Highlights
-
•
PHA bioplastics from food waste offer a sustainable alternative for packaging.
-
•
PHA production achieves up to 87 % yield using optimized fermentation processes.
-
•
Low-cost carbon sources (VFAs, waste oils) reduce PHA production expenses.
-
•
PHA’s biodegradability and biocompatibility make it ideal for replacing plastics.
-
•
Overcoming production costs and scalability can enhance PHA industry adoption.
Keywords: Bio-packaging, Cupriavidus necator, Food waste, Polyhydroxyalkanoate, Volatile fatty acids
Abstract
The growing demand for sustainable food packaging and the increasing concerns regarding environmental pollution have driven interest in biodegradable materials. This paper presents an in-depth review of the production of Polyhydroxyalkanoates (PHA), a biodegradable polymer, from food waste. PHA-based bioplastics, particularly when derived from low-cost carbon sources such as volatile fatty acids (VFAs) and waste oils, offer a promising solution for reducing plastic waste and enhancing food packaging sustainability. Through optimization of microbial fermentation processes, PHA production can achieve significant efficiency improvements, with yields reaching up to 87 % PHA content under ideal conditions. This review highlights the technical advancements in using PHA for food packaging, emphasizing its biodegradability, biocompatibility, and potential to serve as a biodegradable alternative to petroleum-based plastics. However, challenges such as high production costs, mechanical limitations, and the need for scalability remain barriers to industrial adoption. The future of PHA in food packaging hinges on overcoming these challenges through further research and innovation in production techniques, material properties, and cost reduction strategies, along with necessary legislative support to promote widespread use.
1. Introduction
Plastic is a polymer composed of carbon atoms that are linked to each other in a chain structure. Plastic is used in almost every industry because of its low price, easy manufacture, water resistance, high strength properties, and diverse functions (Thiounn and Smith, 2020). Since its invention in the late 19th century, the use of plastic in everyday life has increased rapidly and exponentially. It is estimated that annual plastic production reached 359 million tons in 2018, with about 8.3 billion tons produced over the past 70 years (Filho et al., 2021, Kaushal et al., 2021). As of 2019, plastic waste reached 368 million tons. About 19 to 23 million tons of unmanaged plastic waste moves annually from land to water sources (Veneral et al., 2023). Certainly, since plastic materials are not widely biodegradable, the problem raises environmental concerns (Li et al., 2023). The large-scale use of plastic accumulates large amounts of residue, causing serious environmental problems and adversely affecting the economy, wildlife, and human health (Veneral et al., 2023). In this regard, scientists are working to develop technologies that allow products and processes to have less impact on the environment, greater energy efficiency, and less waste production. These technologies range from the production and optimization of classical recycling processes, such as mechanical and chemical processes, to recycling through bioprocessing or the production of biodegradable bioplastics (Chen et al., 2020, Liu et al., 2021, Shen et al., 2021).
Food waste has become a global focus that requires attention and requires urgent action (Jabeen et al., 2023). Despite high levels of hunger and environmental problems, another worrying thing is that about one-third of food produced for human consumption is wasted (Asioli and Grasso, 2021, Hellali et al., 2023). Where half of all types of food are wasted and 30–50 % of food produced is not eaten, while one-third of edible food is wasted worldwide. In addition, food waste that is increasingly piling up is also caused by the rapid rate of world population growth (Cederberg and Sonesson, 2011, Chong et al., 2021). This not only has an impact on the social side but can affect environmental stability because many smell bad.
At this time, a lot of engineering was done to convert food waste into polyhydroxyalkanoate (PHA). Previously, food waste could be converted into bioactive compounds, biofuels, bioenergy, single-cell protein source fertilizers, and various other products by means such as bioconversion, enzymatic hydrolysis, microbial digestion, and so on (Rifna et al., 2024). PHA is a linear polyester synthesized by various microorganisms and serves as an intracellular carbon and energy reserve (Jendrossek and Pfeiffer, 2014, Prieto et al., 2016, Yadav et al., 2020) PHA is produced by different microorganisms under stress conditions. PHAs are stored as cytoplasmic inclusions and serve as a metabolic strategy for microorganisms to adapt to fluctuations in nutrient availability (Han et al., 2015). PHA synthesis increases in microbial cells when they have an abundance of carbon but a limited supply of nitrogen or phosphorus (Jendrossek and Pfeiffer, 2014).
PHA polymers, as bio-derived products, are widely regarded as the most attractive petrochemical-derived thermoplastic polyolefins (Taguchi and Matsumoto, 2021). This is mainly due to the sustainability of polyhydroxyalkanoates (PHAs) that have similar material properties with widely used polyolefins, such as polyethylene and polypropylene, so that they can attract interest in applications in agricultural plastics, food packaging, biomedical devices, 3D printing, etc (Gupta et al., 2022, Mehrpouya et al., 2021). The use of PHA is very promising as a substitute for petroleum-based plastics due to its higher thermal stability, easy biodegradability ability, and biocompatibility properties (Sirohi et al., 2020, Yadav et al., 2020). Biobased characters and various properties make PHA a superior material to replace oil-based plastics to be made into food packaging (Khatami et al., 2022).
The conversion of food waste into PHA has been extensively explored, recognizing food waste as a promising low-cost carbon source. Given the significant waste streams produced during food production, processing, and consumption, various technologies have been proposed to facilitate the conversion of food waste into PHA (Mustapha and Wan, 2022, Wellenreuther et al., 2022). Food waste can be a cost-effective raw material for PHA production when converted into a substrate form that can be utilized for PHA-producing bacteria (Bhatia et al., 2021, Jõgi and Bhat, 2020, Talan et al., 2022, Varghese et al., 2022). Previous research on the conversion of food waste into PHA is shown in Table 1.
Table 1.
Research on food waste into PHA (Notes: PHB = polyhydroxybutyrate; PHBV = poly(3-hydroxybutyrate-co-3-hydroxyvalerate); P(3HB-co-3HHx) = 3-hydroxybutyrate-co-3-hydroxyhexanoate) (Raunhan et al., 2023).
| Microorganism | Carbon sources |
PHA |
References | ||
|---|---|---|---|---|---|
| Type | Content (wt%) | Production (g/L PHA) | |||
| Bacillus megaterium ATCC 14945 | Mixture of VFAs from the acidogenic fermentation of food waste | PHB | 8.6 | 0.16 | Vu et al. (2021) |
| Bacillus cepacia DSM 50181 | Digestate liquor from food waste and primary sludge | PHB | 77.5 | 1.39 |
Khatami et al. (2022) |
| Cupriavidus necator DSM 545 | PHB | 54.9 | 1.09 | ||
| Cupriavidus necator H16 ATCC 17699 | Mixture of VFAs from food waste fermentate | PHB | 87.0 | − |
Hafuka et al. (2011) |
| Cupriavidus necator H16 (DSM 428) | Waste cooking oil | PHB | 76.9 | 103.8 |
Loan et al. (2022) |
| Waste fish oil | PHB | 72.5 | 83.2 | ||
| Pseudomonas oleovorans ATCC 29347 | Mixture of VFAs from the acidogenic fermentation of potato peels | PHBV | 39.0 | 0.82 | Aremu et al. (2021) |
| Engineered Ralstonia eutropha Re2133/pCB81 | The mixture of VFAs from food waste fermentate | P(3HB-co-3HHx) | 55.0 | 0.67 | Bhatia et al. (2019) |
| Rhodopseudomonas palustris | The mixture of VFAs from food waste fermentate | PHBV | 48.6 | − | Dan et al. (2023) |
| Thauera sp. Sel9 | Alfalfa hydrolysate | PHBV | − | 1.4 | Critelli et al. (2022) |
| Thauera mechernichensis TL1 | Food waste anaerobic digestate | PHBV | 24.0 | 0.52 | Raunhan et al. (2023) |
The purpose of this article is to explain the processing to convert complex compounds present in food waste into precursors of PHA, biodegradable polymers that can be used as basic materials in the manufacture of bioplastics. Research on bioplastics is increasingly gaining attention as a way to create a sustainable and environmentally friendly environment. Biodegradable bioplastics have the same characteristics as conventional plastics. Therefore, the use of organic waste i.e., food waste for bioplastic production not only reduces dependence on the raw materials that plastic uses but can effectively help solid waste management. With food waste research and development for PHA production a sustainable solution, PHA can be a more environmentally friendly alternative to conventional plastic and help address global problems related to food waste.
2. Polyhydroxyalkanoate (PHA)
2.1. Source of PHA
Table 2 shows that the naturally-occurring sources of PHA are diverse and primarily consist of various bacterial strains with the inherent ability to synthesize these biopolymers. Naturally occurring sources of PHAs are diverse and primarily consist of various bacterial strains with the inherent ability to synthesize these biopolymers. These bacteria accumulate PHA as intracellular energy reserves under specific environmental conditions, often using readily available carbon sources. Understanding the range of bacterial PHA producers and their preferred carbon sources is crucial for researchers seeking to optimize PHA production for various applications. Analyzing these natural sources can provide valuable insights into the metabolic pathways involved in PHA biosynthesis and guide the selection of suitable bacterial strains for further study and development. Notably, among these sources, Cupriavidus necator has emerged as a particularly promising candidate due to its exceptional PHA production efficiency. This bacterium’s ability to produce a wide range of PHA titers warrants further investigation for optimizing industrial-scale PHA production processes (Stadio et al., 2024, Subagyo et al., 2021).
Table 2.
Notable wild-type PHA producers (Tan et al., 2014).
| Producer organism(s) | PHA type | Carbon source | Time (h) | Cell titre (g/L) | PHA titre (g/L) | References |
|---|---|---|---|---|---|---|
| Azotobacter vinelandii | scl | Glucose | 28 | 45.5 | 30.3 | Subramanian et al. (2020) |
| Bacillus megaterium | scl | Molasses (sucrose) | 24 | 90.7 | 41.6 | Kanjanachumpol et al. (2013) |
| Burkholderia cepacia | scl | Wood sugars | 96 | 17.0 | 8.7 | Pan et al. (2012) |
| Chelatococcus daeguensis | scl | Glycerol | 40 | 25.4 | 17.4 | Cui et al. (2015) |
| Cupriavidus necator | scl | Palm oil | 72 | 161.0 | 109.5 | Zainab-L and Sudesh (2019) |
| Haloferax Mediterrane | scl | Glucose | 117 | 85.8 | 41.7 | Don et al. (2006) |
| Halomonas sp. | scl | Glucose, corn powder | 56 | 90.5 | 71.3 | Ren et al. (2018) |
| Pseudomonas oleovorans | mcl | Octanoic acid | 38 | 63.0 | 39.0 | Marsudi et al. (2007) |
| Pseudomonas putida | mcl | Glucose, nonanoic acid | 32 | 98.0 | 31.4 | Davis et al. (2015) |
| Paraburkholderia sacchari | scl | Xylose | 70 | 30.0 | 16.0 | Oliveira-Filho et al. (2020) |
2.2. Structures and properties of PHA
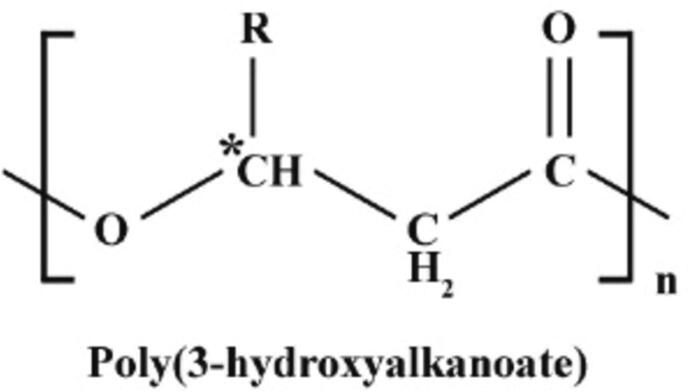
PHA mainly consists of 3-hydroxy and 4-hydroxy fatty (Prieto et al., 2016). PHA synthesis involves the formation of ester bonds between hydroxyl groups on one monomer and carboxyl groups on another (Sehgal and Gupta, 2020). These biopolymers have an R group, with the R group representing a hydrogen atom or alkyl group varying from a methyl group (C1) to a tridecyl (C13), as illustrated in Fig. 1 (Żur-Pińska et al., 2023). In Table 3, if this R group is a methyl group (CH3), which contains only one carbon atom, the resulting bioplastic is named poly(3-hydroxybutyrate) because there are a total of four carbons in the monomer unit (three from the main chain and one from the main chain methyl group). Similarly, if the R group has three carbons (C3H7), the bioplastic is called poly(3-hydroxyhexanoate) because the total number of carbons in the monomer becomes six.
Fig. 1.
PHA backbone structure that can be decorated with various side clusters “R clusters” (Tan et al., 2014).
Table 3.
The specific identity of the R group determines the total number of carbon atoms and the resulting PHA nomenclature (Tan et al., 2014).
| R group | Carbon | PHA polymer |
|---|---|---|
| methyl | C4 | Poly(3-hydroxybutyrate) |
| ethyl | C5 | Poly(3-hydroxyvalerate) |
| propyl | C6 | Poly(3-hydroxyhexanoate) |
| butyl | C7 | Poly(3-hydroxyheptanoate) |
| pentyl | C8 | Poly(3-hydroxyoctanoate) |
| hexyl | C9 | Poly(3-hydroxynonanoate) |
| heptyl | C10 | Poly(3-hydroxydecanoate) |
| octyl | C11 | Poly(3-hydroxyundecanoate) |
| nonyl | C12 | Poly(3-hydroxydodecanoate) |
| decyl | C13 | Poly(3-hydroxytridecanoate) |
| undecyl | C14 | Poly(3-hydroxytetradecanoate) |
| dodecyl | C15 | Poly(3-hydroxypentadecanoate) |
| tridecyl | C16 | Poly(3-hydroxyhexadecanoate) |
Based on the number of carbon atoms in repeating units, PHAs can be categorized into PHAs with short chain length (scl-PHA), PHA with medium chain length (mcl-PHA), and PHA with long chain length (lcl-PHA) (Zhang et al., 2024). Scl-PHA consists of 3 to 5 carbon atoms that have a well-crystallized structure so that they are generally more rigid and have a higher melting temperature than other types of PHA (Liu et al., 2021, Żur-Pińska et al., 2023). On the other hand, mcl-PHA consists of 6 to 14 carbon atoms, while lcl-PHA consists of 15 or more than 15 carbon atoms (Paduvari et al., 2024, Sharma et al., 2021). Mcl-PHA and lcl-PHA have a less crystallized structure so they have a flexible texture and are more elastic compared to scl-PHA (Chacón et al., 2024, Dalton et al., 2022).
Briefly, the biosynthesis approach requires three important steps: (i) catalytic conversion of acetoacetyl-CoA to acetoacetyl-CoA by the enzyme ketothiolase (PhaA); (ii) catalytic hydrogenation of acetoacetyl-CoA by the enzyme PHA reductase (PhaB) to obtain monomers (R)-3-hydroxy-butyryl-CoA; and (iii) catalytic polymerization of (R)-3 hydroxy-butyryl-CoA by the enzyme PHB polymerase (Kinyanjui Muiruri et al., 2023). PHA synthase is categorized into four different classes (I-IV) based on amino acid sequence, subunit composition, and size of constituent material that can be handled (substrate specificity). Class I, III, and IV phases show a preference for substrates with short chain lengths (C3 to C5) and are responsible for the production of PHAs with short chain lengths (scl-PHA). In contrast, Class II PhaC favors substrates with longer chain lengths (C6 to C14) and is used for the biosynthesis of medium chain length PHAs (mcl-PHA). The specific biosynthetic pathway used by microorganisms for PHA production depends on the class of PhaC to which it belongs. Bacteria that store Class I, III, or IV PhaC, such as Cupriavidus necator, Haloferax mediterranei, and Bacillus megaterium, utilize conserved pathways to produce precursors. This pathway involves the sequential action of two enzymes: PhaA (a β-ketothiolase) and PhaB (an acetoacetyl-CoA reductase). They work together to convert acetyl-CoA into direct precursors for the polymerization of poly(3-hydroxybutyrate) (P3HB), (R)-3-hydroxybutyryl-CoA [(R)-HB-CoA)]. Pseudomonas species, known as Class II PhaC depositors, apply a different strategy. These bacteria lack enzymes for conserved pathways and instead divert the intermediate chain precursors (R)-HA-CoA from two sources: β-oxidation, facilitated by PhaJ (a transenoyl-CoA hydratase), and fatty acid synthesis, through a combination of the actions of PhaG ((R)-3-hydroxyacylacyl thioesterase-carrying protein) and AlkK (a mcl-fatty acid-CoA ligase) (Acuña and Poblete-Castro, 2023, Mezzina et al., 2021, Neoh et al., 2022).
Haloferax mediterranei stands out among manufacturers of scl-PHA. These organisms have the unique ability to synthesize the precursor (R)-3-hydroxyalkyl-CoA [(R)-HV-CoA]] de novo, allowing their incorporation into co-polymers with (R)-HB-CoA (P3HBV). In H. Mediterranei, propionyl-CoA, the primary precursor for the synthesis of (R)-HV-CoA, is obtained via one of four endogenous routes and then condensed with acetyl-CoA. This ability highlights the impressive metabolic versatility observed in PHA-producing microorganisms (Han et al., 2013).
2.3. PHA production from food waste streams
Based on various scientific studies, diverse by-products and food processing wastes have emerged as promising raw materials for PHA production. These materials include food and pulp (Acuña and Poblete‐Castro, 2023), molasses (Tyagi et al., 2022), waste oil (Ingram et al., 2022, Pan et al., 2021), animal processing waste (Ali et al., 2023), and even organic fractions of municipal waste (Ji et al., 2023, Li et al., 2023). The composition of these feedstocks determines the level of pre-processing required to convert larger molecules into usable PHA precursors. For example, fatty acids derived from lipids can be directly incorporated into PHAs (Zhang et al., 2023), while other components such as glycerol (from lipids), sugars (derived from starch or lignocellulose), or even aromatics from lignocellulose require metabolic conversion through de novo fatty acid synthesis (Bhatia et al., 2023, Tao et al., 2024). The choice of raw materials greatly affects both the substrate content and the extent of pre-processing required. Starchy waste, such as leftover pasta, is more easily decomposed compared to more complex lignocellulose materials such as grain flour, although both can ultimately produce high amounts of glucose (Brojanigo et al., 2022, Sirohi et al., 2021).
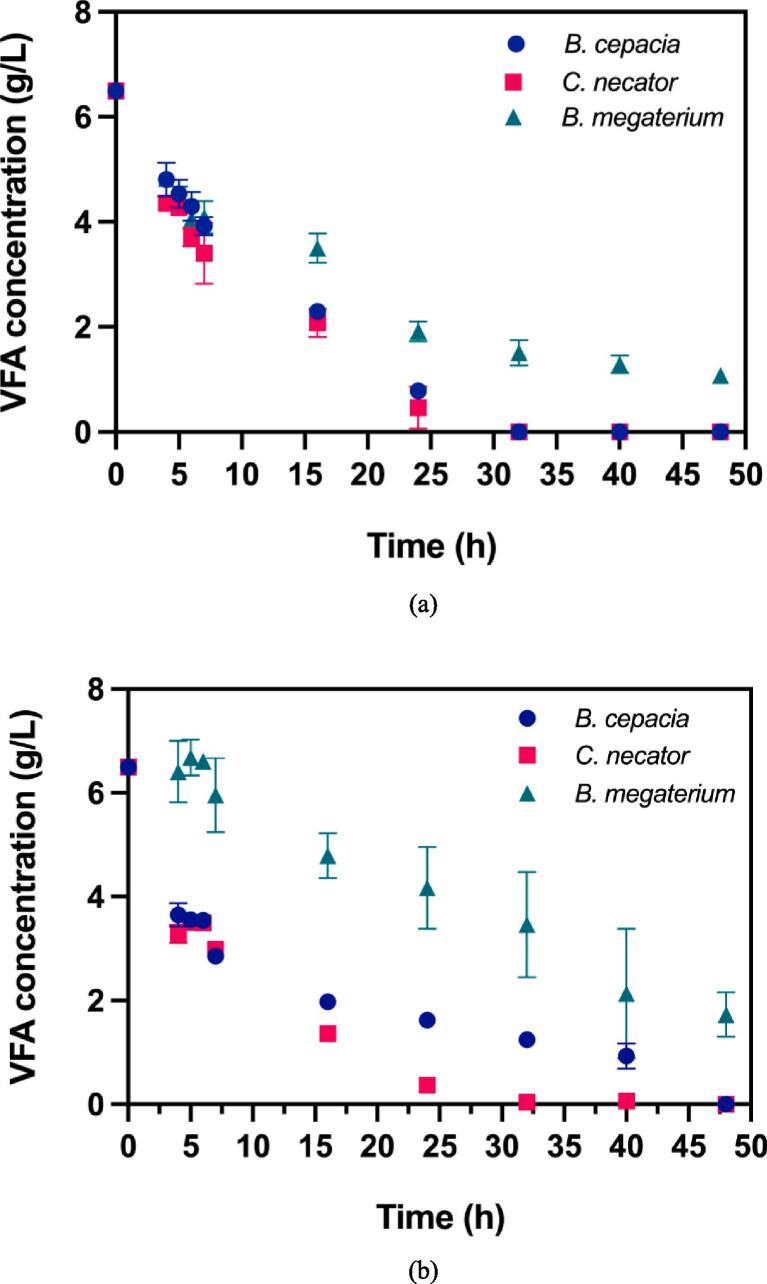
Minimizing processing steps and associated costs is critical to expanding the utilization of diverse raw materials and improving the economic viability of PHA production. Khatami et al. (2022) investigated how food waste-derived volatile fatty acids (VFAs) can be effectively utilized as low-cost carbon sources for bacterial production of PHA. The study highlights that Cupriavidus necator can accumulate up to 77.54 % PHA from VFAs derived from food waste and primary sludge. These findings indicate that waste-derived VFAs, such as acetic and propionic acids, are vital for improving the metabolic conversion into PHA. Fig. 2 illustrates how VFAs are consumed over time in both synthetic medium and digestate liquor, representing the metabolic pathways used by various bacterial strains in PHA biosynthesis (Khatami et al., 2022). This insight into microbial fermentation processes underlines the efficiency of food waste valorization into bioplastics.
Fig. 2.
VFA concentration over time in mono cultures using: (a) synthetic medium and (b) digestate liquor. This figure illustrates the key metabolic pathways and the consumption of VFAs by Cupriavidus necator, Burkholderia cepacia, and Bacillus megaterium, which are essential for PHA accumulation from food waste streams (Khatami et al., 2022).
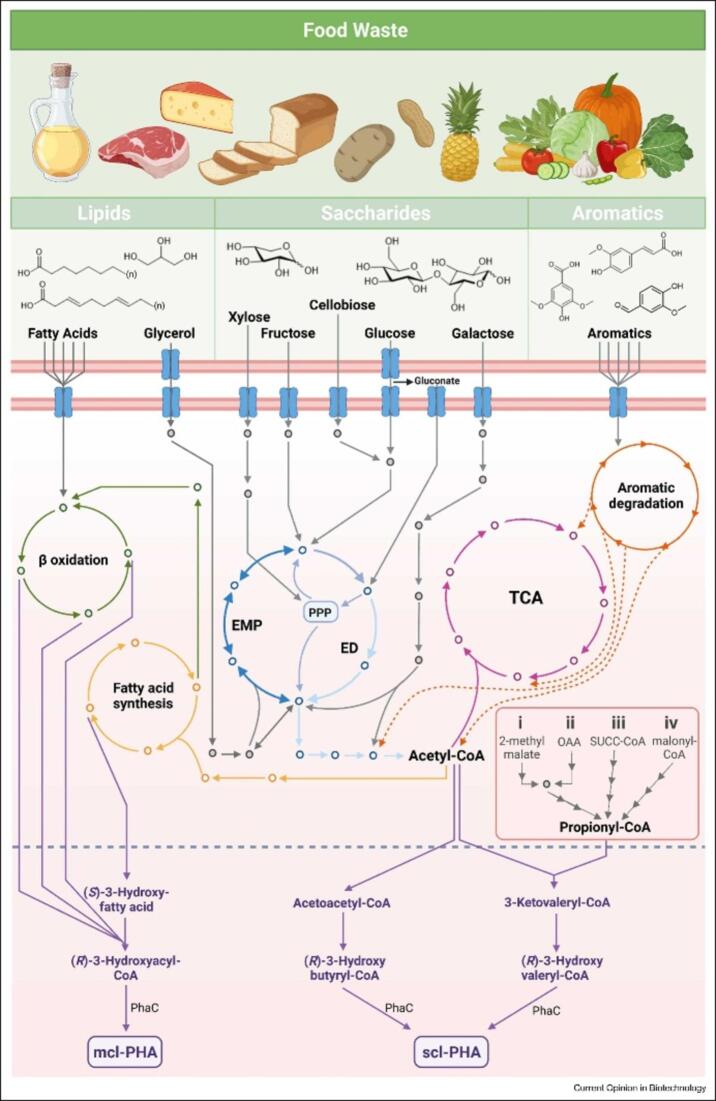
In Fig. 3, the colored pathways visually represent these processes: green for beta-oxidation (fatty acid breakdown), yellow for fatty acid synthesis, and blue for sugar breakdown (dark blue − Embden-Meyerhof-Parnass pathway, medium blue − Entner-Doudoroff pathway, light blue − Pentose Phosphate pathway). The pink pathway represents the tricarboxylic acid cycle (TCA), a cycle for energy production, and the orange pathway highlights the degradation of aromatic compounds. A separate inset details four pathways used by archaeon H. mediterranei to produce propionyl-CoA from food scraps. Finally, the purple pathway for PHA biosynthesis (medium and short chain forms) (Chacón et al., 2024).
Fig. 3.
Various metabolic pathways are utilized by bacteria and archaea to process food waste components (Chacón et al., 2024).
Many hydrolysates derived from food waste can serve as an important source of nitrogen for microbial cell growth in addition to providing a carbon source for PHA production. Research on the utilization of food waste hydrolysate as a source of nitrogen mainly focuses on byproducts with high protein content, such as whey hydrolysate, soybeans, and rapeseed (Alsafadi et al., 2020, Hierro-Iglesias et al., 2022, Wongsirichot et al., 2022). However, it is important to note that the use of these high-nitrogen hydrolysates may require additional strategies. This strategy may involve the addition of hydrolysates with carbon sources or the use of microorganisms with growth-associated PHA production, such as Haloferax mediterranei (Khamplod et al., 2023). This approach ensures that microbes have the building blocks (carbon and nitrogen) needed for cell growth and PHA biosynthesis.
2.3.1. Production of PHA from lipid waste
The production of mcl-PHA from lipids is known as “de novo fatty acid biosynthesis,” offering a unique route for the production of PHAs from unrelated substrates such as glycerol, glucose, or acetate in Pseudomonas (Lim et al., 2023). The main enzyme in this pathway is 3-hydroxy-ACP-CoA transacylase, which is encoded by the PhaG gene (ACP: acyl carrier protein). This enzyme catalyzes the important conversion of (R)-3-hydroxy acyl-ACP to the corresponding CoA derivative, (R)-3-hydroxy acyl-CoA, which serves as a direct precursor to the polymerization of mcl-PHA (Lim et al., 2023). Interestingly, studies have shown that PhaG overexpression occurs in strains of Pseudomonas putida that experience nitrogen-deficient conditions (Mohammad and Bhukya, 2022). The pathway leading to (R)-3-hydroxy acyl-ACP begins with the conversion of acetyl-CoA, a product of glycerol catabolism, to malonyl-CoA. This ATP and CO2-dependent reaction is catalyzed by a biotin-dependent enzyme, acetyl-CoA carboxylase. Malonyl-CoA then reacts with acyl carrier protein (ACP) to form the malonyl-ACP complex, releasing CoA. Furthermore, ketoacyl-ACP synthase condenses one molecule each of acetyl-ACP and malonyl-ACP, producing acetoacetyl-ACP and liberating CO2 And ACP-SH. This intermediate undergoes reduction by ketoacyl-ACP reductase to form (R)-3-hydroxybutyrate-ACP (Morya et al., 2021).
This pathway then proceeds through the removal of water molecules by hydroxyl-ACP-dehydrase, producing unsaturated pontony-ACP. Lastly, enoyl-ACP reductase reduces pontoyl-ACP to butyryl-ACP (Reddy et al., 2020). Chain elongation in the synthesis of longer mcl-PHA building blocks is accomplished by coupling butyryl-ACP with malonyl-CoA, with each cycle contributing two additional carbons to the chain. Specifically, propionyl-CoA acts as the initiating molecule (Reddy et al., 2020). The de novo synthesis of odd-numbered mcl-PHA building blocks instead of acetyl-CoA. This efficient metabolic pathway offers significant advantages to Pseudomonas putida when using glycerol as a substrate. Studies by Beckers et al. show that P. putida KT2440 requires only 0.039 mmol/g carbon dry weight (CDM) hours of glycerol for energy maintenance in continuous culture, a much lower value (about 17-fold) compared to that observed in organisms such as E. coli (Koller and Obruča, 2022). This translates into substantial economic benefits for the biotechnological application of glycerol as a substrate.
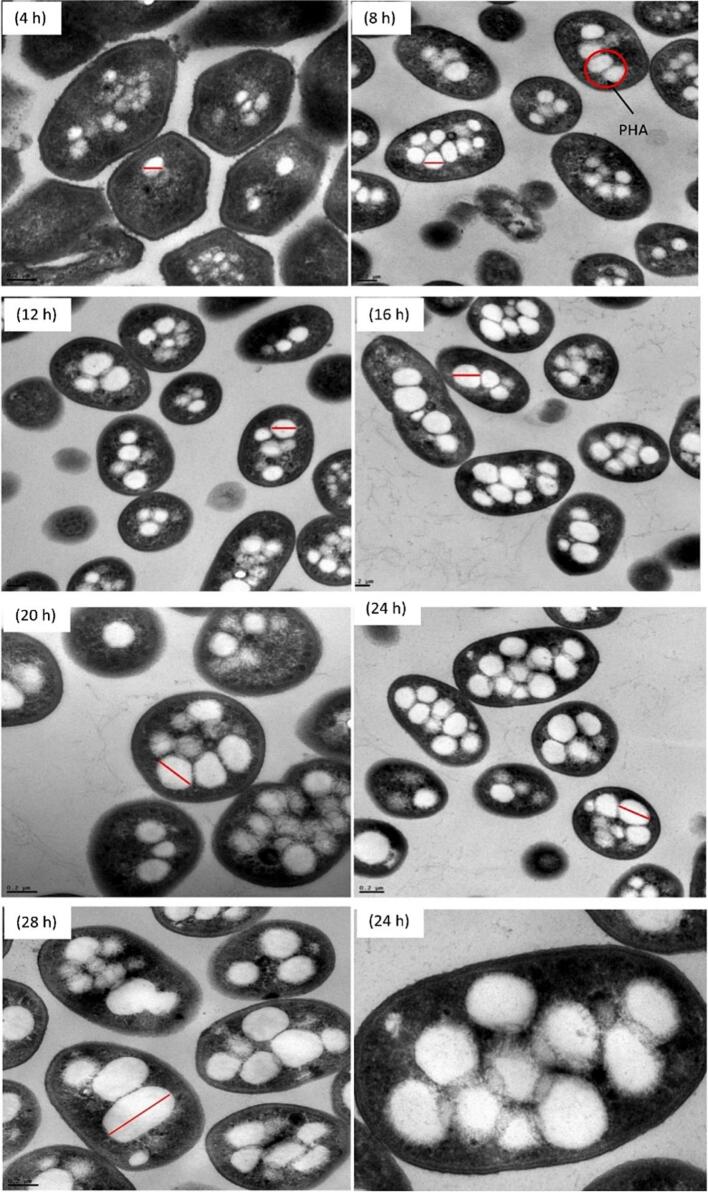
An important factor affecting mcl-PHA biosynthesis is the interaction between carbon and nitrogen availability. In Fig. 4, the transition from glycerol (carbon source) to nitrogen-restricted states triggers modulation of gene expression for processes such as glycerol degradation, cellular respiration chains, energy sensing mechanisms, and PHA biosynthesis. The transition from glycerol (carbon source) to nitrogen-restricted states triggers modulation of gene expression for processes such as glycerol degradation, cellular respiration chains, energy sensing mechanisms, and PHA biosynthesis. This metabolic shift directs the carbon flux toward the formation of PHA precursors (acetyl-CoA derived from the Entner-Doudoroff pathway and pyruvate decarboxylation) while minimizing respiration through glyoxylate shunt. Under nitrogen limitations, and favorable conditions for PHA biosynthesis, Beckers et al. reported a significant increase in PHA content in the dry mass of cells (CDM) to 0.297 g/g at a low dilution rate (D) of 0.044/h compared to 0.128 g/g at a higher dilution rate (D) of 0.205/h. These cultivation conditions also lead to a specific modulation of by-product formation, characterized by reduced levels of succinate and malate, intermediates of the TCA cycle (Wang et al., 2024).
Fig. 4.
Transmission electron micrograph of Priestia megaterium DSM 509 grown on glycerol in a nitrogen-deficient medium (scale bar: 0.2 μm) (Shahid et al., 2024).
Using basic flux mode analysis, the study by Beckers et al. further revealed the remarkable potential of P. putida KT2440 for PHA biosynthesis from glycerol. The analysis also identifies potential targets for metabolic engineering aimed at increasing PHA production capabilities (Liu et al., 2023). These findings highlight the complex interaction between individual gene expression and metabolic flux under conditions that favor or inhibit PHA biosynthesis on glycerol as a carbon source. This knowledge provided a valuable basis for the development of engineered P. putida strains that demonstrated superior PHA production efficiency.
2.3.2. Production of PHA from Saccharide waste
Leftover hydrolysates often contain a mixture of simple sugars and more complex carbohydrates, including disaccharides such as sucrose, lactose, and cellobiose. Direct utilization of these disaccharides for PHA production offers significant advantages by eliminating the need for additional pre-processing steps to convert them into monosaccharides, thereby reducing overall production costs. Several studies have explored this concept by engineering bacteria to express enzymes capable of hydrolyzing certain disaccharides. For example, the strain Pseudomonas putida EM42 was engineered to express the bglC (β-glucosidase-coding) gene of Thermobifida fusca. This modification allows the strain to grow on ac obiose as the sole source of carbon by hydrolyzing glycosidic β-1,4 bonds in the disaccharide (Sachan et al., 2024). Similarly, Cupriavidus necator NCIMB11599 was engineered to secrete ac (β-fructofuranosidase) from Mannheimia succiniciproducens. This recombinant strain showed the ability to accumulate up to 73.2 % PHA (dry weight of cells) when grown on sucrose, a disaccharide that wild-type strains cannot utilize (Sohn et al., 2021).
Another study investigated lactose utilization by C. necator DSM545 expressing the lacZ β-galactosidase gene from Escherichia coli. Although the strain is capable of growing on lactose, it is believed that only the glucose part of the disaccharide is utilized, whereas galactose remains unutilized (Chavan et al., 2024). However, subsequent research by Peabody et al. (2019) succeeded in engineering P. putida KT2440 for galactose growth as the only carbon source. This feat was achieved by introducing the De Ley-Doudoroff galactose catabolic pathway into the bacterial genome (Chacón et al., 2024).
2.3.3. Production of PHA from aromatic waste
Lignin, a complex aromatic polymer that makes up 15–30 % of lignocellulose, provides significant opportunities for valorization. Although some PHA producers may utilize certain lignin-derived aromatic monomers for PHA growth and production (Andhalkar et al., 2024), expanding their metabolic capabilities to cover a wider range of residues will allow for more efficient conversion of lignin feedstock. Ferulic and coumaric acids are the main constituents of lignin. However, Cupriavidus necator H16 shows limited growth on coumaric acid as its sole carbon source and is completely incapable of utilizing ferulic acid (Morya et al., 2021). To overcome these limitations, metabolic engineering approaches are used. Recognition of genes encoding enzymes from Pseudomonas putida (fcs – feruloyl-CoA synthase; ech – enoyl-CoA hydratase/aldolase; vdh – vanillin dehydrogenase) and from Rhodopseudomonas palustris and Sphingomonas paucimobilis (vanAB2/ligM – vanillate-O-demethylase), respectively, allowed strains of C. necator H16 is engineered to utilize ferulic and coumaric acids for the growth and production of P3HB (poly-3-hydroxybutyrate) (Kawaguchi et al., 2022).
Another important strategy to promote microbial growth on mixed substrates involves eliminating carbon catabolite repression (CCR). In P. putida KT2440, this is achieved through the deletion of the crc gene (which codes for the catabolite repression control protein). The resulting strain, P. putida KT2440Δcrc, was further engineered for the utilization of xylose and arabinose. This genetically modified strain shows the ability to co-utilize glucose, xylose, arabinose, coumaric acid, and acetic acid simultaneously, eliminating the phenomenon of diauxic growth and consequently reducing overall cultivation time (Sun et al., 2020).
2.4. Comparative analysis of PHA production methods
PHAs are biodegradable biopolymers synthesized by microorganisms under nutrient-limited conditions. Various methods are employed to produce PHA, each offering unique advantages and facing specific challenges in terms of yield, cost, scalability, and environmental impact. This section presents a comparative analysis of the major PHA production methods, including microbial fermentation, enzymatic production, and the use of mixed waste streams. The choice of bacterial species, substrates, and production techniques significantly influences the feasibility of scaling PHA production for industrial applications.
Microbial fermentation is the most established and widely used method for producing PHA, leveraging bacteria such as Cupriavidus necator, Burkholderia cepacia, and Bacillus megaterium. In this process, microorganisms synthesize PHA from carbon-rich substrates under nutrient-limited conditions, typically when nitrogen or phosphorus is depleted. The carbon source is a critical factor affecting both yield and cost of production.
The study by Khatami et al. (2022) provides a detailed comparison of these bacterial strains using two distinct substrates: synthetic medium and digestate liquor (a byproduct of food waste and primary sludge). The performance of these strains varies significantly based on the substrate used. Cupriavidus necator exhibited the highest PHA yields in both synthetic medium and digestate liquor, accumulating up to 77.54 % of cell dry weight (CDW) in the latter. This highlights the ability of C. necator to efficiently metabolize volatile fatty acids (VFAs) such as acetic acid and propionic acid, which are abundant in digestate liquor derived from food waste. Burkholderia cepacia achieved moderate PHA yields of 54.9 % CDW, suggesting that while it is capable of PHA production, its efficiency is lower compared to C. necator. This reduced efficiency may be attributed to differences in metabolic pathways and substrate preferences between the two species. Bacillus megaterium, despite being known for PHA production, performed poorly in both synthetic medium and digestate liquor, accumulating only 3.42 % CDW in digestate liquor. This could be due to its lower capacity for metabolizing VFAs, particularly in complex substrates like digestate liquor.
The choice of substrate plays a crucial role in determining the cost-effectiveness and scalability of PHA production. Synthetic medium is commonly used in laboratory studies because it allows for precise control over nutrient availability. However, its high cost makes it unsuitable for industrial-scale production. In contrast, digestate liquor, a waste product from anaerobic digestion of food waste, offers a low-cost alternative that aligns with circular economy principles. Khatami et al. (2022) demonstrated that using digestate liquor not only reduces production costs but also leads to higher PHA yields for certain bacterial strains, particularly C. necator.
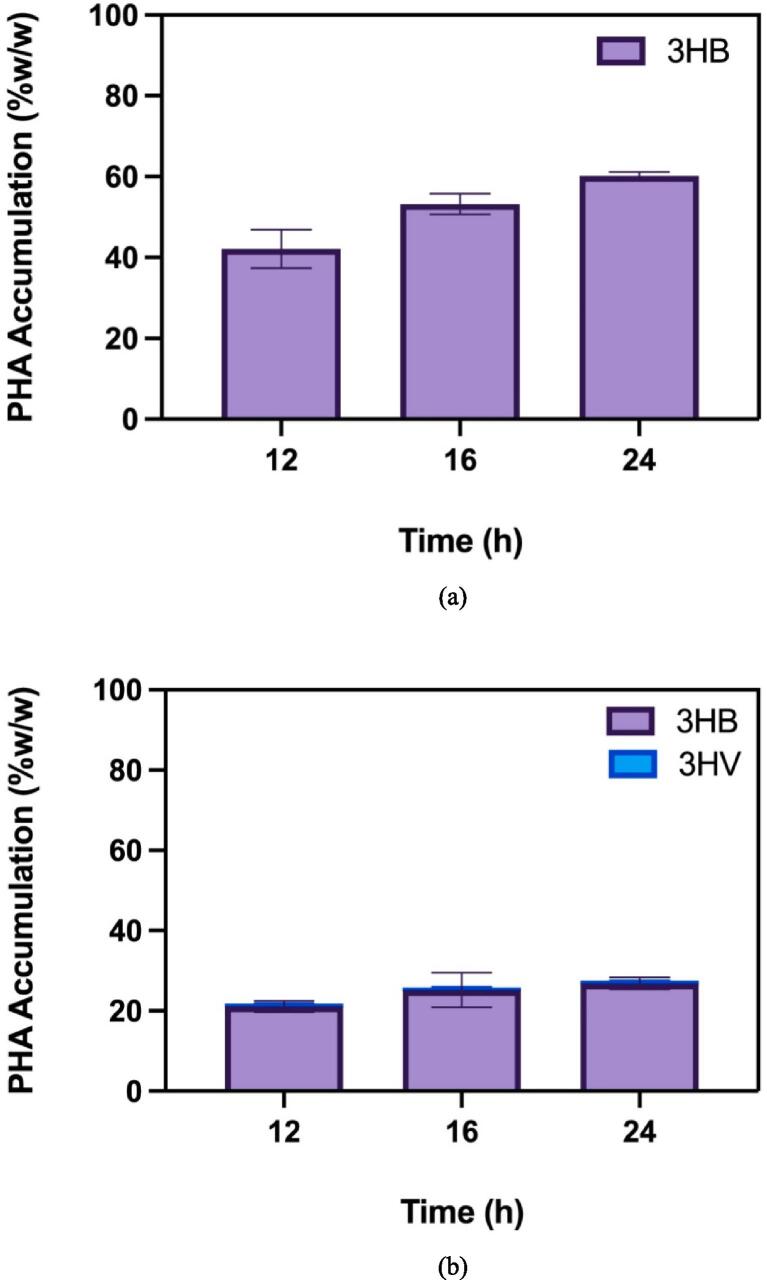
The performance of these bacterial strains in synthetic medium is illustrated in Fig. 5, where Cupriavidus necator outperformed Burkholderia cepacia in terms of PHA accumulation over time. This highlights the superior efficiency of C. necator in controlled environments like synthetic media. However, despite the high yields achieved in synthetic medium, its high cost limits its feasibility for large-scale production.
Fig. 5.
PHA accumulation over time in mono cultures using synthetic medium: (a) Cupriavidus necator and (b) Burkholderia cepacia (Khatami et al., 2022).
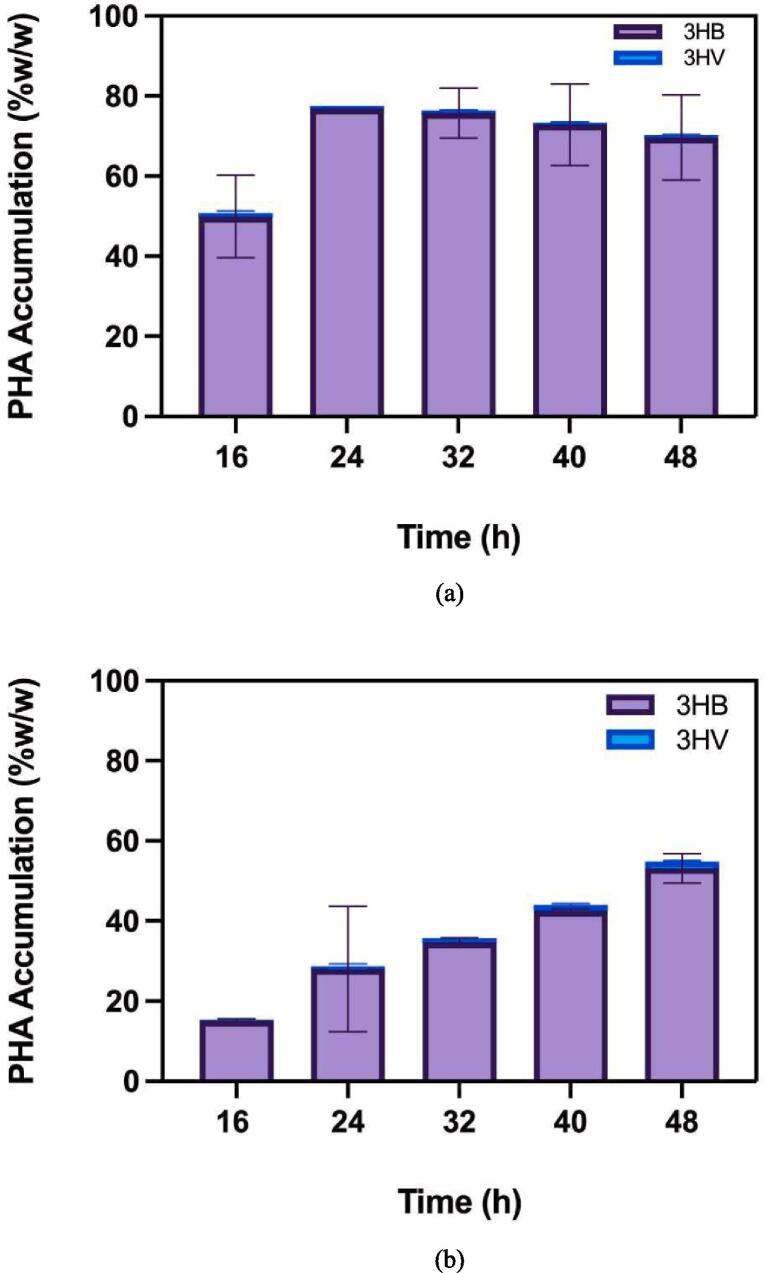
Fig. 6 illustrates the performance of these strains in digestate liquor. As seen in the figure, Cupriavidus necator again exhibited the highest PHA accumulation, followed by Burkholderia cepacia and Bacillus megaterium, which had much lower yields. This shows the potential of using waste-derived substrates like digestate liquor to produce PHA at a lower cost, though variability in the composition of digestate liquor may introduce challenges in process control.
Fig. 6.
PHA accumulation over time in mono cultures using digestate liquor: (a) Cupriavidus necator; (b) Burkholderia cepacian; and (c) Bacillus megaterium (Khatami et al., 2022).
Another method for PHA production involves the use of enzymes to catalyze the polymerization of hydroxyalkanoates (HA) into PHA. Enzymatic production is less commonly used compared to microbial fermentation but offers advantages in producing PHAs with specific molecular structures and properties. This method allows for greater selectivity and customization, making it ideal for producing specialized PHAs tailored to specific industrial applications (Khatami et al., 2022).
Despite these advantages, enzymatic production has several drawbacks. It is generally more expensive due to the cost of enzymes and the complexity of the purification process. Moreover, enzymatic methods are less scalable than microbial fermentation, limiting their use to small-scale or niche applications. As such, enzymatic production is not currently considered viable for large-scale PHA production.
The use of mixed waste streams as feedstock for PHA production is an increasingly popular approach, especially in the context of circular economy principles. Waste streams such as food waste, agricultural residues, and municipal waste are abundant, low-cost carbon sources that can be utilized in microbial fermentation to produce PHA. Khatami et al. (2022) demonstrated that digestate liquor derived from the anaerobic digestion of food waste can be effectively used to produce PHA, with Cupriavidus necator achieving high yields.
The major advantage of using mixed waste streams is the significant reduction in feedstock costs, making PHA production more economically viable. However, the composition of waste streams can vary, introducing variability in microbial growth and PHA synthesis. Pretreatment of waste streams is often necessary to remove contaminants or inhibitory compounds, adding complexity to the production process. The Table 4 summarizes the advantages and disadvantages of the three main PHA production methods.
Table 4.
The advantages and disadvantages of the three main PHA production methods.
| Method | Advantages | Disadvantages |
|---|---|---|
| Microbial Fermentation | High yield, flexible feedstock options, scalable | Requires precise nutrient control, energy-intensive, byproduct purification needed |
| Enzymatic Production | Selective, customizable PHA properties | Expensive, less scalable, complex purification required |
| Mixed Waste Streams | Low-cost feedstock, aligns with circular economy principles | Variable feedstock quality, pretreatment often required |
3. Food packaging
The global shift toward sustainable practices has increased interest in biodegradable materials as alternatives to conventional plastic packaging. Biodegradable biological resources such as proteins, lipids, and polysaccharides are increasingly being used to address plastic pollution concerns (Amin et al., 2021). Plastics remain dominant in food packaging due to their low cost, ease of processing, and versatile production methods (Asdagh et al., 2021), but overuse and poor recycling practices contribute significantly to environmental pollution (Sani et al., 2021). Consequently, biodegradable materials, particularly biopolymers, are being explored as replacements.
PHAs is a type of biodegradable polymer produced by microorganisms, hold significant promise as a sustainable alternative to traditional plastics. PHA’s biodegradability and compatibility with existing plastic-processing techniques make it a strong candidate for various food packaging applications. However, challenges related to production cost, scalability, and material properties limit its large-scale adoption in the food packaging industry. Addressing these barriers is crucial for transitioning PHA from a niche product to a mainstream packaging solution.
This study provides several key insights into how PHA production can be optimized for industrial use, particularly in the food packaging sector. The use of food waste-derived volatile fatty acids (VFAs) as a carbon source for PHA production has been shown to reduce production costs while maintaining high PHA yields. Specifically, Cupriavidus necator was able to produce up to 77.54 % of cell dry weight (CDW) as PHA when grown in digestate liquor, a waste byproduct from food waste and primary sludge processing (Khatami et al., 2022). This finding is critical for industries aiming to adopt sustainable practices, as it demonstrates how waste can be repurposed to produce high-value bioplastics.
The versatility of PHA is another important factor that enhances its industrial potential. PHAs can be processed into various packaging forms, including flexible films, trays, and containers, through standard manufacturing techniques such as injection molding, extrusion, and thermoforming. This adaptability makes PHA suitable for a wide range of food packaging applications, from fresh produce packaging to more rigid containers for processed foods. However, while PHA has advantages in biodegradability, its mechanical and barrier properties need improvement to match those of conventional plastics.
One of the primary limitations of PHA is its relatively high production cost compared to petroleum-based plastics such as polyethylene (PE) or polypropylene (PP). Scaling up production using low-cost feedstocks like food waste is one approach to reducing these costs (Loan et al., 2022, Vu et al., 2021). Additionally, the mechanical properties of PHA—such as flexibility and barrier performance against moisture and gases—are lower than those of traditional plastics. These limitations pose challenges for its widespread adoption in food packaging, where durability and food preservation are critical.
However, research has shown that blending PHA with other biopolymers or incorporating functional additives, such as plasticizers or nanoparticles, can significantly improve these properties (Khatami et al., 2022). Such modifications can increase flexibility, enhance moisture resistance, and improve gas barrier properties, making PHA more competitive for a wider variety of food packaging applications. For instance, biopolymer-based membranes are hydrophilic, which can reduce mechanical strength, but they excel in gas barrier properties, which is beneficial for food preservation (Luangapai et al., 2019).
Moreover, the incorporation of functional active ingredients into PHA-based packaging such as antibacterial agents, antioxidants, and colorants can further enhance the quality and safety of packaged foods (Tavassoli-Kafrani et al., 2022). This capability allows PHA to contribute to extended shelf life, improved food safety, and enhanced consumer appeal through smart packaging innovations. These developments are crucial for industries looking to meet both environmental standards and consumer demands for safer, more sustainable packaging solutions.
The environmental benefits of PHA packaging are considerable. Unlike petroleum-based plastics, PHA is fully biodegradable, breaking down naturally into non-toxic by products, thus reducing long-term environmental impact. Moreover, PHA’s biocompatibility makes it safe for direct contact with food, minimizing the risk of chemical migration that can occur with traditional plastics. These factors make PHA an attractive option for companies aiming to reduce their environmental footprint while ensuring food safety.
In the context of food safety, the ability of PHA to degrade safely without leaving harmful microplastics behind is a key benefit. As public awareness of microplastic contamination grows, packaging materials that do not contribute to this problem are becoming more attractive to both consumers and regulatory bodies.
3.1. Biodegradable food packaging process using PHA
Biodegradation of bio-based polymers utilizes fermentation methods to transform raw biomass into feedstock (e.g., lactic acid for PLA production) and polymers (e.g., polyester PHA synthesized by bacteria under nutrient-limited conditions) (Beltrán et al., 2019). The steps of processing biodegradable food packaging involve the polymerization of the basic constituent materials, the addition of additives and blending with another polymer, and processing include extrusion, injection molding, blow molding, and thermoforming, to achieve the final product (Soroudi and Jakubowicz, 2013).
3.1.1. Film extrusion process
Extrusion techniques have garnered attention in biopolymer film processing research in recent years due to their potential as a low-cost, high-demand alternative (Chevalier et al., 2018). Because it achieves high temperatures quickly and has a very easy operation, the extrusion process is the most popular method for producing industrial plastics at large production levels (Borries-Medrano et al., 2016). The polymer resin is first melted and then forced into an extrusion die to create a film. It then rapidly cools and solidifies on cooling rollers. The thickness of the continuous film extrusion process can be adjusted. The drawback of biopolymers is that, in comparison to traditional plastics, they are more heat-sensitive. Furthermore, during the extrusion process, certain elements including excessive heat, friction force, and pressure break down biopolymers. Screw configuration, temperature profile, duration, and correct fusion and homogenization are important factors to optimize to prevent degradation of biopolymers. Adding plasticizers serves the purpose of boosting process capacity. The twin-screw extrusion approach is recommended, if more costly since it provides superior controllability for naturally occurring biopolymers. Biopolymers such as PLA (polylactic acid), starch, chitosan, and cellulose derivatives are frequently extruded into films. A revolutionary food packaging film based on starch was created by Gutiérrez and Valencia, and it has better qualities than traditional TPS (thermoplastic straw) films (Gutiérrez and Valencia, 2021). The application of starch films in most food products as coatings or packaging without changing their appearance or taste because they are tasteless, odorless, and colorless (Basiak et al., 2017). In this case, the researchers tried to overcome the shortcomings of TPS films, namely reduced sensitivity and brittleness to water (Mendes et al., 2020). The researchers' hypothesis was to use reactive extrusion to link the starch chains with sodium TPP (tripolyphosphate) and modify the structure of regular starch in an attempt to fortify the starch network. In this instance, SNC (ordinary corn starch), which has a greater amylose content, was used to create four film systems. Reactive extrusion was used in the film-making process to progressively alter the starch and cross-linking. Every movie has its unique qualities. Because of the increased hydrogen bonding caused by cross-linking, the resultant starch phosphate plastic is more hydrophilic and has better crystallinity than regular TPS. SNC addition enhanced the crystallinity as well. However, no appreciable adjustments were made to enhance the standard TPS's membrane properties. Although the technical procedure is the same, the problem with water sensitivity persists. The starch-phosphate layer did not release phosphorus as predicted for fertilization of lettuce seedlings. This is probably because of the reduced bioavailability and lower phosphorus concentration. The starch chain structure was altered as a result of this change; however, the plastic properties were not noticeably improved. Reactive extrusion can alter starch-based plastics, as demonstrated by this work; nonetheless, more investigation is required to address TPS materials' drawbacks. Effective mixing, the production of gelatinized starch, and chemical cross-linking are made possible by the extrusion process, which produces packaging that is both high-quality and environmentally friendly (Gutiérrez and Valencia, 2021). One type of packaging material composed of natural polymer compounds is an edible film (Galus and Kadzińska, 2015). The benefits of edible film include non-toxicity, convenience, safety, biodegradability, and hygiene. It may also be utilized as a plastic inner packaging and additive carrier, replacing traditional packaging and offering a wide range of development opportunities (Gómez-Guillén et al., 2011, Limpisophon et al., 2010). The protein-based edible film is easily absorbed and digested by the human body. The intermolecular crosslinking that results from the hydrogen bond, hydrophobic action, and disulfide bond between protein molecules gives the film exceptional mechanical strength and barrier performance. At the moment, a lot of research is being done on edible films based on proteins, including zein, whey protein, gelatin, and soybean protein isolate as the primary raw materials (Jin et al., 2021). Extrusion techniques with temperatures ranging from 85 °C to 150 °C can be used to create edible films (Huntrakul et al., 2020). Nonetheless, an edible film produced by solvent-casting techniques is expected to be more effective than extrusion techniques (Park et al., 2008). Pranata et al. conducted research to produce bioplastic films from Eggs with Protein (EWP) using extrusion and calendering processes, both of which are commonly used methods for producing plastic films in industrial environments (Pranata et al., 2019). Egg white protein (EWP) has the characteristics of a wide source, easy digestibility, high biological valence, rich nutritional value, and excellent functional properties, such as gelling, foaming, and emulsifying properties (Jin et al., 2021). The purpose of this study was to determine whether extrusion and calendering-produced EWP films may be utilized in food packaging as an alternative, sustainable material. Initially, the goal of the study was to determine the minimum temperature needed for the roll calendering and extrusion operations in order to create sustainable and uniform EWP films. Consequently, it was discovered that extruder temperatures between 40 °C and 75 °C and rolling temperatures between 115 °C and 120 °C can be used to manufacture transparent and thin EWP films. The EWP film was exposed to varying temperatures and humidity levels, which affected the food packaging's properties and allowed for further evaluation. The thickness, resilience, flexibility, permeability, color, transparency, and removal of plasticity of EWP films were all found to be impacted by humidity. Temperature, meantime, had an impact on the transparency, brightness, and transmittance of the films. The primary conclusions show that while EWP films have stronger stiffness, heat resistance, and oxygen barrier qualities, they are less flexible and brittle than PLA plastic films that are sold in stores. The clarity, brightness, color, ethanol barrier qualities, and moisture resistance of PLA and EWP films are comparable. The goal of this extrusion technology is to make it possible to generate EWP bioplastic films sustainably in a manner akin to that of plastic films made in factories. This study demonstrated that using extrusion procedures that comply with conventions can result in the production of films based on EWP (Pranata et al., 2019).
3.1.2. Blown film extrusion process
The blown film extrusion process is a conventional plastic process to produce thin films for commercial packaging applications (Khumkomgool et al., 2020). In this process, a liquid biopolymer tube is extruded vertically up through the ring mold and pumped into it with deep air pressure. To saturate it, cooling air is blown into the tubes that are expanding before the tube compresses and rolls. Compared to the printed film, the biaxial orientation of the chain increases the strength of the film and the barrier properties. The comparison between biopolymers is limited to their melting elasticity. Pump films made of aliphatic starch, PLA, and polyester can be made, but low melt strength can cause bubbles to become unstable. When extruding a pump film, using long chain branched material helps improve melt resistance and bubble stability. In this study, the composite film HPDSP (hydroxypropyl distarch phosphate)/PHA was produced using a pump extrusion process in laboratory conditions. Compared to solvent casting, blower extrusion attracts attention because it is highly productive, efficient, and similar to the actual industrial production rate (Gao et al., 2012). As a result, the research concentrated on a blower extrusion film made of starch or a starch/PHA mixture that is ideal for making large-scale packaging films. Using inflatable film extrusion technology, Phothisarattana et al. showed a new biodegradable nanocomposite film containing ZnO nanoparticles as an antimicrobial packaging on meat products (Phothisarattana et al., 2022). Biodegradable polymers such as PBAT (Poly(Butylene Adipate-Co-Terephthalate)) are extremely flexible and easily converted into food and packaging films. However, the high PBAT costs limit its use for commercial packaging applications (Wadaugsorn et al., 2022). However, PBAT cannot prevent food degradation due to its poor barrier properties compared to conventional plastics. To deal with this, ZnO nanoparticles are added, which can have an antimicrobial effect and increase food shelf life (Phothisarattana et al., 2022).
ZnO nanoparticles have high stability as a low-cost nanofiller with beneficial properties, such as high ultraviolet (UV) absorption and strong antibacterial activity on various bacteria (Yu et al., 2021). The scientists used the double screw extrusion method accompanied by pump film extrusion to insert ZnO nanoparticles (1–5 %) into the PBAT/TPS polymer mixture. The pump film extraction method was chosen because it allowed sustainable film production on a commercial scale, unlike the solvent casting method used in most previous experiments. The PBAT/TPS/ZnO nanocomposite film made shows that some ZnO nanoparticles are agglomerated. This leads to a decline in mechanical properties, such as a decrease in traction. Nevertheless, the use of ZnO nanoparticles increased the interaction of hydrogen bonds with the TPS biopolymers domain, which resulted in increased dispersion. FTIR (Fourier Transform Infrared Spectroscopy) shows that ZnO nanoparticles alter the bonding of CO and CO in PBAT. The presence of higher ZnO content, i.e., 5 %, strong interaction between ZnO and TPS increases nanoparticle dispersion and reduces nanoparticle compression along with increased amorphous particle content. When used to store pork in packaging, PBAT/TPS/ZnO nanocomposite films show antimicrobial effects and reduce microbial growth and lipid oxidation in meat when compared to conventional ZnO free films. It also extends the shelf life of pig meat in packing for more than three days during storage in the refrigerator, with ZnO concentrations higher than 3 %. ZnO nanoparticle characteristics, as well as control of oxygen permeability in the film and surface morphology, contribute to the antimicrobial and antioxidant effects (Phothisarattana et al., 2022). Using pump film extrusion techniques, Fehlberg et al. investigated whether there was a mixture of OP powder (Orange Peel) into a polyethylene film, which made the packaging material more environmentally friendly. Using Low Linear Density Polyethylene Polymer Matrix (LLDPE). LLDPE is a cheap conventional plastic that is widely used in food packaging applications due to its good mechanical properties (Panrong et al., 2020). The process is as follows: the OP powder is mixed with LLDPE in a double-screw extrusion machine to produce a base mixture containing 22 % OP. Then, the blown film of the extruder is blended with LLPDE to produce the film with 5–12,5 % OP. The blower film extrusion method is used to process the mixture film up to 11.5 % OP. The loss of the film during the melting process was caused by an operational improvement above 11.5 %. After that, the morphology, thickness, mechanics, barriers, optics, and thermal properties of the made LLDPE/OP film were checked. The results of the research showed that LLDPE/OP films blown with 11.5 % OP had the same crystallinity, melt temperature, and water vapor barrier properties as the unblown LLDPE films. The use of OP increases the barrier capacity of visible light and ultraviolet rays. Traction strength and prolongation break the mixed film, however, decreased (Fehlberg et al., 2023).
3.1.3. Multilayer film process
Multilayer films have emerged as a viable strategy to circumvent the inherent limitations of biopolymers. These engineered systems incorporate multiple layers, each designed to impart specific functional attributes. Typical components include external protective layers, moisture and gas barriers, and heat-sealable or food-contact linings. The fabrication of these multilayer structures commonly involves lamination, extrusion, or coextrusion processes (Morinval et al., 2024). A common approach involves the integration of natural biopolymer films with synthetic polymers. To enhance the overall properties of these composite materials, nanomaterials are often incorporated. Illustrative examples include multilayer inflatable films and thermoformed containers produced from starch (Grzebieniarz et al., 2023), poly(lactic acid) (PLA) (Martin and Avérous, 2001), chitosan (Kruk et al., 2023), and aliphatic polyesters (Yang et al., 2024). Recent research has explored current trends in bioactive multilayer films, which combine natural biopolymers and additives to create sustainable and environmentally friendly food packaging solutions. These films achieve optimal properties, such as mechanical strength, excellent barrier properties, and low hydrophilicity (Fotie et al., 2020, Le Gars et al., 2020). This approach effectively overcomes the limitations inherent in single-layer biopolymer films. Common biopolymers utilized in these multilayer structures include gelatin, chitosan, zein, PLA, cassava starch, potato starch, and soy isolate protein (Martin and Avérous, 2001). These multilayer plastic systems enable the controlled incorporation of naturally derived bioactive compounds, such as plant extracts and essential oils. This approach facilitates the development of active packaging with functionalities including antibacterial, antioxidant, and UV protection (Avila et al., 2023).
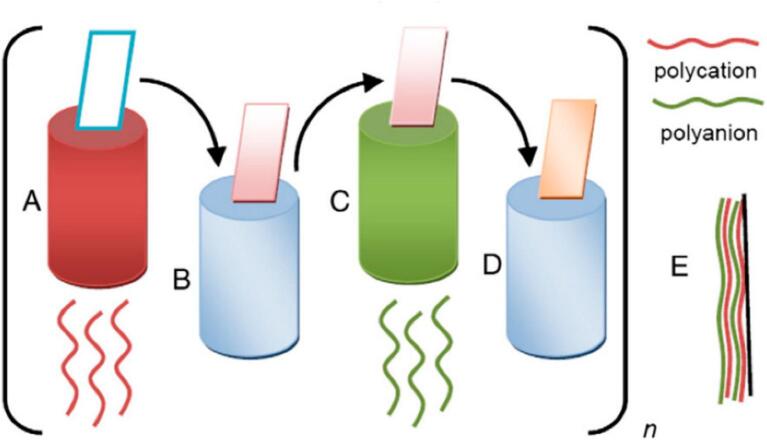
In addition to these methods, electrostatic interactions between polymers with opposite charges offer another approach for creating multilayer films. This process involves immersing a substrate in a polycation solution followed by immersion in a polyanion solution (Fotie et al., 2020, Garrido et al., 2016). This Layer-by-Layer deposition technique, as outlined by De Villiers et al., is advantageous for producing uniform multilayer films with controlled thickness at the nanoscale (Gontard et al., 1992). The process can be divided into several key steps: Step A involves immersing the substrate in a polycation, Step B consists of washing the substrate in a solvent for polycation, Step C involves immersing the substrate in a polyanion, and Step D is washing the substrate in a solvent for polyanion. These steps can be repeated multiple times to build up the desired multilayer system, as represented by Step E (Granda-Restrepo et al., 2009). The schematic process of Layer-by-Layer method is illustrated in Fig. 7.
Fig. 7.
Schematic process of Layer-by-Layer method (Granda-Restrepo et al., 2009).
A study characterized fully biodegradable multilayer films comprising PLA and organoclay nanoparticles for potential food packaging applications (Maleki et al., 2022, Mujtaba et al., 2019). These films were fabricated through an extrusion process resulting in a three-layer structure (Naser et al., 2021). The core layer contained 4 % organoclay nanoparticles, while the outer layers consisted of pure PLA as illustrated in Fig. 8. The incorporation of organoclay notably improved both the oxygen barrier and mechanical properties of the multilayer film compared to a pure PLA film, with a 40 % increase in tear resistance (Scarfato et al., 2017). Moreover, the organoclay layer facilitated heat sealing at higher temperatures (80–100 °C) (Velásquez et al., 2021). Lactic acid migration rates for all multilayer films remained well below the EU’s total food migration limit of 60 mg/kg, despite significant morphological changes observed during the migration test, particularly in the ethanol trial (Velásquez et al., 2021). These findings suggest the potential for developing high-performance, fully biodegradable PLA/organoclay multilayer structures that meet regulatory standards for food packaging (Vidal et al., 2023). However, multilayer plastic packaging-based products are still difficult to recycle and this aspect, besides undermining the sustainability of materials, also slows down the transition towards a circular economy. The main problem blocking the proper recycling path is caused by the fact that standard technologies are currently incapable of identifying, selecting, and easily separating the various layers that comprise these packaging materials (Velásquez et al., 2019). Hence, in European countries, the leading solution for multilayer flexible packaging disposal is represented by its combustion with the recovery of energy. Alternatively, in low-income countries, this waste will be sent to landfills (Costamagna et al., 2023).
Fig. 8.
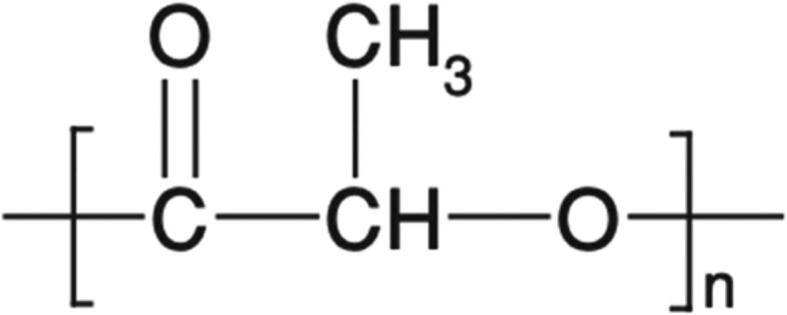
The chemical structure of PLA (Naser et al., 2021).
3.1.4. Film casting process
Solvent casting involves dissolving biopolymers in a solvent and applying the solution to a substrate using a coating blade. The solvent evaporates, leaving behind a biopolymer film (Murueva et al., 2024). Common solvents include dimethyl sulfoxide and dimethylacetamide. Multiple layers can be sequentially applied to create multilayer films. While this method allows for the incorporation of additives like lipids and antimicrobial agents, it is a batch process unsuitable for large-scale production (Rakkan et al., 2024). Edible films offer a sustainable packaging alternative to traditional plastics, reducing waste and extending the shelf life of fresh products like meat (Murueva et al., 2024). These films are produced through wet casting (solvent evaporation) or dry casting (heat and pressure). Biopolymers, such as starch, proteins, and lipids, form the basis of these films. By combining different materials, desired barrier, mechanical, and optical properties can be achieved (Tomietto et al., 2022). Edible films act as protective barriers for meat, preventing moisture loss, gas exchange, lipid oxidation, and microbial growth. Incorporating antibacterial and antioxidant compounds further enhances the film's protective properties. Accurate moisture control, consistent film thickness, and careful biopolymer selection are crucial for successful film production. Although sustainable and biodegradable, edible films can be costly and may alter food texture or flavor (Prakoso et al., 2023).
3.1.5. Thermoforming
A popular manufacturing process named thermal forming is heating a thermoplastic sheet and using a mold to shape it into the desired shape (Leite et al., 2018). The process is categorized into three stages: heating, forming, and cooling. The process begins with heating the thermoplastic sheet until it is soft and forming it using a mold with the help of a vacuum or pressure while cooling it until the desired shape is obtained. The process of pressing results in drying and densification, which increases the density and improves the mechanical properties of the molded product (Pasquier et al., 2023). In this process, there are three types depending on the load applied, namely pressure, vacuum, and contact forming methods. Aliphatic starch, PLA, and polyester materials thermoformed into food packaging, trays, refrigerator doors, tubs, and cups, are some of the thin and thick gauge thermoforming products produced by pressure, vacuum, and contact forming methods. Sheet thickness and temperature must be kept neither too hot nor too cold to prevent product damage. The low melting force of these products limits the pull ratio and therefore plasticizers are often required (Hosseinionari and Seethaler, 2024, Patil et al., 2021).
3.1.6. Compression and injection molding process
Plastic materials in particular are molded through the Injection Process. This process is said to be preferred for the production of a wide variety of products with varied specifications such as shapes and sizes, etc. In fact, the type of raw material varies, depending on the function or service that the final product will provide (Mourya et al., 2023). The cycle time is shorter than thermoforming, but the flexibility of the mold is limited. The injection molding process involves injecting a liquid polymer into a mold that is reinforced under high pressure to create complex shapes. The main injection molding products are Poly Lactic Acid (PLA) tableware and stationery. Starch-like materials can be molded through compression and injection processes using plasticizers. The focus of thermocompression is to make the production method more scalable while saving energy than conventional solvent casting techniques (Lopez et al., 2014). In spite of the fact that infusion molding has higher generation rate, exactness compression molding is competent of manufacturing focal points with higher quality (lower leftover stresses and more precise geometries) (Li et al., 2017).
3.2. Implementation of biodegradable food packaging
3.2.1. Meat and seafood
Biodegradable packaging products manufactured from food waste and by-products offer promising applications for products like meat and seafood. Gelatin-based packaging infused with grapefruit seed extract demonstrated significant antimicrobial effectiveness against several meat spoilage bacteria, including Escherichia coli, Staphylococcus aureus, Salmonella Typhimurium, and Listeria monocytogenes. This packaging extended the storage life of beef by six days compared to conventional packaging (Abreu et al., 2012). In conclusion, biodegradable packaging derived from food waste demonstrates considerable potential for enhancing the quality, safety, and shelf life of perishable meat and seafood products (Bhargava et al., 2020). Scaling up laboratory-based research on biodegradable packaging from food waste to industrial applications requires further investigation. Key areas of focus include plastic formation, mechanical properties, food-packaging interactions, production feasibility, and economic viability. Addressing these challenges is essential for the successful commercialization of such innovative packaging materials (Dilkes-Hoffman et al., 2018).
These packaging materials minimize moisture loss, maintain product texture, and prevent unsightly juice leakage, ultimately reducing food waste. Edible packaging further safeguards food quality by inhibiting biochemical degradation, protecting lipids and proteins from oxidation, delaying rancidity, and preventing discoloration (Yb and Kola, 2019). The application of this food packaging is presented in Table 5.
Table 5.
Applications of edible food packaging on meat and seafood (Petkoska et al., 2021).
| Food product | Edible materials | Beneficial effect | References |
|---|---|---|---|
| Sliced ham | Na-alginate edible films as vehicles for delivering probiotic bacteria | Some probiotic bacteria were successfully delivered into the product using edible film | Pandhi et al., 2019, Pavli et al., 2019 |
| Oregano essential oil (OEO) is incorporated in Na-alginate edible films | Decrease in Listeria population. The presence of OEO on the film resulted in a difference in color compared to the control test sample which showed a decrease in product quality but an increase in aroma | ||
| Sliced cooked ham | Film matrix composed of poly(lactic acid) containing cellulose nanocrystals (PLA-CNC); films converted to bioactive films using nisin as an antimicrobial agent | Ability to inhibit Listeria monocytogenes and its physicochemical and structural properties (stored for 14 days at 4 °C). Significant reduction of L. monocytogenes in ham from day 1 and complete inhibition from day 3. | Salmieri et al. (2014) |
| Muscle foods | Collagen edible films | Lower moisture content, minimize lipid oxidation, prevent discoloration, and reduce water droplets | Pandhi et al. (2019) |
| Shrimps | Bilayer films based on agar and Na-alignate with cinnamon oil | Antioxidant activity; effective against Photobacterium phosphoreum | Arancibia et al. (2014) |
| Lamb meat | Nano-encapsulated Satureja khuzestanica essential oils (SKEO) in chitosan coatings (containing free or nano-encapsulated SKEO) | Effectively inhibited microbial growth and chemical spoilage; the encapsulation process slowed down the release of SKEO and led to longer lasting antimicrobial and antioxidant activities; improved sensory attributes | Pabast et al. (2018) |
| Turkey deli meat | Pullulan films with silver nanoparticles (Ag Nps), zinc oxide nanoparticles (ZnO NPs) oregano oil (OR) 2 % and rosemary oil (RO) 2 % | Ag NPs and OR edible films were more active than ZnO NPs and RO. Films based on Ag NPs, ZnO NPs, OR, and RO had antibacterial activity against pathogens: Listeria monocytogenes and Staphylococcus aureus. | Guo et al., 2014, Khalaf et al., 2013 |
| Edible coatings incorporating chitosan, lauric arginate ester (LAE) and nisin | Reduction of Listeria innocua. Combining antimicrobial coatings or films with flash pasteurization (FP) will reduce L. innocua. | ||
| Roasted turkey | Four polysaccharide-based edible coatings (starch, chitosan, alginate and pectin) incorporating sodium lactate (SL) and sodium diacetate (SD) and commercial preparations Opti.Form PD4, NovaGardTM CB1, Protect-M and GuardianTM NR100 | Pectin coatings using SL/SD, Opti. Form PD4 with or without Protect-M, and NovaGARDTM CB1 showed higher antimicrobial efficacy against L. monocytogenes. Frozen storage significantly increased the anti listeria activity of various coating treatments | Jiang et al. (2011) |
| Harbin red sausage | Edible chitosan (CTS) coatings (0, 1, 2, and 3 %) | Improved storage stability by increasing the concentration of edible CTS coating. Maintaining storage stability (pH and water distribution) and inhibiting microbial growth (total aerobic bacteria and lactic acid bacteria). | Dong et al. (2020) |
| Ham and bologna | Antimicrobial effects of carvacrol and cinnamaldehyde incorporated into apple, carrot, and hibiscus-based edible films | Antimicrobial effect against Listeria monocytogenes. Carvacrol film showed better antimicrobial activity than cinnamaldehyde film. The cream is more effective on ham than bologna. | Ravishankar et al. (2012) |
| Fresh pork | Chitosan-gelatine edible coatings incorporating grape seed extract and/or nisin | Effectively inhibited pork oxidation and microbial spoilage; grape seed extract further strengthened the antioxidant activity against meat oxidation, but incorporating nisin into the coating did not enhance the antimicrobial and antioxidant effects further | Xiong et al. (2020a) |
| Fresh pork loin | Oregano essential oil (OEO) and resveratrol (RES) nanoemulsion loaded in pectin (PEC) edible coating | It can significantly extend the shelf life of pork by minimizing pH and color changes, slowing down lipid and protein oxidation, maintaining meat tenderness, and preventing microbial growth | Xiong et al. (2020b) |
| Beef meat | Antioxidants naturally present in olives, hydroxytyrosol (HT) and 3,4-dihydroxyphenylglycol (DHPG) added to a pectin-fish gelatin edible film. A new composite film included beeswax is also compared. | Films with antioxidants were able to reduce the formation of oxidation products compared to standard films without antioxidants. Lower lipid oxidation. The combined effect of oxygen barrier action and specific antioxidant activity of beeswax-based composite film (lipid oxidation was inhibited and could be stored for 7 days at 40 °C). | Bermúdez-Oria et al. (2019) |
| Sea bass (Dicentrarchus labrax) fillets | A combination of liquid smoke and thymol encapsulated in chitosan nanofibers | Delays the total growth of mesophilic aerobic bacteria, psychrophilic bacteria, as well as yeasts and molds throughout the storage period | Ceylan et al. (2018) |
Table 5 presents several examples of how edible food packaging has been used to preserve the quality and extend the shelf life of meat and seafood products. This table illustrates the practical applications of various biopolymer-based films and coatings, such as those made from chitosan, alginate, and gelatin, which have shown significant antimicrobial and antioxidant activity in preventing spoilage and degradation. While the data in this table comes from previous studies, our contribution lies in expanding on this information by discussing how PHA-based packaging offers a sustainable, scalable solution that can address the limitations of traditional edible coatings in the meat and seafood industry.
Edible coatings for meat and seafood, such as those made from collagen, chitosan, and pectin, have been proven to reduce microbial growth, inhibit lipid oxidation, and improve moisture retention, thereby extending product shelf life (Pandhi et al., 2019, Salmieri et al., 2014). For example, chitosan-based coatings have demonstrated strong antimicrobial effects against pathogens like Listeria monocytogenes and Staphylococcus aureus, which are common in meat products (Guo et al., 2014). However, these edible coatings often face limitations in terms of scalability and industrial applicability, as they require direct application to the product surface, which may not be feasible in large-scale food production (Pabast et al., 2018).
PHA-based films, in contrast, offer several advantages for large-scale meat and seafood packaging. PHA is biodegradable, compostable, and can be processed into rigid or flexible films using conventional plastic-forming techniques, making it suitable for automated packaging systems (Shankar et al., 2021). This is particularly important in the meat and seafood industry, where packaging must not only preserve product quality but also meet high standards for food safety and shelf life extension. Unlike traditional edible coatings, PHA films can incorporate antimicrobial agents such as essential oils or plant extracts directly into the packaging material, providing continuous protection against microbial contamination throughout the storage and distribution process (Kumar et al., 2010).
Maintaining the moisture balance and preventing oxidation are two of the most critical factors in preserving the quality of meat and seafood products. Edible coatings like those made from Na-alginate, chitosan, and collagen have demonstrated effectiveness in preventing moisture loss and minimizing lipid oxidation, thereby extending the shelf life of products like sliced ham, shrimp, and lamb meat (Arancibia et al., 2014, Pandhi et al., 2019). For example, collagen films have been used on muscle foods to reduce lipid oxidation and prevent discoloration (Pandhi et al., 2019). Similarly, chitosan coatings with nano-encapsulated essential oils were shown to enhance the sensory attributes of lamb meat while inhibiting microbial growth (Pabast et al., 2018).
However, while these coatings work effectively on a small scale, PHA-based films provide a more robust solution for industrial-scale packaging. PHA films can be engineered to have superior barrier properties, including moisture resistance and oxygen permeability, making them ideal for preventing lipid oxidation and rancidity in meat and seafood products. Additionally, PHA films can be designed to offer controlled release of antimicrobial agents like essential oils, providing long-term protection against spoilage microorganisms (Shankar et al., 2021). This continuous antimicrobial activity helps maintain the freshness of products like beef, pork, and turkey, while preventing oxidation and microbial growth (Xiong et al., 2020, Xiong et al., 2020).
One of the key advantages of edible coatings highlighted in Table 5 is their ability to act as carriers for antimicrobial agents, such as oregano essential oil and nisin, which have proven effective in inhibiting pathogens like Listeria monocytogenes and Staphylococcus aureus (Guo et al., 2014, Salmieri et al., 2014). However, these coatings often require manual application or surface contact, limiting their scalability for industrial use.
PHA-based packaging can overcome these limitations by integrating antimicrobial agents directly into the packaging material during production. For example, PHA films with embedded essential oils or nanoparticles could provide continuous antimicrobial activity, reducing the need for additional treatments or preservatives. Studies have shown that combining antimicrobial agents like grapefruit seed extract or oregano oil with PHA films can effectively extend the shelf life of pork, turkey, and seafood by inhibiting microbial growth and slowing down oxidation (Guo et al., 2014, Xiong et al., 2020).
By using PHA packaging, manufacturers can achieve the dual benefits of enhanced food safety and environmental sustainability. PHA’s biodegradable nature ensures that these packaging materials can be safely disposed of or composted after use, reducing the environmental impact associated with conventional plastic packaging.
As concerns about plastic pollution and sustainability continue to grow, there is increasing pressure on the food packaging industry to adopt eco-friendly alternatives. Traditional edible coatings, while biodegradable, often come with limitations in terms of cost and scalability (Abreu et al., 2012). In contrast, PHA offers a more sustainable solution by being fully biodegradable and compostable in natural environments. Additionally, PHA can be produced from renewable resources like food waste and agricultural by-products, further reducing its environmental footprint (Kumar et al., 2010).
The meat and seafood industries, which rely heavily on packaging to preserve product freshness and prevent contamination, stand to benefit significantly from the adoption of PHA-based films. By replacing petroleum-based plastics with PHA, these industries can reduce their reliance on non-renewable resources and contribute to the reduction of plastic waste in both land and marine environments (Yb and Kola, 2019).
3.2.2. Fresh food
Fresh produce, including vegetables and fruits, undergoes significant transpiration and respiration, leading to weight loss, sensory quality decline, and microbial contamination. Biodegradable film packaging offers a potential solution by modifying the package atmosphere to accommodate product respiration while minimizing condensation (Shankar et al., 2021). Biopolymers such as polysaccharides, proteins, and PLA can be engineered to create packaging with effective gas and moisture barrier properties. This enables the preservation of crucial quality attributes, including texture, color, flavor, and nutrient content, in fresh fruits and vegetables. Notably, these materials are fully biodegradable upon disposal, minimizing environmental impact (Kumar et al., 2010, Rhim, 2013, Siracusa et al., 2008). The utilization of biodegradable packaging materials for fresh produce aligns with sustainable agricultural practices and contributes to the reduction of plastic waste and environmental pollution (Jafarzadeh et al., 2024).
Edible packaging applied to fresh fruits or vegetables can effectively regulate gas permeability, particularly oxygen diffusion, thereby retarding the ripening process. This extended product freshness and visual appeal by acting as a barrier to gas exchange and preventing the absorption of unwanted odors. Furthermore, edible packaging maintains product moisture, adheres well due to its hydrophilic nature, prevents off-flavor development, enhances visual appearance through a glossy finish, preserves fruit firmness, reduces weight loss, and minimizes liquid leakage (Monteiro Fritz et al., 2019, Rudra et al., 2020, Yousuf and Qadri, 2020). Various food packaging applications for fruits and vegetables are summarized in Table 6.
Table 6.
Applications of edible food packaging from fruits and vegetables (Petkoska et al., 2021).
| Food product | Edible materials | Beneficial effect | References |
|---|---|---|---|
| Cherry tomato | Edible film incorporated with chitosan and liposome encapsulation of Artemisia annua oil. Edible coating based on nanoemulsion-thymol-quinoa protein/chitosan | Inactivation of Escherichia coli O157:H7. Inhibition of Botrytis cinerea. | Cui et al., 2017, Robledo et al., 2018 |
| Tomato | Aloe vera based edible coating | Delays ripening and extends shelf life (up to 39 days compared to 19 days for control sample) | Athmaselvi et al. (2013) |
| Cucumber | Corn starch and mint (Mentha viridis L.) extract edible coating | Improved shelf life and quality stored at room temperature/low temperature (25 °C/10 °C) | Raghav and Saini (2018) |
| Brocolli (fresh cut) | Chitosan coatings with bio actives: tea tree, rosemary, pollen and propolis, pomegranate and resveratrol | Effect of Inhibition on the Survival of Escherichia coli and Listeria monocytogenes | Alvarez et al. (2013) |
| Okra | Alginate coating containing nano-emulsified basil (Ocimum basilicum L.) oil | Potent against spoilage fungi Penicillium chrysogenum and Aspergillus flavus | Gundewadi et al. (2018) |
| Carrots | Protein, polyalcohol and polysaccharide coatings | Extended shelf life | Villafañe (2017) |
| Green beans | Antimicrobial coating formulation consisting of modified chitosan containing a nanoemulsion of mandarin essential oil | Reduces of L. innocua with the storage time, due to strong antimicrobial effect. A strong impact on the firmness of green beans. | Donsì et al. (2015) |
| Blueberries and raspberries | Carrageenan and green tea extract | Antiviral activity against murine norovirus, hepatitis A virus | Falcó et al. (2019) |
| Strawberries and raspberries | Alginate-oleic acid-based coatings with green tea | Antiviral effect against foodborne pathogens | Falcó et al. (2019) |
| Strawberry | Prickly pear cactus mucilage (Opuntia ficus indica) | Extended shelf-life | Del-Valle et al., 2005, Pinzon et al., 2020 |
| Composite films made from banana starch-chitosan and Aloe vera gel (AV) at different concentrations | Reduced mold spoilage, and prolonged shelf life to 15 days when stored at 20 % AV gel, while retaining physicochemical properties (color and firmness). Weight loss reduced by 5 %; limited water vapor transfer. | ||
| Strawberry (fresh cut) | Gellan-based coatings (Gel) with Geraniol (G) and Pomegranate extract (PE) incorporation (in different concentrations) | Gel + G coatings significantly reduced microbial counts; better microbiological stability. The use of PE did not control microbial growth. Samples with coatings + G showed a better firmness loss than standard products. | Tomadoni et al. (2018) |
| Grape berry | Coatings based on carnauba wax-lemongrass oil nanoemulsions | Antimicrobial effect by inhibiting Salmonella typhimurium and Escherichia coli. The potential reduced weight loss, firmness, phenolic compounds, and antioxidant activity. | Kim et al. (2014) |
| Lime fruit | Pectin-based coating | Progressive increase in shriveling or wilting and loss of green color over storage time; higher temperatures accelerate these changes | Maftoonazad and Ramaswamy (2019) |
| Banana | A rice starch edible coating blended with sucrose esters | Prolong postharvest quality during ripening (at 20 ± 2 ◦C); effectively in slowing ethylene biosynthesis and reducing respiration rate. Shelf life prolonged for 12 days | Thakur et al. (2019) |
| Fuji apples (fresh cut) | Nanoemulsion-based edible coatings with lemongrass essential oil | Inactivates of Escherichia coli | Salvia-Trujillo et al. (2015) |
| Apple cv. Golab kohanz | Nanochitosan-based coating | Specially reduced weight loss, respiration rate, ethylene production and peroxidase activity; retarded the tenderization process and improved the flesh color after the climacteric peak | (Gardesh et al., 2016) |
| Papaya (fresh cut) | Psyllium gum alone and in combination with sunflower oil | Maintained quality and increased shelf life | Yousuf and Srivastava (2015) |
| Apricot | Chitosan coatings | Improved total phenolics content and antioxidant activity | Ghasemnezhad et al. (2010) |
| Kiwi fruits slices | Opuntia ficus-indica mucilage edible coating | Considerably higher firmness and lower weight loss than untreated slices | Allegra et al. (2017) |
| Cantaloupe | Edible coating with antimicrobial components: chitosan, pectin, and trans-cinnamaldehyde | Shelf life extension up to 7–9 days compared to the control sample (4 days) at 4 °C. Higher concentrations (2 and 3 g/100 g) of trans-cinnamaldehyde encapsulated were more effective as an antimicrobial i.e., against mesophilic microorganisms. The chitosan coating alone was only effective against yeast and molds at a very low degree. | Martiñon et al. (2014) |
Table 6 outlines various applications of edible food packaging for fresh produce, presenting examples where biopolymer-based films and coatings, such as those made from chitosan, alginate, and polysaccharides, have been successfully used to extend the shelf life of fruits and vegetables. This table draws on research showing how edible packaging improves quality and safety while minimizing spoilage. Our contribution, however, goes beyond summarizing these applications by focusing on how PHA-based packaging offers a scalable, industrial alternative that aligns with sustainability goals and current packaging needs for fresh produce.
Edible packaging materials, while effective at the product level, are often limited in scalability due to challenges related to material handling and cost when applied to large-scale production. These coatings and films, such as those made from chitosan, aloe vera, or starch, are generally applied directly to the surface of the food and have been shown to reduce microbial contamination and slow down the ripening process (Monteiro Fritz et al., 2019, Rudra et al., 2020). However, these applications are more suited for small-scale production and often lack the durability and mechanical properties required for long-term storage and transportation.
PHA-based films, on the other hand, offer several advantages for scalability in industrial food packaging. PHA can be processed using existing plastic-forming technologies such as injection molding, thermoforming, and extrusion, making it compatible with large-scale packaging production lines (Shankar et al., 2021). This is particularly important in the context of the fresh produce industry, where packaging materials must be produced and distributed on a massive scale. PHA’s biodegradability and compatibility with current industrial practices provide an edge over traditional edible coatings, which are less versatile and often require manual application (Kumar et al., 2010).
Fresh fruits and vegetables require packaging materials that can regulate gas exchange—especially oxygen and carbon dioxide—during storage. Edible films, such as those made from chitosan and alginate, have shown significant promise in controlling gas permeability and extending the shelf life of produce. For instance, aloe vera-based coatings were able to delay ripening in tomatoes by slowing down the ethylene production process, thereby extending the shelf life from 19 days (for control samples) to 39 days (Athmaselvi et al., 2013). This aligns with the goal of minimizing post-harvest losses.
While these results are promising, PHA-based packaging has the potential to provide superior gas barrier properties, making it more effective in regulating the respiration of fresh produce during storage and transportation. PHA films have been shown to have excellent oxygen barrier properties, which are crucial for reducing respiration rates and extending shelf life (Rhim, 2013). The ability of PHA to act as a biodegradable barrier aligns with current industry demands for materials that preserve the freshness of perishable products, like fruits and vegetables, without compromising environmental sustainability.
Moreover, as PHA is capable of forming strong and flexible films, it could serve as a better replacement for petroleum-based plastics used in Modified Atmosphere Packaging (MAP), where the control of oxygen and carbon dioxide levels is critical to maintaining product freshness and extending shelf life (Shankar et al., 2021). Unlike traditional edible coatings, which are typically applied at the point of sale or on a small scale, PHA films could be integrated into large-scale packaging systems, offering automated and uniform application across different products (Jafarzadeh et al., 2024).
Moisture regulation is another critical factor in fresh produce packaging, as improper moisture control can lead to weight loss, microbial growth, and decreased product quality. Edible coatings made from starch, polysaccharides, and proteins are effective in maintaining moisture levels and inhibiting microbial growth. For example, chitosan coatings have been widely studied for their antimicrobial properties and their ability to extend the shelf life of products like broccoli and cucumbers (Alvarez et al., 2013, Raghav and Saini, 2018).
However, while these edible coatings offer benefits, they are generally less durable than synthetic or bioplastic films and may degrade faster under fluctuating humidity conditions. PHA-based films, by contrast, can be engineered to offer improved moisture resistance through blending with plasticizers or other biopolymers (Khatami et al., 2022). The incorporation of antimicrobial agents—such as essential oils or natural plant extracts—into PHA films has also been explored as a way to enhance their ability to inhibit the growth of spoilage microorganisms (Monteiro Fritz et al., 2019, Yousuf and Qadri, 2020). This makes PHA packaging a highly versatile and effective solution for both moisture control and microbial inhibition, further supporting its use for fresh produce applications.
One of the most significant advantages of PHA-based films over edible coatings is their environmental sustainability. While edible coatings are biodegradable, they are typically applied in small quantities and often remain limited to niche markets due to higher costs and application challenges. PHA-based packaging offers a scalable alternative that is fully biodegradable and can decompose in both soil and marine environments (Kumar et al., 2010). This makes PHA packaging not only beneficial for extending product freshness but also for reducing plastic waste and minimizing the environmental impact of food packaging (Siracusa et al., 2008).
PHA’s ability to biodegrade in natural environments means it can play a significant role in replacing conventional petroleum-based plastics, which are a major contributor to plastic pollution. In addition, the use of waste-derived feedstocks for producing PHA, such as food waste and agricultural residues, enhances the overall sustainability of the material by promoting circular economy principles (Khatami et al., 2022). This dual benefit—of environmental sustainability and food preservation—makes PHA a more attractive option than current edible coatings for large-scale, sustainable packaging solutions in the fresh produce industry.
3.2.3. Beverages
Biodegradable polymers offer a sustainable alternative to traditional plastic beverage packaging. Polysaccharides derived from plants, such as starches and cellulose, can be utilized to produce biodegradable packaging bottles and films (Kumar et al., 2014). Cellulose nanomaterials, such as cellulose nanofibers (CNF) and cellulose nanocrystals (CNC), derived from wood, cotton, and other plant sources exhibit properties advantageous for packaging applications (Castro et al., 2011). PLA, a commercially viable bioplastic produced through lactic acid polymerization, offers excellent mechanical strength, low toxicity, and effective barrier properties against gases, water vapor, and odors, making it suitable for beverage bottles and films (Muller et al., 2017). PHAs such as PHB and PHBV are polyesters generated by the fermentation of bacteria. The properties are close to polypropylene and polyethylene, so can replace conventional plastics in bottles, films, and other packaging (Bugnicourt et al., 2014). Both PHB and PHBV have excellent impermeability to both air and water. Mixing PHB with naturally occurring additives can increase mechanical stability for use in drink bottles (Getachew and Woldesenbet, 2016). PHA is industrially produced through the bacterial fermentation of sugars and glucose (Sun et al., 2007).
3.2.4. Bakery products
Biodegradable packaging offers a sustainable and environmentally friendly solution to reduce plastic waste in the bakery industry. Research has explored biodegradable materials to extend the shelf life of bakery products (Gonon et al., 2023). PLA packaging incorporating essential oils effectively inhibited the growth of Aspergillus niger and Penicillium corylophilum molds on cakes and bread, extending their storage period beyond 15 days (Debonne et al., 2018). PLA plastic infused with 2 % cinnamon leaf essential oil demonstrated optimal performance. Biopolymer-based edible films and coatings, composed of proteins and polysaccharides, provided effective barriers against water vapor and gas exchange, preserving bread quality. Chitosan-based coatings reinforced with essential oils inhibited mold growth and moisture loss in panettone for 16 days, while enhancing sensory attributes compared to untreated samples (Barbosa et al., 2022). Biodegradable packaging systems incorporating enriched edible films or natural antimicrobial/biopolymer coatings offer a promising approach to enhance product quality, safety, and shelf life within the bakery industry while promoting sustainability (Klinmalai et al., 2021). However, scaling up this technology for industrial applications necessitates further research. Additionally, a comprehensive assessment of consumer acceptance regarding sensory attributes is crucial prior to commercialization (Qian et al., 2021). A summary of edible food packaging applications for bakery products is presented in Table 7.
Table 7.
Applications of edible food packaging on bakery products (Petkoska et al., 2021).
| Food product | Edible materials | Beneficial effect | References |
|---|---|---|---|
| Muffins | Triticale-based edible film coating | Inhibits the spoilage process. | Pandhi et al. (2019) |
| Fruit bars | Edible films based on sodium alignate, carboxymethyl cellulose, and whey protein isolate | No significant effect on chemical characteristics. Maintain textural properties, reduce moisture loss, loss of total phenolic content and prevent radical scavenging activity during storage. | Bilbao-Sainz et al., 2018, Eyiz et al., 2020 |
| Layer-by-layer electrostatic deposition of the polycation chitosan and the polyanion alginate to coat fruit bars enriched with ascorbic acid | Increased ascorbic acid content, antioxidant capacity, firmness and prevention of mold growth during storage | ||
| Bakery product | Antimicrobial activity of essential oils: thyme, cinnamon, oregano, and lemongrass | Preventing the growth of harmful microorganisms and extending shelf life and improving safety | Gavahian et al. (2020) |
| Bread (mini-burger buns) | Sodium alginate, pectin (from citrus peel), whey protein concentrate used as edible coatings | Drying of the coating on the surface of bread | Chakravartula et al. (2019) |
| Sliced wheat bread | Nanocomposite film and coating based on chitosancarboxymethyl cellulose-oleic acid (CMC-CH-OL) incorporated with different concentrations (0.5, 1, and 2 %) of zinc oxide nanoparticles (ZnO NPs) | Microbial storage time was improved from 3 to 35 days for 2 % CMC-CH-OL-ZnO NPs when compared to the standard sample. Reduction in yeast and mold counts over 15 days; improved antimicrobial properties for coatings containing 1 % and 2 % ZnO NPs with no mold growth over 15 days. Better maintenance of moisture content | Noshirvani et al. (2017) |
Table 7 presents various applications of edible packaging in bakery products, demonstrating how biopolymer-based coatings and films have been used to extend shelf life and maintain product quality by inhibiting microbial growth and preventing moisture loss. This table draws on existing studies but our contribution goes further by discussing how PHA-based packaging can offer significant advantages in the industrial packaging of bakery goods, expanding on the limitations of current edible coatings while promoting environmental sustainability.
Bakery products, such as bread and muffins, are particularly vulnerable to spoilage due to mold growth and moisture loss. Edible coatings, such as those made from chitosan, alginate, and starch, have proven effective in delaying spoilage and maintaining product quality (Barbosa et al., 2022). For example, coatings made from chitosan and incorporated with essential oils have been shown to extend the shelf life of panettone for up to 16 days by preventing mold growth and moisture loss (Klinmalai et al., 2021). Similarly, edible films incorporating cinnamon leaf essential oil in PLA-based coatings inhibited mold growth on bread, significantly extending its storage period (Debonne et al., 2018).
PHA-based films, however, offer additional benefits for larger-scale bakery applications. Unlike traditional edible coatings that are often limited to artisanal or small-scale production, PHA films can be applied using existing industrial packaging technologies, allowing for automated processing and uniform application across a wide range of bakery products (Shankar et al., 2021). PHA films are biodegradable and have the potential to incorporate antimicrobial agents, such as essential oils or plant extracts, which can further enhance their ability to inhibit microbial growth (Gonon et al., 2023). This provides a more scalable and sustainable solution for extending the shelf life of bakery goods while reducing the reliance on petroleum-based plastics.
Traditional edible films and coatings, while effective at the product level, are often applied manually and are difficult to scale for industrial production. Edible coatings, such as those used on muffins and fruit bars, are typically added during the final stages of processing, which can complicate the packaging process when it comes to large-scale operations (Eyiz et al., 2020, Pandhi et al., 2019). These coatings are typically effective for short-term protection, but as bakery products are often shipped long distances and stored for extended periods, more durable and scalable solutions are needed.
PHA-based films offer a significant improvement in this regard, as they can be processed into rigid or flexible films using conventional plastic-forming methods, making them suitable for automated packaging systems (Shankar et al., 2021). This allows for consistent application and better control over the packaging environment, which is essential for maintaining the shelf life of bakery products like bread and buns. Moreover, the barrier properties of PHA films—such as their ability to prevent moisture loss and reduce oxygen permeability—are critical for preventing mold growth and ensuring the freshness of baked goods during transportation and storage (Rhim, 2013).
By using PHA in bakery packaging, manufacturers can achieve the dual goals of extending product shelf life and reducing plastic waste, since PHA is fully biodegradable and can be produced from renewable resources like food waste. This makes PHA an attractive alternative to conventional plastic films used in the bakery industry, offering both environmental and economic benefits (Siracusa et al., 2008).
Maintaining moisture balance in bakery products is critical to preserving their texture and preventing spoilage. Edible coatings, such as those made from sodium alginate, have been shown to prevent moisture loss in products like mini-burger buns, helping to maintain freshness over time (Chakravartula et al., 2019). Similarly, nanocomposite films made from chitosan and zinc oxide nanoparticles have been shown to extend the shelf life of sliced wheat bread by preventing microbial growth and controlling moisture levels (Noshirvani et al., 2017).
However, PHA films offer several advantages in moisture regulation compared to traditional edible coatings. While edible films are often hydrophilic and can absorb moisture, PHA-based films can be engineered to have better water resistance, making them more suitable for products that require long-term moisture control. Blending PHA with other biopolymers or adding plasticizers can further improve its barrier properties against water vapor, ensuring that bakery goods remain fresh for longer periods (Khatami et al., 2022). This makes PHA packaging particularly effective for products like bread and muffins, which are prone to spoilage if exposed to excessive moisture. By offering improved moisture control and microbial resistance, PHA-based packaging can help extend the shelf life of bakery products in a way that is both sustainable and scalable, making it a valuable innovation for the food packaging industry (Debonne et al., 2018).
One of the primary advantages of using PHA-based films in bakery packaging is their environmental sustainability. Traditional edible coatings are often biodegradable, but their use is limited due to their high cost and application challenges at the industrial scale (Barbosa et al., 2022). In contrast, PHA can be produced from renewable sources like food waste, making it a fully biodegradable and compostable material (Kumar et al., 2010). This aligns with growing consumer demand for eco-friendly packaging that reduces plastic waste and minimizes environmental impact (Shankar et al., 2021).
Moreover, PHA packaging offers an opportunity for food manufacturers to appeal to sustainability-conscious consumers. By clearly communicating the biodegradable nature of PHA films on packaging labels, companies can enhance their brand image and appeal to consumers who are increasingly aware of the environmental consequences of plastic waste (Gonon et al., 2023). In doing so, PHA packaging can contribute to both sustainability goals and consumer satisfaction, positioning it as a competitive alternative to conventional plastics in the bakery industry (Siracusa et al., 2008).
3.3. Health and environmental benefits of PHA-based packaging
PHA-based packaging offers multiple benefits beyond its biodegradability and compatibility with industrial packaging systems. The impact of PHA on consumer health is one of its most significant advantages over conventional petroleum-based plastics.
One of the main concerns with traditional plastic packaging is the migration of harmful chemicals into food products. Additives like plasticizers, phthalates, and bisphenol A (BPA), commonly used in plastic production, can leach into food under certain conditions, particularly in the presence of fatty or acidic foods (Siracusa et al., 2008). These substances have been associated with health risks such as endocrine disruption and reproductive health issues.
PHA-based packaging, by contrast, is made from natural, renewable materials and does not contain these harmful additives, making it a safer option for direct food contact. This is particularly important for sensitive food products like fresh produce, meat, and dairy, where the integrity of the packaging is critical to food safety.
The non-toxic and biocompatible properties of PHA eliminate the risk of chemical migration into food products, which is a significant concern with traditional plastics such as polyethylene (PE) and polyvinyl chloride (PVC). Research shows that PHA films provide a safer alternative, particularly for fatty and acidic food products that are more likely to induce chemical migration in conventional plastic packaging (Kumar et al., 2010).
Moreover, PHA films do not release toxic substances during use or disposal, making them suitable for packaging applications that prioritize food safety and consumer health. This makes PHA ideal for products requiring long-term storage, such as meat and seafood, where packaging must prevent spoilage without posing health risks (Shankar et al., 2021).
In addition to reducing chemical migration, PHA-based packaging improves overall food safety by offering excellent barrier properties against oxygen and water vapor. These properties are essential for preventing spoilage and extending the shelf life of perishable food products, such as fresh fruits, vegetables, meat, and seafood (Monteiro Fritz et al., 2019). The ability of PHA to maintain food freshness and prevent microbial contamination makes it a valuable solution for manufacturers focused on health-conscious consumers.
The incorporation of antimicrobial agents into PHA packaging provides an added layer of protection, further enhancing food safety by inhibiting the growth of pathogens like Escherichia coli and Listeria monocytogenes (Rhim, 2013). This additional functionality makes PHA-based packaging particularly suitable for high-risk products, such as deli meats, poultry, and seafood, where microbial contamination is a common concern (Yousuf and Qadri, 2020).
Another significant consumer health benefit of PHA-based packaging is its ability to reduce microplastic contamination. Traditional plastics degrade slowly, often breaking down into microplastics that contaminate the environment and food chain, leading to potential health risks such as inflammation, oxidative stress, and hormonal imbalances (Jafarzadeh et al., 2024).
PHA, however, is fully biodegradable and breaks down into harmless by-products, such as water and carbon dioxide, without leaving behind microplastic residues. This reduces the overall environmental exposure to plastics and mitigates the health risks associated with microplastic ingestion. The use of PHA in food packaging can therefore contribute to a healthier ecosystem and minimize the long-term impact of plastic pollution on human health.
Overall, PHA-based packaging aligns with the growing consumer demand for sustainable and safe food packaging solutions. By replacing petroleum-based plastics with biodegradable alternatives like PHA, manufacturers can address both environmental concerns and the need for safe, non-toxic packaging materials. For consumers, this means lower exposure to harmful chemicals and microplastics, while still benefiting from high-performance packaging that preserves food quality and safety (Kumar et al., 2010).
3.4. Biocompatibility and versatility of PHA-based packaging
In addition to its mechanical properties and biodegradability, PHA possesses significant advantages in terms of biocompatibility and versatility, making it a superior alternative to conventional plastics in various industrial applications, including food packaging, medical devices, and environmental products. Biocompatibility refers to the ability of a material to be safely used in contact with living tissues without causing adverse reactions. PHA is a naturally occurring polymer that is synthesized by microorganisms and is inherently biocompatible, meaning it poses no health risks when used in direct contact with food or the human body. This property is particularly important in food packaging, where the packaging material must be safe and non-toxic to prevent any migration of harmful chemicals into the food (Kumar et al., 2010).
In contrast to conventional plastics, which often contain plasticizers and stabilizers that may leach into food products, PHA-based packaging does not pose these risks. Its non-toxic degradation into water and carbon dioxide makes it an ideal material for applications where food safety and consumer health are of paramount importance (Rhim, 2013). This is especially relevant for perishable goods like meat, seafood, dairy products, and fresh produce, where maintaining food safety during packaging and storage is critical (Shankar et al., 2021).
Beyond food packaging, PHA's biocompatibility extends its use to the medical field, where it is employed in drug delivery systems, tissue engineering scaffolds, and biodegradable implants. These applications highlight PHA's ability to interact safely with human tissues, degrade naturally, and avoid complications related to toxicity (Monteiro Fritz et al., 2019).
One of the key strengths of PHA is its versatility, making it a valuable material for a variety of industrial applications, including food packaging, medical devices, and agricultural films. PHA can be processed using a wide range of plastic-forming techniques, such as injection molding, thermoforming, extrusion, and blow molding (Khatami et al., 2022). This flexibility allows PHA to be manufactured into a variety of shapes and structures, such as rigid containers, flexible films, trays, and bottles, depending on the specific requirements of the packaging application (Shankar et al., 2021).
Furthermore, the versatility of PHA allows for the modification of its properties to meet the needs of different applications. By blending PHA with other biopolymers, such as PLA (polylactic acid) or PBAT (polybutylene adipate terephthalate), its mechanical strength, moisture resistance, and gas barrier properties can be enhanced (Vu et al., 2021). For example, blending PHA with PLA can improve its oxygen barrier properties, making it suitable for longer shelf-life packaging for products like dairy and processed foods (Khatami et al., 2022).
Beyond its widespread use in food packaging, PHA is also utilized in the medical field due to its biodegradability and biocompatibility. In medical applications, PHA is used to create biodegradable sutures, wound dressings, and tissue engineering scaffolds (Rhim, 2013). Because PHA degrades safely inside the body without causing harmful side effects, it is commonly used for temporary implants that do not require surgical removal.
In addition to medical uses, PHA's environmental benefits make it a key material for agricultural films, biodegradable bags, and compostable consumer goods. Unlike traditional petroleum-based plastics, which can take hundreds of years to degrade, PHA naturally decomposes in soil, freshwater, and marine environments into non-toxic by-products, making it an environmentally sustainable option for disposable items (Siracusa et al., 2008).
Another significant advantage of PHA is its ability to be customized for specific functions by incorporating functional additives, such as antimicrobial agents, antioxidants, or nanoparticles. For instance, PHA films infused with antimicrobial agents like essential oils or nisin can provide added protection against foodborne pathogens such as Listeria monocytogenes and Salmonella (Xiong et al., 2020b). This makes PHA packaging highly suitable for high-risk food products, such as meat, seafood, and ready-to-eat meals, where microbial contamination is a major concern.
Incorporating plasticizers or nanoparticles can also enhance the flexibility, moisture resistance, and strength of PHA films, enabling the material to meet the demands of a wide range of packaging and industrial applications (Khatami et al., 2022). This ability to tailor PHA’s properties ensures that it can be used effectively in industries beyond packaging, including biomedical and environmentally sustainable products.
4. Conclusion and future prospects
The conversion of food waste into Polyhydroxyalkanoates (PHA) offers a sustainable and innovative solution to two pressing global challenges: the environmental pollution caused by conventional plastics and the massive amounts of food waste generated annually. The research presented in this manuscript demonstrates that PHA-based bioplastics have the potential to transform the food packaging industry, providing a biodegradable alternative to petroleum-based plastics. However, while the biodegradability and biocompatibility of PHA offer significant environmental and health benefits, critical challenges remain that must be addressed to facilitate the broader adoption of PHA at an industrial scale.
The most pressing challenge is the high production cost of PHA, which is largely driven by the complexity of fermentation processes and the expensive substrates required. However, the use of food waste as a carbon source—specifically through the conversion of volatile fatty acids (VFAs)—has emerged as a promising solution. Studies have demonstrated that microbial strains like Cupriavidus necator can achieve high yields of PHA from waste-derived feedstocks, with up to 77.54 % cell dry weight (CDW) produced in digestate liquor derived from food waste. This approach not only reduces production costs but also aligns with the circular economy, turning waste streams into valuable resources. Further research into optimizing fermentation techniques and identifying additional low-cost substrates is essential to reduce costs and improve scalability.
Despite its environmental advantages, PHA faces material limitations when compared to traditional plastics like polyethylene (PE) or polypropylene (PP). PHA films often exhibit inferior mechanical properties, including reduced flexibility, moisture resistance, and gas barrier performance. These limitations restrict PHA’s applicability in certain packaging environments, particularly where durability and moisture control are critical, such as in the packaging of fresh produce or bakery goods. To overcome these challenges, future research must explore polymer blending and the incorporation of functional additives like nanoparticles or plasticizers to enhance PHA’s performance. Blending PHA with biopolymers like PLA (polylactic acid) or PBAT (polybutylene adipate terephthalate) has already shown potential in improving flexibility and mechanical strength, making PHA more suitable for food packaging applications.
In terms of food safety and consumer health, PHA-based packaging offers distinct advantages over conventional plastics. The biocompatibility of PHA ensures that it does not leach harmful chemicals into food products, a concern associated with plastics that contain additives such as phthalates or bisphenol A (BPA). This makes PHA particularly suitable for packaging perishable goods like meat, seafood, and dairy products, where maintaining food quality and extending shelf life are paramount. Additionally, PHA’s biodegradability means it breaks down into non-toxic by-products, such as water and carbon dioxide, minimizing its environmental impact and reducing the risk of microplastic pollution. These properties make PHA an ideal candidate for Modified Atmosphere Packaging (MAP), where gas regulation is crucial to extending the freshness of perishable items.
The environmental benefits of PHA are equally significant. Conventional plastics contribute to long-term pollution, particularly through the formation of microplastics that persist in ecosystems for hundreds of years. In contrast, PHA degrades naturally in marine and terrestrial environments, reducing the overall ecological footprint of packaging waste. This feature makes PHA particularly appealing as a material for addressing global plastic pollution. However, further research is needed to understand the degradation behavior of PHA under different environmental conditions, particularly in industrial composting and natural ecosystems.
The adoption of PHA-based bioplastics will also require legislative support and policy interventions to ensure widespread use. Regulatory bodies can play a critical role by incentivizing the development and adoption of biodegradable materials through subsidies or tax incentives for companies investing in sustainable packaging solutions. Furthermore, the establishment of clear biodegradability standards and certification schemes will be essential in ensuring that PHA-based products meet environmental criteria and are correctly labeled for consumers. Such policies would encourage industries to transition from conventional plastics to biodegradable alternatives, while also ensuring that PHA-based products can compete in the marketplace.
Beyond legislative support, consumer awareness is crucial for driving demand for sustainable packaging. Educating consumers about the environmental and health benefits of PHA-based materials can help shift market preferences toward eco-friendly alternatives. This can be achieved through product labeling that clearly indicates a product’s biodegradability and compostability, helping consumers make informed purchasing decisions. Additionally, collaborative efforts between industry leaders, academia, and government agencies are essential to accelerate the development of next-generation PHA materials that are tailored to the needs of different industries.
Looking ahead, the next steps for advancing PHA adoption in the food industry and beyond include: (a) Optimization of PHA production methods to reduce costs and improve scalability, particularly through the use of waste-derived feedstocks; (b) Enhancing the mechanical and barrier properties of PHA to make it more suitable for packaging applications requiring high durability and moisture control; (c) Developing active packaging solutions that incorporate antimicrobial agents or oxygen scavengers to further extend the shelf life of perishable goods; (d) Fostering legislative and regulatory support to encourage the adoption of biodegradable materials through incentives and policy changes; and (e) Increasing consumer awareness through education and clear product labeling, driving demand for sustainable packaging options.
CRediT authorship contribution statement
Heri Septya Kusuma: Writing – review & editing, Supervision, Methodology, Conceptualization. Atna Sabita: Writing – original draft, Visualization, Validation, Investigation, Formal analysis. Najla Anira Putri: Writing – original draft, Visualization, Validation, Investigation, Formal analysis. Nadhira Azliza: Writing – original draft, Visualization, Validation, Investigation, Formal analysis. Nafisa Illiyanasafa: Writing – original draft, Visualization, Validation, Investigation, Formal analysis. Handoko Darmokoesoemo: Writing – original draft, Visualization, Validation, Investigation, Formal analysis. Andrew Nosakhare Amenaghawon: Writing – original draft, Visualization, Validation, Investigation, Formal analysis. Tonni Agustiono Kurniawan: Writing – original draft, Visualization, Validation, Investigation, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- de Abreu D.A.P., Cruz J.M., Paseiro Losada P. Active and Intelligent Packaging for the Food Industry. Food Reviews International. 2012;28:146–187. doi: 10.1080/87559129.2011.595022. [DOI] [Google Scholar]
- Acuña J.M.B., Poblete-Castro I. Rational engineering of natural polyhydroxyalkanoates producing microorganisms for improved synthesis and recovery. Microbial Biotechnology. 2023;16:262–285. doi: 10.1111/1751-7915.14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N., Rashid M.I., Rehan M., Shah Eqani S.A.M.A., Summan A.S.A., Ismail I.M.I., Koller M., Ali A.M., Shahzad K. Environmental evaluation of polyhydroxyalkanoates from animal slaughtering waste using Material Input Per Service Unit. New Biotechnology. 2023;75:40–51. doi: 10.1016/j.nbt.2023.03.004. [DOI] [PubMed] [Google Scholar]
- Allegra A., Sortino G., Inglese P., Settanni L., Todaro A., Gallotta A. The effectiveness of Opuntia ficus-indica mucilage edible coating on post-harvest maintenance of ‘Dottato’ fig (Ficus carica L.) fruit. Food Packag Shelf Life. 2017;12:135–141. doi: 10.1016/j.fpsl.2017.04.010. [DOI] [Google Scholar]
- Alsafadi D., Ibrahim M.I., Alamry K.A., Hussein M.A., Mansour A. Utilizing the crop waste of date palm fruit to biosynthesize polyhydroxyalkanoate bioplastics with favorable properties. Science of The Total Environment. 2020;737 doi: 10.1016/j.scitotenv.2020.139716. [DOI] [PubMed] [Google Scholar]
- Alvarez M.V., Ponce A.G., Moreira M. del R. Antimicrobial efficiency of chitosan coating enriched with bioactive compounds to improve the safety of fresh cut broccoli. LWT - Food Science and Technology. 2013;50:78–87. doi: 10.1016/j.lwt.2012.06.021. [DOI] [Google Scholar]
- Amin U., Khan M.U., Majeed Y., Rebezov M., Khayrullin M., Bobkova E., Shariati M.A., Chung I.M., Thiruvengadam M. Potentials of polysaccharides, lipids and proteins in biodegradable food packaging applications. International Journal of Biological Macromolecules. 2021;183:2184–2198. doi: 10.1016/j.ijbiomac.2021.05.182. [DOI] [PubMed] [Google Scholar]
- Andhalkar V.V., Montané D., Medina F., Constantí M. Biorefinery design with combined deacetylation and microwave pretreatment for enhanced production of polyhydroxyalkanoates and efficient carbon utilization from lignocellulose. Chemical Engineering Journal. 2024;486 doi: 10.1016/j.cej.2024.149754. [DOI] [Google Scholar]
- Arancibia M., Giménez B., López-Caballero M.E., Gómez-Guillén M.C., Montero P. Release of cinnamon essential oil from polysaccharide bilayer films and its use for microbial growth inhibition in chilled shrimps. LWT - Food Science and Technology. 2014;59:989–995. doi: 10.1016/j.lwt.2014.06.031. [DOI] [Google Scholar]
- Aremu M.O., Ishola M.M., Taherzadeh M.J. Polyhydroxyalkanoates (PHAs) production from volatile fatty acids (VFAs) from organic wastes by Pseudomonas oleovorans. Fermentation. 2021;7:287. doi: 10.3390/fermentation7040287. [DOI] [Google Scholar]
- Asdagh A., Karimi Sani I., Pirsa S., Amiri S., Shariatifar N., Eghbaljoo-Gharehgheshlaghi H., Shabahang Z., Taniyan A. Production and characterization of nanocomposite film based on whey protein isolated/copper oxide nanoparticles containing coconut essential oil and paprika extract. Journal of Polymers and the Environment. 2021;29:335–349. doi: 10.1007/s10924-020-01882-w. [DOI] [Google Scholar]
- Asioli D., Grasso S. Do consumers value food products containing upcycled ingredients? The effect of nutritional and environmental information. Food Quality and Preference. 2021;91 doi: 10.1016/j.foodqual.2021.104194. [DOI] [Google Scholar]
- Athmaselvi K.A., Sumitha P., Revathy B. Development of Aloe vera based edible coating for tomato. International Agrophysics. 2013;27:369–375. doi: 10.2478/intag-2013-0006. [DOI] [Google Scholar]
- Avila L.B., Schnorr C., Silva L.F.O., Morais M.M., Moraes C.C., da Rosa G.S., Dotto G.L., Lima É.C., Naushad Mu. Trends in bioactive multilayer films: Perspectives in the use of polysaccharides, proteins, and carbohydrates with natural additives for application in food packaging. Foods. 2023;12:1692. doi: 10.3390/foods12081692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa, M.H.R., Gonçalves, S. de Á., Marangoni Júnior, L., Alves, R.M.V., Vieira, R.P., 2022. Physicochemical properties of chitosan-based films incorporated with limonene. Journal of Food Measurement and Characterization 16, 2011–2023. https://doi.org/10.1007/s11694-022-01337-x.
- Basiak E., Lenart A., Debeaufort F. Effects of carbohydrate/protein ratio on the microstructure and the barrier and sorption properties of wheat starch–whey protein blend edible films. Journal of the Science of Food and Agriculture. 2017;97:858–867. doi: 10.1002/jsfa.7807. [DOI] [PubMed] [Google Scholar]
- Beltrán F.R., Infante C., de la Orden M.U., Martínez Urreaga J. Mechanical recycling of poly(lactic acid): Evaluation of a chain extender and a peroxide as additives for upgrading the recycled plastic. Journal of Cleaner Production. 2019;219:46–56. doi: 10.1016/j.jclepro.2019.01.206. [DOI] [Google Scholar]
- Bermúdez-Oria A., Rodríguez-Gutiérrez G., Rubio-Senent F., Fernández-Prior Á., Fernández-Bolaños J. Effect of edible pectin-fish gelatin films containing the olive antioxidants hydroxytyrosol and 3,4-dihydroxyphenylglycol on beef meat during refrigerated storage. Meat Science. 2019;148:213–218. doi: 10.1016/j.meatsci.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Bhargava N., Sharanagat V.S., Mor R.S., Kumar K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends in Food Science and Technology. 2020;105:385–401. doi: 10.1016/j.tifs.2020.09.015. [DOI] [Google Scholar]
- Bhatia S.K., Gurav R., Choi T.-R., Jung H.-R., Yang S.-Y., Song H.-S., Jeon J.-M., Kim J.-S., Lee Y.-K., Yang Y.-H. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) production from engineered Ralstonia eutropha using synthetic and anaerobically digested food waste derived volatile fatty acids. International Journal of Biological Macromolecules. 2019;133:1–10. doi: 10.1016/j.ijbiomac.2019.04.083. [DOI] [PubMed] [Google Scholar]
- Bhatia S.K., Hwang J.H., Oh S.J., Kim H.J., Shin N., Choi T.-R., Kim H.-J., Jeon J.-M., Yoon J.-J., Yang Y.-H. Macroalgae as a source of sugar and detoxifier biochar for polyhydroxyalkanoates production by Halomonas sp. YLGW01 under the unsterile condition. Bioresource Technology. 2023;384 doi: 10.1016/j.biortech.2023.129290. [DOI] [PubMed] [Google Scholar]
- Bhatia S.K., Otari S.V., Jeon J.-M., Gurav R., Choi Y.-K., Bhatia R.K., Pugazhendhi A., Kumar V., Rajesh Banu J., Yoon J.-J., Choi K.-Y., Yang Y.-H. Biowaste-to-bioplastic (polyhydroxyalkanoates): Conversion technologies, strategies, challenges, and perspective. Bioresource Technology. 2021;326 doi: 10.1016/j.biortech.2021.124733. [DOI] [PubMed] [Google Scholar]
- Bilbao-Sainz C., Chiou B., Punotai K., Olson D., Williams T., Wood D., Rodov V., Poverenov E., McHugh T. Layer-by-Layer Alginate and Fungal Chitosan Based Edible Coatings Applied to Fruit Bars. Journal of Food Science. 2018;83:1880–1887. doi: 10.1111/1750-3841.14186. [DOI] [PubMed] [Google Scholar]
- Brojanigo S., Gronchi N., Cazzorla T., Wong T.S., Basaglia M., Favaro L., Casella S. Engineering Cupriavidus necator DSM 545 for the one-step conversion of starchy waste into polyhydroxyalkanoates. Bioresource Technology. 2022;347 doi: 10.1016/j.biortech.2021.126383. [DOI] [PubMed] [Google Scholar]
- Bugnicourt E., Cinelli P., Lazzeri A., Alvarez V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polymer Letters. 2014;8:791–808. doi: 10.3144/expresspolymlett.2014.82. [DOI] [Google Scholar]
- Castro C., Zuluaga R., Putaux J.-L., Caro G., Mondragon I., Gañán P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydrate Polymers. 2011;84:96–102. doi: 10.1016/j.carbpol.2010.10.072. [DOI] [Google Scholar]
- Cederberg C., Sonesson U. Causes and Prevention Alexandre Meybeck Center for International Forestry Research; 2011. Global Food Losses and Food Waste-Extent. [Google Scholar]
- Ceylan Z., Unal Sengor G.F., Yilmaz M.T. Nanoencapsulation of liquid smoke/thymol combination in chitosan nanofibers to delay microbiological spoilage of sea bass (Dicentrarchus labrax) fillets. Journal of Food Engineering. 2018;229:43–49. doi: 10.1016/j.jfoodeng.2017.11.038. [DOI] [Google Scholar]
- Chacón M., Wongsirichot P., Winterburn J., Dixon N. Genetic and process engineering for polyhydroxyalkanoate production from pre- and post-consumer food waste. Current Opinion in Biotechnology. 2024;85 doi: 10.1016/j.copbio.2023.103024. [DOI] [PubMed] [Google Scholar]
- Chakravartula S.S.N., Soccio M., Lotti N., Balestra F., Dalla Rosa M., Siracusa V. Characterization of composite edible films based on pectin/alginate/whey protein concentrate. Materials. 2019;12:2454. doi: 10.3390/ma12152454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan S., Yadav B., Tyagi R.D., Wong J.W.C., Drogui P. Comparative study of chemical and enzymatic pre-treatments of kitchen waste (KW) to generate fermentable sugars for the production of polyhydroxyalkanoates (PHA) Biochemical Engineering Journal. 2024;204 doi: 10.1016/j.bej.2024.109240. [DOI] [Google Scholar]
- Chen Z., Wang Y., Cheng Y., Wang X., Tong S., Yang H., Wang Z. Efficient biodegradation of highly crystallized polyethylene terephthalate through cell surface display of bacterial PETase. Science of The Total Environment. 2020;709 doi: 10.1016/j.scitotenv.2019.136138. [DOI] [PubMed] [Google Scholar]
- Chevalier E., Assezat G., Prochazka F., Oulahal N. Development and characterization of a novel edible extruded sheet based on different casein sources and influence of the glycerol concentration. Food Hydrocolloids. 2018;75:182–191. doi: 10.1016/j.foodhyd.2017.08.028. [DOI] [Google Scholar]
- Chong J.W.R., Khoo K.S., Yew G.Y., Leong W.H., Lim J.W., Lam M.K., Ho Y.-C., Ng H.S., Munawaroh H.S.H., Show P.L. Advances in production of bioplastics by microalgae using food waste hydrolysate and wastewater: A review. Bioresource Technology. 2021;342 doi: 10.1016/j.biortech.2021.125947. [DOI] [PubMed] [Google Scholar]
- Costamagna M., Massaccesi B.M., Mazzucco D., Baricco M., Rizzi P. Environmental assessment of the recycling process for polyamides - Polyethylene multilayer packaging films. Sustainable Materials and Technologies. 2023;35:e00562. [Google Scholar]
- Critelli P., Pesante G., Lupinelli S., Modesti M., Zanatta S., Battista F., Bolzonella D., Frison N. Production and characterisation of PHAs by pure culture using protein hydrolysates as sole carbon source. Environmental Technology and Innovation. 2022;28 doi: 10.1016/j.eti.2022.102919. [DOI] [Google Scholar]
- Cui B., Huang S., Xu F., Zhang R., Zhang Y. Improved productivity of poly (3-hydroxybutyrate) (PHB) in thermophilic Chelatococcus daeguensis TAD1 using glycerol as the growth substrate in a fed-batch culture. Applied Microbiology and Biotechnology. 2015;99:6009–6019. doi: 10.1007/s00253-015-6489-1. [DOI] [PubMed] [Google Scholar]
- Cui H., Yuan L., Li W., Lin L. Edible film incorporated with chitosan and Artemisia annua oil nanoliposomes for inactivation of Escherichia coli O157:H7 on cherry tomato. International Journal of Food Science and Technology. 2017;52:687–698. doi: 10.1111/ijfs.13322. [DOI] [Google Scholar]
- Dalton B., Bhagabati P., De Micco J., Padamati R.B., O’Connor K. A review on biological synthesis of the biodegradable polymers polyhydroxyalkanoates and the development of multiple applications. Catalysts. 2022;12:319. doi: 10.3390/catal12030319. [DOI] [Google Scholar]
- Dan T., Jing H., Shen T., Zhu J., Liu Y. Performance of production of polyhydroxyalkanoates from food waste fermentation with Rhodopseudomonas palustris. Bioresource Technology. 2023;385 doi: 10.1016/j.biortech.2023.129165. [DOI] [PubMed] [Google Scholar]
- Davis R., Duane G., Kenny S.T., Cerrone F., Guzik M.W., Babu R.P., Casey E., O’Connor K.E. High cell density cultivation of Pseudomonas putida KT2440 using glucose without the need for oxygen enriched air supply. Biotechnology and Bioengineering. 2015;112:725–733. doi: 10.1002/bit.25474. [DOI] [PubMed] [Google Scholar]
- Debonne E., Van Bockstaele F., De Leyn I., Devlieghere F., Eeckhout M. Validation of in-vitro antifungal activity of thyme essential oil on Aspergillus niger and Penicillium paneum through application in par-baked wheat and sourdough bread. LWT. 2018;87:368–378. doi: 10.1016/j.lwt.2017.09.007. [DOI] [Google Scholar]
- Del-Valle V., Hernández-Muñoz P., Guarda A., Galotto M.J. Development of a cactus-mucilage edible coating (Opuntia ficus indica) and its application to extend strawberry (Fragaria ananassa) shelf-life. Food Chemistry. 2005;91:751–756. doi: 10.1016/j.foodchem.2004.07.002. [DOI] [Google Scholar]
- Dilkes-Hoffman L.S., Lane J.L., Grant T., Pratt S., Lant P.A., Laycock B. Environmental impact of biodegradable food packaging when considering food waste. Journal of Cleaner Production. 2018;180:325–334. doi: 10.1016/j.jclepro.2018.01.169. [DOI] [Google Scholar]
- Don T.-M., Chen C.W., Chan T.-H. Preparation and characterization of poly(hydroxyalkanoate) from the fermentation of Haloferax mediterranei. Journal of Biomaterials Science. Polymer Edition. 2006;17:1425–1438. doi: 10.1163/156856206778937208. [DOI] [PubMed] [Google Scholar]
- Dong C., Wang B., Li F., Zhong Q., Xia X., Kong B. Effects of edible chitosan coating on Harbin red sausage storage stability at room temperature. Meat Science. 2020;159 doi: 10.1016/j.meatsci.2019.107919. [DOI] [PubMed] [Google Scholar]
- Donsì F., Marchese E., Maresca P., Pataro G., Vu K.D., Salmieri S., Lacroix M., Ferrari G. Green beans preservation by combination of a modified chitosan based-coating containing nanoemulsion of mandarin essential oil with high pressure or pulsed light processing. Postharvest Biology and Technology. 2015;106:21–32. doi: 10.1016/j.postharvbio.2015.02.006. [DOI] [Google Scholar]
- Eyiz V., Tontul İ., Türker S. The effect of edible coatings on physical and chemical characteristics of fruit bars. Journal of Food Measurement and Characterization. 2020;14:1775–1783. doi: 10.1007/s11694-020-00425-0. [DOI] [Google Scholar]
- Falcó I., Flores-Meraz P.L., Randazzo W., Sánchez G., López-Rubio A., Fabra M.J. Antiviral activity of alginate-oleic acid based coatings incorporating green tea extract on strawberries and raspberries. Food Hydrocolloids. 2019;87:611–618. doi: 10.1016/j.foodhyd.2018.08.055. [DOI] [Google Scholar]
- Fehlberg J., McKay S., Matuana L.M., Almenar E. Use of orange juice processing waste to produce films using blown film extrusion for food packaging. Journal of Food Engineering. 2023;341 doi: 10.1016/j.jfoodeng.2022.111337. [DOI] [Google Scholar]
- Filho W.L., Salvia A.L., Bonoli A., Saari U.A., Voronova V., Klõga M., Kumbhar S.S., Olszewski K., De Quevedo D.M., Barbir J. An assessment of attitudes towards plastics and bioplastics in Europe. Science of The Total Environment. 2021;755 doi: 10.1016/j.scitotenv.2020.142732. [DOI] [PubMed] [Google Scholar]
- Fotie G., Gazzotti S., Ortenzi M.A., Piergiovanni L. Implementation of high gas barrier laminated films based on cellulose nanocrystals for food flexible packaging. Applied Sciences. 2020;10:3201. doi: 10.3390/app10093201. [DOI] [Google Scholar]
- Fotie G., Limbo S., Piergiovanni L. Manufacturing of food packaging based on nanocellulose: Current advances and challenges. Nanomaterials. 2020;10:1726. doi: 10.3390/nano10091726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galus S., Kadzińska J. Food applications of emulsion-based edible films and coatings. Trends in Food Science and Technology. 2015;45:273–283. doi: 10.1016/j.tifs.2015.07.011. [DOI] [Google Scholar]
- Gao W., Dong H., Hou H., Zhang H. Effects of clays with various hydrophilicities on properties of starch–clay nanocomposites by film blowing. Carbohydrate Polymers. 2012;88:321–328. doi: 10.1016/j.carbpol.2011.12.011. [DOI] [Google Scholar]
- Gardesh A.S.K., Badii F., Hashemi M., Ardakani A.Y., Maftoonazad N., Gorji A.M. Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv. Golab Kohanz. LWT. 2016;70:33–40. doi: 10.1016/j.lwt.2016.02.002. [DOI] [Google Scholar]
- Garrido T., Leceta I., Cabezudo S., Guerrero P., de la Caba K. Tailoring soy protein film properties by selecting casting or compression as processing methods. European Polymer Journal. 2016;85:499–507. doi: 10.1016/j.eurpolymj.2016.11.007. [DOI] [Google Scholar]
- Gavahian M., Chu Y.-H., Lorenzo J.M., Mousavi Khaneghah A., Barba F.J. Essential oils as natural preservatives for bakery products: Understanding the mechanisms of action, recent findings, and applications. Critical Reviews in Food Science and Nutrition. 2020;60:310–321. doi: 10.1080/10408398.2018.1525601. [DOI] [PubMed] [Google Scholar]
- Getachew A., Woldesenbet F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Research Notes. 2016;9:1–9. doi: 10.1186/s13104-016-2321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemnezhad M., Shiri M.A., Sanavi M. Effect of chitosan coatings on some quality indices of apricot (Prunus armeniaca L.) during cold storage. Caspian J Env Sci. 2010;8:25–33. [Google Scholar]
- Gómez-Guillén M.C., Giménez B., López-Caballero M.E., Montero M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocolloids. 2011;25:1813–1827. doi: 10.1016/j.foodhyd.2011.02.007. [DOI] [Google Scholar]
- Gonon H., Srisa A., Promhuad K., Chonhenchob V., Bumbudsanpharoke N., Jarupan L., Harnkarnsujarit N. PLA thermoformed trays incorporated with cinnamaldehyde and carvacrol as active biodegradable bakery packaging. Food Packaging and Shelf Life. 2023;38 doi: 10.1016/j.fpsl.2023.101123. [DOI] [Google Scholar]
- Gontard N., Guilbert S., Cuq J. Edible wheat gluten films: Influence of the main process variables on film properties using response surface methodology. Journal of Food Science. 1992;57:190–195. doi: 10.1111/j.1365-2621.1992.tb05453.x. [DOI] [Google Scholar]
- Granda-Restrepo D.M., Soto-Valdez H., Peralta E., Troncoso-Rojas R., Vallejo-Córdoba B., Gámez-Meza N., Graciano-Verdugo A.Z. Migration of α-tocopherol from an active multilayer film into whole milk powder. Food Research International. 2009;42:1396–1402. doi: 10.1016/j.foodres.2009.07.007. [DOI] [Google Scholar]
- Grzebieniarz W., Biswas D., Roy S., Jamróz E. Advances in biopolymer-based multi-layer film preparations and food packaging applications. Food Packaging and Shelf Life. 2023;35 doi: 10.1016/j.fpsl.2023.101033. [DOI] [Google Scholar]
- Gundewadi G., Rudra S.G., Sarkar D.J., Singh D. Nanoemulsion based alginate organic coating for shelf life extension of okra. Food Packaging and Shelf Life. 2018;18:1–12. doi: 10.1016/j.fpsl.2018.08.002. [DOI] [Google Scholar]
- Guo M., Jin T.Z., Wang L., Scullen O.J., Sommers C.H. Antimicrobial films and coatings for inactivation of Listeria innocua on ready-to-eat deli turkey meat. Food Control. 2014;40:64–70. doi: 10.1016/j.foodcont.2013.11.018. [DOI] [Google Scholar]
- Gupta A., Lolic L., Mekonnen T.H. Reactive extrusion of highly filled, compatibilized, and sustainable PHBV/PBAT – Hemp residue biocomposite. Compos Part A Appl Sci Manuf. 2022;156 doi: 10.1016/j.compositesa.2022.106885. [DOI] [Google Scholar]
- Gutiérrez T.J., Valencia G.A. Reactive extrusion-processed native and phosphated starch-based food packaging films governed by the hierarchical structure. International Journal of Biological Macromolecules. 2021;172:439–451. doi: 10.1016/j.ijbiomac.2021.01.048. [DOI] [PubMed] [Google Scholar]
- Hafuka A., Sakaida K., Satoh H., Takahashi M., Watanabe Y., Okabe S. Effect of feeding regimens on polyhydroxybutyrate production from food wastes by Cupriavidus necator. Bioresource Technology. 2011;102:3551–3553. doi: 10.1016/j.biortech.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Han J., Hou J., Zhang F., Ai G., Li M., Cai S., Liu H., Wang L., Wang Z., Zhang S., Cai L., Zhao D., Zhou J., Xiang H. Multiple propionyl coenzyme A-supplying pathways for production of the bioplastic poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in Haloferax mediterranei. Applied and Environmental Microbiology. 2013;79:2922–2931. doi: 10.1128/AEM.03915-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Wu L.-P., Hou J., Zhao D., Xiang H. Biosynthesis, characterization, and hemostasis potential of tailor-made poly(3-hydroxybutyrate-co-3-hydroxyvalerate) produced by Haloferax mediterranei. Biomacromolecules. 2015;16:578–588. doi: 10.1021/bm5016267. [DOI] [PubMed] [Google Scholar]
- Hellali W., Korai B., Lambert R. Food from waste: The effect of information and attitude towards risk on consumers’ willingness to pay. Food Quality and Preference. 2023;110 doi: 10.1016/j.foodqual.2023.104945. [DOI] [Google Scholar]
- Hierro-Iglesias C., Chimphango A., Thornley P., Fernández-Castané A. Opportunities for the development of cassava waste biorefineries for the production of polyhydroxyalkanoates in Sub-Saharan Africa. Biomass and Bioenergy. 2022;166 doi: 10.1016/j.biombioe.2022.106600. [DOI] [Google Scholar]
- Hosseinionari H., Seethaler R. The integration of model predictive control and deep reinforcement learning for efficient thermal control in thermoforming processes. Journal of Manufacturing Processes. 2024;115:82–93. doi: 10.1016/j.jmapro.2024.01.085. [DOI] [Google Scholar]
- Huntrakul K., Yoksan R., Sane A., Harnkarnsujarit N. Effects of pea protein on properties of cassava starch edible films produced by blown-film extrusion for oil packaging. Food Packaging and Shelf Life. 2020;24 doi: 10.1016/j.fpsl.2020.100480. [DOI] [Google Scholar]
- Ingram H.R., Martin R.J., Winterburn J.B. Optimized cell growth and poly(3-hydroxybutyrate) synthesis from saponified spent coffee grounds oil. Applied Microbiology and Biotechnology. 2022;106:6033–6045. doi: 10.1007/s00253-022-12093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabeen F., Dhir A., Islam N., Talwar S., Papa A. Emotions and food waste behavior: Do habit and facilitating conditions matter? Journal of Business Research. 2023;155 doi: 10.1016/j.jbusres.2022.113356. [DOI] [Google Scholar]
- Jafarzadeh S., Yildiz Z., Yildiz P., Strachowski P., Forough M., Esmaeili Y., Naebe M., Abdollahi M. Advanced technologies in biodegradable packaging using intelligent sensing to fight food waste. International Journal of Biological Macromolecules. 2024;261 doi: 10.1016/j.ijbiomac.2024.129647. [DOI] [PubMed] [Google Scholar]
- Jendrossek D., Pfeiffer D. New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate) Environmental Microbiology. 2014;16:2357–2373. doi: 10.1111/1462-2920.12356. [DOI] [PubMed] [Google Scholar]
- Ji M., Zheng T., Wang Z., Lai W., Zhang L., Zhang Q., Yang H., Meng S., Xu W., Zhao C., Wu Q., Chen G.-Q. PHB production from food waste hydrolysates by Halomonas bluephagenesis Harboring PHB operon linked with an essential gene. Metabolic Engineering. 2023;77:12–20. doi: 10.1016/j.ymben.2023.03.003. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Neetoo H., Chen H. Efficacy of freezing, frozen storage and edible antimicrobial coatings used in combination for control of Listeria monocytogenes on roasted turkey stored at chiller temperatures. Food Microbiology. 2011;28:1394–1401. doi: 10.1016/j.fm.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Jin H., Chen J., Zhang J., Sheng L. Impact of phosphates on heat-induced egg white gel properties: Texture, water state, micro-rheology and microstructure. Food Hydrocolloids. 2021;110 doi: 10.1016/j.foodhyd.2020.106200. [DOI] [Google Scholar]
- Jõgi K., Bhat R. Valorization of food processing wastes and by-products for bioplastic production. Sustainable Chemistry and Pharmacy. 2020;18 doi: 10.1016/j.scp.2020.100326. [DOI] [Google Scholar]
- Kanjanachumpol P., Kulpreecha S., Tolieng V., Thongchul N. Enhancing polyhydroxybutyrate production from high cell density fed-batch fermentation of Bacillus megaterium BA-019. Bioprocess and Biosystems Engineering. 2013;36:1463–1474. doi: 10.1007/s00449-013-0885-7. [DOI] [PubMed] [Google Scholar]
- Kaushal J., Khatri M., Arya S.K. Recent insight into enzymatic degradation of plastics prevalent in the environment: A mini - review. Clean Eng Technol. 2021;2 doi: 10.1016/j.clet.2021.100083. [DOI] [Google Scholar]
- Kawaguchi H., Takada K., Elkasaby T., Pangestu R., Toyoshima M., Kahar P., Ogino C., Kaneko T., Kondo A. Recent advances in lignocellulosic biomass white biotechnology for bioplastics. Bioresource Technology. 2022;344 doi: 10.1016/j.biortech.2021.126165. [DOI] [PubMed] [Google Scholar]
- Khalaf H., Sharoba A., El-Tanahi H., Morsy M. Stability of antimicrobial activity of pullulan edible films incorporated with nanoparticles and essential oils and their impact on turkey deli meat quality. Journal of Food and Dairy Sciences. 2013;4:557–573. doi: 10.21608/jfds.2013.72104. [DOI] [Google Scholar]
- Khamplod T., Wongsirichot P., Winterburn J. Production of polyhydroxyalkanoates from hydrolysed rapeseed meal by Haloferax mediterranei. Bioresource Technology. 2023;386 doi: 10.1016/j.biortech.2023.129541. [DOI] [PubMed] [Google Scholar]
- Khatami K., Perez-Zabaleta M., Cetecioglu Z. Pure cultures for synthetic culture development: Next level municipal waste treatment for polyhydroxyalkanoates production. Journal of Environmental Management. 2022;305 doi: 10.1016/j.jenvman.2021.114337. [DOI] [PubMed] [Google Scholar]
- Khumkomgool A., Saneluksana T., Harnkarnsujarit N. Active meat packaging from thermoplastic cassava starch containing sappan and cinnamon herbal extracts via LLDPE blown-film extrusion. Food Packaging and Shelf Life. 2020;26 doi: 10.1016/j.fpsl.2020.100557. [DOI] [Google Scholar]
- Kim I.-H., Oh Y.A., Lee H., Song K.B., Min S.C. Grape berry coatings of lemongrass oil-incorporating nanoemulsion. LWT - Food Science and Technology. 2014;58:1–10. doi: 10.1016/j.lwt.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Kinyanjui Muiruri, J., Chee Chuan Yeo, J., Yun Debbie Soo, X., Wang, S., Liu, H., Kong, J., Cao, J., Hoon Tan, B., Suwardi, A., Li, Z., Xu, J., Jun Loh, X., Zhu, Q., 2023. Recent advances of sustainable Short-chain length polyhydroxyalkanoates (Scl-PHAs) – Plant biomass composites. Eur Polym J 187, 111882. https://doi.org/10.1016/j.eurpolymj.2023.111882.
- Klinmalai P., Srisa A., Laorenza Y., Katekhong W., Harnkarnsujarit N. Antifungal and plasticization effects of carvacrol in biodegradable poly(lactic acid) and poly(butylene adipate terephthalate) blend films for bakery packaging. LWT. 2021;152 doi: 10.1016/j.lwt.2021.112356. [DOI] [Google Scholar]
- Koller M., Obruča S. Biotechnological production of polyhydroxyalkanoates from glycerol: A review. Biocatalysis and Agricultural Biotechnology. 2022;42 doi: 10.1016/j.bcab.2022.102333. [DOI] [Google Scholar]
- Kruk J., Tkaczewska J., Szuwarzyński M., Mazur T., Jamróz E. Influence of storage conditions on functional properties of multilayer biopolymer films based on chitosan and furcellaran enriched with carp protein hydrolysate. Food Hydrocolloids. 2023;135 doi: 10.1016/j.foodhyd.2022.108214. [DOI] [Google Scholar]
- Kumar A., Negi Y.S., Choudhary V., Bhardwaj N.K. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. Journal of Materials Physics and Chemistry. 2014;2:1–8. doi: 10.12691/jmpc-2-1-1. [DOI] [Google Scholar]
- Kumar P., Sandeep K.P., Alavi S., Truong V.D., Gorga R.E. Preparation and characterization of bio-nanocomposite films based on soy protein isolate and montmorillonite using melt extrusion. Journal of Food Engineering. 2010;100:480–489. doi: 10.1016/j.jfoodeng.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Le Gars M., Dhuiège B., Delvart A., Belgacem M.N., Missoum K., Bras J. High-barrier and antioxidant poly(lactic acid)/nanocellulose multilayered materials for packaging. ACS Omega. 2020;5:22816–22826. doi: 10.1021/acsomega.0c01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite W., Campos Rubio J., Mata Cabrera F., Carrasco A., Hanafi I. Vacuum thermoforming process: An approach to modeling and optimization using artificial neural networks. Polymers (Basel) 2018;10:143. doi: 10.3390/polym10020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Li L., Naples N.J., Roblee J.W., Yi A.Y. Micro-optical fabrication by ultraprecision diamond machining and precision molding. Frontiers of Mechanical Engineering. 2017;12:181–192. doi: 10.1007/s11465-017-0444-z. [DOI] [Google Scholar]
- Li K., Jia W., Xu L., Zhang M., Huang Y. The plastisphere of biodegradable and conventional microplastics from residues exhibit distinct microbial structure, network and function in plastic-mulching farmland. Journal of Hazardous Materials. 2023;442 doi: 10.1016/j.jhazmat.2022.130011. [DOI] [PubMed] [Google Scholar]
- Li R., Huang J., Liu C., Yu K., Guo F., Li Y., Chen Z., Wang X., Zhao R., Zhang J., Liang J., Li Y., Lin L., Sun L., Li X., Li B. Genome-centric metagenomics provides new insights into metabolic pathways of polyhydroxyalkanoates biosynthesis and functional microorganisms subsisting on municipal organic wastes. Water Research. 2023;244 doi: 10.1016/j.watres.2023.120512. [DOI] [PubMed] [Google Scholar]
- Lim S.W., Kansedo J., Tan I.S., Tan Y.H., Nandong J., Lam M.K., Ongkudon C.M. Biosynthesis of polyhydroxyalkanoates (PHAs) from non-edible Cerbera odollam (sea mango) oil. Bioresour Technol Rep. 2023;24 doi: 10.1016/j.biteb.2023.101653. [DOI] [Google Scholar]
- Lim S.W., Kansedo J., Tan I.S., Tan Y.H., Nandong J., Lam M.K., Ongkudon C.M. Microbial valorization of oil-based substrates for polyhydroxyalkanoates (PHA) production – Current strategies, status, and perspectives. Process Biochemistry. 2023;130:715–733. doi: 10.1016/j.procbio.2023.05.013. [DOI] [Google Scholar]
- Limpisophon K., Tanaka M., Osako K. Characterisation of gelatin–fatty acid emulsion films based on blue shark (Prionace glauca) skin gelatin. Food Chemistry. 2010;122:1095–1101. doi: 10.1016/j.foodchem.2010.03.090. [DOI] [Google Scholar]
- Liu C.-H., Chen H.-Y., Chen Y.-L.-L., Sheu D.-S. The polyhydroxyalkanoate (PHA) synthase 1 of Pseudomonas sp. H9 synthesized a 3-hydroxybutyrate-dominant hybrid of short- and medium-chain-length PHA. Enzyme and Microbial Technology. 2021;143 doi: 10.1016/j.enzmictec.2020.109719. [DOI] [PubMed] [Google Scholar]
- Liu H., Chen Y., Wang S., Liu Y., Zhao W., Huo K., Guo H., Xiong W., Wang S., Yang C., Liu R. Metabolic engineering of genome-streamlined strain Pseudomonas putida KTU-U27 for medium-chain-length polyhydroxyalkanoate production from xylose and cellobiose. International Journal of Biological Macromolecules. 2023;253 doi: 10.1016/j.ijbiomac.2023.126732. [DOI] [PubMed] [Google Scholar]
- Liu W., Zhang J., Liu H., Guo X., Zhang X., Yao X., Cao Z., Zhang T. A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environment International. 2021;146 doi: 10.1016/j.envint.2020.106277. [DOI] [PubMed] [Google Scholar]
- Loan T.T., Trang D.T.Q., Huy P.Q., Ninh P.X., Van Thuoc D. A fermentation process for the production of poly(3-hydroxybutyrate) using waste cooking oil or waste fish oil as inexpensive carbon substrate. Biotechnology Reports. 2022;33:e00700. doi: 10.1016/j.btre.2022.e00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez O., Garcia M.A., Villar M.A., Gentili A., Rodriguez M.S., Albertengo L. Thermo-compression of biodegradable thermoplastic corn starch films containing chitin and chitosan. LWT - Food Science and Technology. 2014;57:106–115. doi: 10.1016/j.lwt.2014.01.024. [DOI] [Google Scholar]
- Luangapai F., Peanparkdee M., Iwamoto S. Biopolymer films for food industries: Properties, applications, and future aspects based on chitosan. Reviews in Agricultural Science. 2019;7:59–67. doi: 10.7831/ras.7.0_59. [DOI] [Google Scholar]
- Maftoonazad N., Ramaswamy H.S. Application and evaluation of a pectin-based edible coating process for quality change kinetics and shelf-life extension of lime fruit (Citrus aurantifolium) Coatings. 2019;9:285. doi: 10.3390/coatings9050285. [DOI] [Google Scholar]
- Maleki G., Woltering E.J., Mozafari M.R. Applications of chitosan-based carrier as an encapsulating agent in food industry. Trends in Food Science and Technology. 2022;120:88–99. doi: 10.1016/j.tifs.2022.01.001. [DOI] [Google Scholar]
- Marsudi S., Tan I.K.P., Gan S.-N., Ramachandran K.B. Production of medium chain length polyhydroxyalkanoates from oleic acid using Pseudomonas putida PGA1 by Fed batch culture. Makara Technologi. 2007;11:1–4. doi: 10.7454/mst.v11i1.434. [DOI] [Google Scholar]
- Martin O., Avérous L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer (Guildf) 2001;42:6209–6219. doi: 10.1016/S0032-3861(01)00086-6. [DOI] [Google Scholar]
- Martiñon M.E., Moreira R.G., Castell-Perez M.E., Gomes C. Development of a multilayered antimicrobial edible coating for shelf-life extension of fresh-cut cantaloupe (Cucumis melo L.) stored at 4 °C. LWT - Food Science and Technology. 2014;56:341–350. doi: 10.1016/j.lwt.2013.11.043. [DOI] [Google Scholar]
- Mehrpouya M., Vahabi H., Barletta M., Laheurte P., Langlois V. Additive manufacturing of polyhydroxyalkanoates (PHAs) biopolymers: Materials, printing techniques, and applications. Materials Science and Engineering: C. 2021;127 doi: 10.1016/j.msec.2021.112216. [DOI] [PubMed] [Google Scholar]
- Mendes J.F., Norcino L.B., Martins H.H.A., Manrich A., Otoni C.G., Carvalho E.E.N., Piccoli R.H., Oliveira J.E., Pinheiro A.C.M., Mattoso L.H.C. Correlating emulsion characteristics with the properties of active starch films loaded with lemongrass essential oil. Food Hydrocolloids. 2020;100 doi: 10.1016/j.foodhyd.2019.105428. [DOI] [Google Scholar]
- Mezzina M.P., Manoli M.T., Prieto M.A., Nikel P.I. Engineering native and synthetic pathways in Pseudomonas putida for the production of tailored polyhydroxyalkanoates. Biotechnology Journal. 2021;16:2000165. doi: 10.1002/biot.202000165. [DOI] [PubMed] [Google Scholar]
- Mohammad S.H., Bhukya B. Biotransformation of toxic lignin and aromatic compounds of lignocellulosic feedstock into eco-friendly biopolymers by Pseudomonas putida KT2440. Bioresource Technology. 2022;363 doi: 10.1016/j.biortech.2022.128001. [DOI] [PubMed] [Google Scholar]
- Monteiro Fritz A.R., de Matos Fonseca J., Trevisol T.C., Fagundes C., Valencia G.A. Polymers for Agri-Food Applications. Springer International Publishing; Cham: 2019. Active, Eco-Friendly and Edible Coatings in the Post-Harvest – A Critical Discussion; pp. 433–463. [DOI] [Google Scholar]
- Morinval A., Follain N., Avérous L. Design and analysis of biodegradable and potentially biobased multiphase systems from starch and co-polyesters. European Polymer Journal. 2024;215 doi: 10.1016/j.eurpolymj.2024.113231. [DOI] [Google Scholar]
- Morya R., Kumar M., Kumar V., Thakur I.S. Biovalorization of lignin derived compounds with molasses as co-substrate for polyhydroxyalkanoate production. Environmental Technology and Innovation. 2021;23 doi: 10.1016/j.eti.2021.101695. [DOI] [Google Scholar]
- Morya R., Sharma A., Kumar M., Tyagi B., Singh S.S., Thakur I.S. Polyhydroxyalkanoate synthesis and characterization: A proteogenomic and process optimization study for biovalorization of industrial lignin. Bioresource Technology. 2021;320 doi: 10.1016/j.biortech.2020.124439. [DOI] [PubMed] [Google Scholar]
- Mourya, A., Nanda, A., Parashar, K., Sushant, Kumar, R., 2023. An explanatory study on defects in plastic molding parts caused by machine parameters in injection molding process. Mater Today Proc 78, 656–661. https://doi.org/10.1016/j.matpr.2022.12.070.
- Mujtaba M., Morsi R.E., Kerch G., Elsabee M.Z., Kaya M., Labidi J., Khawar K.M. Current advancements in chitosan-based film production for food technology; A review. International Journal of Biological Macromolecules. 2019;121:889–904. doi: 10.1016/j.ijbiomac.2018.10.109. [DOI] [PubMed] [Google Scholar]
- Muller J., González-Martínez C., Chiralt A. Combination of poly(lactic) acid and starch for biodegradable food packaging. Materials. 2017;10:952. doi: 10.3390/ma10080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murueva A.V., Dudaev A.E., Shishatskaya E.I., Ghorabe F.D.E., Nemtsev I.V., Lukyanenko A.V., Volova T.G. Biodegradable polymer casting films for drug delivery and cell culture. Giant. 2024;19 doi: 10.1016/j.giant.2024.100314. [DOI] [Google Scholar]
- Mustapha S.N.H., Wan J.S. Effect of hybridization composition and glycerin content on novel corn starch/nata de coco plastic film: Thermal, mechanical, and degradation study. Food Chemistry. 2022;373 doi: 10.1016/j.foodchem.2021.131440. [DOI] [PubMed] [Google Scholar]
- Naser A.Z., Deiab I., Darras B.M. Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: A review. RSC Advances. 2021;11:17151–17196. doi: 10.1039/D1RA02390J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoh S.Z., Chek M.F., Tan H.T., Linares-Pastén J.A., Nandakumar A., Hakoshima T., Sudesh K. Polyhydroxyalkanoate synthase (PhaC): The key enzyme for biopolyester synthesis. Curr Res Biotechnol. 2022;4:87–101. doi: 10.1016/j.crbiot.2022.01.002. [DOI] [Google Scholar]
- Noshirvani N., Ghanbarzadeh B., Rezaei Mokarram R., Hashemi M. Novel active packaging based on carboxymethyl cellulose-chitosan-ZnO NPs nanocomposite for increasing the shelf life of bread. Food Packaging and Shelf Life. 2017;11:106–114. doi: 10.1016/j.fpsl.2017.01.010. [DOI] [Google Scholar]
- Oliveira-Filho E.R., Silva J.G.P., de Macedo M.A., Taciro M.K., Gomez J.G.C., Silva L.F. Investigating nutrient limitation role on improvement of growth and poly(3-hydroxybutyrate) accumulation by Burkholderia sacchari LMG 19450 from xylose as the sole carbon source. Frontiers in Bioengineering and Biotechnology. 2020;7:416. doi: 10.3389/fbioe.2019.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabast M., Shariatifar N., Beikzadeh S., Jahed G. Effects of chitosan coatings incorporating with free or nano-encapsulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control. 2018;91:185–192. doi: 10.1016/j.foodcont.2018.03.047. [DOI] [Google Scholar]
- Paduvari R., Subrahmanya Bhat K., Somashekara D.M. Biosynthesis of 3-hydroxydecanoate (3HD) and 3-hydroxydodecanoate (3HDd) in the polyhydroxyalkanoate copolymeric ester chain by Pseudomonas aeruginosa MCC 5300 using oleic acid as a carbon source. Bioresour Technol Rep. 2024;25 doi: 10.1016/j.biteb.2023.101690. [DOI] [Google Scholar]
- Pan L., Li J., Wang R., Wang Yu., Lin Q., Li C., Wang Y. Biosynthesis of polyhydroxyalkanoate from food waste oil by Pseudomonas alcaligenes with simultaneous energy recovery from fermentation wastewater. Waste Management. 2021;131:268–276. doi: 10.1016/j.wasman.2021.06.008. [DOI] [PubMed] [Google Scholar]
- Pan W., Perrotta J.A., Stipanovic A.J., Nomura C.T., Nakas J.P. Production of polyhydroxyalkanoates by Burkholderia cepacia ATCC 17759 using a detoxified sugar maple hemicellulosic hydrolysate. Journal of Industrial Microbiology & Biotechnology. 2012;39:459–469. doi: 10.1007/s10295-011-1040-6. [DOI] [PubMed] [Google Scholar]
- Pandhi S., Kumar A., Alam T. Probiotic edible films and coatings: Concerns, applications and future prospects. Journal of Packaging Technology and Reseach. 2019;3:261–268. doi: 10.1007/s41783-019-00069-6. [DOI] [Google Scholar]
- Panrong T., Karbowiak T., Harnkarnsujarit N. Effects of acetylated and octenyl-succinated starch on properties and release of green tea compounded starch/LLDPE blend films. Journal of Food Engineering. 2020;284 doi: 10.1016/j.jfoodeng.2020.110057. [DOI] [Google Scholar]
- Park J.W., Scott Whiteside W., Cho S.Y. Mechanical and water vapor barrier properties of extruded and heat-pressed gelatin films. LWT - Food Science and Technology. 2008;41:692–700. doi: 10.1016/j.lwt.2007.04.015. [DOI] [Google Scholar]
- Pasquier E., Skunde R., Ruwoldt J. Influence of temperature and pressure during thermoforming of softwood pulp. Journal of Bioresources and Bioproducts. 2023;8:408–420. doi: 10.1016/j.jobab.2023.10.001. [DOI] [Google Scholar]
- Patil J.P., Nandedkar V., Mishra S., Saha S.K. Transient thermal analysis of close pressure thermoforming process. Journal of Manufacturing Processes. 2021;62:513–522. doi: 10.1016/j.jmapro.2020.12.057. [DOI] [Google Scholar]
- Pavli F., Argyri A.A., Skandamis P., Nychas G.-J., Tassou C., Chorianopoulos N. Antimicrobial activity of oregano essential oil incorporated in sodium alginate edible films: Control of Listeria monocytogenes and spoilage in ham slices treated with high pressure processing. Materials. 2019;12:3726. doi: 10.3390/ma12223726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody G.L., Elmore J.R., Martinez-Baird J., Guss A.M. Engineered Pseudomonas putida KT2440 co-utilizes galactose and glucose. Biotechnology for Biofuels. 2019;12:295. doi: 10.1186/s13068-019-1627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkoska A.T., Daniloski D., D’Cunha N.M., Naumovski N., Broach A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Research International. 2021;140 doi: 10.1016/j.foodres.2020.109981. [DOI] [PubMed] [Google Scholar]
- Phothisarattana D., Wongphan P., Promhuad K., Promsorn J., Harnkarnsujarit N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids and Surfaces. B, Biointerfaces. 2022;214 doi: 10.1016/j.colsurfb.2022.112472. [DOI] [PubMed] [Google Scholar]
- Pinzon M.I., Sanchez L.T., Garcia O.R., Gutierrez R., Luna J.C., Villa C.C. Increasing shelf life of strawberries (Fragaria ssp) by using a banana starch-chitosan-Aloe vera gel composite edible coating. International Journal of Food Science and Technology. 2020;55:92–98. doi: 10.1111/ijfs.14254. [DOI] [Google Scholar]
- Prakoso F.A.H., Indiarto R., Utama G.L. Edible film casting techniques and materials and their utilization for meat-based product packaging. Polymers (Basel) 2023;15:2800. doi: 10.3390/polym15132800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata M.P., González-Buesa J., Chopra S., Kim K., Pietri Y., Ng P.K.W., Matuana L.M., Almenar E. Egg white protein film production through extrusion and calendering processes and its suitability for food packaging applications. Food and Bioprocess Technology. 2019;12:714–727. doi: 10.1007/s11947-019-2248-0. [DOI] [Google Scholar]
- Prieto A., Escapa I.F., Martínez V., Dinjaski N., Herencias C., de la Peña F., Tarazona N., Revelles O. A holistic view of polyhydroxyalkanoate metabolism in Pseudomonas putida. Environmental Microbiology. 2016;18:341–357. doi: 10.1111/1462-2920.12760. [DOI] [PubMed] [Google Scholar]
- Qian M., Liu D., Zhang X., Yin Z., Ismail B.B., Ye X., Guo M. A review of active packaging in bakery products: Applications and future trends. Trends in Food Science and Technology. 2021;114:459–471. doi: 10.1016/j.tifs.2021.06.009. [DOI] [Google Scholar]
- Raghav P.K., Saini M. Development of mint (Mentha viridis L.) herbal edible coating for shelf life enhancement of cucumber (Cucumis sativus) International Journal of Green and Herbal Chemistry. 2018;7:379–391. doi: 10.24214/ijghc/gc/7/2/37991. [DOI] [Google Scholar]
- Rakkan T., Zhang S., Lehner S., Hufenus R., Sangkharak K., Ren Q. Bio-based modification of polyhydroxyalkanoates (PHA) towards increased antimicrobial activities and reduced cytotoxicity. International Journal of Biological Macromolecules. 2024;275 doi: 10.1016/j.ijbiomac.2024.133132. [DOI] [PubMed] [Google Scholar]
- Raunhan R., Jantharadej K., Mhuantong W., Chanprateep Napathorn S., Boonchayaanant Suwannasilp B. Valorization of food waste derived anaerobic digestate into polyhydroxyalkanoate (PHA) using Thauera mechernichensis TL1. Waste Management. 2023;171:248–258. doi: 10.1016/j.wasman.2023.08.039. [DOI] [PubMed] [Google Scholar]
- Ravishankar S., Jaroni D., Zhu L., Olsen C., McHugh T., Friedman M. Inactivation of Listeria monocytogenes on ham and bologna using pectin-based apple, carrot, and hibiscus edible films containing carvacrol and cinnamaldehyde. Journal of Food Science. 2012;77:M377–M382. doi: 10.1111/j.1750-3841.2012.02751.x. [DOI] [PubMed] [Google Scholar]
- Reddy M.V., Watanabe A., Onodera R., Mawatari Y., Tsukiori Y., Watanabe A., Kudou M., Chang Y.-C. Polyhydroxyalkanoates (PHA) production using single or mixture of fatty acids with Bacillus sp. CYR1: Identification of PHA synthesis genes. Bioresour Technol Rep. 2020;11 doi: 10.1016/j.biteb.2020.100483. [DOI] [Google Scholar]
- Ren Y., Ling C., Hajnal I., Wu Q., Chen G.-Q. Construction of Halomonas bluephagenesis capable of high cell density growth for efficient PHA production. Applied Microbiology and Biotechnology. 2018;102:4499–4510. doi: 10.1007/s00253-018-8931-7. [DOI] [PubMed] [Google Scholar]
- Rhim J.-W. Effect of PLA lamination on performance characteristics of agar/κ-carrageenan/clay bio-nanocomposite film. Food Research International. 2013;51:714–722. doi: 10.1016/j.foodres.2013.01.050. [DOI] [Google Scholar]
- Rifna E.J., Dwivedi M., Seth D., Pradhan R.C., Sarangi P.K., Tiwari B.K. Transforming the potential of renewable food waste biomass towards food security and supply sustainability. Sustainable Chemistry and Pharmacy. 2024;38 doi: 10.1016/j.scp.2024.101515. [DOI] [Google Scholar]
- Robledo N., Vera P., López L., Yazdani-Pedram M., Tapia C., Abugoch L. Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films; antifungal effect in cherry tomatoes. Food Chemistry. 2018;246:211–219. doi: 10.1016/j.foodchem.2017.11.032. [DOI] [PubMed] [Google Scholar]
- Rudra, S.G., Gundewadi, G., Sharma, R.R., 2020. Natural Additives with Antimicrobial and Flavoring Potential for Fresh-Cut Produce, in: Fresh-Cut Fruits and Vegetables. Elsevier, pp. 165–182. https://doi.org/10.1016/B978-0-12-816184-5.00008-2.
- Sachan R.S.K., Devgon I., Karnwal A., Mahmoud A.E.D. Valorization of sugar extracted from wheat straw for eco-friendly polyhydroxyalkanoate (PHA) production by Bacillus megaterium MTCC 453. Bioresour Technol Rep. 2024;25 doi: 10.1016/j.biteb.2024.101770. [DOI] [Google Scholar]
- Salmieri S., Islam F., Khan R.A., Hossain F.M., Ibrahim H.M.M., Miao C., Hamad W.Y., Lacroix M. Antimicrobial nanocomposite films made of poly(lactic acid)–cellulose nanocrystals (PLA–CNC) in food applications—part B: Effect of oregano essential oil release on the inactivation of Listeria monocytogenes in mixed vegetables. Cellulose. 2014;21:4271–4285. doi: 10.1007/s10570-014-0406-0. [DOI] [Google Scholar]
- Salvia-Trujillo L., Rojas-Graü M.A., Soliva-Fortuny R., Martín-Belloso O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biology and Technology. 2015;105:8–16. doi: 10.1016/j.postharvbio.2015.03.009. [DOI] [Google Scholar]
- Sani I.K., Geshlaghi S.P., Pirsa S., Asdagh A. Composite film based on potato starch/apple peel pectin/ZrO2 nanoparticles/ microencapsulated Zataria multiflora essential oil; investigation of physicochemical properties and use in quail meat packaging. Food Hydrocolloids. 2021;117 doi: 10.1016/j.foodhyd.2021.106719. [DOI] [Google Scholar]
- Scarfato P., Di Maio L., Milana M.R., Giamberardini S., Denaro M., Incarnato L. Performance properties, lactic acid specific migration and swelling by simulant of biodegradable poly(lactic acid)/nanoclay multilayer films for food packaging. Food Additives & Contaminants: Part A. 2017;34:1730–1742. doi: 10.1080/19440049.2017.1321786. [DOI] [PubMed] [Google Scholar]
- Sehgal R., Gupta R. Polyhydroxyalkanoate and its efficient production: An eco-friendly approach towards development. 3 Biotech. 2020;10:549. doi: 10.1007/s13205-020-02550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid S., Mosrati R., Corroler D., Amiel C., Gaillard J.-L. Bioconversion of glycerol into polyhydroxyalkanoates through an atypical metabolism shift using Priestia megaterium during fermentation processes: A statistical analysis of carbon and nitrogen source concentrations. International Journal of Biological Macromolecules. 2024;256 doi: 10.1016/j.ijbiomac.2023.128116. [DOI] [PubMed] [Google Scholar]
- Shankar S., Khodaei D., Lacroix M. Effect of chitosan/essential oils/silver nanoparticles composite films packaging and gamma irradiation on shelf life of strawberries. Food Hydrocolloids. 2021;117 doi: 10.1016/j.foodhyd.2021.106750. [DOI] [Google Scholar]
- Sharma V., Sehgal R., Gupta R. Polyhydroxyalkanoate (PHA): Properties and modifications. Polymer (Guildf) 2021;212 doi: 10.1016/j.polymer.2020.123161. [DOI] [Google Scholar]
- Shen M., Hu T., Huang W., Song B., Zeng G., Zhang Y. Removal of microplastics from wastewater with aluminosilicate filter media and their surfactant-modified products: Performance, mechanism and utilization. Chemical Engineering Journal. 2021;421 doi: 10.1016/j.cej.2021.129918. [DOI] [Google Scholar]
- Siracusa V., Rocculi P., Romani S., Rosa M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci Technol. 2008;19:634–643. doi: 10.1016/j.tifs.2008.07.003. [DOI] [Google Scholar]
- Sirohi R., Pandey J.P., Tarafdar A., Agarwal A., Chaudhuri S.K., Sindhu R. An environmentally sustainable green process for the utilization of damaged wheat grains for poly-3-hydroxybutyrate production. Environmental Technology and Innovation. 2021;21 doi: 10.1016/j.eti.2020.101271. [DOI] [Google Scholar]
- Sirohi R., Prakash Pandey J., Kumar Gaur V., Gnansounou E., Sindhu R. Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB) Bioresource Technology. 2020;311 doi: 10.1016/j.biortech.2020.123536. [DOI] [PubMed] [Google Scholar]
- Sohn Y.J., Son J., Jo S.Y., Park S.Y., Yoo J.I., Baritugo K.-A., Na J.G., Choi J., Kim H.T., Joo J.C., Park S.J. Chemoautotroph Cupriavidus necator as a potential game-changer for global warming and plastic waste problem: A review. Bioresource Technology. 2021;340 doi: 10.1016/j.biortech.2021.125693. [DOI] [PubMed] [Google Scholar]
- Soroudi A., Jakubowicz I. Recycling of bioplastics, their blends and biocomposites: A review. European Polymer Journal. 2013;49:2839–2858. doi: 10.1016/j.eurpolymj.2013.07.025. [DOI] [Google Scholar]
- Stadio G.D., Orita I., Nakamura R., Fukui T. Gas fermentation combined with water electrolysis for production of polyhydroxyalkanoate copolymer from carbon dioxide by engineered Ralstonia eutropha. Bioresource Technology. 2024;394 doi: 10.1016/j.biortech.2023.130266. [DOI] [PubMed] [Google Scholar]
- Subagyo D.C.H., Shimizu R., Orita I., Fukui T. Isopropanol production with reutilization of glucose-derived CO2 by engineered Ralstonia eutropha. Journal of Bioscience and Bioengineering. 2021;132:479–486. doi: 10.1016/j.jbiosc.2021.08.004. [DOI] [PubMed] [Google Scholar]
- Subramanian A.M., Nanjan S.E., Prakash H., Santharam L., Ramachandran A., Sathyaseelan V., Ravi D.P., Mahadevan S. Biokinetics of fed-batch production of poly (3-hydroxybutyrate) using microbial co-culture. Applied Microbiology and Biotechnology. 2020;104:1077–1095. doi: 10.1007/s00253-019-10274-7. [DOI] [PubMed] [Google Scholar]
- Sun H., Zhang X., Wang D., Lin Z. Insights into the role of energy source in hormesis through diauxic growth of bacteria in mixed cultivation systems. Chemosphere. 2020;261 doi: 10.1016/j.chemosphere.2020.127669. [DOI] [PubMed] [Google Scholar]
- Taguchi S., Matsumoto K. Evolution of polyhydroxyalkanoate synthesizing systems toward a sustainable plastic industry. Polymer Journal. 2021;53:67–79. doi: 10.1038/s41428-020-00420-8. [DOI] [Google Scholar]
- Talan A., Pokhrel S., Tyagi R.D., Drogui P. Biorefinery strategies for microbial bioplastics production: Sustainable pathway towards Circular Bioeconomy. Bioresour Technol Rep. 2022;17 doi: 10.1016/j.biteb.2021.100875. [DOI] [Google Scholar]
- Tan G.-Y., Chen C.-L., Li L., Ge L., Wang L., Razaad I., Li Y., Zhao L., Mo Y., Wang J.-Y. Start a research on biopolymer polyhydroxyalkanoate (PHA): A review. Polymers (Basel) 2014;6:706–754. doi: 10.3390/polym6030706. [DOI] [Google Scholar]
- Tao Q., Huang H., Yang M., Zou Y., Harder M.K., Yan Q., Liang B., Ntaikou I., Antonopoulou G., Lyberatos G., Zhang Y. Comparison of batch, fed-batch and continuous operation modes for scalable polyhydroxyalkanoate (PHA) production and carbon sequestration from phenol. Journal of Water Process Engineering. 2024;60 doi: 10.1016/j.jwpe.2024.105147. [DOI] [Google Scholar]
- Tavassoli-Kafrani E., Gamage M.V., Dumée L.F., Kong L., Zhao S. Edible films and coatings for shelf life extension of mango: A review. Critical Reviews in Food Science and Nutrition. 2022;62:2432–2459. doi: 10.1080/10408398.2020.1853038. [DOI] [PubMed] [Google Scholar]
- Thakur R., Pristijono P., Bowyer M., Singh S.P., Scarlett C.J., Stathopoulos C.E., Vuong Q.V. A starch edible surface coating delays banana fruit ripening. LWT. 2019;100:341–347. doi: 10.1016/j.lwt.2018.10.055. [DOI] [Google Scholar]
- Thiounn T., Smith R.C. Advances and approaches for chemical recycling of plastic waste. Journal of Polymer Science. 2020;58:1347–1364. doi: 10.1002/pol.20190261. [DOI] [Google Scholar]
- Tomadoni B., Moreira M.R., Pereda M., Ponce A.G. Gellan-based coatings incorporated with natural antimicrobials in fresh-cut strawberries: Microbiological and sensory evaluation through refrigerated storage. LWT. 2018;97:384–389. doi: 10.1016/j.lwt.2018.07.029. [DOI] [Google Scholar]
- Tomietto P., Russo F., Galiano F., Loulergue P., Salerno S., Paugam L., Audic J.-L., De Bartolo L., Figoli A. Sustainable fabrication and pervaporation application of bio-based membranes: Combining a polyhydroxyalkanoate (PHA) as biopolymer and CyreneTM as green solvent. J Memb Sci. 2022;643 doi: 10.1016/j.memsci.2021.120061. [DOI] [Google Scholar]
- Tyagi B., Gupta B., Khatak D., Meena R., Shekhar Thakur I. Genomic analysis, simultaneous production, and process optimization of extracellular polymeric substances and polyhydroxyalkanoates by Methylobacterium sp. ISTM1 by utilizing molasses. Bioresource Technology. 2022;354 doi: 10.1016/j.biortech.2022.127204. [DOI] [PubMed] [Google Scholar]
- Varghese S., Dhanraj N.D., Rebello S., Sindhu R., Binod P., Pandey A., Jisha M.S., Awasthi M.K. Leads and hurdles to sustainable microbial bioplastic production. Chemosphere. 2022;305 doi: 10.1016/j.chemosphere.2022.135390. [DOI] [PubMed] [Google Scholar]
- Velásquez E., Patiño Vidal C., Rojas A., Guarda A., Galotto M.J., López de Dicastillo C. Natural antimicrobials and antioxidants added to polylactic acid packaging films. Part I: Polymer processing techniques. Comprehensive Reviews in Food Science and Food Safety. 2021;20:3388–3403. doi: 10.1111/1541-4337.12777. [DOI] [PubMed] [Google Scholar]
- Velásquez E., Rojas A., Piña C., Galotto M.J., López de Dicastillo C. Development of bilayer biodegradable composites containing cellulose nanocrystals with antioxidant properties. Polymers (Basel) 2019;11:1945. doi: 10.3390/polym11121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneral C., Camargo A.F., Klein G.H., Veneral J., Treichel H. From macro to micro: Plastic as an environmental problem. Environ Nanotechnol Monit Manag. 2023;20 doi: 10.1016/j.enmm.2023.100906. [DOI] [Google Scholar]
- Vidal C.P., Velásquez E., Gavara R., Hernández-Muñoz P., Muñoz-Shugulí C., Galotto M.J., López de Dicastillo C. Modeling the release of an antimicrobial agent from multilayer film containing coaxial electrospun polylactic acid nanofibers. Journal of Food Engineering. 2023;352 doi: 10.1016/j.jfoodeng.2023.111524. [DOI] [Google Scholar]
- Villafañe F. Edible coatings for carrots. Food Reviews International. 2017;33:84–103. doi: 10.1080/87559129.2016.1150291. [DOI] [Google Scholar]
- von Borries-Medrano E., Jaime-Fonseca M.R., Aguilar-Méndez M.A. Starch–guar gum extrudates: Microstructure, physicochemical properties and in-vitro digestion. Food Chemistry. 2016;194:891–899. doi: 10.1016/j.foodchem.2015.08.085. [DOI] [PubMed] [Google Scholar]
- Vu D.H., Wainaina S., Taherzadeh M.J., Åkesson D., Ferreira J.A. Production of polyhydroxyalkanoates (PHAs) by Bacillus megaterium using food waste acidogenic fermentation-derived volatile fatty acids. Bioengineered. 2021;12:2480–2498. doi: 10.1080/21655979.2021.1935524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadaugsorn K., Panrong T., Wongphan P., Harnkarnsujarit N. Plasticized hydroxypropyl cassava starch blended PBAT for improved clarity blown films: Morphology and properties. Industrial Crops and Products. 2022;176 doi: 10.1016/j.indcrop.2021.114311. [DOI] [Google Scholar]
- Wang H., Li H., Lee C.K., Mat Nanyan N.S., Tay G.S. A systematic review on utilization of biodiesel-derived crude glycerol in sustainable polymers preparation. International Journal of Biological Macromolecules. 2024;261 doi: 10.1016/j.ijbiomac.2024.129536. [DOI] [PubMed] [Google Scholar]
- Wellenreuther C., Wolf A., Zander N. Cost competitiveness of sustainable bioplastic feedstocks – A Monte Carlo analysis for polylactic acid. Clean Eng Technol. 2022;6 doi: 10.1016/j.clet.2022.100411. [DOI] [Google Scholar]
- Wongsirichot P., Gonzalez-Miquel M., Winterburn J. Recent advances in rapeseed meal as alternative feedstock for industrial biotechnology. Biochemical Engineering Journal. 2022;180 doi: 10.1016/j.bej.2022.108373. [DOI] [Google Scholar]
- Xiong Y., Chen M., Warner R.D., Fang Z. Incorporating nisin and grape seed extract in chitosan-gelatine edible coating and its effect on cold storage of fresh pork. Food Control. 2020;110 doi: 10.1016/j.foodcont.2019.107018. [DOI] [Google Scholar]
- Xiong Y., Li S., Warner R.D., Fang Z. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control. 2020;114 doi: 10.1016/j.foodcont.2020.107226. [DOI] [Google Scholar]
- Yadav B., Pandey A., Kumar L.R., Tyagi R.D. Bioconversion of waste (water)/residues to bioplastics- A circular bioeconomy approach. Bioresource Technology. 2020;298 doi: 10.1016/j.biortech.2019.122584. [DOI] [PubMed] [Google Scholar]
- Yang J., Punia Bangar S., Rizwan Khan M., Hammouda G.A., Alam P., Zhang W. Biopolymer-based packaging films/edible coatings functionalized with ε-polylysine: New options for food preservation. Food Research International. 2024;187 doi: 10.1016/j.foodres.2024.114390. [DOI] [PubMed] [Google Scholar]
- Yb B., Kola M. Influence of composite edible coating systems on preservation of fresh meat cuts and products: A brief review on their trends and applications, Article. International Food Research Journal. 2019 [Google Scholar]
- Yousuf, B., Qadri, O.S., 2020. Preservation of Fresh-Cut Fruits and Vegetables by Edible Coatings, in: Fresh-Cut Fruits and Vegetables. Elsevier, pp. 225–242. https://doi.org/10.1016/B978-0-12-816184-5.00011-2.
- Yousuf B., Srivastava A.K. Psyllium (Plantago) gum as an effective edible coating to improve quality and shelf life of fresh-cut papaya (Carica papaya) International Journal of Bioengineering and Life Sciences. 2015;9:765–770. [Google Scholar]
- Yu F., Fei X., He Y., Li H. Poly(lactic acid)-based composite film reinforced with acetylated cellulose nanocrystals and ZnO nanoparticles for active food packaging. International Journal of Biological Macromolecules. 2021;186:770–779. doi: 10.1016/j.ijbiomac.2021.07.097. [DOI] [PubMed] [Google Scholar]
- Zainab-L I., Sudesh K. High cell density culture of Cupriavidus necator H16 and improved biological recovery of polyhydroxyalkanoates using mealworms. Journal of Biotechnology. 2019;305:35–42. doi: 10.1016/j.jbiotec.2019.09.001. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Lin Y., Wu S., Li X., Cheng J.J., Yang C. Effect of composition of volatile fatty acids on yield of polyhydroxyalkanoates and mechanisms of bioconversion from activated sludge. Bioresource Technology. 2023;385 doi: 10.1016/j.biortech.2023.129445. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wang Y., Wang X., Zhang Y., Zhu T., Peng L., Xu Y., Chen X., Wang D., Ni B.-J., Liu Y. Towards scaling-up implementation of polyhydroxyalkanoate (PHA) production from activated sludge: Progress and challenges. Journal of Cleaner Production. 2024;447 doi: 10.1016/j.jclepro.2024.141542. [DOI] [Google Scholar]
- Żur-Pińska J., Gładysz M.Z., Ubels D., Siebring J., Włodarczyk-Biegun M.K. Smart and sustainable: Exploring the future of PHAs biopolymers for 3D printing in tissue engineering. Sustainable Materials and Technologies. 2023;38:e00750. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.