Abstract
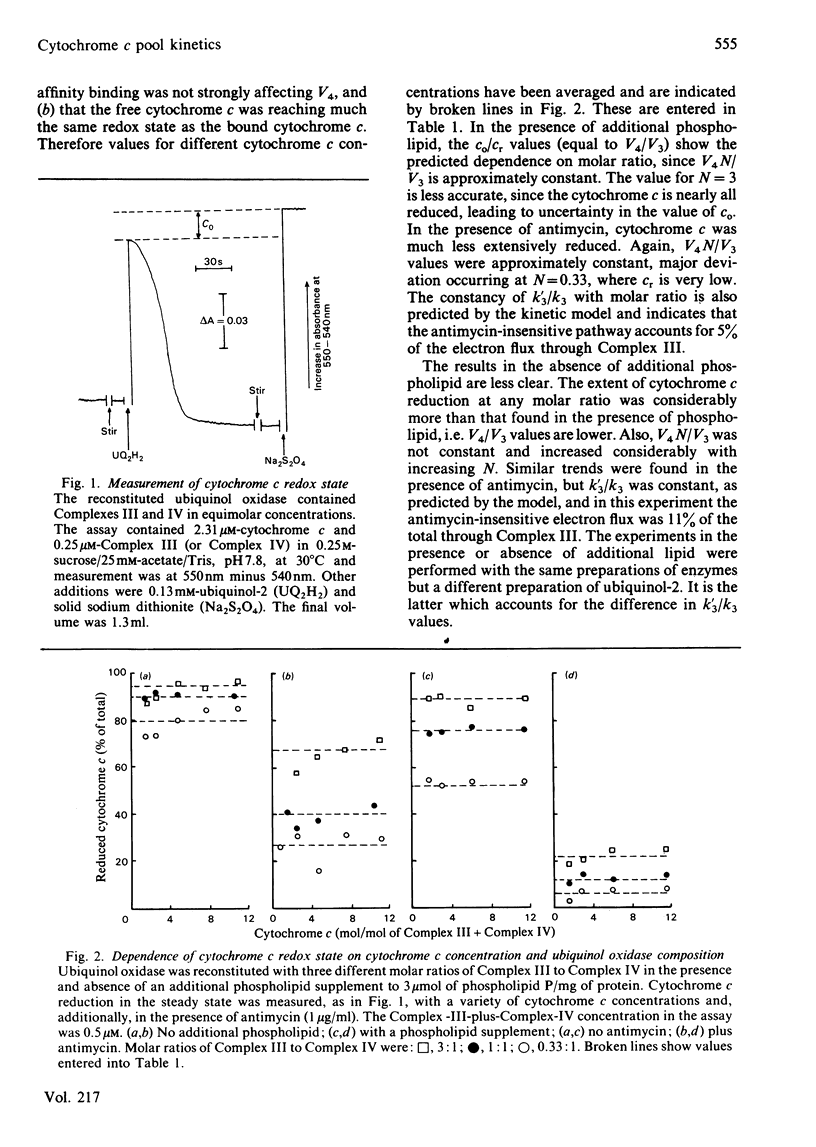
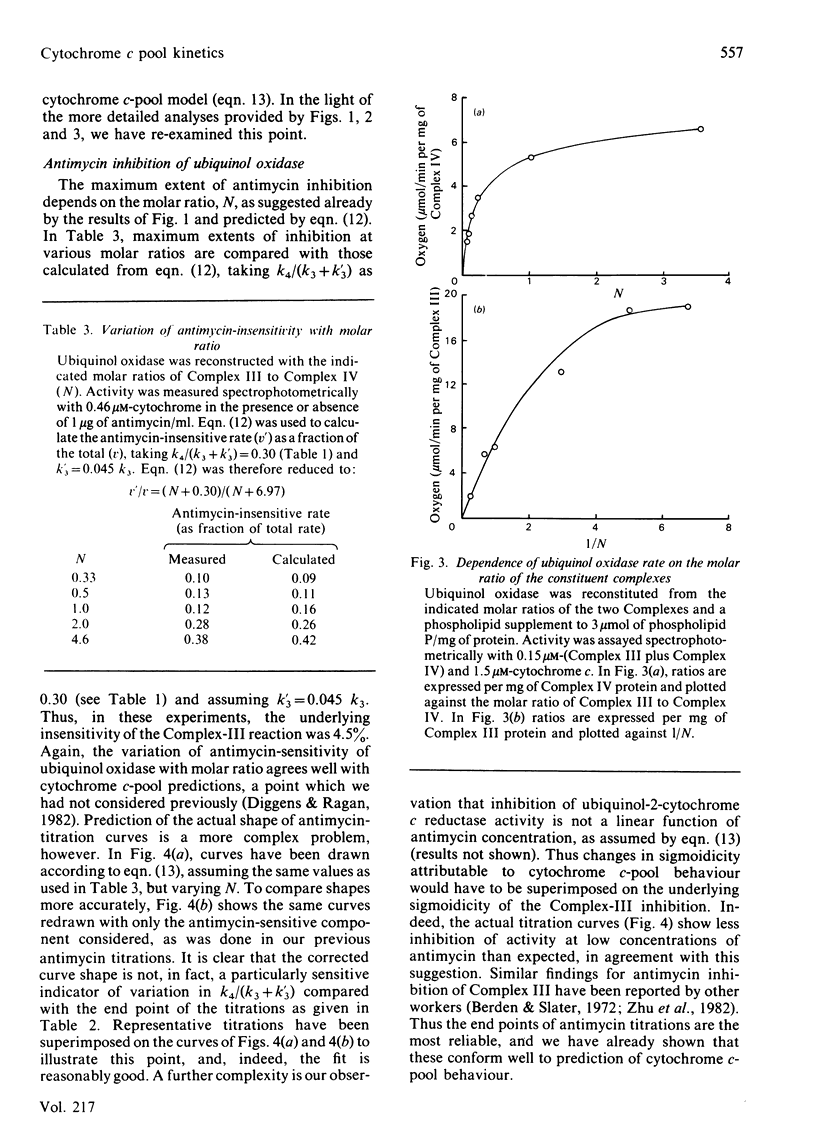
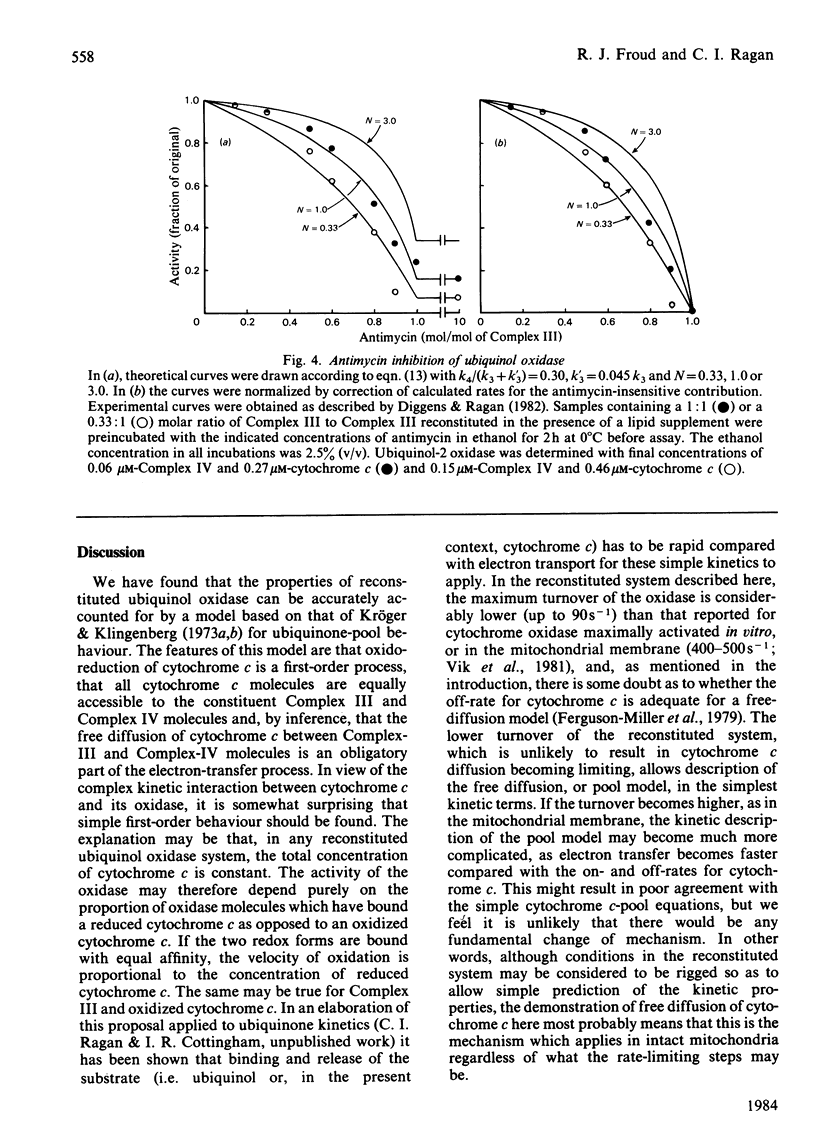
Ubiquinol oxidase has been reconstituted from ubiquinol-cytochrome c reductase (Complex III), cytochrome c and cytochrome c oxidase (Complex IV). The steady-state level of reduction of cytochrome c by ubiquinol-2 varies with the molar ratios of the complexes and with the presence of antimycin in a way that can be quantitatively accounted for by a model in which cytochrome c acts as a freely diffusible pool on the membrane. This model was based on that of Kröger & Klingenberg [(1973) Eur. J. Biochem. 34, 358-368] for ubiquinone-pool behaviour. Further confirmation of the pool model was provided by analysis of ubiquinol oxidase activity as a function of the molar ratio of the complexes and prediction of the degree of inhibition by antimycin.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berden J. A., Slater E. C. The allosteric binding of antimycin to cytochrome b in the mitochondrial membrane. Biochim Biophys Acta. 1972 Feb 28;256(2):199–215. doi: 10.1016/0005-2728(72)90053-9. [DOI] [PubMed] [Google Scholar]
- Bernardi P., Azzone G. F. Cytochrome c as an electron shuttle between the outer and inner mitochondrial membranes. J Biol Chem. 1981 Jul 25;256(14):7187–7192. [PubMed] [Google Scholar]
- Capaldi R. A., Darley-Usmar V., Fuller S., Millett F. Structural and functional features of the interaction of cytochrome c with complex III and cytochrome c oxidase. FEBS Lett. 1982 Feb 8;138(1):1–7. doi: 10.1016/0014-5793(82)80382-7. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Hayashi H. The polypeptide composition of cytochrome oxidase from beef heart mitochondria. FEBS Lett. 1972 Oct 1;26(1):261–263. doi: 10.1016/0014-5793(72)80587-8. [DOI] [PubMed] [Google Scholar]
- Diggens R. J., Ragan C. I. Properties of ubiquinol oxidase reconstituted from ubiquinol-cytochrome c reductase, cytochrome c and cytochrome c oxidase. Biochem J. 1982 Feb 15;202(2):527–534. doi: 10.1042/bj2020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit B. P., Waring A. J., Wells K. O., 3rd, Wong P. S., Woodrow G. V., 3rd, Vanderkooi J. M. Rotational motion of cytochrome c derivatives bound to membranes measured by fluorescence and phosphorescence anisotropy. Eur J Biochem. 1982 Aug;126(1):1–9. doi: 10.1111/j.1432-1033.1982.tb06737.x. [DOI] [PubMed] [Google Scholar]
- Errede B., Kamen M. D. Comparative kinetic studies of cytochromes c in reactions with mitochondrial cytochrome c oxidase and reductase. Biochemistry. 1978 Mar 21;17(6):1015–1027. doi: 10.1021/bi00599a012. [DOI] [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Correlation of the kinetics of electron transfer activity of various eukaryotic cytochromes c with binding to mitochondrial cytochrome c oxidase. J Biol Chem. 1976 Feb 25;251(4):1104–1115. [PubMed] [Google Scholar]
- Froud R. J., Ragan C. I. Cytochrome c mediates electron transfer between ubiquinol-cytochrome c reductase and cytochrome c oxidase by free diffusion along the surface of the membrane. Biochem J. 1984 Jan 15;217(2):561–571. doi: 10.1042/bj2170561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron C., Ragan C. I., Trumpower B. L. The interaction between mitochondrial NADH-ubiquinone oxidoreductase and ubiquinol-cytochrome c oxidoreductase. Restoration of ubiquinone-pool behaviour. Biochem J. 1978 Sep 15;174(3):791–800. doi: 10.1042/bj1740791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höchli M., Hackenbrock C. R. Lateral translational diffusion of cytochrome c oxidase in the mitochondrial energy-transducing membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1236–1240. doi: 10.1073/pnas.76.3.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato S., Sigel E., Carafoli E., Cherry R. J. Rotation of cytochrome oxidase in phospholipid vesicles. Investigations of interactions between cytochrome oxidases and between cytochrome oxidase and cytochrome bc1 complex. J Biol Chem. 1981 Jul 25;256(14):7518–7527. [PubMed] [Google Scholar]
- King T. E. Cytochrome c1 from mammalian heart. Methods Enzymol. 1978;53:181–191. doi: 10.1016/s0076-6879(78)53023-1. [DOI] [PubMed] [Google Scholar]
- Koppenol W. H., Margoliash E. The asymmetric distribution of charges on the surface of horse cytochrome c. Functional implications. J Biol Chem. 1982 Apr 25;257(8):4426–4437. [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. Further evidence for the pool function of ubiquinone as derived from the inhibition of the electron transport by antimycin. Eur J Biochem. 1973 Nov 15;39(2):313–323. doi: 10.1111/j.1432-1033.1973.tb03129.x. [DOI] [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur J Biochem. 1973 Apr;34(2):358–368. doi: 10.1111/j.1432-1033.1973.tb02767.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ludwig B. Heme aa3-type cytochrome c oxidases from bacteria. Biochim Biophys Acta. 1980 Dec;594(2-3):177–189. doi: 10.1016/0304-4173(80)90008-7. [DOI] [PubMed] [Google Scholar]
- Poore V. M., Fitzsimons J. T., Ragan C. I. The effects of lipid fluidity on the rotational diffusion of complex I and complex III in reconstituted NADH-cytochrome c oxidoreductase. Biochim Biophys Acta. 1982 Dec 8;693(1):113–124. doi: 10.1016/0005-2736(82)90477-1. [DOI] [PubMed] [Google Scholar]
- Ragan C. I., Heron C. The interaction between mitochondrial NADH-ubiquinone oxidoreductase and ubiquinol-cytochrome c oxidoreductase. Evidence for stoicheiometric association. Biochem J. 1978 Sep 15;174(3):783–790. doi: 10.1042/bj1740783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan C. I., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 28. The reconstitution of the first site of energy conservation. J Biol Chem. 1973 Apr 10;248(7):2563–2569. [PubMed] [Google Scholar]
- Rich P. R. A generalised model for the equilibration of quinone pools with their biological donors and acceptors in membrane-bound electron transfer chains. FEBS Lett. 1981 Aug 3;130(2):173–178. doi: 10.1016/0014-5793(81)81113-1. [DOI] [PubMed] [Google Scholar]
- Rieder R., Bosshard H. R. Comparison of the binding sites on cytochrome c for cytochrome c oxidase, cytochrome bc1, and cytochrome c1. Differential acetylation of lysyl residues in free and complexed cytochrome c. J Biol Chem. 1980 May 25;255(10):4732–4739. [PubMed] [Google Scholar]
- Smith L., Davies H. C., Nava M. E. Studies of the kinetics of oxidation of cytochrome c by cytochrome c oxidase: comparison of spectrophotometric and polarographic assays. Biochemistry. 1979 Jul 10;18(14):3140–3146. doi: 10.1021/bi00581a035. [DOI] [PubMed] [Google Scholar]
- Smith L., Davies H. C., Nava M. Oxidation and reduction of soluble cytochrome c by membrane-bound oxidase and reductase systems. J Biol Chem. 1974 May 10;249(9):2904–2910. [PubMed] [Google Scholar]
- Speck S. H., Ferguson-Miller S., Osheroff N., Margoliash E. Definition of cytochrome c binding domains by chemical modification: kinetics of reaction with beef mitochondrial reductase and functional organization of the respiratory chain. Proc Natl Acad Sci U S A. 1979 Jan;76(1):155–159. doi: 10.1073/pnas.76.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vik S. B., Georgevich G., Capaldi R. A. Diphosphatidylglycerol is required for optimal activity of beef heart cytochrome c oxidase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1456–1460. doi: 10.1073/pnas.78.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms J., Veerman E. C., König B. W., Dekker H. L., van Gelder B. F. Ionic strength effects on cytochrome aa3 kinetics. Biochim Biophys Acta. 1981 Mar 12;635(1):13–24. doi: 10.1016/0005-2728(81)90003-7. [DOI] [PubMed] [Google Scholar]
- Yu C. A., Yu L., King T. E. Kinetics of electron transfer between cardiac cytochrome c 1 and c. J Biol Chem. 1973 Jan 25;248(2):528–533. [PubMed] [Google Scholar]
- Zhu Q. S., Berden J. A., De Vries S., Slater E. C. On the role of ubiquinone in the respiratory chain. Biochim Biophys Acta. 1982 Apr 19;680(1):69–79. doi: 10.1016/0005-2728(82)90317-6. [DOI] [PubMed] [Google Scholar]


