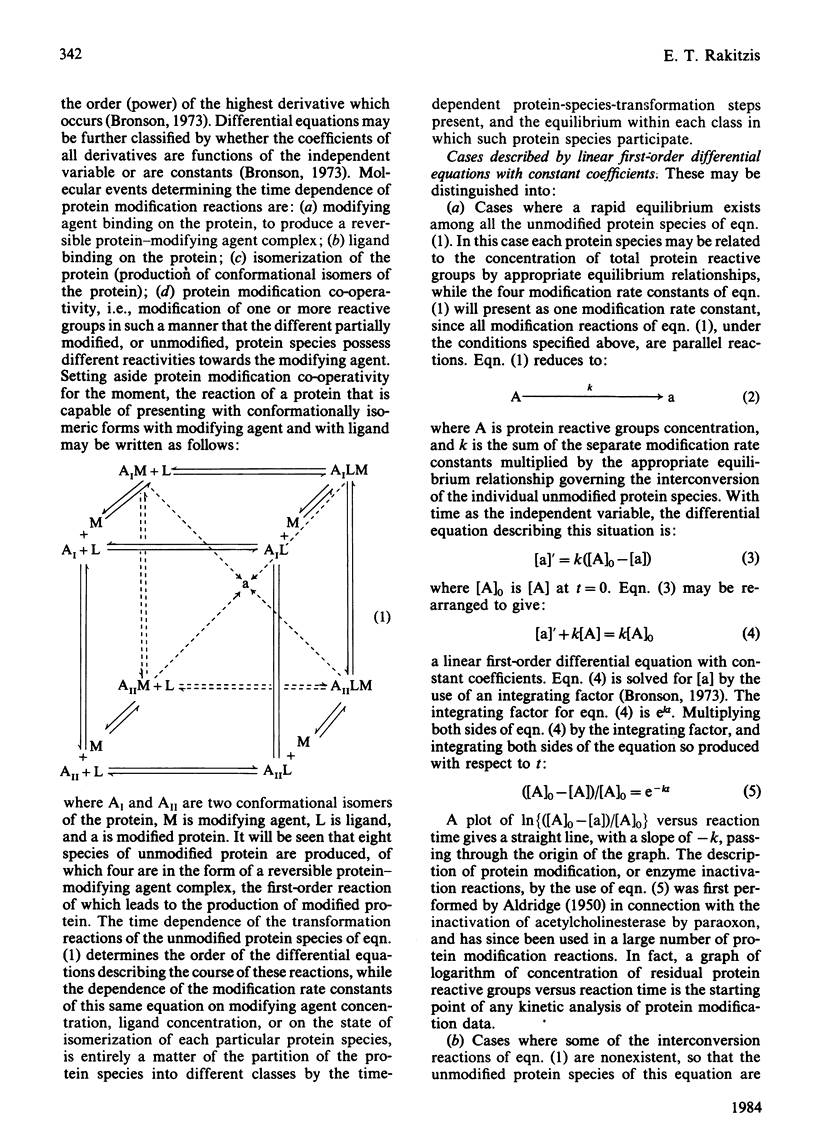
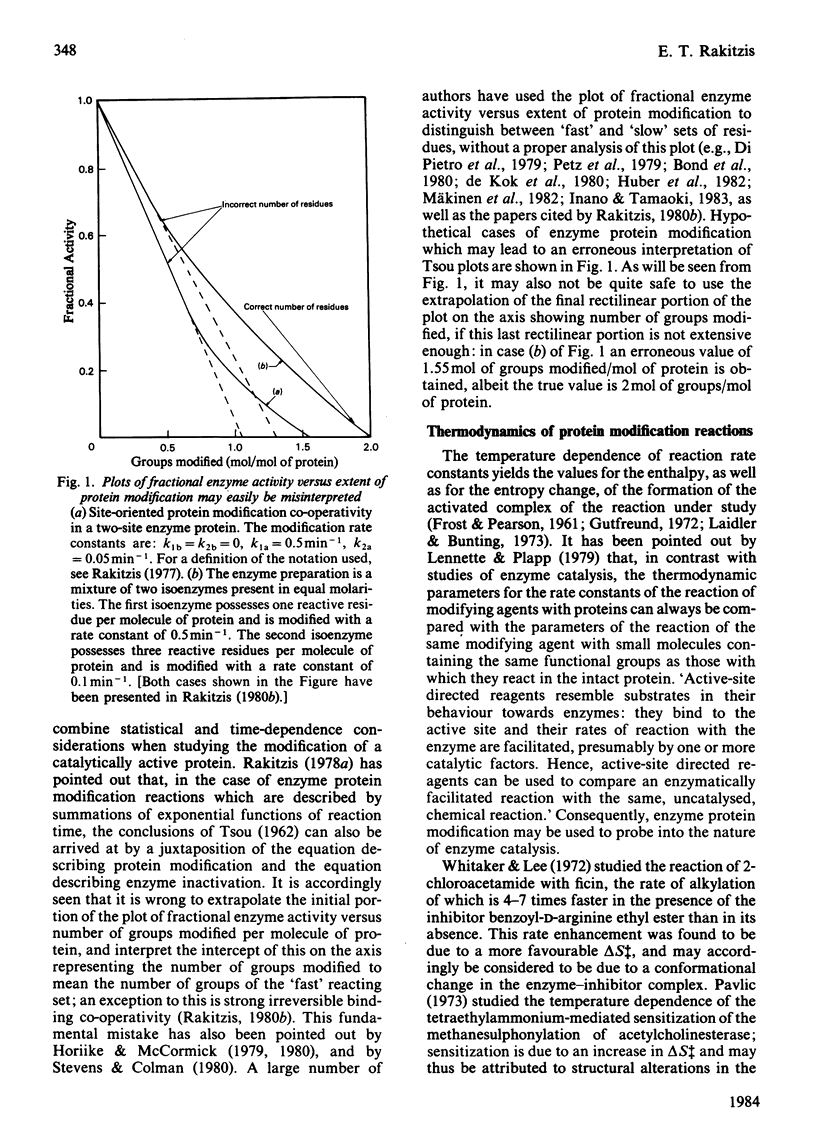
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. Some properties of specific cholinesterase with particular reference to the mechanism of inhibition by diethyl p-nitrophenyl thiophosphate (E 605) and analogues. Biochem J. 1950 Apr;46(4):451–460. doi: 10.1042/bj0460451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai G., Ashani Y., Grunfeld Y., Kalir A., Cohen S. Synthesis and properties of 2-S-(N,N-dialkylamino)ethyl)thio-1,3,2-dioxaphosphorinane 2-oxide and of the corresponding quaternary derivatives as potential nontoxic antiglaucoma agents. J Med Chem. 1976 Jun;19(6):810–813. doi: 10.1021/jm00228a600. [DOI] [PubMed] [Google Scholar]
- Ashani Y., Wins P., Wilson I. B. The inhibition of cholinesterase by diethyl phosphorochloridate. Biochim Biophys Acta. 1972 Oct 12;284(2):427–434. doi: 10.1016/0005-2744(72)90139-8. [DOI] [PubMed] [Google Scholar]
- Barnett P., Rosenberry T. L. Inactivation of Electrophorus electricus acetylcholinesterase by benzenemethane sulfonylfluoride. Arch Biochem Biophys. 1978 Sep;190(1):202–205. doi: 10.1016/0003-9861(78)90268-0. [DOI] [PubMed] [Google Scholar]
- Belfort M., Maley G. F., Maley F. A single functional arginyl residue involved in the catalysis promoted by Lactobacillus casei thymidylate synthetase. Arch Biochem Biophys. 1980 Oct 1;204(1):340–349. doi: 10.1016/0003-9861(80)90042-9. [DOI] [PubMed] [Google Scholar]
- Bing D. H., Mernitz J. L., Spurlock S. E. Inactivation of the first component of human complement by m-(o-(2-chloro-5-fluorosulfonylphenylureido)-phenoxybutoxy)benzamidine. Biochemistry. 1972 Nov 7;11(23):4263–4268. doi: 10.1021/bi00773a011. [DOI] [PubMed] [Google Scholar]
- Bond M. W., Chiu N. Y., Cooperman B. S. Identification of an arginine important for enzymatic activity within the covalent structure of yeast inorganic pyrophosphatase. Biochemistry. 1980 Jan 8;19(1):94–102. doi: 10.1021/bi00542a015. [DOI] [PubMed] [Google Scholar]
- Borders C. L., Jr, Patrick S. L., Davis T. L., Mézes P. S., Viswanatha T. Inactivation of beta-lactamase I from B. cereus 569/H with phenylglyoxal, an arginine-selective reagent. Biochem Biophys Res Commun. 1982 Nov 16;109(1):242–249. doi: 10.1016/0006-291x(82)91591-1. [DOI] [PubMed] [Google Scholar]
- Brake A. J., Weber B. H. Evidence for an essential carboxyl group for yeast phosphoglycerate kinase. Reaction with Woodward's reagent K. J Biol Chem. 1974 Sep 10;249(17):5452–5457. [PubMed] [Google Scholar]
- Brocklehurst K. Specific covalent modification of thiols: applications in the study of enzymes and other biomolecules. Int J Biochem. 1979;10(4):259–274. doi: 10.1016/0020-711x(79)90088-0. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K. The equilibrium assumption is valid for the kinetic treatment of most time-dependent protein-modification reactions. Biochem J. 1979 Sep 1;181(3):775–778. doi: 10.1042/bj1810775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K. Two-protonic-state electrophiles as probes of enzyme mechanisms. Methods Enzymol. 1982;87:427–469. doi: 10.1016/s0076-6879(82)87026-2. [DOI] [PubMed] [Google Scholar]
- Cardemil E., Eyzaguirre J. Evidence of essential arginyl residues in rabbit muscle pyruvate kinase. Arch Biochem Biophys. 1979 Feb;192(2):533–538. doi: 10.1016/0003-9861(79)90123-1. [DOI] [PubMed] [Google Scholar]
- Childs R. E., Bardsley W. G. Time-dependent inhibition of enzymes by active-site-directed reagents. A theoretical treatment of the kinetics of affinity labelling. J Theor Biol. 1975 Sep;53(2):381–394. doi: 10.1016/s0022-5193(75)80010-5. [DOI] [PubMed] [Google Scholar]
- Chu F. S., Bergdoll M. S. Studies on the chemical modification of staphylococcal enterotoxin B. I. Alkylation and oxidation of methionine residues. Biochim Biophys Acta. 1969 Nov 11;194(1):279–286. doi: 10.1016/0005-2795(69)90204-9. [DOI] [PubMed] [Google Scholar]
- Coan C., Keating S. Reactivity of sarcoplasmic reticulum adenosinetriphosphatase with iodoacetamide spin-label: evidence for two conformational states of the substrate binding sites. Biochemistry. 1982 Jun 22;21(13):3214–3220. doi: 10.1021/bi00256a028. [DOI] [PubMed] [Google Scholar]
- Connolly B. A., Trayer I. P. Affinity labelling of rat-muscle hexokinase type II by a glucose-derived alkylating agent. Eur J Biochem. 1979 Jan 15;93(2):375–383. doi: 10.1111/j.1432-1033.1979.tb12833.x. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. Validity of a 'steady-state' treatment of inactivation kinetics. Eur J Biochem. 1979 Jan 15;93(2):383–385. doi: 10.1111/j.1432-1033.1979.tb12834.x. [DOI] [PubMed] [Google Scholar]
- Di Pietro A., Godinot C., Martin J. C., Gautheron D. C. Affinity labeling of catalytic and regulatory sites of pig heart mitochondrial F1-ATPase by 5'-p-fluorosulfonylbenzoyladenosine. Biochemistry. 1979 May 1;18(9):1738–1745. doi: 10.1021/bi00576a016. [DOI] [PubMed] [Google Scholar]
- Fields R. The measurement of amino groups in proteins and peptides. Biochem J. 1971 Sep;124(3):581–590. doi: 10.1042/bj1240581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Arányi P., Keleti T. Mechanisms of irreversible modifications of isomerisable and dissociable-associable oligomeric proteins. Acta Biol Med Ger. 1973;31(2):153–174. [PubMed] [Google Scholar]
- Freedman R. B., Radda G. K. The reaction of 2,4,6-trinitrobenzenesulphonic acid with amino acids, Peptides and proteins. Biochem J. 1968 Jul;108(3):383–391. doi: 10.1042/bj1080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. R., Brubacher L. J., Leggett D. J. A graphical method for extracting rate constants from some enzyme-catalyzed reactions not monitored to completion. Can J Biochem. 1978 Nov;56(11):1055–1057. doi: 10.1139/o78-166. [DOI] [PubMed] [Google Scholar]
- Grouselle M., Pudles J. Chemical studies on yeast hexokinase. Specific modification of a single tyrosyl residue with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide. Eur J Biochem. 1977 Apr 15;74(3):471–480. doi: 10.1111/j.1432-1033.1977.tb11414.x. [DOI] [PubMed] [Google Scholar]
- Halász P., Polgár L. Use of methyl iodide for probing the polarity of the immediate environment of --SH groups in thiolenzymes. Reaction of methyl iodide with thiosubtilisin. Eur J Biochem. 1976 Dec 11;71(2):563–569. doi: 10.1111/j.1432-1033.1976.tb11146.x. [DOI] [PubMed] [Google Scholar]
- Hill A. V. The Combinations of Haemoglobin with Oxygen and with Carbon Monoxide. I. Biochem J. 1913 Oct;7(5):471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollaway M. R., Clarke P. H., Ticho T. Chloroacetone as an active-site-directed inhibitor of the aliphatic amidase from Pseudomonas aeruginosa. Biochem J. 1980 Dec 1;191(3):811–826. doi: 10.1042/bj1910811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiike K., McCormick D. B. Correlations between biological activity and the number of functional groups chemically modified. J Theor Biol. 1979 Aug 7;79(3):403–414. doi: 10.1016/0022-5193(79)90355-2. [DOI] [PubMed] [Google Scholar]
- Horiike K., McCormick D. B. Effect of ligand on chemical modification of proteins. Graphical determinations of dissociation constant and number of essential residues affected by ligand binding. J Theor Biol. 1980 Jun 21;84(4):691–708. doi: 10.1016/s0022-5193(80)80028-2. [DOI] [PubMed] [Google Scholar]
- Huber R. E., Fowler A. V., Zabin I. Inactivation of beta-Galactosidase by iodination of tyrosine-253. Biochemistry. 1982 Sep 28;21(20):5052–5055. doi: 10.1021/bi00263a031. [DOI] [PubMed] [Google Scholar]
- Höltje H. D. Structure-activity studies of enzyme substrates using model interaction calculations. J Theor Biol. 1974 Nov;48(1):197–205. doi: 10.1016/0022-5193(74)90190-8. [DOI] [PubMed] [Google Scholar]
- Ikai A., Fish W. W., Tanford C. Kinetics of unfolding and refolding of proteins. II. Results for cytochrome c. J Mol Biol. 1973 Jan 10;73(2):165–184. doi: 10.1016/0022-2836(73)90321-5. [DOI] [PubMed] [Google Scholar]
- Ikai A., Tanford C. Kinetics of unfolding and refolding of proteins. I. Mathematical analysis. J Mol Biol. 1973 Jan 10;73(2):145–163. doi: 10.1016/0022-2836(73)90320-3. [DOI] [PubMed] [Google Scholar]
- Inano H., Tamaoki B. Affinity labeling of arginyl residues at the catalytic region of estradiol 17 beta-dehydrogenase from human placenta by 16-oxoestrone. Eur J Biochem. 1983 Jan 1;129(3):691–695. doi: 10.1111/j.1432-1033.1983.tb07104.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. L., Poisner A. M. Inactivation of amniotic prorenin by ethyl diazoacetylglycinate. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1404–1409. doi: 10.1016/s0006-291x(80)80053-2. [DOI] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Keleti T. Simple mechanisms for the modification of homotetrameric proteins. J Theor Biol. 1971 Mar;30(3):545–551. doi: 10.1016/0022-5193(71)90007-5. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Schlegel W. Size determination of enzymes by radiation inactivation. Anal Biochem. 1979 Jan 1;92(1):2–10. doi: 10.1016/0003-2697(79)90617-1. [DOI] [PubMed] [Google Scholar]
- Kenyon G. L., Bruice T. W. Novel sulfhydryl reagents. Methods Enzymol. 1977;47:407–430. doi: 10.1016/0076-6879(77)47042-3. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- LEVY H. M., LEBER P. D., RYAN E. M. INACTIVATION OF MYOSIN BY 2,4-DINITROPHENOL AND PROTECTION BY ADENOSINE TRIPHOSPHATE AND OTHER PHOSPHATE COMPOUNDS. J Biol Chem. 1963 Nov;238:3654–3659. [PubMed] [Google Scholar]
- Lennette E. P., Plapp B. V. Transition-state analysis of the facilitated alkylation of ribonuclease A by bromoacetate. Biochemistry. 1979 Sep 4;18(18):3938–3946. doi: 10.1021/bi00585a015. [DOI] [PubMed] [Google Scholar]
- Levilliers N., Péron-Renner M., Pudles J. Role of serine 195 in the stabilization of beta-trypsin and beta-trypsin-ligand complexes. J Mol Biol. 1977 Apr 15;111(3):279–303. doi: 10.1016/s0022-2836(77)80052-1. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Maglothin J. A., Wilson I. B. Equilibrium constants for the phosphorylation of acetylcholinesterase by some diethyl phosphorothiolates and phosphates. Biochemistry. 1974 Aug 13;13(17):3520–3527. doi: 10.1021/bi00714a017. [DOI] [PubMed] [Google Scholar]
- Maglothin J. A., Wins P., Wilson I. B. Reactivation and aging of diphenyl phosphoryl acetylcholinesterase. Biochim Biophys Acta. 1975 Oct 22;403(2):370–387. doi: 10.1016/0005-2744(75)90066-2. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Malcolm A. D. Identification of a class of lysines within the non-specific DNA-binding site of RNA polymerase core enzyme from Escherichia coli. Eur J Biochem. 1980 May;106(1):313–320. doi: 10.1111/j.1432-1033.1980.tb06025.x. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Malcolm A. D. Properties of methyl acetimidate and its use as a protein-modifying reagent. Biochem J. 1981 Jan 1;193(1):245–249. doi: 10.1042/bj1930245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm A. D., Radda G. K. The reaction of glutamate dehydrogenase with 4-iodoacetamido salicylic acid. Eur J Biochem. 1970 Sep;15(3):555–561. doi: 10.1111/j.1432-1033.1970.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Carrion M., Turano C., Riva F., Fasella P. Evidence of a critical histidine residue in soluble aspartic aminotransferase. J Biol Chem. 1967 Apr 10;242(7):1426–1430. [PubMed] [Google Scholar]
- Mäkinen K. K., Mäkinen P. L., Wilkes S. H., Bayliss M. E., Prescott J. M. Chemical modification of Aeromonas aminopeptidase. Evidence for the involvement of tyrosyl and carboxyl groups in the activity of the enzyme. Eur J Biochem. 1982 Nov;128(1):257–265. [PubMed] [Google Scholar]
- Pauling L. The Oxygen Equilibrium of Hemoglobin and Its Structural Interpretation. Proc Natl Acad Sci U S A. 1935 Apr;21(4):186–191. doi: 10.1073/pnas.21.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlic M. R. On the nature of the acceleration of the methanesulfonylation of acetylcholinesterase by tetraethylammonium. Biochim Biophys Acta. 1973 Dec 19;327(2):393–397. doi: 10.1016/0005-2744(73)90422-1. [DOI] [PubMed] [Google Scholar]
- Pavlic M. R., Wilson I. B. On the mechanism of the acceleration of methanesulfonylation of acetylcholinesterase with cationic accelerators. Biochim Biophys Acta. 1978 Mar 14;523(1):101–108. doi: 10.1016/0005-2744(78)90013-x. [DOI] [PubMed] [Google Scholar]
- Pavlic M. R., Zorko M. The acceleration of methanesulfonylation of acetylcholinesterase with cationic accelerators as an electrostatic effect. Biochim Biophys Acta. 1978 Jun 9;524(2):340–348. doi: 10.1016/0005-2744(78)90170-5. [DOI] [PubMed] [Google Scholar]
- Pedersen A. O., Jacobsen J. Reactivity of the thiol group in human and bovine albumin at pH 3--9, as measured by exchange with 2,2'-dithiodipyridine. Eur J Biochem. 1980 May;106(1):291–295. doi: 10.1111/j.1432-1033.1980.tb06022.x. [DOI] [PubMed] [Google Scholar]
- Petz D., Löffler H. G., Schneider F. Inhibition of E. coli L-Asparaginase by reaction with 2,3-butanedione. Chemical modification of arginine and histidine residues. Z Naturforsch C. 1979 Sep-Oct;34(9-10):742–746. doi: 10.1515/znc-1979-9-1015. [DOI] [PubMed] [Google Scholar]
- Purdie J. E., Heggie R. M. The kinetics of the reaction of N,N-dimethyl-2-phenylaziridinium ion with bovine erythrocyte acetylcholinesterase. Can J Biochem. 1970 Mar;48(3):244–250. doi: 10.1139/o70-044. [DOI] [PubMed] [Google Scholar]
- RAY W. J., Jr, KOSHLAND D. E., Jr A method for characterizing the type and numbers of groups involved in enzyme action. J Biol Chem. 1961 Jul;236:1973–1979. [PubMed] [Google Scholar]
- Rakitzis E. T., Gilligan P. J., Hoffman J. F. Kinetic analysis of the inhibition of sulfate transport in human red blood cells by isothiocyanates. J Membr Biol. 1978 Jun 28;41(2):101–115. doi: 10.1007/BF01972628. [DOI] [PubMed] [Google Scholar]
- Rakitzis E. T. Kinetic analysis of biphasic protein modification reactions. Cooperative effects. Biophys Chem. 1983 Sep;18(2):133–137. doi: 10.1016/0301-4622(83)85007-8. [DOI] [PubMed] [Google Scholar]
- Rakitzis E. T. Kinetic analysis of biphasic protein modification reactions. J Math Biol. 1980 Aug;10(1):79–87. doi: 10.1007/BF00276397. [DOI] [PubMed] [Google Scholar]
- Rakitzis E. T. Kinetics of irreversible enzyme inhibition by an unstable inhibitor. Biochem J. 1974 Aug;141(2):601–603. doi: 10.1042/bj1410601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakitzis E. T. Kinetics of irreversible enzyme inhibition by an unstable inhibitor. Biochem J. 1981 Nov 1;199(2):462–463. doi: 10.1042/bj1990462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakitzis E. T. Kinetics of irreversible enzyme inhibition by an unstable inhibitor: co-operative effects. J Theor Biol. 1978 Nov 21;75(2):239–243. doi: 10.1016/0022-5193(78)90233-3. [DOI] [PubMed] [Google Scholar]
- Rakitzis E. T. Kinetics of irreversible enzyme inhibition: co-operative effects. J Theor Biol. 1977 Jul 7;67(1):49–59. doi: 10.1016/0022-5193(77)90184-9. [DOI] [PubMed] [Google Scholar]
- Rakitzis E. T. Kinetics of irreversible enzyme inhibition: the Tsou plot in irreversible binding co-operativity. J Theor Biol. 1978 Feb 20;70(4):461–465. doi: 10.1016/0022-5193(78)90253-9. [DOI] [PubMed] [Google Scholar]
- Rakitzis E. T. Kinetics of irreversible enzyme inhibition: the interpretation of the fractional enzyme activity vs. extent of protein modification plot. J Theor Biol. 1980 Aug 7;85(3):553–560. doi: 10.1016/0022-5193(80)90328-8. [DOI] [PubMed] [Google Scholar]
- Rando R. R. Beta, gamma unsaturated amino acids as irreversible enzyme inhibitors. Nature. 1974 Aug 16;250(467):586–587. doi: 10.1038/250586a0. [DOI] [PubMed] [Google Scholar]
- Rando R. R. Chemistry and enzymology of kcat inhibitors. Science. 1974 Jul 26;185(4148):320–324. doi: 10.1126/science.185.4148.320. [DOI] [PubMed] [Google Scholar]
- Redkar V. D., Kenkare U. W. Effect of ligands on the reactivity of essential sulfhydryls in brain hexokinase. Possible interaction between substrate binding sites. Biochemistry. 1975 Oct 21;14(21):4704–4712. doi: 10.1021/bi00692a022. [DOI] [PubMed] [Google Scholar]
- Roberts M. F., Switzer R. L., Schubert K. R. Inactivation of Salmonella phosphoribosylpyrophosphate synthetase by oxidation of a specific sulfhydryl group with potassium permanganate. J Biol Chem. 1975 Jul 25;250(14):5364–5369. [PubMed] [Google Scholar]
- Sanner T., Tron L. Properties of the highly reactive SH groups of phosphorylase b. Biochemistry. 1975 Jan 28;14(2):230–235. doi: 10.1021/bi00673a006. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Schwartz M. A., Odin E. Enzyme inhibitors. XVII. Kinetic studies on the irreversible inhibition of adenosine deaminase. J Med Chem. 1967 Jul;10(4):686–689. doi: 10.1021/jm00316a036. [DOI] [PubMed] [Google Scholar]
- Schirmer R. H., Schirmer I., Noda L. Studies on the histidine residues of rabbit muscle myokinase. Biochim Biophys Acta. 1970 Apr 28;207(1):165–177. doi: 10.1016/0005-2795(70)90148-0. [DOI] [PubMed] [Google Scholar]
- Schnackerz K. D., Noltmann E. A. Carboxamidomethylation and inactivation of rabbit muscle phosphoglucose isomerase. J Biol Chem. 1970 Dec 10;245(23):6417–6423. [PubMed] [Google Scholar]
- Singer S. J. Covalent labeling of active sites. Adv Protein Chem. 1967;22:1–54. doi: 10.1016/s0065-3233(08)60040-6. [DOI] [PubMed] [Google Scholar]
- Stevens E., Colman R. F. Chemical modification of enzymes: critical evaluation of the graphical correlation between residual enzyme activity and number of groups modified. Bull Math Biol. 1980;42(2):239–255. doi: 10.1007/BF02464640. [DOI] [PubMed] [Google Scholar]
- Söderling E., Mäkinen K. K. Modification of the Cl- -activated arginine aminopeptidases from rat liver and human erythrocytes: a comparative study. Arch Biochem Biophys. 1983 Jan;220(1):11–21. doi: 10.1016/0003-9861(83)90381-8. [DOI] [PubMed] [Google Scholar]
- TSOU C. L. Relation between modification of functional groups of proteins and their biological activity. I.A graphical method for the determination of the number and type of essential groups. Sci Sin. 1962 Nov;11:1535–1558. [PubMed] [Google Scholar]
- Tanford C., Aune K. C., Ikai A. Kinetics of unfolding and refolding of proteins. 3. Results for lysozyme. J Mol Biol. 1973 Jan 10;73(2):185–197. doi: 10.1016/0022-2836(73)90322-7. [DOI] [PubMed] [Google Scholar]
- Tatsunami S., Yago N., Hosoe M. Kinetics of suicide substrates. Steady-state treatments and computer-aided exact solutions. Biochim Biophys Acta. 1981 Dec 15;662(2):226–235. doi: 10.1016/0005-2744(81)90034-6. [DOI] [PubMed] [Google Scholar]
- Tian W. X., Tsou C. L. Determination of the rate constant of enzyme modification by measuring the substrate reaction in the presence of the modifier. Biochemistry. 1982 Mar 2;21(5):1028–1032. doi: 10.1021/bi00534a031. [DOI] [PubMed] [Google Scholar]
- Tomich J. M., Marti C., Colman R. F. Modification of two essential cysteines in rabbit muscle pyruvate kinase by the guanine nucleotide analogue 5'[p-(fluorosulfonyl) benzoyl] guanosine. Biochemistry. 1981 Nov 10;20(23):6711–6720. doi: 10.1021/bi00526a029. [DOI] [PubMed] [Google Scholar]
- Vas M., Boross L. Heat inactivation of D-glyceraldehyde-3-phosphate dehydrogenase apoenzyme. Acta Biochim Biophys Acad Sci Hung. 1972;7(2):105–114. [PubMed] [Google Scholar]
- Waley S. G. Kinetics of suicide substrates. Biochem J. 1980 Mar 1;185(3):771–773. doi: 10.1042/bj1850771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J. R., Lee L. S. Ficin- and papain-catalyzed reactions. Effect of temperature on reactivity of the essential sulfhydryl group of ficin in the presence and absence of competitive inhibitors. Arch Biochem Biophys. 1972 Jan;148(1):208–216. doi: 10.1016/0003-9861(72)90133-6. [DOI] [PubMed] [Google Scholar]
- de Kok A., Kornfeld S., Benziman M., Milner Y. Subunit composition and partial reactions of the 2-oxoglutarate dehydrogenase complex of Acetobacter xylinum. Eur J Biochem. 1980 May;106(1):49–58. doi: 10.1111/j.1432-1033.1980.tb05996.x. [DOI] [PubMed] [Google Scholar]


