Abstract
This study investigates the prevalence of dyslipidemia and its association with disease activity in children with Juvenile Dermatomyositis (JDM). A retrospective chart review of 142 JDM patients who had fasting lipid profiles was conducted. Clinical, and laboratory indicators of disease activity at the time of lipid assessment were obtained. JDM patients displayed a high prevalence (72%) of abnormal or borderline fasting lipid profiles, particularly involving HDL and triglycerides. Treatment-naïve patients exhibited the most significant dyslipidemia, with significantly lower median HDL levels compared to those on medication (30 vs. 49 mg/dL, p < 0.0001). HDL levels inversely correlated with various disease activity measures, including disease activity score (DAS) total (r= -0.38, p < 0.001), DAS muscle weakness (r= -0.5, p < 0.001), DAS skin (r= -0.25, p = 0.003), neopterin (r= -0.41, p < 0.001), ESR (r= -0.25, p = 0.006), and vWF Ag (r= -0.21, p = 0.02). In conclusion, JDM patients have a high prevalence of dyslipidemia, especially low HDL and elevated triglycerides. The severity of dyslipidemia (low HDL) correlates with disease activity, with treatment-naïve patients demonstrating the lowest HDL levels. These findings suggest the importance of annual lipid profile monitoring in JDM patients, potentially followed by early interventions such as dietary adjustments and exercise programs.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77985-4.
Keywords: Juvenile Dermatomyositis, Dyslipidemia, Disease Activity scores, HDL, Neopterin
Subject terms: Rheumatic diseases, Idiopathic inflammatory myopathies, Paediatric rheumatic diseases, Vasculitis syndromes, Dyslipidaemias
Introduction
Juvenile Dermatomyositis (JDM) is a rare autoimmune disorder characterized by chronic muscle and skin inflammation1, with an estimated annual incidence of 2.7–3.4 cases per million children2. The hallmark clinical features of JDM include muscle weakness and distinctive skin rashes such as Gottron papules and heliotrope rash1,3. Adult patients with systemic autoimmune myopathies are at increased risk of developing atherosclerotic coronary artery disease and display coronary artery disease, diastolic dysfunction, myocarditis, and ECG abnormalities4. Cardiovascular damage is a major cause of mortality for adult patients with systemic autoimmune myopathies4,5. Older patients who had JDM in childhood have a higher risk of atherosclerosis, documented by increased intima-media thickness6. This heightened risk is likely due to a combination of factors: traditional risk factors like hypertension, dyslipidemia, and obesity7,8 in addition to ongoing inflammation, endothelial injury9,10, and the prolonged use of corticosteroid therapy8. Early identification and management of modifiable cardiovascular risk factors, such as hypertension and dyslipidemia, could improve long-term outcomes for JDM patients.
Dyslipidemia, an abnormal lipid profile, is associated with many rheumatological disorders such as systemic lupus erythematosus (SLE)11, and rheumatoid arthritis12,13. Dyslipidemia in SLE is characterized by decreased high-density lipoprotein (HDL), increased triglycerides (TG), and low-density lipoprotein (LDL)14,15. In addition, SLE disease activity correlated with the degree of dyslipidemia11. While smaller studies in JDM have shown a link to dyslipidemia16, larger cohort studies are needed. The aim of this study is to assess the prevalence of dyslipidemia in JDM and its relation to disease activity and chronic inflammation.
Methods
Study design
This retrospective chart review study, approved by Ann & Robert H. Lurie Children’s Hospital of Chicago Institutional Review Board (IRB# 2012–14858), was conducted at Lurie Children’s spanning the years 1980 to 2021. The study included all children who met the Bohan and Peter criteria17 for JDM and underwent fasting lipid profile assessment in the course of clinical care. Only fasting lipid profiles were included in the analysis due to the potential impact of recent meals on triglyceride levels18. Therefore, patients with only random lipid profiles were excluded. The lipid profile was assessed by Lurie Children’s Laboratory, which included measurements for total cholesterol, HDL cholesterol, TGs, and calculated LDL cholesterol. Based on the 2011 AAP guidelines for cardiovascular health, the lipid profile data were categorized into three groups: acceptable, borderline, and abnormal (Supplemental Table 1)19.
Study population
This study included 142 pediatric patients diagnosed with JDM. Females predominated the study population, comprising 75.4% of the total. Racial and ethnic distribution revealed 70% white (non-Hispanic), 20% Hispanic (white), 4% African American, and 6% other races/ethnicities (Table 1). The distribution of myositis-specific antibodies (MSAs) was as follows: 35% anti-P155/140+, 20% anti-MJ+, 4% anti-MDA-5+, 3% anti-Mi-2+, and 11% multiple MSAs or other, and 26% MSA negative (Table 1). 5.6% of the subjects had other antibodies suggestive of overlap syndrome (3.5% anti-U1RNP and 2.1% anti-U5RNP), though the majority of these antibodies exhibited weak expression. At the time of lipid assessment, the average age of the JDM children was 11.9 ± 5.2 years. A majority of the study subjects (68.3%) were undergoing immunosuppressive medication, with details available in Supplemental Table 2. Of note, 43% of the total study subjects (63% of the patients in the medication group) were taking corticosteroids at the time of the lipid profile. The mean dose of corticosteroids was 12 ± 12.4 mg, with a wide range from 1 mg to 80 mg per day. Nineteen of the JDM children were treatment-naïve and sub-analysis was done on this group to evaluate the impact of the disease process on dyslipidemia in isolation of the medication confounding effect.
Table 1.
Demographic and disease characteristics of the JDM cohort.
| Frequency (n) | Percentage | |
|---|---|---|
| Sample size | 142 | |
| Sex | ||
| Female | 107 | 75.4% |
| Male | 35 | 24.6% |
| Race/Ethnicity | ||
| White, non-Hispanic | 99 | 69.7% |
| White, Hispanic | 29 | 20.4% |
| African American | 6 | 4.2% |
| Others | 8 | 5.6% |
| Myositis Specific Antibodies | ||
| P155/140 | 49 | 34.5% |
| MJ | 28 | 19.7% |
| Mi2 | 4 | 2.8% |
| MDA5 | 6 | 4.2% |
| Others or multiple MSAs | 15 | 10.6% |
| Negative | 37 | 26.1% |
| Not done | 3 | 2.1% |
| Treatment Status | ||
| Treatment-naïve | 19 | 13.4% |
| On Medication | 97 | 68.3% |
| Off Medication | 26 | 18.3% |
Disease activity assessment
JDM disease activity was evaluated clinically using disease activity scores (DAS) for skin (S), muscle weakness (M), and total (T)20, as well as Childhood Myositis Assessment Scale (CMAS)21 and nailfold capillary end row loops (ERL) count9. One experienced observer examined ERL images, counting capillaries per 3 mm section in each finger (excluding thumbs). The average number of capillaries per millimeter (ERL/mm) was then calculated by averaging the values from all eight fingers9. Additionally, laboratory tests to assess disease activity were conducted at the time of the lipid profile assessment. These laboratory tests included muscle enzyme (creatine phosphokinase [CK], aspartate aminotransferase [AST], lactate dehydrogenase [LDH], and aldolase), von Willebrand factor antigen (vWF Ag), erythrocyte sedimentation rate (ESR) and serum neopterin. Serum neopterin was measured using an enzyme-linked immunosorbent assay (ELISA) method22. MSAs were assessed through immunoprecipitation and immunodiffusion at the Oklahoma Medical Research Foundation23.
Statistical analysis
The Mann-Whitney U test was used to compare the median levels of non-parametric variables, such as TG and HDL cholesterol levels. For categorical value, The Chi-square test was utilized. Spearman’s correlation coefficient was employed to explore the association between HDL level and various disease activity indicators in JDM subjects. Statistical analyses were performed using IBM SPSS Statistics®, while GraphPad Prism® version 10.2 was used to generate the corresponding figures.
Results
Fasting lipids
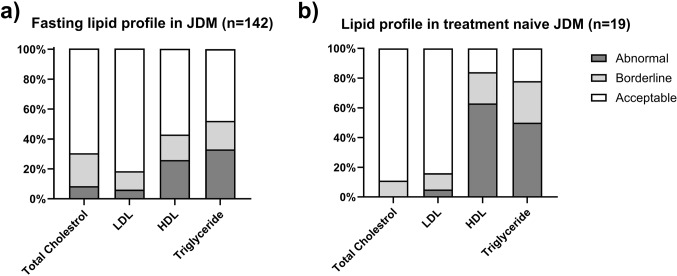
The analysis of lipid profiles in JDM patients revealed significant abnormalities in TG and HDL levels. Over half (52%) of participants had elevated or borderline-high fasting TG, while nearly half (43%) had low or borderline-low HDL. These dyslipidemia features were more pronounced in treatment-naïve JDM patients, with only 22% and 16% showing normal TG and HDL levels, respectively. Conversely, total cholesterol and LDL cholesterol remained within the normal range for most treatment-naïve individuals (70% and 82%, respectively) (Fig. 1). Treatment status (treatment-naïve, on medication, off medication) significantly impacted HDL levels with the treatment-naïve group showing a significantly lower level of HDL than the “on medication” group (median level 30 vs. 49, p < 0.0001). LDL and TG levels did not show significant differences between treatment groups (Fig. 2).
Figure 1.
The prevalence of abnormal lipid profile in juvenile dermatomyositis (JDM). (A) 43% of JDM patients exhibited low or borderline-low HDL, while only 18% of them had elevated or borderline-high fasting LDL levels. Additionally, 52% of JDM patients displayed elevated or borderline-high fasting triglyceride levels. (B) Treatment-naïve JDM patients showed a higher prevalence of lipid profile abnormalities, with only 22% and 16% having normal triglyceride (TG) and HDL levels, respectively. However, total cholesterol and LDL cholesterol levels remained within the normal range for the majority of treatment-naïve individuals (70% and 82%, respectively).
Figure 2.
Lipid profile in JDM based on the treatment status. (A) Treatment status (treatment-naïve, on medication, off medication) significantly impacted HDL levels with the treatment-naïve group displaying significantly lower HDL levels compared to the “on medication” group (median level 30 vs. 49, p < 0.0001). (B) There were no significant differences observed in low-density lipoprotein (LDL) among the different treatment groups. (C) TG levels did not show significant differences between the different treatment groups.
Clinical disease activity markers and HDL level
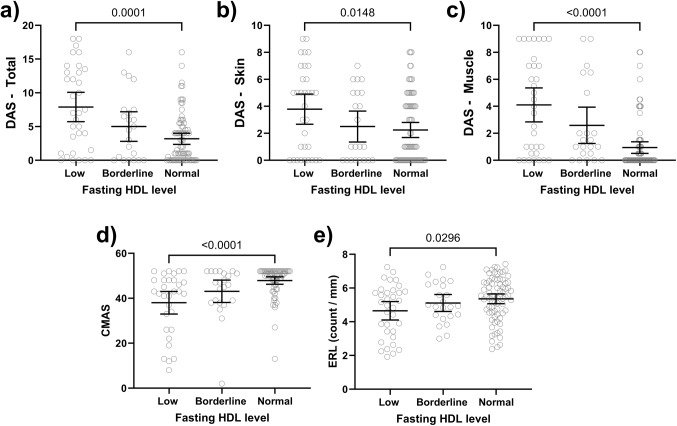
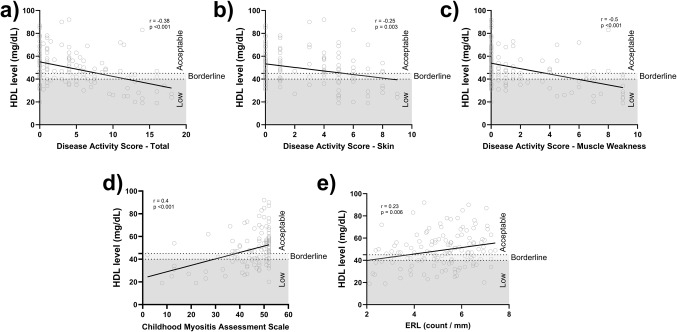
Detailed information regarding disease activity indicators for JDM children at the time of lipid profile assessment, including DAS (skin, muscle, total), CMAS, nailfold ERL count, neopterin, ESR, vWF Ag, muscle enzymes, and flow cytometry data, are presented in Table 2. Patients with low HDL exhibited more active disease compared to the normal HDL group (Fig. 3). This was evidenced by significantly higher median scores for DAS-T (p = 0.001), DAS-S (p = 0.01), and DAS-M (p < 0.001) (Table 3). Furthermore, the low HDL group had a lower CMAS than the normal HDL group (p < 0.001) which is consistent with more muscle weakness (Table 3). Nailfold capillary assessment revealed a lower ERL in the low HDL group (5.1 vs. 5.5 capillaries/mm, p = 0.03) (Table 3). We also investigated the correlation between HDL levels and various disease activity markers. HDL levels demonstrated significant correlations with several clinical disease activity indicators: DAS-T (r= -0.38, p < 0.001), DAS-M (r= -0.5, p < 0.001), DAS-S (r= -0.25, p = 0.003), CMAS (r = 0.4, p < 0.001), and ERL (r = 0.23, p = 0.006) (Fig. 4).
Table 2.
Disease activity assessment of the JDM cohort at the time of lipid profile.
| Reference Range | Mean ± SD | Median (range) | |
|---|---|---|---|
| Age at time of lipid assessment (yr) | 11.0 ± 5.1 | 10.7 (2.4–23.2) | |
| Age at time of JDM diagnosis (yr) | 6.9 ± 3.8 | 6.2 (1–18) | |
| Duration of untreated disease (mo) | 8.8 ± 11.2 | 4.9 (0.5–73) | |
| Clinical disease activity indicator | |||
| DAS total | 0 | 4.6 ± 4.9 | 4.0 (0–18) |
| DAS skin | 0 | 2.6 ± 2.7 | 1.0 (0–9) |
| DAS muscle weakness | 0 | 2.0 ± 2.9 | 0.5 (0–9) |
| CMAS | 52 | 44.7 ± 10.4 | 48 (2–52) |
| ERL (#/mm) | > 7 | 5.1 ± 1.3 | 5.3 (1.9–7.4) |
| Laboratory disease activity indicators | |||
| Neopterin (nmol/L) | < 10 | 9.0 ± 7.5 | 6.2 (3.4–49.3) |
| ESR (mm/hr) | < 20 | 12 ± 16.3 | 8 (1-120) |
| vWF Antigen | 127 ± 64 | 110 (36–411) | |
| Muscle enzymes | |||
| CK (IU/L) | 26–27 | 442 ± 1921 | 85 (19-18947) |
| AST (IU/L) | 17–96 | 40.3 ± 62.6 | 27 (12–575) |
| LDH (IU/L) | 147–463 | 258 ± 157.7 | 217 (7-982) |
| Aldolase (U/L) | 3.4–8.6 | 7.8 ± 9.7 | 5.7 (2.5–87.6) |
| Flow cytometry | |||
| Total T cells (CD3+) | 70.9 ± 10.6 | 72.5 (40–95) | |
| T helper cells (CD3 + CD4+) | 47.5 ± 35.2 | 46 (16–67) | |
| T cytotoxic cells (CD3 + CD8+) | 23.7 ± 7.1 | 24 (6–48) | |
| B cells (CD19+) | 19.6 ± 10.2 | 18 (1–53) | |
| NK cells (CD16+/CD56+) | 8.3 ± 5.4 | 7 (1–39) |
Figure 3.
Disease activity indicators in JDM based on the HDL level. JDM patients with low HDL exhibited significantly higher median scores for DAS-T (A), DAS-S (B), and DAS-M (C) compared to the normal HDL group D) The low HDL group had a lower CMAS than the normal HDL group. E) Nailfold capillary assessment revealed a lower ERL in the low HDL.
Table 3.
Disease activity markers and flow cytometry results in JDM children with low and normal HDL.
| Low HDL median (IQR) | Normal HDL median (IQR) | p value | |
|---|---|---|---|
| Sample size | 35 | 78 | |
| Clinical disease activity indicators | |||
| DAS total | 8.2 (5.9–13.4) | 1.8 (0–5) | 0.0001 |
| DAS skin | 5 (0-6.5) | 1 (0–4) | 0.01 |
| DAS muscle weakness | 4 (0.5–7.25) | 0 (0–1) | < 0.0001 |
| CMAS | 43 (26–48) | 51 (46–52) | < 0.0001 |
| ERL (#/mm) | 5.1 (3.3–5.8) | 5.5 (4.5–6.3) | 0.03 |
| Laboratory disease activity indicators | |||
| Neopterin (nmol/L) | 11.9 (6.1–21) | 6 (5.2–6.6) | < 0.0001 |
| ESR (mm/h) | 9.5 (6–23) | 7 (4–12) | 0.01 |
| vWF Ag (%) | 127 (107–189) | 100.5 (77.3-143.3) | 0.008 |
| Muscle enzymes | |||
| CK (IU/L) | 88.5 (67.3-136.8) | 82 (59.5–107) | 0.33 |
| AST (IU/L) | 30 (24.5–47) | 26 (20–32) | 0.005 |
| LDH (IU/L) | 273.5 (224.5-330.3) | 195 (163–239) | < 0.0001 |
| Aldolase (U/L) | 6.2 (5.2–7.9) | 5.5 (4.5–6.6) | 0.03 |
| Flow cytometry (percentage) | |||
| T cells (CD3+) | 67 (62–75) | 75 (70–81) | 0.0004 |
| T helper cells (CD3 + CD4+) | 44 (37–49) | 46 (40.8–52) | 0.077 |
| T cytotoxic cells (CD3 + CD8+) | 23 (18–25) | 24.5 (20.8–30) | 0.058 |
| B cells (CD19+) | 22 (16–29) | 15 (10–20) | 0.0007 |
| NK cells (CD16+/CD56+) | 8 (5–11) | 7 (4–12) | 0.54 |
Figure 4.
Correlation analysis between HDL levels and JDM disease activity indicators. There was a negative correlation between HDL levels and disease activity scores, including DAS-T (A), DAS-S (B), and DAS-M (C). There was a positive correlation between HDL levels and CMAS (D) as well as ERL capillary count (E).
Laboratory disease activity indicators and HDL level
The low HDL group exhibited significantly higher inflammatory markers (such as neopterin, ESR, and vWF Ag) than the normal HDL group (Table 3). Given the association between HDL and muscle weakness, we further evaluated muscle enzymes. The low HDL group showed significantly elevated AST, LDH, and aldolase levels (Table 3). Additionally, HDL levels correlated with inflammatory markers, including neopterin (r= -0.41, p < 0.001), ESR (r= -0.25, p = 0.006), and vWF Ag (r= -0.21, p = 0.02). We examined the prevalence of abnormal HDL in JDM-MSA subgroups (Supplemental Fig. 1). Anti-MDA5 and anti-P155/140 groups had the highest prevalence of abnormal HDL, but the difference wasn’t statistically significant.
Finally, we explored the relationship between HDL and lymphocyte subsets. An inverse correlation was found between HDL level and the percentage of B cells (r= -0.37, p < 0.001), while a positive correlation was observed with T cell percentage (r = 0.35, p < 0.001). Consistent with this finding, the low HDL group had a significantly higher median B cell percentage compared to the normal HDL group (Table 3).
Discussion
This study investigated the prevalence of dyslipidemia and its association with disease activity in a large cohort of JDM patients. Our findings demonstrate an increased prevalence of dyslipidemia, particularly low HDL levels and elevated TG, especially before treatment. This pattern of dyslipidemia differs somewhat from that observed in SLE patients, who often exhibit elevated LDL in addition to low HDL and elevated TG levels14,24,25. Studies have shown that SLE patients on prednisone therapy experience increased LDL and TG levels, while HDL levels remain relatively unaffected26. JDM patients, particularly those newly diagnosed and treatment-naïve, had more significant dyslipidemia, with a low prevalence of normal TG (22%) and HDL (16%) levels, respectively. This observation suggests a potential link between this dyslipidemia pattern and the underlying disease pathophysiology of JDM, rather than a consequence of treatment. Furthermore, patients demonstrated improved HDL levels following treatment initiation which supports a link between disease activity and HDL levels in JDM. On the other hand, triglyceride levels (TG) were elevated in both treatment-naïve JDM patients and on medication group (Fig. 2), suggesting a possible impact of the treatment, such as corticosteroids, in maintaining higher than normal TG levels even after treatment. Overall, dyslipidemia is relatively common in JDM patients, and different mechanisms might be driving dyslipidemia in JDM.
Patients with low HDL exhibited significantly higher scores on disease activity measures like DAS (total, skin, muscle) and lower CMAS scores, indicative of greater muscle weakness and skin rash. Similar observations have been reported in other rheumatological disorders such as SLE11,27 and rheumatoid arthritis28. Lower HDL in treatment-naïve JDM patients is likely multifactorial from ongoing inflammation, endothelial cell dysfunction, and decreased physical activity due to muscle weakness. For example, pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), have been demonstrated to suppress lecithin cholesterol acyltransferase (LCAT) activity, an enzyme critical for HDL maturation29. Additionally, JDM has been linked to the downregulation of microRNA-10a (miR-10a)30, which regulates the expression of ATP-binding cassette subfamily G member 1 (ABCG1), a protein essential for lipid transport30. Of note, miR-10a downregulation is correlated with increased VCAM-1 gene expression and vWF Ag, a marker of endothelial cell injury31 and vascular inflammation30,32. Endothelial cell dysfunction is a hallmark of JDM pathophysiology, as evidenced by the significant reduction in nailfold ERL count9,33 and increased serum vWF Ag31. In this study, we found a correlation between HDL level and both of these markers. Interestingly, the anti-P155/140 subgroups had the highest prevalence of abnormal HDL, which is the same subgroup with a more pronounced reduced nailfold capillary count34.
Emerging evidence suggests a direct role of HDL on various immune cells. For instance, HDL has been shown to suppress dendritic cell (DC) function by decreasing the expression of MHC-II and co-stimulatory molecules, thereby affecting T-cell activation35. HDL decreases TLR signaling and pro-inflammatory cytokine production in macrophages by reducing cholesterol from cell membranes through lipid transporters ABCG1 and ABCA136,37. Therefore, low HDL might lead to increased macrophage activation, which is consistent with our finding that the low HDL group has elevated neopterin. Macrophage activation is an important part of JDM pathophysiology as more than 80% of untreated JDM has elevated serum neopterin, a metabolite secreted from macrophages after exposure to interferon-gamma from activated T cells and natural killer (NK) cells22,38,39. It is important to note that while the low HDL group had a slightly higher ESR than the normal HDL group, the mean ESR levels in both groups remained within the normal range (0–20 mm/hr). This is not unexpected, as ESR is generally not a sensitive marker of inflammation in JDM22.
In this study, HDL level also correlated with the percentage of B cells which is another important immune cell in the JDM pathophysiology as evidenced by the role of autoantibodies (MSAs) in the disease phenotype3,40. Furthermore, treatment-naïve JDM patients have been shown to have a unique B cell population (expressing otoferlin) with the percentage of B cells correlating with disease activity40,41. Lastly, B cell-targeted therapies such as rituximab (a monoclonal antibody targeting CD20) and intravenous immune globulin (IVIG) have demonstrated effectiveness in JDM treatment42–44.
This study, while one of the largest to investigate dyslipidemia and disease activity in JDM, has limitations inherent to its retrospective design. Retrospective analysis restricts our ability to definitively establish causal relationships between dyslipidemia and disease activity. The inclusion of treatment-naïve patients offers valuable insights into the potential independent effect of JDM on lipid profiles, isolating the disease process from the confounding influence of medication. However, the sample size within this important subgroup is limited due to ethical considerations regarding delayed treatment initiation for fasting lipid profiles. Additionally, we were unable to provide longitudinal data on lipid profiles for the same patients over time. Future studies are needed to explore this aspect, which could enhance our understanding of the causal relationship between dyslipidemia and medication use. Lastly, although significant correlations between dyslipidemia and disease activity markers were identified, the study did not examine the specific mechanisms underlying these associations. Future studies with prospective designs and mechanistic investigations are warranted.
In conclusion, JDM patients have a high prevalence of dyslipidemia, especially low HDL and elevated triglycerides. The severity of dyslipidemia (low HDL) correlates with disease activity, with treatment-naïve patients demonstrating the lowest HDL levels. These findings suggest the importance of annual lipid profile monitoring in JDM patients, potentially followed by early interventions such as dietary adjustments and exercise programs.
Supplementary Information
Acknowledgements
The authors acknowledge and appreciate the contributions of the many Pachman Lab members and the Division of Pediatric Rheumatology who enabled the data collection: Dr. Klein-Gitelman cared for the JDM patients over the past 6 years. Drs. Megan Curran, Kaveh Ardalan, and Christopher Costin were also involved. Dr. Amer Khojah, MD analyzed the de-identified data while he was on the staff of Ann & Robert H. Lurie Children’s Hospital of Chicago, with manuscript completion while at Umm Al-Qura University, College of Medicine, Makkah, Saudi Arabia.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Ann & Robert H. Lurie Children’s Hospital of Chicago (IRB# 2012–14858).
Informed consent
Signed informed consent was obtained from all subjects involved in the study.
Abbreviations
- JDM
Juvenile Dermatomyositis
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- TG
Triglyceride
- DAS
Disease Activity Score
- CMAS
Childhood Myositis Assessment Scale
- ESR
Erythrocyte sedimentation rate
- vWF Ag
von Willebrand factor antigen
- ECG
Electrocardiogram
- SLE
Systemic lupus erythematosus
- AAP
American Academy of Pediatrics
- MSA
Myositis-specific antibodies
- ERL
Nailfold capillary end row loops
- LDH
Lactate dehydrogenase
- AST
Aspartate aminotransferase
- CK
Creatine phosphokinase
- ELISA
Enzyme-linked immunosorbent assay
- DC
Dendritic cell
- NK
Natural killer cell
- TNF-α
tumor necrosis factor-alpha
- LCAT
lecithin cholesterol acyltransferase
- miR-10a
microRNA-10a
- ABCG1
ATP-binding cassette subfamily G member 1
- VCAM-1
vascular cell adhesion molecule 1
- DC
dendritic cell
- ABCA1
ATP-binding cassette transporter A1
- IVIG
intravenous immune globulin
Author contributions
All authors have contributed to the manuscript. Conception and design: AmK and LMP. Acquisition of data: GM, ArK, LMP. Analysis and interpretation of data: AmK, MK and LMP. Manuscript writing and review: AmK, GM, ArK, MK, and LMP.
Funding
Supported in part by the CureJM Foundation Center of Excellence; R-21 AR077565 (both to LMP). The data in this project is entered in REDCap, supported by NUCATS, and funded in part by a Clinical and Translational Science Award (CTSA) grant from the National Institutes of Health (NIH), UL1TR001422.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request by contacting the corresponding author, Dr. Lauren Pachman, at Pachman@northwestern.edu.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pachman, L. M., Nolan, B. E., DeRanieri, D. & Khojah, A. M. Juvenile Dermatomyositis: New clues to diagnosis and therapy. Curr. Treatm Opt. Rheumatol.7, 39–62. 10.1007/s40674-020-00168-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendez, E. P. et al. US incidence of juvenile dermatomyositis, 1995–1998: results from the National Institute of Arthritis and Musculoskeletal and skin diseases Registry. Arthritis Rheum.49, 300–305. 10.1002/art.11122 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Pachman, L. M. & Khojah, A. M. Advances in Juvenile Dermatomyositis: Myositis specific antibodies aid in understanding Disease Heterogeneity. J. Pediatr.195, 16–27. 10.1016/j.jpeds.2017.12.053 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz, T., Diederichsen, L. P., Lundberg, I. E., Sjaastad, I. & Sanner, H. Cardiac involvement in adult and juvenile idiopathic inflammatory myopathies. RMD Open.2, e000291. 10.1136/rmdopen-2016-000291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marie, I. Morbidity and mortality in adult polymyositis and dermatomyositis. Curr. Rheumatol. Rep.14, 275–285. 10.1007/s11926-012-0249-3 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Eimer, M. J. et al. Clinical status and cardiovascular risk profile of adults with a history of juvenile dermatomyositis. J. Pediatr.159, 795–801. 10.1016/j.jpeds.2011.05.015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyle, K. et al. Metabolic abnormalities and cardiovascular risk factors in children with myositis. J. Pediatr.155, 882–887. 10.1016/j.jpeds.2009.06.009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khojah, A., Liu, V., Morgan, G., Shore, R. M. & Pachman, L. M. Changes in total body fat and body mass index among children with juvenile dermatomyositis treated with high-dose glucocorticoids. Pediatr. Rheumatol. Online J.19, 118. 10.1186/s12969-021-00622-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pachman, L. M., Morgan, G., Klein-Gitelman, M. S., Ahsan, N. & Khojah, A. Nailfold capillary density in 140 untreated children with juvenile dermatomyositis: an indicator of disease activity. Pediatr. Rheumatol.21, 118. 10.1186/s12969-023-00903-x (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barth, Z. et al. Association between Nailfold Capillary Density and Pulmonary and Cardiac involvement in medium to Longstanding Juvenile Dermatomyositis. Arthritis Care Res. (Hoboken). 71, 492–497. 10.1002/acr.23687 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Atta, A. M. et al. Clinical and laboratory aspects of dyslipidemia in Brazilian women with systemic lupus erythematosus. Clin. Rheumatol.37, 1539–1546. 10.1007/s10067-018-4051-0 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Batún Garrido, J. A. & Olán, F. Hernández Núñez, É. [Dyslipidemia and atherogenic risk in patients with rheumatoid arthritis]. Clin. Investig Arterioscler.28, 123–131. 10.1016/j.arteri.2016.02.002 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Schimmel, E. K. & Yazici, Y. Increased lipid levels but unchanged atherogenic index in rheumatoid arthritis patients treated with biologic disease modifying antirheumatic drugs: published experience. Clin. Exp. Rheumatol.27, 446–451 (2009). [PubMed] [Google Scholar]
- 14.Olusi, S. O. & George, S. Prevalence of LDL atherogenic phenotype in patients with systemic lupus erythematosus. Vasc Health Risk Manag. 7, 75–80. 10.2147/vhrm.S17015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamal, S. M. et al. Immunological profile and dyslipidemia in Egyptian systemic lupus erythematosus patients. Egypt. Rheumatologist. 39, 89–92. 10.1016/j.ejr.2016.05.007 (2017). [Google Scholar]
- 16.Kozu, K. T. et al. Dyslipidaemia in juvenile dermatomyositis: the role of disease activity. Clin. Exp. Rheumatol.31, 638–644 (2013). [PubMed] [Google Scholar]
- 17.Leclair, V. & Lundberg, I. E. New Myositis classification criteria-what we have learned since Bohan and Peter. Curr. Rheumatol. Rep.20. 10.1007/s11926-018-0726-4 (2018). [DOI] [PMC free article] [PubMed]
- 18.Keirns, B. H., Sciarrillo, C. M., Koemel, N. A. & Emerson, S. R. Fasting, non-fasting and postprandial triglycerides for screening cardiometabolic risk. J. Nutr. Sci.10, e75. 10.1017/jns.2021.73 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Expert panel on integrated guidelines for cardiovascular. Health and risk reduction in children and adolescents: summary report. Pediatrics. 128 (Suppl 5), 213–256. 10.1542/peds.2009-2107C (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bode, R. K., Klein-Gitelman, M. S., Miller, M. L., Lechman, T. S. & Pachman, L. M. Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis Rheum.49, 7–15. 10.1002/art.10924 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Rider, L. G. et al. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: physician and Patient/Parent global activity, manual muscle testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity score (DAS), short form 36 (SF-36), Child Health Questionnaire (CHQ), physician global damage, myositis damage index (MDI), quantitative muscle testing (QMT), Myositis Functional Index-2 (FI-2), Myositis activities Profile (MAP), inclusion body myositis functional rating scale (IBMFRS), cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), cutaneous Assessment Tool (CAT), Dermatomyositis skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI). Arthritis Care Res. (Hoboken). 63 (Suppl 11), 118–157. 10.1002/acr.20532 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khojah, A., Morgan, G. & Pachman, L. M. Clues to Disease Activity in Juvenile Dermatomyositis: neopterin and other biomarkers. Diagnostics (Basel). 1210.3390/diagnostics12010008 (2021). [DOI] [PMC free article] [PubMed]
- 23.Tansley, S. L. et al. Autoantibodies in juvenile-onset myositis: their diagnostic value and associated clinical phenotype in a large UK cohort. J. Autoimmun.84, 55–64. 10.1016/j.jaut.2017.06.007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, S. Y. et al. High-density lipoprotein in Lupus: disease biomarkers and potential therapeutic strategy. Arthritis Rheumatol.72, 20–30. 10.1002/art.41059 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang, S. et al. Dyslipidemia is associated with inflammation and organ involvement in systemic lupus erythematosus. Clin. Rheumatol.42, 1565–1572. 10.1007/s10067-023-06539-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ettinger, W. H., Goldberg, A. P., Applebaum-Bowden, D. & Hazzard, W. R. Dyslipoproteinemia in systemic lupus erythematosus. Effect of corticosteroids. Am. J. Med.83, 503–508. 10.1016/0002-9343(87)90762-5 (1987). [DOI] [PubMed] [Google Scholar]
- 27.Yuan, J., Li, L. I., Wang, Z., Song, W. & Zhang, Z. Dyslipidemia in patients with systemic lupus erythematosus: Association with disease activity and B-type natriuretic peptide levels. Biomed. Rep.4, 68–72. 10.3892/br.2015.544 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attar, S. M. Hyperlipidemia in rheumatoid arthritis patients in Saudi Arabia. Correlation with C-reactive protein levels and disease activity. Saudi Med. J.36, 685–691. 10.15537/smj.2015.6.10557 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francone, O. & Feingold, K. Endotoxin and TNF lead to reduced plasma LCAT activity and decreased hepatic LCAT mRNA levels in Syrian hamsters. J. Lipid Res.36, 1254–1263 (1995). [PubMed] [Google Scholar]
- 30.Xu, D. et al. MicroRNA-10a regulation of Proinflammatory mediators: an important component of untreated juvenile Dermatomyositis. J. Rheumatol.43, 161–168. 10.3899/jrheum.141474 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Gibbs, E., Khojah, A., Morgan, G., Ehwerhemuepha, L. & Pachman, L. M. The von Willebrand factor antigen reflects the Juvenile dermatomyositis disease activity score. Biomedicines 11, (2023). 10.3390/biomedicines11020552 [DOI] [PMC free article] [PubMed]
- 32.Xu, D., Kachaochana, A., Morgan, G. A., Huang, C. C. & Pachman, L. M. Arthritis and rheumatism. S890-S890 (Wiley-Blackwell, Hoboken).
- 33.Khojah, A., Morgan, G., Klein-Gitelman, M. S. & Pachman, L. M. Juvenile dermatomyositis: association between nail fold capillary end row loop–area under the curve–and disease damage indicators. Pediatr. Rheumatol.21, 137 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khojah, A. et al. Association of p155/140 Autoantibody with loss of Nailfold Capillaries but not generalized Lipodystrophy: a study of ninety-six children with Juvenile Dermatomyositis. Arthritis Care Res. (Hoboken). 74, 1065–1069. 10.1002/acr.24535 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Westerterp, M. et al. Cholesterol accumulation in dendritic cells links the inflammasome to acquired immunity. Cell Metabol.25, 1294–1304 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yvan-Charvet, L. et al. Increased inflammatory gene expression in ABC transporter–deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 118, 1837–1847 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Nardo, D. et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol.15, 152–160 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khojah, A. et al. Increased percentage of HLA-DR T cells in untreated juvenile dermatomyositis. Clin. Immunol. Commun.5, 20–25. 10.1016/j.clicom.2024.02.002 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khojah, A. et al. Decreased peripheral blood natural killer cell count in untreated juvenile dermatomyositis is Associated with muscle weakness. Int. J. Mol. Sci.25, 7126 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costin, C. et al. B cell lymphocytosis in Juvenile Dermatomyositis. Diagnostics. 13, 2626 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bukhari, A. et al. Increased otoferlin expression in B cells is Associated with muscle weakness in untreated juvenile dermatomyositis: a pilot study. Int. J. Mol. Sci.24, 10553 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ochfeld, E. et al. Coding joint: kappa-deleting recombination excision circle ratio and B cell activating factor level: predicting juvenile dermatomyositis rituximab response, a proof-of-concept study. BMC Rheumatol.6, 36. 10.1186/s41927-022-00265-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oddis, C. V. et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum.65, 314–324. 10.1002/art.37754 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aggarwal, R. et al. Trial of Intravenous Immune Globulin in Dermatomyositis. N Engl. J. Med.387, 1264–1278. 10.1056/NEJMoa2117912 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request by contacting the corresponding author, Dr. Lauren Pachman, at Pachman@northwestern.edu.