Abstract
Background
One of the main causes of death in the world is chronic obstructive pulmonary disease (COPD) with partially reversible airflow limitation, which is defined as a preventable and treatable pathological condition. Anti-inflammatory and antioxidant properties of camel milk (CM) were indicated previously. The effect of CM in cigarette smoke induced-COPD in rats was evaluated in this study.
Methods
Five groups of rats including a) control, b) chronic obstructive pulmonary diseases (COPD, cigarette smoke exposed), c) COPD group treated with dexamethasone, d) COPD group treated with low dose of camel milk (CM) and e) COPD group treated with high dose of CM by gavage during the cigarette smoke exposure period (n = 7) were studied.
Results
In the COPD group, total and differential white blood cells (WBC) count in the bronchoalveolar fluid (BALF), tumor necrosis factor-alpha (TNF-α) level in the lung tissue and malondialdehyde (MDA) level in the BALF and lung tissue, lung pathological changes and tracheal responsiveness to methacholine were significantly increased, but catalase (CAT) and superoxide dismutase (SOD) activities and the level of thiol in the BALF and lung tissue were significantly decreased compared to the control group (all, p < 0.001). However, in the COPD groups treated with both doses of CM and dexamethasone, most variable did not achieved to the control levels and were significantly different with the control group (p < 0.05 to p < 0.001). In the COPD group treated with both doses of CM (dose dependently) and dexamethasone, almost all measured variables were significantly improved (p < 0.05 to p < 0.001).
Conclusion
The potential effect of CM on lung inflammation and oxidative stress in a rat model of COPD comparable to dexamethasone was demonstrated.
Keywords: Camel milk, COPD, Inflammation, Oxidative stress
1. Introduction
One of the leading causes of death in addition to non-communicable diseases (NCDs) including several types of cancer, ischaemic heart disease, cirrhosis, and Alzheimer's disease in the world is chronic obstructive pulmonary disease (COPD) [1] with the main characteristic of partially reversible airflow limitation, which is defined as a preventable and treatable pathological condition. In industrialized nations collectively (regions with very low child and adult mortality), COPD was responsible for approximately 300,000 fatalities, which accounts for 10 % of the worldwide total. In humans, COPD develops slowly and progressively and irritants such as harmful gases including tobacco smoke, urban dust, or occupational factors [1], bacteria or viruses [2] cause its occasional exacerbation. Cigarette smoking (CS) is used to induce animal COPD models [3,4], which are considered the most common and significant risk factors for COPD [5]. Also, other toxic gases such as nitrogen dioxide, sulfur dioxide [6] and Pneumocystis carinii infection [7] are also used to induce the animal model of COPD.
Emphysema, small airway remodeling, chronic bronchitis, and pulmonary hypertension are the four abnormalities in chronic and persistent COPD [1]. Increased mucus production, pulmonary inflammatory infiltrate and pulmonary edema have been observed in short-term (days) induction protocols [8], while long-term induction protocols (weeks or months), in addition to inflammatory infiltration, pulmonary regeneration and emphysema are also seen, which are characterized by fibrosis and thickening of bronchioles and arterial walls [9].
The production of approximately 30 million tons of camel milk (CM) annually in the Middle East is reported by FAOSTAT [10]. CM with a sweet and spicy taste (sometimes salty) [11] contains 3.00–3.90 % protein, 0.50–2.50 % vitamins and 0.60–0.90 % minerals. Each 100 g CM contains 0.21–0.29 mg iron, 58–59 mg sodium, 0.19–0.53 mg zinc, 156–179 mg potassium and 10.40–14.00 mg magnesium [12]. There is a higher concentration of linoleic acid and polyunsaturated acids in CM fat compared to buffalo, ewe and cow's milk [13]. In addition, CM has higher levels of iron, potassium, manganese, magnesium, zinc, copper, sodium and vitamins, especially vitamin C, and lower levels of lactose and cholesterol compared to cow's milk [13].
Hypoglycemic (rats, 250 ml of CM, 21 days) [14], anti-diabetic (rats, 35 mL/rat/day CM, 8 week; clinical trial, 500 mL/day CM, 3 month) [15], anti-cancer (colorectal cancer HCT 116 and breast cancer MCF-7, 25–250 μl/ml) [16], anti-fungal (HepG2, CaCo2, and Vero cells, 24.68, 14.40, 301.40 μg/ml), antioxidant (rats, 10 ml/kg, 3 week) [17] and anti-hypertensive (rats, 1200 mg/kg, 20 day) [18] effects were reported for CM and it is also used in treating of food allergies in children, in a 22-year-old white male with Crohn's disease [19], children with autism [20,21]. The possible role of CM in enhancing immune defense mechanism [22] and the antibacterial and antiviral activities of camel milk has been stated due to the presence of protective proteins [23].
Therefore, in this study, the effect of CM on a rat model of COPD induced by CS was investigated by measuring the total and differential white blood cells (WBC) in the bronchoalveolar fluid (BALF), cytokine in the homogenized lung tissue, oxidative stress markers in the BALF and lung tissue, pathological changes of the lung and tracheal responsiveness to methacholine.
2. Research methods
2.1. Camel milk preparation
After purchasing CM from Asayesh Company in Gonbad Kavous City, Iran it was turned into powder form using the lyophilization method [24]. Then, it was mixed with drinking water (0.2 g/ml) and given to rats through gavage.
2.2. Experimental animals
Thirty-five male Wistar rats (200–250 g) were purchased and kept at the animal house of Mashhad University of Medical Sciences under 12 h light/dark cycle, 22 ± 2 °C, humidity 54 ± 2 % and free access to food and water. This study was approved by the ethics committee of Mashhad University of Medical Sciences in animal experiments (Code 981778). Rats were randomly devoted to five groups (n = 7 in each group) as described in Table 1.
Table 1.
Studied groups, cigarette smoke (CS) exposure and treatment with camel milk (CM) and dexamethasone (Dexa).
| Group | CS exposure | Treatment |
|---|---|---|
| Control | – | water |
| COPD group | CS exposure for 3 months | water |
| COPD groups-treated with CM | ” | 4 ml/kg |
| ” | 8 ml/kg | |
| COPD group-treated with dexamethasone | ” | 1 mg/kg |
Non-treated and treated groups were exposed to CS as described in the method section. In treated groups CM or dexamethasone were add to the drinking water of animals during cigarette smoke period (3 months).
2.3. Chronic obstructive pulmonary disease model induction
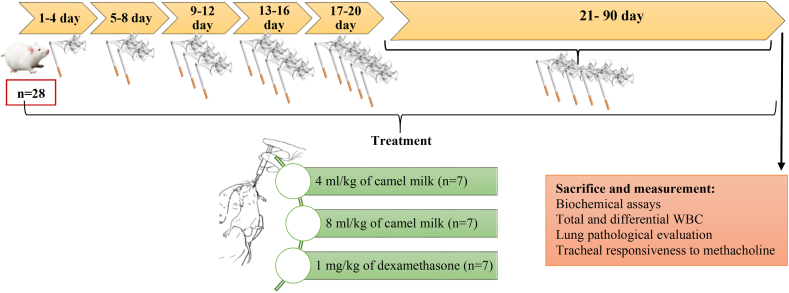
In all experimental groups except control, each rat was exposed to 5 unfiltered cigarettes six days a week for three consecutive months. To adapt the animals to CS, rats were first exposed to the smoke of 1 cigarette for four days. Then, the number of cigarettes was increased every four days, and after about 20 days, the number of cigarettes reached 5/day. Each cigarette produced about 20 puffs of smoke, which was drawn into the 20 ml syringe by changing the direction of the three-way valve, and by reversing the direction of the three-way valve, the smoke was injected into the chamber where the animal's head was located. Animals are exposed to each cigarette for about 10 min. The time between each two cigarettes was 10 min, i.e., the animals were out of the smoky environment for 10 min (Fig. 1). The cigarettes were from PINE brand and their filter were removed [25].
Fig. 1.
Flowchart of the study deign of the animal model of COPD induced by cigarette smoke exposure, their treatments and evaluated parameters.
The control rats were not exposed to smoke and were kept in the animal room of the Department of Physiology and benefited from normal air for three months. To protect the examiner, a mask and proper ventilation were used at the test site.
2.4. Biochemical assays
At the end of the COPD induction and treatment period (3 months), rats were sacrificed by intraperitoneal (i.p) injection of ketamine (50 mg/kg). Using phosphate buffered saline (PBS), the right lung was washed (five times with 1 ml, 5 ml in total). Four ml of the prepared BALF was centrifuged at 4 °C and 2500 rpm for 10 min. Therefore, the supernatant was collected and stored at −80 °C. Then, based on previous studies, the levels of malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT) and thiol in BALF and the homogenized lung tissue were measured [26,27].
Tumor necrosis factor-α (TNF-a) concentration was also measured in the homogenized lung tissue using a double sandwich enzyme-linked immunosorbent assay (ELISA) kit (Karmania pars, Kerman, Iran) as described in a previous study [26].
2.5. Counting the total and differential of white blood cells
Based on previous studies and using Turk solution and Neobayer chamber, the total and using Wright Giemsa staining of a smear of the BALF, differential WBC was assessed [28].
2.6. Pathological lung changes
After removing the lung tissue from the chest, the left lung tissue was fixed in 10 % formalin solution for 2–3 days. The lung tissue was placed in different solutions of formalin, alcohol (with different grades, 70 %, 80 %, 90 % and 100 % alcohol), and xylol for 18 h in the etcotechnikum machine. Then, paraffin blocks were prepared, cut into thin slices and stained with hematoxylin and eosin. The changes in lung pathology in terms of emphysema, fibrosis, interstitial tissue inflammation, granuloma formation and lymphoid accumulation in lung tissue were evaluated according the method of a previous study [29].
2.7. Measurement of the tracheal responsiveness to methacholine
According to previous studies, after sacrificing the animals and removing the tracheal, 3–4 cartilaginous rings were cut and placed on the organ bath. Then, by adding cumulative concentrations of methacholine, the contraction-response curve to methacholine was prepared to determine the concentration causing 50 % of the response (EC50) as a response criterion of the tracheal chain [30,31].
2.8. Statistical calculations
Statistical comparisons were done by Instat software. All data were quoted as the arithmetic mean ± standard error. Then the data between control and un-treated COPD and between un-treated and treated COPD groups were compared using one-way analysis of variance (ANOVA) with Tukey-Kramer's post-test. p < 0.05 was considered statistically significant.
3. Results
3.1. Oxidant and antioxidant markers in the BALF and lung tissue
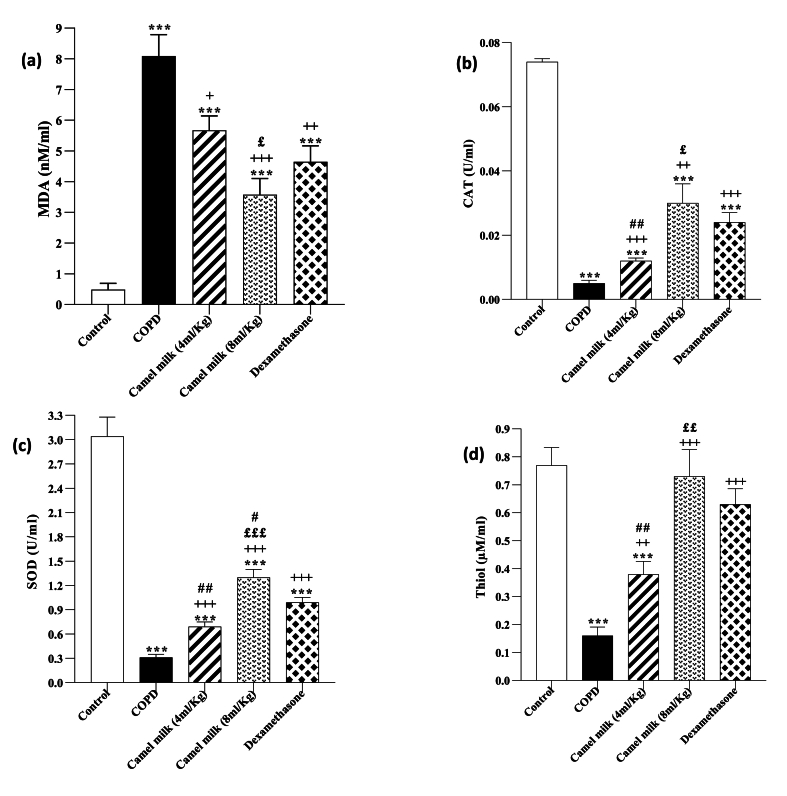
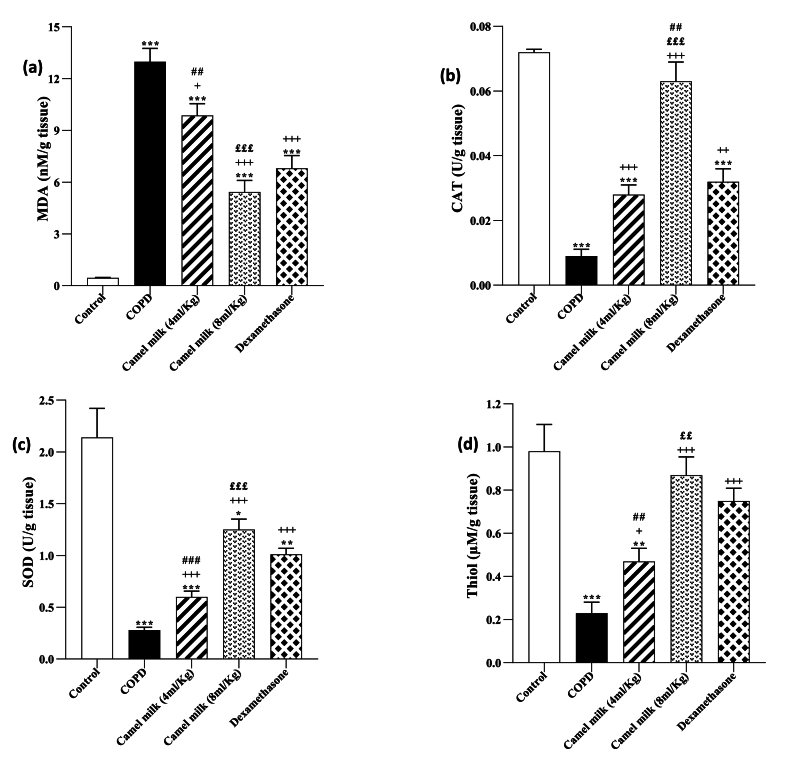
In the COPD group, the BALF MDA level and lung tissue was significantly increased but CAT and SOD activities and thiol levels were significantly decreased compared to the control group (p < 0.001 for all cases, Fig. 2, Fig. 3).
Fig. 2.
The BALF levels of MDA (a), CAT (b), SOD (c) and thiol (d) in control, COPD, COPD groups-treated with 4 and 8 ml/kg camel milk (CM) and dexamethasone (Dexa), (n = 7). Data are presented as mean ± SEM. ∗∗∗P < 0.001; compared to the control group. +P < 0.05, ++P < 0.01 and +++P < 0.001; compared to untreated COPD group. £P < 0.05, ££P < 0.01 and £££P < 0.001; compared to COPD group-treated with 4 ml/kg CM. #P < 0.05 and ##P < 0.01; compared to Dexa group. Statistical analyses were performed using one-way analysis of variance (ANOVA) with Tukey-Kramer's post-test.
Fig. 3.
The lung tissue levels of MDA (a), CAT (b), SOD (c) and thiol (d) in control, COPD, COPD groups-treated with 4 and 8 ml/kg CM and dexamethasone (n = 7). Data are presented as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001; compared to the control group. +P < 0.05, ++P < 0.01 and +++P < 0.001; compared to COPD. ££P < 0.01, £££P < 0.001; compared to COPD group-treated with 4 ml/kg of CM group. ##P < 0.01 and ###P < 0.001; compared to Dexamethasone. Statistical analyses were performed using one-way analysis of variance (ANOVA) with Tukey-Kramer's post-test.
In the COPD groups treated with 4 and 8 ml/kg CM and 1 mg/kg dexamethasone, the BALF MDA level and lung tissue was significantly decreased, but CAT and SOD activities and thiol level were significantly increased compared to the COPD group (p < 0.01 to 0.001, Fig. 2, Fig. 3). However, the COPD groups treated with both doses of CM and dexamethasone, the MDA level in the BALF and lung tissue were significantly higher (both, p < 0.001) but, CAT and SOD activates and thiol level were significantly lower than the control group (p < 0.01 to 0.001, Fig. 2, Fig. 3).
In the COPD treated with a high dose of CM (8 ml/kg), improvement of all measured oxidative stress markers in the BALF and lung tissue were significantly higher than its low dose (4 ml/kg) (p < 0.05 to p < 0.001, Fig. 2, Fig. 3).
In the treated COPD groups, the effects of 4 ml/kg CM, on all anti-oxidant markers in the BALF and its impacts on MDA, SOD and thiol in the lung tissue were lower than dexamethasone (p < 0.001 for SOD in the lung tissue and p < 0.01 for other cases). However, the effects of a high dose of CM on all oxidative stress markers both in the BALF and lung tissue were higher than dexamethasone but these differences were statistically significant for CAT and SOD activities in the lung tissue and BALF respectively (p < 0.05 for SOD and p < 0.01, for CAT, Fig. 2, Fig. 3).
3.2. The level of TNF-α in the homogenized lung tissue
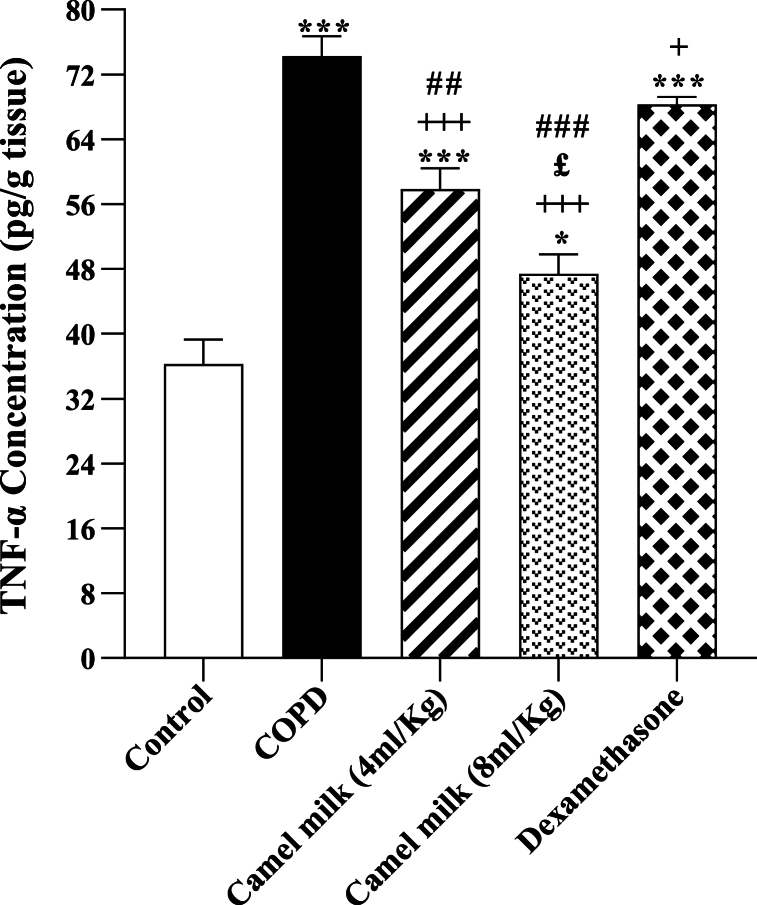
In the COPD group, the level of TNF-α in the homogenized lung tissue was significantly increased compared to the control group (p < 0.001, Fig. 4). In the COPD groups treated with 4 and 8 ml/kg of CM and 1 mg/kg dexamethasone, the level of TNF-α in the homogenized lung tissue was significantly decreased compared to the COPD group (p < 0.05 for dexamethasone and p < 0.001 for other cases, Fig. 4). However, in the COPD groups-treated with 4 and 8 ml/kg CM and 1 mg/kg dexamethasone, the level of TNF-α in the homogenized lung tissue were significantly higher compared to the control group (all, p < 0.001, Fig. 4).
Fig. 4.
The lung tissue levels of TNF-α in control, COPD, COPD groups-treated with 4 and 8 ml/kg CM and dexamethasone (n = 7). Data are presented as mean ± SEM. ∗P < 0.05 and ∗∗∗P < 0.001; compared to the control group. +P < 0.05 and +++P < 0.001; compared to COPD. £P < 0.05; compared to COPD group-treated with 4 ml/kg CM group. ##P < 0.01 and ###P < 0.001; compared to Dexamethasone. Statistical analyses were performed using one-way analysis of variance (ANOVA) with Tukey-Kramer's post-test.
In the treated COPD groups the effects of 4 and 8 ml/kg CM on TNF-α level in the homogenized lung tissue were significantly higher than the effect of dexamethasone (p < 0.01 for low and p < 0.001 high dose of CM, Fig. 4). In addition, the effect of 8 ml/kg CM on TNF-α level was significantly higher than its 4 ml/kg dose (p < 0.05, Fig. 4).
3.3. Total and differential WBC count in the BALF
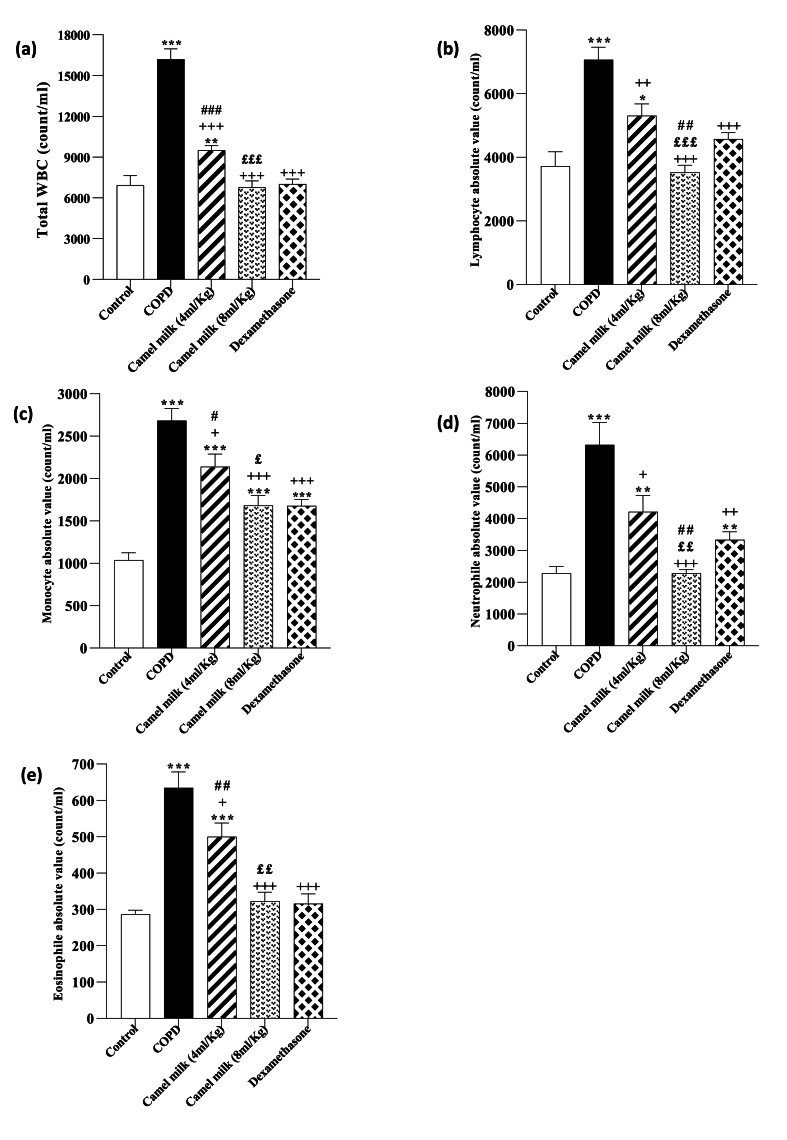
In the COPD group, total WBC, lymphocyte, monocyte, neutrophil and eosinophil counts in the BALF were significantly increased compared to the control group (all, p < 0.001 Fig. 5).
Fig. 5.
Total WBC (a), lymphocyte (b), monocyte (c), Neutrophil (d) and eosinophil (e) counts in the BALF in control, COPD, COPD groups-treated with 4 and 8 ml/kg CM and dexamethasone (n = 7). Data are presented as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001; compared to the control group. +P < 0.05, ++P < 0.01 and +++P < 0.001; compared to COPD. £P < 0.05, ££P < 0.01 and £££ P < 0.001; compared to COPD group-treated with 4 ml/kg CM group. #P < 0.05, ##P < 0.01 and ###P < 0.001; compared to Dexamethasone. Statistical analyses were performed using one-way analysis of variance (ANOVA) with Tukey-Kramer's post-test.
In the COPD groups treated with 4 and 8 ml/kg CM and 1 mg/kg dexamethasone, total WBC, lymphocyte, monocyte, neutrophil and eosinophil counts in the BALF were significantly decreased compared to the COPD group (p < 0.05 to p < 0.01, Fig. 5). However, total WBC, lymphocyte, monocyte, neutrophil and eosinophil counts in the BALF in the treated group with 4 ml/kg CM, monocyte in the treated group with 8 ml/kg CM, and monocyte and neutrophil in in the treated group with dexamethasone were significantly higher than the control group (p < 0.05 to p < 0.001, Fig. 5).
In the COPD group treated with 8 ml/kg CM, total WBC, lymphocyte, monocyte, neutrophil and eosinophil counts in the BALF were significantly lower than the COPD group treated with 4 ml/kg of CM (p < 0.05 for monocyte, p < 0.01 for neutrophil and eosinophil, and p < 0.001 for total WBC and lymphocyte, Fig. 5).
In the COPD group-treated with 4 ml/kg CM, total WBC, monocyte, and eosinophil counts in the BALF were significantly higher than the group treated with dexamethasone (p < 0.05 for monocyte, p < 0.01 for eosinophil, and p < 0.001 for total WBC, Fig. 5). However, in the COPD group-treated with 8 ml/kg CM, lymphocyte and neutrophil counts in the BALF were significantly lower than group-treated with dexamethasone (both, p < 0.01, Fig. 5).
3.4. Pathological changes in the lung tissue
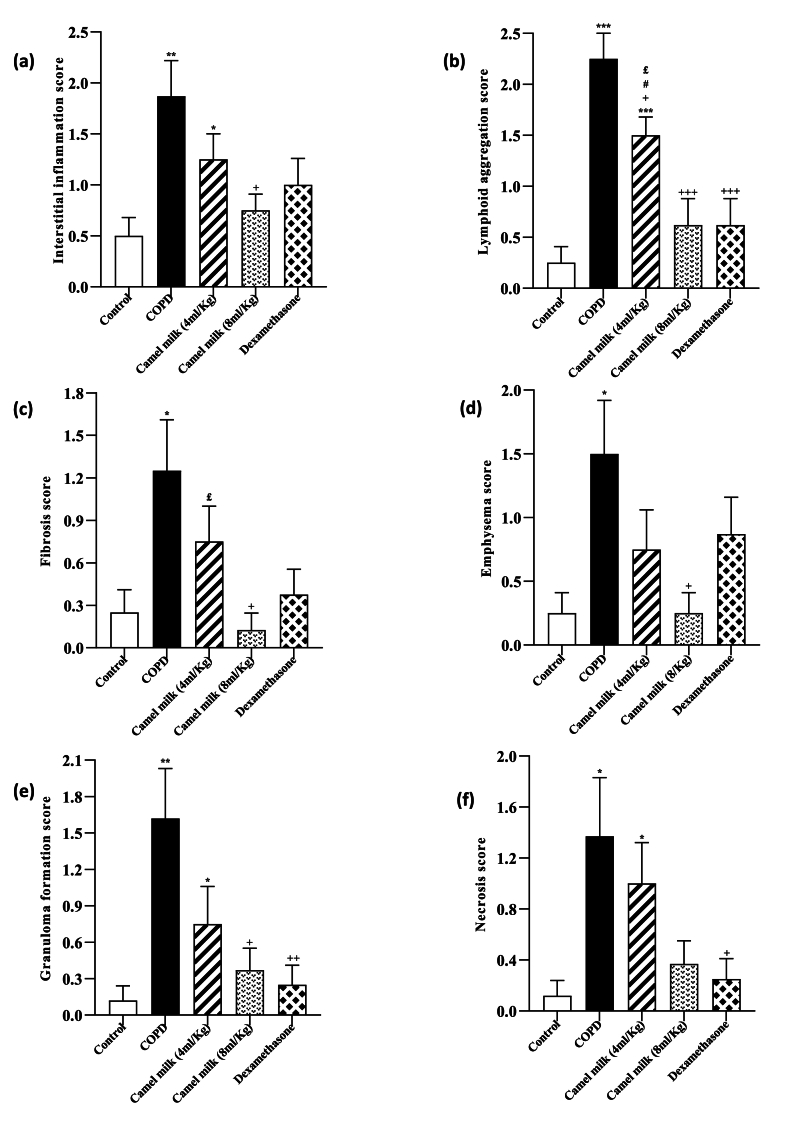
In the COPD group, pathology changes of lung tissue showed that the interstitial inflammation, emphysema, lymphoid accumulation, granuloma formation, tissue necrosis and fibrosis were significantly increased compared to the control group (p < 0.05 to p < 0.001, Fig. 6, Fig. 7).
Fig. 6.
Interstitial tissue inflammation (a), Lymphoid accumulation (b), fibrosis (c), emphysema (d), granuloma formation (e) and tissue necrosis (f) score in the lung tissue in control, COPD, COPD groups-treated with 4 and 8 ml/kg CM and dexamethasone (n = 7). Data are presented as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001; compared to the control group. +P < 0.05, +++P < 0.001; compared to COPD. £P < 0.05; compared to COPD group-treated with 4 ml/kg CM group. #P < 0.05; compared to Dexamethasone. Statistical analyses were performed using one-way analysis of variance (ANOVA) with Tukey-Kramer's post-test.
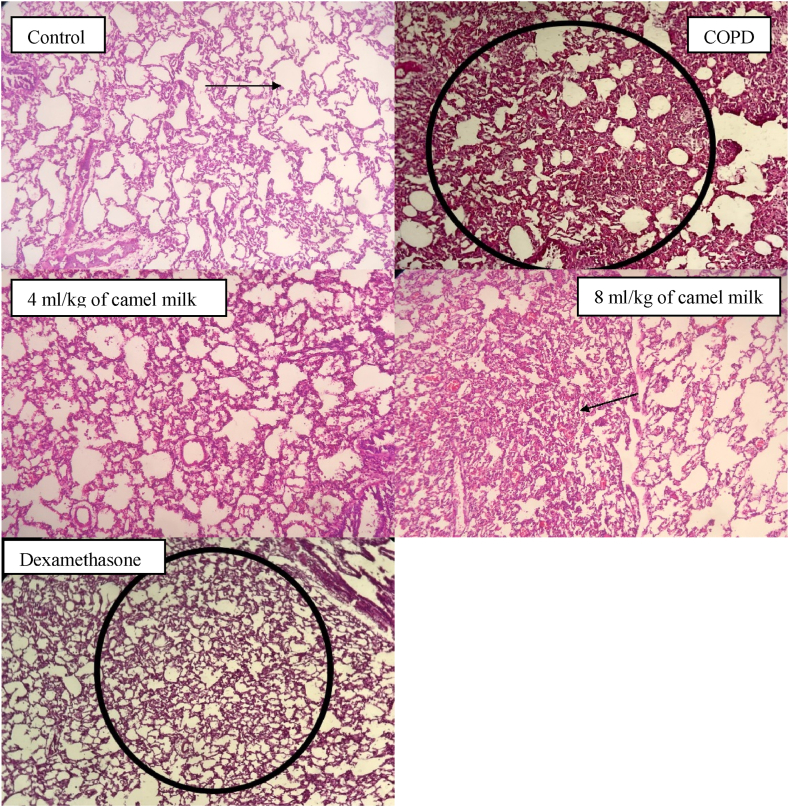
Fig. 7.
Pathological studies of lung specimens under a light microscope (X40), in the lung tissue in control, COPD, COPD groups-treated with 4 and 8 ml/kg CM and dexamethasone (n = 7) with interstitial tissue inflammation, emphysema, Lymphoid accumulation, granuloma formation, fibrosis and tissue necrosis.
In the COPD groups treated with both doses of CM and dexamethasone, the interstitial inflammation, emphysema, lymphoid accumulation, granuloma formation, tissue necrosis and fibrosis were significantly reduced compared to the COPD group (p < 0.05 to p < 0.001, Fig. 6). However, the pathological changes in the lung tissue in the treated groups were higher than the control group, but this deference's were statistically significant in the groups treated with 4 ml/kg CM (p < 0.05 to p < 0.001, Fig. 6).
In the COPD group-treated with 8 ml/kg CM, lymphoid accumulation and fibrosis were lower than the COPD group-treated with 4 ml/kg CM (p < 0.05 for lymphoid accumulation, Fig. 6).
In the COPD group-treated with 4 ml/kg of camel milk, the interstitial inflammation, lymphoid accumulation, granuloma formation, tissue necrosis and fibrosis expect for emphysema were higher than the group treated with dexamethasone (both, p < 0.05, Fig. 6).
In the COPD group treated with 4 ml/kg CM, lymphoid accumulation was higher than in the group treated with dexamethasone (p < 0.05, Fig. 6).
3.5. Tracheal responsiveness to methacholine
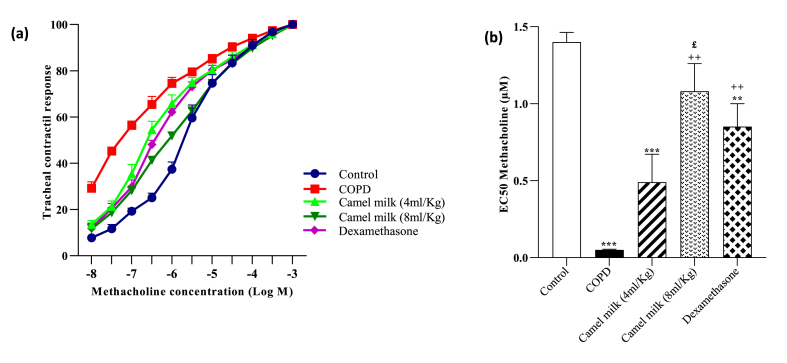
In the COPD group, the cumulative concentration-response curve to methacholine was shifted to the left and the level of EC50 was significantly decreased compared with the control group (p < 0.001 Fig. 8).
Fig. 8.
Cumulative concentration-response to methacholine-induced tracheal smooth muscle contraction (a) and the values of EC50 (concentration of methacholine-induced 50 % contractile response) (b), in control, COPD, COPD groups-treated with 4 and 8 ml/kg CM and dexamethasone (n = 7). Data are presented as mean ± SEM. ∗∗P < 0.01 and ∗∗∗P < 0.001; compared to the control group. ++P < 0.01 and +++P < 0.001; compared to COPD. ###P < 0.001; compared to Dexamethasone. Statistical analyses were performed using one-way analysis of variance (ANOVA) with Tukey-Kramer's post-test.
In the COPD groups treated with 4 and 8 ml/kg CM and 1 mg/kg dexamethasone, the cumulative concentration-response curve to methacholine was shifted to the right and the levels of EC50 were significantly increased compared with the COPD group (p < 0.01 for 8 ml/kg CM and dexamethasone, Fig. 8). However, in COPD groups treated with 4 and 1 mg/kg dexamethasone, the levels of EC50 were significantly lower than the control group (p < 0.01 for dexamethasone, p < 0.001 for 4 ml/kg camel milk, Fig. 8). The level of EC50 in the COPD groups-treated with 8 ml/kg CM was higher than the COPD group treated with 4 ml/kg CM (p < 0.01, Fig. 8).
4. Discussion
In this study, the results of COPD group were compared to those of the control group to show the induction of animal model of COPD. The results of treated groups with dexamethasone, low and high doses of CM were compared to those of untreated COPD to show the efficacy of treatment. In addition the results of treated groups with low and high doses of CM were compared to those dexamethasone, to compare the effects of CM to a standard anti-inflammatory drug. In addition, the results of treated groups were compared to the control group to ensure that values fully returned to normal level or not. Total and differential WBC counts in the BALF and MDA level in the BALF and the homogenized lung tissue, the level of TNF-α in the homogenized lung tissue, lung pathological changes and TR to methacholine were increased but CAT and SOD activities and the level of thiol in the BALF and the homogenized lung tissue were decreased in the COPD group compared to the control group were observed. In addition untreated COPD group experienced weight loss, chronic cough, tired look, slowed movement, poor feeding, dull fur, yellowish urine, and dry stool. These results indicate the development of COPD in the rat model due to CS, which are confirmed by the previous studies [25,32] that the induction of COPD due to CS was reported indicated by lung injury, increased total and differential WBC counts, the change in the levels of oxidant and antioxidant markers in the BALF, change in inflammatory cytokines in the BALF and increased lung pathological changes [25].
One of the important features of COPD pathogenesis is oxidative stress. There is a significant increase in oxidant load in patients with COPD. Lung oxidative stress causes inactivation of antiproteinases, mucus hypersecretion, epithelial injury of the air space, activation of transcription factor and gene expression of pro-inflammatory mediators and increase of neutrophils influx to the lungs. Therefore, the harmful effects of oxidants may change the inflammatory events and induce pathogenesis in COPD [33]. In fact, an increased serum level of MDA in the guinea pigs model of COPD caused by CS was seen [25]. The results of the present study also indicate an increase in the level of MDA and a decrease in the level of SOD and CAT and thiol in the BALF and the lung tissue in the COPD group which is supported by previous studies.
Treatment with 4 and 8 ml/kg CM improved the consequences of CS exposure and reduced MDA level and increased SOD, CAT and thiol levels in the BALF and the lung tissue. Hence, this result indicates the potential effect of CM on the treatment of COPD by improving oxidative stress markers; which was found to be more effective than dexamethasone. In Rats and MCF7 breast cancer cells, administration of CM (1 ml/rat, orally) reduced MDA and increased SOD and CAT were shown [34]. Moreover, in the C57BL/6J mice with radiation-induced intestinal damage, administration of 0.4 ml/day CM reduced the serum MDA but increased the serum SOD levels [35].
In the COPD group, the level of TNF-α in the lung tissue was significantly increased, but in the COPD groups treated with both doses of CM (4 and 8 ml/kg) and COPD groups treated with dexamethasone, the level of TNF- α in the lung tissue was significantly reduced. However, in the treated COPD groups the effects of 4 and 8 ml/kg CM on TNF-α level in the homogenized lung tissue were significantly higher than the effect of dexamethasone. In the guinea pig model of lung injury caused by repeated exposure to CS, the TNF-α mRNA expression, accumulation of macrophages and neutrophils, protease activity, lung structural changes and airway resistance were significantly increased [36]. In a study conducted on patients with COPD, the increased level of TNF-α was shown in lung biopsy [37]. In another study conducted on rat model of COPD, it was found that the level of TNF-α significantly increased in the lung tissue [38]. Administrating 35 ml CM in type 2 diabetic rats reduced the production of TNF-α, thus indicating its anti-inflammatory activity [39]. Several studies show the reduction of TNF-α level following the consumption of CM, indicating its anti-inflammatory effect [40]. Therefore, the results of the present study regarding increased TNF-α in COPD and its reduction by CM are supported by several previous studies.
Total and differential WBC counts were also significantly increased in the COPD group, however, in the COPD groups treated with both doses of CM and COPD groups treated with dexamethasone, total and differential WBC counts were significantly reduced compared to the COPD group. In a study on Sprague-Dawley rats, the CS-induced COPD, total WBC, neutrophils, and macrophages in the BALF were significantly increased and lung injury indicated by alveolar space enlargement, severe airway inflammation and mitochondrial damage of the airway epithelium. Increased total and differential WBC, MDA and TNF-α in the animal model of COPD were also reported [41]. Therefore, the increased total and differential WBC in this study was supported by previous studies in animal model of COPD. In addition, the current study showed that the effect of CM on reduction of total and differential WBC in a rat model of COPD were significantly higher than the effect of dexamethasone.
In the COPD groups, treated with 4 and 8 ml/kg of CM and dexamethasone, the cumulative concentration-response curve to methacholine was shifted to the right and the levels of EC50 were significantly increased compared with the COPD group. In addition, treatment with both doses of CM and dexamethasone reduced lung pathological changes induced by CS. These results indicate the prophylactic effect of CM on nonspecific TR (methacholine response) and lung pathological changes, which are the most critical features of COPD.
CM is rich in lactoferrin, which exhibits strong antimicrobial and anti-inflammatory properties. It also contributes to the enhancement of lymphocyte maturation and function. TNF-a is a crucial immuno-modulatory cytokine that amplifies the inflammatory response by triggering the production of reactive oxygen species (ROS), arachidonic acid metabolites, proteases, and certain cytokines. Upon activation, inflammatory cells such as neutrophils, macrophages, and lymphocytes generate oxidative stress by releasing reactive oxygen species such as superoxide anions, hydrogen peroxide, and hydroxyl radicals. The consumption of camel milk mitigates the oxidative stress generated by the immune system's anti-inflammatory response [17]. Therefor CM possibly ameliorate COPD by these mechanisms.
In the present study, the nutritional profile of the powdered CM mixed with water was not provided which should be performed in further studies.
The other findings in the current study were the similar effects of CM and dexamethasone as a known anti-inflammatory drug. Although the effects of a low dose of CM on some parameters, were lower than dexamethasone, the effects of almost all variables in the treated group with its high dose were higher than the dexamethasone-treated group. This finding also confirmed the anti-inflammatory notion of the CM.
5. Conclusion
The results of the present study expressed the potential effect of CM on measured variables including total and differential WBC in the BALF, oxidant and antioxidant level in the BALF and lung tissue, TNF-α level and pathological changes in lung tissue and TR level in a rat model of COPD comparable to the effects of dexamethasone. These results indicate the potential therapeutic effect of CM in COPD, however, it's suggested that more studies in this field including clinical trials.
CRediT authorship contribution statement
Sepide Behrouz: Writing – original draft, Resources, Investigation, Formal analysis, Data curation, Conceptualization. Mahla Mohammadi: Writing – original draft, Resources, Investigation, Data curation, Conceptualization. Hadi Sarir: Writing – review & editing. Nema Mohammadian Roshan: Formal analysis. Mohammad Hossein Boskabady: Writing – review & editing, Validation, Supervision, Project administration, Funding acquisition, Conceptualization.
Additional information
No additional information is available for this paper.
Data availability
The data will be made available upon request from the corresponding author, Mohammad Hossein Boskabady.
Funding
Open access funding provided by Mashhad University of Medical Sciences. This study was approved by the ethics committee of Mashhad University of Medical Sciences in animal experiments (Code 981778) on February 28, 2021.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviation:
- BALF
bronchoalveolar fluid
- CAT
catalase
- COPD
chronic obstructive pulmonary disease
- CM
camel milk
- CS
cigarette smoking
- ELISA
enzyme-linked immunosorbent assay
- i.p
intraperitoneal
- MDA
malondialdehyde
- PBS
phosphate buffer solution
- SOD
superoxide dismutase
- TNF-α
tumor necrosis factor-alpha
- WBC
white blood cells
References
- 1.Li Y., Li S.Y., Li J.S., Deng L., Tian Y.G., Jiang S.L., Wang Y., et al. A rat model for stable chronic obstructive pulmonary disease induced by cigarette smoke inhalation and repetitive bacterial infection. Biol. Pharm. Bull. 2012;35(10):b12–b407. doi: 10.1248/bpb.b12-00407. [DOI] [PubMed] [Google Scholar]
- 2.Kościuch J., Chazan R. The role of viruses in the pathogenesis of obstructive lung diseases. Pol Merkur Lekarsk. 2003;15(87):292–295. [PubMed] [Google Scholar]
- 3.Wright J.L., Churg A. Cigarette smoke causes physiologic and morphologic changes of emphysema in the Guinea pig1–3. Am. Rev. Respir. Dis. 1990;142:1422–1428. doi: 10.1164/ajrccm/142.6_Pt_1.1422. [DOI] [PubMed] [Google Scholar]
- 4.Wright J.L., Churg A. Animal models of cigarette smoke-induced chronic obstructive pulmonary disease. Expert Rev. Respir. Med. 2010;4(6):723–734. doi: 10.1586/ers.10.68. [DOI] [PubMed] [Google Scholar]
- 5.Kianmeher M., Ghorani V., Boskabady M.H. Animal model of asthma, various methods and measured parameters: a methodological review. Iran. J. Allergy, Asthma Immunol. 2016;15(6):445–465. [PubMed] [Google Scholar]
- 6.Murărescu E.D., Eloae-Zugun F., Mihailovici M.S. Experimental COPD induced by solid combustible burn smoke in rats: a study of the emphysematous changes of the pulmonary parenchyma. Rom. J. Morphol. Embryol. 2008;49(4):495–505. [PubMed] [Google Scholar]
- 7.Christensen P.J., Preston A.M., Ling T., Du M., Fields W.B., Curtis J.L., Beck J.M. Pneumocystis murina infection and cigarette smoke exposure interact to cause increased organism burden, development of airspace enlargement, and pulmonary inflammation in mice. Infect. Immun. 2008;76(8):3481–3490. doi: 10.1128/iai.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardaker E., Freeman M., Dale N., Bahra P., Raza F., Banner K., Poll C. Exposing rodents to a combination of tobacco smoke and lipopolysaccharide results in an exaggerated inflammatory response in the lung. Br. J. Pharmacol. 2010;160(8):1985–1996. doi: 10.1111/j.1476-5381.2010.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J., Zhao M., Liao S. Establishment and pathological study of models of chronic obstructive pulmonary disease by S02 inhalation method. Chin Med J. 2000;113(3):213–2166. [PubMed] [Google Scholar]
- 10.Almoraie N.M., Shatwan I.M. Effect of camel milk on the physicochemical, rheological, and sensory qualities of bread. J. Food Qual. 2021;2021 doi: 10.1155/2021/8889406. [DOI] [Google Scholar]
- 11.Rao M., Gupta R., Dastur N. Camels' milk and milk products. Ind. J. Dairy Sci. 1970;23(2):71–78. [Google Scholar]
- 12.Zibaee S., Yousefi M., Taghipour A., Kiani M.A., Noras M.R. Nutritional and therapeutic characteristics of camel milk in children: a systematic review. Electron. Physician. 2015;7(7):1523. doi: 10.19082/1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nahar N., Khan W.I., Shoeb M. Physico-Chemical analysis and composition of camel milk of Bangladesh. J. Basic Appl. Sci. 2016;12:231–235. doi: 10.6000/1927-5129.2016.12.35. [DOI] [Google Scholar]
- 14.Agrawal R.P., Kochar D.K., Sahani M.S., Tuteja F.C., Ghorui S.K. Hypoglycemic activity of camel milk in streptozotocin induced diabetic rats. Int J Diab Dev. 2004;24:47–49. . [Google Scholar]
- 15.Agrawal R.P., Saran S., Sharma P., Gupta R.P., Kochar D.K., Sahani M.S. Effect of camel milk on residual β-cell function in recent onset type 1 diabetes. Diabetes Res. Clin. Pract. 2007;77(3):494–495. doi: 10.1016/j.diabres.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Krishnankutty R., Iskandarani A., Therachiyil L., Uddin S., Azizi F., Kulinski M., Ahmad Bhat A., et al. Anticancer activity of camel milk via induction of autophagic death in human colorectal and breast cancer cells. Asian Pac J Cancer Prev. 2018;19(12):3501–3509. doi: 10.31557/APJCP.2018.19.12.3501. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behrouz S., Saadat S., Memarzia A., Sarir H., Folkerts G., Boskabady M.H. The antioxidant, anti-inflammatory and immunomodulatory effects of camel milk. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.855342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yahya M.A., Alhaj O.A., Al-Khalifah A.S. Antihypertensive effect of fermented skim camel (Camelus dromedarius) milk on spontaneously hypertensive rats. Nutr Hos. 2017;34(2):416–421. doi: 10.20960/nh.1163. . [DOI] [PubMed] [Google Scholar]
- 19.Rosenheck D., Ravee Y., Yagil R. Camel milk as an alternative treatment for Crohn's disease: 1264. Am. J. Gastroenterol. 2012;107:S503. . [Google Scholar]
- 20.El Sayed I., Ruppanner R., Ismail A., Champagne C.P., Assaf R. Antibacterial and antiviral activity of camel milk protective proteins. J. Dairy Res. 1992;59(2):169–175. doi: 10.1017/S0022029900030417. [DOI] [PubMed] [Google Scholar]
- 21.Al-Ayadhi L., Alhowikan A.M., Bhat R.S., El-Ansary A. Comparative study on the ameliorating effects of camel milk as a dairy product on inflammatory response in autism spectrum disorders. Neurochem. J. 2022;16(1):99–108. doi: 10.1134/S1819712422010020. [DOI] [Google Scholar]
- 22.Yagil R. Publications Division, Food and Agriculture Organization of the United Nations; Rome, Italy: 1982. Camels and Camel Milk: FAO Animal Production and Health; p. 26. [Google Scholar]
- 23.Shabo Y., Yagil R. Etiology of autism and camel milk as therapy. Int. J. Disabil. Hum. Dev. 2005;4(2):67–70. doi: 10.1515/IJDHD.2005.4.2.67. [DOI] [Google Scholar]
- 24.Tang X.C., Pikal M.J. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm. Res. (N. Y.) 2004;21(2):191–200. doi: 10.1023/B:PHAM.0000016234.73023.75. [DOI] [PubMed] [Google Scholar]
- 25.Mahtaj L.G., Feizpour A., Kianmehr M., Soukhtanloo M., Boskabady M.H. The effect of carvacrol on systemic inflammation in Guinea pigs model of COPD induced by cigarette smoke exposure. Pharmacol. Rep. 2015;67(1):140–145. doi: 10.1016/j.pharep.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Ghasemi S.Z., Memarzia A., Behrouz S., Gholamnezhad Z., Boskabady M.H. Comparative effects of Curcuma longa and curcumin on paraquat-induced systemic and lung oxidative stress and inflammation in rats. Avicenna J Phytomed. 2022;12(4):414. doi: 10.22038/AJP.2022.19713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behrouz S., Beigoli S., Ghalibaf M.H.E., Ghasemi S.Z., Boskabady M., Roshan N.M., Boskabady M.H. The effect of varasurf surfactant on respiratory distress syndrome in rats. Toxicol. Environ. Health Sci. 2024:1–11. doi: 10.1007/s13530-024-00232-w. [DOI] [Google Scholar]
- 28.Saadat S., Beheshti F., Askari V.R., Hosseini M., Mohamadian Roshan N., Boskabady M.H. Aminoguanidine affects systemic and lung inflammation induced by lipopolysaccharide in rats. Respir. Res. 2019;20(1):1–13. doi: 10.1186/s12931-019-1054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boskabady M.H., Jalali S. Effect of carvacrol on tracheal responsiveness, inflammatory mediators, total and differential WBC count in blood of sensitized Guinea pigs. Exp. Biol. Med. 2013;238(2):200–208. doi: 10.1177/1535370212474604. [DOI] [PubMed] [Google Scholar]
- 30.Boskabady M.H., Tabatabaee A., Jalali S. Potential effect of the extract of Zataria multiflora and its constituent, carvacrol, on lung pathology, total and differential WBC, IgE and eosinophil peroxidase levels in sensitized Guinea pigs. J. Funct.Foods. 2014;11:49–61. doi: 10.1016/j.jff.2014.08.021. [DOI] [Google Scholar]
- 31.Memarzia A., Ghasemi S.Z., Behrouz S., Boskabady M.H. The effects of Crocus sativus extract on inhaled paraquat-induced lung inflammation, oxidative stress, pathological changes and tracheal responsiveness in rats. Toxicon. 2023;235 doi: 10.1016/j.toxicon.2023.107316. [DOI] [PubMed] [Google Scholar]
- 32.Zhang M., Zhang Y., Roth M., Zhang L., Shi R., Yang X., Li Y., et al. Sirtuin 3 inhibits airway epithelial mitochondrial oxidative stress in cigarette smoke-induced COPD. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/7582980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacNee W., Rahman I. Is oxidative stress central to the pathogenesis of chronic obstructive pulmonary disease? Trends Mol. Med. 2001;7(2):55–62. doi: 10.1016/S1471-4914(01)01912-8. [DOI] [PubMed] [Google Scholar]
- 34.Badawy A.A., El-Magd M.A., AlSadrah S.A. Therapeutic effect of camel milk and its exosomes on MCF7 cells in vitro and in vivo. Integr. Cancer Ther. 2018;17(4):1235–1246. doi: 10.1177/1534735418786000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y.Z., Li C., Gu J., Lv S.C., Song J.Y., Tang Z.B., Duan G.X., et al. Anti-oxidative and immuno-protective effect of camel milk on radiation-induced intestinal injury in C57BL/6 J mice. Dose Response. 2021;19(1) doi: 10.1177/15593258211003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubo S., Kobayashi M., Masunaga Y., Ishii H., Hirano Y., Takahashi K., Shimizu Y. Cytokine and chemokine expression in cigarette smoke-induced lung injury in Guinea pigs. Eur. Respir. J. 2005;26(6):993–1001. doi: 10.1183/09031936.05.00042405. [DOI] [PubMed] [Google Scholar]
- 37.Mueller R., Chanez P., Campbell A., Bousquet J., Heusser C., Bullock G. Different cytokine patterns in bronchial biopsies in asthma and chronic bronchitis. Respir. Med. 1996;90(2):79–85. doi: 10.1016/S0954-6111(96)90202-4. [DOI] [PubMed] [Google Scholar]
- 38.Sun T., Wang X., Liu Z., Liu S., Zhang J. Patterns of cytokine release and evolution of remote organs from proximal femur fracture in COPD rats. Injury. 2011;42(8):825–832. doi: 10.1016/j.injury.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 39.Cui C., Lu Y., Yue Y., Wu S., Wang S., Yu M., Sun Z. Camel milk regulates T‐cell proliferation to alleviate dextran sodium sulphate‐induced colitis in mice. Int. J. Food Sci. Technol. 2020;55(4):1648–1660. doi: 10.1111/ijfs.14434. [DOI] [Google Scholar]
- 40.Abd-Elhakim Y.M., El-Sharkawy N.I., Mohammed H.H., Ebraheim L.L., Shalaby M.A. Camel milk rescues neurotoxic impairments induced by fenpropathrin via regulating oxidative stress, apoptotic, and inflammatory events in the brain of rats. Food Chem. Toxicol. 2020;135 doi: 10.1016/j.fct.2019.111055. [DOI] [PubMed] [Google Scholar]
- 41.Liu C.Y., Wu J.H., Chen Z.Y., Zhang Y., Huang C.L., Lin A.M., Xu X.T., et al. Effect of doxofylline on reducing the inflammatory response in mechanically ventilated rats with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2021;16:2375. doi: 10.2147/COPD.S315639. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be made available upon request from the corresponding author, Mohammad Hossein Boskabady.