Highlights
-
•
The American Heart Association’s Life Essential 8 (LE8) includes physical activity as a component, which is often self-reported and less accurate than device measures.
-
•
We measured LE8 calculated using both self-reported and accelerometer-measured physical activity and tested the associations with incident cardiovascular disease (CVD).
-
•
In a diverse group of 4,000+ older women, LE8 scores and incident CVD associations varied by physical activity measurement type, yet both showed reduced risk.
Keywords: Accelerometer, Steps, Women’s health, Cardiovascular health, Questionnaire, Device
Abstract
Objective
The American Heart Association’s Life’s Essential 8 (LE8) metric includes self-reported physical activity as one of the metrics for assessing cardiovascular health. Self-reported physical activity is prone to misclassification, whereas accelerometer measures are less biased. We examined associations of LE8 and incident cardiovascular disease (CVD) using self-reported and accelerometer-measured physical activity.
Methods
Participants in the Women’s Health Initiative (WHI) Objective Physical Activity and Cardiovascular Health Study (n = 4,243; mean age = 79 ± 7 years) with no CVD history completed the WHI physical activity questionnaire and the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire prior to wearing a hip-worn accelerometer for up to seven days in 2012–2014. LE8 components (physical activity, diet, sleep, body mass index, smoking, blood pressure, non-HDL cholesterol, and glucose) were scored according to guidelines. Scores were created using five physical activity measures: WHI questionnaire (LE8WHI), CHAMPS (LE8CHAMPS), accelerometer-measured physical activity (LE8A), and sample quantiles of accelerometer-measured physical activity (LE8AQ) and daily steps (LE8STEPS). Hazard ratios (HR) for physician-adjudicated CVD were estimated using Cox regression.
Results
707 incident CVD events occurred over an average 7.5 years. Multivariable HRs (95 % CI) comparing women in the highest vs. lowest quartiles of LE8 scores were: LE8WHI = 0.53 (0.43–0.67), LE8CHAMPS = 0.47 (0.38–0.60), LE8A = 0.44 (0.36–0.56), LE8AQ = 0.44 (0.35–0.55), and LE8STEPS = 0.45 (0.35–0.57).
Conclusions
The LE8-incident CVD association varies by physical activity measurement, however all methods showed reduced risk. Device-measures of physical activity may be more accurate in the LE8, but when impractical to implement, also support use of self-reported measures.
1. Introduction
In 2010, the American Heart Association (AHA) established the Life’s Simple 7 (LS7), a set of modifiable risk factors used to indicate ideal, intermediate, or poor levels of cardiovascular health (Lloyd-Jones et al., 2010). The LS7 metric comprises seven behavioral and health factors related to diet, physical activity, body mass index (BMI), smoking, blood pressure, fasting glucose, and total cholesterol (Lloyd-Jones et al., 2010, U.S. Department of Health and Human Services, 2018). A number of studies have shown a consistent linear decrease in risk of cardiovascular disease (CVD) and cardiovascular and all-cause mortality with increasing LS7 score (Aneni et al., 2017, Guo and Zhang, 2017, Fang et al., 2016). However, the LS7 has several documented limitations, including a crude additive scoring system, weak sensitivity to individual-level change, and the omission of sleep as a component of the metric (Lloyd-Jones et al., 2022). As such, in 2022, the AHA updated LS7 to address these limitations and established the Life’s Essential 8 (LE8) (Lloyd-Jones et al., 2022).
In 2017–2020, the prevalence of CVD among older women ages 60–79 years in the United States (US) was 19.6 % and increased to 32.8 % for women ages ≥ 80 years (Tsao et al., 2023). Given that the burden of CVD in older women increases in populations with increasing age, characterizing levels of cardiovascular health among older women is of public health importance. A study of LE8 using data from the National Health and Nutrition Examination Survey (NHANES) from 2013 to 2018 found that women had significantly higher scores (indicating more favorable cardiovascular health) than men, and scores decreased with increasing age for both sexes (Lloyd-Jones et al., 2022). Characterizing the extent to which the LE8 score predicts incident CVD in older women can help individuals, public health practitioners, and healthcare providers better strategize risk assessment and prevention efforts.
The physical activity component of the LE8 is based on meeting the minimally recommended amount of at least 150 min/week of moderate-to-vigorous physical activity (MVPA) (U.S. Department of Health and Human Services, 2018). Most studies of the association between LS7 or LE8 scores and incident CVD have relied on self-reported data for categorizing the physical activity component. However, compared to self-report, accelerometers allow for a more accurate assessment of all physical activity (leisure-time and otherwise) and accelerometer-measured physical activity is associated with lower CVD risk in older women (LaMonte et al., 2017). Device-based measurements of physical activity, such as those produced by accelerometers, may be able to reduce the exposure misclassification that occurs in self-reported measures (Lee and Shiroma, Feb 2014, Wijndaele et al., Oct 2015). In addition, while self-reported questionnaires can provide information on domains (e.g., leisure, transportation, occupational) and type (e.g., sports, bicycling, muscle-strengthening) of physical activities, device-based measures allow for measurement of daily step counts which are not captured with self-reported methods. To our knowledge, no study has calculated the LE8 score using accelerometer-measured physical activity for comparison against the LE8 score using self-reported measures.
Thus, the purpose of our study was three-fold: (1) to describe the distribution of the summary LE8 score using both self-reported and accelerometer-measured physical activity in a large community-based cohort of ambulatory older women; (2) to determine the association of LE8 score with incident CVD; and (3) to assess the extent to which classification of the LE8 physical activity component by self-report compared with accelerometer affects the calculation of LE8 score and its impact on CVD.
2. Methods
2.1. Study population
The Objective physical activity and Cardiovascular Health (OPACH) is an ancillary, nested cohort study within the Women’s Health Initiative (WHI) program (LaCroix et al., 2017). Details of OPACH have been published elsewhere (LaCroix et al., 2017). Briefly, OPACH collected accelerometry data from 6,489 ambulatory community-living women from May 2012 to April 2014 and were followed for incident CVD through February 2023. In this study, we excluded 530 women with prevalent CVD at OPACH baseline, 326 who did not meet the criteria for adherent accelerometer wear (≥4 days with ≥ 10 h/day), 145 with missing self-reported physical activity data, 1,245 with missing any other LE8 component data, and which left an analytic sample of 4,243 women. Excluded participants were more likely to be Black, be a non-drinker, and have two or more comorbidities than those included (Supplemental Table 1). All women provided informed written consent and the WHI Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle, WA) approved of the study protocol and ethical compliance.
2.2. Exposure ascertainment
2.2.1. Physical activity component
Participants were asked to wear the ActiGraph GT3X + accelerometer (ActiGraph, Pensacola, FL) over their right hip 24 h/day for seven consecutive days, removing devices only when showering or swimming. The Choi algorithm identified periods of accelerometer non-wear (Choi et al., 2011). Participants self-reported in-bed and out-of-bed times using sleep logs on days when the accelerometer was worn. Missing sleep time was imputed using the participants average sleep time, if available, or the OPACH overall average otherwise. These sleep logs were used to remove periods of accelerometer wear while in bed so that only movement during out-of-bed awake wear time was used. Accelerometer data was processed using the protocol determined in the OPACH calibration study (Evenson et al., 2015) and a vector magnitude cut point of > 518 counts/15-seconds was applied for determining time spent in MVPA. Daily steps were determined by calculating steps for each 15-second epoch using ActiLife’s proprietary algorithm and dividing the total number of steps by the number of adherent wear days (LaCroix, 2017, Lee et al., 2019). Differences in accelerometer wear time were accounted for using the residuals method (Willett and Stampfer, 1986), which is done by fitting a linear regression model where physical activity was the dependent variable and wear time was the independent variable. The residuals from the model were calculated and then added to the estimated sample mean physical activity measure to obtain the residualized value (Willett and Stampfer, Jul 1986, Healy et al., Mar 2011).
Self-reported physical activity data were based on responses to the WHI physical activity questionnaire (PAQ) (Meyer et al., 2009) and the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire (Stewart et al., 2001). Data from the physical activity compendium (Ainsworth et al., Jan 1993, Ainsworth et al., Aug 2011) was used to assign energy expenditure values to the activities queried on the WHI and CHAMPS questionnaire. Activities of 3.0–5.9 metabolic equivalents (METs) were considered moderate intensity and those ≥ 6.0 METs were considered vigorous intensity. Both the WHI PAQ and the CHAMPS questionnaire asked questions pertaining to frequency (episodes per week) and duration (time per episode) of participation in usual recreational and leisure-time activities. Time per week in MVPA was computed using responses on frequency and duration of physical activities of ≥ 3 METs (LaMonte et al., 2019).
2.2.2. Remaining LE8 components
Information on sleep, smoking status, medications, and diet (Healthy Eating Index-2015 (Beasley et al., 2020) were collected by interview and/or self-administered questionnaires at OPACH baseline. As part of the WHI Long Life Study in-home examination protocol, baseline data collection included an assessment of participant’s height, weight, systolic blood pressure, and diastolic blood pressure. In addition, a fasting blood draw was collected during the participant’s home visit (≤6 months within accelerometer wear). Glucose, non-HDL, and total cholesterol were measured at the University of Minnesota (LaCroix et al., 2017).
2.2.3. Computation of the LE8
A modified version of the original AHA LE8 score was developed for this study (Supplemental Table 2). In brief, classifications for diet, BMI, blood pressure, and blood lipids were treated as per the original definition. Classifications for smoking, sleep, and fasting blood glucose could not be directly matched with the AHA definitions due to unmeasured variables (i.e., years since quitting smoking, HbA1c) or misaligned questionnaire responses (i.e., sleep hours/night). For physical activity, the AHA LE8 definition (Lloyd-Jones et al., 2022) based on a continuous scale was used for the WHI PAQ, CHAMPS questionnaire, and accelerometer measures. However, to assess the robustness of the classification of the accelerometer-measured minutes per week of MVPA, two additional scoring methods (one for minutes per week of MVPA and one for average steps/day) classified by quantiles corresponding to the point values were used (Supplemental Table 2).
Each LE8 component was scored from 0 to 100, with a lower score indicating the least healthy while a higher score indicating the healthiest, in accordance with AHA definitions (Supplemental Table 2) (Lloyd-Jones et al., 2022). The mean LE8 overall score for each participant was calculated by summing the eight LE8 component scores and dividing by eight. For each participant, the LE8 score is calculated five times using five different measures for the physical activity component: (1) the WHI PAQ (LE8WHI); (2) the CHAMPS questionnaire (LE8CHAMPS); (3) accelerometer-measured minutes/week of MVPA (LE8A); (4) accelerometer-measured minutes/week of MVPA (LE8AQ) and (5) average steps/day (LE8STEPS) where point values correspond to sample quantiles instead of the AHA definition (Supplemental Table 2). The following description is how women were scored for LE8AQ and LE8STEPS: women in the > 90th sample percentile of MVPA time or daily steps were assigned a score of 100 points, women in the 80th–<90th sample percentiles were assigned 90 points, and so forth (Supplemental Table 2).
2.3. Outcome ascertainment
The primary outcome for this study is incident clinical CVD events, defined as the first occurrence of a myocardial infarction, stroke, or death attributable to CVD. Incident CVD ascertainment methods are described elsewhere (LaCroix et al., 2017). Each woman returned an annual medical history update, which included reports of newly physician diagnosed disease and hospitalizations. Each reported CVD event was adjudicated by trained study physicians who reviewed relevant medical records to confirm whether the outcome met the strict defining criteria (Supplemental Table 3) (Curb et al., 2003). For the present study, data from incident CVD events occurred between OPACH baseline (2012–2014) and February 19, 2023.
2.4. Covariates
At WHI baseline, information on age, race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Hispanic), and educational attainment (high school or less, some college, college graduate or more) was obtained by questionnaire. Self-reported alcohol consumption (non-drinker,< 1 drink/week, ≥1 drinks/week) was measured by questionnaire nearest to the OPACH baseline. Multimorbidity was quantified as the number of comorbidities (cancer, hip fracture, osteoarthritis, depression, chronic obstructive pulmonary disease, cognitive impairment, and frequent falls) present at OPACH baseline (0, 1, 2, or ≥ 3). Missingness was present for education (n = 23) and alcohol intake (n = 305). Multiple imputation was performed using chained Markov regression equations for five iterations and 100 imputations.
2.5. Statistical analysis
The prevalence of each LE8 metric at baseline across all study participants were calculated. Descriptive baseline characteristics were presented by LE8 quartiles using means and standard deviation (SD) for continuous variables and frequencies (%) for categorical variables. Associations between LE8 score, both as a continuous variable and as quartiles, and incident CVD were investigated using multivariable Cox proportional hazards regression models. Time to event was defined as the number of days from accelerometer-measured physical activity (OPACH baseline) to the date of first incident CVD event, loss to follow-up, date of last obtained annual WHI medical update, death from non-CVD cause, or the end of follow up, whichever came first. Model 1 adjusted for age and race and ethnicity. Model 2 additionally contains potential confounders: education level, alcohol use, and multimorbidity. The proportional hazards assumption was assessed by inspecting plots of Schoenfeld residuals and no major violations were observed. To characterize the dose–response characteristics of the LE8-CVD association and to evaluate the linearity of associations, we modeled LE8 scores and CVD incidence using restricted cubic spline regressions with knots placed at the 10th, 50th, and 90th percentiles and computed a Wald test for the nonlinear spline components. The referent value was placed at LE8 score = 50, which corresponds to “low” cardiovascular health as defined by AHA (Lloyd-Jones et al., 2022). We examined the consistency of associations using analyses stratified across categories of age, race/ethnicity, and education level. Effect modification was explored by running models with cross-product terms between continuous LE8 and stratification variables. In sensitivity analyses, we evaluated the potential for reverse-causation bias by repeating the Cox quartile and continuous models and excluding data from women who had incident CVD within the first two years of follow-up. To examine the influence of excluding 1,716 women with missing LE8 data from the analytic sample, we repeated the primary analysis and included these women after performing multiple imputation for missing LE8 data. All analyses were conducted using R version 4.3.1.
3. Results
In our sample, there were 707 incident CVD events over an average 7.5 years of follow-up. The overall average LE8 scores were LE8WHI = 61.8 (SD = 13.6), LE8CHAMPS = 65.0 (SD = 13.2), LE8A = 68.2 (SD = 11.6), LE8AQ = 63.8 (SD = 12.3), and LE8STEPS = 63.8 (SD = 12.5). All Pearson correlation coefficients comparing the five LE8 measures were ≥ 0.91. Women in quartile 4 (highest) of LE8 were more likely to be White, have higher educational attainment, have ≥ 1 alcoholic drinks/week, and have a lower number of comorbidities than those in quartile 1 (Table 1).
Table 1.
Baseline characteristics overall and by quartile of Life’s Essential 8 score calculated using accelerometer-measured minutes per week of moderate-to-vigorous physical activity in the Women’s Health Initiative Objective Physical Activity and Cardiovascular Health Study in 40 United States clinical sites, 2012–2014.
| Characteristic | Total |
LE8A Score Quartile (Range) |
|||
|---|---|---|---|---|---|
| Q1 (26.3–60.0) | Q2 (60.1–68.8) | Q3 (68.9–76.3) | Q4 (76.4–100) | ||
| N (%) | 4,243 (100) | 1,063 (25.1) | 1,136 (26.8) | 1,001 (23.6) | 1,043 (24.6) |
| LE8A metric, mean (SD) | |||||
| Physical activity | 94.8 (15.8) | 89.2 (23.1) | 94.8 (15.4) | 96.8 (11.0) | 98.4 (6.8) |
| Diet | 39.8 (31.3) | 23.2 (26.4) | 34.5 (29.1) | 44.0 (29.2) | 58.3 (29.6) |
| Smoking | 77.1 (27.1) | 66.1 (28.2) | 73.8 (27.3) | 80.1 (25.7) | 88.9 (21.0) |
| BMI | 66.1 (31.6) | 43.1 (30.6) | 60.8 (30.4) | 73.9 (26.2) | 87.7 (19.1) |
| Lipids | 61.3 (28.1) | 51.6 (29.6) | 59.7 (27.4) | 62.6 (26.7) | 71.5 (25.1) |
| Glucose | 76.4 (32.9) | 55.0 (35.1) | 72.2 (33.8) | 84.4 (27.7) | 95.2 (16.3) |
| Blood pressure | 56.8 (28.9) | 39.9 (25.9) | 52.8 (27.4) | 60.4 (26.9) | 74.8 (23.4) |
| Sleep | 73.8 (30.6) | 56.3 (32.5) | 71.3 (30.8) | 79.8 (27.5) | 88.6 (19.9) |
| Age, years, n (%) | |||||
| 63–69 | 430 (10.1) | 114 (10.7) | 113 (9.9) | 106 (10.6) | 97 (9.3) |
| 70–79 | 1,675 (39.5) | 438 (41.2) | 467 (41.1) | 386 (38.6) | 384 (36.8) |
| 80–89 | 1,963 (46.3) | 470 (44.2) | 509 (44.8) | 468 (46.8) | 516 (49.5) |
| 90+ | 175 (4.1) | 41 (3.9) | 47 (4.1) | 41 (4.1) | 46 (4.4) |
| Mean (SD) | 78.7 (6.6) | 78.3 (6.7) | 78.6 (6.6) | 78.9 (6.6) | 79.2 (6.6) |
| Race and ethnicity, n (%) | |||||
| Non-Hispanic White | 2,206 (52.0) | 505 (47.5) | 573 (50.4) | 530 (52.9) | 598 (57.3) |
| Non-Hispanic Black | 1,285 (30.3) | 399 (37.5) | 362 (31.9) | 279 (27.9) | 245 (23.5) |
| Hispanic | 752 (17.7) | 159 (15.0) | 201 (17.7) | 192 (19.2) | 200 (19.2) |
| Highest education level, n (%) | |||||
| High school or less | 840 (19.9) | 263 (24.9) | 217 (19.3) | 194 (19.5) | 166 (15.9) |
| Some college | 1,625 (38.5) | 424 (40.1) | 491 (43.6) | 366 (36.8) | 344 (33.0) |
| College graduate or more | 1,755 (41.6) | 370 (35.0) | 419 (37.2) | 435 (43.7) | 531 (51.0) |
| Missing, n (%) | 23 (0.5) | 6 (0.6) | 9 (0.8) | 6 (0.6) | 2 (0.2) |
| Alcohol intake, n (%) | |||||
| Non-drinker | 1,424 (36.2) | 409 (43.0) | 387 (36.7) | 307 (32.7) | 321 (32.4) |
| <1 drink/week | 1,344 (34.1) | 331 (34.8) | 364 (34.5) | 311 (33.1) | 338 (34.1) |
| ≥1 drinks/week | 1,170 (29.7) | 212 (22.3) | 303 (28.7) | 322 (34.3) | 333 (33.6) |
| Missing, n (%) | 305 (7.2) | 111 (10.4) | 82 (7.2) | 61 (6.1) | 51 (4.9) |
| Multimorbiditya, n (%) | |||||
| 0 | 1,283 (30.2) | 274 (25.8) | 332 (29.2) | 298 (29.8) | 379 (36.3) |
| 1 | 1,969 (46.4) | 491 (46.2) | 519 (45.7) | 482 (48.2) | 477 (45.7) |
| 2 | 779 (18.4) | 225 (21.2) | 227 (20.0) | 176 (17.6) | 151 (14.5) |
| ≥3 | 212 (5.0) | 73 (6.9) | 58 (5.1) | 45 (4.5) | 36 (3.5) |
| Accelerometer awake wear, mean (SD) | |||||
| Hours/day | 14.9 (1.3) | 14.8 (1.4) | 14.8 (1.3) | 15.0 (1.2) | 15.1 (1.1) |
Abbreviations: LE8A, Life’s Essential 8 score calculated using accelerometer-measured minutes per week of moderate-to-vigorous physical activity; SD, standard deviation.
Comorbidities include cancer, hip fracture, osteoarthritis, depression, chronic obstructive pulmonary disease, cognitive impairment, and frequent falls.
The distribution of LE8 physical activity component scores varied considerably by measurement method (Table 2). When using self-report for the physical activity component, 25.1 % of women met criteria for 100 points using the WHI PAQ and 53.4 % when using the CHAMPS questionnaire. When using accelerometer-measured min/week of MVPA, 81.9 % of women met the 100-point criteria. For a score of 0 points, 43.6 % met the criteria when using the WHI physical activity questionnaire, followed by 23.2 % for the CHAMPS questionnaire, and 0 % when using accelerometers.
Table 2.
Prevalence of Life’s Essential 8 physical activity component score by measurement type in the Women’s Health Initiative Objective Physical Activity and Cardiovascular Health Study in 40 United States clinical sites, 2012–2014.
| Points | Definition | WHI PAQ | CHAMPS | Accelerometer MVPA | Accelerometer Quantilesa |
|---|---|---|---|---|---|
| 100 | ≥150 min/week of MVPA | 1,066 (25.1) | 2,265 (53.4) | 3,476 (81.9) | 425 (10.0) |
| 90 | 120–149 | 196 (4.6) | 216 (5.1) | 227 (5.3) | 424 (10.0) |
| 80 | 90–119 | 341 (8.0) | 273 (6.4) | 201 (4.7) | 848 (20.0) |
| 60 | 60–89 | 293 (6.9) | 194 (4.6) | 160 (3.8) | 849 (20.0) |
| 40 | 30–59 | 272 (6.4) | 310 (7.3) | 136 (3.2) | 848 (20.0) |
| 20 | 1–29 | 225 (5.3) | 0 (0.0) | 43 (1.0) | 849 (20.0) |
| 0 | None | 1,850 (43.6) | 985 (23.2) | 0 (0.0) | 0 (0.0) |
Abbreviations: CHAMPS, Community Healthy Activities Model Program for Seniors questionnaire; MVPA, moderate-to-vigorous intensity physical activity; WHI PAQ, Women’s Health Initiative physical activity questionnaire.
Values presented as n (%).
Accelerometer-measured MVPA (min/week) and steps (steps/day) were classified by quantiles corresponding to the point values (e.g., women in the > 90th percentile were assigned a score of 100 points and those in the 80th–<90th percentiles were assigned 90 points).
Regardless of the measurement method used for the physical activity component of the LE8 score, the crude CVD rates were progressively lower with increasing quartiles of LE8 score (Table 3). Compared to women in the lowest quartile of LE8 score, women in the highest quartile had 47 % lower risk for incident CVD when using the LE8WHI (HR = 0.53; 95 % CI = 0.43, 0.67), 53 % lower risk for the LE8CHAMPS (HR = 0.47; 95 % CI = 0.38, 0.60), 56 % lower risk for the LE8A (HR = 0.44; 95 % CI = 0.36, 0.56) and LE8AQ (HR = 0.44; 95 % CI = 0.35, 0.55), and 55 % lower risk for the LE8STEPS (HR = 0.45; 95 % CI = 0.35, 0.57). In confounder adjusted models, a 10-point higher LE8 score at baseline was associated with decreased risks of incident CVD ranging from 17 % for the LE8WHI (HR = 0.83; 95 % CI = 0.79, 0.88) to 22 % for LE8AQ (HR = 0.78; 95 % CI = 0.74, 0.83). Results from sensitivity analyses evaluating reverse causation where data were excluded from 132 women with incident CVD that occurred within the first two years of follow‐up were generally consistent with the main analyses (Supplemental Table 4). The estimates from sensitivity analyses that included women with imputed LE8 data were modestly attenuated compared the primary results, though patterns of associations were similar (Supplemental Table 5).
Table 3.
Associations of Life’s Essential 8 score with incident cardiovascular disease by method of physical activity measurement using multivariable Cox regression models in the Women’s Health Initiative Objective Physical Activity and Cardiovascular Health Study in 40 United States clinical sites, 2012–2014.
|
LE8 Score Quartile |
Continuous LE8 Scorea | ||||
|---|---|---|---|---|---|
| Q1 (Least Healthy) | Q2 | Q3 | Q4 (Healthiest) | ||
| LE8WHI | |||||
| Average MVPA min/week (SD) | 19.9 (62.8) | 57.0 (109.7) | 109.5 (136.5) | 190.6 (171.8) | |
| CVD events [rate]b | 223 [28.6] | 204 [25.8] | 159 [19.4] | 121 [5.1] | – |
| Model 1 HR (95 % CI)c | 1 (REF) | 0.85 (0.70, 1.03) | 0.63 (0.51, 0.77) | 0.49 (0.39, 0.62) | 0.81 (0.77, 0.86) |
| Model 2 HR (95 % CI)c | 1 (REF) | 0.88 (0.73, 1.07) | 0.66 (0.54, 0.81) | 0.53 (0.43, 0.67) | 0.83 (0.79, 0.88) |
| LE8CHAMPS | |||||
| Average MVPA min/week (SD) | 123.8 (279.4) | 262.9 (345.0) | 357.7 (388.8) | 471.8 (446.4) | |
| CVD events [rate]b | 238 [29.9] | 193 [25.1] | 162 [20.2] | 114 [13.8] | – |
| Model 1 HR (95 % CI)c | 1 (REF) | 0.83 (0.68, 1.00) | 0.65 (0.53, 0.80) | 0.44 (0.35, 0.55) | 0.80 (0.76, 0.85) |
| Model 2 HR (95 % CI)c | 1 (REF) | 0.85 (0.70, 1.03) | 0.68 (0.56, 0.84) | 0.47 (0.38, 0.60) | 0.82 (0.77, 0.87) |
| LE8A | |||||
| Average MVPA min/week (SD) | 296.1 (215.9) | 356.9 (223.5) | 391.7 (236.8) | 443.8 (258.8) | |
| CVD events [rate]b | 228 [30.0] | 203 [23.9] | 155 [20.3] | 121 [14.8] | – |
| Model 1 HR (95 % CI)c | 1 (REF) | 0.75 (0.62, 0.91) | 0.61 (0.50, 0.75) | 0.42 (0.33, 0.52) | 0.77 (0.72, 0.82) |
| Model 2 HR (95 % CI)c | 1 (REF) | 0.76 (0.63, 0.92) | 0.64 (0.52, 0.78) | 0.44 (0.36, 0.56) | 0.79 (0.74, 0.84) |
| LE8AQ | |||||
| Average MVPA min/week (SD) | 257.3 (166.0) | 329.9 (221.7) | 392.9 (225.9) | 511.8 (262.6) | |
| CVD events [rate]b | 250 [31.4] | 185 [24.9] | 160 [19.6] | 112 [13.4] | – |
| Model 1 HR (95 % CI)c | 1 (REF) | 0.74 (0.61, 0.89) | 0.58 (0.47, 0.70) | 0.41 (0.33, 0.51) | 0.77 (0.72, 0.82) |
| Model 2 HR (95 % CI)c | 1 (REF) | 0.75 (0.62, 0.91) | 0.60 (0.49, 0.74) | 0.44 (0.35, 0.55) | 0.78 (0.74, 0.83) |
| LE8STEPS | |||||
| Average daily steps (SD) | 2630 (1317) | 3310 (1749) | 4048 (3340) | 5383 (5133) | |
| CVD events [rate]b | 235 [31.1] | 209 [26.8] | 156 [18.2] | 107 [13.4] | – |
| Model 1 HR (95 % CI)c | 1 (REF) | 0.80 (0.66, 0.96) | 0.55 (0.45, 0.67) | 0.42 (0.33, 0.52) | 0.77 (0.72, 0.82) |
| Model 2 HR (95 % CI)c | 1 (REF) | 0.82 (0.68, 0.99) | 0.58 (0.47, 0.71) | 0.45 (0.35, 0.57) | 0.79 (0.74, 0.84) |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; LE8, Life’s Essential 8; LE8A, Life’s Essential 8 score calculated using accelerometer-measured minutes per week of moderate-to-vigorous physical activity; LE8AQ, Life’s Essential 8 score calculated using sample-based quantiles accelerometer-measured minutes per week of moderate-to-vigorous physical activity; LE8CHAMPS, Life’s Essential 8 score calculated using the Community Healthy Activities Model Program for Seniors questionnaire; LE8STEPS, Life’s Essential 8 score calculated using sample-based quantiles accelerometer-measured average steps per day; LE8WHI, Life’s Essential 8 score calculated using the Women’s Health Initiative’s physical activity questionnaire; MVPA, moderate-to-vigorous physical activity; SD, standard deviation.
Data are from models including LE8 score in models in continuous form expressed per 10-point increase in LE8 score.
Crude incidence rate per 1000 person-years.
Model 1 adjusted for age and race and ethnicity. Model 2 included Model 1 and education level, alcohol use, and multimorbidity.
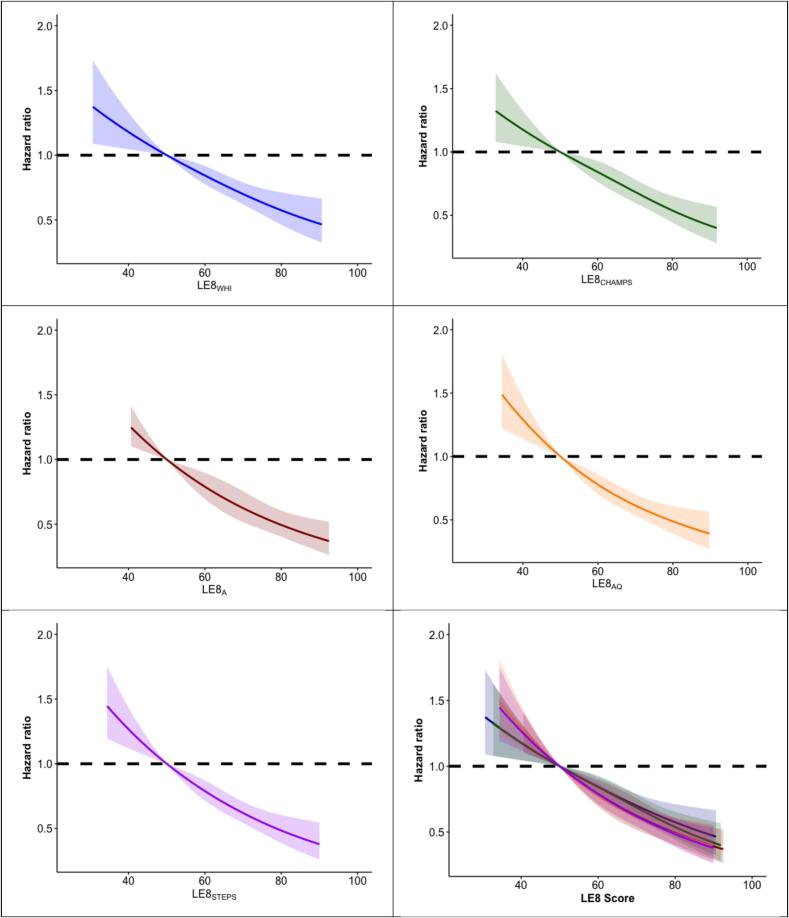
Overall, dose–response trajectories showing higher LE8 scores associated with lower CVD risk in a linear fashion (Fig. 1; p-non-linear tests all > 0.50) consistent in direction with our primary analyses. In stratified analyses, there was no evidence of effect modification by age, race and ethnicity, or education level for associations between all LE8 scores and incident CVD (Table 4; all interaction p-values > 0.05).
Fig. 1.
Dose-response associations of Life’s Essential 8 score and incident cardiovascular disease, by Life’s Essential 8 score separately and combined using restricted cubic spline models in the Women’s Health Initiative Objective Physical Activity and Cardiovascular Health Study in 40 United States clinical sites, 2012–2014. Abbreviations: LE8, Life’s Essential 8; LE8A, Life’s Essential 8 score calculated using accelerometer-measured minutes per week of moderate-to-vigorous physical activity; LE8AQ, Life’s Essential 8 score calculated using sample-based quantiles accelerometer-measured minutes per week of moderate-to-vigorous physical activity; LE8CHAMPS, Life’s Essential 8 score calculated using the Community Healthy Activities Model Program for Seniors questionnaire; LE8STEPS, Life’s Essential 8 score calculated using sample-based quantiles accelerometer-measured average steps per day; LE8WHI, Life’s Essential 8 score calculated using the Women’s Health Initiative’s physical activity questionnaire. Models adjusted for age, race/ethnicity, education, alcohol use, and multimorbidity. Results were trimmed at the 1st and 99th percentiles. Associations were modeled using restricted cubic splines with knots placed at the 10th, 50th, and 90th percentiles, with the referent value placed at a score of 50. All associations were linear only (all p-non-linear > 0.50).
Table 4.
Associations of a 10-point increment in Life’s Essential 8 score with incident cardiovascular disease by physical activity measurement stratified by age, race and ethnicity, and education level using multivariable Cox regression models in the Women’s Health Initiative Objective Physical Activity and Cardiovascular Health Study in 40 United States clinical sites, 2012–2014.
| N | N events [rate]a |
LE8WHI |
LE8CHAMPS |
LE8A |
LE8AQ |
LE8STEPS |
|
|---|---|---|---|---|---|---|---|
| HR (95 % CI)b | HR (95 % CI)b | HR (95 % CI)b | HR (95 % CI)b | HR (95 % CI)b | |||
| Overall | 4,243 | 707 [22.1] | 0.84 (0.79, 0.89) | 0.82 (0.77, 0.87) | 0.79 (0.74, 0.84) | 0.78 (0.74, 0.83) | 0.79 (0.74, 0.84) |
| Age | |||||||
| <80 years | 2,105 | 188 [11.1] | 0.78 (0.70, 0.87) | 0.78 (0.70, 0.87) | 0.72 (0.64, 0.82) | 0.75 (0.66, 0.84) | 0.76 (0.67, 0.85) |
| ≥80 years | 2,138 | 519 [34.8] | 0.85 (0.80, 0.91) | 0.83 (0.78, 0.89) | 0.81 (0.75, 0.88) | 0.80 (0.74, 0.86) | 0.80 (0.74, 0.86) |
| P-valuec | − | − | 0.09 | 0.25 | 0.07 | 0.26 | 0.25 |
| Race and ethnicity | |||||||
| Non-Hispanic White | 2,206 | 488 [30.9] | 0.83 (0.77, 0.89) | 0.82 (0.76, 0.88) | 0.79 (0.73, 0.85) | 0.79 (0.73, 0.85) | 0.79 (0.73, 0.85) |
| Non-Hispanic Black | 1,285 | 146 [14.5] | 0.81 (0.71, 0.92) | 0.79 (0.70, 0.89) | 0.75 (0.65, 0.86) | 0.73 (0.63, 0.84) | 0.75 (0.65, 0.86) |
| Hispanic | 752 | 73 [12.0] | 0.88 (0.73, 1.04) | 0.86 (0.71, 1.03) | 0.83 (0.67, 1.02) | 0.84 (0.69, 1.03) | 0.84 (0.69, 1.03) |
| P-valuec | − | − | 0.35 | 0.34 | 0.34 | 0.21 | 0.34 |
| Highest education level | |||||||
| High school or less | 840 | 149 [23.9] | 0.84 (0.74, 0.96) | 0.86 (0.75, 0.98) | 0.80 (0.69, 0.93) | 0.81 (0.70, 0.93) | 0.80 (0.69, 0.92) |
| Some college | 1,625 | 293 [24.0] | 0.79 (0.73, 0.87) | 0.79 (0.72, 0.86) | 0.74 (0.66, 0.82) | 0.74 (0.67, 0.81) | 0.74 (0.67, 0.82) |
| College graduate | 1,755 | 260 [19.5] | 0.84 (0.78, 0.94) | 0.86 (0.75, 0.91) | 0.80 (0.74, 0.92) | 0.81 (0.73, 0.89) | 0.80 (0.74, 0.90) |
| P-valuec | − | − | 0.46 | 0.51 | 0.22 | 0.29 | 0.33 |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; LE8A, Life’s Essential 8 score calculated using accelerometer-measured minutes per week of moderate-to-vigorous physical activity; LE8AQ, Life’s Essential 8 score calculated using sample-based quantiles accelerometer-measured minutes per week of moderate-to-vigorous physical activity; LE8CHAMPS, Life’s Essential 8 score calculated using the Community Healthy Activities Model Program for Seniors questionnaire; LE8STEPS, Life’s Essential 8 score calculated using sample-based quantiles accelerometer-measured average steps per day; LE8WHI, Life’s Essential 8 score calculated using the Women’s Health Initiative’s physical activity questionnaire.
Crude rate per 1,000 person-years.
Models adjusted for age, race/ethnicity, education level, alcohol use, and multimorbidity. Variables were not included in the model when stratifying by the variable.
P-value from for the cross‐product interaction terms between continuous LE8 score and stratification variables.
4. Discussion
Compared with women in the lowest quartile of LE8 score, women in the highest quartile had a 47 % to 56 % lower risk of incident CVD depending on physical activity measurement. Regardless of how physical activity was measured, we observed significant inverse linear dose–response associations between LE8 scores and incident CVD indicating that higher LE8 scores predicted lower risk of CVD. The associations between LE8 and CVD were robust to sensitivity analyses and associations did not differ by stratification variables. In general, point estimates from accelerometer measures were more protective (i.e., lower) compared to self-report, however all methods of scoring physical activity had strong associations with CVD.
Our study builds upon previous work examining the association of ideal cardiovascular health and incident CVD in WHI. In 2016, Foraker et al. found that women with the lowest LS7 scores (i.e., 0–1 ideal metrics) had a nearly seven-fold higher risk of incident CVD (HR = 6.83, 95 % CI = 5.83, 8.00) than women with the highest LS7 scores (i.e., 6–7 ideal metrics) (Foraker et al., 2016). Several other studies have reported strong, inverse associations between ideal cardiovascular health and CVD risk. In a meta-analysis of nine prospective cohort studies, having ideal LS7 metrics (generally ≥ 5 versus 0 to 2) was associated with relative risk for CVD of 0.20 (95 % CI: 0.11–0.37) (Fang et al., 2016). In terms of LE8, a recent study using UK Biobank data reported participants (mean age = 55.9 years) in the highest quartile of LE8 had a 52 % reduced risk of incident CVD compared to those in the lowest quartile, which is similar to our findings (Petermann-Rocha et al., 2023). Another recent study using data from the Framingham Heart Study measured the LE8 at two different examination periods and reported participants who maintained a high LE8 score were at the lowest risk of CVD, compared with other groups (Rempakos et al., 2023). Two studies using a Chinese population-based cohort found that high LE8 scores were significantly associated with 4.8 additional adjusted years without incident CVD (Xia et al., 2023) and high LE8 score was associated with decreased 10 year and lifetime risk of atherosclerotic CVD (Jin et al., 2023).
Our study is novel in examining how physical activity measurement by various self-reported and accelerometer-based methods influences the association between cardiovascular health metrics and incident CVD. In each study, including the previous WHI analysis, physical activity was assessed using only self-reported measures. Given that there is no guidance for incorporating device-based physical activity measures into the LE8, we elected to apply several different scoring methods. As shown in Table 2, the different physical activity measurement methods used for the LE8 resulted in varying distributions of points for the physical activity component. Despite this, estimates for the association between LE8 scores calculated using accelerometry (i.e., LE8A, LE8AQ, LE8STEPS) and incident CVD were similar. While the score distribution for the physical activity component of the LE8A was heavily skewed (e.g., 81.9 % of the sample scored 100 points), estimates for the association with CVD were comparable to those from the quantile-based methods used for LE8AQ, which suggests the AHA’s scoring method for the physical activity component using minutes/week of MVPA is robust for classifying CVD risk. In addition, our results also indicate that using daily steps as a measure of physical activity for the LE8 is a conceivable alternative to using minutes/week of MVPA as estimates of associations with CVD were similar for LE8STEPS and LE8A/LE8AQ. Given these findings, the ubiquity of devices for measuring physical activity, and the interest in step counting as a simple and accessible means of tracking physical activity specifically (Bassett et al., 2017, Kraus et al., Jun 2019), complementing LE8 self-reported physical activity criteria with device-based guidance such as daily step targets may increase the effectiveness and uptake of the LE8 from both the public health and clinical application perspectives.
For self-reported measures, the distribution of the physical activity component scores for LE8WHI and LE8CHAMPS differed. Compared to women in the lowest quartile, women in the highest quartile of LE8WHI had 47 % lower risk for incident CVD compared to 53 % lower risk when using LE8CHAMPS. A possible explanation for the different associations and score distributions is the way in which physical activity participation was queried. The WHI PAQ asks participations to report frequency and duration of participation in moderate or strenuous exercise whereas the CHAMPS has respondents report time spent in 35 specific activities that are commonly performed by older adults. The detailed, age-relevant CHAMPS questionnaire may have enabled improved recall of physical activity participation among the older women in our sample, leading to more accurate classification of CVD risk. When device-based measures of physical activity aren’t practical or available, using questionnaires that are tailored to the population of interest (e.g., CHAMPS and older women) may more accurately reflect cardiovascular health than questionnaires that are not as targeted.
Our study is the first to calculate LE8 scores using a variety of physical activity measures and subsequently assess their prospective associations with incident CVD in a cohort of older women. Despite the distributions of the LE8 physical activity component scores varying considerably across all LE8 measures, the associations of each LE8 score with incident CVD displayed similar patterns, thus demonstrating the robustness of the LE8 in quantifying cardiovascular health. The present study has several strengths, starting with the large racially and ethnically diverse study population of older women, an understudied population. In OPACH, 94 % met the criteria for adherent accelerometer wear. We applied calibrated accelerometer vector magnitude cutpoints to classify MVPA. We note several limitations to our study. Over 20 % of the original OPACH sample was excluded from our analysis for missing LE8 data, and participants who were excluded were less healthy in general than the analytic sample. However, a previous NHANES study that included participants with a wider range of health status reported similar LE8 score distributions (Lloyd-Jones et al., 2022). It is highly likely that applying the LE8 to other external cohorts will involve similar challenges. In addition, we imputed missing LE8 data and found comparable results (Supplemental Table 5). Next, we used a modified version of AHA’s scoring criteria for the LE8 due to data availability and a lack of widely accepted guidance for scoring accelerometer-measured physical activity, though associations between each LE8 score and incident CVD in our study are similar to other published estimates (Petermann-Rocha et al., Apr 2023, Xia et al., 2023, Jin et al., Jun 2023, Li et al., Jul 2023). Dietary self-report data used in this study may be systematically biased (Neuhouser et al., 2008, Prentice et al., 2011, Lampe et al., Feb 2017, Freedman et al., Nov 2015, Freedman et al., 2014, Freedman et al., 2015). Not all studies may have detailed physical activity assessment measures such as the WHI PAQ and CHAMPS and results may not be directly transferrable, nonetheless compatibility of results between this study’s self-report and accelerometer measured physical activity are encouraging. Lastly, our findings should be replicated among men and younger populations to establish generalizability.
5. Conclusion
Our study showed that among older women, higher LE8 scores calculated using either self-reported or accelerometer measures of physical activity were strongly associated with lower CVD incidence. Using device-based measures of physical activity like daily steps may be increasingly feasible for practitioners to implement. Updating AHA guidance to incorporate these types of measures may improve the practical utility of the LE8.
CRediT authorship contribution statement
Eric T. Hyde: Writing – original draft, Writing – review & editing, Methodology, Software, Visualization, Formal analysis, Data curation, Supervision. Steve Nguyen: Writing – review & editing, Visualization, Validation, Software, Formal analysis. Michael J. LaMonte: Writing – review & editing, Supervision, Methodology. Chongzhi Di: Writing – review & editing, Formal analysis. John Bellettiere: Writing – review & editing, Supervision, Methodology, Formal analysis, Conceptualization. Lesley F. Tinker: Writing – review & editing, Data curation. Randi E. Foraker: Writing – review & editing, Methodology. Hilary A. Tindle: Writing – review & editing, Methodology. Marcia L. Stefanick: Writing – review & editing, Supervision. Andrea Z. LaCroix: Writing – review & editing, Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the WHI participants, staff, and investigators. The short list of WHI investigators can be found at: https://www-whi-org.s3.us-west-2.amazonaws.com/wp-content/uploads/WHI-Investigator-Short-List.pdf. The full list of WHI Investigators can be found at the following site: http://www.whi.org/researchers/Documents%2520%2520Write%2520a%2520Paper/WHI%2520Investigator%2520Long%2520List.pdf.
Sources of Funding
This work was supported by the National Institute on Aging (P01 AG052352), National Heart, Lung, and Blood Institute (HL105065, HL153462, HL151885, HL150170, HL130591, T32HL079891, 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005, R01HL105065), the National Cancer Institute (1R01CA226078), and the Tobacco-Related Disease Research Program (T31KT1501).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2024.102904.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Ainsworth B.E., Haskell W.L., Leon A.S., et al. Compendium of physical activities: classification of energy costs of human physical activities. Med. Sci. Sports Exerc. Jan 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- Ainsworth B.E., Haskell W.L., Herrmann S.D., et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med. Sci. Sports Exerc. Aug 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- Aneni E.C., Crippa A., Osondu C.U., et al. Estimates of Mortality Benefit From Ideal Cardiovascular Health Metrics: A Dose Response Meta-Analysis. J. Am. Heart Assoc. 2017;6(12):e006904. doi: 10.1161/JAHA.117.006904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D.R., Toth L.P., LaMunion S.R., Crouter S.E. Step Counting: A Review of Measurement Considerations and Health-Related Applications. Sports Med. 2017;47(7):1303–1315. doi: 10.1007/s40279-016-0663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley J.M., Rillamas-Sun E., Tinker L.F., et al. Dietary Intakes of Women's Health Initiative Long Life Study Participants Falls Short of the Dietary Reference Intakes. J. Acad. Nutr. Diet. Sep 2020;120(9):1530–1537. doi: 10.1016/j.jand.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi L., Liu Z., Matthews C.E., Buchowski M.S. Validation of Accelerometer Wear and Nonwear Time Classification Algorithm. Med. Sci. Sports Exerc. 2011;43(2):357–364. doi: 10.1249/MSS.0b013e3181ed61a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curb J.D., McTiernan A., Heckbert S.R., et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann. Epidemiol. Oct 2003;13(9 Suppl):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- Evenson K.R., Wen F., Herring A.H., et al. Calibrating physical activity intensity for hip-worn accelerometry in women age 60 to 91 years: The Women's Health Initiative OPACH Calibration Study. Prev. Med. Rep. 2015;2:750–756. doi: 10.1016/j.pmedr.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang N., Jiang M., Fan Y. Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: A meta-analysis. Int. J. Cardiol. 2016;214:279–283. doi: 10.1016/j.ijcard.2016.03.210. [DOI] [PubMed] [Google Scholar]
- Foraker R.E., Abdel-Rasoul M., Kuller L.H., et al. Cardiovascular Health and Incident Cardiovascular Disease and Cancer: The Women's Health Initiative. Am. J. Prev. Med. Feb 2016;50(2):236–240. doi: 10.1016/j.amepre.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L.S., Commins J.M., Moler J.E., et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am. J. Epidemiol. 2014;180(2):172–188. doi: 10.1093/aje/kwu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L.S., Commins J.M., Moler J.E., et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for potassium and sodium intake. Am. J. Epidemiol. 2015;181(7):473–487. doi: 10.1093/aje/kwu325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L.S., Midthune D., Carroll R.J., et al. Application of a New Statistical Model for Measurement Error to the Evaluation of Dietary Self-report Instruments. Epidemiology. Nov 2015;26(6):925–933. doi: 10.1097/ede.0000000000000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: A meta-analysis of prospective studies. Clin. Cardiol. 2017;40(12):1339–1346. doi: 10.1002/clc.22836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy G.N., Matthews C.E., Dunstan D.W., Winkler E.A., Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur. Heart J. Mar 2011;32(5):590–597. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Li J., Liu F., et al. Life's Essential 8 and 10-Year and Lifetime Risk of Atherosclerotic Cardiovascular Disease in China. Am. J. Prev. Med. Jun 2023;64(6):927–935. doi: 10.1016/j.amepre.2023.01.009. [DOI] [PubMed] [Google Scholar]
- Kraus W.E., Janz K.F., Powell K.E., et al. Daily Step Counts for Measuring Physical Activity Exposure and Its Relation to Health. Med. Sci. Sports Exerc. Jun 2019;51(6):1206–1212. doi: 10.1249/mss.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCroix AZ, Rillamas-Sun E, Buchner D, et al. The Objective Physical Activity and Cardiovascular Disease Health in Older Women (OPACH) Study. BMC Public Health. Feb 14 2017;17(1):192. doi:10.1186/s12889-017-4065-6. [DOI] [PMC free article] [PubMed]
- LaMonte M.J., Lee I.-M., Rillamas-Sun E., et al. Comparison of Questionnaire and Device Measures of Physical Activity and Sedentary Behavior in a Multi-Ethnic Cohort of Older Women. J. Meas. Phys. Behav. 2019;2(2):82. doi: 10.1123/jmpb.2018-0057. [DOI] [Google Scholar]
- LaMonte MJ, Lewis CE, Buchner DM, et al. Both Light Intensity and Moderate-to-Vigorous Physical Activity Measured by Accelerometry Are Favorably Associated With Cardiometabolic Risk Factors in Older Women: The Objective Physical Activity and Cardiovascular Health (OPACH) Study. J Am Heart Assoc. Oct 17 2017;6(10)doi:10.1161/jaha.117.007064. [DOI] [PMC free article] [PubMed]
- Lampe J.W., Huang Y., Neuhouser M.L., et al. Dietary biomarker evaluation in a controlled feeding study in women from the Women's Health Initiative cohort. Am. J. Clin. Nutr. Feb 2017;105(2):466–475. doi: 10.3945/ajcn.116.144840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.M., Shiroma E.J. Using accelerometers to measure physical activity in large-scale epidemiological studies: issues and challenges. Br. J. Sports Med. Feb 2014;48(3):197–201. doi: 10.1136/bjsports-2013-093154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.-M., Shiroma E.J., Kamada M., Bassett D.R., Matthews C.E., Buring J.E. Association of Step Volume and Intensity With All-Cause Mortality in Older Women. JAMA Intern. Med. 2019;179(8):1105–1112. doi: 10.1001/jamainternmed.2019.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Ma H., Wang X., Feng H., Qi L. Life's Essential 8, Genetic Susceptibility, and Incident Cardiovascular Disease: A Prospective Study. Arterioscler. Thromb. Vasc. Biol. Jul 2023;43(7):1324–1333. doi: 10.1161/atvbaha.123.319290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D.M., Hong Y., Labarthe D., et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D.M., Allen N.B., Anderson C.A.M., et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation. 2022;146(5):e18–e43. doi: 10.1161/CIR.0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D.M., Ning H., Labarthe D., et al. Status of Cardiovascular Health in US Adults and Children Using the American Heart Association's New “Life's Essential 8” Metrics: Prevalence Estimates From the National Health and Nutrition Examination Survey (NHANES), 2013 Through 2018. Circulation. 2022;146(11):822–835. doi: 10.1161/circulationaha.122.060911. [DOI] [PubMed] [Google Scholar]
- Meyer A.-M., Evenson K.R., Morimoto L., Siscovick D., White E. Test-Retest Reliability of the Women's Health Initiative Physical Activity Questionnaire. Med. Sci. Sports Exerc. 2009;41(3):530–538. doi: 10.1249/MSS.0b013e31818ace55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhouser M.L., Tinker L., Shaw P.A., et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women's Health Initiative. Am. J. Epidemiol. 2008;167(10):1247–1259. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
- Petermann-Rocha F., Deo S., Celis-Morales C., et al. An Opportunity for Prevention: Associations Between the Life's Essential 8 Score and Cardiovascular Incidence Using Prospective Data from UK Biobank. Curr. Probl. Cardiol. Apr 2023;48(4) doi: 10.1016/j.cpcardiol.2022.101540. [DOI] [PubMed] [Google Scholar]
- Prentice R.L., Mossavar-Rahmani Y., Huang Y., et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am. J. Epidemiol. 2011;174(5):591–603. doi: 10.1093/aje/kwr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempakos A., Prescott B., Mitchell G.F., Vasan R.S., Xanthakis V. Association of Life's Essential 8 With Cardiovascular Disease and Mortality: The Framingham Heart Study. J. Am. Heart Assoc. 2023;12(23):e030764. doi: 10.1161/JAHA.123.030764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A.L., Mills K.M., King A.C., Haskell W.L., Gillis D., Ritter P.L. CHAMPS Physical Activity Questionnaire for Older Adults: outcomes for interventions. Med. Sci. Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart Disease and Stroke Statistics—2023 Update: A Report From the American Heart Association. Circulation. 2023;147(8):e93–e621. doi: 10.1161/CIR.0000000000001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. Washington, DC: U.S. Department of Health and Human Services; 2018.
- Wijndaele K., Westgate K., Stephens S.K., et al. Utilization and Harmonization of Adult Accelerometry Data: Review and Expert Consensus. Med. Sci. Sports Exerc. Oct 2015;47(10):2129–2139. doi: 10.1249/mss.0000000000000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett W., Stampfer M.J. Total energy intake: implications for epidemiologic analyses. Am. J. Epidemiol. Jul 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- Xia X., Chen S., Tian X., et al. Association of Cardiovascular Health Assessed by the New Life's Essential 8 Metrics With Years Lived Without Cardiovascular Disease. J. Am. Heart Assoc. 2023;12(11) doi: 10.1161/jaha.122.029241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.