Abstract
Emerging insights in osteoimmunology have enabled researchers to explore in depth the role of immune modulation in regulating bone health. Bone is one of the common sites of metastasis notably in case of breast cancer, prostate cancer and several other cancer types. High calcium ion concentration and presence of several factors within the mineralized bone matrix including TGF-β, BMP etc., aid in tumor growth and proliferation. Accumulating evidence has substantiated the role of the gut-microbiota (GM) in tumorigenesis, further providing a strong impetus for the growing “immune-cancer-gut microbiota” relationship. Recent advancements in research further highlight the importance of the intra-tumor microbiota in conjunction with GM in cancer metastasis. Intratumoral microbiota owing to their ability to cause genetic instability, mutations, and epigenetic modifications within the tumor microenvironment, has been recognized to affect cancer cell physiology. The host microbiota and immune system crosstalk shapes the innate and adaptive arms of the immune system, which is the key player in cancer progression. In this review, we aim to decipher the role of microorganisms mediating bone metastasis by shedding light on the immuno-onco-microbiome (IOM) axis. We discussed the feasible cancer therapeutic interventions based on the modulation of the microbiome-immune cell axis which includes prebiotics, probiotics, and postbiotics. Here, we leverage the conceptual framework based on the published articles on microbiota-based therapies to target bone metastases. Understanding this complicated nexus will provide insights into fundamental factors governing bone metastases which will subsequently help in managing this malignancy with better efficacy.
Keywords: Gut microbiota, Bone metastases, Bone cancer, Immune system, Osteo-immuno-oncology, Biotics, Intratumoral microbiota
1. Introduction
The majority of deaths due to cancer are a result of incurable metastases, which occur when genetically unstable cancer cells disseminate to a site distant from the primary tumor (Fares et al., 2020; Mani et al., 2024). The roaming tumor cells interact with their receptive target tissues, but colonization occurs only at particular organ sites, which provides a microenvironment that is conducive to the growth and proliferation of tumor cells (Valastyan and Weinberg, 2011). Disseminated tumor cells (DTCs) are believed to remain dormant even after migrating to distant organs until an unknown trigger drives them to interact favorably with the new environment to obtain an advantage for growth and survival (Fares et al., 2020).
Bone is one of the common sites for the metastasis of solid tumors (Coleman et al., 2020). Estimates from the available literature suggest that the highest incidence of metastatic bone disease is observed in cancers arising from the breast (75 %) (Zhong et al., 2023) and prostate (70 %) (Zhou et al., 2024), and non-small-cell lung cancer (20–50 %) (Gong et al., 2024), kidney (40 %) (Coleman et al., 2020) and thyroid (∼40 %) (Yao et al., 2024). The bone's unique microenvironment characterized by high abundance of calcium ions and the chemo-attractiveness fueled by the release of growth factors from the bone matrix and prostaglandins or other factors from different bone cells, including stromal cells, osteoblasts, osteocytes, and osteoclasts, make it an excellent place for tumor cells to colonize (Coleman et al., 2010, Coleman et al., 2020; Elaasser et al., 2024). Intriguingly, bone is a unique environment that contains a wide range of immune cells and bone cells. These cells are known for their reciprocal relationships with the tumor and bone-resident cells, which facilitate the effective colonization of DTCs in the bone (Coleman et al., 2010; Ryan et al., 2022). Hence, this crosstalk between the bone and immune system is well established which further discerns the interaction of immune cells and cancer cells during bone metastases.
The role of gut microbiota (GM) in shaping both the adaptive and innate arms of the immune system has been mapped which further sheds light on the immuno-onco-microbiome (IOM) axis to derive insights into the microbial population modulating tumorigenesis (Sepich-Poore et al., 2021). However, it is unclear whether the bacteria inhabit a hospitable microenvironment within the tumor to multiply, or whether they are the main agents of carcinogenesis and are thus responsible for the persistence of the tumor and its resistance to anticancer therapies. For identifying host-centric tumor characteristics, it is essential to dissect the complex interplay between the GM, anti-tumor activity, and immunosurveillance. Recent research has witnessed a surge in investigations understanding IOM axis (Li et al., 2024b; Sepich-Poore et al., 2021), particularly in malignancies such as colon cancer (Jia et al., 2024a; Zhang et al., 2023a) melanoma (Bender et al., 2023a; Björk et al., 2024; Lee et al., 2023) and breast cancer (Jia et al., 2024a; Lee et al., 2023; Viswanathan et al., 2023). It can thus be hypothesized that the gut may also have direct or indirect effects on secondary cancers, such as secondary bone metastasis. This can thus provide more insights about the relationship between GM and the initiation, progression, prognosis, and treatment of bone cancer. In this review, we comprehensively discuss the likely mechanisms through which GM can regulate the onset and progression of bone cancer and how intra-tumor microbiota can affect the host's response to the therapies. Furthermore, we examine the underlying strengths of modulating the microbial profiles and immune-oncosystem through microbiological interventions to serve as a feasible therapeutic intervention in cancers.
2. Bone cancer and metastases
Bone is a rigid yet extremely dynamic, metabolically active organ, that serves a structural purpose by supporting vital internal organs and bone marrow simultaneously providing the site for muscle attachment for movement. Also, bone is a reservoir of minerals and energy that contributes to the maintenance of serum homeostasis via buffering action. To sustain skeletal strength and integrity, bone undergoes a continuous remodeling process within the basic multicellular unit. Resorption, reversal, and formation are the three consecutive steps that constitute the bone remodeling process as reviewed in detail in other reviews (Bolamperti et al., 2022; Han et al., 2018; Wang et al., 2022b). Depending on the site of origin, bone cancers are classified into primary or secondary.
The primary bone malignancies, such as osteosarcoma, chondrosarcoma, and Ewing sarcoma are rare, however, the risk of developing bone metastases in advanced cancers is quite high. Although the incidence of bone metastases varies among different cancer types, primary tumors originating from the breast and prostate have the highest relative incidence of bone colonization (Ibraheem, 2022; Macedo et al., 2017).
2.1. Primary bone cancer
Primary bone tumors are a rare and heterogeneous group of mesenchyme-derived neoplasms that account for only 0.2 % of all human neoplasia (Franchi, 2012; Sung et al., 2021). The latest fifth edition of the World Health Organization (WHO) classifies bone tumors into cartilage tumors, osteogenic tumors, fibrogenic tumors, vascular tumors of bone, osteoclastic giant cell-rich tumors, notochordal tumors, hematopoietic neoplasms of bone, and other mesenchymal tumors of bone (Choi and Ro, 2021). These tumors emerge due to close interactions with the cells of the local bone microenvironment including osteoblasts, osteoclasts, macrophages, and tumor-infiltrating lymphocytes, etc. (Heymann et al., 2019). Three commonly known subtypes of primary bone cancers – osteosarcoma, Ewing sarcoma, and chondrosarcoma – are briefly reviewed below (Brown et al., 2018).
Osteosarcoma (OS) is a highly malignant bone tumor and the third most common cancer in adolescents worldwide, with an OS incidence of 3.4 per million people per year (Mirabello et al., 2009). Due to the sporadic origin of OS, the majority of cases exhibit features of chromosomal abnormality involving genetic alteration or inactivation of germline tumor protein 53 (TP53), retinoblastoma protein (RB1), RecQ like helicase 4 (RECQL4), bloom syndrome gene (BLM) and Werner syndrome ATP-dependent helicase (WRN) (Martin et al., 2012).
Ewing sarcoma (EWS) is the second most common primary bone tumor affecting children and adolescents. Molecular analysis revealed the presence of a characteristic chromosomal translocation and the resulting EWS-FLI1 gene fusion in nearly 85 % of the examined patients (Balamuth and Womer, 2010). EWS-FLI-1 fusions have been reported to form R-loops that block the replication machinery in cells and consequently lead to high levels of DNA damage (Gorthi et al., 2018). Although much is known about the diverse roles of EWS-FLI-1 in epigenetic remodeling, non-coding RNA regulation, oncogene activation, and tumor suppressor repression, the origin cell of EWS is still unknown (Pachva et al., 2021).
Chondrosarcoma (CS) represents a group of tumors that predominate in the cartilage and range from low-grade to high-grade tumors depending upon their metastatic potential. These tumors account for nearly 10–20 % of all primary malignant bone tumors (Thorkildsen et al., 2019). Perturbations in the Hedgehog signaling pathway, tumor suppressor pathways, and PI3K-Akt-mTOR pathway have been reported to contribute to the development of cartilaginous neoplasms (Chow, 2018). Mutations in the exostosin glycosyltransferase (EXT1 or EXT2) and isocitrate dehydrogenase (IDH) 1 and 2 genes are also found in such tumors (Amary et al., 2011).
2.2. Secondary bone cancer
Advanced-stage tumor with their origins mapped to the breast, prostate, and lung have a fairly high probability of metastasizing to the bone (Table 1). In body, cancers spread to the bone marrow via the hematogenous or lymphatic system (Coleman, 2001; Coleman et al., 2010).
Table 1.
Cancers with high potential for bone metastasis and their respective tumor microbiome.
| Cancer type | % Bone metastasis | Underlying mechanisms for bone metastases | Tumor microbiome profile | References |
|---|---|---|---|---|
| Breast cancer | ∼75 % | RANKL-dependent and independent osteoclastic differentiation of HSCs | Pseudomonas, Proteus, Sphingomonas yanoikuyae, Enterococcus, Cladosporium, Lactobacillus, Methylobacterium radiotolerans, Streptococcus | (Fu et al., 2022; Lucas et al., 2018; Ma et al., 2020b; Tzeng et al., 2021) |
| Parathyroid hormone-related protein (PTH-rP) | ||||
| connexin-43 gap junctions mediated regulation of intracellular Ca concentrations | ||||
| heterotypic adherent junctions (E-cadherin and osteogenic N-cadherin and OB-cadherin) | ||||
| Prostate Cancer | ∼70 % | PCa cells secrete OB-stimulating GFs: ET-1, adrenomedullin, FGFs, PDGFs, and BMPs | Cutibacterium acnes, Staphylococcus aureus, Listeria Monocytogenes, Xanthomonas albilineans, Pseudomonas, Escherichia, Acinetobacter | (Jiang et al., 2023; Kustrimovic et al., 2023; Ma et al., 2020a) |
| loss of tumor-intrinsic type-I IFN driving cancer cell progression | ||||
| Upregulates RANK-L expression in bone stromal cells | ||||
| Increased Activin A expression and thus expression of osteoblastic lesions | ||||
| Lung Cancer | ∼25–40 % | Increased EGFR expression and osteolytic factors: PTHrP, IL-11 | Modestobacter, Propionibacterium, Acinetobacter, Cyanobacteria, Enterobacteriaceae, Klebsiella, Veillonella, Megasphaera | (Greathouse et al., 2018; Zhou et al., 2017) |
| OCs support DTCs, proliferation via IL-19/IL-20RB/STAT3 axis | ||||
| OBs express SDF-1 that increases MMP-9 expression through CXCR4 mediated activation of MAPK/ERK pathway | ||||
| Renal cancer | ∼20–40 % | RC cells secrete PTHrP, TGF-β, increase RANKL production | Blautia, Streptococcus, Ruminococcus, Romboutsia, Eubacterium | (Piao et al., 2023; Umer et al., 2018) |
| Bone cells secrete PDGF, FGF, IGF therefore promoting RC cells migration | ||||
| increase expression of calcium sensing receptors |
HSCs: Hematopoietic stem cells, PTH-rP: Parathyroid hormone-related protein, Ca: Calcium, PCa: Prostrate cancer, GFs: Growth factors, ET-1: Endothelin-1, FGF: Fibroblast growth factor, PDGF: Platelet-derived growth factor, BMP: Bone morphogenic protein, IFN: Interferon, RANK-L: Receptor activator for nuclear factor kappa-B ligand, EGFR: Epidermal growth factor receptor, IL- Interleukin, OC: Osteoclast, DTC: Disseminated tumor cells, STAT3: Signal transducer and activator of transcription 3, OB: Osteoblast, SDF-1: Stromal cell derived factor-1, MMP: Matrix metalloproteinase 9, CXCR4: C-X-C Chemokine Receptor 4, MAPK/ERK: Mitogen-activated protein kinases/Extracellular signal-regulated kinase, RC: Renal cancer, TGF: Transforming growth factor, IGF: Insulin growth factor.
Tumor cells predominantly interact with bone marrow stromal cells (BMSCs) via CXCL12 that assist in homing of DTCs to the bone (Elaasser et al., 2024). The unique physicochemical properties of bone – hypoxia, intense vascularization, high local calcium concentrations, abundance of immobilized growth factors and acidosis – provide an optimal niche for DTCs to adapt, colonize, and proliferate, as stated by Stephen Paget's “seed and soil” hypothesis (Paget, 1889, Paget, 1989).
The presence of growth factors like IGF, VEGF aid in invasion, growth and survival of metastatic tumor cells while the calcium ions interact with extracellular calcium receptors on the cancer cells and trigger a cascade of downstream signaling events to promote tumor growth and proliferation (Coleman et al., 2020; Elaasser et al., 2024). DTCs from cancers, seed this fertile environment. Furthermore, a recent study suggests that initiation of bone metastasis is coupled with bone remodeling. It has been demonstrated that pathological fractures increase metastatic colonization around the injury. NG2+ Bone marrow mesenchymal stem cells are the key players serving dual purposes of bone remodeling and metastasis. These promote metastasis initiation via N-cadherin-mediated cell-cell interaction (Coleman, 2001; Coleman et al., 2020; Elaasser et al., 2024; Zhang et al., 2023b).
Prior to the dissemination of the tumor cells from the primary site, secretion of some systemic factors and micro vesicles (e.g. exosomes) direct the conversion of incipient metastatic sites into compatible ‘pre-metastatic niches’ (Peinado et al., 2017). This prepares the recipient site to undergo extracellular matrix remodeling, increase vascular permeability, and suppress the immune microenvironment. Upon entering the bone microenvironment, DTCs encounter perivascular niche which is a heterogenous network of blood vessels and perivascular cells that decides their ultimate fate. Several factors promote dormancy of DTCs and their retention in perivascular niche while other facilitate their migration to the endosteal niche (Satcher and Zhang, 2022). Vascular E-selectins stimulate mesenchymal to epithelial transition through Wnt signaling and NG2+ cells reinforce dormancy via TGF-beta secretion however, endothelial derived E-selectin stimulates the migration of DTCs to endosteal surface which harbours osteoblasts, MSCs and osteoprogenitors that modulate cancer proliferation via several mechanisms involving mTOR signaling, calcium signaling, epigenomic modification etc., subsequently leading to bone metastasis (Satcher and Zhang, 2022).
Bone metastases can manifest either as osteolytic, osteoblastic, or mixed, depending upon the primary mechanism by which tumor cells alter the normal bone remodeling process (Coleman, 2001; Coleman et al., 2020). Osteolytic metastasis is characterized by a lytic and destructive lesion, primarily mediated by osteoclasts, and is not a direct effect of tumor cells on bone. It is predominantly observed in solid tumors of the breast, thyroid, lung, and kidney wherein the cooperation of several genes is required for the formation of aggressive bone lesions (Macedo et al., 2017). Kang et al. reported that elevated expression of pre-metastatic genes - C-X-C chemokine receptor 4 (CXCR4), matrix metalloproteinase (MMP)-1, connective tissue growth factor (CTGF), fibroblast growth factor (FGF5), interleukin (IL)-11, and osteopontin (OPN) – are collectively responsible for the manifestation of osteolytic breast cancer metastasis (Kang et al., 2003).
Osteoblastic metastasis, also known as sclerotic metastasis, is associated with the production of bone-synthesizing factors by tumor cells, which promote the stimulation and proliferation of osteoblasts, resulting in the deposition of new bone (Macedo et al., 2017). Although the underlying mechanisms of osteoblastic bone metastasis are poorly understood, studies have shown indications of tumor osteomimicry and tumor-induced endothelial-to-osteoblast conversion that may provide insights into this domain. Tumor osteomimicry is defined as the ability of cancer cells to express a bone-specific protein profile, including osteocalcin, OPN, and receptor activator of nuclear factor kappa-Β ligand (RANKL), which induce seeding of cancer cells in bone microenvironment (Macedo et al., 2017). Endothelin-1 (ET-1) (Guise et al., 2003), bone morphogenetic proteins (BMPs) (Sun et al., 2020), and growth differentiation factor 15 (GDF15) (Siddiqui et al., 2022) have been reported as signaling osteoblastic factors for bone metastasis.
3. Nexus between bone metastases and the immune system: osteo-immuno-oncology
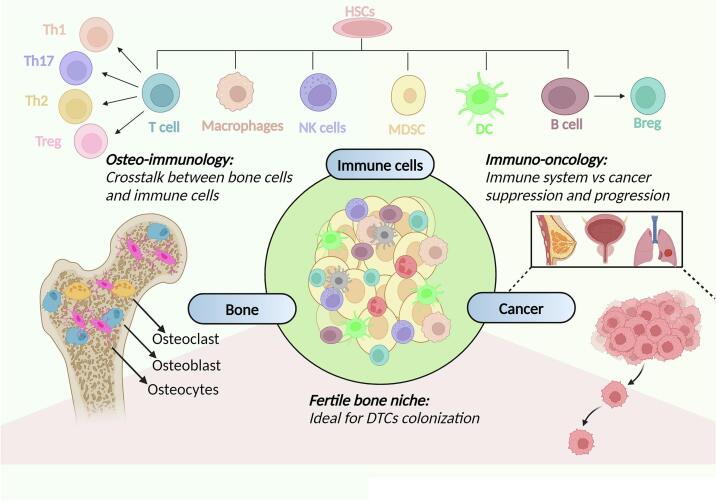
The existence of dynamic cross-communication between the immune system and the skeleton has sparked the emergence of a novel field of immunology known as “Osteoimmunology” (Fig. 1). It specifically deals with the study of the reciprocal regulation of immune cells by the bone cells and their intimate interactions in the bone microenvironment (Guder et al., 2020; Srivastava, 2018). Crosstalk between the skeletal system and immune system is essential for maintaining both osseous homeostasis and immune function. It is well recognized that lymphocytes and immune factors including cytokines, chemokines, and exosomes can promote or antagonize bone remodeling depending upon the external stimuli and the microenvironment as reviewed in detail elsewhere (Guder et al., 2020; Huppert et al., 2022). Now that we can appreciate the mutual dependency of the two systems and the underlying cross-regulation, we are further trying to examine osteoimmune communication in the context of bone metastases.
Fig. 1.
The osteo-immuno-oncology triad. Development of immune cells from hematopoietic stem cells (HSCs) in bone marrow indicates that bone and immune system are in constant interaction (Ponzetti and Rucci, 2019). Interaction of bone resident cells (osteoclasts, osteoblasts, osteocytes) with the immune cells is crucial to maintain bone homeostasis. Further, establishment of an immunosuppressive microenvironment and failed anti-tumor immune responses promote bone colonization of tumor cells from advanced cancers of breast, prostate, and lung.
It is an important domain that needs to be addressed particularly because we lack a comprehensive understanding of osteo-immuno-oncology which is essential for developing innovative treatments that specifically target bone metastases. A variety of immune cells including T cells, natural killer cells (Roato and Vitale, 2019), macrophages (Di Mitri et al., 2023) and myeloid-derived suppressor cells (MDSCs) are involved in bone metastases providing an immune-privileged habitat for DTCs to infiltrate and proliferate (Chen et al., 2024) (Fig. 2). Transforming growth factor (TGF)-β-released during osteoclastic bone resorption, promotes tumor growth by interfering with immune defenses because of its inherent immunosuppressive capabilities. In a study by Jiao et al. (2019), TGF-β blockade promotes clonal expansion of CD8+ T cells and provide a conducive environment for T cell activation in murine models of bone castrate resistant prostate cancer (Clambey et al., 2012; Jiao et al., 2019).
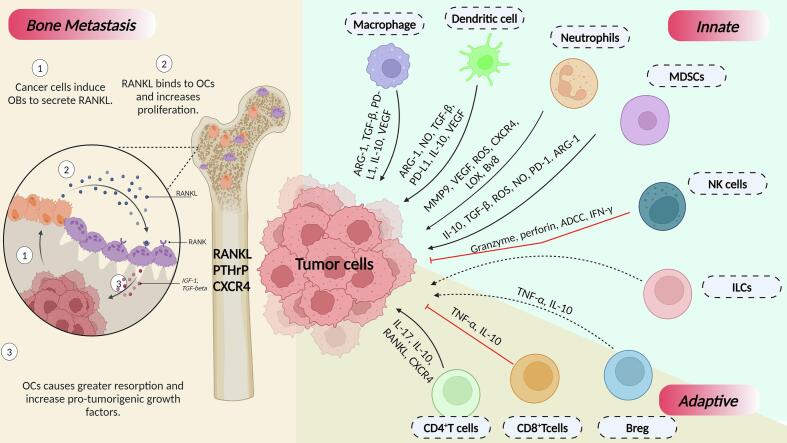
Fig. 2.
The interaction between immune cells and tumor cells during bone metastasis. Under the innate arm of the immune system, macrophages, dendritic cells (DCs), neutrophils, and myeloid-derived suppressor cells (MDSCs) release pro-tumorigenic cytokines that aid in the successful establishment of disseminated tumor cells (DTCs) in bone. Natural killer (NK) cells, however, deploy anti-tumorigenic cytokines that promote tumor regression. On the other hand, CD4 + T cells and Bregs release certain cytokines that stimulate cancer cells to metastasize to bone. Differentiation of CD8 + T cells into cytotoxic T lymphocytes (CTLs) exert anti-tumoral attack by secretion of proinflammatory cytokines- TNF-α and IFN-γ, consequently an anti-metastatic effect. As shown in the figure, the osteolytic tumor cells majorly release pro-osteoclastogenic cytokines such as receptor activator for nuclear factor kappa-B ligand (RANKL), parathyroid hormone-related protein (PTHrP), and C-X-C Chemokine Receptor 4 (CXCR4) which activate osteoclastogenesis and drive formation of osteolytic lesion.
Treg cells play a crucial role in promoting metastatic tumor growth in the bone microenvironment either by modulating RANK/RANKL axis or by interacting with and suppressing other immune cells including CD8+ T cells and helper T cells (Chen et al., 2024). Tregs play a pivotal role in promoting the formation of osteoblastic bone lesions by tilting the balance between osteoclasts and osteoblasts towards osteogenesis by suppressing osteoclast differentiation and proliferation (Huppert et al., 2022). Similar effects have been observed in prostate cancer metastasis to bone wherein increased Treg infiltration often leads to a poor prognosis and increased bone metastasis (Chen et al., 2024). In the immune microenvironment of bone metastasis from prostate cancer, Tregs can translocate to the bone marrow through (CXCR4)/(CXCL12) and can trigger the formation of osteoblastic lesions (Zou et al., 2004). In a mouse breast cancer model, overexpression of cyclooxygenase 2 (COX2) resulted in an increased recruitment of Tregs to the tumor and increased bone metastasis indicating the role of Tregs in promoting colonization and proliferation of breast cancer cells to the bone. This was further substantiated by decreased bone metastasis of breast cancer (Huppert et al., 2022) cells upon inhibition of CXCL12/CXCR4 axis and indoleamine 2,3-dioxygenase (IDO1) expression (Huppert et al., 2022).
Furthermore, it is anticipated that the bone microenvironment fosters a pro-metastatic niche feedback loop as under metastatic conditions (Huppert et al., 2022), recruitment of Tregs to the BM is increased via the action of the CXCR4/CXCL12 signaling pathway. Within the bone, RANK + DCs induce Treg cell expansion, which themselves serve as the key source for RANKL production in the presence of tumors, further inducing bone resorption that leads to local acidification of the bone microenvironment thereby activating TGF-β and generating more Tregs which promote tumor cell proliferation and growth within the bones (Huppert et al., 2022).
MDSCs owing to their remarkable immunosuppressive capabilities, which include the inhibition of T cell proliferation and antigen-specific T cell responses and decreased cytokine production intricately contribute to cancer progression including secondary bone metastasis (Li et al., 2024a). However, their role in breast cancer-associated osteolysis and bone metastasis extends beyond immunosuppression, as evident by increased MDSCs expansion and bone destruction in intraorthotopic and intracardiac breast cancer models of nude mice (Danilin et al., 2012). It has been hypothesized that MDSCs serve as the growth and invasion factors creating a locally favorable microenvironment in the bone to promote tumor growth (Danilin et al., 2012). Notably, in tumor-bearing mice, MDSCs in bone marrow exhibit increased expression of TGF-β1 that stimulates breast cancer cells to express certain factors e.g., parathyroid hormone-related protein (PTHrP) and GLI2 that are essential for breast cancer-induced osteolysis. Furthermore, a vicious cycle leads to MDSC expansion during breast cancer and increased dissemination of the breast cancer cells to the bone (Liu et al., 2023a). The tumor-derived factors including macrophage colony-stimulating factor (MCSF), COX-2, interleukins like IL-6, 13, 17 promote the expansion of MDSCs exhibiting increased expression of TGF-β (Liu et al., 2023a). In addition, MDSCs promote tumor angiogenesis via increased MMP9 secretion which further increases VEGF bioavailability, ultimately leading to TFG-β overexpression which is associated with bone destruction (Han et al., 2024). Besides, there is compelling anticipation that MDSCs possess the capacity to differentiate into cell types deemed essential for tumor growth. This proposition was substantiated by the observed ability of Gr1+CD11b+ cells to differentiate into osteoclast precursors both in vitro and in-vivo. Notably, in orthotopic mice models, the augmentation of MDSCs expansion was positively correlated with an increased population of OC precursors (Danilin et al., 2012; Sawant et al., 2013).
Bone-marrow-associated macrophages (BMMs) derived from the classical monocytes promote breast cancer metastasis to bones in an IL4R+ dependent manner which is anticipated to be crucial for the polarization and pro-tumor function of BMMs (Chen et al., 2024; Ma et al., 2020b). As a point of evidence, mosaic mice bearing IL4ra−/− exhibited decreased bone colonization of breast cancer cells compared with IL4ra+/− mosaic mice. It is suggested that IL4R regulates tumor-promoting functions rather than BMMs recruitment as substantiated by the comparable abundance of monocytes and macrophages in both the mice groups. The comparable abundance of osteoclasts in mosaic mice suggested the significance of IL4R exclusively in promoting BMMs-induced bone metastasis with no direct effect on bone remodeling (Ma et al., 2020b). Moreover, it has been elucidated that macrophages derived from inflammatory monocytes rather than the BMMs are responsible for bone metastasis. This assertion was corroborated by a notable reduction in bone metastatic growth in mice deficient in chemokine (C-C motif) ligand-2 (CCL2), an important factor responsible for the recruitment of inflammatory monocytes to the bone (Ma et al., 2020b). In contrast, no such effects were observed upon ablation of bone marrow resident macrophages via diphtheria toxin (DT) treatment in transgenic mice expressing the DT receptor gene under the control of CD169 promoter- a cell surface marker specific to BMMs (Ma et al., 2020b).
4. Gut microbiome and bone homeostasis
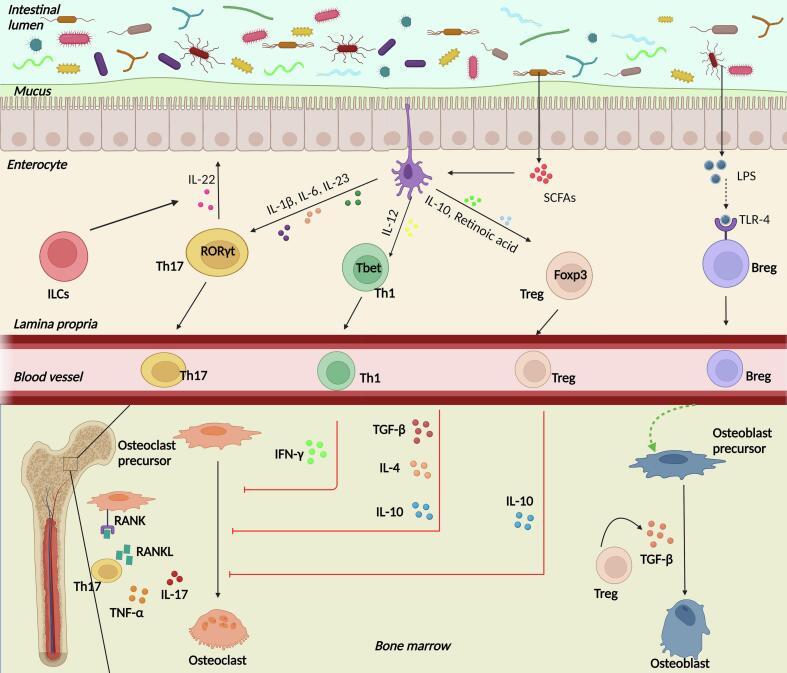
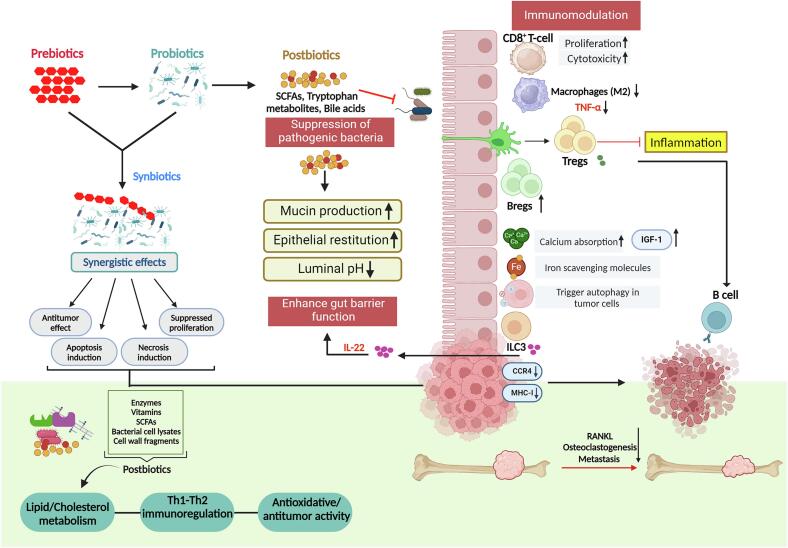
The colonization of GM begins at birth when the neonate is exposed to vaginal and environmental microbial flora. GM changes with age and exhibits high variability in elderly individuals compared to younger adults (Claesson et al., 2011). Recently, osteomicrobiology has emerged as a novel interdisciplinary field that dictates the role of GM in bone homeostasis, combining bone physiology, gastroenterology, immunology, and microbiology. The human GM collectively refers to the trillions of microorganisms diversifying from acellular to cellular prokaryotes (bacteria and archaea) and eukaryotic microbes that inhabit the gastrointestinal tract (Shreiner et al., 2015). The first evidence of the association between GM and bone was elucidated in a study by Sjögren et al. where they found a significantly higher trabecular bone volume and reduced level of osteoclastogenesis in bones of germ-free (GF) mice as compared to that of conventionally raised (ConvR) mice (Sjögren et al., 2012). Dysregulated bone remodeling because of GM dysbiosis has been observed in patients with bone pathologies. Based on the results of 16S rRNA gene sequencing in patients with osteoporosis and primary osteopenia, it was hypothesized that the immune-inflammatory axis act as a bridge between GM and bone metabolism (Wang et al., 2017). This may be attributed to altered levels of insulin growth factor (IGF)-1, tumor necrosis factor (TNF)-α, and IL-1β or alterations in relative abundances of osteoclasts, osteoclast precursor cells, CD4+ T cells, and inflammatory cytokines (Fig. 3) (Yan et al., 2016).
Fig. 3.
The nexus between gut microbiota (GM) and bone. The GM maintains bone homeostasis and under conditions of dysbiosis, results in abnormal bone remodeling contributing towards osteo-pathologies. A leaky gut allows the translocation of GM species from the intestinal lumen into the lamina propria which hosts a multitude of immune cells. GM species and derived short chain fatty acids (SCFAs) induce the dendritic cells (DCs) to stimulate Th17 and Th1 cells which further participate in inhibiting osteoclastogenesis via the production of cytokines such as interferon (IFN)-γ, interleukin (IL)-10, IL-4, and transforming growth factor (TGF)-β. Secretion of a variety of interleukins drive activation of Th17 cells which promote osteoclastogenesis via tumor necrosis factor (TNF)-α, IL-17, and receptor activator for nuclear factor kappa-B (RANK)- receptor activator for nuclear factor kappa-B ligand (RANKL) signaling. Also, production of IL-22 by Th17 and innate lymphoid cells (ILCs) maintains gut membrane integrity. Bregs are activated directly by the bacteria-derived lipopolysaccharide (LPS) via toll like receptor-4 (TLR4) signaling which inhibits maturation of osteoclast precursors directly and indirectly by inducing Tregs to suppress bone resorption. Secretion of TGF-β by Tregs function to convert pre-osteoblasts into osteoblasts, thus inducing bone formation.
On the contrary, short-chain fatty acids (SCFAs) derived from the GM exert anti-inflammatory effects in the intestinal mucosa by histone deacetylases (HDACs) inhibition and G-protein-coupled receptors (GPCR) activation in IECs. This in turn affects gene regulation of cell proliferation, differentiation, and inflammatory response thus protecting from diseases.
A study by Lucas et al. revealed propionate and butyrate-induced metabolic reprogramming of osteoclasts and subsequent downregulation of essential osteoclast genes- TNF receptor-associated factor 6 (TRAF6) and nuclear factor of activated T cells (NFATc1), thus acting as potent regulators of bone homeostasis (Lucas et al., 2018). In the case of microbial dysbiosis, altered immune responses lead to increased bacterial translocation from the intestinal lumen into the lamina propria accompanied by the release of pro-inflammatory cytokines (Lyu et al., 2023). A prolonged state of inflammation often results in chronic inflammatory diseases that are associated with bone destruction, such as inflammatory bowel disease (IBD). Certain SCFAs e.g., acetate assist in ameliorating ovariectomy (ovx)-induced bone loss by decreasing the number of osteoclasts in a T-cell and B-cell dependent manner. A study identified that nucleotide-binding oligomerization domain (NOD1) and NOD2 signaling affects bone mass in response to external stimuli, like GM. Bacterial ligand stimulation of NODs resulted in osteoclastogenesis via increased RANKL and TNF-α in the bone of ConvR mice (Ohlsson and Sjögren, 2018). From such studies, it is derived that colonization with GM leads to activation of the immune system, especially CD4+ T cells which directs osteoclastogenesis via increased levels of pro-inflammatory cytokines in bone (Ohlsson et al., 2017).
4.1. GM Dysbiosis and bone metastases
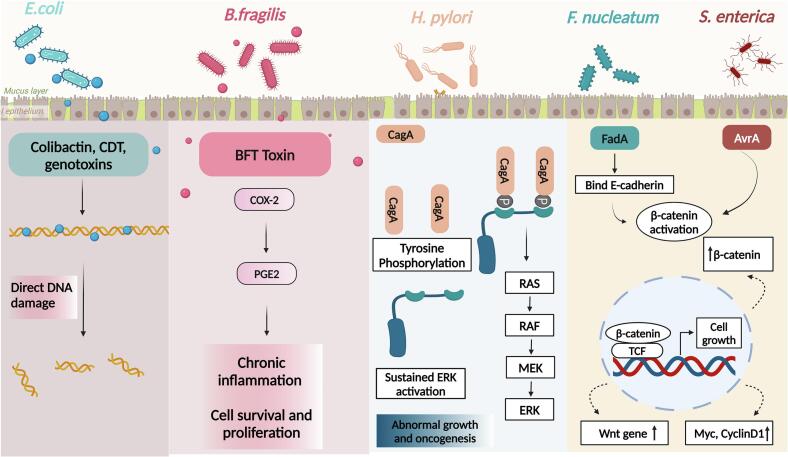
GM has been recognized to play a crucial role in cancer progression (Balkwill and Mantovani, 2001; Lu et al., 2014; Tjalsma et al., 2012). Primarily, certain microorganisms such as Escherichia coli and Shigella dysenteriae are known to secrete DNA-damaging toxins e.g., colibactin and cytolethal distending toxin (CDT), which are known to participate in chronic inflammation and tumor progression (Guerra et al., 2011). Additionally, microbes secrete certain pro-tumorigenic metabolites e.g., lithocholic acid (LCA) which have a role in promoting colorectal cancer in an IL-8-dependent manner (Fig. 4) (Ivleva and Grivennikov, 2022).
Fig. 4.
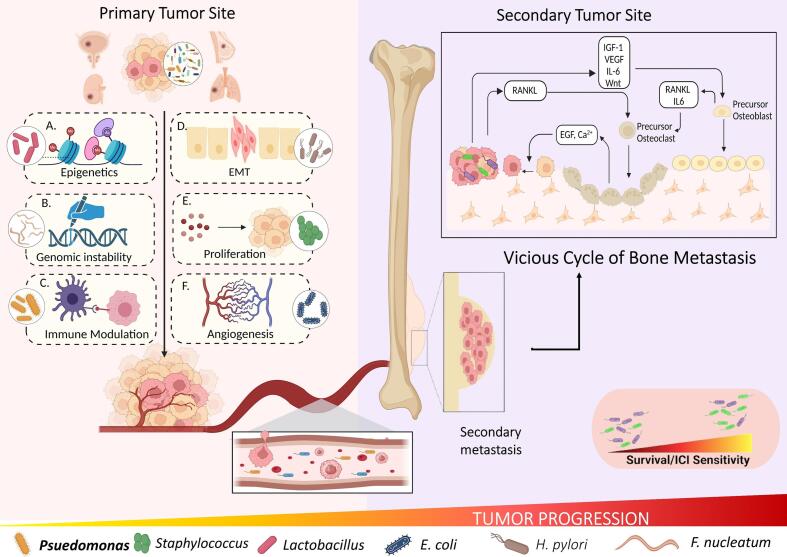
Microbial inducers of carcinogenesis. Genotoxin-producing bacteria E. coli and B. fragilis drive carcinogenesis by inducing direct DNA damage and cyclooxygenase (COX)-2 mediated signaling, respectively. H. pylori produces cytotoxin-associated gene A (CagA) bacterial protein which bind to the phosphorylated Src homology 2 (SH2) domain leading to an increase in ERK levels driving oncogenesis. The virulence factors produced by F. nucleatum and S.enterica induce activation of β-catenin which upon translocation to the nucleus results in overexpression of a variety of genes involved in cell proliferation, such as Myc, CycinD1, Wnt etc.
To begin with, altered GM profiles in osteosarcoma mice models provide a proof of concept for establishing a cross talk between the gut microbiome and bone malignancy. The OS mouse models exhibited higher alpha diversity than controls, characterized by a significant decrease in the relative abundance of Lachnospiraceae spp. and an increased Firmicutes/Bacteroidetes (F/B) ratio (Le et al., 2023). This shift was largely driven by a decrease in the Bacteroides spp. and a simultaneous increase in Firmicutes specifically Roseburia spp. and Akkermansia. The increased Roseburia population likely triggers butyrate production in the gut subsequently enhancing mucin production by colonocytes which upon degradation by Akkermansia spp. enhances the alpha diversity thereby establishing a favorable gut environment as a response to this malignant condition. This corroborates with previous findings showing a considerable association of high alpha diversity with patients' response to chemotherapy. In melanoma mouse models, the GM has been recognized to play a pivotal role in restraining the progression of tumor-induced bone metastasis, as it regulates the immune cells' trafficking to the bone. To investigate the role of the GM, the resident microbes were ablated to mimic dysbiosis using broad-spectrum antibiotics, and B16-F10 melanoma cells were administered. Antibiotic induced-GM depletion significantly enhanced intraosseous tumor growth and osteolysis as revealed by increased tumor burden and decreased perforation number, volume, thickness, and ectopic bone volume (BV) compared to mice with intact GM. This finding was in corroboration with earlier literature demonstrating enhanced breast cancer growth upon antibiotic manipulation of the GM in mice (Le et al., 2023).
Furthermore, the antibiotic-induced GM depletion also prevented intestinal egress of anti-tumorigenic natural killer (NK and type 1 T helper (Th1)) cells to the bone marrow. In the case of breast cancer, the GM analysis revealed that certain microbial species were particularly abundant in patients with breast cancer bone metastasis such as Streptococcus, Campylobacter, Moraxellaceae, etc. These patients also lack the presence of certain beneficial bacterial species (Megamonas and Akkermansia) (Wenhui et al., 2022). Another shotgun metagenomic analysis by Zhu and colleagues shows the relationship between GM dysbiosis and breast cancer development in post-menopausal patients, in which it was observed that E. coli, Klebsiella, Prevotella amnii, Enterococcus gallinarum, Actinomyces sp., etc. were enriched in post-menopausal-breast cancer (PMOB) patients (Zhu et al., 2018). Additionally, a mouse model study showed that administering antibiotics led to faster tumor growth that is anticipated to be caused due to mast cell homing and/or function (McKee et al., 2021). Breast cancer commonly metastasizes to the bone as mentioned previously, which has a rich environment of calcium and minerals. It can thus be hypothesized that GM dysbiosis leads to breast cancer development which is eventually metastasized to bones.
16S rRNA sequencing reveals gut dysbiosis in prostate cancer patients leading to tumor progression and its metastasis. It is observed that Ruminococcus species is extensively upregulated in human clinical samples as well as mouse models of prostate cancer (Liu et al., 2021). The Fecal microbial transplantation of castration-resistant prostate cancer mice feces into TRAMP (Transgenic adenocarcinoma of the mouse prostate) mice resulted in an enhanced tumor progression. The gut microbiota dysbiosis is associated with prostate cancer progression potentially by the “LFCAT1-DNA repair pathway” (Liu et al., 2021). The bacterial sps i.e. Ruminococcus, Alistipes, and Phascolarctobacterium also release certain GAMs (Gut-associated metabolites) such as Short-chain Fatty Acids (SCFAs) like acetate and butyrate that regulate prostate cancer progression by many pathways. These SCFAs induce autophagy in cancer cells followed by NF-kB and MAP-kinase pathway activation along with macrophages subsequently promoting tumor growth (Liu et al., 2023b). Androgen deprivation in mice and humans has been found to promote expansion of defined commensal microbiota triggering the onset of castration resistant prostate cancer (Terrisse et al., 2022). These findings suggest that either extraneous supplementation of androgen or use of some GM modulation strategies to increase the number of androgen synthesizing flora may contribute to bone metastases.
In the aforementioned studies, the common thread of gut dysbiosis alongside its association with bone metastasis either directly or via immune modulation, implies a potential mechanistic link between the two. In forthcoming sections, we will discuss specific immune components governing bone metastasis that can be targeted via microbiological interventions, further establishing an interplay between GM and bone metastasis.
4.2. Intratumoral microbiome and metastases
Intratumoral microbiome (ITM), characterized by its dynamic and diverse composition, has recently been recognized as an integral and intrinsic component of the tumor tissue instead of an incidental presence. The tumor microenvironment owing to the hypoxic, nutrient-enriched, and immunosuppressive conditions, provides a favorable niche for microbial colonization (Lu et al., 2024; Yang et al., 2023). Destructed mucosal barriers, dysbiotic gut, adjacent tissue migration, and hematogenic invasion are considered to be the sources of microorganisms in tumor tissues wherein these organisms either directly or indirectly influence cancer initiation and progression or modulate the immune response by different signaling pathways (Cao et al., 2024; Yang et al., 2023; Yousefi et al., 2024).
ITM directly or via secreted metabolites affects epigenetic modifications that aberrantly activate oncogenes or suppress tumor suppressor genes thereby affecting cancer progression (Wu et al., 2024). For instance, SCFAs modulate the activity of histone acetylase and deacetylase, subsequently triggering genomic epigenetic changes. As a point of evidence, a study by Ma et al. suggested that butyrate promotes lung cancer metastasis and decreases the likelihood of recurrence-free survival in patients. This was evidenced by a higher abundance of butyrate and butyrate-producing microbiota e.g., Roseburia in the tumor tissues of individuals with reoccurring lung cancer than the non-recurring group. Microbiome-derived butyrate, when present in low concentration in the tumor site, inhibited the expression of HDAC, a common deacetylating agent. This inhibition promoted histone H3K27 acetylation at the H19 promoter which upon activation instigates downstream factors viz., MMP15, pivotal in regulating tumor metastasis and progression. Additionally, butyrate promoted secretion of the various cytokines notably IL-10 and IL-13, thereby triggering polarization of M2 macrophages. This facilitates immune evasion, promotes angiogenesis, and remodels local tissue, consequently establishing a tumorigenic milieu. Intriguingly, this metabolite elicited an upregulation in the expression of genes crucially implicated in cell adhesion and connection e.g., neuroactive ligand-receptor interaction and extracellular matrix interaction thereby promoting metastasis (Ma et al., 2024). Conclusively, this study provides a cross-talk between intratumor microbiome and cancer metastasis. Along similar lines, L johnsonii in collaboration with C. sporogenes converts dietary tryptophan to Indole-3-propionic acid that promotes the infiltration of CD8+ T cells into tumor sites and enhances the H3K27 acetylation in the Tcf7 gene thereby modulating the stemness of these cells and facilitating the generation of progenitor exhausted CD8+ T cells (Tpex) subsequently ameliorating the efficacy of anti-PD-1 immunotherapy (Jia et al., 2024b). The above two studies indicate the potential of intratumoral microbiota-derived metabolites to be utilized as drug adjuvants for patients receiving personalized cancer immunotherapy.
Besides, ITM interacts with the immune system and can foster an immunosuppressive microenvironment and immune cell inactivation. F. nucleatum colonization in breast cancer tissues, facilitated by high Gal-GalNAc levels on breast cancer cells, supports metastasis by suppressing the accumulation of tumor-infiltrating T cells (Parhi et al., 2020). Alternatively, ITM can interact with pattern recognition receptors in the tumor microenvironment thereby activating inflammatory pathways and cascade. The circulating tumor cells are characterized by the presence of microorganisms suggesting the ability of ITM together with the host tumor cells; to travel through the circulation system to the distal organ wherein these can alter internal features of oncocytes and the external conditions to establish a microenvironment conducive to metastasis. With the progression of metastatic growth, the microbiota is influenced by the distal organ environment, which explains the different ITM profiles in the primary tumor tissue and the site of metastasis. Moreover, ITM can modulate the cellular cytoskeletal system and significantly relieve the mechanical stress-induced contractile forces on the tumor cells thereby enhancing tumor cells' viability during metastasis. As a point of evidence, Staphylococcus, Lactobacillus, and Streptococcus residing in breast cancer tissues are known for restraining the RhoA/ROCK signaling pathway to remodel the actin skeleton, increasing tumor cells' resistance to fluid shear stress that is commonly experienced by circulating cells after intravasation responsible for their apoptosis (Fu et al., 2022). A similar observation has been made with respect to colorectal cancer and could hold for other cancer types as well. Certain microorganisms can promote cancer cell adhesion to endothelial cells by triggering NF-κB signaling pathway and upregulating intercellular adhesion molecule (ICAM)-1. Recent studies have unveiled a positive association between tumor microbial diversity and infiltrating neutrophils and macrophages suggesting that bacterial colonization within the tumor tissues contributes to an immunosuppressive environment by establishing neutrophil extracellular traps (NET)-based shielding of tumor cells from cytotoxic killing (Battaglia et al., 2024; Blake et al., 2024).
Furthermore, ITM-derived metabolites have a significant impact on tumor immunity and response to immune checkpoint inhibitors (ICI) therapy (Table 2). As a point of evidence, the presence of Clostridiales derived trimethylamine N-oxide in the breast cancer tissues triggered gasdermin E mediated pyroptosis in tumor cells via activation of endoplasmic reticulum stress kinase PERK (Wang et al., 2022a). Another in-vivo experiment unveiled that Lactobacillus reutri (LR) colonizes the tumor microenvironment and via secretion of an immunomodulatory, tryptophan catabolite i.e., indole-3-aldehyde drives spontaneous antitumor immunity and potentiates ICI therapy (Bender et al., 2023b). I3A being an aryl hydrocarbon receptor (AhR) agonist, directly acts upon CD8+ T cells and promotes IFN-γ production. It was reported that I3A-induced immunity was constrained to the tumor microenvironment, thereby suggesting the importance of the local environment in governing the sensitivity of tumor-infiltrating CD8+ T cells to type 1 CD8+ T cells (Tc1) cell-promoting factors (I3A in this case). Additionally, LR treatment elevated the recruitment of CD8+ T cells to the tumor site by promoting the expression of Ccl5 and Ccl4 genes. I3A simultaneously increased the cytotoxic granzyme B production in the recruited CD8+ T cells via the upregulation of essential transcription factors Blimp1 and lfng. Combinatorial treatment including LR along with anti-PD-L1 or anti-CTLA-4 therapy exhibited pronounced cytotoxic T-cell response in the tumor (Bender et al., 2023b) microenvironment and better control than the ICI therapies given individually. Lastly, I3A was found in higher abundance in the ICI responder melanoma patients thus indicating that the metabolites derived from intratumoral microbiota have the potential to serve as biomarkers for predicting ICI sensitivity (Fig. 5) (Bender et al., 2023b).
Table 2.
The ability of microbial species to modulate anti-tumor responses and bone homeostasis.
| Microorganism | Cancer targeted | Impact on ICI therapy/anti-tumor response |
Additional role in bone health/homeostasisa | References | |
|---|---|---|---|---|---|
| Immunotherapy given | MOA of ICI promotion/anti-tumor activity | ||||
| Bifidobacteria | Breast | Anti-PD-1 therapy | Increased NK cell tumor infiltration, decreased production of pro-tumor macrophages | Suppresses differentiation and functional activity of RANKL-induced osteoclaststs | (Di Modica et al., 2021; Kim et al., 2021; Sapra et al., 2022) |
| Lactobacillus | Breast | anti-PD-1/PD-L1 | Indole acetic acid production and CD8+ T cell activation via AhR signaling | Improve osteogenesis and inhibit osteoclastogenesis | (Peng et al., 2020; Sapra et al., 2021) |
| Prevotella | Prostate | – | Decrease levels of androgens and delayed onset of cancer | Inhibits osteoclastic bone resorption and slows down bone loss in PMO | (Chen et al., 2023; Fujita et al., 2023; Lee et al., 2014; Pernigoni et al., 2021; Ponzetti and Rucci, 2019) |
| Akkermansia | Lung cancer | – | Enhanced CD8+ cytotoxicity, granzyme and IFN production, supressed PDL-1 expression | Increase in osteoblast population and improves bone quality | (Keshavarz Azizi Raftar et al., 2021; Xu et al., 2024) |
PD-1: Programmed Cell Death Protein 1, PD-L1: Programmed Cell Death Ligand 1, DC: Dendritic cell, AhR: Aryl hydrocarbon receptor, IL: Interleukin, pTregs: Peripherally derived Tregs, IFN: Interferon, MDSCs: Myeloid-derived suppressor cells, RANK-L: Receptor activator for nuclear factor kappa-B ligand, PMO: Post-menopausal osteoporosis.
This column is unrelated from the cancer-studies mentioned in rest of the columns of the table but instead it includes independent studies demonstrating the contributory role of corresponding bacterial species in maintaining bone homeostasis. Thereby suggesting the prospect of using these bacterial species for managing the metastasis of the primary malignancies like breast and prostate to the bone.
Fig. 5.
Intratumoral microbiota governing cancer progression: Microbiota residing within the tumor tissues affect the cancer progression by several mechanisms A. Epigenetic modifications involving histone acetylation; B. Genetic modification; C. Immunomodulation; D. Epithelial to Mesenchymal transition; E. Increased cancer cell proliferation; F. Increased Angiogenesis. Either of these mechanisms individually or collectively, allows the tumor cells to metastasize which is usually to bone in case of Breast, Prostate, Lung and Renal cancer. Within the bone, microbiome along with the tumor cells travel to the site of secondary tumor and create microenvironment conducive for metastasis. The translocated microbiota in conjunction with the resident microbiota of that tissue decide the survival of the patient and their response to immune checkpoint inhibitors (ICI) therapy.
While the aforementioned studies may not directly align with bone metastasis, we took into consideration the malignancies with a higher likelihood of metastasizing to bone as the former is still unexplored. This broader perspective will facilitate the extrapolation of relevant principles and methodologies for better understanding and management of bone metastasis. By examining analogous scenarios and identifying how the Intratumoral microbiome interacts with the osteo-immune system, we stand to deepen our understanding of the disease progression and concurrently design a robust framework for future investigations aimed at identifying novel therapeutic targets and strategies.
5. Targeting “microbiome-immune-bone axis” in metastases
The conventional treatment for primary bone cancer involves surgical resection followed by chemotherapy and radiotherapy, but these treatments have limited efficacy against secondary bone cancers like those from the breast and prostate (Ferguson and Turner, 2018). Currently, anti-resorptive agents like bisphosphonates and denosumab are used to prevent skeletal-related events (SREs) in patients with bone metastases (Hofbauer et al., 2021). Despite advancements, managing bone metastases remains challenging. However, recent research suggests the potential of GM in improving the cancer treatment outcomes. Clinical and pre-clinical studies provide strong evidence that modulating the gut microbiome to reverse the established microbial dysbiosis can improve patient outcomes. This can be achieved by developing novel therapeutic strategies including fecal dietary interventions such as prebiotics probiotics, etc. (Knisely et al., 2023).
Probiotics, like Lactobacillus species have shown anticancer effects in murine breast cancer model by immune response modulation via cytokines viz., IFNγ, TGF-β etc., (Maroof et al., 2012). Besides, Lactobacillus rhamnosus GG (LGG) supplementation in sex-steroid deficient female mice improved the intestinal permeability and simultaneously reduced the levels of osteoclastogenic cytokines (Li et al., 2016a). Lactobacillus reutri have been shown to promote anti-tumor immunity in breast cancer by promoting interferon-γ-producing CD8+T cells and its derived tryptophan metabolite was demonstrated to enhance ICI therapy (Bender et al., 2023a, Bender et al., 2023b). Taken together, these studies present the prospect of extrapolating these benefits of probiotics for managing bone metastasis.
Prebiotics, such as oligofructose and inulin, promote beneficial bacteria growth while reducing inflammation and cell proliferation linked to colorectal cancer. Rivera-Huerta et al. demonstrated that inulin-type fructans (ITF), derived from chicory and Mexican blue agave has a significant effect on bone metrics and gut health. Their findings unveiled that ITF supplementation enhanced calcium absorption, which holds significant implications for bone health. Furthermore, following ITF therapy, examinations of bone densitometry showed a noteworthy improvement, indicating possible advantages for bone strength and density. The significant reduction in TNFα levels, a key proinflammatory cytokine, coupled with elevated IL-10 levels following ITF therapy, underscores its potential in cancer treatment. Given the intricate interplay between inflammation and cancer progression, the anti-inflammatory properties of ITF could extend to bone metastasis management (Rivera-Huerta et al., 2017).
Postbiotics, derived from beneficial gut bacteria, have shown potential in cancer therapy. Compounds like butyrate and tributyrin induce tumor cell apoptosis, while hydrogen sulphide provides cytoprotective effects. SCFAs, produced by gram-positive, gut-resident bacteria are presumed to mediate bone homeostasis by restoring IGF-1 levels (Yan et al., 2016). IGF-1 has been hypothesized to play a crucial role in mediating DTCs' interaction with the bone microenvironment in various types of cancers e.g., neuroblastoma, breast cancer and prostate cancer (Rieunier et al., 2019). This hypothesis is supported by decreased bone metastases in mice BC models transfected with 486STOP (dominant negative IGF-1R). Similarly, non-obese diabetic-severe combined immunodeficient (NOD-SCID) mice implanted with human adult bone and injected with MDA PCa 2b cells, exhibited decreased PCa induced bone metastases upon treatment with anti-IGF-1R antibody (Goya et al., 2004). Conclusively, IGF-1R appears as a promising target for developing therapeutic interventions for managing bone metastasis.
Altogether, the above-mentioned studies, alongside the existing literature on microbial interventions in cancer metastasis (Table 3 & Fig. 6), lay a compelling foundation suggesting the commendable role of microbiota in modulating immune cells' behaviour or influencing bone cells involved in bone remodeling.
Table 3.
Microbiological-based interventions in treating various cancer types.
| Nutritional supplement | Example | Cancer type targeted | Mode of action | Reference |
|---|---|---|---|---|
| Probiotics | Lactobacillus species | Murine breast cancer model | Activate NK cells. | (Aragón et al., 2015; Hibberd et al., 2017; Li et al., 2016b; Sapra et al., 2021, Sapra et al., 2022; Zitvogel et al., 2017) |
| Promote dendritic cell maturation. | ||||
| Release iron-scavenging molecules. | ||||
| Lactobacillus acidophilus and Bifidobacterium animalis | Colon Tumors | Modify the microbiota favoring butyrate-producing bacteria. | ||
| Bifidobacterium longum | Colorectal Cancer | Butyrate in turn, inhibits cell growth, induces apoptosis, and diminishes inflammation | ||
| Lactobacillus rhamnosus GG (LGG) | Colorectal Cancer | Suppresses RANKL-induced osteoclastogenesis | ||
| Enhances gut permeability. | ||||
| Reduces Osteoclastogenic cytokines | ||||
| Prebiotics | Non-digestible polysaccharides | Ulcerative colitis patients with a risk of colorectal cancer | Increase SCFAs and SCFA receptor expression, reducing inflammation and cell proliferation. | (Hu et al., 2016; Li et al., 2020; Taper and Roberfroid, 2000; Zitvogel et al., 2017) |
| Oligofructose or Inulin | Breast, Colon and Lung Cancer | Enhance the curative properties of many cytotoxic medications, thus improving chemotherapy effectiveness. | ||
| Mucin or inulin polysaccharides | Melanoma mice models | Alter tumor microenvironment leading to tumor ablation. | ||
| Synbiotics | Lactobacillus casei spp. along with dextran | Colorectal Cancer | Increased NK cell activity. | (Ogawa et al., 2005; Rafter et al., 2007; Saito et al., 2019) |
| Lower production of inflammation mediators like COX-2, STAT3, IL-6. | ||||
| Decreased cell proliferation and improved mucosal structure. | ||||
| Postbiotics | Tributyrin | Trigger autophagy, preventing tumor cells from metastasizing. | (Vrzáčková et al., 2021) | |
| Indole-3-Lactic acid | Colorectal Cancer | epigenetically induced increased IL12a production in DCs, subsequently enhancing CD8+ T cell function | ||
| H2S | Causes Tissue damage at very high concentrations yet at low level, acts as a cytoprotective agent. |
NK: Natural Killer, RANK-L: Receptor activator for nuclear factor kappa-B ligand, SCFA: Short chain fatty acids, COX-2: Cyclooxygenase-2, STAT3: Signal transducer and activator of transcription 3, IL: Interleukin, DC: Dendritic cells.
Fig. 6.
Modulation of gut microbiota (GM) for therapeutic purposes. Prebiotics and probiotics, including the gut-associated short-chain fatty acids (SCFAs) exert anti-cancer effects by preventing the epithelial breach by pathogenic bacteria. This is mediated by activation of the immune machinery to enhance the integrity of gut barrier function by release of interleukin (IL)-22 from innate lymphoid cell (ILC)3s. Activation of dendritic cells (DC)s stimulate Th17 cells to produce IL-17A which has an anti-tumor effect. SCFAs also play a crucial role in maintaining luminal pH and mucin secretions, keeping up with the epithelial integrity. Synbiotics and postbiotics, which include bacterial cell lysates, enzymes, vitamins, SCFAs, and cell wall fragments, also have anti-cancer effects resulting in tumor regression.
6. Challenges and future perspectives
The above-mentioned studies highlight a striking variability in effects observed with microorganisms and their metabolites, which intricately depends upon the specific bacterial strain and cancer type taken into consideration. Notably, butyrate, recently recognized for its role in maintaining bone health, serves as a prime example of this variability. While it markedly attenuated prostate cancer progression by inducing autophagy, its presence within the lung tumor tissues paradoxically facilitated metastatic dissemination via epigenetic modifications, both of which have a substantial risk of bone invasion. Furthermore, it has been observed that at low concentrations, butyrate significantly promoted cancer cell proliferation, whereas at high concentrations, it unexpectedly suppressed cell proliferation (Oncel et al., 2024). This divergence underscores a complex interplay between microbial-derived metabolites, their localization, cancer type, and their progression. Furthermore, the bacterial composition and richness of different cancer types are highly heterogeneous. Given this heterogeneity of microbiota and their divergent roles, it is crucial to acknowledge that the metabolites' effects are highly context-dependent, and benefits observed in one type of malignancy may not seamlessly extrapolate to another. Though recent studies have started to recognize the presence of microorganisms in tumor sites as well, the gut still stands to be the biggest reservoir for colonising microorganisms with access to all the other organs of the body. Thus, there is a high possibility that the GM and immune system may interact with intratumor microbiota to determine cancer progression. Drawing from these points, it can be concluded that the effectiveness of microbiota-focused treatments significantly depends on the interplay between the functioning of the gut and tumor-resident microbiota with the immune system and their influence on the local and systemic environment. Investigating these facets concurrently would unveil novel approaches for developing prevention and treatment modalities to manage bone metastasis. The ideal course of action is to investigate microbiota-based techniques targeting both gut and tumor microbes in larger patient groups (Wang et al., 2023).
An in-depth understanding of the interplay between intestinal and intratumor microbiomes in conjunction with the immune system holds the promise to stratify patients into responders and non-responders. Given the heterogeneity of the microbiome, this approach will allow for the development of personalized therapeutic interventions tailored to individual microbiome profiles. Microbiological-based interventions, including probiotics, prebiotics, and postbiotics, will assist in altering gut or intra-tumor microbiota for reverting non-responder patients to a responsive state and enhancing the efficacy of administered therapies. Considering all these aspects, we propose leveraging microbiota-based interventions to reconfigure the immune environment in bone tissue. However, the microbiomes, being complex ecosystems, are rapidly evolving, and so are the tumor cells and the tumor microenvironment. Hence, it is difficult to effectively comprehend the intricate relationship between tumor and GM in the context of cancer progression. The efforts are further complicated by the accessibility constraints associated with low biomass microbial components and ethical challenges associated with handling human tissues. The utilization of interdisciplinary strategies involving bioinformatics and multi-omics approach such as metagenomics, meta-transcriptomics, and metabolomics, will foster a deeper understanding of the mechanisms underlying microbiome-tumor interactions. In conjunction with computational approaches for high-dimensional analyses of the established bacterial genera in the tumor microenvironment on a large scale, advanced techniques involving multiple integrated imaging and analysis methods, spatial and cellular localization of the tumor-resident microorganisms will deepen our comprehension of host-microbiome interactions (Battaglia et al., 2024; Cao et al., 2024; Fu et al., 2022). The incorporation of synthetic biology techniques will enable the rational engineering of microorganisms for the prevention and treatment of metastasis. Furthermore, artificial intelligence and machine learning approaches can be explored for designing models trained with large, multidimensional datasets obtained from the muti-omics and sequencing studies. This will streamline the patient stratification based on state-specific microbial signatures, thereby precisely and non-invasively predicting the patients' sensitivity to immunotherapy. Furthermore, machine learning platforms will assist in the expansion of public repositories that will provide more human microbiome-omics data and enable researchers to identify new associations between the microbiome and malignancy (Novielli et al., 2024). Owing to its ability to provide, high-confidence taxonomic and functional characterization of tumor and gut resident bacteria, this approach will bring about a fundamental paradigm shift in the field of oncology from observational association studies to experimental causal inference and clinical intervention (Battaglia et al., 2024; Li et al., 2022). It is anticipated that machine learning and artificial intelligence will instigate the development of microbe-based or microbe-directed clinical interventions, especially when used in combination with emerging technologies such as cultivation-free genome sequencing and the manipulation of gut and tumor microbial genes. We believe that this approach holds great promise for the future of immune-onco-microbiome research (Giuffrè et al., 2023). With the standardization of protocols, development of robust sequence analysis strategies, and development of reliable databases, these strategies hold the potential to revolutionize the management of bone metastatic cancer. This promotes an immunostimulatory landscape while strategically regulating the bone remodeling process. Diverging from conventional research paradigms that often focus on singular aspects, this novel approach utilizing microorganisms concurrently targets immune modulation and bone remodeling, ultimately maintaining skeletal stability amidst metastatic cancers. The convergence of these scientific shreds of evidence and clinical insights represents an opportunity for a paradigm shift in managing bone metastasis. Exploring combination therapy strategies based on microbial interventions to improve the clinical effect will provide promising research direction.
7. Conclusion
In a nutshell, the increased propensity of bone metastasis in breast and prostate cancer patients with dysbiotic gut, alongside altered GM profiles in osteosarcoma mouse models and increased bone metastasis in melanoma mice models with depleted GM, shed light on the intriguing interplay between gut microbiota and bone metastasis. Dysbiotic gut could potentially foster an environment conducive to the proliferation and survival of metastatic tumor cells within the bone via multiple mechanisms e.g., regulation of critical immunological components including IGF-1 levels, IL-6, Tregs, etc. Besides, tumor tissues harbor distinct microbial communities that are either the inherent residents of that organ or originate from different sources e.g., disrupted mucosal barriers. As an integral part of tumor tissues, these microorganisms significantly affect cancer physiology via various mechanisms involving epigenetic modifications, immune evasion, etc. Not only do they modulate cancer progression, but also have the potential to influence patients' sensitivity to immunotherapies. Since majority of the crucial pathways are closely linked to the immune system components, in the crosstalk between the human microbiome and metastatic environment, one thing that stays constant is the osteoimmune framework linking bone metastases and microbiome. Identifying this convoluted connection will provide important information about possible treatments for bone metastases. The integration of microbial interventions with immunotherapy or chemotherapy has the potential to navigate drug resistance and augment anticancer efficacy. The existing studies offer a theoretical framework that requires the utilization of similar approaches to identify the individual bacterial mediators and metabolites involved in osteo-carcinogenesis and delineate their interaction with osteometabolites and osteoimmune systems to subsequently decipher the cumulative role of gut and tumor microbiota on bone metastasis. This field has immense flourishing potential yet interdisciplinary comprehensive approaches are required for further exploration. Transformative therapeutic modalities can be established by fostering synergy across diverse fields which can revolutionize the landscape of bone metastasis.
List of abbreviations
- ICI
immune check Inhibition
- NET
neutrophil extracellular traps
- ITM
intra-tumor microbiota
- ICAM
intercellular adhesion molecule
- GM
gut microbiota
- IOM
immuno-onco-microbiome
- OS
osteosarcoma
- TP53
tumor protein 53
- RB1
retinoblastoma protein
- RECQL4
RecQ like helicase 4
- BLM
bloom syndrome gene
- WRN
Werner syndrome ATP-dependent helicase
- EWS
Ewing sarcoma
- CS
chondrosarcoma
- EXT
exostosin glycosyltransferase
- IDH
isocitrate dehydrogenase
- CXCR4
C-X-C chemokine receptor 4
- MMP
matrix metalloproteinase
- CTGF
connective tissue growth factor
- FGF5
fibroblast growth factor
- IL
interleukin
- OPN
osteopontin nuclear factor kappa-Β ligand (RANKL)
- ET-1
endothelin-1
- BMP
bone morphogenetic proteins
- GDF15
growth differentiation factor 15,
- MDSCs
myeloid-derived suppressor cells
- ILCs
innate lymphoid cells
- (TGF)-b
transforming growth factor
- COX2
cyclooxygenase 2
- IDO1
indoleamine 2,3-dioxygenase
- PTHrP
parathyroid hormone-related protein
- VEGF
vascular endothelial growth factor
- MCSF
macrophage colony-stimulating factor
- BMMs
bone-marrow-associated macrophages
- CCL2
chemokine (C-C motif) ligand-2
- DT
diphtheria toxin
- GF
germ-free
- ConvR
conventionally raised
- IGF
insulin growth factor
- TNF
tumor necrosis factor
- SCFAs
short-chain fatty acids
- HDACs
histone deacetylases
- GPCR
G-protein-coupled receptors
- NFaTc
nuclear factor of activated T cells
- IBD
inflammatory bowel disease
- CDT
cytolethal distending toxin
- LCA
lithocholic acid
- BV
bone volume
- PMOB
post-menopausal-breast cancer
CRediT authorship contribution statement
Shreya Kapoor: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation. Muskan Gupta: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Leena Sapra: Writing – review & editing, Methodology, Investigation, Formal analysis, Conceptualization. Taranjeet Kaur: Writing – review & editing. Rupesh K. Srivastava: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization.
Funding
This work was financially supported by projects: DBT (BT/PR41958/MED/97/524/2021), ICMR (61/05/2022-IMM/BMS) and an Intramural Grant (AC-73) sanctioned to RKS.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
SK, MG, LS, TK, and RKS acknowledge the Department of Biotechnology, AIIMS, New Delhi-India for providing infrastructural facilities. SK thank DBT for the research fellowship. MG and TKS thank Department of Biotechnology for the post-graduate fellowship. LS thanks UGC for research fellowship. Figures are created with the help of BioRender.
Data availability
No data was used for the research described in the article.
References
- Amary M.F., Bacsi K., Maggiani F., Damato S., Halai D., Berisha F., Pollock R., O’Donnell P., Grigoriadis A., Diss T., Eskandarpour M., Presneau N., Hogendoorn P.C., Futreal A., Tirabosco R., Flanagan A.M. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J. Pathol. 2011;224:334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- Aragón F., Carino S., Perdigón G., de Moreno de LeBlanc A. Inhibition of growth and metastasis of breast cancer in mice by milk fermented with Lactobacillus casei CRL 431. J. Immunother. 2015;38:185–196. doi: 10.1097/CJI.0000000000000079. [DOI] [PubMed] [Google Scholar]
- Balamuth N.J., Womer R.B. Ewing’s sarcoma. Lancet Oncol. 2010;11:184–192. doi: 10.1016/S1470-2045(09)70286-4. [DOI] [PubMed] [Google Scholar]
- Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Battaglia T.W., Mimpen I.L., Traets J.J.H., van Hoeck A., Zeverijn L.J., Geurts B.S., de Wit G.F., Noë M., Hofland I., Vos J.L., Cornelissen S., Alkemade M., Broeks A., Zuur C.L., Cuppen E., Wessels L., van de Haar J., Voest E. A pan-cancer analysis of the microbiome in metastatic cancer. Cell. 2024;187:2324–2335. doi: 10.1016/j.cell.2024.03.021. e19. [DOI] [PubMed] [Google Scholar]
- Bender M.J., McPherson A.C., Phelps C.M., Pandey S.P., Laughlin C.R., Shapira J.H., Medina Sanchez L., Rana M., Richie T.G., Mims T.S., Gocher-Demske A.M., Cervantes-Barragan L., Mullett S.J., Gelhaus S.L., Bruno T.C., Cannon N., McCulloch J.A., Vignali D.A.A., Hinterleitner R., Joglekar A.V., Pierre J.F., Lee S.T.M., Davar D., Zarour H.M., Meisel M. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. 2023;186:1846–1862. doi: 10.1016/j.cell.2023.03.011. e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M.J., McPherson A.C., Phelps C.M., Pandey S.P., Laughlin C.R., Shapira J.H., Medina Sanchez L., Rana M., Richie T.G., Mims T.S., Gocher-Demske A.M., Cervantes-Barragan L., Mullett S.J., Gelhaus S.L., Bruno T.C., Cannon N., McCulloch J.A., Vignali D.A.A., Hinterleitner R., Joglekar A.V., Pierre J.F., Lee S.T.M., Davar D., Zarour H.M., Meisel M. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. 2023;186:1846–1862. doi: 10.1016/j.cell.2023.03.011. e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk J.R., Bolte L.A., Maltez Thomas A., Lee K.A., Rossi N., Wind T.T., Smit L.M., Armanini F., Asnicar F., Blanco-Miguez A., Board R., Calbet-Llopart N., Derosa L., Dhomen N., Brooks K., Harland M., Harries M., Lorigan P., Manghi P., Marais R., Newton-Bishop J., Nezi L., Pinto F., Potrony M., Puig S., Serra-Bellver P., Shaw H.M., Tamburini S., Valpione S., Waldron L., Zitvogel L., Zolfo M., de Vries E.G.E., Nathan P., Fehrmann R.S.N., Spector T.D., Bataille V., Segata N., Hospers G.A.P., Weersma R.K. Longitudinal gut microbiome changes in immune checkpoint blockade-treated advanced melanoma. Nat. Med. 2024;30:785–796. doi: 10.1038/s41591-024-02803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake S.J., Wolf Y., Boursi B., Lynn D.J. Role of the microbiota in response to and recovery from cancer therapy. Nat. Rev. Immunol. 2024;24:308–325. doi: 10.1038/s41577-023-00951-0. [DOI] [PubMed] [Google Scholar]
- Bolamperti S., Villa I., Rubinacci A. Bone remodeling: an operational process ensuring survival and bone mechanical competence. Bone Res. 2022;10:48. doi: 10.1038/s41413-022-00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H.K., Schiavone K., Gouin F., Heymann M.-F., Heymann D. Biology of bone sarcomas and new therapeutic developments. Calcif. Tissue Int. 2018;102:174–195. doi: 10.1007/s00223-017-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Xia H., Tan X., Shi C., Ma Y., Meng D., Zhou M., Lv Z., Wang S., Jin Y. Intratumoural microbiota: a new frontier in cancer development and therapy. Signal Transduct. Target. Ther. 2024;9:15. doi: 10.1038/s41392-023-01693-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zhou G., Han H., Jin J., Li Z. Causal effects of specific gut microbiota on bone mineral density: a two-sample Mendelian randomization study. Front. Endocrinol. (Lausanne) 2023;14 doi: 10.3389/fendo.2023.1178831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Lei J., Mou H., Zhang W., Jin L., Lu S., Yinwang E., Xue Y., Shao Z., Chen T., Wang F., Zhao S., Chai X., Wang Z., Zhang J., Zhang Z., Ye Z., Li B. Multiple influence of immune cells in the bone metastatic cancer microenvironment on tumors. Front. Immunol. 2024;15 doi: 10.3389/fimmu.2024.1335366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.H., Ro J.Y. The 2020 WHO classification of tumors of bone: an updated review. Adv. Anat. Pathol. 2021;28:119–138. doi: 10.1097/PAP.0000000000000293. [DOI] [PubMed] [Google Scholar]
- Chow W.A. Chondrosarcoma: biology, genetics, and epigenetics. F1000Res. 2018;7:1826. doi: 10.12688/f1000research.15953.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson M.J., Cusack S., O’Sullivan O., Greene-Diniz R., de Weerd H., Flannery E., Marchesi J.R., Falush D., Dinan T., Fitzgerald G., Stanton C., van Sinderen D., O’Connor M., Harnedy N., O’Connor K., Henry C., O’Mahony D., Fitzgerald A.P., Shanahan F., Twomey C., Hill C., Ross R.P., O’Toole P.W. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. 2011;108:4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clambey E.T., McNamee E.N., Westrich J.A., Glover L.E., Campbell E.L., Jedlicka P., de Zoeten E.F., Cambier J.C., Stenmark K.R., Colgan S.P., Eltzschig H.K. Hypoxia-inducible factor-1 alpha–dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl. Acad. Sci. 2012;109 doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- Coleman R.E., Lipton A., Roodman G.D., Guise T.A., Boyce B.F., Brufsky A.M., Clézardin P., Croucher P.I., Gralow J.R., Hadji P., Holen I., Mundy G.R., Smith M.R., Suva L.J. Metastasis and bone loss: advancing treatment and prevention. Cancer Treat. Rev. 2010;36:615–620. doi: 10.1016/j.ctrv.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R.E., Croucher P.I., Padhani A.R., Clézardin P., Chow E., Fallon M., Guise T., Colangeli S., Capanna R., Costa L. Bone metastases. Nat. Rev. Dis. Primers. 2020;6:83. doi: 10.1038/s41572-020-00216-3. [DOI] [PubMed] [Google Scholar]
- Danilin S., Merkel A.R., Johnson J.R., Johnson R.W., Edwards J.R., Sterling J.A. Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. Oncoimmunology. 2012;1:1484–1494. doi: 10.4161/onci.21990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mitri D., Conforti F., Mantovani A. Macrophages and bone metastasis. J. Exp. Med. 2023;220 doi: 10.1084/jem.20222188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Modica M., Gargari G., Regondi V., Bonizzi A., Arioli S., Belmonte B., De Cecco L., Fasano E., Bianchi F., Bertolotti A., Tripodo C., Villani L., Corsi F., Guglielmetti S., Balsari A., Triulzi T., Tagliabue E. Gut microbiota condition the therapeutic efficacy of trastuzumab in HER2-positive breast cancer. Cancer Res. 2021;81:2195–2206. doi: 10.1158/0008-5472.CAN-20-1659. [DOI] [PubMed] [Google Scholar]
- Elaasser B., Arakil N., Mohammad K.S. Bridging the gap in understanding bone metastasis: a multifaceted perspective. Int. J. Mol. Sci. 2024;25:2846. doi: 10.3390/ijms25052846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares J., Fares M.Y., Khachfe H.H., Salhab H.A., Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J.L., Turner S.P. Bone cancer: diagnosis and treatment principles. Am. Fam. Physician. 2018;98:205–213. [PubMed] [Google Scholar]
- Franchi A. Epidemiology and classification of bone tumors. Clin. Cases Miner. Bone Metab. 2012;9:92–95. [PMC free article] [PubMed] [Google Scholar]
- Fu A., Yao B., Dong T., Chen Y., Yao J., Liu Y., Li H., Bai H., Liu X., Zhang Y., Wang C., Guo Y., Li N., Cai S. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022;185:1356–1372. doi: 10.1016/j.cell.2022.02.027. e26. [DOI] [PubMed] [Google Scholar]
- Fujita K., Matsushita M., De Velasco M.A., Hatano K., Minami T., Nonomura N., Uemura H. The gut-prostate axis: a new perspective of prostate cancer biology through the gut microbiome. Cancers (Basel) 2023;15:1375. doi: 10.3390/cancers15051375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrè M., Moretti R., Tiribelli C. Gut microbes meet machine learning: the next step towards advancing our understanding of the gut microbiome in health and disease. Int. J. Mol. Sci. 2023;24:5229. doi: 10.3390/ijms24065229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Hu S., Shan Q., Qin J., Han N., Xie F., Lu H. Bone metastasis in non-small-cell lung cancer: genomic characterization and exploration of potential targets. Ther. Adv. Med. Oncol. 2024;16 doi: 10.1177/17588359241239293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorthi A., Romero J.C., Loranc E., Cao L., Lawrence L.A., Goodale E., Iniguez A.B., Bernard X., Masamsetti V.P., Roston S., Lawlor E.R., Toretsky J.A., Stegmaier K., Lessnick S.L., Chen Y., Bishop A.J.R. EWS–FLI1 increases transcription to cause R-loops and block BRCA1 repair in Ewing sarcoma. Nature. 2018;555:387–391. doi: 10.1038/nature25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goya M., Miyamoto S., Nagai K., Ohki Y., Nakamura K., Shitara K., Maeda H., Sangai T., Kodama K., Endoh Y., Ishii G., Hasebe T., Yonou H., Hatano T., Ogawa Y., Ochiai A. Growth inhibition of human prostate cancer cells in human adult bone implanted into nonobese diabetic/severe combined Immunodeficient mice by a ligand-specific antibody to human insulin-like growth factors. Cancer Res. 2004;64:6252–6258. doi: 10.1158/0008-5472.CAN-04-0919. [DOI] [PubMed] [Google Scholar]
- Greathouse K.L., White J.R., Vargas A.J., Bliskovsky V.V., Beck J.A., von Muhlinen N., Polley E.C., Bowman E.D., Khan M.A., Robles A.I., Cooks T., Ryan B.M., Padgett N., Dzutsev A.H., Trinchieri G., Pineda M.A., Bilke S., Meltzer P.S., Hokenstad A.N., Stickrod T.M., Walther-Antonio M.R., Earl J.P., Mell J.C., Krol J.E., Balashov S.V., Bhat A.S., Ehrlich G.D., Valm A., Deming C., Conlan S., Oh J., Segre J.A., Harris C.C. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol. 2018;19:123. doi: 10.1186/s13059-018-1501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guder C., Gravius S., Burger C., Wirtz D.C., Schildberg F.A. Osteoimmunology: a current update of the interplay between bone and the immune system. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra L., Guidi R., Frisan T. Do bacterial genotoxins contribute to chronic inflammation, genomic instability and tumor progression? FEBS J. 2011;278:4577–4588. doi: 10.1111/j.1742-4658.2011.08125.x. [DOI] [PubMed] [Google Scholar]
- Guise T.A., Yin J.J., Mohammad K.S. Role of endothelin-1 in osteoblastic bone metastases. Cancer. 2003;97:779–784. doi: 10.1002/cncr.11129. [DOI] [PubMed] [Google Scholar]
- Han Y., You X., Xing W., Zhang Z., Zou W. Paracrine and endocrine actions of bone—the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018;6:16. doi: 10.1038/s41413-018-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Song X., Xiao Z., Zhu G., Gao R., Ni B., Li J. Study on the mechanism of MDSC-platelets and their role in the breast cancer microenvironment. Front. Cell Dev. Biol. 2024;12 doi: 10.3389/fcell.2024.1310442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann M.-F., Lézot F., Heymann D. The contribution of immune infiltrates and the local microenvironment in the pathogenesis of osteosarcoma. Cell. Immunol. 2019;343 doi: 10.1016/j.cellimm.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Hibberd A.A., Lyra A., Ouwehand A.C., Rolny P., Lindegren H., Cedgård L., Wettergren Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017;4 doi: 10.1136/bmjgast-2017-000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer L.C., Bozec A., Rauner M., Jakob F., Perner S., Pantel K. Novel approaches to target the microenvironment of bone metastasis. Nat. Rev. Clin. Oncol. 2021;18:488–505. doi: 10.1038/s41571-021-00499-9. [DOI] [PubMed] [Google Scholar]
- Hu Y., Le Leu R.K., Christophersen C.T., Somashekar R., Conlon M.A., Meng X.Q., Winter J.M., Woodman R.J., McKinnon R., Young G.P. Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats. Carcinogenesis. 2016;37:366–375. doi: 10.1093/carcin/bgw019. [DOI] [PubMed] [Google Scholar]
- Huppert L.A., Green M.D., Kim L., Chow C., Leyfman Y., Daud A.I., Lee J.C. Tissue-specific Tregs in cancer metastasis: opportunities for precision immunotherapy. Cell. Mol. Immunol. 2022;19:33–45. doi: 10.1038/s41423-021-00742-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraheem A. Bone metastasis as the initial presentation of a hidden bronchogenic carcinoma. Cureus. 2022 doi: 10.7759/cureus.28081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva E.A., Grivennikov S.I. Microbiota-driven mechanisms at different stages of cancer development. Neoplasia. 2022;32 doi: 10.1016/j.neo.2022.100829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D., Wang Q., Qi Y., Jiang Y., He J., Lin Y., Sun Y., Xu J., Chen W., Fan L., Yan R., Zhang W., Ren G., Xu C., Ge Q., Wang Lan, Liu W., Xu F., Wu P., Wang Y., Chen S., Wang Liangjing. Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer. Cell. 2024;187:1651–1665. doi: 10.1016/j.cell.2024.02.022. e21. [DOI] [PubMed] [Google Scholar]
- Jia D., Wang Q., Qi Y., Jiang Y., He J., Lin Y., Sun Y., Xu J., Chen W., Fan L., Yan R., Zhang W., Ren G., Xu C., Ge Q., Wang Lan, Liu W., Xu F., Wu P., Wang Y., Chen S., Wang Liangjing. Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer. Cell. 2024;187:1651–1665. doi: 10.1016/j.cell.2024.02.022. e21. [DOI] [PubMed] [Google Scholar]
- Jiang M., Yang Z., Dai J., Wu T., Jiao Z., Yu Y., Ning K., Chen W., Yang A. Intratumor microbiome: selective colonization in the tumor microenvironment and a vital regulator of tumor biology. MedComm (Beijing) 2023;4 doi: 10.1002/mco2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao S., Subudhi S.K., Aparicio A., Ge Z., Guan B., Miura Y., Sharma P. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell. 2019;179:1177–1190. doi: 10.1016/j.cell.2019.10.029. e13. [DOI] [PubMed] [Google Scholar]
- Kang Y., Siegel P.M., Shu W., Drobnjak M., Kakonen S.M., Cordón-Cardo C., Guise T.A., Massagué J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/S1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Keshavarz Azizi Raftar S., Hoseini Tavassol Z., Amiri M., Ejtahed H.-S., Zangeneh M., Sadeghi S., Ashrafian F., Kariman A., Khatami S., Siadat S.D. Assessment of fecal Akkermansia muciniphila in patients with osteoporosis and osteopenia: a pilot study. J. Diabetes Metab. Disord. 2021;20:279–284. doi: 10.1007/s40200-021-00742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Oh R., Park S., Ji G.E., Park M.S., Kim S.-E. Combination therapy of Bifidobacterium longum RAPO with anti-PD-1 treatment enhances anti-tumor immune response in association with gut microbiota modulation. Curr. Dev. Nutr. 2021;5:1131. doi: 10.1093/cdn/nzab061_015. [DOI] [Google Scholar]
- Knisely A., Seo Y.D., Wargo J.A., Chelvanambi M. Monitoring and modulating diet and gut microbes to enhance response and reduce toxicity to cancer treatment. Cancers (Basel) 2023;15:777. doi: 10.3390/cancers15030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustrimovic N., Bombelli R., Baci D., Mortara L. Microbiome and prostate cancer: a novel target for prevention and treatment. Int. J. Mol. Sci. 2023;24:1511. doi: 10.3390/ijms24021511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D., Chambers M.M., Mercado K., Gutowski C.J. Characterization of the gut microbiome in an osteosarcoma mouse model. J. Orthop. Res. 2023;41:2730–2739. doi: 10.1002/jor.25635. [DOI] [PubMed] [Google Scholar]
- Lee K.M., Stott R.T., Zhao G., SooHoo J., Xiong W., Lian M.M., Fitzgerald L., Shi S., Akrawi E., Lei J., Deng S., Yeh H., Markmann J.F., Kim J.I. TGF-β-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur. J. Immunol. 2014;44:1728–1736. doi: 10.1002/eji.201344062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-C., Yang H.-W., Liu C.-J., Lee F., Ko W.-C., Chang Y.-C., Yang P.-S. Unraveling the connections between gut microbiota, stress, and quality of life for holistic care in newly diagnosed breast cancer patients. Sci. Rep. 2023;13 doi: 10.1038/s41598-023-45123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-Y., Chassaing B., Tyagi A.M., Vaccaro C., Luo T., Adams J., Darby T.M., Weitzmann M.N., Mulle J.G., Gewirtz A.T., Jones R.M., Pacifici R. Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Invest. 2016;126:2049–2063. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-Y., Chassaing B., Tyagi A.M., Vaccaro C., Luo T., Adams J., Darby T.M., Weitzmann M.N., Mulle J.G., Gewirtz A.T., Jones R.M., Pacifici R. Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Invest. 2016;126:2049–2063. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Elmén L., Segota I., Xian Y., Tinoco R., Feng Y., Fujita Y., Segura Muñoz R.R., Schmaltz R., Bradley L.M., Ramer-Tait A., Zarecki R., Long T., Peterson S.N., Ronai Z.A. Prebiotic-induced anti-tumor immunity attenuates tumor growth. Cell Rep. 2020;30:1753–1766. doi: 10.1016/j.celrep.2020.01.035. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Luo H., Ji B., Nielsen J. Machine learning for data integration in human gut microbiome. Microb. Cell Factories. 2022;21:241. doi: 10.1186/s12934-022-01973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Zhi, Xia Q., He Y., Li L., Yin P. MDSCs in bone metastasis: mechanisms and therapeutic potential. Cancer Lett. 2024;592 doi: 10.1016/j.canlet.2024.216906. [DOI] [PubMed] [Google Scholar]
- Li Zehua, Xiong W., Liang Z., Wang J., Zeng Z., Kołat D., Li X., Zhou D., Xu X., Zhao L. Critical role of the gut microbiota in immune responses and cancer immunotherapy. J. Hematol. Oncol. 2024;17:33. doi: 10.1186/s13045-024-01541-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang C., Zhang Z., Jiang H. Gut microbiota dysbiosis accelerates prostate cancer progression through increased LPCAT1 expression and enhanced DNA repair pathways. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.679712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang Z., Zhou Y., Yang Y. MDSCs in breast cancer: an important enabler of tumor progression and an emerging therapeutic target. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1199273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhou Q., Ye F., Yang C., Jiang H. Gut microbiota-derived short-chain fatty acids promote prostate cancer progression via inducing cancer cell autophagy and M2 macrophage polarization. Neoplasia. 2023;43 doi: 10.1016/j.neo.2023.100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Wu S., Zhang Y., Xia Y., Liu X., Zheng Y., Chen H., Schaefer K.L., Zhou Z., Bissonnette M., Li L., Sun J. Enteric bacterial protein AvrA promotes colonic tumorigenesis and activates colonic beta-catenin signaling pathway. Oncogenesis. 2014;3:e105. doi: 10.1038/oncsis.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.-Q., Qiao H., Tan X.-R., Liu N. Broadening oncological boundaries: the intratumoral microbiota. Trends Microbiol. 2024 doi: 10.1016/j.tim.2024.01.007. [DOI] [PubMed] [Google Scholar]
- Lucas S., Omata Y., Hofmann J., Böttcher M., Iljazovic A., Sarter K., Albrecht O., Schulz O., Krishnacoumar B., Krönke G., Herrmann M., Mougiakakos D., Strowig T., Schett G., Zaiss M.M. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018;9:55. doi: 10.1038/s41467-017-02490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Z., Hu Y., Guo Y., Liu D. Modulation of bone remodeling by the gut microbiota: a new therapy for osteoporosis. Bone Res. 2023;11:31. doi: 10.1038/s41413-023-00264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Gnanasekar A., Lee A., Li W.T., Haas M., Wang-Rodriguez J., Chang E.Y., Rajasekaran M., Ongkeko W.M. Influence of intratumor microbiome on clinical outcome and immune processes in prostate cancer. Cancers (Basel) 2020;12:2524. doi: 10.3390/cancers12092524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R.-Y., Zhang H., Li X.-F., Zhang C.-B., Selli C., Tagliavini G., Lam A.D., Prost S., Sims A.H., Hu H.-Y., Ying T., Wang Z., Ye Z., Pollard J.W., Qian B.-Z. Monocyte-derived macrophages promote breast cancer bone metastasis outgrowth. J. Exp. Med. 2020;217 doi: 10.1084/jem.20191820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Chen H., Li H., Zheng M., Zuo X., Wang W., Wang S., Lu Y., Wang Jun, Li Y., Wang Jie, Qiu M. Intratumor microbiome-derived butyrate promotes lung cancer metastasis. Cell Rep. Med. 2024;5 doi: 10.1016/j.xcrm.2024.101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo F., Ladeira K., Pinho F., Saraiva N., Bonito N., Pinto L., Gonçalves F. Bone metastases: an overview. Oncol. Rev. 2017 doi: 10.4081/oncol.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani K., Deng D., Lin C., Wang M., Hsu M.L., Zaorsky N.G. Causes of death among people living with metastatic cancer. Nat. Commun. 2024;15:1519. doi: 10.1038/s41467-024-45307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroof H., Hassan Z.M., Mobarez A.M., Mohamadabadi M.A. Lactobacillus acidophilus could modulate the immune response against breast cancer in murine model. J. Clin. Immunol. 2012;32:1353–1359. doi: 10.1007/s10875-012-9708-x. [DOI] [PubMed] [Google Scholar]
- Martin J.W., Squire J.A., Zielenska M. The genetics of osteosarcoma. Sarcoma. 2012;2012:1–11. doi: 10.1155/2012/627254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A.M., Kirkup B.M., Madgwick M., Fowler W.J., Price C.A., Dreger S.A., Ansorge R., Makin K.A., Caim S., Le Gall G., Paveley J., Leclaire C., Dalby M., Alcon-Giner C., Andrusaite A., Feng T.-Y., Di Modica M., Triulzi T., Tagliabue E., Milling S.W.F., Weilbaecher K.N., Rutkowski M.R., Korcsmáros T., Hall L.J., Robinson S.D. Antibiotic-induced disturbances of the gut microbiota result in accelerated breast tumor growth. iScience. 2021;24 doi: 10.1016/j.isci.2021.103012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabello L., Troisi R.J., Savage S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int. J. Cancer. 2009;125:229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novielli P., Romano D., Magarelli M., Di Bitonto P., Diacono D., Chiatante A., Lopalco G., Sabella D., Venerito V., Filannino P., Bellotti R., De Angelis M., Iannone F., Tangaro S. Explainable artificial intelligence for microbiome data analysis in colorectal cancer biomarker identification. Front. Microbiol. 2024;15 doi: 10.3389/fmicb.2024.1348974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Asai Y., Tamai R., Makimura Y., Sakamoto H., Hashikawa S., Yasuda K. Natural killer cell activities of synbiotic Lactobacillus casei ssp. casei in conjunction with dextran. Clin. Exp. Immunol. 2005;143:103–109. doi: 10.1111/j.1365-2249.2005.02975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C., Sjögren K. Osteomicrobiology: a new cross-disciplinary research field. Calcif. Tissue Int. 2018;102:426–432. doi: 10.1007/s00223-017-0336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C., Nigro G., Boneca I.G., Bäckhed F., Sansonetti P., Sjögren K. Regulation of bone mass by the gut microbiota is dependent on NOD1 and NOD2 signaling. Cell. Immunol. 2017;317:55–58. doi: 10.1016/j.cellimm.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Oncel S., Safratowich B.D., Lindlauf J.E., Liu Z., Palmer D.G., Briske-Anderson M., Zeng H. Efficacy of butyrate to inhibit colonic cancer cell growth is cell type-specific and apoptosis-dependent. Nutrients. 2024;16:529. doi: 10.3390/nu16040529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachva M.C., Lai H., Jia A., Rouleau M., Sorensen P.H. Extracellular vesicles in reprogramming of the Ewing sarcoma tumor microenvironment. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.726205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. doi: 10.1016/S0140-6736(00)49915-0. [DOI] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- Parhi L., Alon-Maimon T., Sol A., Nejman D., Shhadeh A., Fainsod-Levi T., Yajuk O., Isaacson B., Abed J., Maalouf N., Nissan A., Sandbank J., Yehuda-Shnaidman E., Ponath F., Vogel J., Mandelboim O., Granot Z., Straussman R., Bachrach G. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020;11:3259. doi: 10.1038/s41467-020-16967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H., Zhang H., Matei I.R., Costa-Silva B., Hoshino A., Rodrigues G., Psaila B., Kaplan R.N., Bromberg J.F., Kang Y., Bissell M.J., Cox T.R., Giaccia A.J., Erler J.T., Hiratsuka S., Ghajar C.M., Lyden D. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer. 2017;17:302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- Peng Z., Cheng S., Kou Y., Wang Z., Jin R., Hu H., Zhang X., Gong J., Li J., Lu M., Wang X., Zhou J., Lu Z., Zhang Q., Tzeng D.T.W., Bi D., Tan Y., Shen L. The gut microbiome is associated with clinical response to anti–PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol. Res. 2020;8:1251–1261. doi: 10.1158/2326-6066.CIR-19-1014. [DOI] [PubMed] [Google Scholar]
- Pernigoni N., Zagato E., Calcinotto A., Troiani M., Mestre R.P., Calì B., Attanasio G., Troisi J., Minini M., Mosole S., Revandkar A., Pasquini E., Elia A.R., Bossi D., Rinaldi A., Rescigno P., Flohr P., Hunt J., Neeb A., Buroni L., Guo C., Welti J., Ferrari M., Grioni M., Gauthier J., Gharaibeh R.Z., Palmisano A., Lucchini G.M., D’Antonio E., Merler S., Bolis M., Grassi F., Esposito A., Bellone M., Briganti A., Rescigno M., Theurillat J.-P., Jobin C., Gillessen S., de Bono J., Alimonti A. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science (1979) 2021;374:216–224. doi: 10.1126/science.abf8403. [DOI] [PubMed] [Google Scholar]
- Piao X.-M., Byun Y.J., Zheng C.-M., Song S.J., Kang H.W., Kim W.T., Yun S.J. A new treatment landscape for RCC: association of the human microbiome with improved outcomes in RCC. Cancers (Basel) 2023;15:935. doi: 10.3390/cancers15030935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetti M., Rucci N. Updates on osteoimmunology: what’s new on the cross-talk between bone and immune system. Front. Endocrinol. (Lausanne) 2019;10 doi: 10.3389/fendo.2019.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafter J., Bennett M., Caderni G., Clune Y., Hughes R., Karlsson P.C., Klinder A., O’Riordan M., O’Sullivan G.C., Pool-Zobel B., Rechkemmer G., Roller M., Rowland I., Salvadori M., Thijs H., Van Loo J., Watzl B., Collins J.K. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007;85:488–496. doi: 10.1093/ajcn/85.2.488. [DOI] [PubMed] [Google Scholar]
- Rieunier G., Wu X., Macaulay V.M., Lee A.V., Weyer-Czernilofsky U., Bogenrieder T. Bad to the bone: the role of the insulin-like growth factor axis in osseous metastasis. Clin. Cancer Res. 2019;25:3479–3485. doi: 10.1158/1078-0432.CCR-18-2697. [DOI] [PubMed] [Google Scholar]
- Rivera-Huerta M., Lizárraga-Grimes V.L., Castro-Torres I.G., Tinoco-Méndez M., Macías-Rosales L., Sánchez-Bartéz F., Tapia-Pérez G.G., Romero-Romero L., Gracia-Mora M.I. Functional effects of prebiotic fructans in colon cancer and calcium metabolism in animal models. Biomed. Res. Int. 2017;2017:1–10. doi: 10.1155/2017/9758982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roato I., Vitale M. The uncovered role of immune cells and NK cells in the regulation of bone metastasis. Front. Endocrinol. (Lausanne) 2019;10 doi: 10.3389/fendo.2019.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C., Stoltzfus K.C., Horn S., Chen H., Louie A.V., Lehrer E.J., Trifiletti D.M., Fox E.J., Abraham J.A., Zaorsky N.G. Epidemiology of bone metastases. Bone. 2022;158 doi: 10.1016/j.bone.2020.115783. [DOI] [PubMed] [Google Scholar]
- Saito Y., Hinoi T., Adachi T., Miguchi M., Niitsu H., Kochi M., Sada H., Sotomaru Y., Sakamoto N., Sentani K., Oue N., Yasui W., Tashiro H., Ohdan H. Synbiotics suppress colitis-induced tumorigenesis in a colon-specific cancer mouse model. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapra L., Dar H.Y., Bhardwaj A., Pandey A., Kumari S., Azam Z., Upmanyu V., Anwar A., Shukla P., Mishra P.K., Saini C., Verma B., Srivastava R.K. Lactobacillus rhamnosus attenuates bone loss and maintains bone health by skewing Treg-Th17 cell balance in Ovx mice. Sci. Rep. 2021;11:1807. doi: 10.1038/s41598-020-80536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapra L., Shokeen N., Porwal K., Saini C., Bhardwaj A., Mathew M., Mishra P.K., Chattopadhyay N., Dar H.Y., Verma B., Srivastava R.K. Bifidobacterium longum ameliorates ovariectomy-induced bone loss via enhancing anti-osteoclastogenic and immunomodulatory potential of regulatory B cells (Bregs) Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.875788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satcher R.L., Zhang X.H.-F. Evolving cancer–niche interactions and therapeutic targets during bone metastasis. Nat. Rev. Cancer. 2022;22:85–101. doi: 10.1038/s41568-021-00406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant A., Deshane J., Jules J., Lee C.M., Harris B.A., Feng X., Ponnazhagan S. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res. 2013;73:672–682. doi: 10.1158/0008-5472.CAN-12-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepich-Poore G.D., Zitvogel L., Straussman R., Hasty J., Wargo J.A., Knight R. The microbiome and human cancer. Science (1979) 2021;371 doi: 10.1126/science.abc4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreiner A.B., Kao J.Y., Young V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015;31:69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui J.A., Seshacharyulu P., Muniyan S., Pothuraju R., Khan P., Vengoji R., Chaudhary S., Maurya S.K., Lele S.M., Jain M., Datta K., Nasser M.W., Batra S.K. GDF15 promotes prostate cancer bone metastasis and colonization through osteoblastic CCL2 and RANKL activation. Bone Res. 2022;10:6. doi: 10.1038/s41413-021-00178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren K., Engdahl C., Henning P., Lerner U.H., Tremaroli V., Lagerquist M.K., Bäckhed F., Ohlsson C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012;27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R.K. Osteoimmunology the i Nexus i between bone and immune system. Front. Biosci. 2018;23:4600. doi: 10.2741/4600. [DOI] [PubMed] [Google Scholar]
- Sun Z., Cai S., Zabkiewicz C., Liu C., Ye L. Bone morphogenetic proteins mediate crosstalk between cancer cells and the tumour microenvironment at primary tumours and metastases (review) Int. J. Oncol. 2020 doi: 10.3892/ijo.2020.5030. [DOI] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Taper H.S., Roberfroid M.B. Nontoxic potentiation of cancer chemotherapy by dietary oligofructose or inulin. Nutr. Cancer. 2000;38:1–5. doi: 10.1207/S15327914NC381_1. [DOI] [PubMed] [Google Scholar]
- Terrisse S., Zitvogel L., Kroemer G. Effects of the intestinal microbiota on prostate cancer treatment by androgen deprivation therapy. Microbial Cell. 2022;9:190–194. doi: 10.15698/mic2022.12.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorkildsen J., Taksdal I., Bjerkehagen B., Haugland H.K., Børge Johannesen T., Viset T., Norum O.-J., Bruland Ø., Zaikova O. Chondrosarcoma in Norway 1990–2013; an epidemiological and prognostic observational study of a complete national cohort. Acta Oncol. (Madr) 2019;58:273–282. doi: 10.1080/0284186X.2018.1554260. [DOI] [PubMed] [Google Scholar]
- Tjalsma H., Boleij A., Marchesi J.R., Dutilh B.E. A bacterial driver–passenger model for colorectal cancer: beyond the usual suspects. Nat. Rev. Microbiol. 2012;10:575–582. doi: 10.1038/nrmicro2819. [DOI] [PubMed] [Google Scholar]
- Tzeng A., Sangwan N., Jia M., Liu C.-C., Keslar K.S., Downs-Kelly E., Fairchild R.L., Al-Hilli Z., Grobmyer S.R., Eng C. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 2021;13:60. doi: 10.1186/s13073-021-00874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umer M., Mohib Y., Atif M., Nazim M. Skeletal metastasis in renal cell carcinoma: a review. Ann. Med. Surg. 2018;27:9–16. doi: 10.1016/j.amsu.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastyan S., Weinberg R.A. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan S., Parida S., Lingipilli B.T., Krishnan R., Podipireddy D.R., Muniraj N. Role of gut microbiota in breast cancer and drug resistance. Pathogens. 2023;12:468. doi: 10.3390/pathogens12030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrzáčková N., Ruml T., Zelenka J. Postbiotics, metabolic signaling, and cancer. Molecules. 2021;26:1528. doi: 10.3390/molecules26061528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang Y., Gao W., Wang B., Zhao H., Zeng Y., Ji Y., Hao D. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ. 2017;5 doi: 10.7717/peerj.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Rong X., Zhao G., Zhou Yifan, Xiao Y., Ma D., Jin X., Wu Y., Yan Y., Yang H., Zhou Yuan, Qian M., Niu C., Hu X., Li D.-Q., Liu Q., Wen Y., Jiang Y.-Z., Zhao C., Shao Z.-M. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 2022;34:581–594. doi: 10.1016/j.cmet.2022.02.010. e8. [DOI] [PubMed] [Google Scholar]
- Wang L., You X., Zhang L., Zhang C., Zou W. Mechanical regulation of bone remodeling. Bone Res. 2022;10:16. doi: 10.1038/s41413-022-00190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wu W., Lin X., Zhang K.M., Wu Q., Luo M., Zhou J. Predictive and prognostic biomarkers of bone metastasis in breast cancer: current status and future directions. Cell Biosci. 2023;13:224. doi: 10.1186/s13578-023-01171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenhui Y., Zhongyu X., Kai C., Zhaopeng C., Jinteng L., Mengjun M., Zepeng S., Yunshu C., Peng W., Yanfeng W., Huiyong S. Variations in the gut microbiota in breast cancer occurrence and bone metastasis. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.894283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Zhang P., Mei W., Zeng C. Intratumoral microbiota: implications for cancer onset, progression, and therapy. Front. Immunol. 2024;14 doi: 10.3389/fimmu.2023.1301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Tan X., Yang Q., Fang Z., Chen W. Akkermansia muciniphila outer membrane protein regulates recruitment of CD8+T cells in lung adenocarcinoma and through JAK–STAT signalling pathway. Microb. Biotechnol. 2024;17 doi: 10.1111/1751-7915.14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Herzog J.W., Tsang K., Brennan C.A., Bower M.A., Garrett W.S., Sartor B.R., Aliprantis A.O., Charles J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. 2016;113 doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Li A., Wang Y., Zhang Y. Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy. Signal Transduct. Target. Ther. 2023;8:35. doi: 10.1038/s41392-022-01304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z., Yao Y., Zhou X., Shen S., Hu X., Gao Q. Prognostic factors of patients with thyroid cancer and bone metastasis at presentation. Front. Endocrinol. (Lausanne) 2024;15 doi: 10.3389/fendo.2024.1344795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi Y., Baines K.J., Maleki Vareki S. Microbiome bacterial influencers of host immunity and response to immunotherapy. Cell Rep. Med. 2024;5 doi: 10.1016/j.xcrm.2024.101487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhao Q., Li T., Lu L., Wang F., Zhang H., Liu Z., Ma H., Zhu Q., Wang J., Zhang X., Pei Y., Liu Q., Xu Y., Qie J., Luan X., Hu Z., Liu X. Lactobacillus plantarum-derived indole-3-lactic acid ameliorates colorectal tumorigenesis via epigenetic regulation of CD8+ T cell immunity. Cell Metab. 2023;35:943–960. doi: 10.1016/j.cmet.2023.04.015. e9. [DOI] [PubMed] [Google Scholar]
- Zhang W., Xu Z., Hao X., He T., Li J., Shen Y., Liu K., Gao Y., Liu J., Edwards D.G., Muscarella A.M., Wu L., Yu L., Xu L., Chen X., Wu Y.-H., Bado I.L., Ding Y., Aguirre S., Wang H., Gugala Z., Satcher R.L., Wong S.T.C., Zhang X.H.-F. Bone metastasis initiation is coupled with bone remodeling through osteogenic differentiation of NG2+ cells. Cancer Discov. 2023;13:474–495. doi: 10.1158/2159-8290.CD-22-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Lin Y., Zhang W., Bi Q. Predicting diagnosis and survival of bone metastasis in breast cancer using machine learning. Sci. Rep. 2023;13 doi: 10.1038/s41598-023-45438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Yu Q.-F., Peng A.-F., Tong W.-L., Liu J.-M., Liu Z.-L. The risk factors of bone metastases in patients with lung cancer. Sci. Rep. 2017;7:8970. doi: 10.1038/s41598-017-09650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Zhang W., Yan S., Zhang K., Wu H., Chen H., Shi M., Zhou T. Novel therapeutic targets on the horizon: an analysis of clinical trials on therapies for bone metastasis in prostate cancer. Cancers (Basel) 2024;16:627. doi: 10.3390/cancers16030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Liao M., Yao Z., Liang W., Li Q., Liu J., Yang H., Ji Y., Wei W., Tan A., Liang S., Chen Y., Lin H., Zhu X., Huang S., Tian J., Tang R., Wang Q., Mo Z. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome. 2018;6:136. doi: 10.1186/s40168-018-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L., Daillère R., Roberti M.P., Routy B., Kroemer G. Anticancer effects of the microbiome and its products. Nat. Rev. Microbiol. 2017;15:465–478. doi: 10.1038/nrmicro.2017.44. [DOI] [PubMed] [Google Scholar]
- Zou L., Barnett B., Safah H., LaRussa V.F., Evdemon-Hogan M., Mottram P., Wei S., David O., Curiel T.J., Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.