Abstract
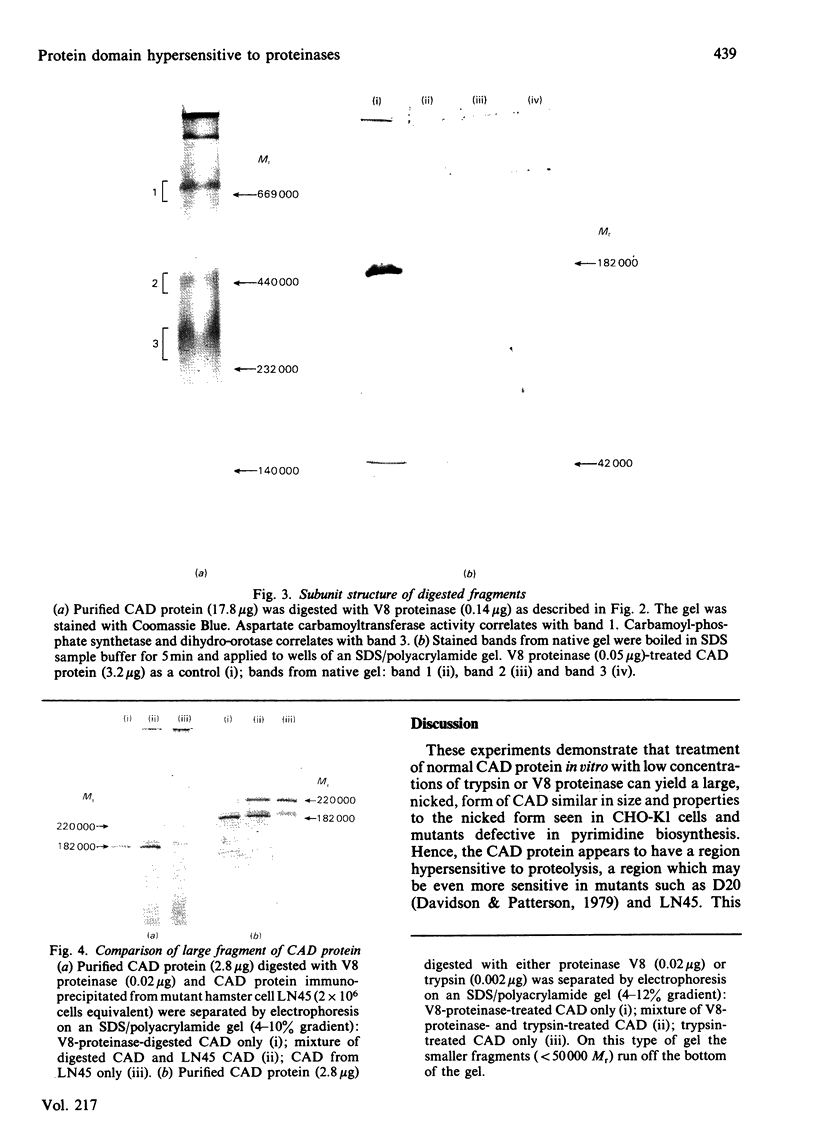
When the multifunctional protein that catalyses the first three steps of pyrimidine biosynthesis in hamster cells is treated with staphylococcal V8 proteinase, a single cleavage takes place. The activities of carbamoyl-phosphate synthetase (EC 6.3.5.5), aspartate carbamoyltransferase (EC 2.1.3.2) and dihydro-orotase (EC 3.5.2.3) and the allosteric inhibition by UTP are unaffected. One fragment, of Mr 182000, has the first and third enzyme activities, whereas the other fragment, of Mr 42000, has aspartate carbamoyltransferase activity and an aggregation site. A similar small fragment is observed in protein digested with low concentrations of trypsin. A similar large fragment is seen after digestion with trypsin and as the predominating form of this protein in certain mutants defective in pyrimidine biosynthesis. These results indicate that a region located adjacent to the aspartate carbamoyltransferase domain is hypersensitive to proteinase action in vitro and may also be sensitive to proteolysis in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Coleman P. F., Suttle D. P., Stark G. R. Purification from hamster cells of the multifunctional protein that initiates de novo synthesis of pyrimidine nucleotides. J Biol Chem. 1977 Sep 25;252(18):6379–6385. [PubMed] [Google Scholar]
- Davidson J. N., Carnright D. V., Patterson D. Biochemical genetic analysis of pyrimidine biosynthesis in mammalian cells: III. Association of carbamyl phosphate synthetase, aspartate transcarbamylase, and dihydroorotase in mutants of cultured Chinese hamster cells. Somatic Cell Genet. 1979 Mar;5(2):175–191. doi: 10.1007/BF01539159. [DOI] [PubMed] [Google Scholar]
- Davidson J. N., Patterson D. Alteration in structure of multifunctional protein from Chinese hamster ovary cells defective in pyrimidine biosynthesis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1731–1735. doi: 10.1073/pnas.76.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. N., Rumsby P. C., Tamaren J. Organization of a multifunctional protein in pyrimidine biosynthesis. Analyses of active, tryptic fragments. J Biol Chem. 1981 May 25;256(10):5220–5225. [PubMed] [Google Scholar]
- Jarry B. P. Purification of aspartate transcarbamylase from Drosophila melanogaster. Eur J Biochem. 1978 Jul 3;87(3):533–540. doi: 10.1111/j.1432-1033.1978.tb12404.x. [DOI] [PubMed] [Google Scholar]
- Kempe T. D., Swyryd E. A., Bruist M., Stark G. R. Stable mutants of mammalian cells that overproduce the first three enzymes of pyrimidine nucleotide biosynthesis. Cell. 1976 Dec;9(4 Pt 1):541–550. doi: 10.1016/0092-8674(76)90036-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mally M. I., Grayson D. R., Evans D. R. Catalytic synergy in the multifunctional protein that initiates pyrimidine biosynthesis in Syrian hamster cells. J Biol Chem. 1980 Dec 10;255(23):11372–11380. [PubMed] [Google Scholar]
- Mori M., Tatibana M. Multi-enzyme complex of glutamine-dependent carbamoyl-phosphate synthetase with aspartate carbamoyltransferase and dihydroorotase from rat ascites-hepatoma cells. Purification, molecular properties and limited proteolysis. Eur J Biochem. 1978 May 16;86(2):381–388. doi: 10.1111/j.1432-1033.1978.tb12320.x. [DOI] [PubMed] [Google Scholar]
- Mori M., Tatibana M. Purification of homogeneous glutamine-dependent carbamyl phosphate synthetase from ascites hepatoma cells as a complex with aspartate transcarbamylase and dihydroorotase. J Biochem. 1975 Jul;78(1):239–242. [PubMed] [Google Scholar]
- Patterson D., Carnright D. V. Biochemical genetic analysis of pyrimidine biosynthesis in mammalian cells: I. Isolation of a mutant defective in the early steps of de novo pyrimidine synthesis. Somatic Cell Genet. 1977 Sep;3(5):483–495. doi: 10.1007/BF01539120. [DOI] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. A structural model of human erythrocyte spectrin. Alignment of chemical and functional domains. J Biol Chem. 1982 Aug 10;257(15):9093–9101. [PubMed] [Google Scholar]
- Strålfors P., Belfrage P. Electrophoretic elution of proteins from polyacrylamide gel slices. Anal Biochem. 1983 Jan;128(1):7–10. doi: 10.1016/0003-2697(83)90336-6. [DOI] [PubMed] [Google Scholar]