Abstract
Background
Tuberculosis (TB) remains a significant cause of mortality globally, with India accounting for 27% of the estimated number of people with TB. Multidrug-resistant TB (MDR-TB) and isoniazid (INH) resistance pose additional challenges to effective treatment. We aimed to describe treatment outcomes of INH mono-resistant TB patients under programmatic conditions in Mumbai, India.
Methods
This retrospective cohort study was conducted at Shatabdi Hospital in Mumbai between 2019–2021.We described the clinical and demographic characteristics, treatment outcomes, and risk factors for unfavourable outcomes among patients with INH mono-resistant TB treated with rifampicin, ethambutol, pyrazinamide, and levofloxacin (LfxREZ) for a duration of 6 months.
Results
Among 3105 patients with drug-resistant TB initiated on treatment, 217 (7 %) had INH mono-resistant TB. Of these, 54 % (117/217) were female, with a median age of 26 years (interquartile range: 20–40). The majority (88 %; 191/217) presented with pulmonary TB, and most (87 %; 188/217) had favourable treatment outcomes, including treatment completion (52 %; 112/217) and cure (35 %; 76/217). Unfavourable outcomes, including treatment failure (2.3 %; 5/217), loss to follow-up (9.2 %; 20/217), or death (1.8 %; 4/217), were observed in 13 % (29/217) of patients. A total of ten (5 %) patients experienced at least one non-severe adverse drug reaction. Factors associated with unfavourable outcomes included severe thinness (p = 0.019) and male gender (p = 0.012).
Conclusion
Treating INH mono-resistant patients with LfxREZ resulted in satisfactory outcomes and low toxicity. It is important to rule out drug resistance to INH while determining the treatment regimen.
Keywords: Tuberculosis, INH mono-resistant, Isoniazid resistance, Drug resistance, India
1. Introduction
Worldwide, an estimated 10.6 million people developed tuberculosis (TB) and 1.3 million died of TB in 2022, India accounting for 27 % of the estimated number of people with TB [1].
Globally, an estimated 410,000 people developed multidrug-resistant (MDR) or rifampicin-resistant TB in 2022 [1]. Isoniazid (INH) is an important first-line anti-TB drug because of its potent early bactericidal activity against rapidly dividing cells. There were an estimated 1.3 million incident cases of isoniazid-resistant TB in 2022, including people with both rifampicin-susceptible (INH mono-resistance) and rifampicin-resistant TB (MDR) [1]. The National Drug Resistance Survey 2014–16 in India reported any isoniazid resistance, including INH mono-resistance in 11.1 % (95 % confidence interval (CI): 10.0–12.2) of new and 25.1 % (95 % CI: 23.1–27.1) of previously treated TB patients, while INH mono-resistance was observed in 3.9 % (95 % CI: 3.2–4.6) and 7.6 % (95 % CI: 6.5–8.9) of new and previously treated TB patients, respectively [2].
Detecting drug resistance early in TB patients is essential for starting the right treatment and improving treatment success rates. In India, the National TB Elimination Programme (NTEP) has developed an integrated algorithm for identifying and managing drug-resistant TB, relying on drug susceptibility testing (DST). For patients with confirmed TB, a first-line line-probe assay (LPA) is conducted to identify INH resistance. Subsequently, a second-line LPA is conducted to identify resistance to fluoroquinolones (FQ) and aminoglycosides [3], [4]. However, the lack of access to Cartridge Based Nucleic Acid Amplification Test (CBNAAT) and LPA in most diagnostic centers, peripheral laboratories, and the large private sector market has resulted in big diagnostic gap and consequently, a low detection rate of MDR-TB cases in India [5].
Updated World Health Organization (WHO) 2022 guidelines recommend treating INH-mono resistant patients with rifampicin, ethambutol, pyrazinamide, and levofloxacin (LfxREZ) for a duration of 6 months [6]. Correspondingly, the Indian guidelines for the programmatic management of drug-resistant tuberculosis (DR-TB) also recommend the use of LfxREZ for 6 or 9 months in the management of INH-mono resistant patients [4].
Patients with INH mono-resistance TB exhibited notably poor treatment outcomes. A meta-analysis including 25 studies from 47 countries showed that the pooled successful treatment rate among patients with INH mono-resistance was 78 % (95 % Confidence Interval (95 % CI); 74 % − 83 %) [7]. Whereas the pooled treatment success rates from the three Southeast Asian countries was 62 % (95 % CI: 56–69) and the pooled success rates from the African region, Region of the Americas, Eastern Mediterranean Region, European Region, West Pacific Region was 67 %, 84 %, 75 %, 84 %, and 82 % respectively [7].
According to the India TB Report 2023, the treatment success rate among 16,186 patients with INH mono/poly resistance treated with LfxREZ in 2021 was 82 % [8]. Similarly, a study conducted in India showed an 82 % treatment success rate among patients with pulmonary isoniazid-resistant TB treated with a levofloxacin-based regimen [9].
In three separate studies conducted in India by Garg et al., Hymn et al., and Nagar et al., the treatment success rate among patients with INH mono-resistance TB treated with levofloxacin-based regimen was observed to be 65.4 % (34/52), 75.9 % (41/54), and 57.4 % (144/251), respectively [10], [11], [12]. Moreover, in a recent conference presentation, the LfxREZ regimen demonstrated efficacy with an 83.7 % (82/98) cure rate in patients with INH mono-resistance [13].
In this retrospective cohort study, we describe the demographic characteristics, clinical profile, treatment outcomes and risk factors for unfavourable treatment outcomes among patients with INH mono-resistant TB initiated on treatment between 2019–2021 at Shatabdi hospital, M−east ward, Mumbai, India.
2. Methods
2.1. Study design
This is a retrospective cohort study using routinely collected programmatic data.
2.2. Study setting
Mumbai, home to 12 % of Maharashtra's population, accounts for 22 % of total TB cases in the state, with M−east ward being one of the hotspots for TB and drug-resistant TB (DR-TB) [14], [15]. This study was done at the nodal DR-TB centre located in Shatabdi hospital M−east ward, Mumbai, India which functions as a collaboration site between the national TB programme and Médecins Sans Frontières (MSF), to provide high quality diagnosis, treatment, and follow-up services for DR-TB patients.
2.3. Diagnosis and treatment
Diagnosis and treatment were according to the Integrated drug-resistant TB algorithm described in the Guidelines for the Programmatic Management of DR-TB in India [4]. Presumptive TB patients underwent the Xpert MTB/RIF assay, followed by first and second-line probe assay (LPA) for samples with confirmed Mycobacterium tuberculosis. All patients who were rifampicin-susceptible on Xpert MTB/RIF assay and isoniazid-resistant on LPA were diagnosed to have INH mono-resistant TB and were initiated on a standard 6-month regimen of LfxREZ. The treatment was monitored with sputum smear and microscopy conducted at months 3, 4, 5, 6 and sputum culture at month 3 and 6 following treatment initiation.
At the MSF outreach clinics patients were assessed for adverse drug reactions (ADRs) and were referred to Shatabdi out-patient department in case of severe ADRs.
2.4. Operational definitions and data management and analysis
Routinely collected patient data were entered from patients' treatment files into a Microsoft Excel spread sheet by trained data entry operators. Demographic and clinical characteristics were summarized using frequencies and percentages for categorical variables, and median and interquartile ranges (IQRs) for continuous variables.
Favourable treatment outcomes were defined as ‘cured’ or ‘completed treatment’. Unfavourable outcomes were defined as ‘treatment failure’, ‘loss to follow-up’ or ‘death’.
Risk factors for unfavourable treatment outcomes, including age, sex, TB history, TB site, severe thinness (defined as body mass index (BMI)-for-age Z-score < -3 SD for 11–19 years and BMI of < 16 kg/m2 for > 19 years), diabetes status, and type of mutation in clinical isolates were explored in a univariate analysis. Risk factors with P < 0.2 in univariate analysis were further analysed in a multivariable logistic regression model. All statistical differences were tested using Chi Square, Fisher Exact, or Kruskal-Wallis rank sum tests, as appropriate. All estimates were reported with their respective 95 % confidence intervals. P-values below 0.05 were considered statistically significant. All analyses were performed using R software (version 4.3.2; The R Foundation, Vienna, Austria).
2.5. Ethics
The study received ethics approval from Institutional Ethics Committee for Research (IECR) of State Health Systems Resource Centre (SHSRC), Pune, Maharashtra (SHSRC/IECR-4 Approval/743/19). The study fulfilled the exemption criteria set by the Médecins Sans Frontières (MSF) Ethics Review Board for a posteriori analysis of routinely collected clinical data and thus did not require MSF ERB full review. Permission was also sought from the India National Tuberculosis Elimination Programme (NTEP).
3. Results
Between January 2019 to December 2021, 3105 DR-TB patients were initiated on treatment, of whom 217 (7 %) were diagnosed INH-Mono-resistant TB; the median (IQR) age was 26 (20–40) years and females constituted 54 % (117) of the study population. Baseline clinical and demographic characteristics along with the risk factors for unfavourable treatment outcomes are described in Table 1.
Table 1.
Risk factors for unfavourable treatment outcomes among patients with isoniazid mono-resistant tuberculosis, Mumbai India, 2019–2021.
| Characteristic |
Overall, N = 2171 |
Favourable outcomes2, N = 1881 |
Unfavourable outcomes3, N = 291 |
OR (95 % CI) | p-value4 | aOR (95 % CI) | p-value4 |
|---|---|---|---|---|---|---|---|
| Age group (in years) | |||||||
| <15 | 20 (9.2 %) | 20 (11 %) | 0 (0 %) | 0 | >0.9 | ||
| 15-<30 | 106 (49 %) | 93 (49 %) | 13 (45 %) | Ref | |||
| 30-<45 | 48 (22 %) | 41 (22 %) | 7 (24 %) | 1.2 (0.4,3.2) | 0.7 | ||
| 45-<60 | 28 (13 %) | 21 (11 %) | 7 (24 %) | 2.4 (0.8,6.6) | 0.1 | ||
| ≥ 60 | 15 (6.9 %) | 13 (6.9 %) | 2 (6.9 %) | 1.1 (0.2,4.6) | >0.9 | ||
| Median age in years (IQR) | 26 (20, 40) | 26 (20, 40) | 34 (22, 45) | 1.02 (0.99,1.04) | 0.2 | 1 (0.97,1.03) | 0.8 |
| Gender | |||||||
| Female | 117 (54 %) | 109 (58 %) | 8 (28 %) | Ref | |||
| Male | 100 (46 %) | 79 (42 %) | 21 (72 %) | 3.6 (1.6,9.1) | 0.004 | 3.5 (1.4, 10.1) | 0.012 |
| Tuberculosis history | |||||||
| New | 133 (61 %) | 118 (63 %) | 15 (52 %) | Ref | |||
| Previously Treated | 84 (39 %) | 70 (37 %) | 14 (48 %) | 1.6 (0.7,3.5) | 0.3 | ||
| Type of tuberculosis | |||||||
| Pulmonary | 191 (88 %) | 165 (88 %) | 26 (90 %) | Ref | |||
| Extrapulmonary | 25 (12 %) | 22 (12 %) | 3 (10 %) | 0.9 (0.2,2.7) | 0.8 | ||
| Disseminated | 1 (0.5 %) | 1 (0.5 %) | 0 (0 %) | 0 | >0.9 | ||
| Mutation | |||||||
| Only KatG | 141 (67 %) | 124 (69 %) | 17 (61 %) | Ref | |||
| Only InhA | 63 (30 %) | 52 (29 %) | 11 (39 %) | 1.5 (0.7,3.5) | 0.3 | ||
| Both | 5 (2.4 %) | 5 (2.8 %) | 0 (0 %) | 0 | >0.9 | ||
| Missing data | 8 | 7 | 1 | ||||
| Diabetes | |||||||
| No | 194 (89 %) | 170 (90 %) | 24 (83 %) | Ref | |||
| Yes | 23 (11 %) | 18 (9.6 %) | 5 (17 %) | 2 (0.6,5.5) | 0.2 | 1.4 (0.4, 4.9) | 0.6 |
| HIV status | |||||||
| Negative | 215 (99 %) | 186 (99 %) | 29 (100 %) | Ref | |||
| Positive | 2 (0.9 %) | 2 (1.1 %) | 0 (0 %) | 0 | >0.9 | ||
| Nutritional status | |||||||
| No Severe thinness | 151 (74 %) | 136 (77 %) | 15 (54 %) | Ref | |||
| Severe thinness | 54 (26 %) | 41 (23 %) | 13 (46 %) | 2.9 (1.3,6.6) | 0.012 | 2.8 (1.2, 6.7) | 0.019 |
| Missing data | 12 | 11 | 1 |
OR: odds ratio; aOR: adjusted odds ratio; IQR: Interquartile range; Ref: reference category.
n (%); Median (IQR).
Favourable outcomes: cure and treatment completion
Unfavourable outcomes: death, loss to follow-up, treatment failure.
Fisher’s exact test; Wilcoxon rank sum test; Pearson’s Chi-squared test.
The majority (n = 191, 88 %) of patients had pulmonary TB. A total of 84 (39 %) patients had a previous history of TB including 80 (37 %) drug-sensitive TB and 4 (2 %) INH mono-resistant TB.
Of the 217 INH mono-resistant TB patients, 141 (65 %) had katG mutation, 63 (29 %) had InhA mutation, and 5 (2 %) had both mutations. Mutations of 8 (4 %) isolates were unknown.
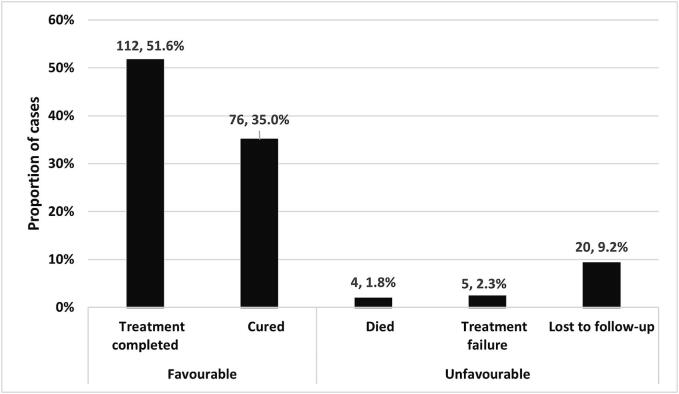
Of the 217 INH mono-resistant patients, 188 (87 %) had favourable treatment outcomes, including 112 (52 %) completed treatment and 76 (35 %) cured while 29 (13 %) patients had unfavourable treatment outcomes; 20 (9.2 %) were lost to follow-up, 5 (2.3 %) failed treatment and 4 (1.8 %) died. Treatment outcomes are shown in Fig. 1.
Fig. 1.
Of the 20 lost to follow-up patients, 14 (70 %) were re-engaged and treated based on drug susceptibility testing (DST) results. The majority (86 %%; 12/14) of re-engaged patients were again found to be isoniazid-resistant while the remaining 2 (14 %) had amplification of resistance with new fluoroquinolone resistance.
Out of 191 pulmonary TB patients, 66 (35 %) had a baseline culture report, with 55 (83 %) positive at baseline. Of the 55 culture positive patients, 43 (78 %) had negative culture by six months, 6(11 %) were lost to follow-up and 6 (11 %) had no culture reports.
Overall, 10 (5 %) patients developed at least one ADR during the treatment course. Nine patients had musculoskeletal or joint pain, one vomiting, and one developed peripheral neuropathy.
In the multivariable model, severe thinness (adjusted odd ratio (aOR) 2.8, 95 % CI: 1.2–6.7; p = 0.019) and male gender (aOR 3.5, 95 % CI: 1.4–10.1; p = 0.012) were significantly associated with unfavourable outcomes (Table 1).
4. Discussion
Our study demonstrated that the LfxREZ regimen is both safe and effective in treating patients with isoniazid (INH) mono-resistant tuberculosis (TB) within routine program settings. We found that 87 % of patients achieved favourable treatment outcomes, surpassing the pooled treatment success rate of 78 % reported in a meta-analysis of 25 studies [7]. Furthermore, our treatment success rates were considerably better than those reported in three studies from Southeast Asia, which showed pooled success rates of 62 % [7]. However, it is important to interpret the comparison cautiously because the meta-analysis includes studies published from year 2009 to 2022, with varying treatment regimens used. Among the three Southeast Asian studies, two used the LfxREZ regimen, while regimen 2HRZE/4HR was initially administered to all patients in one study.
We observed higher treatment success rates compared to three independent studies conducted in India, which reported treatment outcomes among patients with INH mono-resistance treated with LfXREZ [10], [11], [12]. The difference in treatment success rates can be attributed to small sample size, varying levels of lost to follow-up, and differing mortality rates across the independent studies.
Our treatment success rates were comparable to those reported in studies by Velayutham et al., with an 82 % treatment success rate, and Munje et al., with an 84 % treatment success rate [9], [13]. Both of these studies included INH- resistant patients treated with levofloxacin-based regimens for six months [9].
Notably, in line with findings from other studies conducted in India, our study also indicated that male sex was associated with unfavourable treatment outcomes [9], [10], [11], [12], [13].
According to the 2023 India TB report, among the INH mono/poly resistant TB patients who began treatment under the national program in 2021, 82 % achieved treatment success, 7 % died, 5 % were lost to follow-up, and 2 % had treatment failure [8]. Our findings indicated a lower mortality rate (1.8 %).
Our study has several limitations. Primarily, its reliance on program data from a single nodal DR-TB centre in Mumbai, India, constrains the generalizability of our findings to other settings or populations. The retrospective design of the study introduces inherent challenges related to data completeness and integrity, potentially affecting the robustness of our conclusions. Furthermore, the limited number of participants with HIV (n = 2) and the absence of data on other potential risk factors, such as alcohol and tobacco use, preclude a comprehensive analysis of their association with unfavourable outcomes. Nonetheless, despite these limitations, our study makes a valuable contribution to the field by providing real-world programmatic data on this relatively understudied aspect of DR-TB management. Our study reported higher favourable outcome rates compared to the reported success rate in previous studies on INH mono-resistant patients given injectable based regimen. This study adds to the limited evidence on treatment outcomes among INH-mono resistant patients treated with LfxREZ and highlights the need to give special attention to males and severely malnourished patients who were more likely to experience unfavourable treatment outcomes.
Undernutrition and tuberculosis (TB) form a mutually reinforcing cycle. Undernutrition increases TB risk, while active TB can induce malnutrition. Moreover, undernutrition increases the risk of drug toxicity, relapse, and mortality in TB patients [16]. In 2013, WHO released operational guidelines for the nutritional care and support of patients with TB[17]. India adapted these guidelines to suit its specific needs in 2017, introducing India-specific criteria for hospitalization, expanding nutritional support to patients with moderately severe undernutrition and drug-susceptible TB, and addressing food insecurity in households affected by TB guidance [18].
Recent trial conducted in India have demonstrated that integrating nutritional support into patient-centred care for TB improves treatment outcomes [19].
Our study also found association of severe thinness with the unfavourable outcome. Therefore, we also recommend integration of nutrition support in the management of TB, especially in countries with high levels of undernutrition like India.
The 2021 Indian guidelines on the Programmatic Management of Drug-Resistant Tuberculosis (DR-TB) recommend using a first-line line-probe assay (FL-LPA) to identify INH resistance, and a second-line LPA to detect resistance to fluoroquinolones (FQ) and aminoglycosides [3], [4]. However, there may be several gaps at the national level that need to be addressed [5].
The widespread adoption of the new Xpert MTB/XDR assay, with integrated INH resistance testing, promises to significantly enhance the efficiency of TB diagnosis and improve the management of TB cases, particularly those involving INH mono-resistance [20], [21]. Identifying INH mono-resistance early allows healthcare providers to tailor treatment regimens more precisely, ensuring patients receive effective treatment while minimizing the risk of treatment failure and the further development of drug resistance.
In conclusion, we strongly recommend adopting the LfxREZ regimen as the standard treatment for INH mono-resistant TB patients due to its proven effectiveness with high favourable outcome and safety with very low adverse drug reaction. Integrating nutritional support into TB management protocols is crucial for improving patient outcomes. Special attention should be given to male patients, who are more likely to experience unfavourable outcomes. Additionally, scaling up the use of the Xpert MTB/XDR assay in routine TB programs is essential for efficient diagnosis and early identification of INH mono-resistance, enabling more precise and effective treatment regimens.
CRediT authorship contribution statement
Sumaiya Khan: Writing – original draft, Formal analysis, Data curation, Conceptualization. Arunima Silsarma: Writing – review & editing, Writing – original draft, Formal analysis, Data curation, Conceptualization. Raman Mahajan: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Data curation. Shahid Khan: Writing – review & editing, Data curation, Conceptualization. Praveen Davuluri: Writing – review & editing, Data curation. Narendra Sutar: Writing – review & editing, Validation. Aparna Iyer: Writing – review & editing, Supervision, Resources, Project administration. Shubhangi Mankar: Writing – review & editing, Validation. Vikas Oswal: Writing – review & editing, Validation, Investigation. Varsha Puri: Writing – review & editing, Validation, Supervision. Daksha Shah: Writing – review & editing, Validation, Supervision. Vijay Chavan: Writing – review & editing, Validation, Supervision, Investigation, Conceptualization. Hannah Spencer: Writing – review & editing, Validation, Supervision, Resources, Project administration. Petros Isaakidis: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to acknowledge our patients and their families, and the MSF project team involved in providing care to the TB patients.
Author contributions
SK, AS, AI, PI and VC conceived and designed the study. SK, PD and NS provided clinical services and SM, VO, VP and DS collaborated in the implementation of clinical activities. SK, AS and RM collected and analysed the study data. SK, AS, RM, VC, HS and PI interpreted the results and drafted the manuscript. All the authors have contributed to the revisions of the manuscript and approved the final manuscript.
Ethics statement
The study received ethics approval from Institutional Ethics Committee for Research (IECR) of State Health Systems Resource Centre (SHSRC), Pune, Maharashtra (SHSRC/IECR-4 Approval/743/19). The study fulfilled the exemption criteria set by the Médecins Sans Frontières (MSF) Ethics Review Board for a posteriori analysis of routinely collected clinical data and thus did not require MSF ERB full review. Permission was also sought from the India National Tuberculosis Elimination Programme (NTEP).
Funding
This research received no external funding.
References
- 1.World Health Organization Global TB Rep. 2023:2023. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023 accessed December 15, 2023. [Google Scholar]
- 2.Ministry of Health and Family Welfare. Report of the first national anti-tuberculosis drug resistance survey India: 2014-16. 2018. https://tbcindia.gov.in/showfile.php?lid=3315 (accessed December 15, 2023).
- 3.Gupta M., Ish P., Malhotra N. Recent updates in diagnosis and management of drug-resistant tuberculosis in India: A paradigm shift and the way ahead during the COVID-19 crisis. Indian Journal of Tuberculosis. 2022;69:264–267. doi: 10.1016/j.ijtb.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Central TB division M of H and FW. Guidelines for Programmatic Management of Drug Resistant Tuberculosis in India-2021. 2021. https://tbcindia.gov.in/showfile.php?lid=3590 (accessed December 15, 2023).
- 5.Husain A.A., Kupz A., Kashyap R.S. Controlling the drug-resistant tuberculosis epidemic in India: Challenges and implications. Epidemiol. Health. 2021;43 doi: 10.4178/EPIH.E2021022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment, 2022 update. 2022. https://www.who.int/publications/i/item/9789240063129 (accessed December 15, 2023). [PubMed]
- 7.Alemu A., Bitew Z.W., Diriba G., Seid G., Moga S., Abdella S., et al. Poor treatment outcome and associated risk factors among patients with isoniazid mono-resistant tuberculosis: A systematic review and meta-analysis. PLoS One. 2023;18 doi: 10.1371/journal.pone.0286194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Health and Family Welfare GOI Leading the way: India TB Report. 2023:2023. https://tbcindia.gov.in/showfile.php?lid=3680 accessed December 15, 2023. [Google Scholar]
- 9.Velayutham B., Shah V., Mythily V., Gopalaswamy R., Kumar N., Mandal S., et al. Factors influencing treatment outcomes in patients with isoniazid-resistant pulmonary TB. International Journal of Tuberculosis and Lung Disease. 2022;26:1033–1040. doi: 10.5588/IJTLD.21.0701. [DOI] [PubMed] [Google Scholar]
- 10.Garg K., Saini V., Dhillon R., Agarwal P. Isoniazid mono-resistant tuberculosis: Time to take it seriously. Indian J Tuberc. 2019;66:247–252. doi: 10.1016/J.IJTB.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Hymn P.K., Gurjar Y., Savani N.M. A Retrospective Analysis of Clinico-Demographic and Genetic Characteristics and Treatment Outcomes in Isoniazid Mono-Resistant Tuberculosis Patients: A Single-Center Study. Cureus. 2023 doi: 10.7759/cureus.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagar J.G., Rami K.C., Patel M.M., Bhavsar K.M. Treatment outcomes of patients with isoniazid resistant tuberculosis under National Tuberculosis Elimination Programme in Ahmedabad city: a retrospective study. Int J Res Med Sci. 2022;10:678. doi: 10.18203/2320-6012.ijrms20220517. [DOI] [Google Scholar]
- 13.Munje R., Mishra G., Agrawal K., Khateeb S., Jichkar S., Gour S., et al. of the international union against tuberculosis and lung disease (The Union); Paris France 15–18 November 2023. Paris. 2023;2023:113. [Google Scholar]
- 14.Mistry N., Tolani M., Osrin D. Drug-resistant tuberculosis in Mumbai, India: An agenda for operations research. Oper Res Health Care. 2012;1:45–53. doi: 10.1016/j.orhc.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardeshi P., Jadhav B., Singh R., Kapoor N., Bardhan R., Jana A., et al. Association between architectural parameters and burden of tuberculosis in three resettlement colonies of M-East Ward, Mumbai. India Cities Health. 2020;4:303–320. doi: 10.1080/23748834.2020.1731919. [DOI] [Google Scholar]
- 16.Padmapriyadarsini C., Shobana M., Lakshmi M., Beena T., Swaminathan S. Undernutrition & tuberculosis in India: Situation analysis & the way forward. Indian J Med Res. 2016;144:11–20. doi: 10.4103/0971-5916.193278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. WHO Guideline: Nutritional care and support for patients with tuberculosis 2013. https://iris.who.int/bitstream/handle/10665/94836/9789241506410_eng.pdf?sequence=1 (accessed July 10, 2024). [PubMed]
- 18.Ministry of Health and Family Welfare Government of India. Guidance Document: Nutritional care and support for patients with Tuberculosis in India. 2017. https://tbcindia.gov.in/WriteReadData/Guidance%20Document%20-%20Nutritional%20Care%20%26%20Support%20for%20TB%20patients%20in%20India.pdf (accessed July 10, 2024).
- 19.Bhargava A., Bhargava M., Meher A., Teja G.S., Velayutham B., Watson B., et al. Nutritional support for adult patients with microbiologically confirmed pulmonary tuberculosis: outcomes in a programmatic cohort nested within the RATIONS trial in Jharkhand. India Lancet Glob Health. 2023;11:e1402–e1411. doi: 10.1016/S2214-109X(23)00324-8. [DOI] [PubMed] [Google Scholar]
- 20.Bachir M., Guglielmetti L., Tunesi S., Billard-Pomares T., Chiesi S., Jaffré J., et al. Molecular detection of isoniazid monoresistance improves tuberculosis treatment: A retrospective cohort in France. J Infect. 2022;85:24–30. doi: 10.1016/J.JINF.2022.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Naidoo K., Dookie N. Can the GeneXpert MTB/XDR deliver on the promise of expanded, near-patient tuberculosis drug-susceptibility testing? Lancet Infect Dis. 2022;22:e121–e127. doi: 10.1016/S1473-3099(21)00613-7. [DOI] [PubMed] [Google Scholar]