Abstract
A leaf spot disease affecting Curcuma kwangsiensis (Zingiberaceae) has been observed in Qinzhou City, Guangxi Province. Infected leaves exhibit yellow-brown spots that progressively expand and eventually lead to leaf death. Curvularia isolates were obtained from the diseased leaves with tissue isolation and single spore purification methods. To accurately identify these isolates, we analyzed their morphological characteristics and phylogenetic relationships using combinations of ITS, GAPDH, and EF-1α gene sequences. Phylogenetic analysis showed that the investigated strains formed a distinct clade separate from other recognized Curvularia species. Furthermore, the strains exhibited differences in conidiophore size and conidia shape/size. Based on phylogenetic studies, morphology, and pathogenicity tests, the pathogen was identified as a new species named Curvularia qinzhouensis. Optimal conditions for mycelial growth were observed at 30 °C and pH 8. The sensitivity of the pathogen to various phytochemicals was also examined. Honokiol, thymol, and citral demonstrated effective antifungal effects, with EC50 values of 6.72 ± 1.75, 25.74 ± 4.30, and 54.24 ± 4.69 µg/ml, respectively. The present investigation provides the first report of leaf spot disease on C. kwangsiensis caused by C. qinzhouensis, and valuable insights for the prevention and control of this disease.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77524-1.
Keywords: Curvularia qinzhouensis, Leaf spot, Curvularia, Molecular identification
Subject terms: Microbiology, Plant sciences
Introduction
Curcuma kwangsiensis S. G. Lee et C. F. Liang is a perennial herbaceous plant belonging to the Zingiberaceae family, commonly grown in the southwestern region of China. It holds significant medicinal value in traditional medicine, with recent studies confirming its efficacy in liver protection, tumor inhibition, antioxidant activity, blood lipid reduction, and immune system modulation1. Apart from its medicinal properties, its attractive thin green leaves and vibrant cylindrical flower clusters make it a popular choice for ornamental purposes in flower beds2. However, with the increasing demand and expansion of cultivation areas, various diseases have emerged, posing a threat to yield and quality. For example, Epicoccum latusicollum has been identified as a pathogen causing leaf blight and defoliation in C. kwangsiensis3. Nonetheless, many pathogens responsible for diseases in C. kwangsiensis remain unidentified.
Curvularia encompassing a diverse range of species including plant, animal, and human pathogenic fungi, is a genus belonging to the Pleosporalean order4. The distinguishing feature of Curvularia is the formation of brown distoseptate conidia, typically with lighter end cells and enlarged middle cells. However, due to morphological similarities, the identification of Curvularia species cannot be accurately achieved solely through morphological features and analyses of the ITS sequence5,6. Consequently, the investigation of species diversity in Curvularia has been conducted using multi-locus sequence analysis. Scientists have employed multi-locus sequence analysis, utilizing the internal transcribed spacer (ITS) region of rDNA, as well as the protein-coding loci glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and translation elongation factor 1-a (EF-1α), to investigate species diversity in Curvularia and its phylogenetic relationships with related genera7,8. To date, approximately 130 species have been recognized within the genus Curvularia based on DNA sequences4,9.
Certain species of Curvularia are responsible for leaf spot in various crops, such as C. asianensis and C. eragrostidis in Sansevieria trifasciata, while C. lunata is a pathogen of sunflower (Helianthus annuus)10,11. Effective management of the leaf spot disease in C. kwangsiensis requires identification of the pathogen and implementation of control measures. In addition, determining the optimal growth temperature and pH for these pathogenic fungi is crucial for understanding their ecological characteristics, developing control strategies, preventing disease outbreaks, and ensuring the successful cultivation of C. kwangsiensis. One area of current research focuses on the use of phytochemicals to manage plant diseases. For instance, carvacrol has shown inhibitory effects on the mycelial growth of three leaf pathogens (Xanthomonas perforans, Alternaria tomatophila, and Podosphaera xanthii)12. Similarly, thymol exhibits strong antifungal properties against Fusarium graminearum by damaging the cell membrane through lipid peroxidation13. The objective of this study was to identify the causative agent of leaf spot disease in C. kwangsiensis and investigate potential phytochemical compounds for controlling the potentially economically important agricultural pathogen.
Results
Sampling, fungal isolation and pathogenicity assay
Curcuma kwangsiensi, cultivated in plantations (∼1.3 ha) in Qinzhou City, Guangxi Province (21° 51’ 00′′ N, 108° 44’ 00′′ E), experienced an outbreak of leaf spot disease in May 2021. Approximately 15% (n = 200) of the plants were infected. Initial symptoms included yellow, watery, irregular lesions on young leaves, which progressed to affect entire tender leaves. Eventually, the leaves turned yellow, withered, and led to plant death (Fig. 1). In this study, twenty-six isolates resembling the genus Curvularia were obtained from symptomatic plants. Three representative isolates (CK43.1, CK56.3, and CK64.5) were randomly selected for further investigation. After being inoculated for three days, the leaves of C. kwangsiensi exhibited similar symptoms to those initially observed in the field, while no symptoms were observed on the control plants (Fig. 1). The fungi inoculated were consistently re-isolated from the necrotic tissue of the inoculated plants and identified based on their morphological characteristics and DNA sequence analysis (Additional file 1). No fungi were isolated from the plants in the negative control group.
Fig. 1.
(a–d) Symptoms of leaf spot disease on Curcuma kwangsiensis under natural conditions. (e–h) Pathogenicity testing on C. kwangsiensi seedlings. (e, f) Control; (g) Symptoms of the disease caused by strain CK56.3 on C. kwangsiensi after 3 days of inoculation; (h) Symptoms of the disease caused by strain CK56.3 on C. kwangsiensi after 9 days of inoculation.
Phylogenetic analyses
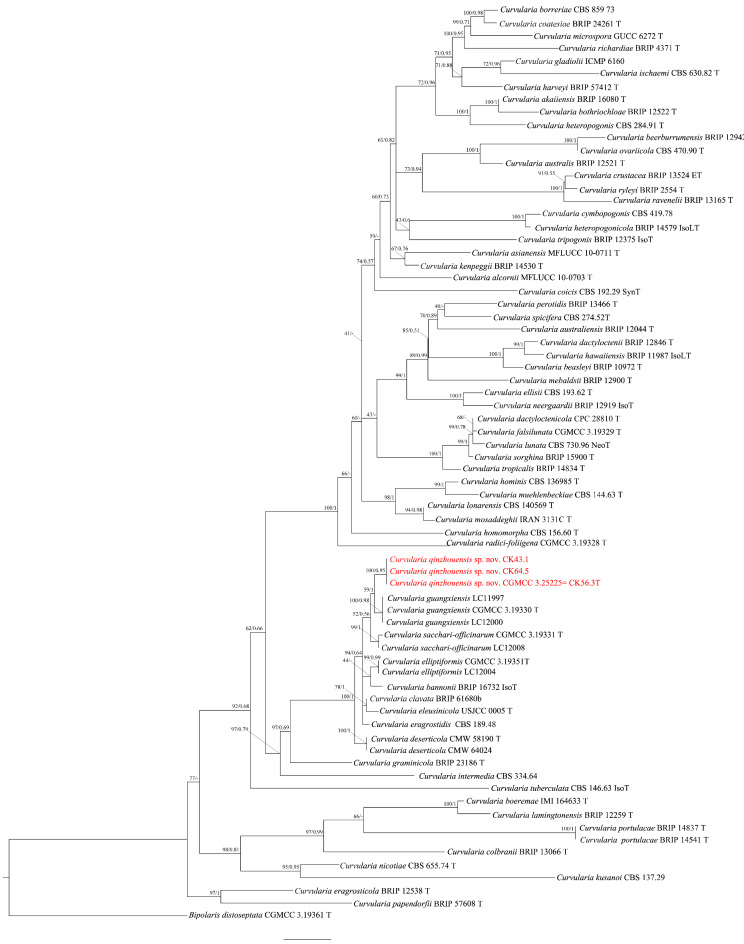
In this study, nine (9) new sequences of ITS, GAPDH and EF-1α were generated. The estimated sizes of individual alignments for ITS, GAPDH, and EF-1α were approximately 590 bp, 600 bp, and 990 bp correspondingly. To determine the phylogenetic position of our new isolates within the Curvularia genus, we conducted an analysis using a concatenated sequence dataset consisting of ITS, GAPDH, and EF-1α. In the phylogenetic analysis, the final concatenated alignment was 2180 bp. The topologies obtained from ML and BI analyses of the concatenated dataset were congruent (Fig. 2). The multilocus phylogenetic analysis showed the isolates CK43.1, CK56.3, CK64.5 are closely associated with C. bannonii but differ from it and other closely related taxa (Table 1). In the gene region of the ITS, the base sequence of C. sacchari-officinarum and C. guangxiensis was the same as our isolates, while C. bannonii, C. elliptiformis, C. clavata, and C. eleusinicola had 2 differences. Only C. eragrostidis had 3 base differences compared to our strains. In the gene region of EF-1α, the base sequence of C. bannonii matched our strains exactly, while there were 2 variations in C. elliptiformis and C. clavata, 3 variations in C. sacchari-officinarum, and 4 variations in C. eleusinicola and C. guangxiensis. GAPDH exhibited a range of 1 to 8 variations at the base level. In Curvularia, it appears that the mutation rate of DNA bases in the GAPDH gene region was evidently higher compared to the ITS and EF-1α gene regions.
Fig. 2.
The combined dataset of ITS, GAPDH, and EF-1α sequences was used to infer the phylogenetic tree of the genus Curvularia using Maximum Likelihood (ML) and Bayesian inference (BI). The tree was rooted with Bipolaris distoseptata (CGMCC 3.19361). The Ultrafast bootstrap values (left) greater than 70% and Bayesian posterior probabilities (right) exceeding 0.7 are indicated at the nodes. The examined isolates in present study are identified as the new species C. qinzhouensis, highlighted in red.
Table 1.
Pairwise comparison of the examined loci of newly identified species with closely related taxa.
| Novel species | Closely related taxa | Loci, identity and percentage and gaps | |||||
|---|---|---|---|---|---|---|---|
| ITS | GAPDH | EF-1α | |||||
| Identity and percentage | Gaps | Identity and percentage | Gaps | Identity and percentage | Gaps | ||
|
Curvularia qinzhouensis sp. nov. (CGMCC3.25225) |
C. bannonii | 395/397 (99.50%) | 1/397 (0%) | 581/586 (99.15%) | 0/586 (0%) | 804/804 (100%) | 0/804 (0%) |
| C. elliptiformis | 539/541 (99.63%) | 1/541 (0%) | 591/599 (98.66%) | 0/599 (0%) | 924/926 (99.78%) | 0/926 (0%) | |
| C. eragrostidis | 571/574 (99.48%) | 2/574 (0%) | 553/554 (99.82%) | 0/554 (0%) | – | – | |
| C. sacchari-officinarum | 537/537 (100%) | 0/537 (0%) | 592/599 (98.83%) | 0/599 (0%) | 923/926 (99.68%) | 0/926 (0%) | |
| C. clavata | 378/380 (99.47%) | 1/380 (0%) | 491/496 (98.99%) | 0/496 (0%) | 802/804 (99.75%) | 0/804 (0%) | |
| C. eleusinicola | 565/567 (99.65%) | 1/567 (0%) | 577/585 (98.63%) | 0/585 (0%) | 895/899 (99.56%) | 0/899 (0%) | |
| C. guangxiensis | 531/531 (100%) | 0/531 (0%) | 594/599 (99.17%) | 0/599 (0%) | 922/926 (99.57%) | 0/926 (0%) | |
| C. deserticola | 588/592 (99.32%) | 3/592(0%) | 572/580 (98.62%) | 0/580(0%) | 913/916 (99.67%) | 0/916(0%) | |
Taxonomy
Curvularia qinzhouensis H. Zhou & H.Y. Wang, sp. nov. (Fig. 3).
Fig. 3.
Morphological characteristics of the strain C. qinzhouensis CGMCC 3.25225: (a, b) Colony of the strain C. qinzhouensis on PDA; (c, d) Colony of the strain C. qinzhouensis on OA; (e, f) Colony of the strain C. qinzhouensis on MEA. (g, c–i) Conidia with conidiophore. (j) Conidia. Scale bars: g-i = 20 μm; j = 10 μm.
MycoBank accession number: MB# 849959.
Etymology. Name refers to the city in Guangxi Province where the strain was isolated.
Type. CHINA, Guangxi Province, Qinzhou City, on Curcuma kwangsiensis, May. 2021.
Hyphae branched, septate, smooth, 1.5–4 μm diam. Conidiophores arising singly or in groups, branched, straight or flexuous, septate, asperulate or smooth, with a thicker cell compared to vegetative hyphae, pale brown to dark brown in color, measuring 35.1-113.3 × 3.8–5.6 μm (average ± SD: = 72.6 ± 22.1 × 4.6 ± 0.5 μm, n = 30). Conidiogenous cells intercalary or terminal, sympodially proliferating, subcylindrical, smooth-walled, light brown to brown, measuring 4.8–32.1 × 3.2–6.7 μm (average ± SD: 17.6 ± 7.7 × 4.7 ± 0.7 μm, n = 50) Conidia usually ellipsoidal, 2–3 septate, central cells slightly larger, apical and basal cells slightly paler compared to the central cells, middle septum truly median, often thick and dark, smooth, dark brown, size 15.5–24.1 × 7.4–12.8 μm (average ± SD: 19.3 ± 1.9 × 9.9 ± 1.1 μm, n = 60). Chlamydospores not observed. Hilum slightly protruding thickened, darkened.
Colonies on PDA grown in the dark exhibited rapid growing, attaining 8.2 cm diam after 7 days at 28 °C, colony flat, entire edge, aerial mycelia dense, greenish black; reverse blackish green. Colonies on OA grown in the dark reaching 8.4 cm diam after 7 days at 28 °C, flat, dense, circular with entire edge, gray-green; reverse olive-green. Colonies on MEA grown in the dark reaching 7.9 cm diam in after days at 28 °C, flat, dense, circular with entire edge, gray-brown; reverse blackish green.
Notes: Curvularia qinzhouensis is most closely associated with C. bannonii [MB#135463] and other members within the sibling clade, i.e. C. elliptiformis [MB#556658], C. eragrostidis [MB#296246], C. sacchari-officinarum [MB#556670], C. eleusinicola [MB#835142], C. deserticola [MB#853988] and C. clavata [MB#329439]14–17. While, C. qinzhouensis differs from C. bannonii and C. eragrostidis in having shorter conidiophore (35.1–113.3 μm vs. about 950 μm and 90–710 μm, respectively)15,18. C. qinzhouensis can be distinguished by the shape or size of its conidia (15.5–24.1 × 7.4–12.8 μm for ellipsoidal shape) compared to those in C. bannonii (24-34.9 × 13–17 μm) and C. clavata (14.5–29 × 4–9 μm for clavate shape)15. C. qinzhouensis differs from C. guangxiensis in conidia size and colony appearance. C. qinzhouensis has ellipsoidal conidia measuring 15.5–24.1 × 7.4–12.8 μm, while C. guangxiensis has ovoid or globose/subglobose conidia ranging from 12 to 24 μm in length and 9–18 μm in width. Moreover, C. qinzhouensis forms dense, greenish-black colonies on PDA and flat, gray-green colonies on OA, while C. guangxiensis has sparse, light grey colonies on PDA and raised, light grey colonies on OA. C. qinzhouensis differs from C. elliptiformis and C. sacchari-officinarum in its conidial and colony characteristics. Specifically, C. qinzhouensis has smooth conidia with paler apical and basal cells, a thick, dark middle septum, and a slightly protruding hilum. C. elliptiformis has conidia with one pale brown cell at the apex or base, three noticeably brown cells, a lighter, thinner middle septum, and a non-protruding hilum. C. sacchari-officinarum has slightly verruculose conidia with a lighter, thinner middle septum and a non-protruding hilum15. On PDA, C. qinzhouensis forms flat, greenish black colonies, C. elliptiformis has slightly radiating margins and color variation, and C. sacchari-officinarum shows slightly raised colonies with varying colors. On OA, C. qinzhouensis forms flat, gray-green colonies, C. elliptiformis forms crateriform white colonies, and C. sacchari-officinarum forms gray-white colonies15. C. qinzhouensis differs from C. eleusinicola in its shorter conidiophore (35.1–113.3 μm vs. ~150 μm). Additionally, C. qinzhouensis has entire edges and greenish-black colonies, while C. eleusinicola has undulate margins, moderate aerial mycelia, a dark brown center, pale brown periphery, and dark brown concentric rings18. C. qinzhouensis differs from C. deserticola by its longer conidiophore cells (4.8–32.1 μm vs. 4–6 μm). Moreover, C. qinzhouensis forms dense, greenish-black colonies on PDA and gray-green colonies on OA, while C. deserticola has olive to silver colonies with sparse aerial mycelia on PDA and brown colonies with olive-colored centers on OA17. C. clavata has been documented on various plant species in different regions, including Eleusine coracana in Sri Lanka, Dactyloctenium aegyptium in Australia, Oryza sativa in India, Saccharum officinarum in Guyana, Curcuma wenyujin in China, and Tobacco in China18–20.C. elliptiformis and C. sacchari-officinarum have been reported on Saccharum officinarum in China15. C. bannonii has been found on Stipagrostis ciliate and Jacquemontia tamnifolia in the USA14,17. C. eragrostidis has been observed on Sorghum seed in Java21.
Biological characterization of C. Qinzhouensis
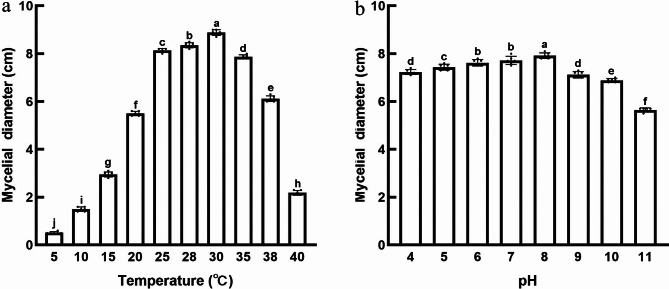
Previous studies have demonstrated the significant impact of various factors, such as temperature and acidity, on the proliferation and virulence of pathogens22,23. In the case of C. qinzhouensis, Fig. 4a shows that it can grow within a temperature range of 10–40 °C. Among these temperatures, the highest growth rate was observed at 30 °C, resulting in colony diameters of approximately 89.0 ± 1.00 mm after 6 days of incubation. Slower colony growth was observed at 15 °C, 20 °C, 38 °C, and 40 °C. The slowest growth occurred at 10 °C, with colony diameters of approximately 15.6 ± 0.60 mm after 6 days of incubation. These findings indicate that C. qinzhouensis thrives best within the temperature range of 25–35 °C, which is considered the most favorable temperature range.
Fig. 4.
Mycelial growth of C. qinzhouensis under different pH and temperature on PDA. (a) Temperature. (b) pH. The data are presented as the mean ± SD (n = 3 biologically replicates). The different letters above the columns signify a significant difference (p < 0.05, one-way ANOVA with Duncan’s multiple range test) in the data among the groups.
This study reveals that C. qinzhouensis is capable of growing within a pH range of 4 to 11, as depicted in Fig. 4b. At pH 11, the mycelia exhibited slow growth, resulting in small and scattered colony diameters measuring 56.5 ± 0.84 mm. Conversely, the colony displayed the highest growth rate at pH 8, with diameters of 79.2 ± 1.17 mm. There were no significant differences in growth between the experimental groups at pH values of 6 and 7. These findings suggest that C. qinzhouensis thrives in both acidic and mildly alkaline environments but experiences hindered growth under highly alkaline conditions.
Antimicrobial activity of phytochemicals against mycelial growth
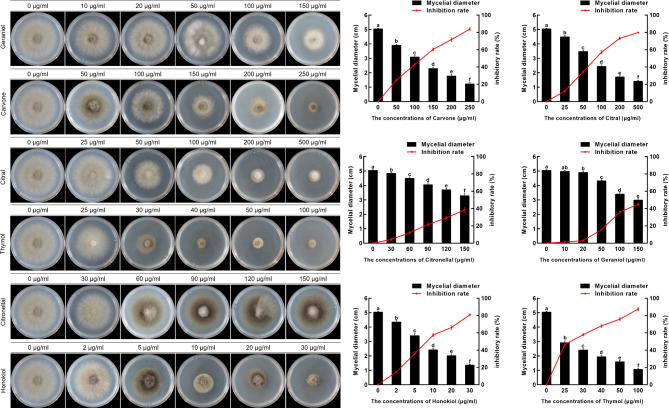
This study highlights the potential benefits of examining phytochemicals for the development of environmentally friendly and non-toxic fungicides to effectively manage C. qinzhouensis. Results from sensitivity testing, presented in Table 2; Fig. 5, indicate a strong correlation (correlation coefficients > 0.90) between phytochemical dosage and inhibition of C. qinzhouensis. Notably, Honokiol exhibited significant antifungal activity with half-maximal effective concentration (EC50) value of 6.72 ± 1.75 µg/ml. Thymol and citral demonstrated EC50 values of 25.74 ± 4.30 µg/ml and 54.24 ± 4.69 µg/ml, respectively. These were followed by geraniol, with EC50 values of 77.09 ± 10.83 mg/l. Conversely, carvone and citronellal displayed the lowest antifungal activity against the pathogen, with EC50 values of 161.53 ± 22.47 mg/ml and 134.00 ± 30.37 mg/ml, respectively.
Table 2.
The activity of phytochemicals in inhibiting the growth of C. Qinzhouensis.
| Phytochemicals | Concentration (µg/ml) | Regression equation | EC50 (µg/mL) | Coefficient of determination (R2) |
|---|---|---|---|---|
| Geraniol | 10, 20, 50, 100, 150 | y = 2.077x + 0.4268 | 77.09 ± 10.83 | 0.9805 |
| Carvone | 50, 100, 150, 200, 250 | y = 2.322x + 0.2854 | 161.53 ± 22.47 | 0.9649 |
| Citral | 25, 50, 100, 200, 500 | y = 1.550x + 1.883 | 54.24 ± 4.69 | 0.9302 |
| Thymol | 25, 30, 40, 50, 100 | y = 2.039x + 2.201 | 25.74 ± 4.30 | 0.9795 |
| Citronellal | 30, 60, 90, 120, 150 | y = 2.031x + 0.2508 | 134.00 ± 30.37 | 0.9698 |
| Honokiol | 2, 5, 10, 20, 30 | y = 1.557x + 3.530 | 6.72 ± 1.75 | 0.9801 |
Fig. 5.
Effects of different phytochemicals on the growth of C. qinzhouensis. (a) Mycelial growth inhibition of C. qinzhouensis CK56.3 after the application of different phytochemicals under a series of concentrations. (b) Effects of six phytochemicals on the mycelial growth of C. qinzhouensis after 3 days in vitro. The data are presented as the mean ± SD (n = 3 biologically replicates). The different letters above the columns signify a significant difference (p < 0.05, one-way ANOVA with Duncan’s multiple range test) in the data among the groups.
Discussion
The three isolates in this study exhibited morphological similarities with five known Curvularia species: C. bannonii, C. elliptiformis, C. eragrostidis, C. radici-foliigena, and C. clavata14–16. However, they also displayed slight differences that did not correspond to any of these species. The key morphological characteristics that distinguish our strains from related species were the dimensions of conidiophores and conidia. The conidial length of C. qinzhouensis was very close to C. eragrostidis (15.5–24.1 × 7.4–12.8 μm vs. 13.8–24.0 × 7.6–13.6 μm), while C bannonii produced the larger conidia (24-34.9 × 13–17 μm). Conidiophores of C. qinzhouensis were shorter than those of C. bannonii and C. eragrostidis14. Nevertheless, accurate microscopic identification of these organisms remains challenging due to overlapping physical characteristics. To address this, we employed a multi-locus analysis utilizing ITS, GAPDH, and EF-1α gene sequences, which also confirmed that these isolates belong to a novel species with strong bootstrap support (Fig. 2). Furthermore, we conducted a comparison of the DNA sequence similarity of the ITS, GAPDH, and EF-1α gene regions (Table 1). The EF-1α gene of C. bannonii had the same base sequence as our strains, except for a single bp difference in the ITS gene region. However, in the GAPDH gene regions, there were 5 bp difference characters observed between C. qinzhouensis and C. bannonii. Notably, within the Curvularia genus, GAPDH exhibits a higher rate of evolution compared to ITS and EF-1α.
Curvularia is a widely distributed genus known for causing various plant diseases4. Curvularia infection are common fungal diseases. For instance, C. lunata infection causes leaf spots on maize24 and root rot on Strawberry25. Leaf spot on Sansevieria trifasciata11 and postharvest rot on Pineapple26 can be caused by C. eragrostidis, while C. hawaiiensis infection leads to leaf spot on rice27. In this study, the pathogenicity analysis revealed that diseased seedlings exhibited yellow, watery, and irregular lesions on their leaves. As the infection progressed, the entire leaf dried up, turned yellow, and eventually perished. To our knowledge, this is the first report of leaf spot on C. kwangsiensi caused by C. qinzhouensis worldwide. Investigating the optimal growth temperature and pH for pathogenic fungi helps understand their ecological characteristics, transmission pathways, and aids in the development of effective control measures. The pathogen thrives and grows rapidly in warmer environments (25–35 °C), suggesting a higher likelihood of causing plant diseases during warmer seasons. The fungus shows adaptability to higher pH levels (pH 8), indicating its ability to survive and reproduce better in alkaline soil or alkaline environments. As a newly discovered species of Curvularia, further investigation is needed to determine the host range of C. qinzhouensis.
Phytochemicals derived from natural sources have gained attention for their potential in managing plant diseases. These compounds offer advantages such as minimal toxicity, low potential for fungicide resistance, and suitability for organic agriculture28. In our study, we tested six phytochemical agents for their antifungal properties against C. qinzhouensis. Honokiol, thymol, and citral were found to effectively suppress the growth of the fungus. On the other hand, carvone and citronellal showed the least effective control. Further research is needed to fully understand the antifungal effects of these phytochemical agents, and their efficacy in controlling leaf diseases should be evaluated under field conditions.
Materials and methods
Sample collection and fungal isolation
We obtained the permission to collect symptomatic plants of C. kwangsiensisi from private plantations, Qinzhou City, Guangxi Province, China in 2021. The plants were identified by Dr. Qi Gao (Professor, Plant resources Conservation and Utilization Laboratory, Guangxi Minzu University, Nanning, China), assigned voucher no. CK # 2021050022 and were deposited in the Virtual Herbarium, Plant resources Conservation and Utilization Laboratory, Guangxi Minzu University, Nanning, China. The latitude of the sampling place is 21.51°N and longitude is 108.44°E. To investigate the disease, six randomly selected symptomatic plants were subsequently transported to the laboratory for analysis. Tissue isolation and single spore purification methods were employed to obtain isolates associated with leaf spot. In brief, plant samples were cleaned and cut into small pieces (~ 5 mm), followed by surface sterilization using 75% ethanol for 2 min. Subsequently, they were soaked in a 1% sodium hypochlorite solution for 60 s and rinsed three times with sterile distilled water for 60 s each. The tissues were placed on potato dextrose agar (PDA, containing 200 g/L potato, 20 g/L glucose and 20 g/L agar) and incubated in the dark at 28 °C for 3 days. Cultures were obtained by transferring hyphal tips from edge of colonies to fresh PDA. For single spore purification, the fungal material was collected using a sterilized inoculating needle and placed in sterilized distilled water. The material was then mechanically disrupted to obtain a suspension of spores. After being spread on water agar (WA, containing 20 g/L agar), the spore suspension was incubated overnight at 28 °C. Germinated spores were marked under a microscope and then moved to PDA and cultivated at 28 °C to obtain pure cultures29,30. The pure cultures were stored at 4 °C. in the Microbiology laboratory, Guangxi Minzu University, China.
Pathogenicity assay
To confirm the pathogenicity of the fungi, nine healthy seedlings of C. kwangsiensi that had been growing for four weeks, were inoculated with mycelial plugs (5 mm in diameter) cut from the edges of actively growing cultures cultivated on WA in the dark at 28 °C for 5 days. As a control, three plants were treated with sterile WA. The greenhouse maintained a temperature of 35 °C with a 12-hour photoperiod and around 90% relative humidity for all the inoculated plants. Following a three-day period of incubation, the inoculated plants were monitored for the development of symptoms. The experiments were repeated three times. Infected leaves were collected and the pathogens were re-isolated using the tissue isolation method. Morphological and sequencing analyses were conducted to compare the re-isolated pathogens with the original strains.
DNA extraction, sequencing and phylogenetic analyses
Genomic DNA was extracted from freshly mycelia grown on PDA using the CTAB method31, with a slight modification. The extracted DNA was then stored at -20 °C. PCR amplification of ITS region was performed using the primer pair ITS1 and ITS4. For GAPDH amplification, the primer pair GPD-1 and GPD-2 was utilized, while EF-1α was amplified with the primer pair EF1-983 F and EF1-2218R, as specified in Table 3.
Table 3.
Primers and references used in this study.
The PCR reaction mixture was prepared with a total volume of 25 µL. It contained 2.5 µL of 10 × PCR reaction Buffer, 125 µM of each dNTP, 0.4 µM of each primer, 0.1 µL of EasyTaq DNA polymerase (Trans gene, Beijing, China), and 1 ng of genomic DNA. The PCR parameters for amplifying ITS, GAPDH, and EF-1α were as follows: an initial denaturation at 95 °C for 4 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 60 s, and extension at 72 °C for 90 s, with a final elongation step of 10 min at 72 °C. The final PCR products were analyzed by electrophoresis in 1% agarose gel. The PCR samples were sent to a commercial provider (Audio Codes Dingsheng Biotechnology Co., Ltd, Wuhan) for Sanger sequencing.
In this study, the phylogenetic analysis comprised the isolates CK43.1, CK56.3, CK64.5, as well as reference isolates representing a total of 64 Curvularia species (Table 4). Strain CGMCC 3.19361 of Bipolaris distoseptata was used as the outgroup. Phylogenetic tree construction was performed using PhyloSuite software35 through Bayesian inference (BI) and Maximum Likelihood (ML) methods, based on a combined dataset of ITS, GAPDH, and EF-1α sequences. Nodes are indicated with Ultrafast bootstrap values (left) that are higher than 70% and Bayesian posterior probabilities (right) that surpass 0.7.
Table 4.
Strains and their GenBank accession numbers used in the present study.
| Species | Strain accession | GenBank accession | ||
|---|---|---|---|---|
| ITS | GAPDH | EF-1α | ||
| Curvularia akaiiensis | BRIP 16,080 T | KJ415539 | KJ415407 | KJ415453 |
| Curvularia alcornii | MFLUCC 10–0703 T | JX256420 | JX276433 | JX266589 |
| Curvularia asianensis | MFLUCC 10–0711 T | JX256424 | JX276436 | JX266593 |
| Curvularia australiensis | BRIP 12,044 T | KJ415540 | KJ415406 | KJ415452 |
| Curvularia australis | BRIP 12,521 T | KJ415541 | KJ415405 | KJ415451 |
| Curvularia bannonii | BRIP 16,732 IsoT | KJ415542 | KJ415404 | KJ415450 |
| Curvularia beasleyi | BRIP 10,972 T | MH414892 | MH433638 | MH433654 |
| Curvularia beerburrumensis | BRIP 12,942 T | MH414895 | MH433634 | MH433657 |
| Curvularia boeremae | IMI 164,633 T | MH414911 | MH433641 | – |
| Curvularia borreriae | CBS 859.73 | HE861848 | HF565455 | – |
| Curvularia bothriochloae | BRIP 12,522 T | KJ415543 | KJ415403 | KJ415449 |
| Curvularia clavata | BRIP 61680b | KU552205 | KU552167 | KU552159 |
| Curvularia coatesiae | BRIP 24,261 T | MH414897 | MH433636 | MH433659 |
| Curvularia coicis | CBS 192.29 SynT | AF081447 | AF081410 | JN601006 |
| Curvularia colbranii | BRIP 13,066 T | MH414898 | MH433642 | MH433660 |
| Curvularia cymbopogonis | CBS 419.78 | HG778985 | HG779129 | – |
| Curvularia crustacea | BRIP 13,524 ET | KJ415544 | KJ415402 | KJ415448 |
| Curvularia dactyloctenii | BRIP 12,846 T | KJ415545 | KJ415401 | KJ415447 |
| Curvularia deserticola | CMW 58,190 T | ON074985 | ON355399 | ON355360 |
| Curvularia deserticola | CMW 64,024 | ON075008 | ON355400 | ON355361 |
| Curvularia dactyloctenicola | CPC 28,810 T | MF490815 | MF490837 | MF490858 |
| Curvularia eleusinicola | USJCC-0005 T | MT262877 | MT393583 | MT432925 |
| Curvularia elliptiformis | CGMCC 3.19351 T | MN215656 | MN264091 | MN263950 |
| Curvularia elliptiformis | LC12004 | MN215659 | MN264092 | MN263953 |
| Curvularia ellisii | CBS 193.62 T | JN192375 | JN600963 | JN601007 |
| Curvularia eragrostidis | CBS 189.48 | HG778986 | HG779154 | |
| Curvularia eragrosticola | BRIP 12,538 T | MH414899 | MH433643 | MH433661 |
| Curvularia falsilunata | CGMCC 3.19329 T | MN215660 | MN264093 | MN263954 |
| Curvularia gladiolii | ICMP 6160 | JX256426 | JX276438 | JX266595 |
| Curvularia graminicola | BRIP 23,186 T | JN192376 | JN600964 | JN601008 |
| Curvularia guangxiensis | CGMCC 3.19330 T | MN215667 | MN264100 | MN263961 |
| Curvularia guangxiensis | LC11997 | MN215668 | MN264104 | MN263965 |
| Curvularia guangxiensis | LC12000 | MN215671 | MN264138 | MN264000 |
| Curvularia harveyi | BRIP 57,412 T | KJ415546 | KJ415400 | KJ415446 |
| Curvularia hawaiiensis | BRIP 11,987 IsoLT | KJ415547 | KJ415399 | KJ415445 |
| Curvularia heteropogonicola | BRIP 14,579 IsoLT | KJ415548 | KJ415398 | KJ415444 |
| Curvularia heteropogonis | CBS 284.91 T | KJ415549 | JN600969 | JN601013 |
| Curvularia hominis | CBS 136,985 T | HG779011 | HG779106 | – |
| Curvularia homomorpha | CBS 156.60 T | JN192380 | JN600970 | JN601014 |
| Curvularia intermedia | CBS 334.64 | HG778991 | HG779155 | – |
| Curvularia ischaemi | CBS 630.82 T | HG778992 | HG779131 | – |
| Curvularia kenpeggii | BRIP 14,530 T | MH414900 | MH433644 | MH433662 |
| Curvularia kusanoi | CBS 137.29 | JN192381 | – | JN601016 |
| Curvularia lamingtonensis | BRIP 12,259 T | MH414901 | MH433645 | MH433663 |
| Curvularia lonarensis | CBS 140,569 T | KT315408 | KY007019 | – |
| Curvularia lunata | CBS 730.96 NeoT | JX256429 | JX276441 | JX266596 |
| Curvularia mebaldsii | BRIP 12,900 T | MH414902 | MH433647 | MH433664 |
| Curvularia microspora | GUCC 6272 T | MF139088 | MF139106 | MF139115 |
| Curvularia mosaddeghii | IRAN 3131 C T | MG846737 | MH392155 | MH392152 |
| Curvularia muehlenbeckiae | CBS 144.63 T | HG779002 | HG779108 | – |
| Curvularia neergaardii | BRIP 12,919 IsoT | KJ415550 | KJ415397 | KJ415443 |
| Curvularia nicotiae | CBS 655.74 T | KJ909772 | KM083614 | – |
| Curvularia sacchari-officinarum | CGMCC 3.19331 T | MN215705 | MN264137 | MN263998 |
| Curvularia sacchari-officinarum | LC12008 | MN215707 | MN264138 | MN264000 |
| Curvularia ovariicola | CBS 470.90 T | JN192384 | JN600976 | JN601020 |
| Curvularia papendorfii | BRIP 57,608 T | KJ415552 | KJ415395 | KJ415441 |
| Curvularia perotidis | BRIP 13,466 T | JN192385 | KJ415394 | JN601021 |
| Curvularia portulacae | BRIP 14,541 T | KJ415553 | KJ415393 | KJ415440 |
| Curvularia portulacae | BRIP 14,837 T | KJ415554 | KJ415392 | KJ415439 |
| Curvularia qinzhouensis sp. nov. | CGMCC 3.25225 = CK56.3 | OR575729 | OR576898 | OR576895 |
| Curvularia qinzhouensis sp. nov. | CK43.1 | OR575728 | OR576897 | OR576894 |
| Curvularia qinzhouensis sp. nov. | CK64.5 | OR575730 | OR576899 | OR576896 |
| Curvularia radici-foliigena | CGMCC3.19328 T | MN215695 | MN264127 | MN263988 |
| Curvularia ravenelii | BRIP 13,165 T | JN192386 | JN600978 | JN601024 |
| Curvularia richardiae | BRIP 4371 T | KJ415555 | KJ415391 | KJ415438 |
| Curvularia ryleyi | BRIP 12,554 T | KJ415556 | KJ415390 | KJ415437 |
| Curvularia sorghina | BRIP 15,900 T | KJ415558 | KJ415388 | KJ415435 |
| Curvularia spicifera | CBS 274.52 T | JN192387 | JN600979 | JN601023 |
| Curvularia tripogonis | BRIP 12,375 IsoT | JN192388 | JN600980 | JN601025 |
| Curvularia tropicalis | BRIP 14,834 T | KJ415559 | KJ415387 | KJ415434 |
| Curvularia tuberculata | CBS 146.63 IsoT | JX256433 | JX276445 | JX266599 |
| Bipolaris distoseptata | CGMCC 3.19361 T | MN215628 | MN264064 | MN263922 |
‘–’ indicates the absence of GAPDH or EF-1α genes in the GenBank accession, where T represents the Ex-type.
Morphological characterization
The examined isolates were cultured on PDA at 28 °C for 7 days in darkness. Round mycelial discs with a diameter of 5 mm were then obtained from the colony periphery and transferred to various fresh media for morphological examinations. The cultural characteristics of the isolates were analyzed by incubating them for 7 days at 28 °C in darkness on PDA, malt extract agar (MEA, containing 30 g/L malt extract and 20 g/L agar), and oatmeal agar (OA, containing 30 g/L oatmeal and 20 g/L agar). Growth rate determination was performed by measuring the radial colonial diameters in at least four different directions after 7 days of incubation at 28 °C in darkness36. Conidia were induced on WA medium, and the features of both conidia and conidiophores were observed and documented using a digital microscope (DM2000, Leica, Germany).
Biological characteristics of the pathogen
The isolates were cultured on PDA with pH values ranging from 4 to 11 at 28 °C. The pathogen-inoculated PDA (pH 7.0) plates were incubated at different temperatures in an artificial climate chamber, including 5 °C, 10 °C, 15 °C, 20 °C, 25 °C, 28 °C, 30 °C, 35 °C, and 38 °C. The growth of the colonies was monitored over a period of 6 days, with colony diameters measured using the crossover method and documented through photography37. Each experiment was repeated three times with two replicates in each.
Antimicrobial activity of phytochemicals against mycelial growth
To identify phytochemicals with effective control against the pathogen, various phytochemicals were screened. Six plant compounds provided by Macklin Biochemical Technology Co., Ltd. (China), including geraniol, citral, thymol, citronellal (dissolved in ethanol), honokiol (dissolved in dimethyl sulfoxide), and carvone/magnolol (dissolved in acetone), were used as the test substances38. Initially, the phytochemicals were dissolved in 1 ml of the suitable solvent, subsequently mixed with water, and introduced into the PDA medium (cooled to approximately 50 °C) to prepare a range of concentration gradients. Each treatment had three replicates. Mycelial disks (5 mm in diameter) were transferred from the colony edge to the center of the PDA plates, which were then incubated in the dark at 28 °C. After 3 days, the colony diameter was measured using a ruler. The experiments were repeated three times with two replicates in each.
The inhibition rate I (%) was then calculated using the provided formula, where C (cm) and T (cm) indicate the tested fungi diameters of the control and treated PDA plates, respectively. The regression equation, determination coefficient (R2), EC50 values of inhibiting mycelial growth for various phytochemicals were determined using the software GraphPad Prism 8.0.
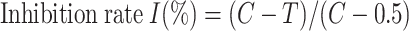 |
Statistical analysis
Data analyses were conducted using Excel 2016 and GraphPad Prism 8. GraphPad Prism 8 was employed to perform normality and homogeneity of variance tests. To determine the significance of differences, a one-way ANOVA with Duncan’s multiple range test was conducted in GraphPad Prism 8, with a significance level of p < 0.05. Graphs were generated using GraphPad Prism 8.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization and methodology, H.W. and R.W.; validation and formal analysis, J.W. and J.S.; investigation, H.W., J.X., M.X., and D.T.; writing—original draft preparation, H.W.; writing—review and editing, H.W. and H.Z.; project administration and funding acquisition, H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China, grant number 32060599; the National Natural Science Foundation of China, grant number 81860678; and the Natural Science Foundation of Guangxi Province, grant number 2021GXNSFAA075023.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hanyi Wang and Rongchang Wei contributed equally to this work
References
- 1.ZeYu, L. et al. Research progress of Curcuma kwangsiensis root tubers and analysis of liver protection and anti-tumor mechanisms based on Q-markers. Zhongguo Zhong yao za zhi Zhongguo zhongyao zazhi China journal of Chinese materia medica47, 1739–1753 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Feng, X., Zhou, L., Sheng, A., Lin, L. & Liu, H. Comparative transcriptome analysis on drought stress-induced floral formation of Curcuma kwangsiensis. Plant Signal Behav.17, 2114642. 10.1080/15592324.2022.2114642 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang, H. et al. First report of Epicoccum latusicollum causing leaf blight on Curcuma kwangsiensis in China. Plant Dis.107, 2546. 10.1094/pdis-11-22-2668-pdn (2023). [Google Scholar]
- 4.Marin-Felix, Y., Hernández-Restrepo, M. & Crous, P. W. Multi-locus phylogeny of the genus Curvularia and description of ten new species. Mycological Progress19, 559–588. 10.1007/s11557-020-01576-6 (2020). [Google Scholar]
- 5.da Cunha, K. C. et al. In vitro antifungal susceptibility and molecular identity of 99 clinical isolates of the opportunistic fungal genus Curvularia. Diagn Microbiol Infect Dis76, 168–174. 10.1016/j.diagmicrobio.2013.02.034 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Tan, Y. P., Crous, P. W. & Shivas, R. G. Cryptic species of Curvularia in the culture collection of the queensland plant pathology herbarium. MycoKeys10.3897/mycokeys.35.25665 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manamgoda, D. S. et al. A phylogenetic and taxonomic re-evaluation of the Bipolaris - Cochliobolus - Curvularia Complex. Fungal Diversity56, 131–144. 10.1007/s13225-012-0189-2 (2012). [Google Scholar]
- 8.Hernandez-Restrepo, M. et al. Multi-locus phylogeny and taxonomy of Exserohilum. Persoonia41, 71–108. 10.3767/persoonia.2018.41.05 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iturrieta-Gonzalez, I., Gene, J., Wiederhold, N. & Garcia, D. Three new Curvularia species from clinical and environmental sources. MycoKeys68, 1–21. 10.3897/mycokeys.68.51667 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Addrah, M. E. et al. Fungicide treatments to control seed-borne fungi of sunflower seeds. Pathogens9, 10.3390/pathogens9010029 (2019). [DOI] [PMC free article] [PubMed]
- 11.Kee, Y. J., Zakaria, L. & Mohd, M. H. Curvularia asianensis and Curvularia eragrostidis associated with leaf spot of Sansevieria trifasciata in Malaysia. Journal of Phytopathology168, 290–296. 10.1111/jph.12890 (2020). [Google Scholar]
- 12.Liu, Q., Qiao, K. & Zhang, S. Potential of a small molecule carvacrol in management of vegetable diseases. Molecules24, 10.3390/molecules24101932 (2019). [DOI] [PMC free article] [PubMed]
- 13.Gao, T. et al. The fungicidal activity of thymol against Fusarium graminearum via inducing lipid peroxidation and disrupting ergosterol biosynthesis. Molecules21, 10.3390/molecules21060770 (2016). [DOI] [PMC free article] [PubMed]
- 14.Tan, Y. P., Madrid, H., Crous, P. W. & Shivas, R. G. Johnalcornia gen. et. comb. nov. and nine new combinations in Curvularia based on molecular phylogenetic analysis. Australasian Plant Pathology43, 589–603. 10.1007/s13313-014-0315-6 (2014). [Google Scholar]
- 15.Raza, M., Zhang, Z.-F., Hyde, K. D., Diao, Y.-Z. & Cai, L. Culturable plant pathogenic fungi associated with sugarcane in southern China. Fungal Diversity99, 1–104. 10.1007/s13225-019-00434-5 (2019). [Google Scholar]
- 16.Su, H.-Y. et al. Hyphomycetes from aquatic habitats in Southern China: Species of Curvularia ( Pleosporaceae ) and Phragmocephala (Melannomataceae ). Phytotaxa226, 201–216. 10.11646/PHYTOTAXA.226.3.1 (2015). [Google Scholar]
- 17.van Vuuren, N., Yilmaz, N., Wingfield, M. J. & Visagie, C. M. Five novel Curvularia species (Pleosporaceae, Pleosporales) isolated from fairy circles in the Namib desert. Mycological Progress23, 39. 10.1007/s11557-024-01977-x (2024). [Google Scholar]
- 18.Ferdinandez, H. S. et al. Molecular phylogeny and morphology reveal three novel species of Curvularia (Pleosporales, Pleosporaceae) associated with cereal crops and weedy grass hosts. Mycological Progress20, 431–451. 10.1007/s11557-021-01681-0 (2021). [Google Scholar]
- 19.Cao, Y. et al. Genome sequence resource of Curvularia clavata causing leaf spot disease on tobacco by oxford Nanopore promethion. Plant Disease107, 1916–1919. 10.1094/pdis-09-22-2283-a (2023). [DOI] [PubMed] [Google Scholar]
- 20.Chen, X. Y., Feng, J. D., Su, Z., Sui, C. & Huang, X. First report of Curvularia leaf blight on Curcuma wenyujin caused by Curvularia clavata in China. Plant Disease97, 138–138. 10.1094/pdis-04-12-0392-pdn (2013). [DOI] [PubMed] [Google Scholar]
- 21.Madrid, H. et al. Novel Curvularia species from clinical specimens. Persoonia33, 48–60. 10.3767/003158514x683538 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur, S., Barakat, R., Kaur, J. & Epstein, L. The effect of temperature on disease severity and growth of Fusarium oxysporum f. sp. apii Races 2 and 4 in Celery. Phytopathology®112, 364–372. 10.1094/phyto-11-20-0519-r (2022). [DOI] [PubMed] [Google Scholar]
- 23.Wan, W. et al. Responses of the rhizosphere bacterial community in acidic crop soil to pH: Changes in diversity, composition, interaction, and function. Sci Total Environ700, 134418. 10.1016/j.scitotenv.2019.134418 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Shaoqing, W. et al. Curvularia lunata and Curvularia leaf spot of maize in China. ACS Omega7, 47462–47470. 10.1021/acsomega.2c03013 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verma, V. S. & Gupta, V. K. First report of Curvularia lunata causing root rot of strawberry in India. Plant Dis94, 477. 10.1094/pdis-94-4-0477c (2010). [DOI] [PubMed] [Google Scholar]
- 26.Ferreira, A. P. S., Pinho, D. B., Machado, A. R. & Pereira, O. L. First report of Curvularia eragrostidis causing postharvest rot on pineapple in Brazil. Plant Dis98, 1277. 10.1094/pdis-03-14-0288-pdn (2014). [DOI] [PubMed] [Google Scholar]
- 27.Imran, M., Khanal, S., Zhou, X.-G., Antony-Babu, S. & Atiq, M. First Report of brown leaf spot of rice caused by Curvularia hawaiiensis in the United States. Plant Disease106, 2527. 10.1094/pdis-10-21-2253-pdn (2022). [Google Scholar]
- 28.Lamichhane, J. R., Dachbrodt-Saaydeh, S., Kudsk, P. & Messéan, A. Toward a reduced reliance on conventional pesticides in European agriculture. Plant Disease100, 10–24. 10.1094/pdis-05-15-0574-fe (2016). [DOI] [PubMed] [Google Scholar]
- 29.Zhang, K., Su, Y.-Y. & Cai, L. An optimized protocol of single spore isolation for fungi. Cryptogamie Mycologie34, 349–356. 10.7872/crym.v34.iss4.2013.349 (2013). [Google Scholar]
- 30.Phookamsak, R. et al. Towards a natural classification of Astrosphaeriella-like species; introducing Astrosphaeriellaceae and Pseudoastrosphaeriellaceae fam. Nov. and Astrosphaeriellopsis, gen. nov.. Fungal Diversity74, 143–197. 10.1007/s13225-015-0352-7 (2015). [Google Scholar]
- 31.Porebski, S., Bailey, L. G. & Baum, B. R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Molecular Biology Reporter15, 8–15. 10.1007/BF02772108 (1997). [Google Scholar]
- 32.White, T. J., Bruns, T. D., Lee, S. & Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. 315–322 (Academic Press, 1990).
- 33.Berbee, M. L., Pirseyedi, M. & Hubbard, S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia91, 964–977. 10.2307/3761627 (1999). [Google Scholar]
- 34.Carbone, I., Anderson, J. & Kohn, L. Patterns of descent in clonal lineages and their multilocus fingerprints are resolved with combined gene genealogies. Evolution53, 11–21. 10.2307/2640916 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Zhang, D. et al. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour20, 348–355. 10.1111/1755-0998.13096 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Sandoval-Denis, M., Lombard, L. & Crous, P. W. Back to the roots: a reappraisal of Neocosmospora. Persoonia43, 90–185. 10.3767/persoonia.2019.43.04 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui, L. et al. Identification and biological characterization of a new pathogen that causes potato scab in Gansu Province. China. Microb Pathog161, 105276. 10.1016/j.micpath.2021.105276 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Wei, Z., Anlong, H., Mingjian, R., Guoyu, W. & Huayang, X. First report on Colletotrichum fructicola causing anthracnose in chinese sorghum and its management using phytochemicals. J Fungi (Basel)9, 279. 10.3390/jof9020279 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.