity in vitro (DPPH assay) | Schmeda-Hirschmann et al. (2015) | |
| H. multifolius | aerial parts | infusion, extract (MeOH) | Antioxidant capacity in vitro (DPPH assay) | Schmeda-Hirschmann et al. (2015) | |
| aerial parts | quercetin (Flv1); isorhamnetin (Flv18); prenyletin (Cum3); haplopinol (Cum4); 6-hydroxy-7-(5′-hydroxy-3′,7′-dimethylocta-2′,6′-dien)-oxycoumarin (Cum6); 6-hydroxy-7-(7′-hydroxy-3′,7′-dimethylocta-2′,5′-dien)-oxycoumarin (Cum7); 6-hydroxy-7-[(E,E)-3′,7′-dimethyl-2′,4′,7′-octatrienyloxy] coumarin (Cum8) | Antioxidant capacity in vitro (DPPH assay) | Torres et al. (2006) | ||
| leaves | infusion, extract (MeOH), resin | Antioxidant capacity in vitro (DPPH assay) | Vogel et al. (2005a) | ||
| H. remyanus | leaves | infusion, extract (MeOH), resin | Antioxidant capacity in vitro (DPPH assay) | Vogel et al. (2005b) | |
| H. rigidus | aerial parts | sternbin (Flv70) | Antioxidant capacity in vitro (TEAC – ABTS, DPPH assay) | Morales et al. (2009) | |
| aerial parts | infusion, extract (MeOH) | Antioxidant capacity in vitro (DPPH assay) | Schmeda-Hirschmann et al. (2015) | ||
| H. taeda | resin, aerial parts | 9-trans-p-coumaroyloxy-α-terpineol (Cin7); 7-trans-p-coumaroyloxy-taedol (Cin8) | Antioxidant capacity in vitro (DPPH assay) | Faini et al. (2007) | |
| aerial parts | infusion; extract (MeOH) | Antioxidant capacity in vitro (DPPH assay) | Schmeda-Hirschmann et al. (2015) | ||
| leaves | infusion, extract (MeOH), resin | Antioxidant capacity in vitro (DPPH assay) | Vogel et al. (2005b) | ||
| Antitumoral | H. remyanus | resin | extract (CH2Cl2) | Cytotoxic effect against T-lymphoblastic leukemia cell line (CCRF-CEM) | Faini et al. (2011) |
| H. rigidus | aerial parts | rigidusol (Dit100) | Cytotoxic effect against human breast adenocarcinoma cell line (MCF-7) | Morales et al., 2000a; Vogel et al., 2005a | |
| sternbin (Flv70) | Cytotoxic effect against human breast adenocarcinoma (MCF-7), human lung carcinoma (A-549) and human colon adenocarcinoma (HT–29) cell lines | Morales et al. (2009) | |||
| Diuretic | H. baylahuen | leaves | extract (EtOH) | Diuretic effect on Wistar rats | Méttola et al. (2018) |
| Hepatoprotective | H. baylahuen | aerial parts | infusion; 7-O-methylaromadenrin (Flv76) | Decrease of glutamic pyruvic transaminase (GTP) levels in serum of rats under CCl4-induced liver injury | Nuñez-Alarcon et al. (1993) |
| infusion | Reduction of serum bilirubin concentration, bromosulfophthalein and alanine aminotransferase activity in dogs under CCl4-induced liver injury | Martin et al. (1988) | |||
| Inhibitory of lipid peroxidation | H. baylahuen | leaves | infusion; extracts (MeOH, EtOH) | Inhibition of lipid peroxidation in vitro and in erythrocyte membranes | Vogel et al., 2005; Méttola et al., 2018 |
| H. multifolius | leaves | infusion; extract (MeOH) | Inhibition of lipid peroxidation in erythrocyte membranes | Vogel et al. (2005a) | |
| H. remyanus | leaves | infusion; extract (MeOH) | Inhibition of lipid peroxidation in erythrocyte membranes | Vogel et al. (2005a) | |
| H. rigidus | aerial parts | sternbin (Flv70) | Inhibition of iron/ascorbate-induced lipid peroxidation in rat cells | Morales et al. (2009) | |
| H. taeda | leaves | infusion; extract (MeOH) | Inhibition of lipid peroxidation in erythrocyte membranes | Vogel et al. (2005a) | |
| Muscle relaxant | H. rigidus | aerial parts | extracts (H2O, MeOH, CH2Cl2) | Relaxation of L-phenylephrine precontracted corpus cavernosum smooth muscles of Guinea pigs | Hnatyszyn et al. (2003) |
| Inhibition of GLUT1 transporter | H. baylahuen | leaves | rhamnetin (Flv17) | Inhibition of GLUT1 transporter in human myeloid HL-60 cells, in transfected Chinese hamster ovary cells overexpressing GLUT1, and in normal human erythrocytes; inhibition of binding of cytochalasin B to GLUT1 in erythrocyte ghosts | Vera et al. (2001) |
| isorhamnetin (Flv18) |
5.2.1 H. anthylloides meyen & walp
Although the bioactivity of the species H. anthylloides has not been extensively studied, it is reported that dichloromethane extracts of its resinous exudates present antibacterial effects, inhibiting the in vitro growth of several human pathogenic bacteria (Urzúa et al., 1995a).
5.2.2 H. baylahuen remy
Haplopappus baylahuen is the species with the highest number of bioactivity studies. Extracts and decoctions of its aerial parts are reported to have antibacterial and bactericide effects against Staphylococcus aureus, Bacillus subtilis, Acremonium falciforme (Lazo, 1990) and Salmonella enteritidis (Elgueta et al., 2021). Moreover, emulsions of its resin have been successfully used to treat the symptoms of dysentery in affected individuals (Fingland, 1903), while extracts of the aerial parts of H. baylahuen have shown anti-inflammatory (Adzet and Gene, 1991), diuretic (Méttola et al., 2018) and hepatoprotective (Nuñez-Alarcon et al., 1993) effects in rat models and hepatoprotective activity in dog models (Martin et al., 1988). The hepatoprotective effect in rats under CCl4-induced liver injury has also been confirmed in the case of 7-O-methylaromadenrin (Flv76) isolated from the aerial parts of the plant (Nuñez-Alarcon et al., 1993). Moreover, rhamnetin (Flv17) and isorhamnetin (Flv18) isolated from the leaves of H. baylahuen have been found to inhibit in a dose-dependent manner the glucose transporter GLUT1 in human cell lines and in vivo in hamsters (Vera et al., 2001). Finally, extracts of the aerial parts of this species have demonstrated significant antioxidant capacity as measured by various in vitro assays (Vogel et al., 2005a; Speisky et al., 2006; Alarcón et al., 2008; Schmeda-Hirschmann et al., 2015; Méttola et al., 2018; Elgueta et al., 2021), while also inhibiting lipid peroxidation in vitro and in erythrocyte membranes (Vogel et al., 2005a; Méttola et al., 2018).
5.2.3 H. chrysanthemifolius (Less.) DC
In the case of H. chrysanthemifolius, scientific evidence supports the antibacterial effect of the methanolic extracts of its resinous exudates, as this has been demonstrated through the in vitro growth inhibition of several Gram-positive human pathogenic bacterial strains (Urzúa et al., 2004b; 2012).
5.2.4 H. deserticola Phil
The diterpene 18-acetoxy-cis-cleroda-3,13(E)-dien-15-oic acid (Dit99) isolated from the resin of H. deserticola presented a bactericidal effect against Streptococcus mutans (Urzúa Moll et al., 1997), while the in vitro antioxidant capacity of the infusion and methanolic extract of the plant’s aerial parts has also been documented (Schmeda-Hirschmann et al., 2015).
5.2.5 H. diplopappus Remy subsp. diplopappus
The resin of H. diplopappus subsp. diplopappus, as well as the isolated diterpenoid 13-O-β-xylopyranosyl-ent-manool (Dit8) present antibacterial effect against various Gram-positive and Gram-negative human pathogenic bacteria (Urzúa et al., 1995a).
5.2.6 H. foliosus (Hook. & Arn.) Hook. & Arn
Scientific evidence supports the antibacterial effect of the resinous exudate of H. foliosus against several Gram-positive and Gram-negative human pathogenic bacteria (Urzúa et al., 1995a; 2003; Urzúa and Mendoza, 2001). Similar bioactivity has been attributed to the diterpenes 2α-hydroxy-cis-clero-3,13(Z),8(17)-trien-15-oic acid (Dit87) and 2α-acetoxy-cis-clero-3,13(Z),8(17)-trien-15-oic acid (Dit88) which were isolated from the resin of H. foliosus (Urzúa et al., 2003).
5.2.7 H. litoralis Phil
In the case of H. litoralis, it has been reported that its resinous exudate inhibits the in vitro growth of Bacillus cereus, B. subtilis, Enterococcus faecalis, Listeria monocytogens, Micrococcus luteus, S. aureus (Urzúa et al., 2004b; 2012).
5.2.8 H. multifolius reiche
Scientific literature provides evidence that support the antibacterial effect of H. multifolius resin and aerial parts extracts against a wide spectrum of Gram-positive and Gram-negative human pathogenic bacteria (Urzúa et al., 1995a; Padilla et al., 2021). Moreover, similar antibacterial activity has been documented for the coumarins esculetin (Cum1), prenyletin (Cum3) and haplopinol (Cum4) isolated from the aerial parts of this species (Chiang et al., 1982). Regarding the in vitro antioxidant capacity of H. multifolius, this has been demonstrated in the case of extracts, aerial parts infusions and resin (Vogel et al., 2005a; Schmeda-Hirschmann et al., 2015), as well as for the isolated compounds quercetin (Flv1), isorhamnetin (Flv18), prenyletin (Cum3), haplopinol (Cum4), 6-hydroxy-7-(5′-hydroxy-3′,7′-dimethylocta-2′,6′-dien)-oxycoumarin (Cum6), 6-hydroxy-7-(7′-hydroxy-3′,7′-dimethylocta-2′,5′-dien)-oxycoumarin (Cum7) and 6-hydroxy-7-[(E,E)-3′,7′-dimethyl-2′,4′,7′-octatrienyloxy] coumarin (Cum8) (Torres et al., 2006). Furthermore, the isolated compounds esculetin (Cum1), esculin (Cum2), prenyletin (Cum3), 6-hydroxy-7-(5′-hydroxy-3′,7′-dimethylocta-2′,6′-dien)-oxycoumarin (Cum6), 6-hydroxy-7-(7′-hydroxy-3′,7′-dimethylocta-2′,5′-dien)-oxycoumarin (Cum7), umbelliferone (Cum14) and O-prenylumbelliferone (Cum15) have demonstrated an anti-inflammatory effect associated to the in vitro inhibition of soybean 15-lipoxygenase (Torres et al., 2013). Finally, methanolic extracts and infusions of H. multifolius leaves inhibited the lipid peroxidation in erythrocyte membranes (Vogel et al., 2005a).
5.2.9 H. remyanus wedd
Infusions, methanolic extracts and resin from the leaves of H. remyanus demonstrated a significant antioxidant capacity in vitro, while also inhibiting lipid peroxidation in erythrocyte membranes (Vogel et al., 2005a). Furthermore, the resinous exudates of the plant exhibited an anti-inflammatory effect in mice (Faini et al., 2011) and a cytotoxic effect against T-lymphoblastic leukemia cell line (CCRF-CEM) (Faini et al., 2011).
5.2.10 H. rigidus Phil
Extracts of the aerial parts of H. rigidus have effectively inhibited the in vitro growth of several Gram-positive bacterial strains (Morales et al., 2003; Ortiz et al., 2019), presented a significant in vitro antioxidant capacity (Schmeda-Hirschmann et al., 2015) and also acted as muscle relaxants in Guinea pig models (Hnatyszyn et al., 2003). The isolated flavanone sternbin (Flv70) presented high in vitro antioxidant capacity, lipid peroxidation inhibitory effects in rat cells and also antitumoral effect against the human breast adenocarcinoma (MCF-7), human lung carcinoma (A-549) and human colon adenocarcinoma (HT–29) cell lines (Morales et al., 2009). The isolated diterpene rigidusol (Dit100) also had a cytotoxic effect on human breast adenocarcinoma cells line (MCF-7) (Morales et al., 2000b).
5.2.11 H. schumannii (Kuntze) G.K. Br. & W.D. Clark
The resinous exudates of H. schumannii inhibited the in vitro growth of several Gram-positive bacterial human pathogens (Urzúa et al., 1995a).
5.2.12 H. scrobiculatus (Nees) DC
Similarly, the only known bioactivity regarding the resin of H. scrobiculatus is that of the in vitro antibacterial effect against several Gram-positive bacteria (Urzúa et al., 1995a; 2004b; 2012).
5.2.13 H. taeda reiche
Ethanolic extracts and infusions of aerial parts of H. taeda successfully inhibited the in vitro growth of several Bacillus and Staphylococcus bacterial strains (Padilla et al., 2021). Regarding the in vitro antioxidant capacity of the species, this has been shown to be significant in the case of aerial parts infusions, extracts and resinous exudates (Vogel et al., 2005a; Schmeda-Hirschmann et al., 2015), as well as for the isolated compounds 9-trans-p-coumaroyloxy-α-terpineol (Cin7) and 7-trans-p-coumaroyloxy-taedol (Cin8) (Faini et al., 2007). Moreover, leaf infusions and methanolic extracts of H. taeda inhibited lipid peroxidation in erythrocyte membranes (Vogel et al., 2005a). Ethanolic extracts, as well as the isolated compounds taedol (Mon41), 18-acetoxy-cis-cleroda-3,13(E)-dien-15-oic acid (Dit99), and sakuranetin (Flv67) exhibited an anti-inflammatory effect against arachidonic acid-induced ear edema in mice (Faini et al., 2008).
5.2.14 H. uncinatus Phil
Extracts of the aerial parts and resinous exudates of H. uncinatus, as well as the isolated diterpenoid 18-acetoxy-cis-cleroda-3-en-15-oic acid (10βH, 16ξ, 19β, 17β, 20α form) (Dit84) have been reported to inhibit in vitro the growth of various Gram-positive human pathogenic bacteria (Urzúa et al., 1995a; 2006; Urzúa and Mendoza, 2001).
5.2.15 H. velutinus remy, H. velutinus Remy subsp. illinitus (Phil.) Klingenb
Dichloromethane extracts of the resinous exudates of H. velutinus and its subspecies H. velutinus subsp. illinitus inhibited in vitro the growth of various Gram-positive and Gram-negative human pathogenic bacteria (Urzúa et al., 1995a).
6 Non-human health related bioactivity and toxicity
Among the pharmacological activities attributed to Haplopappus species and not related to the human health, the most studied is the antimicrobial effect against plant pathogens. The essential oil of the leaves of H. baylahuen inhibited the in vitro growth of the fungi Aspergillus nigra and Fusarium oxysporum (Becerra et al., 2010). Moreover, the diterpenoid 7,13-(E)-labdadien-15,18-dioic acid 18-methyl ester (Dit45) was isolated from the resinous exudate of Haplopappus velutinus and inhibiting in vitro the mycelial growth of Botrytis cinerea (Echeverría et al., 2019). In the case of the phytopathogenic bacterium Clavibacter michiganensis subsp. michiganensis, its in vitro growth was inhibited by the resin (Urzúa and Mendoza, 2001) and the isolated diterpene 18-acetoxy-cis-cleroda-3-en-15-oic acid (10βH, 16ξ, 19β, 17β, 20α form) (Dit84) (Urzúa et al., 2006) from H. uncinatus, as well as by the methanolic extract of the resin of H. foliosus (Urzúa and Mendoza, 2001).
The essential oil of H. foliosus also exhibited insecticide effects against house flies (Musca domestica) (Urzúa et al., 2010), while hydroethanolic and chloroform extracts of H. rigidus presented biotoxic activity against Artemia salina (Morales et al., 2003).
7 Concluding remarks and future perspectives
The available scientific literature on the genus Haplopappus can be said to support, although partially, its widespread and longstanding use as a medicinal plant. However, the results of the present review highlight several limitations that need to be addressed.
Firstly, phytochemical and bioactivity research of the genus Haplopappus is largely concentrated in the 1990s and 2000s, with almost 80% of the investigation having been performed before 2010. Therefore, a revival of scientific interest and the application of modern, more advanced and diverse analytical and biological techniques can further elucidate the composition and bioactivity of Haplopappus plant species, thus broadening the existing knowledge and promoting its potential uses.
Furthermore, phytochemical and pharmacological evidence is available only for the 40% and 23%, respectively, of the botanical taxa of the genus Haplopappus, while for many of the studied taxa, the available information is rather limited. Similarly, terpenoids and phenolics correspond to approximately 70% of the compounds reported in Haplopappus spp., suggesting that scientific investigation up to date has possibly understudied other chemical groups. It is, therefore, suggested to extend the focus of scientific research to more, if not all, Haplopappus species and groups of chemical compounds, thus permitting to fully explore its promising chemical and biological prospects.
Based on the available bioactivity and pharmacological evidence, Haplopappus species can be considered as a valuable plant resource for health-promoting applications. However, the majority of the investigation provides evidence associated to the in vitro antibacterial and antioxidant activity of the genus Haplopappus. In contrast, there is a lack of scientific evidence to support or refute various traditional uses, while, at the same time, the limited number of in vivo studies and/or clinical trials hinders its wider human health-promoting application and secure use.
In this context, the information presented in the present review supports the ethnopharmacological, phytochemical and bioactive potential of the genus Haplopappus, while addressing the aforementioned limitations could further promote and broaden both scientific research and future applications and uses.
Acknowledgments
CM was supported by the Scholarship Program of the Agencia Nacional de Investigación y Desarrollo de Chile (ANID Doctorado Nacional 2022/21220376).
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. We gratefully acknowledge support from Comisión Nacional de Investigación Científica y Tecnológica—CONICYT PAI/ACADEMIA No. 79160109 and from Dirección de Investigación Científica y Tecnológica—DICYT USACH 022341EM_Ayudante.
Author contributions
CM: Writing–review and editing, Writing–original draft, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization. JE: Writing–review and editing, Writing–original draft, Supervision, Project administration, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1490243/full#supplementary-material
References
- Adzet T. E., Gene R. M. (1991). Estudio de la actividad antiinflamatoria de especies vegetales de origen centro y sudamericano. Dominguezia 9, 17–23. [Google Scholar]
- Alarcón E., Campos A. M., Edwards A. M., Lissi E., López-Alarcón C. (2008). Antioxidant capacity of herbal infusions and tea extracts: a comparison of ORAC-fluorescein and ORAC-pyrogallol red methodologies. Food Chem. 107, 1114–1119. 10.1016/j.foodchem.2007.09.035 [DOI] [Google Scholar]
- Aldunate C., Armesto J., Castro V., Villagrán C. (1981). Estudio etnobotánico en una comunidad precordillerana de Antofagasta: Toconce. Bol. del Mus. Nac. Hist. Nat. 38, 183–223. 10.54830/bmnhn.v38.1981.473 [DOI] [Google Scholar]
- Alonso J. (2005). Monografía: Baylahuén. Bol. Latinoam. del Caribe plantas aromáticas 4, 60–62. [Google Scholar]
- Arzneibuch H. (2006). Amtliche deutsche Ausgabe. Stuttgart, Germany: Deutscher Apotheker Verlag. [Google Scholar]
- Ates N., Ulubelen A., Clark W. D., Brown G. K., Mabry T. J., Dellamonica G., et al. (1982). Flavonoids of haplopappus scrobiculatus and haplopappus sericeus. J. Nat. Prod. 45, 189–190. 10.1021/np50020a014 [DOI] [Google Scholar]
- Ayanoglu E., Ulubelen A., Clark W. D., Brown G. K., Kerr R. R., Mabry T. J. (1981). Myricetin and quercetin methyl ethers from Haplopappus integerrimus var. punctatus. Phytochemistry 20, 1715–1717. 10.1016/s0031-9422(00)98561-3 [DOI] [Google Scholar]
- Becerra J., Bittner M., Hernandez V., Brintrup C., Silva M. (2010). Activity of essential oils of Canelo, Queule, Bailahuen y Culen against phythopatogenic fungi. Bol. Latinoam. del Caribe Plantas Aromat. 9, 212–215. [Google Scholar]
- Cárdenas U. (1998). Entre el tolar y el pajonal: Percepción ambiental y uso de plantas en la comunidad atacameña de Talabre, II Región, Chile. Estud. Atacameños 16, 251–282. 10.22199/s07181043.1998.0016.00010 [DOI] [Google Scholar]
- Chiang M. T., Bittner M., Silva M., Mondaca A., Zemelman R., Sammes P. G. (1982). A prenylated coumarin with antimicrobial activity from Haplopappus multifolius. Phytochemistry 21, 2753–2755. 10.1016/0031-9422(82)83119-1 [DOI] [Google Scholar]
- Del Vitto L. A., Petenatti E. M., Petenatti M. E. (2010). “Ethnomedical plants from Cuyo region, Argentina: uses and conservational status,” in Tradiciones & transformaciones en etnobotánica. Editors Pochettino M. L., Ladio A. H., Arenas P. M. (Jujuy, Argentina: CYTED - Programa Iberoamericano Ciencia y Tecnología para el Desarrollo; ), 223–229. [Google Scholar]
- de Mösbach E. W. (1992). “Botánica indígena de Chile,”. Chile: Editorial Andrés Bello. [Google Scholar]
- Echeverría J., González-Teuber M., Urzúa A. (2019). Antifungal activity against Botrytis cinerea of labdane-type diterpenoids isolated from the resinous exudate of Haplopappus velutinus Remy (Asteraceae). Nat. Prod. Res. 33, 2408–2412. 10.1080/14786419.2018.1443093 [DOI] [PubMed] [Google Scholar]
- Elgueta E., Mena J., Orihuela P. A. (2021). Hydroethanolic extracts of Haplopappus baylahuen Remy and Aloysia citriodora Palau have bactericide activity and inhibit the ability of Salmonella Enteritidis to form biofilm and adhere to human intestinal cells. Biomed. Res. Int. 2021, 3491831. 10.1155/2021/3491831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza E. (1897). Plantas medicinales de Chile: Fragmento de la cuarta edición de la Jeografía Descriptiva de la República de Chile. Santiago de Chile: Imprenta i Encuadernación Barcelona. [Google Scholar]
- Faini F., Labbé C., Delporte C., Backhouse N., Castro C., Torres R. (2008). Anti-inflammatory and antioxidant activities of Haplopappus taeda extracts. Planta Med. 74, PA78. 10.1055/s-0028-1084076 [DOI] [Google Scholar]
- Faini F., Labbé C., Torres R., Delle Monache F., Delle Monache G. (1999). Diterpenes from haplopappus chrysanthemifolius. Phytochemistry 52, 1547–1550. 10.1016/s0031-9422(99)00395-7 [DOI] [Google Scholar]
- Faini F., Labbé C., Torres R., Monache G. D., Monache F. D. (2002). Labdane diterpenes from Haplopappus illinitus. Nat. Prod. Lett. 16, 223–228. 10.1080/10575630290020460 [DOI] [PubMed] [Google Scholar]
- Faini F., Labbé C., Torres R., Rodilla J. M., Silva L., Delle Monache F. (2007). New phenolic esters from the resinous exudate of Haplopappus taeda. Fitoterapia 78, 611–613. 10.1016/j.fitote.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Faini F., Torres R., Rodilla J. M., Labbé C., Delported C., Jañaa F. (2011). “Chemistry and bioactivity of haplopappus remyanus (“bailahuen”), a chilean medicinal plant,” in J. Braz. Chem. Soc. 2344–2349. 10.1590/S0103-50532011001200015 [DOI] [Google Scholar]
- Fingland W. (1903). The successful treatment of sporadic dysentery by Aplopappus baylahuen. Lancet 162, 456–457. 10.1016/s0140-6736(00)67384-1 [DOI] [Google Scholar]
- García N., Cádiz-Véliz A., Villalobos M., Morales V. (2024). Taxonomic novelties in haplopappus (Asteraceae, Astereae) from Chile. PhytoKeys 237, 201–218. 10.3897/phytokeys.237.114461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia N., Medina P., Morales V. (2018). Haplopappus mieresii sp. nov.(Asteraceae) and the reinstatement of H. reicheanus from central Chile. Phytotaxa 376, 103–113. 10.11646/phytotaxa.376.2.4 [DOI] [Google Scholar]
- Gómez D., Ahumada J., Necul E. (1997). Medicina tracional atacameña. Santiago, Chile: Ministerio de Educación. [Google Scholar]
- Gómez-Parra D., Siarez Flores E. (1995). “Alimentación tradicional atacameña,” in Antofagasta, Chile: NORprint. [Google Scholar]
- Hnatyszyn O., Moscatelli V., Garcia J., Rondina R., Costa M., Arranz C., et al. (2003). Argentinian plant extracts with relaxant effect on the smooth muscle of the corpus cavernosum of Guinea pig. Phytomedicine 10, 669–674. 10.1078/0944-7113-00261 [DOI] [PubMed] [Google Scholar]
- Hoffmann A., Farga C., Lastra J., Veghazi E. (1992). Plantas medicinales de uso común en Chile. Santiago Fund. Claudio Gay c1992, 2nd. [Google Scholar]
- Hörhammer L., Wagner H., Wilkomirsky M. T., Iyengar M. A. (1973). Flavonoide in einigen chilenischen heilpflanzen. Phytochemistry 12, 2068–2069. 10.1016/s0031-9422(00)91548-6 [DOI] [Google Scholar]
- Houghton P. J., Manby J. (1985). Medicinal plants of the Mapuche. J. Ethnopharmacol. 13, 89–103. 10.1016/0378-8741(85)90063-7 [DOI] [PubMed] [Google Scholar]
- Jakupovic J., Baruah R. N., Zdero C., Eid F., Pathak V. P., Chau-Thi T. V., et al. (1986). Further diterpenes from plants of the Compositae, subtribe Solidagininae. Phytochemistry 25, 1873–1881. 10.1016/s0031-9422(00)81166-8 [DOI] [Google Scholar]
- Kim H. W., Wang M., Leber C. A., Nothias L.-F., Reher R., Kang K. B., et al. (2021). NPClassifier: a deep neural network-based structural classification tool for natural products. J. Nat. Prod. 84, 2795–2807. 10.1021/acs.jnatprod.1c00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg L. (2007). Monographie der südamerikanischen Gattungen Haplopappus Cass. und Notopappus L. Klingenberg (Asteraceae-Astereae). Stuttgart, Germany: Schweizerbart’sche Verlagsbuchhandlung. [Google Scholar]
- Labbé C., Faini F., Coll J., Carbonell P. (1998). Guaiane sesquiterpenoids from Haplopappus foliosus. Phytochemistry 49, 793–795. 10.1016/s0031-9422(97)00871-6 [DOI] [Google Scholar]
- Latorre I., Peña R. C., Erazo S. (1990). Ensayos farmacognósticos de tres compuestas usadas en medicina popular en Chile Central. An. la Real Acad. Nac. Farm. 56, 359–366. [Google Scholar]
- Laval E. (1957). Medicina aborigen tradicional atacameña. Rev. del Serv. Nac. Salud 2. [Google Scholar]
- Lazo W. (1990). Acción antimicrobiana de algunas plantas de uso medicinal en Chile: I. Bol. Micol. 5, 25–28. 10.22370/bolmicol.1990.5.1-2.1587 [DOI] [Google Scholar]
- Maatooq G. T., Gohar A. A., Hoffmann J. J. (2002). New terpenoids from Haplopappus multifolius. Pharmazie 57, 282–285. [PubMed] [Google Scholar]
- Madaleno I. M., Delatorre-Herrera J. (2013). Medicina popular de Iquique, Tarapacá. Idesia (Arica) 31, 67–78. 10.4067/s0718-34292013000100009 [DOI] [Google Scholar]
- Maldonado Z., Hoeneisen M., Silva M. (1993). Constituents of Haplopappus bezanillanus and H. hirtellus. Bol. la Soc. Chil. Quim. 38, 43–48. [Google Scholar]
- Marambio O., Silva M. (1989). New compounds isolated from Haplopappus taeda Reiche. Bol. la Soc. Chil. Quim. 34, 105–113. [Google Scholar]
- Marambio O., Silva M. (1996). Compuestos fenólicos y triterpenos aislados desde Haplopappus velutinus Remy y H. glutinosus Cass. Bol. la Soc. Chil. Quím. 41, 199–200. [Google Scholar]
- Martin R., Wittwer F., Núñez J. (1988). Effect of an infusion of Haploppapus baylahuen in dogs with liver damage. J. Vet. Med. Ser. A Ger. Fr. [PubMed] [Google Scholar]
- Mellado Campos V. (1996). Herbolaria médica de Chile: diagnóstico de su estado actual y perspectivas futuras para la medicina oficial chilena. Ministerio: Santiago de Chile. [Google Scholar]
- Méttola R. M., Brodkewics I. Y., Vera N. R., Reynoso M. A. (2018). Actividad diurética y antioxidante de los extractos etanólicos de especies vegetales usadas en la medicina popular argentina. Rev. Cuba. Plantas Med. 23. [Google Scholar]
- Ministerio de Salud (2010). MHT. Medicamentos herbarios tradicionales. 103 especies vegetales. Santiago, Chile: Ministerio de Salud. [Google Scholar]
- Monterrey N. (1996). Hierbas medicinales andinas de la 2a región. Santiago, Chile: Ministerio de Educación. [Google Scholar]
- Montes M., Wilkomirsky T. (1987). “Medicina tradicional chilena,” in Editiorial de la Universidad de Concepción. [Google Scholar]
- Morales G., Paredes A., Sierra P., Loyola L. A. (2009). Cytotoxicity, scavenging and lipid peroxidation - inhibiting activities of 5,3´,4´-trihy-droxy - 7 - methoxyflavanone isolated from haplopappus rigidus. J. Chil. Chem. Soc. 54, 105–107. 10.4067/S0717-97072009000200001 [DOI] [Google Scholar]
- Morales G., Sierra P., Borquez J., Loyola L. A. (2000a). Rigidúsido, un nuevo glicoditerpenoide de Haplopappus rigidus. Bol. la Soc. Chil. Quím. 45, 611–614. 10.4067/s0366-16442000000400014 [DOI] [Google Scholar]
- Morales G., Sierra P., Loyola L. A., Borquez J. (2000b). Diterpenoids from haplopappus rigidus. Phytochemistry 55, 863–866. 10.1016/s0031-9422(00)00321-6 [DOI] [PubMed] [Google Scholar]
- Morales G., Sierra P., Mancilla A., Paredes A., Loyola L. A., Gallardo O., et al. (2003). Secondary metabolites from four medicinal plants from northern Chile: antimicrobial activity and biotoxicity against Artemia salina. J. Chil. Chem. Soc. 48, 13–18. 10.4067/s0717-97072003000200002 [DOI] [Google Scholar]
- Mostny G., Jeldes F., González R., Oberhauser F., Gonzàlez R. (1954). “Peine, un pueblo atacameño,” in Instituto de Geografia, Facultad de Filosofia. Santiago, Chile: Universidad de Chile. [Google Scholar]
- Munizaga C. (1963). “Un medico herbolario de la actualidad en el norte de Chile,” 26. Santiago, Chile: Rev. Univ. la Univ. Católica Chile. [Google Scholar]
- Munizaga C., Gunkel H. (1958). “Notas etnobotanicas del pueblo atacameno de Socaire,” in Centro de Estudios Antropológicos. Santiago, Chile: Universidad de Chile. [Google Scholar]
- Muñoz M., Barrera E., Meza I. (1981). El uso medicinal y alimenticio de plantas nativas y naturalizadas en Chile. Mus. Nac. Hist. Nat. 33, 91. [Google Scholar]
- Murillo A. (1861). Memoria sobre las plantas medicinales de Chile y el uso medicinal que de ellas se hace en el país. Santiago, Chile: Impr. de Ferrocarril. [Google Scholar]
- Murillo A. (1889). Plantes médicinales du Chili. Paris: Exposition Universelle de Paris, Section chilienne. [Google Scholar]
- Nuñez-Alarcon J., Dolz H., Quiñones M. H., Carmona M. T. (1993). Epicuticular flavonoids from Haplopappus baylahuen and the hepatoprotective effect of the isolated 7-methyl aromadendrin. Bol. la Soc. Chil. Quim. 38, 15–22. [Google Scholar]
- Nuñez-Alarcón J., Quiñones M. (1995). Flavonoids and coumarins of Haplopappus multifolius. Biochem. Syst. Ecol. 23, 453–454. 10.1016/0305-1978(95)00019-Q [DOI] [Google Scholar]
- Oksuz S., Ulubelen A., Clark W. D., Brown G. K., Brown T. J. (1981). Flavonoids of haplopappus canescens. Rev. Latinoam. Quim 12, 12. [Google Scholar]
- Ortiz S., Lecsö-Bornet M., Bonnal C., Houze S., Michel S., Grougnet R., et al. (2019). Bioguided identification of triterpenoids and neolignans as bioactive compounds from anti-infectious medicinal plants of the Taira Atacama’s community (Calama, Chile). J. Ethnopharmacol. 231, 217–229. 10.1016/j.jep.2018.10.029 [DOI] [PubMed] [Google Scholar]
- Padilla C., Lobos O., Poblete-Tapia P., Carrasco-Sánchez V. (2021). Antimicrobial activity of bioactive compounds of Haplopappus multifolius and Haplopappus taeda against human pathogenic microorganisms. Iran. J. Microbiol. 13, 98–103. 10.18502/ijm.v13i1.5498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer J. (2022). Haplopappus multifolius. The IUCN Red List of Threatened Species. 2022: e.T184927307A188497867. [Google Scholar]
- POWO (2024). Plants of the World online. Kew: Facilitated by the Royal Botanic Gardens. Available at: http://www.plantsoftheworldonline.org/RetrievedDDMonth2021. [Google Scholar]
- Ratera E., Ratera M. (1980). “Plantas de la flora argentina empleadas en medicina popular,”. Buenos Aires, Argentina: Hemisferio Sur. [Google Scholar]
- J., Remington,, H. Woods, (1918). The dispensatory of the United States of America. 20th edition (USA: Lippincott; ). [Google Scholar]
- Rodriguez R., Marticorena C., Alarcón D., Baeza C., Cavieres L., Finot V. L., et al. (2018). Catálogo de las plantas vasculares de Chile. Gayana Bot. 75, 1–430. 10.4067/s0717-66432018000100001 [DOI] [Google Scholar]
- Rossomando P. C., Guerreiro E., Giordano O. S. (1995). “Diterpenes and other constituents from Argentinean Haplopappus species,” in Anales de la Asociación Química Argentina, 55–58. [Google Scholar]
- Schmeda-Hirschmann G., Quispe C., González B. (2015). Phenolic profiling of the South American “baylahuen” tea (Haplopappus spp., Asteraceae) by HPLC-DAD-ESI-MS. Molecules 20, 913–928. 10.3390/molecules20010913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrickel S., Bittner M. (2001). “La salud en nuestras manos. Plantas medicinales en Chile, Riqueza Natural y Científica,” in Concepción, Chile. Concepción, Chile: Lamas. [Google Scholar]
- Schwenker G., Kloss P., Engels W. (1967). On the isolation of prenyletin from Haplopappus baylahuen. Pharmazie 22, 724–725. [PubMed] [Google Scholar]
- Serracino G., Stehberg R., Liberman G. (1974). Informe etnobotánico de Guatin (San Pedro de Atacama). Antropol. Nueva Epoca 1, 55–65. [Google Scholar]
- Silva M., Sammes P. G. (1973). A new diterpenic acid and other constituents of Haplopappus foliosus and H. angustifolius. Phytochemistry 12, 1755–1758. 10.1016/0031-9422(73)80397-8 [DOI] [Google Scholar]
- Speisky H., Rocco C., Carrasco C., Lissi E. A., López‐Alarcón C. (2006). Antioxidant screening of medicinal herbal teas. Phyther. Res. 20, 462–467. 10.1002/ptr.1878 [DOI] [PubMed] [Google Scholar]
- Steinmetz E. F. (1954). Materia medica vegetabilis . Amsterdam, The Nethelands. [Google Scholar]
- Tojo E., Rial M. E., Urzúa A., Mendoza L. (1999). Clerodane diterpenes from Haplopappus deserticola. Phytochemistry 52, 1531–1533. 10.1016/s0031-9422(99)00193-4 [DOI] [Google Scholar]
- Torres R., Faini F., Delle Monache F., Delle Monache G. (2004). Two new O-geranyl coumarins from the resinous exudate of Haplopappus multifolius. Fitoterapia 75, 5–8. 10.1016/j.fitote.2003.06.003 [DOI] [PubMed] [Google Scholar]
- Torres R., Faini F., Modak B., Urbina F., Labbé C., Guerrero J. (2006). Antioxidant activity of coumarins and flavonols from the resinous exudate of Haplopappus multifolius. Phytochemistry 67, 984–987. 10.1016/j.phytochem.2006.03.016 [DOI] [PubMed] [Google Scholar]
- Torres R., Faini F., Rodilla J. M. L., Silva L. A., Sanz F. (2005). Crystal structure of taedol, C10H16O2, from Haplopappus taeda. Z. für Krist. Cryst. Struct. 220, 537–538. 10.1524/ncrs.2005.220.4.537 [DOI] [Google Scholar]
- Torres R., Mascayano C., Nunez C., Modak B., Faini F. (2013). Coumarins of Haplopappus multifolius and derivative as inhibitors of LOX: evaluation in-vitro and docking studies. J. Chil. Chem. Soc. 58, 2027–2030. 10.4067/s0717-97072013000400027 [DOI] [Google Scholar]
- Tschesche R., Sualaheen Delhvi M., Sepúlveda‐Boza S., Zilliken F., Kirfel A., Will G. (1985). Haplopappin, ein 8‐(α‐Methylbenzyl)flavonoid aus Haplopapus foliosus. Liebigs Ann. Chem. 1985, 2465–2471. 10.1002/jlac.198519851215 [DOI] [Google Scholar]
- Ulubelen A., Ayanoglu E., Clark W. D., Brown G. K., Mabry T. J. (1982). Flavonoids from haplopappus foliosus. J. Nat. Prod. 45, 363–364. 10.1021/np50021a021 [DOI] [Google Scholar]
- Ulubelen A., Clark W. D., Brown G. K., Mabry T. J. (1981). Flavonoids of haplopappus rengifoanus Remy in Gay. J. Nat. Prod. 44, 294–295. 10.1021/np50015a010 [DOI] [Google Scholar]
- Urzúa A. (2004). Secondary metabolites in the epicuticle of Haplopappus foliosus DC. (Asteraceae). J. Chil. Chem. Soc. 49, 137–141. 10.4067/S0717-97072004000200006 [DOI] [Google Scholar]
- Urzúa A., Andrade L., Jara F. (2000). Comparative chemical composition of the resinous exudates from Haplopappus foliosus and H. uncinatus. Biochem. Syst. Ecol. 28, 491–493. 10.1016/s0305-1978(99)00080-0 [DOI] [PubMed] [Google Scholar]
- Urzúa A., Contreras R., Jara P., Avila F., Suazo M. (2004a). Comparative chemical composition of the trichome secreted exudates and of the waxy coating from Haplopappus velutinus, H. illinitus, H. shumanni and H. uncinatus . Biochem. Syst. Ecol. 32 (2), 215–218. 10.1016/S0305-1978(03)00157-1 [DOI] [Google Scholar]
- Urzúa A., Cuadra P., Rodriguez R., Mendoza L. (1991). Flavonoid aglycones in the resinous exudate of some Chilean plants. Fitoterapia 62, 358. [Google Scholar]
- Urzúa A., Echeverría J., Espinoza J. (2012). Lipophilicity and antibacterial activity of flavonols: antibacterial activity of resinous exudates of haplopappus litoralis, H. chrysantemifolius and H. scrobiculatus. Bol. Latinoam. del Caribe Plantas Aromat. 11, 369–376. [Google Scholar]
- Urzúa A., Iturra B., Sebastián B., Muñoz M. (2007a). Chemical components from the surface of Haplopappus bustillosianus. Biochem. Syst. Ecol. 35, 794–796. 10.1016/j.bse.2007.03.020 [DOI] [Google Scholar]
- Urzúa A., Jara F., Tojo E., Wilkens M., Mendoza L., Rezende M. C. (2006). A new antibacterial clerodane diterpenoid from the resinous exudate of Haplopappus uncinatus. J. Ethnopharmacol. 103, 297–301. 10.1016/j.jep.2005.09.042 [DOI] [PubMed] [Google Scholar]
- Urzúa A., Mendoza L. (1989). 19-Metil éster del ácido dehidropinifólico en la resina de Haplopappus velutinus. Bol. la Soc. Chil. Quim. 34, 221–223. [Google Scholar]
- Urzua A., Mendoza L. (1993). Chemical composition of the resinous exudate of Haplopappus velutinus. Bol. la Soc. Chil. Quim. 38, 89–93. [Google Scholar]
- Urzúa A., Mendoza L. (2001). Antibacterial activity of the resinous exudates from Haplopappus uncinatus and Haplopappus foliosus. Fitoterapia 72, 418–420. 10.1016/s0367-326x(00)00332-4 [DOI] [PubMed] [Google Scholar]
- Urzúa A., Mendoza L., Andrade L., Miranda B. (1997). Diterpenoids in the trichome resinous exudate from Haplopappus shumannii. Biochem. Syst. Ecol. 25, 683–684. 10.1016/s0305-1978(97)00071-9 [DOI] [Google Scholar]
- Urzúa A., Santander R., Echeverria J., Cabezas N., Palacios S. M., Rossi Y. (2010). Insecticide properties of the essential oils from haplopappus foliosus and Bahia Ambrosoides against the house fly, Musca Domestica L. J. Chil. Chem. Soc. 55, 392–395. 10.4067/S0717-97072010000300026 [DOI] [Google Scholar]
- Urzúa A., Santander R., Echeverría J., Rezende M. C. (2007b). Secondary metabolites in the flower heads of Haplopappus berterii (Asteraceae) and its relation with insect-attracting mechanisms. J. Chil. Chem. Soc. 52, 1142–1144. 10.4067/s0717-97072007000200005 [DOI] [Google Scholar]
- Urzua A., Tojo E., Soto J. (1995a). A diterpene xyloside from the resinous exudate of Haplopappus diplopappus. Phytochemistry 38, 555–556. 10.1016/0031-9422(94)00590-p [DOI] [Google Scholar]
- Urzúa A., Torres R., Mendoza L., Delle Monache F. (2003). Antibacterial new clerodane diterpenes from the surface of Haplopappus foliosus. Planta Med. 69, 675–677. 10.1055/s-2003-41118 [DOI] [PubMed] [Google Scholar]
- Urzúa A., Torres R., Muñoz M., Palacios Y. (1995b). Comparative antimicrobial study of the resinous exudates of some Chilean Haplopappus (Asteraceae). J. Ethnopharmacol. 45, 71–74. 10.1016/0378-8741(94)01196-7 [DOI] [PubMed] [Google Scholar]
- Urzúa A., Vines M., Echeverría J., Ibacache A., Iturra B., Sebastián B. (2004b). “Actividad antibacteriana y composición química de los extractos superficiales de Haplopappus berteri, H. cuneifolius, H. litoralis, H. scrobiculatus y H. sp. (“Bailahuen”),” in Segundo Simposium Internacional de Plantas Medicinales y Fitoterapia. [Google Scholar]
- Urzúa Moll A., Caroli Rezende M., Beezer A., Mitchell J. (1997). A calorimetric bioassay of diterpenoids from Chilean medicinal plants against Streptococcus mutans. Bol. la Soc. Chil. Quim. 43, 81–85. [Google Scholar]
- Valant-Vetschera K. M., Wollenweber E. (2007). Chemodiversity of exudate flavonoids in seven tribes of Cichorioideae and Asteroideae (Asteraceae). Z. für Naturforsch. C 62, 155–163. 10.1515/znc-2007-3-401 [DOI] [PubMed] [Google Scholar]
- Vera J. C., Reyes A. M., Velásquez F. V., Rivas C. I., Zhang R. H., Strobel P., et al. (2001). Direct inhibition of the hexose transporter GLUT1 by tyrosine kinase inhibitors. Biochemistry 40, 777–790. 10.1021/bi001660j [DOI] [PubMed] [Google Scholar]
- Villagra C., Vera W., Lenitz S., Bergmann J. (2021). Differences in volatile emissions between healthy and gall-induced branches of Haplopappus foliosus (Asteraceae). Biochem. Syst. Ecol. 98, 104309. 10.1016/j.bse.2021.104309 [DOI] [Google Scholar]
- Villagrán C., Castro V., Sánchez G., Romo M., Latorre C., Hinojosa L. F. (1998). La tradición surandina del desierto: Etnobotánica del área del Salar de Atacama (Provincia de El Loa, Región de Antofagasta, Chile). Estud. atacameños, 7–105. [Google Scholar]
- Villagrán C., Romo M., Castro V. (2003). Etnobotánica del sur de losAndes de la primera región de Chile: Un enlace entre las culturas altiplánicas y las de quebradas altas del Loa Superior. Chungara, Rev. Antropol. Chil. 35, 73–124. 10.4067/s0717-73562003000100005 [DOI] [Google Scholar]
- Vogel H., González B., San Martín J., Razmilic I., Villalobos P., Schneider E. (2007). Study of the marketing and sustainability of the forest collection of bailahuén, a Chilean medicinal plant. Med. Plant Conserv. 13, 15–21. [Google Scholar]
- Vogel H., González M., Faini F., Razmilic I., Rodríguez J., San Martín J., et al. (2005a). Antioxidant properties and TLC characterization of four Chilean Haplopappus-species known as bailahuen. J. Ethnopharmacol. 97, 97–100. 10.1016/j.jep.2004.10.027 [DOI] [PubMed] [Google Scholar]
- Vogel H., Razmilic I., San Martín J., Doll U., González B. (2005b). Plantas medicinales chilenas. Experiencias de domesticación y cultivo de boldo, matico, bailahuén, canelo, peumo y maqui. Talca, Chile. Editor. Univ. Talca. [Google Scholar]
- Wickens G. E. (1993). Vegetation and ethnobotany of the Atacama Desert and adjacent Andes in northern Chile. Opera Bot. 121, 291–307. [Google Scholar]
- Zdero C., Bohlmann F., Niemeyer H. M. (1990). Diterpenes and umbelliferone derivatives from Haplopappus deserticola. Phytochemistry 29, 326–329. 10.1016/0031-9422(90)89064-g [DOI] [Google Scholar]
- Zdero C., Bohlmann F., Niemeyer H. M. (1991a). Friedolabdanes and other constituents from chilean Haplopappus species. Phytochemistry 30, 3669–3677. 10.1016/0031-9422(91)80089-j [DOI] [Google Scholar]
- Zdero C., Bohlmann F., Niemeyer H. M. (1991b). Seco-nor-normal and rearranged labdanes from Haplopappus parvifolius. Phytochemistry 30, 3683–3691. 10.1016/0031-9422(91)80091-e [DOI] [Google Scholar]
- Zuloaga F. O., Belgrano M. J., Zanotti C. A. (2019). Actualización del Catálogo de las Plantas Vasculares del Cono Sur. Darwiniana, Nueva Ser. 7, 208–278. 10.14522/darwiniana.2019.72.861 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









