Abstract
Cancer cells maintain high levels of reactive oxygen species (ROS) to drive their growth, but ROS can trigger cell death through oxidative stress and DNA damage. To survive enhanced ROS levels, cancer cells activate their antioxidant defenses. One such defense is MTH1, an enzyme that prevents the incorporation of oxidized nucleotides into DNA, thus preventing DNA damage and allowing cancer to proliferate. MTH1 levels are often elevated in many cancers, and thus, inhibiting MTH1 is an attractive strategy for suppressing tumor growth and metastasis. Targeted MTH1 inhibition can induce DNA damage in cancer cells, exploiting their vulnerability to oxidative stress and selectively targeting them for destruction. Targeting MTH1 is promising for cancer treatment because normal cells have lower ROS levels and are less dependent on these pathways, making the approach both effective and specific to cancer. This review aims to investigate the potential of MTH1 as a therapeutic target, especially in cancer treatment, offering detailed insights into its structure, function, and role in disease progression. We also discussed various MTH1 inhibitors that have been developed to selectively induce oxidative damage in cancer cells, though their effectiveness varies. In addition, this review provide deeper mechanistic insights into the role of MTH1 in cancer prevention and oxidative stress management in various diseases.
Keywords: MTH1 enzyme, Therapeutic target, Oxidative stress, Reactive oxygen species, MTH1 inhibitors, Cancer therapy
Graphical abstract
Highlights
-
•
MTH1 is an attractive drug target for cancer therapy and other diseases.
-
•
MTH1 hydrolyzes oxidized dNTPs, preventing their incorporation and allowing cancer cells to proliferate.
-
•
MTH1 functions as a molecular rheostat in oncogenic transformation.
-
•
Inhibitors of MTH1 are assemble for their potential to selectively kill cancer cells by increasing ROS induced DNA damage.
1. Introduction
The human body requires redox homeostasis for vital physiological functioning, and its imbalance is a key contributor to several frequently occurring diseases, including type 2 diabetes, atherosclerosis, chronic obstructive pulmonary disease, Alzheimer's disease (AD), and cancer [1]. A hallmark in the pathogenesis of cancer is oxidative stress, which is favored by redox imbalance. It gives rise to numerous malignant behaviors in cancer cells [2,3].
Reactive oxygen species (ROS) are the main mediators in redox reactions, often considered as by-products of cellular respiration. The reactions involving cyclooxygenase, NADPH oxidase, xanthine oxidase, lipogenesis, and the Fenton reaction, generating hydroxyl radicals, superoxide radicals, and hydrogen peroxide, give rise to ROS [4,5]. Physiologically, ROS in low to moderate concentrations aid in maintaining intracellular activities and signaling pathways. However, elevated levels promote malignant transformation, cellular damage, and even cell death. Excessive accumulation of ROS creates a highly reactive cellular environment, which leads to DNA damage via alkylation, deamination, oxo-group modification, methylation, or halogenation of bases, thereby altering the morphology of bases. This further leads to the improper incorporation of bases into DNA and subsequent DNA damage, thus promoting disease pathology [6].
Redox reactions play key roles in cellular respiration, particularly in ATP production within mitochondria. ROS act as signaling molecules, regulating cellular processes like proliferation, differentiation, and apoptosis. Maintaining redox homeostasis is crucial for normal cellular functions, supported by antioxidant systems (e.g., superoxide dismutase and glutathione peroxidase) that neutralize excess ROS to protect cells from oxidative damage. In immune responses, ROS help kill pathogens, but excessive ROS can cause tissue damage and chronic inflammation. An imbalance in redox homeostasis leads to oxidative stress, characterized by elevated ROS levels, which can cause DNA damage, mutations, and genomic instability, promoting cancer development [7]. Cancer cells exploit this by using high ROS levels for growth and survival. Redox imbalances are also implicated in neurodegenerative diseases like AD and Parkinson's disease (PD), where oxidative stress contributes to neuronal damage and inflammation [8,9]. In cardiovascular diseases, such as atherosclerosis and heart failure, oxidative stress causes endothelial dysfunction and vascular damage [10]. Diabetes is linked to increased ROS, impairing insulin signaling and promoting insulin resistance [11]. Chronic inflammation can sustain oxidative stress, worsening conditions like rheumatoid arthritis and inflammatory bowel disease, and tissue damage [12].
Mammalian cells have enzymes like MutT Homolog1 (MTH1), which play a critical role in maintaining the integrity of DNA by countering such acts of DNA damage. Also, tumor cells rely much on MTH1 to support various biological properties required for their existence. Removal of oxidized nucleotides by MTH1 may relieve the proliferative stress of cancer cells and thereby represent a vulnerability factor and a competent target for anticancer compounds. Increased MTH1 expression has also been correlated with several other diseases like cardiovascular diseases, neurological diseases, skin and immune system diseases, and inflammation-related conditions [13].
Despite the growing interest in MTH1 as a therapeutic target, several gaps persist in our understanding and application of MTH1 inhibitors. Many reported inhibitors exhibit inconsistent efficacy, with some demonstrating significant cytotoxic effects against cancer cells while others fall short, suggesting a lack of comprehensive understanding of the underlying mechanisms of action. Additionally, the molecular dynamics and interactions of these inhibitors with MTH1 have not been fully elucidated, which hampers the rational design of more effective compounds. The variability in responses across different cancer types further complicates the development of universally effective therapies. Moreover, while some studies have highlighted MTH1 role in cellular processes and tumorigenesis, there remains a scarcity of integrated research focusing on its comprehensive biological functions and the consequences of its inhibition in diverse pathological contexts.
This review is aimed to bridge research gaps and to advance the understanding of MTH1 in cancer biology. The potential of MTH1 as a promising drug target, particularly in the context of tumor metabolism and oxidative stress management, which are increasingly recognized as pivotal in cancer progression. By synthesizing existing knowledge and identifying unexplored avenues for research, this review aims to stimulate interest in developing next-generation MTH1 inhibitors that are more effective and selective.
This review is to provide a comprehensive overview of MTH1 molecular structure, biological functions, and its implications in disease and tumor biology, thereby setting a foundation for future research. By consolidating findings from recent advancements in medicinal chemistry and related fields, this review seeks to highlight the therapeutic potential of MTH1 while outlining the challenges that need to be addressed. Furthermore, identifying key areas for further investigation will help guide researchers in the development of more potent and selective MTH1 inhibitors. Ultimately, the goal is to enhance our understanding of MTH1 role in cancer and to catalyze the development of effective therapeutic strategies targeting this enzyme.
2. MTH1 from NUDIX hydrolase superfamily
The ROS generates a highly reactive environment, often altering the cellular pool of normal bases and producing noncanonical versions. Because different DNA polymerase variants cannot differentiate between normal and altered bases, these noncanonical bases can be incorporated into DNA, leading to DNA damage [14]. Sanitization enzymes play a crucial role by hydrolyzing and removing noncanonical dNTPs from the precursor pool, preventing their integration into newly formed DNA strands. They also help maintain a balanced pool of standard dNTPs, as having all four classical base precursors is essential for the proper proofreading function of DNA polymerases. In proliferating cancer cells, which exhibit high ROS levels, sanitization enzymes are overexpressed to meet the significant energy demands required for tumor growth [15]. Targeting these enzymes could increase noncanonical dNTPs, causing DNA damage and cancer cell death.
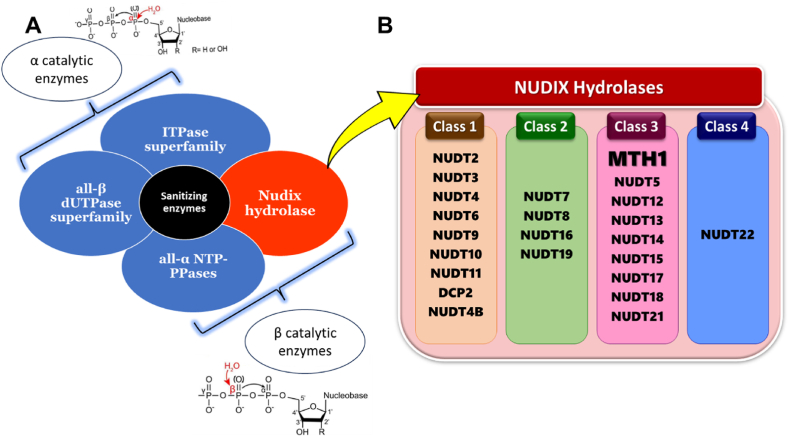
ROS create various noncanonical bases based on their structural diversity, there are different classes of sanitizing enzymes designed to prevent their incorporation into DNA. Specifically, these enzymes belong to four main superfamilies: all-β dUTPases, ITPases, all-α NTP-PPases, and NUDIX hydrolases (Fig. 1). Based on their mechanism of action, the four superfamilies of sanitizing enzymes can be divided into two categories: alpha category which includes all-β dUTPases and ITPases and beta category which includes all-α NTP-PPases and NUDIX hydrolases. The classification is based on the specific phosphorus atom in the noncanonical nucleotide that these enzymes target for nucleophilic attack. This action leads to the breakage of the phosphoanhydride bond in the nucleotide during a process called pyrophosphorolysis [16].
Fig. 1.
A. Protein superfamilies which function either by a nucleophilic attack on α-phosphorus (top) or β-phosphorus (bottom) of noncanonical dNTPs. B. NUDIX family members were categorized into four structural classes based on structure-based sequence alignments and their specific enzymatic activities against diverse substrates.
The all-β dUTPase superfamily forms trimeric structures to prevent the incorporation of dUTPs into DNA, as uracil in DNA can cause double-stranded breaks and compromise genomic stability. These enzymes hydrolyze dUTP into dUMP and pyrophosphate, with dUMP serving as a substrate for thymidylate synthase, thereby preventing dUTP integration and maintaining genetic integrity [17,18]. The ITPase superfamily includes enzymes that hydrolyze deaminated purine nucleoside triphosphates (NTPs) like inosine triphosphate (ITP) and xanthosine triphosphate (XTP), along with their deoxygenated forms. These enzymes preferentially act on non-canonical NTPs such as dITP and dXTP, distinguishing them from canonical guanosine triphosphate (GTP) due to structural differences in the purine ring 2-amino group. This specificity allows ITPases to regulate levels of these modified nucleotides effectively [19]. The all-α NTP-PPases are a diverse group of enzymes with a common structural motif found across various life forms, including humans (dCTP, pyrophosphatase 1), archaea (MazG, NTP pyrophosphatase), phages (HisE, phosphoribosyl-ATP pyrophosphatase), and protozoans (dimeric GTPases). They are involved in hydrolyzing NTPs and related molecules, playing crucial roles in nucleotide metabolism and maintaining genomic integrity during replication and transcription processes [20].
The NUDIX hydrolases represent a distinct superfamily of sanitization enzymes, functionally related to other major superfamilies but with unique structural characteristics. They are increasingly recognized for their importance in human health, particularly in the context of cancer research, due to their roles in nucleotide metabolism and cellular signaling pathways. The original prototypical NUDIX hydrolase MutT from E. coli was discovered almost 70 years ago and is recognized for its role as an antimutator and a nucleoside triphosphatase that protects the accuracy of DNA synthesis. It functions by breaking down noncanonical nucleotides into their monophosphate forms, preventing their incorporation into DNA strands [21].
The term NUDIX hydrolases refers to a family of enzymes that are named based on their preferred substrate structure, which is ‘nucleoside diphosphate linked to another moiety, X. These enzymes are found across all forms of life, including eukaryotes, bacteria, archaea, and viruses, indicating their evolutionary conservation and widespread importance [[22], [23], [24]]. Humans have around 23 unique NUDIX proteins, all of which feature a highly conserved primary sequence called the ‘NUDIX box’ motif (GX (5)EX (7)REUXEEXGU), where “U" denotes an aliphatic hydrophobic amino acid (such as valine, leucine, or isoleucine), and “X" represents any amino acid [25]. Members of the NUDIX superfamily catalyze various substrates, including both canonical and noncanonical dNTPs, methylated nucleotides, non-nucleoside and dinucleotide polyphosphates, nucleotide sugars, alcohols, and capped mRNAs. Their high specificity allows them to recognize noncanonical dNTPs among many canonical dNTPs [18,26].
The NUDIX family members were classified into four structural classes based on structure-based sequence alignments and their specific enzymatic activities against various substrates (Fig. 1). Carreras-Puigvert and colleagues developed a framework to physiologically profile human NUDIX proteins. They started with affinity purification of different human NUDIX hydrolases, then assessed their functions and aligned their sequences based on structural features to link specific enzymatic activities to particular structural elements within the enzymes [18,25]. The first class of NUDIX hydrolases includes nine enzymes, of which four are specifically identified as diphosphoinositol polyphosphate phosphohydrolases. These enzymes are grouped together based on similarities in their structure, function, and sequence alignments.
The second class of NUDIX hydrolases includes, enzymes like NUDT7, NUDT8, NUDT16, and NUDT19. These enzymes, such as NUDT7, function as CoA diphosphatases, meaning they break down CoA (coenzyme A), oxidized CoA, and CoA esters into 3', 5'-ADP and a corresponding 4'-phosphopantetheine derivative [27]. The third class of NUDIX hydrolases includes NUDT1 (MTH1), NUDT15 (MTH2), and NUDT18 (MTH3), as well as NUDT12, NUDT13, NADH diphosphatases, and others [24]. The fourth class of NUDIX hydrolases has the outlier enzyme NUDT22, which has a distinctive protein structure and a modified NUDIX box. Unlike other NUDIX enzymes, NUDT22 specifically hydrolyzes UDP-glucose and UDP-galactose into their respective sugar 1-phosphates and UMP [28].
All NUDIX hydrolases share the conserved ‘NUDIX box’ motif, but their diverse enzymatic activities are influenced by their overall protein structures. For example, MTH1, NUDT15 (MTH2), and NUDT18 (MTH3) specialize in hydrolyzing mutagenic substrates like oxidized guanine and deoxy guanine. In contrast, NUDT5 and NUDT14 are involved in breaking down ADP-ribose and ADP-glucose [24,25]. In model organisms, certain NUDIX hydrolases were found to be upregulated during oxidative stress. This suggests that NUDIX hydrolases play a crucial role in helping cells survive under stress conditions, indicating a stress defense mechanism dependent on these enzymes [25]. This observation prompted researchers to examine the levels of NUDIX hydrolases in cancer tissues. They discovered that MTH1 was consistently upregulated in all cancer tissues, while other NUDIX hydrolases were only upregulated in specific types of cancer tissues [15].
Human cells contain three types of MutT homolog proteins: MTH1 (NUDT1), MTH2 (NUDT15), and MTH3 (NUDT18). These proteins are members of the NUDIX hydrolase family, class 3, and share the conserved NUDIX motif, a sequence crucial for their hydrolase activity. This motif is essential for the binding of substrates and the catalysis of their hydrolysis. While the core residues of the active site are conserved across these proteins, differences in the surrounding amino acids result in variations in how each enzyme binds substrates and their catalytic efficiency. In laboratory experiments, it has been demonstrated that each of these NUDIX enzymes can break down different oxidized nucleotides [[29], [30], [31]]. For example, MTH2 is known to convert 8-oxo-dGTP and 8-oxo-dGDP into 8-oxo-dGMP. In contrast, MTH3 hydrolyses 8-oxo-dGDP and 8-oxo-GDP into their respective monophosphate analogues, but it cannot hydrolyze their triphosphate counterparts. Despite these abilities, the hydrolytic activity of MTH2 and MTH3 on 8-oxo-dGTP and 8-oxo-dGDP is extremely weak compared to MTH1, suggesting that their physiological relevance is limited [32,33].
Among the MutT homologs, MTH2 exhibits the second-highest activity towards 8-oxo-dGTP but shows significantly higher activity towards the canonical nucleotide dGTP. MTH2 displays a nearly 10-fold preference for dGTP over 8-oxo-dGTP as a substrate. MTH1, on the other hand, is highly specific and efficient in hydrolyzing 8-oxo-dGTP due to its precisely configured active site. MTH2 and MTH3, while capable of hydrolyzing a broader range of substrates, do so with less efficiency compared to MTH1 [33,34].
Structurally, although these proteins share a similar overall fold, there are notable differences. For instance, in MTH2, a shift in the helix α2 distorts the substrate binding pocket compared to MTH1. Additionally, MTH1 and MTH2 have different amino acids, making up the base of their active sites, which confers different substrate recognition properties. Unlike MTH1, MTH2 lacks hydrogen bonding capabilities and does not have an equivalent amino acid to Trp117, which is crucial for the base-stacking interactions necessary for binding 8-oxo-dGTP effectively. This deficiency means MTH2 cannot recognize and bind 8-oxo-dGTP as effectively as MTH1 [34].
Compared to MTH1, other NUDIX enzymes are much less efficient at catalyzing reactions with noncanonical substrates. For instance, NUDT18 (MTH3) exhibits significantly lower catalytic activity towards O6-methyl-dGTP compared to MTH1 [35]. Furthermore, MTH2 has shown higher activity towards dGTP, 6-thio-dGTP, and 6-thio-GTP. The activity of NUDT15 (MTH2) towards 6-thioguanine species is particularly interesting, given that a mutation in NUDT15 has been recently identified as a sensitivity factor in thiopurine therapy [36]. Despite the differential substrate preferences among the NUDIX hydrolases, MTH1 remains the most studied protein within this family, likely due to its high specificity and efficiency in hydrolyzing oxidized nucleotides, which plays a crucial role in preventing mutagenesis and maintaining genomic stability.
Moreover, potential evolutionary relatives of MTH1 are obtained by BLAST, and sequences with good resemblance were analyzed. The evolutionary relationship of MTH1 with other related proteins was obtained by the Fast Minimum Evolution method using BLAST pairwise alignments covering all 156 amino acid residues. The phylogenetic evolutionary relationship of MTH1 with related proteins showed the division of proteins into two major clusters. Cluster I consist of closely related subtypes, including MTH1 from Chaetorhynchus papuensis (Papuan whipbird) and Panurus biarmicus (Bearded reedling). Instead Cluster II comprises a diverse set of closely related homologs, including MTH1 from Homo sapiens (Human) and Pongo abelii (Sumatran orangutan), Plecturocebus cupreus (Coppery titi monkey) and Saguinus oedipus (Cotton-top tamarin), Balaenoptera physalus (Fin whale) and Eschrichtius robustus (Gray whale). MTH1_Homo_sapiens (Human) is closely related to two subtypes namely Pongo abelii and Macaca mulatta whereas it is most distant to Asarcornis scutulata (White-winged Duck). This comparison of Cluster I and Cluster II reveals a wide phylogenetic distribution of the MTH1 gene, from birds to primates and marine mammals, underscoring its fundamental role in cellular processes across diverse taxa (Fig. 2).
Fig. 2.
Phylogenetic evolutionary tree of MTH1 Kinase: Evolutionary relationship of MTH1 with other related proteins obtained by Fast Minimum Evolution method using BLAST pairwise alignments covering all the 156 amino acid residues. MTH1_Homo_sapiens (Human) is closely related to two subtypes namely, Pongo abelii and Macaca mulatta; whereas it is most distant to Asarcornis scutulata (White-winged Duck).
3. Molecular genetics of MTH1
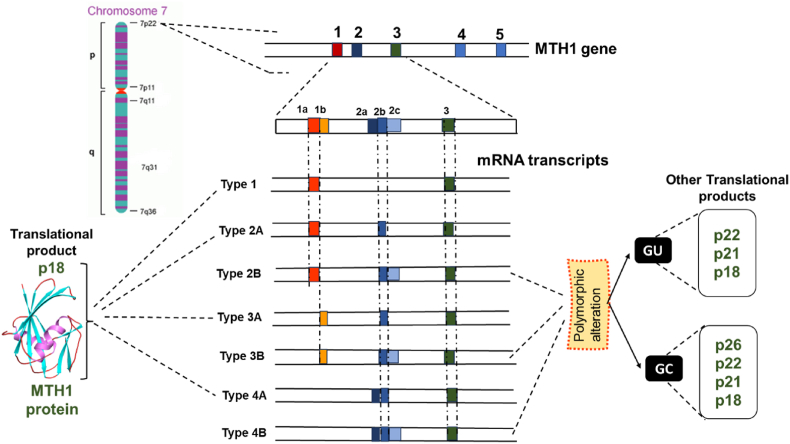
The protein MTH1 or MutT homolog 1 is a member of the NUDIX hydrolase family, also termed 7,8-dihydro-8-oxoguanine triphosphatase hydrolase. The chromosomal location of the hMTH1 gene is 7p22, spanning approximately 9 kb. Due to the existence of various sites of alternative RNA splicing and transcription initiation, it consists of 5 exons that can generate a maximum of seven different mRNA kinds. Amongst the five exons in the MTH1 protein, two exons have alternative sequences. They are Exon 1, which has two alternative sequences, i.e., exon 1a and exon 1 b, and secondly, Exon 2, which is alternatively spliced, has three contiguous segments, i.e., exon 2a, exon 2 b, and exon 2c. The resulting seven different kinds of mRNAs are type 1, 2 A, 2 B, 3 A, 3 B, 4 A, and 4 B. A majority of human cells and tissues have a significant amount of type 1 mRNA, which lacks exon 2 and is basically transcribed from exon 1a. The remaining six variants of MTH1 mRNA have not less than one exon 2 segment, showing that exon 2 selection is regulated during the pre-MTH1 mRNA processing. The majority of the 5′ region of Type 4 A and Type 4 B mRNA correlates to the exon 2a segment, and the exon 2a segment is probably the first exon for both types of MTH1 mRNA (Fig. 3). Both the types lack sequences that correlate to exon 1a and exon 1 b in its 5’ region. The mRNA variants that have one of the exon 1 sequences of any type will not have the exon 2a segment [37,38].
Fig. 3.
Seven types of MTH1 mRNA are generated through alternative transcription initiation and splicing, as depicted along with part of the genomic structure. Exons 3–5 do not undergo alternative splicing. Types 1, 2 A, 3 A, and 4 A mRNA translate into p18, the major form of the MTH1 protein. B-type mRNAs (2 B, 3 B, and 4 B) produce three polypeptides: p22, p21, and p18. Polymorphic changes (GT to GC) create an additional AUG codon upstream, leading to the polypeptide p26. Thus, GC allele homozygotes produce four polypeptides (p26, p22, p21, and p18), while GU allele individuals form three (p22, p21, and p18).
The hMTH1 protein exists in its four isolated forms, i.e., p18, p21, p22, and p26. Type 1, 2 A, 3 A, and 4 A mRNA translates into p18, which is also the major form of MTH1 protein, as shown in Fig. 3. The B-type mRNAs (2 B, 3 B, and 4 B) give rise to three polypeptide forms, p22, p21, and p18, where two additional AUG initiation codons are present in between exon 2 b-2c fragments. A case of polymorphic alteration where GU bases are replaced by GC bases at the anterior of exon 2c sequence, giving rise to another AUG codon upstream, yielding another polypeptide p26. Hence, homozygotes with GC allele produce four kinds of peptides: p26, p22, p21, and p18, whereas GU allelic individuals form p22, p21, and p18. Thus, synthesis of hMTH1 is dependent on alternative splicing and alternative initiation during transcription and translation, respectively, where additional alterations are supported by SNP [39].
MTH1 is a housekeeping gene that is inferred by the presence of Sp1 binding site at 50 and 160 bases, which is highly rich in its GC content and is located upstream of the initiator sequence. MTH1 has TCACTTCC as the initiator sequence, found present at the 5’ end on exon 1a. The human MTH1 gene has a 13bp sequence spanning from positions −17 to −29, and within this 13bp sequence lies a consensus binding site for Ets family proteins. Given the established role of Ets family proteins in regulating transcription in response to various developmental and mitogenic signals, it is plausible that these sites play a crucial role in inducing MTH1 gene expression during the proliferative activation of peripheral blood lymphocytes by PHA and IL-2. Also, the MTH1 gene has sequences like NF-kB and AP-1, which upregulates the MTH1 gene expression under oxidative stress-prone conditions [37,40].
4. Structural analysis of MTH1 protein
Human MTH1, a. k.a NUDT1, is an 18 kDa Nudix pyrophosphatase that exists as a monomer with α-β- α scaffold. Structurally, MTH1 acquires seven β strands and two α helices along with four long loops. The acquired structure is as follows βA (residues 5–11), βB (17–22), βC (67–74), βD (80–87), βE (102–107), βF (132–139), βG (146–153), αI (44–58) and αII (120–130) where loop 4 (L4) links βE with αII and loop (L1) links βB and αI (Fig. 4). The main body of the protein consists of 5 β stands (βA, βD, βC, βF, and βG) with αII, whereas an additional lobe emerges with helix αI, L1, and two antiparallel β strands (βB and βE). This additional lobe is attached to the main body at the βA strand, resulting in the formation of a deep and narrow pocket, demarking the main body of the protein. The h3JNC’ couplings revealed hydrogen bond formation between Val 85 and Val 67, leading to a small β-bulge structure causing a bend at Gly 68 in the main protein chain [38,41].
Fig. 4.
A. Structural representation of the amino acid sequence of human MTH1. The MTH1 protein is a 156 long polypeptide chain with a Nudix hydrolase domain from A3 to K132 and a 23-amino acid Nudix box from G37-L59. B. Three-dimensional structure of the human MTH1. The chain of MTH1 is coloured based on secondary structural elements, including Helices-magenta, Sheets-cyan, and Loops-red. Structurally MTH1 exist as a monomer and acquire seven β strands and two α helices along with four long loops. Secondary structure elements (αI, αII, and βA to βG) and loop regions (L1 to L4) are labeled.
The 156-amino acid MTH1 contains a highly conserved primary sequence termed the ‘Nudix box’ motif or mutT box, which is a 23-amino acid region with a sequence G37-X5-E43-X7-R51-E52-L53-X-E55-E56-X-G58-L59 (Fig. 4). It features an amphipathic helix of roughly three turns (α1) and a preceding loop (L1) that takes on a clearly defined hairpin-like shape. Saturation mutagenesis studies revealed that out of these 23 residues, 14 residues (39, 41, 43, 45, 48, 51, 52, 53, 55, 56, and 57) are of prime importance as they facilitate catalysis by MTH1. The residues Ala 49, Leu 53, and Leu 59 are critical in aligning the Nudix helix against the β-sheet and increase the hydrophobicity of the core formed by Thr8, Val 10, and Val 12 from strand βA, Phe 86 from strand βD, and Phe 35 from loop L1. The residues 37–43 appeared to be crucial for MTH1 activity. The residue Gly42 has a β-turn getting stabilized by a hydrogen bond between Glu 43 and Gln 40. Both Gly42 and Gly 43 are highly conserved throughout the NUDIX family, and alteration in them reduces the catalytic activity of MTH1. The other loop-forming residues, Val 39 and Ile 45, bond hydrophobically, giving the loop the β-hairpin conformation along with β-turn-forming residues. The highly conserved Gly37 is crucial for catalytic activity, with its Hα1 proton positioned close to the side chain of the metal-coordinating residue Glu 52. Consequently, substituting Gly37 likely alters the local structure of the catalytic site residues, resulting in a loss of enzyme activity [32,34].
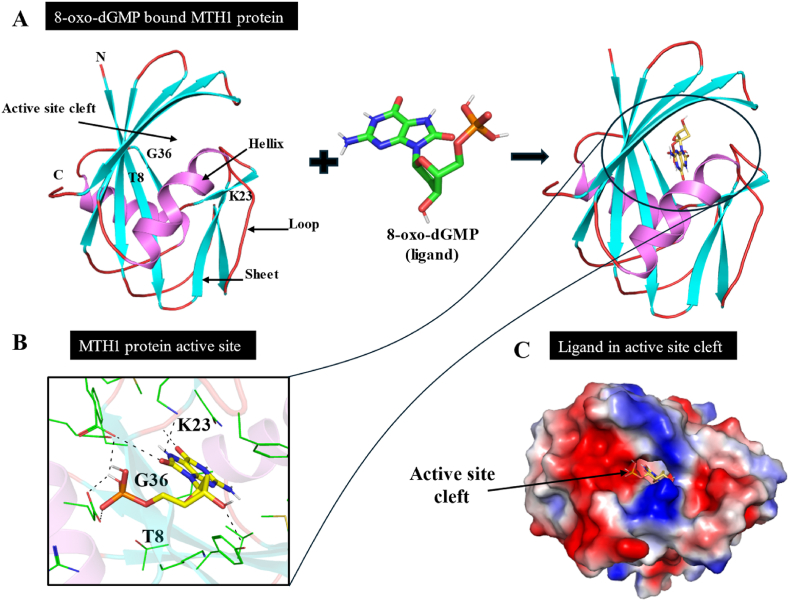
Apart from the Nudix motif, there are several other important residues present for the recognition of the oxidized bases. The pocket-forming residues Trp117 and Asp119 are involved in substrate recognition. Trp117 is located in close proximity to the binding site. Asn33 is a key residue with its side chain exposed in the binding pocket, and its mutation has been observed to have a detrimental effect on MTH1 catalytic activity. MTH1 exhibits broad substrate specificity, hydrolyzing a range of oxidized nucleotides such as 8-oxo-dGTP, 8-oxo-dATP, 2-OH-dATP, 8-oxo-rGTP, 8-oxo-rATP, 2-OH-rATP, 8-Cl-dGTP, and 8-Br-dGTP [42]. To gain deeper insight into the recognition mechanisms and hydrolysis by MTH1, researchers solved the X-ray crystal structure of the complex formation between 8-oxo-dGMP and MTH1 (Fig. 5).
Fig. 5.
Structural insights into MTH1 protein: A. Binding mode of complex formation between 8-oxo-dGMP and MTH1. Cartoon representation showing docked 8-oxo-dGMP in the active site cavity of MTH1. B. 3D illustration showing the interaction between 8-oxo-dGMP with MTH1, with a focus on the functionally critical amino acid residues. C. Surface view of 8-oxo-dGMP in the active site cavity of MTH1.
On entering into the active site, 8-oxo-dGMP embrace antipodal conformation, following π-stacking interaction with Trp117 and Phe72, while Asp119 and Asp120 recognize its Watson-Crick face through hydrogen bonds (6-O, 1-NH and 2-NH2 groups of 8-oxo-dGMP). Asn33, located at the base of the purine ring, forms additional hydrogen bonds. Mutations such as Trp117Ala and Asn33Glu significantly reduce or abolish MTH1 hydrolytic activity, highlighting the crucial roles of Trp117, Asp119, Asp120, and Asn33 in substrate recognition and excision. These residues are also key targets for MTH1 inhibition by specific inhibitors [32,34,43].
The structural basis of MTH1 activity is defined by its ability to hydrolyze oxidized nucleotides, particularly 8-oxo-dGTP, preventing their incorporation into DNA and ensuring genomic stability. Its substrate-binding pocket is specialized for oxidized purine nucleotides like 8-oxo-dGTP and 2-OH-dATP. While MTH1 focuses on sanitizing these damaged nucleotides, other NUDIX hydrolases, such as NUDT5, NUDT15, and NUDT9, have broader roles in metabolic processes like poly-ADP-ribose signaling and drug metabolism. MTH1 hydrolyzes 8-oxo-dGTP by cleaving the phosphate bonds, whereas other NUDIX hydrolases differ in the types of bonds they cleave, depending on their substrates. MTH1 primarily serves a preventive role, unlike other NUDIX enzymes, which function in diverse cellular pathways [41,[44], [45], [46]].
5. Biological role of MTH1
MTH1 derives its name from its initial discovery in E. coli as the product of the MutT gene, known for its role as a mutator gene. This gene was later cloned and identified as a dNTPase in humans. In a similar manner to the bacterial MutT, human MTH1 hydrolyses oxidized nucleotides [47]. MTH1 hydrolyses oxidized purine deoxyribonucleotides, such as 8-oxo-dGTP, 2-OH-dATP, and 8-oxo-dATP, along with their ribonucleotide analogues, converting them into monophosphate forms. These oxidized nucleotides, if left unchecked, can get incorporated into DNA or RNA during replication and transcription processes, eventually leading to genome instability and mutations. For example, 8-oxo-dGTP can form Watson–Crick base pairs with dCTP as well as Hoogsteen base pairs with dATP, potentially leading to GC to TA transversion mutations during replication. MTH1 prevents this mis-incorporation by catalyzing such nucleotides, of which enzymatic by-products are subsequently degraded to prevent their re-incorporation by deoxyribonucleotide salvage pathways. This process is crucial for avoiding the integration of oxidized nucleotides into genomic DNA during replication, thereby maintaining normal cellular function [47].
Additionally, MTH1 serves as a defense mechanism against DNA methylation by eliminating methylated purine triphosphates like O6-methyl-dGTP from the nucleotide pool. This specific capability to hydrolyze O6-methyl-dGTP efficiently is highly conserved throughout evolution and is unique to MTH1 among all human NUDIX enzymes. DNA is highly vulnerable to damage from ROS. When oxidative stress occurs, ROS can easily damage DNA bases, threatening genomic stability. Guanine (G) is especially prone to oxidation due to its low redox potential, frequently getting oxidized to 8-oxoG by ROS. During DNA replication, 8-oxoG can incorrectly pair with cytosine (C) or adenine (A), leading to potential mutations and functional impairments in the DNA. The presence of 8-oxoG is a recognized indicator of oxidative DNA damage, highlighting the severity of such damage [35,48].
In both prokaryotic and eukaryotic cells, 8-oxoG is primarily removed via the base excision repair pathway, which involves the DNA glycosylase OGG1. In E coli, the ‘GO repair system’—comprising DNA glycosylases MutM and MutY, along with 8-oxoGTPase MutT—works together to prevent mutations from 8-oxoG. In human and mouse cells, MTH1 holds significant importance as it collaborates with the enzyme OGG1 in a repair mechanism to mitigate mutations induced by 8-oxoG. This has led to thorough research focusing on the role of MTH1 in various diseases, particularly cancer, given their tendency for high oxidant levels and susceptibility to DNA damage. These conditions increase reliance on MTH1 for protection, driving efforts to develop therapeutic approaches targeting this enzyme.
MTH1 plays an important role in protecting the genome and epigenome from the damaging effects of oxidative stress, which can significantly impact their stability. ROS can directly damage DNA, leading to mutations, strand breaks, and base modifications like 8-oxoG, resulting in faulty transcription, replication errors, and genomic instability. Beyond direct DNA damage, oxidative stress also disrupts the epigenome by altering DNA methylation patterns, histone modifications, and chromatin structure, potentially causing abnormal gene expression. These epigenetic changes can have long-term consequences, contributing to diseases such as cancer, neurodegenerative disorders, and aging-related conditions. While the direct role of MTH1 in epigenetic regulation is still under investigation, its ability to prevent the incorporation of oxidized nucleotides into DNA indirectly helps maintain epigenetic stability. In the absence of MTH1, oxidative stress can worsen DNA damage, trigger faulty repair mechanisms, and lead to epigenetic dysregulation, increasing the risk of cancer and other related diseases. As a result, pharmacological inhibition of MTH1 has gained attention as a potential cancer therapy by targeting cells under high oxidative stress that depend on MTH1 for survival [49,50].
6. Role of MTH1 in cancer
Tumor cells exhibit high metabolic activity and oxidative stress, leading them to express elevated levels of MTH1 which degrades oxidized nucleotides, preventing their incorporation into DNA and RNA. Tumor cells experience heightened oxidative stress, leading to a dependence on MTH1 for proper cellular function. Mouse embryonic fibroblasts (MEFs) from MTH1 germline-null animals show increased sensitivity to hydrogen peroxide (H2O2)-induced oxidative DNA damage and cell death compared to wildtype MEFs, even though MTH1-null animals do not exhibit developmental defects. This suggests that MTH1 plays a crucial role in protecting cells from oxidative stress, which is uncommon in healthy tissues but prevalent and exploitable in many cancers [51].
MTH1 plays a pivotal role in reducing the toxicity of ROS, thereby aiding the proliferation, migration, and invasion of tumor cells. This function suggests that MTH1 serves as a crucial survival mechanism, enabling tumor cells to withstand and thrive despite oxidative stress [52]. Tumor cells, thus, express higher levels of MTH1 compared to normal cells. Elevated MTH1 expression has been found to play a direct role in the growth of various types of tumors. MTH1 holds potential as a biomarker for tracking cancer progression and assessing its role in targeted therapies.
6.1. Elevated MTH1 regulates mitotic activity in tumors, limiting 8-oxodGTP availability
The link between high 8-oxoGTP levels and increased MTH1activity is particularly important in cancer cells, where MTH1 is often overexpressed to protect against oxidative damage. However, while MTH1 helps prevent immediate oxidative damage, its over-activity can lead to imbalances in nucleotide pools, contributing to the complexity of cancer progression. Thus, understanding the dual role of 8-oxoGTP and MTH1 in genomic stability and carcinogenesis is crucial for developing targeted cancer therapies [53]. Scientists have designed a novel probe called ARGO (ATP-releasing guanine oxidation probe), which integrates 8-oxodGTP with ATP, to measure MTH1 enzyme activity in different cancers, such as colorectal cancer (CRC), non-small cell lung cancer (NSCLC), and pancreatic ductal adenocarcinoma (PDAC), along with their normal tissue counterparts [54]. Their findings revealed that tumor tissues from these cancers exhibited significantly higher levels of MTH1 and 8-oxodGTPase activity compared to normal tissues, highlighting the potential of MTH1 as a cancer biomarker.
Mechanistically, Patterson et al. [55] discovered that combining an MTH1 inhibitor with an antimitotic drug, such as a PLK1 inhibitor, leads to cancer-specific mitotic vulnerability. Using the VISAGE strategy, they found that MTH1 interacts with β-microtubule proteins, causing changes in microtubule assembly. When a PLK1 inhibitor is present, this interaction impairs the proper localization of mitotic chromosomes to the spindle midzone, leading to cancer cell death [55]. MTH1 inhibitor, TH1579 led to eradication of primary Acute Myeloid Leukemia cells and chemotherapy-resistant leukemic stem cells by causing mitotic arrest, increasing ROS levels, and enhancing DNA damage [56].
6.2. MTH1 maintains telomerase activity and telomere stability in tumor
Impact of 8-oxoG Lesions and 8-oxoGTP: 8-oxoG lesions in telomeres can prompt aging and cell death. Telomerase, which maintains telomere length, can be impaired by oxidative stress, leading to dysfunctional telomeres and genomic instability. The MTH1 enzyme, which degrades 8-oxoGTP to prevent oxidative damage, does not protect against aging when inhibited. This suggests that MTH1 depletion increases 8-oxoGTP incorporation, impairing telomerase function and telomere maintenance [57]. Expression of the catalytic domain of telomerase, hTERT, does not prevent early aging in BJ skin fibroblasts due to MTH1 depletion, suggesting that 8-oxodG or 8-oxodGTP disrupt telomerase function [58]. Studies show that MTH1 reduction impairs telomerase activity in cancer cells. Research by Fouquerel et al. [59] revealed that 8-oxodGTP acts as a chain terminator for telomerase in HeLa cells, indicating that oxidized nucleotides damage telomeric DNA primarily through telomerase. Furthermore, cells with suppressed MTH1 activity and very short telomeres exhibit enhanced markers levels of irreparable telomere breaks and telomere-induced damage foci, leading to loss of viability, while cells with long telomeres are unaffected by MTH1 loss. Given that many tumors exhibit varying telomere lengths, including very short ones despite active telomerase, telomere length could potentially serve as a prognostic marker for the targeted application of MTH1 inhibitors in clinical treatments. Hence, MTH1 can also be a mediator of telomerase efficiency and telomere maintenance.
MTH1 inhibition can damage mammalian telomeric repeats (TTAGGG) due to their high guanine content, resulting in telomere dysfunction and genomic instability, contributing to cellular aging and tumor suppression. The extensive vulnerability of telomeric DNA to oxidative damage prevents cells from achieving immortality despite telomerase expression [60]. During the S-phase, the antioxidant protein peroxiredoxin-1 (PRDX1) associates with telomeres, and its depletion increases ROS and 8-oxodGTP levels, leading to premature chain termination by telomerase. Co-deletion of PRDX1 and MTH1 in colon cancer cells showed compensatory interactions, increasing expression of the remaining gene, and resulting in greater telomere shortening compared to single gene loss [57]. MTH1 loss-induced telomere shortening was most pronounced at 40 % O2 levels but absent at 5 % O2, with combined PRDX1 and MTH1 loss reducing telomere elongation rates in rapid telomerase-mediated lengthening models under specific conditions. PRDX1/MTH1-deleted cells show reduced telomerase activity at chromosome ends during oxidative stress, indicating MTH1 role in safeguarding telomeres from shortening by maintaining nucleotide substrate quality and preserving telomerase function [61]. Further validation in cancer models and in vivo studies is necessary. Nevertheless, these findings propose potential therapeutic strategies, like combining telomerase inhibitors with thiol antioxidants, to enhance MTH1 inhibition for cancer therapy.
6.3. Elevated MTH1 regulates DNA repair pathways in cancer
Elevated genomic 8-oxodG, resulting from MTH1 functional loss, likely increases engagement of several repair pathways, including BER, producing DNA nicks as repair intermediates. The higher pro-oxidant environment in cancer and the higher oxidation potential of 8-oxoGTP may promote secondary oxidative lesions in tumors, which are in charge of transforming integrated oxidized guanine moieties into DNA strand breaks. Therefore, the reliance on MTH1 in many cancer cells is crucial, as it provides an essential protective measure to avoid the need for these repair processes and their associated risk of DNA breaks by ensuring better quality control in the nucleotide pool [62]. Several tumors, including NSCLC, have shown high dependency on MTH1 to control the levels of 8-oxodG and also exhibited low levels or reduced activity of OGG1. Therefore, MTH1 becomes crucial for these tumors to manage oxidative stress and maintain genomic stability in the absence of adequate OGG1 function [63]. Deleting MTH1 or OGG1 individually results in a delayed onset of lung cancer, whereas co-deleting both OGG1 and MTH1 prevents lung cancer occurrence altogether [64]. One possible explanation for this finding is that blocking OGG1 repair, in combination with MTH1 loss, may avert the genomic instability that leads to malignancy (Fig. 6).
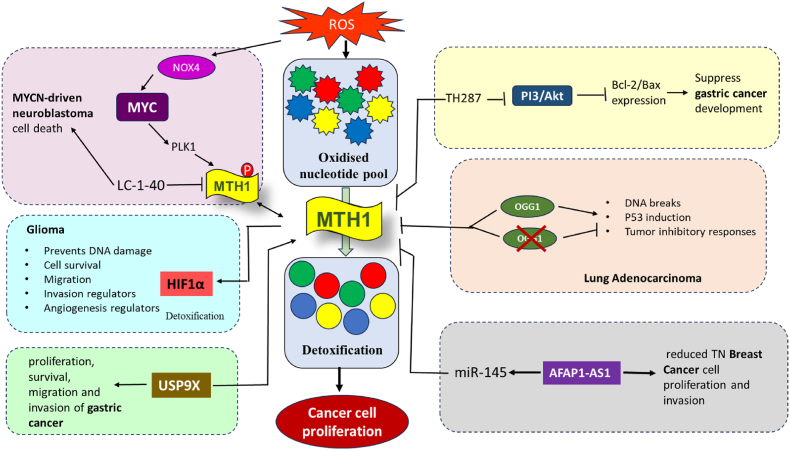
Fig. 6.
Physiological role of MTH1 and the possible effect of the therapeutic use of it. MTH1 inhibition is selectively toxic to cancer cells, which have higher ROS levels than normal cells. ROS oxidizes dNTPs, and these damaged nucleotides can be incorporated into DNA, causing instability. MTH1 hydrolyses oxidized dNTPs, preventing their incorporation and allowing cancer cells to proliferate. MYC balances the NOX4-ROS and PLK1-MTH1 pathways, and LC-1-40 induces MYC-driven tumor cell death by degrading MTH1 and disrupting this balance. MTH1, under HIF1α regulation, performs various functions promoting glioma. USP9X stabilizes MTH1, enhancing the proliferation, survival, migration, and invasion of gastric cancer cells. TH287 exhibits anticancer effects in gastric cancer cells by targeting mitochondrial function and inhibiting PI3K/AKT signaling, highlighting its potential as a novel therapeutic option. OGG1 co-inhibition counteracts the anticancer effects of MTH1 inhibition by reducing the accumulation of unrepaired oxidative DNA damage in lung adenocarcinoma. AFAP1-AS1 promotes TNBC cell proliferation and invasion by regulating MTH1 expression through targeting miR-145, which is typically downregulated in these cells. See text for more details.
In a separate investigation, MTH1 knockout did not exhibit a significant mutagenic burden, whereas the simultaneous knockout of MTH1 and MSH2 showed a marked increase in G- > T transversions compared to the former. This indicates an interaction between mismatch repair (MMR) and the mechanisms regulating genomic 8-oxodG levels. Many tumors, including colorectal cancer, display MMR deficiencies, resulting in high rates of hypermutation and microsatellite instability (MSI). Initially not assumed to depend on internal oxidative stress, research by Russo and colleagues demonstrated that elevated MTH1 levels effectively reduced the HPRT mutator phenotype in MMR-deficient MSH2-null mouse cells and colorectal DLD-1 human cells, and decreased MSI incidence in the latter. These findings suggest that heightened levels of oxidized nucleotides contribute to genomic instability in MMR-deficient cancer cells, underscoring the critical role of elevated MTH1 expression and function in mismatch-repair-deficient tumor cells [65,66].
6.4. MTH1 maintains DNA and RNA polymerase fidelity in cancer cells
DNA and RNA polymerase required balanced supply of dNTP and NTP while carrying out replication and transcription in order to prevent mutations. Among the nucleotides, GTP and dGTP are particularly prone to oxidation due to high redox potential of guanine. Recent research highlights that high 8-oxoGTP levels lead to polymerase errors, polymerase slippage, and reduced repair efficiency. Cancer cells require elevated levels of MTH1 in order to restrict the availability of 8-oxodGTP to polymerases, thereby ensuring that replication and transcription processes proceed without introducing breakage errors resulting in cancer cell proliferation. In mitochondrial DNA from rat tissue, minimal 8-oxodGTP levels (<1 % of total dGTP) significantly reduce mitochondrial DNA polymerase (Polγ) fidelity [67]. This effect is heightened in tissues with imbalanced nucleotide pools, like cardiac subsarcolemmal tissue with high dGTP levels, where 8-oxodGTP competes with dTTP for incorporation opposite dA. Even in rat liver, with balanced dGTP levels, 2.4 nM 8-oxodGTP raises A- > C transversions about 7-fold compared to levels without 8-oxodGTP [[67], [68], [69]].
RNA polymerase II can incorporate 8-oxoGTP instead of GTP during transcription, albeit at a low rate [70]. This suggests that MTH1 activity not only prevents mutations during DNA replication by maintaining balanced GTP/dGTP pools but also limits mutational errors during transcription processes. MTH1 plays a vital role in removing oxidized guanine ribonucleotides from the nucleotide pool. Without MTH1, there is a significant risk of genomic instability caused by an imbalanced GTP and dGTP pool, leading to the incorporation of damaged nucleotides into DNA and RNA, which can result in mutations and termination of replication and transcription processes, thereby suppressing cancer cell proliferation [71].
6.5. MTH1 as a combinatorial therapy to treat cancer
Despite its known role in promoting cancer invasion, there are studies indicating that MTH1 may also have a synergistic effect in inhibiting the growth of cancer cells. In human breast cancer cells, specifically MCF7-R, reducing MTH1 expression resulted in the activation of p21, a protein that inhibits cell cycle progression. This activation made cells more responsive to gemcitabine, indicating that suppressing the MTH1 gene could increase the effectiveness of treatment in drug-resistant breast tumors. This approach suggests a new strategy for enhancing the treatment of resistant cancers with adjuvant therapies [72]. ROS-inducing agents can induce parthanatos in cervical cancer cells, and it was observed that MTH1 inhibition synergizes with low doses of ROS-inducing agents by increasing the presence of 8-oxoG in DNA. In vivo experiments showed that this drug combination effectively inhibited the growth of tumor xenografts [73].
Mechanistically, MTH1 deficiency appears to downregulate factors involved in the glutathione-dependent oxidative stress defense system [74]. This observation aligns with studies in thyroid cancer. Downregulating MTH1 expression, particularly in combination with drugs that modulate the glutathione pool, leads to elevated ROS levels in cancer cells. This increase in ROS disrupts the proliferation of thyroid cancer cells and enhances the effectiveness of MTH1 inhibitors as an anticancer therapy [75].
The combination of TH1579, MTH1 inhibitor with radiation therapy is making strides in cancer treatment by enhancing cell sensitivity to radiation and overcoming resistance caused by hypoxic stress. This development could significantly improve radiation therapy efficacy. Furthermore, piperlongumine disrupts redox balance in cancer cells, boosting their sensitivity to MTH1 inhibitors and suggesting a promising strategy to enhance MTH1 inhibition effectiveness in cancer treatment [76]. In the realm of strengthening photodynamic therapy (PDT), Chen et al. [77] introduced a novel nano amplifier, which is a combination of amphiphilic iridium complex with the MTH1 inhibitor TH287. It operates by triggering ROS-induced damage upon light activation, facilitating increased accumulation of 8-oxo-dGTP through MTH1 inhibition. This strategy effectively enhances oxidative stress, promoting cell death and thereby maximizing the therapeutic effectiveness of PDT.
In another study, TH1579 administration enhances M1 macrophage polarization, stimulates CD8 expansion, and promotes the activation of DC and T cells. Co-administration of PD-L1 and MTH1 inhibitors enhances the disruption of mesothelioma tumor growth and accumulation of mesothelioma-associated pleural effusion as compared to monotherapy alone [78]. Similarly, TH1579 has been shown to synergize effectively with immune checkpoint inhibitors or MAPKi therapy by downregulating PMS2 and inducing cytotoxicity in both ICI-sensitive and resistant patient-derived cells from cutaneous malignant melanoma (CMM). This demonstrates that simultaneous silencing of MTH1 and PMS2 results in significant cancer cell death compared to either treatment alone in CMM treatment [79].
6.6. MTH1 maintains multiple KRAS-driven pro-malignant pathways
RAS oncoproteins induce oxidative stress, leading to DNA lesions like 8-oxoG and genomic instability. Cancer cells with RAS mutations often express MTH1 to counteract ROS-induced damage, aiding in their growth. Reducing MTH1 in these cells lowers 8-oxoG levels and ROS, thereby suppressing oncogenic signaling and transformation. Thus, MTH1 protects against DNA damage and maintains the oncogenic phenotype in RAS-driven cancers [80,81]. This is achieved by maintaining tumor-promoting phenotypes such as anoikis resistance and epithelial-to-mesenchymal transition (EMT), which depend on ROS-generating pathways like Akt or Rac 1 signaling [29,82]. Suppressing MTH1 in NSCLC cells with KRAS mutations reduces tumor formation, eliminates high oncogenic RAS-expressing cells, and decreases cellular ROS levels and Akt signaling. MTH1 functions as a non-oncogene addiction in RAS-driven cancers [29]. This term describes an essential pathway that is not directly involved in tumor initiation and becomes crucial for the survival and progression of cancer cells. MTH1 becomes an addiction for RAS-harboring cells, as its inhibition eliminates their pro-tumorigenic abilities [29,80].
A positive correlation between MTH1 and KRAS mRNA levels in early-stage human NSCLC tumors was also observed. Additionally, introducing oncogenic RAS into normal cells was sufficient to induce MTH1 upregulation at both the mRNA and protein level [29,83]. These findings collectively suggest that RAS mutations not only drive tumorigenesis but also create a specific dependence on MTH1 activity for the cancer cells to maintain their malignant potential. Given the current limitations of directly targeting oncogenic RAS in clinical settings, MTH1 inhibition could represent a promising alternative therapeutic strategy. Targeting MTH1 may show a potential to eliminate RAS-driven tumor cells, as well as other oncogene-dependent tumors exhibiting elevated levels of ROS [29,80]. This approach holds particular promise for cancers where RAS mutations are present.
6.7. MTH1 regulates signaling pathways within tumors
MTH1 inhibition might not be exclusive to RAS-driven cancers. Cells harboring other ROS-generating oncogenes, such as NF-κB, Myc, and PI3K, might also be susceptible to MTH1-targeting therapies due to their vulnerability to oxidative DNA damage. In NSCLC, MTH1 has been implicated in driving disease progression and metastasis by influencing critical pathways such as PI3K/AKT and MAPK/ERK. MTH1 controls cell growth pathways, influencing proliferation, cell cycle, and apoptosis while also promoting EMT essential for cancer cell movement and metastasis in NSCLC. Inhibition of MTH1 has been shown to mitigate NSCLC metastasis [84]. MTH1 inhibition in gastric cancer triggers anticancer effects by reducing mitochondrial membrane potential and suppressing Bcl-2/Bax expression through inhibition of pro-oncogenic PI3K/AKT signaling pathways [85]. Furthermore, studies on gliomas have associated MTH1 expression with the hypoxia-inducible factor HIF1α [86]. The signaling of the same is given in Fig. 6.
A study using zebrafish embryos investigated the effects of hypoxia and HIF-1α activation on MTH1 function. Hypoxia-induced HIF-1α activation, achieved through DMOG treatment or VHL factor mutation, led to glutathione oxidation and increased sensitivity to the MTH1 inhibitor TH588. Injecting oxidized nucleotides further confirmed the link between oxidative stress and MTH1 inhibitor sensitivity. The research underscores the importance of understanding the interplay between ROS-generating signaling and DNA repair for the potential clinical use of MTH1 inhibitors [87]. NUDT1 (MTH1) was also identified to have MYC-driven dependency, as shown in Fig. 6.
MYC regulates two parallel metabolic pathways to maintain tumor cell survival: the NOX4-ROS pathway, which generates ROS, and the PLK1-NUDT1 pathway, which sanitizes nucleotide pools by removing oxidized nucleotides. NOX1 and NOX2 primarily generate superoxide, and NOX4 and mitochondria play a pivotal role in the direct and secondary production of hydrogen peroxide, contributing to both oxidative stress and redox signaling. The compartmentalization and regulation of these ROS species are essential in maintaining cellular homeostasis and determining cell fate [88,89]. To elaborate further, ROS such as superoxide (O₂⁻) and H₂O₂, produced by NOX enzymes, are key players in oxidative stress, with the potential to cause significant damage to cellular components like proteins, lipids, and DNA. The findings from the EU-ROS study emphasize the need to identify the specific sources and types of ROS in different cellular contexts, further reinforcing the importance of targeting these species to mitigate their harmful effects in diseases like cancer, cardiovascular disorders, and neurodegeneration [90]. The role of MYC in balancing these processes ensures that ROS levels are kept in check while maintaining the integrity of the nucleotide pools, crucial for tumor cell survival. This MYC-directed mechanism highlights the intricate regulation of ROS production and nucleotide sanitation in cancer cells [91].
7. MTH1 in different cancer types
Cancer cells, faced with the cytotoxic effects of elevated ROS, adopt adaptive strategies to ensure survival. Research indicates a critical role for MTH1, a Nudix phosphor hydrolase, in this defense mechanism [47]. MTH1 functions within the base excision repair pathway, specifically targeting oxidized nucleoside triphosphates like 8-oxo-dGTP, 2-OH-dATP, and N6-methyl-dATP. By catalyzing their hydrolysis into monophosphates, MTH1 prevents their incorporation into DNA during replication. This crucial action minimizes genotoxicity, the introduction of mutations caused by these damaged nucleotides, and promotes genome stability, a hallmark that cancer cells strive to maintain despite the mutagenic environment they have created [14,35].
MTH1 overexpresses in various cancers as depicted in Fig. 7 including breast, brain, lung, gastric, renal, and colorectal cancers [14,84,92]. Even within breast cancer subtypes, MTH1 expression remains high irrespective of the molecular and clinical characteristics of the tumor [93]. This overexpression is closely linked to cellular ROS levels. Cancer cells burdened with high ROS rely more heavily on MTH1 for survival. The intensified oxidative stress triggers a cellular response that upregulates MTH1 expression, highlighting its importance in mitigating ROS-induced DNA damage and promoting cancer cell survival [94].
Fig. 7.
Schematic representation of MTH1 and its associated diseases highlithing the probable role of MTH1 in augmentation of these diseases.
7.1. Breast cancer
Breast tumor tissues display significantly higher MTH1 protein and mRNA levels compared to healthy controls. MTH1 expression did not vary between different breast cancer subtypes, nor was it correlated with patient age, tumor size, or lymph node metastasis. Functionally, inhibiting MTH1 resulted in a dose-dependent decrease in cancer cell viability and their ability to form colonies, highlighting its importance for their survival [93]. Research has shed light on a novel regulatory network involving competing endogenous RNAs that influences proliferation and invasion in triple-negative breast cancer (TNBC) cells. A critical role for the long non-coding RNA, AFAP1-AS1 was identified, in promoting TNBC cell proliferation as shown in Fig. 6. This function is mediated by ability of AFAP1-AS1 to target miR-145, which in turn regulates MTH1 expression in breast cancer cells [95].
A study investigated the potential of targeting MTH1 to overcome gemcitabine resistance in breast cancer. Using MCF7-R cells, a gemcitabine-resistant invasive breast cancer cell line, they explored the effects of silencing the MTH1 gene. Their findings demonstrate that MTH1 gene silencing has a cytotoxic effect on these resistant cancer cells in vitro, suggesting its potential as a therapeutic strategy, particularly in combination with gemcitabine [15].
Stable depletion of MTH1 in breast cancer cell lines, specifically MCF7 and T47D, resulted in a marked increase in the ratio of CD44+CD24−/low subpopulations, indicating an enrichment of breast cancer stem cells. Furthermore, MTH1 inhibition enhanced the formation of tumor spheroids, determining its role in promoting stemness properties in MCF7 cells. These findings suggest that MTH1 suppression is associated with the expansion of BCSC populations and the potential for increased tumorigenic capacity in breast cancer [96]. Silencing the MTH1 gene in gemcitabine-resistant MCF7-R breast cancer cells using specific siRNA resulted in a 77 % reduction in MTH1 protein and a 1.75-fold increase in sensitivity to gemcitabine, alongside elevated p21 expression, suggesting induced cell death [72]. MA-24 is an MTH1 inhibitor that exhibits potent antitumor activity against breast cancer. It induces DNA strand breaks and apoptosis through the cleaved-caspase 3–cleaved-PARP pathway, with tumor growth inhibition rate of 61.8 % in vivo [97].
7.2. Lung cancer
NSCLC cells and tissues displayed elevated MTH1 levels, and the upregulation is correlated with increased metastasis and poorer prognosis for patients. Suppressing MTH1 not only inhibited the hallmarks of cancer progression, like cell proliferation and cell cycle progression in vitro, but also promoted apoptosis. The in vivo experiments of MTH1 suppression restrained tumor growth and lung metastasis [84]. A study suggests MTH1 expression levels could be a valuable marker for NSCLC prognosis. NSCLC patients with high MTH1 expression exhibited more aggressive tumors and a poorer overall prognosis. MTH1 positivity was linked to smoking history and higher oxidative stress levels in these patients. This association between MTH1 and aggressive tumor characteristics suggests that MTH1 expression analysis could help identify patients at higher risk and potentially those who might benefit from targeted therapies against MTH1 [82].
Researchers found that knocking down of MTH1 in NSCLC tumors activating mutations in the KRAS gene cells, significantly reduced their ability to form tumors. This effect seems to be due to the elimination of the subpopulation within the tumor that expresses the highest levels of the RAS oncoprotein. MTH1 knockdown also led to a decrease in both ROS levels and Akt signaling activity. This suggests a possible mechanism by which MTH1 inhibition suppresses tumor formation in KRAS-mutant NSCLC [83].
Disruption of the MTH1 gene inhibited the growth of NSCLC, promoted apoptosis in tumor tissues, and reduced liver metastasis. This study presents a potential therapeutic strategy targeting the MTH1 gene for NSCLC treatment [98]. Elevated levels of MTH1 have been linked to increased resistance to oxidative stress and enhanced survival of cancer cells. Targeting MTH1 in lung cancer may sensitize tumors to oxidative damage induced by chemotherapy, presenting a potential strategy for treatment enhancement. MTH1 and NUDT5 are upregulated in NSCLC and are linked to tumor metastasis and poor patient prognosis. Their suppression inhibits tumor growth and promotes apoptosis while enhancing cell migration and invasion. Both of these proteins activate the MAPK and PI3K/AKT pathways and induce EMT that can be potentially used as prognostic markers and therapeutic targets in NSCLC [84].
MTH1 knockdown in lung cancer cell lines led to increased oxidatively damaged DNA and altered DNA damage signaling, resulting in reduced proliferation of H23 cells. However, this knockdown did not induce apoptosis or enhance the effectiveness of gemcitabine, cisplatin, or radiation in combination treatments, indicating complex interactions in lung cancer biology [99]. A multifunctional non-viral vector has been developed that is capable of delivering CRISPR/Cas9 plasmids to the nuclei of tumor cells, effectively targeting NSCLC. Disruption of the MTH1 gene via this vector inhibited NSCLC growth, promoted apoptosis in tumor tissues, and reduced liver metastasis, offering a promising therapeutic strategy for NSCLC [98].
7.3. Gastric cancer
Gastric cancer development is heavily influenced by factors that generate excessive ROS and DNA damage. These include dietary choices, alcohol and tobacco use, and chronic inflammation triggered by Helicobacter pylori infection [100]. Human DNA repair genes, particularly MTH1, have been found to be highly expressed in digestive tract tumors [101]. A specific inhibitor of MTH1 blocks MTH1 activity in both cell cultures and in vivo, but it also disrupts the normal function of enzyme, leading to a buildup of 8-oxo-dG inside cancer cells. This accumulation of 8-oxo-dG ultimately triggers apoptosis in gastric cancer cells. The study suggests that targeting the overactive MTH1 and the resulting surge of 8-oxo-dG is a critical mechanism by which MI-743 kills gastric cancer cells [102]. MTH1 inhibitor TH287 disrupts cancer cells in several ways. It disrupts the mitochondria and interferes with a PI3K/AKT signaling pathway, which is important for cell survival and growth, as shown in Fig. 6. This attack on cancer cells ultimately leads to a range of anti-cancer effects, potentially offering a new therapeutic avenue for gastric cancer [85].
7.4. Osteosarcoma
Osteosarcoma stands as the predominant primary malignancy within bone tumors. These tumors localize predominantly within the long bones [103]. The pathogenesis of osteosarcoma centers around a dysregulation in the tightly coupled interplay between bone formation and resorption [104]. A potential link between oxidative stress and MTH1 activity in osteosarcoma is indicated. Tumors displayed elevated levels of ROS, suggesting that MTH1 within these cells may be constitutively activated. This hypothesis is further supported by MTH1 overexpression in both osteosarcoma biopsies and cell lines, encompassing a wide range of genetic alterations. When compared to healthy mesenchymal stem cells, the MTH1 levels in the tumor samples were found to be significantly higher [105]. Specific knockdown of MTH1 in the tumor cells by employing siRNA or shRNA techniques resulted in a demonstrably reduced viability of the tumor cells.
Treatment with TH1579, an MTH1 inhibitor, significantly reduced the invasive and migratory capabilities of osteosarcoma cells [106]. A study revealed that (S)-crizotinib, another MTH1 inhibitor alongside TH1579, exhibited the capacity to reduce the migratory ability of osteosarcoma cells in vitro experiments. These findings strengthen the evidence supporting the role of MTH1 in osteosarcoma cell migration [107].
7.5. Pancreatic cancer
Pancreatic cancer presents a formidable therapeutic challenge due to its aggressive nature. A key factor contributing to this aggressiveness might be the high prevalence of K-Ras mutations (over 90 %) [108]. Research suggests that these mutations in Ras proteins make cancer cells more vulnerable to a type of cell death triggered by ROS [109]. The role of MTH1 in KRAS-driven pancreatic ductal adenocarcinoma (PDAC) development was investigated. MTH1 depletion via shRNA significantly reduced the growth of KRAS-driven PDAC cells [110].
In the germline, MTH1 deficiency led to reduced tumor burden only in the short-latency PKT mice, not the long-latency KPC mice. These results suggest a potential role for MTH1 in KRAS-driven PDAC development, particularly in fast-growing tumors [110]. To quantify MTH1 enzyme activity in PDAC tissues, a novel tool called the chimeric ARGO probe was used. This probe combines the substrate for MTH1 that is 8-oxodGTP with ATP. The results revealed a significant increase in MTH1 8-oxodGTPase activity within tumor samples compared to healthy tissues obtained from the same patients, suggesting that MTH1 activity is elevated in PDAC tumors [54].
8. MTH1 associated with other diseases
The production of ROS during oxidative phosphorylation leads to a dual outcome for cancer cells. ROS signaling promotes cancer cell survival, proliferation, and metastasis. However, this very ROS production, if excessive, can trigger tumor suppression by damaging cancer cell DNA [111]. The rapid proliferation and metabolic abnormalities inherent to these cells elevate ROS levels, leading to potentially lethal damage to their own genetic material. This vulnerability presents a therapeutic opportunity, as normal cells tolerate physiological ROS levels much better [112]. To counteract this ROS-induced DNA damage, cancer cells often upregulate DNA repair proteins like MTH1, highlighting a potential target for novel cancer therapies [113].
8.1. MTH1 in neurodegenerative diseases
In the context of neurodegenerative diseases, MTH1 takes on a neuro-protective role (Fig. 7). By effectively reducing its substrate, 8-oxoG, within the nuclear and mitochondrial DNA of Alzheimer patients, helping in minimizing the accumulation of detrimental mutations. This function extends to protecting neurons, as MTH1 activity also inhibits glial cell proliferation, a process sometimes linked to neuronal damage. Overall, the ability of MTH1 to safeguard against DNA damage and potentially harmful glial over activity, positions it as a contributor to neuronal integrity in neurodegenerative diseases [114].
AD, the leading cause of dementia is recognized by the distinct pathologies in the brain. These signs include amyloid plaques, which are abnormal clusters of protein fragments accumulating between nerve cells. Another hallmark is neurofibrillary tangles, twisted fibers formed by the protein tau that build up inside nerve cells. The most devastating consequence of these pathologies is neuronal loss or neurodegeneration, referring to the death or deterioration of brain cells. This progressive loss of neurons disrupts communication within the brain, ultimately leading to the characteristic cognitive decline associated with AD [115].
Oxidative stress is one of the major causes in the development of AD. Within the brains of AD patients, a specific marker of oxidative damage, 8-oxoG, accumulates significantly. MTH1 acts as a cellular defense system, minimizing the accumulation of 8-oxoG in DNA. However, MTH1 expression itself is down regulated in the AD brain, hindering its ability to combat oxidative damage and potentially contributing to disease progression [116].
Studies have shown that mice lacking MTH1 enzymes are more susceptible to neuro degeneration when exposed to oxidative stress [117,118]. Isolated cortical neurons from adult mice lacking MTH1 enzyme, when exposed to oxidative stress displayed accumulation of 8-oxoG, specifically in their mitochondrial DNA but not in their nuclear DNA [52]. Despite this mitochondrial dysfunction and impaired development of neurites, the neurons did not die. These findings suggest that mitochondrial dysfunction alone may not be enough to trigger neuro degeneration [114]. MTH1 plays a critical role in safeguarding the brain during progression of AD. This enzyme helps keep levels of a harmful DNA molecule, 8-oxoG, in check, particularly within the DNA of immune cells called microglia. By maintaining low 8-oxoG levels, MTH1 helps prevent the detrimental activation of microglia and subsequent neurodegeneration [114].
Microglia help in clearing damaged neurons. However, chronically activated microglia can contribute to neuro degeneration triggered by excessive DNA damage, which is marked by the presence of 8-oxoG. Studies suggest that deficiencies in MTH1 could create a vicious cycle [119]. During phagocytosis, activated microglia produce ROS, further promoting 8-oxoG accumulation within their DNA. This, in turn, fuels their chronic activation, a state known as microgliosis [120]. Microgliosis is associated with increased intracellular amyloid beta accumulation, another hallmark of AD. This rise in amyloid beta could further exacerbate neurodegeneration.
PD is a chronic and progressive neurodegenerative disease relentlessly attacking the brain by targeting dopamine-producing neurons. This loss of dopamine signaling disrupts critical pathways in the brain, leading to the hallmark symptoms of PD, such as tremors, stiffness, and difficulty with movement [121]. A growing body of evidence suggests a link between DNA repair capacity and susceptibility to PD. This neurodegenerative disease might have a genetic component, with variations (polymorphisms) in genes coding for DNA repair enzymes potentially playing a role. Analysis of these polymorphisms can help in understanding the underlying genetic vulnerability to oxidative stress, a cellular process that damages DNA [122].
PD research points to a potential role for MTH1, an enzyme involved in DNA repair. Studies have revealed that MTH1 is overexpressed in the neurons of patients with PD. This overexpression suggests that MTH1 activity might be dysregulated in PD, potentially impacting how neurons handle DNA damage [123]. PD patients exhibit a fingerprint of oxidative stress. The evidence to this is consistently elevated levels of a DNA damage marker, 8-OHdG, in their cerebrospinal fluid and urine [124,125]. In a study found that this increase in 8-OHdG levels occurs independent of polymorphisms in genes.
PD patients have also displayed higher levels of NADPH compared to healthy controls. NADPH is a cellular molecule crucial for various processes, including antioxidant defenses. While the precise cause-and-effect relationship between these findings needs further investigation, it suggests a potential link between oxidative stress, DNA damage, and NADPH metabolism in the development of PD [126]. In cells lacking functional MTH1, 8-oxo-dGTP accumulated. This accumulation coincided with mitochondrial dysfunction, characterized by the presence of electron-dense deposits within the mitochondria, and ultimately led to cell death in mouse fibroblasts [127]. However, the exact mechanisms by which these oxidized molecules, whether simply accumulated or incorporated into mitochondrial DNA, cause mitochondrial dysfunction and cell death remain unclear.
The overexpression of MTH1 in dopamine neurons of PD patients hints at a possible protective mechanism. The increased presence of MTH1 might be an attempt by the neurons to counteract the detrimental effects of DNA damage [118]. By targeting or manipulating MTH1 activity, there is a possibility to slow or prevent the progressive degeneration of dopamine neurons, a hallmark of PD, and ultimately improve patient outcomes. This can lead to exciting avenues for developing novel therapeutic strategies for PD.
Multiple Sclerosis (MS) is a neurodegenerative disease where oxidative stress significantly contributes to nerve cell and fiber damage, along with inflammation in the central nervous system. This is supported by the presence of markers indicating oxidative stress, such as damage to DNA itself, and the presence of 8-oxoG, which have been found in MS patients [128]. Studies support the role of oxidative stress in MS. Higher levels of 8-oxoG were found in patients with relapsing-remitting MS (RR-MS) compared to healthy controls. Similarly, a significant increase in overall DNA damage within leukocytes of RR-MS patients was observed [129,130].
RR-MS patients had shown lower levels of MTH1 gene expression compared to healthy controls in spite of the increased levels of oxidative DNA damage. The oxidative damage leads to genomic DNA damage and does not just affect the temporary nucleotide pool in the blood. This is interesting because MTH1 and another gene, OGG1, typically work together to repair DNA damage, as shown in Fig. 6. Surprisingly, OGG1 expression was actually higher in MS patients. This seemingly contradictory finding suggests a potential compensatory mechanism. The increased OGG1 expression might be an attempt to make up for the reduced function of MTH1 in these patients, aiming to maintain overall DNA integrity despite the challenges posed by MS [129].
8.2. MTH1 in cardiovascular disease
Understanding of complex interplay between oxidative stress and heart health is rapidly evolving. Oxidative stress is intricately linked to various cardiac abnormalities and diseases, including cardio toxicity [131], ischemic heart disease [132], pathological cardiac hypertrophy [133], heart failure (HF) [134], and diabetic cardiomyopathy [135]. A significant rise in 8-oxo-dG levels within patients suffering from coronary heart disease, stroke, and heart failure was observed, highlighting 8-oxo-dG as a promising biomarker for both diagnosing and predicting the course of these cardiovascular diseases [136]. A comprehensive analysis of studies investigating the link between 8-oxo-dG and cardiovascular disease revealed a consistent trend. All 13 case-control studies included showed a strong positive correlation between higher 8-oxo-dG levels and cardiovascular disease. Additionally, two prospective studies found a significant association between 8-oxo-dG and heart failure, suggesting the potential for this marker in predicting disease progression [137].
A study in the rat model sheds light on the contribution of oxidative stress and DNA damage to hypertension by examining the endothelial cells lining the aorta. Firstly, the increased 8-oxo-dG accumulation was observed during hypertension in the ECs. Secondly, elevated MTH1 protein expression was noted, indicating the endothelial cells might be trying to compensate for the oxidative stress by ramping up their DNA repair mechanisms. However, in the smooth muscle cells of the aorta, a decrease in MTH1 protein expression was observed. This potential impairment in DNA repair within smooth muscle cells could be a significant contributing factor to hypertension [138].
Oxidative stress and its damaging effects on DNA have been shown as a potential culprit in heart failure. Elevated levels of 8-oxoG were observed in both the urine and heart tissue samples of HF patients. It was correlated with the activation of the ERK-MAPK pathway and increased MTH1 expression (Fig. 7). While MTH1 usually acts as a protector but the simultaneous rise in MTH1 expression and DNA damage (8-oxoG) hints that MTH1 activity might be dysregulated in failing hearts. This could lead to MTH1 unintentionally contributing to higher 8-oxoG levels, potentially worsening damage to cardiomyocytes [139]. Further research is crucial to understand the exact mechanisms at play and explore whether targeting MTH1 activity could be a new strategy to combat HF.
8.3. MTH1 in skin diseases
Chronic inflammation is thought to be perpetuated by the continuous generation of ROS [140]. During inflammation, the actions of infiltrating immune cells like neutrophils and macrophages lead to a significant increase in the production of pro-oxidant molecules. A growing body of evidence suggests a critical link between disrupted cellular balance (redox regulation), impaired repair mechanisms, and DNA damage in various inflammatory diseases. However, despite this established connection, the potential of inhibiting MTH1, as a therapeutic approach for inflammatory conditions remains unexplored [141].
Psoriasis is a chronic inflammatory condition affecting the skin characterized by the appearance of red, thickened patches with flaky white scales. This happens because of the proliferation of keratinocytes [142]. Psoriasis is a non-cancerous condition with maintained cellular control and exhibits some characteristics similar to cancer. These shared features include abnormal cell differentiation, increased cell proliferation, and angiogenesis. This heightened cellular activity within psoriatic lesions disrupts the local balance between oxidants and antioxidants, leading to a state of redox imbalance. Studies have suggested that elevated ROS levels play a significant role in the development of psoriasis [143].
In a study, MTH1 inhibition was investigated on skin inflammation driven by a specific type of immune cell (T helper type 17 cells) by using a well-established mouse model for psoriasis induced by a molecule called IMQ [144]. This model mimics human psoriasis because it depends on specific immune signaling molecules (IL-17 and/or IL-23) and closely resembles the characteristics of human psoriatic lesions. It was found that inhibiting MTH1 with TH1827 significantly improved the psoriatic features in mice (Fig. 7).
Specifically, it reduced the characteristic histological signs of psoriasis in the skin and dramatically decreased the number of neutrophils that had infiltrated the deeper layers of the skin and lymph nodes [145]. The role of MTH1 in a mouse model of psoriasis was investigated in a recent study. MTH1 levels were found to be elevated in psoriatic skin lesions and inhibiting MTH1 reduced both ROS and oxidative stress, along with IL-17-mediated inflammation [146]. These findings suggest that MTH1 inhibition might be a promising therapeutic strategy for treating psoriasis.
CMM stands out as the type of cancer with the most mutations in its genes on average. This high burden of mutations, along with how the DNA damage response functions in these cells, have been shown to influence how CMM progresses and how well it responds to treatment [147]. MTH1 is mostly overexpressed in various cancers, including CMM [148]. In a recent study, it was found that combining an MTH1 inhibitor, TH1579, with a BRAF inhibitor led to a more significant increase in cell death in CMM cells with the BRAFV600 mutation compared to using either inhibitor by itself [149]. Melanoma cells with higher sensitivity to MTH1 inhibitor-induced apoptosis also had higher levels of naturally occurring ROS within the cells. This suggests that the overexpression of MTH1 in cancer cells, like melanoma, might be a mechanism for these cells to protect their genetic integrity under conditions of oxidative stress [150].
A protein complex called the Skp1-Cullin-F-box (SCF) ubiquitin ligase mediates the key mechanism behind the upregulation of MTH1 in melanoma cells. Specifically, the F-box protein Skp2 within this complex promotes a specific type of protein modification (K63-linked polyubiquitination) that stabilizes MTH1. It utilizes MTH1 to safeguard the integrity of the melanoma cell genome during oxidative stress. The activation of the MAPK pathway protects melanoma cells from oxidative stress by promoting the Skp2/MTH1 axis [151]. TH1579, a powerful MTH1 inhibitor, has been studied as a promising therapeutic strategy for CMM. TH1579 effectiveness goes beyond just inhibiting MTH1.
It also triggers the downregulation of key proteins (AXL and CAV-1) involved in resistance to BRAF inhibitors. This combined effect leads to a multi-pronged attack on cancer cells: increased incorporation of damaged nucleotides, a rise in intracellular ROS, and mitotic arrest [149].
9. Inhibitors to MTH1
MTH1 is crucial in cancer research due to its role in countering stress damage caused from ROS and maintaining gene stability. This has given rise to the development of various MTH1 inhibitors and new treatments based on these inhibitors. With significantly higher expression in tumor cells than in normal cells, MTH1 is a key target for cancer therapy, offering potential for targeted treatments that disrupt cancer cell growth while sparing healthy tissues. These targeted therapies aim to create more potent and less harmful treatments for different cancer types. Ongoing research is actively investigating MTH1 inhibitors as a promising approach to disrupt cancer cell growth and improve treatment effectiveness.
In the same line, FDA-approved PARP inhibitor Olaparib in 2014, an antitumor drug targeting DNA damage repair pathway, emerged as a notable step towards advancing such targeted therapies in cancer treatment [152]. Since 2014, a variety of MTH1 inhibitors have emerged from different origins. Here we have consolidated the inhibitors along with their half-maximal inhibitory concentrations (IC50s), which were based on their inhibitory potency against 8-oxo-dGTP hydrolyzed by MTH1. This data facilitates a clear comparison of inhibitor activities.
9.1. Natural products as MTH1 inhibitors
Natural compounds are promising sources of anticancer agents, providing viable alternatives to synthetic drugs in cancer therapy. These substances can enhance the efficacy of synthetic treatments by lowering dosage requirements and reducing harmful side effects. Inhibitors of MTH1 have also been identified among natural products.
Dong et al. [153] identified echinacoside, a phenol-containing natural compound isolated from medicinal plants such as Cistanche and Echinacea, as an effective inhibitor of MTH1 with an IC50 value of 7 μmol/L (Table 1). The application of echinacoside led to increased levels of oxidative damage in various human cancer cell lines, resulting in a sudden rise in DNA damage markers and the induction of p21 overexpression. This cascade led to significant apoptotic cell death and cell cycle arrest, specifically in cancer cells, demonstrating echinacoside selectivity against cancerous cells while sparing normal cells [153]. In another study, eight farnesyl phenolic (FP) compounds (FP-1 to 8) containing a coumaroyl moiety were extracted from the fruiting bodies of Ganoderma sinense. These compounds exhibited inhibitory activity against MTH1, with IC50 values in the range of 8.6–28.5 μmol/L (Table 1). They displayed strong specificity towards MTH1 and showed cytotoxic effects and selectivity against various carcinoma cell lines [154].
Table 1.
List of MTH1 inhibitors.
| S. No. | Inhibitors | Class | IC50 values | Treatment time and cell types | Methods | Functions | References |
|---|---|---|---|---|---|---|---|
| 1. | Echinacoside | phenol | 7 μmol/L | 12 h s and Human MG63 osteosarcoma, SK-HEP-1 hepatocarcinoma, MCF7 breast cancer, SW480 colorectal cancer | Colony formation assay, MTT assay, Western blot, DAPI-stained nuclei | Increased oxidative damage and P21 overexpression | [153] |
| 2. | FP 1-8 | farnesyl phenolic | 8.6–28.5 μmol/L | 48 h s, U2OS, SW620, ES-2, | CCK8 assay method, Cellular thermal shift assay (CETSA) | Induced DNA damage and obstruct DNA replication in cancer cells | [154] |
| 3. | α-mangostin | xanthone | 0.47 μmol/L | 30 min s | Malachite green inhibition assay | Binds to the active site of MTH1 and inhibits its activity | [155] |
| 4. | 3-isomangostin | xanthone | 52 nmol/L | Possess strong MTH1 inhibitory potency | [155] | ||
| 5. | Thymoquinone | flavonoids | 13.5 μM | 72 h s, MCF7 breast cancer | MTT assay, annexin-V and Calcein-AM/PI double staining and MitoSOXTM Red staining | Impose oxidative stress leading to cell death. | [71] |
| 6. | Baicalin | flavonoids | 38.9 μM | Binds to the active site and inhibits MTH1 activity | [71] | ||
| 7. | Fisetin | flavonoids | 33.67 μM | 24 h s, squamous cell carcinoma cell line A431 | MTT assay, colony formation assay, Cell migration assay, DCFH-DA dye, TMRM dye | Induces apoptosis by downregulating MTH1 | [156] |
| 8. | Emodin | hydroxy-anthraquinone | 32.71 μM | 72 h, NSCLC and A549 | Mitochondrial Membrane Potential (ΔΨM) Assay, Annexin V/PI Apoptosis Analysis, Comet Assay, TUNEL assay | Elevate ROS burden and inhibit dNTP pool sanitization | [157] |
| 9. | TH287 | Pyrimidine Analogues | 0.8 nmol/L | 24 h, BGC-823 and SGC-7901 | Transwell and scratch assays, JC-1 staining, | Affect mitochondrial function and block PI3K/AKT signaling | [14,85,[158], [159], [160]] |
| 10. | TH588 | 7.0 nmol/L | Human pancreatic carcinoma (MIAPaCa-2 and Panc-1), lymphoma (BALM3), and leukemia (NB4) | MTT assay, FITC-labeled avidin immunofluorescence experiments | Inhibit microtubule polymerization | [14,85,158,160] | |
| 11. | TH1579 | obstructs mitosis and trigger spindle assembly checkpoint | [56,105,149,161,162] | ||||
| 12. | Crizotinib | Small molecule inhibitors | 1.4–4.3 μmol/L | 24–72 h, A549, HCC and H1975 | MTT, XTT, or WST-1 assay, Annexin V/PI Staining, TUNEL Assay |
targets ALK, HGFR, c-MET), ROS1 (c-ros) | [[163], [164], [165]] |
| 13. | (S)-Crizotinib | 330 nmol/L | induces the accumulation of DNA single-strand breaks | [107,166,167] | |||
| 14. | SCH 5 1344 | 410 nmol/L | 2–3 days, KRAS tumor cells | Silencing of MTH1 by siRNA | inhibit RAS-overexpressing fibroblast growth | [15] | |
| 15. | Imiquimod (TLR7 agonist) | 0.65 μmol/L | 72 h s, precancerous dysplastic oral keratinocyte (DOK) cell line, squamous cell carcinoma cell line A431 | MTT viability assay, Annexin V and 7-aminoactinomycin D (7AAD) double staining, | Induces cytokine production | [15,145] | |
| 16. | Resiquimod (TLR7 agonist) | produce antiviral cytokines, including IFN-α and TNF-α | [15,168] | ||||
| 16. | Amino pyrimidines, AP1-12 | Pyrimidine Analogues | 0.2 nmol/L to 4 μmol/L | dGTP was used to determine the IC50 values. | Inhibit MTH1 by binding to the active site of MTH1 | [15,169] | |
| 17. | MI-743 | 91.44 nmol/L | 72 h s, gastric cancer cell lines MGC-803 and HGC-27 cells, | cellular thermal shift assay (CETSA) | Trigger DDR and apoptosis | [13,102,170] | |
| 18. | MI-401 | 461.32 nmol/L | build-up of 8-oxo-dG lesions | [13,102,170] | |||
| 19. | purinone (P) analogues, P1-2 | Purine analogues | 2.8 μmol/L | P-2, PM-1 and PM-3 tested in K562 cells by CETSA. | dGTP was used for determining the IC50 values | Inhibit MTH1 activity | [15,168] |
| 20. | purinone macrocycle analog, PM1-5 | 0.3 nmol/L to 1.4 nmol/L | increase inhibitory effectiveness against MTH1 | [15,168] | |||
| 21. | fragments docked against MTH1 binding site, F1-5 | 6–79 μmol/L | Binds to the active site and inhibit MTH1 activity | [15,171] | |||
| 22. | Purine derivatives Pu1-8 | 3.3 to 0.21 μmol/L | Pu-3 and Pu-5: HeLa cells | target engagement assay | Binds MTH1 and induce DNA damage | [15,172,173] | |
| 23. | 3-amidoquinolines (AQ), AQ1-12 | Quinoline and quinazoline analoguesa | 0.9 nmol/L to 9 nmol/L | dGTP was used for determining the IC50 values | Enhanced inhibitory potency against MTH1 | [15,168] | |
| 24. | 2-aminoquinazoline (AZ), AZ1-4 | 3.32 μmol/L to 9 nmol/L | Binds to the active site and inhibit MTH1 activity | [15,168] | |||
| 25. | 7-azaindole (AI), AI1-15 | Indole and indazole analogues | 5 nmol/L to 23 μmol/L | AI-13 (BAY-707) | Caco-2 Permeability Assay | Binds to the active site and inhibit MTH1 activity | [15,174] |
| 26. | 7-azaindazole (AID) analogues, AID1-5 | 1–30 nmol/L | Accumulation of 8-oxoG concentrations, DNA damage, ensuing cell death | [15,174] | |||
| 27. | 7-deazadGTP | Nucleotide analogues, 8-halogenated 7-deazadGTP analogues |
1.57 μmol/L | Competitive inhibition with 8-oxo-dGTP, | [175,176] | ||
| 28. | 7-I-7-deazadGTP | 2.62 μmol/L | 24 h s, HeLa cells | cell titer assay | |||
| 29. | 8-Cl-7-deazadGTP | 0.857 μmol/L | dGTP was used for determining the IC50 values | ||||
| 30. | 8-Br-7-deazadGTP | 0.496 μmol/L | |||||
| 31. | 8-I-7-deazadGTP | 0.415 μmol/L | |||||
| 32. | Cd (II) and Cu (II) | Metalated analogues | 30 and 17 μmol/L | 2 h s, CHO cells | HPLC | Enhanced levels of 8-oxo-dG lesion in DNA | [15,177] |
| 33. | CR1-4 | 151 μmol/L to 6 nmol/L | 8-oxo-dGTP was used for determining the IC50 values | HPLC | Binds to the active site and suppress MTH1 activity | [178] |
Interestingly, X-ray crystallographic screening discovered α-mangostin, a natural xanthone extracted from mangosteen pericarp, as an MTH1 inhibitor, binding to its active site and inhibiting its activity with an IC50 value of 0.47 μmol/L. An inhibition assay later found that 3-isomangostin, a cyclized derivative of α-mangostin, was the most effective MTH1 inhibitor, with an IC50 of 52 nmol/L (Table 1), displaying a novel binding mode. Researchers speculated that mangostins could exert their antitumor effects across various cancer cells by suppressing MTH1 activity [155]. In the same line, we have investigated a few phytochemicals and discovered thymoquinone and baicalin as potent inhibitors to MTH1 with IC50 values of 13.5 μM and 38.9 μM. Both flavonoids, thymoquinone and baicalin, displayed stable complex formation with MTH1, as observed by MD simulations. Both compounds exhibited superior cytotoxic and anti-mitotic behavior towards breast cancer cells [71]. Fig. 8 contains the structures of all the mentioned natural products as MTH1 inhibitors.
Fig. 8.
Natural Inhibitors of MTH1. (A) Echinacoside, (B) alpha-mangostin, (C) Baicalin, (D) Thymoquinone, (E) 3-isomangostin, (F) farnesyl phenolic scaffold. (G) Fisetin, (H) Emodin.
Another study reports Fisetin, a natural flavonoid, demonstrating anticancer properties against skin cancer cell lines A375 and A431. Fisetin induces apoptosis and cell cycle arrest at the G0/G1 phase by increasing ROS, decreasing mitochondrial membrane potential, and elevating apoptotic cell counts. In-silico studies confirmed strong binding affinity for MTH1. These findings showed downregulation of MTH1 and upregulation of ATM and p53, suggesting anticancer effects of Fisetin [179]. Similarly, Emodin, natural hydroxy-anthraquinone isolated from the roots and rhizomes of a number of medicinal plants, has been shown to inhibit MTH1 in NSCLC cells, leading to impaired cell growth and inducing senescence. Treatment with emodin increases cellular ROS levels, damages dNTP pools, and inhibits MTH1 function. These findings suggest the anticancer effects of emodin hold clinical potential for NSCLC treatment [157].
9.2. Synthetic Inhibitors of MTH1
9.2.1. Pyrimidine analogues as MTH1 inhibitors
Petrocchi et al. developed the aminopyrimidine (AP) analog AP-1 as an MTH1 inhibitor (IC50 = 4 μmol/L). Adding a dimethyl group to AP-1 created AP-2 (IC50 = 0.14 μmol/L) with 26-fold enhanced potency. AP-3 (IC50 = 53 nmol/L), lacking the terminal hydroxyl group of AP-2, showed improved potency. Introduction of a methyl group at the 5-position increased potency, seen in AP-4 (IC50 = 0.2 nmol/L) compared to AP-3, and AP-5 (IC50 = 0.6 nmol/L) compared to AP-2. However, chloride (AP-6, IC50 = 22 nmol/L) or methoxyl (AP-7, IC50 = 10 nmol/L) substitutions at the 5-position in AP-2 resulted in poorer inhibitors. Replacement of the alkoxy chain with an alkyl amine in AP-4 and AP-5 led to AP-8 (IC50 = 0.5 nmol/L) and AP-9 (IC50 = 3.1 nmol/L), respectively, demonstrating slightly lower but comparable potencies. Compounds like AP-10 (IC50 = 0.5 nmol/L), AP-11 (IC50 = 0.2 nmol/L), and AP-12 (IC50 = 1.8 nmol/L) with tertiary amines or carbon chains at the 4-position showed similar potency. AP-5 was highly selective for MTH1 without off-target kinase activity in a panel of 97 kinases. Despite excellent characteristics in permeability, solubility, and stability in rat and human systems, AP-5 and AP-4 demonstrated minimal anticancer effects in various human cell lines, even at concentrations up to 50 μmol/L, compared to TH287 and TH588 13,15,158.
Another study with 1000 compounds screened to yield two potential MTH1 inhibitors with 5-cyano-6-phenylpyrimidine structure, namely MI-743 (IC50 = 91.44 nmol/L) and MI-401 (IC50 = 461.32 nmol/L) (Fig. 9). MI-743 was found to have cellular-level interaction with MTH1. Also, MI-743 caused cytotoxicity and triggered the build-up of 8-oxo-dG lesions, DNA damage, and apoptosis in gastric cancer cell lines MGC-803 and HGC-27, showing anti-proliferative effects (IC50 = 2.91 μmol/L and 1.14 μmol/L, respectively) with minimal impact on normal cell lines (Table 1). Moreover, MI-743 triggered a DNA damage response and apoptosis, likely due to MTH1 inhibition. MI-743 showed significant antitumor effects in MGC-803 xenografts in mice without evident toxicity, indicating its potential as a gastric cancer treatment targeting MTH1 [13,102,170].
Fig. 9.
Synthetic Inhibitors to MTH1: (A) Aminopyrimidines analogues, AP1-7, (B) AP8-12, (C) MI-743, (D) MI-401, (E) Purinone macrocycle analog, PM1-5, (F) purinone (P) analogues, P1-2, (G) fragments docked against MTH1 binding site, F1 and F2, (H) F3 and F4, (I) Purine derivatives Pu1-8, (J) 3-amidoquinolines (AQ), AQ1-7, (K) 2-aminoquinazoline (AZ), AZ1-4, (L) 7-azaindole (AI), AI1-15, (M) 7-azaindazole (AID) analogues, AID1-5, (N) 8-halogenated 7-deazadGTP analogues, (O) Metalated analogues, CR1, (P) CR2-4.
9.2.2. Purine analogues as MTH1 inhibitors
A series of purinone (P) analogues were designed as MTH1 inhibitors with P-1 inhibiting MTH1 activity at IC50 value of 2.8 μmol/L which was further enhanced by P-2 i.e. N-methylation of P-1 13,15,160. Another monologue, PM-1 i.e. P-2 macrocyclization by n-pentyl bis-ether, exhibit 1700-fold increase inhibitory effectiveness against MTH1 (IC50 = 0.3 nmol/L). However, macrocyclization increased lipophilicity, improving membrane permeability but also raising in vitro clearance. Shortening the n-pentyl bis-ether in PM-1 to an n-butyl bis-ether linker in PM-2 reduced lipophilicity with a slight potency decrease (IC50 = 0.8 nmol/L). PM-2 with polar ether attached to all carbon side chain introduces PM-3 having similar potency (IC50 = 0.5 nmol/L) but reduced lipophilicity. PM-4 with amide group (IC50 = 1.4 nmol/L) exhibits enhanced polarity, low logD (1.8) but was not stable in human plasma same as tertiary amide analog PM-5. Compounds PM-1 and PM-3 showed strong correlation between MTH1 inhibitory potency and target engagement in K562 cells. PM-3, tested against 267 kinases and 153 pharmacology targets, displayed high selectivity for MTH1 with no notable off-target effects. PM-1 exhibited some activity in a few cell lines at high concentrations, while PM-3 had minimal impact on cell viability [13,15,168].
Another study applied structure-based virtual screening with 0.3 million fragments docked against the MTH1 binding site and discovered 22 commercially available fragment ligands. Experimental evaluation found five fragments, including purine analogues that inhibited MTH1 with IC50 values between 6 and 79 μmol/L (Table 1). Optimization of these fragments based on predicted binding modes led to several potential inhibitors. For example, F-1 (IC50 = 79 μmol/L) was optimized to F-2 (IC50 = 170 nmol/L), and F-3 (IC50 = 24 μmol/L) was optimized to F-4 (IC50 = 3.5 μmol/L). Notably, one fragment matched a component of a compound discovered by Kettle et al. [13,171], demonstrating the strategy reliability.
Furthermore, Kumar and colleagues [171,173] utilized chemical array platforms to discover several purine (Pu) derivatives as MTH1 inhibitors from a chemical library of the RIKEN Natural Products Depository (Fig. 9). Pu-1 (NPD15095) inhibited MTH1 with an IC50 of 3.3 μmol/L. Further testing of 131 related compounds yielded seven more active inhibitors (Pu-2 to Pu-8) better than Pu-1. Pu-3 (NPD7155), Pu-5 (NPD9948), Pu-6, and Pu-8 were the most potent with IC50 values of 0.21, 0.29, 0.21, and 0.23 μmol/L, respectively (Table 1). Pu-3 and Pu-5 specifically stabilized MTH1 in HeLa cells but showed only weak cytotoxicity (IC50 = 65 and 35 μmol/L, respectively), likely due to inducing DNA damage only at higher concentrations.
9.2.3. Quinoline and quinazoline analogues as MTH1 inhibitors
The high-throughput screening was conducted and identified 3-amido quinolines (AQ) as potent MTH1 inhibitors [13,168], such as AQ-1 (IC50 = 0.9 nmol/L) and AQ-2 (IC50 = 12.5 nmol/L). The dimethyl analog AQ-3 showed significantly lower activity. Removing both halogens from AQ-1 resulted in AQ-4, which was more potent (IC50 = 0.5 nmol/L) and less lipophilic. Adding a basic group to AQ-4 generates AQ-5 with improved potency to 80 pmol/L and increased solubility. Removing both methoxy groups from AQ-1 created AQ-6 with slightly reduced potency (IC50 = 2 nmol/L), while further truncated quinoline, pyridyl AQ-7 retained some potency (IC50 = 9 nmol/L) (Fig. 9).
Likewise, 2-aminoquinazoline (AZ) was identified as MTH1 inhibitor. The initial hit compound, AZ-1, showed moderate potency with an IC50 of 3.32 μmol/L (Table 1). Dimethylation of the amine to produce AZ-2 reduced potency four-fold. Interestingly, removing the methyl group in AZ-1 to create AZ-3 significantly enhanced potency against MTH1 (IC50 = 21 nmol/L). Furthermore, the dimethylamide AZ-4 exhibited even higher potency against MTH1 (IC50 = 9 nmol/L) and favorable physicochemical properties. Furthermore, three representative inhibitors (AQ-1, AQ-4, and AZ-4) demonstrated effective engagement with MTH1 in K562 cells and high specificity. However, AQ-1 exhibited only minimal antiproliferative effects at high concentrations in some tumor cell lines, while AQ-4 showed nearly no activity. Further studies revealed that AQ-1 did not induce an increase in DDR signaling markers, which may explain its limited effectiveness [13,15,168].
9.2.4. Indole and indazole analogues as MTH1 inhibitors
Rahm et al. identified a 7-azaindole (AI) fragment hit through fragment-based screening, with cyclic amines at the 4-position using structure-based drug design and medicinal chemistry approaches. Compounds AI-1 to AI-5 were developed, with AI-2 featuring a 2-phenylpyrrolidine showing high potency (IC50 = 5 nmol/L). AI-2 underwent secondary amide substitution, creating AI-6 with notable potency (IC50 < 0.1 nmol/L). AI-7, the replacement of the phenyl group with pyridine in AI-6, exhibits enhanced hydrophilicity and microsomal stability. Further modifications with pyrrolidine-based and secondary amide substituents led to AI-8 to AI-11 (Fig. 9), where AI-10 and AI-11 showed high potency, moderate microsomal stability, and medium permeability. Also, AI-10 displayed 1000-fold selectivity against other tested targets including kinases and ATPases. Four new compounds, AI-12 to AI-15, were designed to improve cell permeability and metabolic stability, showcasing picomolar potency, high solubility, permeability, and acceptable metabolic stability. AI-13 was selected for further in vivo studies, but did not demonstrate anticancer efficacy in vitro or in vivo, alone or in combination therapies [13,174].
Alongside the above purine fragments, fragment F-5 was designed (IC50 value 23 μmol/L), optimizing it further to F-6 with an IC50 of 120 nmol/L. The SB2001, an indazole analog, inhibits MTH1 (IC50 = 3.57 μmol/L), inducing oxidative damage and cytotoxicity in defective HeLa cells. Another MTH1 inhibitor, 7-azaindazole (AID) analogues showed a similar activity (IC50 3.0 μmol/L) to SB2001, and AID-2 to AID-5 exhibiting stronger inhibitory activities with IC50 values in the range 1–30 nmol/L [13,174].
9.2.5. Nucleotide analogues as MTH1 inhibitors
While MTH1 degrades 8-oxo-dGTP and 2-OH-dATP, it cannot hydrolyze their diphosphate forms, which can instead bind and inhibit MTH1 activity. However, other human Nudix proteins like MTH2 and MTH3 can hydrolyze these diphosphates, reducing their potential as antitumor agents. Nucleotide analogues were used as MTH1 inhibitors where 8-halogenated 7-deazadGTP (mimics of 8-oxo-dGTP), inhibited MTH1 despite minor differences at the 7- and 8-positions. 7-deazadGTP and 7-I-7-deazadGTP had an IC50 value of 1.57 μmol/L and 2.62 μmol/L respectively, while 8-Cl-7-deazadGTP, 8-Br-7-deazadGTP, and 8-I-7-deazadGTP showed enhanced inhibitory activities with IC50 values of 0.857, 0.496, and 0.415 μmol/L, respectively (Table 1), attributed to increased π−π interactions in MTH1 active site. Further research could explore their anticancer potential through nucleoside kinase activation or efficient delivery. The potency of 7-deazadGTP analogues is likely due to enhanced π−π interactions with Trp117 and Phe72 in MTH1 active site, facilitated by the 7-CH substitution. Activating 8-halogenated 7-deazadG analogues through nucleoside kinases or effective delivery could enhance their anticancer efficacy [13,175,176].
9.2.6. Metalated analogues as MTH1 inhibitors
Transition metal ions like Cd (II) and Cu (II) were identified as MTH1 inhibitors [15,177] with IC50 values of 30 and 17 μmol/L, respectively (Table 1). Streib et al. [178] developed unconventional cyclometalated ruthenium (CR) half-sandwich complexes that showed potent inhibition against MTH1 with low-nanomolar IC50 values, demonstrating specificity through testing with a diverse panel of proteins. Initially, CR-1 bound to MTH1 with an IC50 of 151 μmol/L, but this was enhanced by over 100-fold in CR-2 by substituting its 8-(pyridin-2-yl) adenine ligand with cyclometalated 4-amino-6-(pyridin-2-yl) quinazoline, achieving an IC50 of 1.1 μmol/L (Fig. 9). Derivatizing the cyclopentadienyl moiety yielded complex CR-3 (IC50 35 nmol/L), and introducing a methyl group at the 2-position of the quinazoline in CR-3 led to CR-4 (IC50 = 6 nmol/L) MTH1 inhibitor [13,178].
9.3. MTH1 inhibitors under clinical studies
9.3.1. TH287 and TH588
The efficacy of MTH1 inhibition in cancer management was observed by using siRNA technology, where knocking down MTH1 inhibited the proliferation of U2OS cells and had little to no effect on VH10 cells [14]. This discovery led to the identification of TH287 and TH588 as potent inhibitors, with IC50 values of 0.8 nmol/L and 7.0 nmol/L, respectively (Table 1). Overcoming the poor stability of TH287 involved substituting a methyl group with cyclopropyl in TH588, enhancing selectivity while slightly reducing MTH1 inhibitory potency. Both TH287 and TH588 induced the accumulation of oxidized dNTPs and exhibited cytotoxic effects in cancer cells sparing normal cells [14,15]. In vivo studies revealed that TH588 effectively halted the growth of subcutaneously transplanted melanoma, overcoming resistance to carboplatin, azelnimidamide, and verofenib treatments [15]. The crystal structures demonstrate that TH287 and TH588 binds to the binding pocket of the MTH1 in the same manner as 8-oxo-dGTP and interact with the key residues of the binding site of MTH1 [158]. Also, TH287 and TH588 demonstrated specific selectivity for MTH1 on evaluation for inhibition against other nudix proteins (MTH2, NUDT5, NUDT12, NUDT14, and NUDT16), nucleoside triphosphate pyrophosphatases (dCTPase, dUTPase, and ITPA), and a broad panel of 87 enzymes including GPCRs, kinases, ion channels, and transporters [13].
TH287 and TH588 selectively targeted SV40-large-T-cells and Ras-expressing BJ cells, suggesting a crucial role of MTH1 in early transformation due to its increased expression [13]. TH287 has demonstrated significant potential in gastric cancer research by affecting mitochondrial function and blocking the PI3K/AKT signaling pathway, making it a promising candidate for targeted treatment [168]. TH588 was tested in mice with BRAFV600E-mutated melanoma, SW480 colorectal, or MCF7 breast tumor xenografts, resulting in reduced tumor growth rates. This indicates that MTH1 is a promising target for various tumors [14]. TH588 also acts as a microtubule-targeting agent, inhibiting microtubule polymerization and reducing microtubule movement during cell division. This delay in cell division contributes significantly to its anticancer properties [180]. Both TH287 and TH588 have advanced into clinical trials, signifying a significant milestone in their development as potential cancer treatments. This progression highlights their potential to provide novel therapeutic avenues by targeting specific molecular pathways crucial in cancer advancement [14].
9.3.2. TH1579 (karonudib)
TH1579 (Karonudib) is an enhanced version of TH588 with superior pharmacokinetics and oral bioavailability, advancing cancer therapeutics. Currently undergoing phase I clinical trials, TH1579 aims to validate its efficacy against various solid tumors [NCT03036228] and hematologic cancers [NCT04077307], marking a pivotal stage in evaluating its clinical potential [169]. TH1579 has exhibited exceptional selectivity, promoting the buildup of oxidative DNA damage through MTH1-dependent mechanisms. Its effectiveness has been validated across diverse models, including human colon cancer and mouse xenografts derived from chemotherapy-resistant malignant melanoma, affirming TH1579 as a potent therapeutic agent for cancer treatment [181].
Mechanistically, TH1579 operates like TH588 where it obstructs mitosis and trigger spindle assembly checkpoint leading to enhanced ROS levels which oxidizes dGTP pool causing an accumulation of 8-oxo-dGTP. During mitotic replication, this oxidized nucleotide is incorporated into DNA, effectively targeting and eliminating cancer cells. TH1579 has shown synergistic effects when combined with BRAF inhibitors, particularly in cells harboring BRAF mutations, where it has shown advancing tumor suppressive effects in vivo and ex vivo studies, which collectively indicate a promising combinatorial approach in cancer therapeutics [79,149].
In immunotherapy, TH1579 does not compromise anti-CTLA-4 antibody-induced antitumor responses in melanoma, nor does it interfere with tumor suppressor activity from autologous tumor-infiltrating lymphocytes (TILs) in a melanoma PDX model. This suggests TH1579 does not disturb T-cell-mediated cytotoxicity in vivo, which is crucial for combined immunotherapy strategies [182]. In summary, the progression and clinical trials of TH1579 signify a significant breakthrough in oncology, promising novel therapeutic avenues for patients with diverse cancer types.
9.3.3. Crizotinib
A study highlighted the role of MTH1 in KRAS mutant tumor cells and utilized a chemical proteomic approach to identify it as a primary cellular target of SCH 5 1344 (IC50 410 nmol/L), a compound previously shown to inhibit the growth of RAS-overexpressing fibroblasts. SCH 5 1344 led to the identification of Crizotinib, a kinase inhibitor widely used as a dual c-MET/ALK inhibitor. Clinically approved in 2011 for treating EML4-ALK-positive NSCLC, Crizotinib was also found to inhibit MTH1 at nanomolar concentrations [170]. Conversely, it is (S)-crizotinib rather than (R)-crizotinib that specifically inhibits MTH1 catalytic activity with an IC50 value of 330 nmol/L (Table 1). This is because (S)-crizotinib forms fewer hydrogen bonds with amino acid residues within the MTH1 active pocket. This selective interaction increases its specificity efficacy in targeting tumor cells with RAS mutations, presenting a potential therapeutic approach for such mutations-associated cancers [15].
(S)-crizotinib, with its high selectivity for MTH1, has been shown to effectively inhibit colony formation in SW480 cells and KRAS-mutated PANC1 cells. Similarly, in human colon carcinoma cells, (S)-crizotinib induces the accumulation of DNA single-strand breaks, activating DNA repair mechanisms and efficiently inhibiting tumor proliferation in mouse xenograft studies with SW480 cells [13]. In another study some agonists of Toll-like receptor 7 (TLR7) were identified that can act as MTH1 inhibitors. Notably, imiquimod, the only small-molecule TLR7 agonist approved by the FDA, displayed substantial submicromolar inhibitory potency towards MTH1 with IC50 0.65 μmol/L. In comparison, another TLR7 agonist, resiquimod, was found to be three times less potent [171].
The fundamental and clinical prospects of MTH1 interventions described above represent a promising frontier in cancer therapy. Ongoing clinical trials of new MTH1 inhibitors are critical to assess their safety, tolerability, and preliminary efficacy in cancer patients. Positive outcomes could pave the way for further development and wider clinical use. Exploring MTH1 inhibitors in combination with existing chemotherapies or targeted therapies may enhance therapeutic outcomes, particularly in tumors with high oxidative stress. Clinical trials investigating these combinations could provide valuable data on synergistic effects. Integrating genetic profiling into clinical practice can help identify patients who are more likely to benefit from MTH1 inhibition. This stratification could lead to more successful outcomes and reduce unnecessary exposure to ineffective treatments.
Recent advancements in the development of MTH1 inhibitors have yielded several promising compounds and highlighted important considerations for future research directions. Researchers have identified novel purine-based MTH1 inhibitors that demonstrate selective targeting of the enzyme. For example, compounds have been developed that are structurally distinct from earlier inhibitors, offering new avenues for exploration. Enhanced understanding of the structure-activity relationship has allowed scientists to refine existing inhibitors, improving their potency and specificity for MTH1. This could lead to more effective therapeutic options with fewer off-target effects. There has been an increasing focus on combining MTH1 inhibitors with other treatment modalities, such as chemotherapy or immunotherapy. These combinations aim to exploit synergistic effects, potentially overcoming resistance mechanisms in cancer cells. Some recent studies have focused on tailoring MTH1 inhibitors to specific cancer types, identifying biomarkers that may predict sensitivity to MTH1 inhibition. This personalized approach could enhance treatment efficacy. Advances in high-throughput screening and better in vitro models have facilitated the discovery of more selective MTH1 inhibitors, allowing for more rigorous evaluation of their therapeutic potential.
Future studies should prioritize validating the specificity of new MTH1 inhibitors to ensure that observed therapeutic effects are indeed due to MTH1 modulation, not off-target actions. Understanding the mechanisms that allow cancer cells to survive despite MTH1 inhibition is crucial. Future research should investigate how alternative DNA repair pathways compensate for MTH1 loss. Continued emphasis on clinical trials for new MTH1 inhibitors is essential. Identifying biomarkers for patient selection can optimize therapeutic strategies and improve outcomes. Further exploration of combination therapies involving MTH1 inhibitors alongside other treatments may reveal more effective strategies to combat cancer and overcome resistance. Detailed studies examining the interactions between MTH1 and various cellular signaling pathways will provide deeper insights into its role in cancer biology, potentially unveiling new therapeutic targets.
While significant advancements in MTH1 inhibitor development have been made, aligning these efforts with strategic future research directions is essential for maximizing their therapeutic potential in cancer treatment. By addressing specificity, resistance mechanisms, and combination strategies, researchers can better harness the potential of MTH1 as a therapeutic target.
Addressing challenges in targeting MTH1 is essential for understanding its therapeutic potential. This includes resistance mechanisms where cancer cells may activate alternative DNA repair pathways or other nudix hydrolase enzymes in response to MTH1 inhibition, which could reduce treatment effectiveness [14]. The tumor microenvironment is where factors secreted by stromal or immune cells may enhance cancer cell survival, complicating MTH1 inhibitor efficacy [183]. The off-target effects and toxicity of MTH1 inhibitors may affect normal tissues, particularly in high-turnover or oxidative stress environments, leading to cytotoxicity [169]. Balancing tumor suppression with minimal damage to healthy tissues is critical. Also, many MTH1 inhibitors may have unintended effects on other pathways, leading to non-specific toxicity. Developing more selective inhibitors is crucial for minimizing off-target interactions and improving safety [166].
10. Conclusion and future prospects
MTH1 plays a significant role in cancer development and various other diseases. Targeting the MTH1 signaling pathway emerges as a promising therapeutic approach. This review discusses recent discoveries that emphasize the potential of MTH1 involvement in tumorigenesis, positioning it as a new target for cancer treatment. It also provides a summary of various compounds that inhibit MTH1, including both naturally occurring and synthetic ones, with some currently undergoing different phases of clinical trials.
However, recent studies with the first wave of MTH1 inhibitors have produced conflicting results regarding their cytotoxicity in cancer cells, leading to questions regarding the validity of MTH1 as a chemotherapeutic target.
For instance, Kawamura et al. [173] identified new purine-based MTH1 inhibitors that targeted cellular MTH1 but had weak cytotoxic effects on cancer cells compared to existing first-in-class inhibitors. The antitumor effects were due to their action on tubulin, not MTH1 inhibition. Overexpression of MTH1 did not protect cells from inhibitor-induced death, and MTH1 knockdown did not affect cancer cell growth. Thus, the cytotoxicity of MTH1 inhibitors was due to off-target effects, and MTH1 is not essential for cancer cell survival. Similarly, kettle et al. [168] observed a significant difference in responses between their MTH1 inhibitors and previously reported tool compounds, indicating that the cytotoxic effects observed with the latter may be due to off-target activities rather than specific inhibition of MTH1. Therefore, the precise role of MTH1 in cancer development and the therapeutic potential of its inhibition remain uncertain and require further investigation.
Also, it remains unclear at what concentrations oxidized nucleotides become lethal to cells, partly due to the lack of reliable methods to measure cellular levels of these free oxidized nucleotides. Consequently, direct evidence connecting MTH1 function to the effects of incorporating these oxidized nucleotides into the DNA of cancer cells is still not well understood. In fact, deleting the bacterial or mouse MTH1 homolog does not result in cellular death, even though it leads to the accumulation of thousands of mutations [184]. This could be because the Nudix family of enzymes, which includes MTH1, comprises over 20 genes, and there are other DNA damage repair pathways, such as base-excision repair, that can repair oxidized bases in DNA. This redundancy may enable cellular survival and nullify the claimed role of MTH1 being essential for cancer-cell survival. Cancer is a multifaceted disease involving dysfunction across multiple systems. Even when MTH1 is inhibited, cancer cells might continue to survive due to other signaling pathways that can bypass the effects of MTH1 inhibition. Therefore, it's crucial to pharmacologically validate the specific roles of MTH1, other Nudix enzymes, and DNA damage repair pathways in cancer progression. Comprehensive studies on MTH1 and its related signaling networks in cancer and other diseases are needed to provide solid evidence for considering MTH1 as a viable drug target.
This review highlights significant advancements in understanding MTH1 as a therapeutic target; however, it is not without limitations. First, the review primarily synthesizes existing literature, which may lead to potential biases in interpreting conflicting results regarding MTH1 inhibitors. Additionally, the variability in experimental conditions, such as cell types and assay methodologies, complicates the generalization of findings across studies. The review also emphasizes the need for reliable methods to measure cellular levels of oxidized nucleotides, yet it does not explore potential technological advancements that could address this gap. Furthermore, while this review discusses various MTH1 inhibitors, it lacks a detailed analysis of their pharmacokinetics and pharmacodynamics, which are crucial for understanding their clinical applicability.
In addition to MTH1, various other enzymes, and signaling pathways play vital roles in regulating oxidative stress. While MTH1 prevents oxidized nucleotides from being incorporated into DNA, other antioxidant enzymes are known to regulate or maintain the redox balance. Some of these enzymes include superoxide dismutase, which converts superoxide radicals into hydrogen peroxide [185], and catalase, which breaks down hydrogen peroxide [186]. Glutathione peroxidase reduces hydrogen peroxide and lipid peroxides using glutathione [187], while peroxiredoxins and the thioredoxin system repair oxidized proteins [188,189]. The Nrf 2 pathway regulates antioxidant gene expression, and glutathione acts as a major intracellular antioxidant, preserving redox homeostasis [190]. Thus, MTH1 primarily prevents oxidized nucleotides from entering DNA, and it operates within a broader network of antioxidant enzymes that regulate the cellular response to oxidative stress. Further research is required to fully understand this comprehensive network.
Future research should focus on the comprehensive characterization of MTH1 and its signaling networks within the context of cancer biology. There is an urgent need for conducting a rigorous validation of MTH1 inhibitors to ascertain their true mechanism of action and off-target effects and investigating the interplay between MTH1 and other DNA repair pathways in cancer cells to identify critical nodes for intervention. It is important to focus the research into the development of next-generation MTH1 inhibitors that could achieve greater specificity and efficacy by expanding research into the development of next-generation MTH1 inhibitors that could achieve greater specificity and efficacy. Developing advanced methodologies for accurately measuring cellular oxidized nucleotide levels will be crucial in elucidating precise role of MTH1 in DNA repair and mutation accumulation. By addressing these limitations and pursuing these future directions, the potential of MTH1 as a viable therapeutic target can be better evaluated, for innovative cancer treatments.
CRediT authorship contribution statement
Aaliya Taiyab: Writing – original draft, Visualization, Funding acquisition, Formal analysis, Data curation, Conceptualization. Anam Ashraf: Investigation, Funding acquisition, Formal analysis, Data curation. Md Nayab Sulaimani: Writing – original draft, Validation, Software, Methodology, Investigation. Aanchal Rathi: Writing – review & editing, Software, Resources, Project administration, Methodology, Conceptualization. Anas Shamsi: Writing – review & editing, Software, Methodology, Investigation, Funding acquisition, Formal analysis. Md Imtaiyaz Hassan: Writing – review & editing, Visualization, Resources, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
Not required.
Funding
This research has been funded by the Ministry of Human Resource Development (MHRD), under the Prime Minister Research Fellowship Scheme (F.No.4l–1l2018-TS-1).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
MIH acknowledges the Council of Scientific and Industrial Research for financial support [Project No. 27(0368)/20/EMR-II]. AS thanks to the Ajman University for the payment of APC.
Contributor Information
Anas Shamsi, Email: anas.shamsi18@gmail.com.
Md Imtaiyaz Hassan, Email: mihassan@jmi.ac.in.
Data availability
No data was used for the research described in the article.
References
- 1.Zuo J., Zhang Z., Luo M., et al. Redox signaling at the crossroads of human health and disease. MedComm. 2020;3(2):e127. doi: 10.1002/mco2.127. Jun 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forman H.J., Zhang H. Author Correction: targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. Aug 2021;20(8):652. doi: 10.1038/s41573-021-00267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris I.S., DeNicola G.M. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. Jun 2020;30(6):440–451. doi: 10.1016/j.tcb.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Tejero J., Shiva S., Gladwin M.T. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol. Rev. Jan 1 2019;99(1):311–379. doi: 10.1152/physrev.00036.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. Jun 20 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 6.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. Jul 2020;21(7):363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 7.Rose Li Y., Halliwill K.D., Adams C.J., et al. Mutational signatures in tumours induced by high and low energy radiation in Trp 53 deficient mice. Nat. Commun. Jan 20 2020;11(1):394. doi: 10.1038/s41467-019-14261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sung H.Y., Choi E.N., Ahn Jo S., Oh S., Ahn J.H. Amyloid protein-mediated differential DNA methylation status regulates gene expression in Alzheimer's disease model cell line. Biochem. Biophys. Res. Commun. Nov 4 2011;414(4):700–705. doi: 10.1016/j.bbrc.2011.09.136. [DOI] [PubMed] [Google Scholar]
- 9.Gonchar O., Mankovska I., Rozova K., Bratus L., Karaban I. Nonel approaches to correction of mitochondrial dysfunction and oxidative disorders in Parkinson's disease. Fiziolohichnyi zhurnal. 06/10. 2019;65:61–72. doi: 10.15407/fz65.03.061. [DOI] [Google Scholar]
- 10.Poznyak A.V., Grechko A.V., Orekhova V.A., Khotina V., Ivanova E.A., Orekhov A.N. NADPH oxidases and their role in atherosclerosis. Biomedicines. Jul 10 2020;8(7) doi: 10.3390/biomedicines8070206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho C., Cardoso S. Diabetes-Alzheimer's disease link: targeting mitochondrial dysfunction and redox imbalance. Antioxidants Redox Signal. Mar 10 2021;34(8):631–649. doi: 10.1089/ars.2020.8056. [DOI] [PubMed] [Google Scholar]
- 12.Pradhan A., Bagchi A., De S., et al. Role of redox imbalance and cytokines in mediating oxidative damage and disease progression of patients with rheumatoid arthritis. Free Radic. Res. Jul 2019;53(7):768–779. doi: 10.1080/10715762.2019.1629586. [DOI] [PubMed] [Google Scholar]
- 13.Yin Y., Chen F. Targeting human MutT homolog 1 (MTH1) for cancer eradication: current progress and perspectives. Acta Pharm. Sin. B. 2020/12/01/2020;10(12):2259–2271. doi: 10.1016/j.apsb.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gad H., Koolmeister T., Jemth A.S., et al. MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool. Nature. Apr 10 2014;508(7495):215–221. doi: 10.1038/nature13181. [DOI] [PubMed] [Google Scholar]
- 15.Ding Y., Liu Q. Targeting the nucleic acid oxidative damage repair enzyme MTH1: a promising therapeutic option. Review. Front. Cell Dev. Biol. 2024-January-31 2024:12doi. doi: 10.3389/fcell.2024.1334417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagy G.N., Leveles I., Vértessy B.G. Preventive DNA repair by sanitizing the cellular (deoxy)nucleoside triphosphate pool. FEBS J. 2014;281(18):4207–4223. doi: 10.1111/febs.12941. [DOI] [PubMed] [Google Scholar]
- 17.Nyíri K., Mertens H.D.T., Tihanyi B., et al. Structural model of human dUTPase in complex with a novel proteinaceous inhibitor. Sci. Rep. Mar 12 2018;8(1):4326. doi: 10.1038/s41598-018-22145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broderick K., Moutaoufik M.T., Aly K.A., Babu M. Sanitation enzymes: exquisite surveillance of the noncanonical nucleotide pool to safeguard the genetic blueprint. Semin. Cancer Biol. Sep 2023;94:11–20. doi: 10.1016/j.semcancer.2023.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Zamzami M.A. Inosine triphosphate pyrophosphatase (ITPase): functions, mutations, polymorphisms and its impact on cancer therapies. Cells. Jan 24 2022;11(3) doi: 10.3390/cells11030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moroz O.V., Murzin A.G., Makarova K.S., Koonin E.V., Wilson K.S., Galperin M.Y. Dimeric dUTPases, HisE, and MazG belong to a new superfamily of all-alpha NTP pyrophosphohydrolases with potential "house-cleaning" functions. J. Mol. Biol. Mar 25 2005;347(2):243–255. doi: 10.1016/j.jmb.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 21.Treffers H.P., Spinelli V., Belser N.O. A factor (or mutator gene) influencing mutation rates in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. Nov 1954;40(11):1064–1071. doi: 10.1073/pnas.40.11.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koonin E.V. A highly conserved sequence motif defining the family of MutT-related proteins from eubacteria, eukaryotes and viruses. Nucleic Acids Res. Oct 11 1993;21(20):4847. doi: 10.1093/nar/21.20.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabelli S.B., Bianchet M.A., Bessman M.J., Amzel L.M. The structure of ADP-ribose pyrophosphatase reveals the structural basis for the versatility of the Nudix family. Nat. Struct. Biol. May 2001;8(5):467–472. doi: 10.1038/87647. [DOI] [PubMed] [Google Scholar]
- 24.Broderick K., Moutaoufik M.T., Aly K., Babu M. 94doi. 2023. Sanitation enzymes: exquisite surveillance of the noncanonical nucleotide pool to safeguard the genetic blueprint. (Seminars in Cancer Biology). 05/01. [DOI] [PubMed] [Google Scholar]
- 25.Carreras-Puigvert J., Zitnik M., Jemth A.S., et al. A comprehensive structural, biochemical and biological profiling of the human NUDIX hydrolase family. Nat. Commun. Nov 16 2017;8(1):1541. doi: 10.1038/s41467-017-01642-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mildvan A.S., Xia Z., Azurmendi H., et al. Structures and mechanisms of Nudix hydrolases. Arch. Biochem. Biophys. 2005;433:129–143. doi: 10.1016/j.abb.2004.08.017. 02/01. [DOI] [PubMed] [Google Scholar]
- 27.Kulikova V.A., Nikiforov A.A. Role of NUDIX hydrolases in NAD and ADP-ribose metabolism in mammals. Biochemistry (Mosc). Aug 2020;85(8):883–894. doi: 10.1134/s0006297920080040. [DOI] [PubMed] [Google Scholar]
- 28.Walter M., Mayr F., Hanna B.M.F., et al. NUDT22 promotes cancer growth through pyrimidine salvage. Oncogene. 2023/04/01 2023;42(16):1282–1293. doi: 10.1038/s41388-023-02643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samaranayake G.J., Huynh M., Rai P. MTH1 as a chemotherapeutic target: the elephant in the room. Cancers. May 8 2017;9(5) doi: 10.3390/cancers9050047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai J.P., Ishibashi T., Takagi Y., Hayakawa H., Sekiguchi M. Mouse MTH2 protein which prevents mutations caused by 8-oxoguanine nucleotides. Biochem. Biophys. Res. Commun. Jun 13 2003;305(4):1073–1077. doi: 10.1016/s0006-291x(03)00864-7. [DOI] [PubMed] [Google Scholar]
- 31.Takagi Y., Setoyama D., Ito R., Kamiya H., Yamagata Y., Sekiguchi M. Human MTH3 (NUDT18) protein hydrolyzes oxidized forms of guanosine and deoxyguanosine diphosphates: comparison with MTH1 and MTH2. J. Biol. Chem. Jun 15 2012;287(25):21541–21549. doi: 10.1074/jbc.M112.363010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter M., Jemth A.-S., Hagenkort A., et al. Crystal structure, biochemical and cellular activities demonstrate separate functions of MTH1 and MTH2. Nat. Commun. 2015/08/04 2015;6(1):7871. doi: 10.1038/ncomms8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashiguchi K., Hayashi M., Sekiguchi M., Umezu K. The roles of human MTH1, MTH2 and MTH3 proteins in maintaining genome stability under oxidative stress. Mutat. Res. Mar 2018;808:10–19. doi: 10.1016/j.mrfmmm.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Svensson L.M., Jemth A.-S., Desroses M., et al. Crystal structure of human MTH1 and the 8-oxo-dGMP product complex. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2011/08/19/2011;585(16):2617–2621. doi: 10.1016/j.febslet.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Scaletti E.R., Vallin K.S., Bräutigam L., et al. MutT homologue 1 (MTH1) removes N6-methyl-dATP from the dNTP pool. J. Biol. Chem. Apr 10 2020;295(15):4761–4772. doi: 10.1074/jbc.RA120.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamashita N., Kawahara M., Imai T., Tatsumi G., Asai-Nishishita A., Andoh A. Loss of Nudt15 thiopurine detoxification increases direct DNA damage in hematopoietic stem cells. Sci. Rep. Jul 24 2023;13(1) doi: 10.1038/s41598-023-38952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakabeppu Y. Molecular genetics and structural biology of human MutT homolog, MTH1. Mutat. Res., Fundam. Mol. Mech. Mutagen. 2001/06/02/2001;477(1):59–70. doi: 10.1016/S0027-5107(01)00096-3. [DOI] [PubMed] [Google Scholar]
- 38.Mishima M., Sakai Y., Itoh N., et al. Structure of human MTH1, a Nudix family hydrolase that selectively degrades oxidized purine nucleoside triphosphates. J. Biol. Chem. Aug 6 2004;279(32):33806–33815. doi: 10.1074/jbc.M402393200. [DOI] [PubMed] [Google Scholar]
- 39.Oda H., Taketomi A., Maruyama R., et al. Multi-forms of human MTH1 polypeptides produced by alternative translation initiation and single nucleotide polymorphism. Nucleic Acids Res. Nov 15 1999;27(22):4335–4343. doi: 10.1093/nar/27.22.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oda H., Nakabeppu Y., Furuichi M., Sekiguchi M. Regulation of expression of the human MTH1 gene encoding 8-oxo-dGTPase. Alternative splicing of transcription products. J. Biol. Chem. Jul 11 1997;272(28):17843–17850. doi: 10.1074/jbc.272.28.17843. [DOI] [PubMed] [Google Scholar]
- 41.Waz S., Nakamura T., Hirata K., et al. Structural and kinetic studies of the human nudix hydrolase MTH1 reveal the mechanism for its broad substrate specificity. J. Biol. Chem. Feb 17 2017;292(7):2785–2794. doi: 10.1074/jbc.M116.749713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakai Y., Furuichi M., Takahashi M., et al. A molecular basis for the selective recognition of 2-hydroxy-dATP and 8-oxo-dGTP by human MTH1. J. Biol. Chem. Mar 8 2002;277(10):8579–8587. doi: 10.1074/jbc.M110566200. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura T., Hirata K., Fujimiya K., et al. X-Ray structure analysis of human oxidized nucleotide hydrolase MTH1 using crystals obtained under microgravity. International Journal of Microgravity Science and Application. 2019;36(1) doi: 10.15011//jasma.36.360103. [DOI] [Google Scholar]
- 44.Carreras-Puigvert J., Zitnik M., Jemth A.-S., et al. A comprehensive structural, biochemical and biological profiling of the human NUDIX hydrolase family. Nat. Commun. 2017/11/16 2017;8(1):1541. doi: 10.1038/s41467-017-01642-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svensson L.M., Jemth A.S., Desroses M., et al. Crystal structure of human MTH1 and the 8-oxo-dGMP product complex. FEBS Lett. Aug 19 2011;585(16):2617–2621. doi: 10.1016/j.febslet.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura T., Koga-Ogawa Y., Fujimiya K., et al. Protonation states of Asp residues in the human Nudix hydrolase MTH1 contribute to its broad substrate recognition. FEBS Lett. Jul 2023;597(13):1770–1778. doi: 10.1002/1873-3468.14611. [DOI] [PubMed] [Google Scholar]
- 47.Helleday T., Rudd S.G. Targeting the DNA damage response and repair in cancer through nucleotide metabolism. Mol. Oncol. Nov 2022;16(21):3792–3810. doi: 10.1002/1878-0261.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jemth A.S., Gustafsson R., Bräutigam L., et al. MutT homologue 1 (MTH1) catalyzes the hydrolysis of mutagenic O6-methyl-dGTP. Nucleic Acids Res. Nov 16 2018;46(20):10888–10904. doi: 10.1093/nar/gky896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mikhed Y., Görlach A., Knaus U.G., Daiber A. Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair. Redox Biol. Aug 2015;5:275–289. doi: 10.1016/j.redox.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mognato M., Burdak-Rothkamm S., Rothkamm K. Interplay between DNA replication stress, chromatin dynamics and DNA-damage response for the maintenance of genome stability. Mutat. Res. Rev. Mutat. Res. Jan-Jun 2021;787 doi: 10.1016/j.mrrev.2020.108346. [DOI] [PubMed] [Google Scholar]
- 51.Samaranayake G.J., Troccoli C.I., Zhang L., et al. The existence of MTH1-independent 8-oxodGTPase activity in cancer cells as a compensatory mechanism against on-target effects of MTH1 inhibitors. Mol. Cancer Therapeut. Feb 2020;19(2):432–446. doi: 10.1158/1535-7163.Mct-19-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barguilla I., Barszczewska G., Annangi B., et al. MTH1 is involved in the toxic and carcinogenic long-term effects induced by zinc oxide and cobalt nanoparticles. Arch. Toxicol. Jun 2020;94(6):1973–1984. doi: 10.1007/s00204-020-02737-y. [DOI] [PubMed] [Google Scholar]
- 53.Hahm J.Y., Park J., Jang E.S., Chi S.W. 8-Oxoguanine: from oxidative damage to epigenetic and epitranscriptional modification. Exp. Mol. Med. Oct 2022;54(10):1626–1642. doi: 10.1038/s12276-022-00822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McPherson L.A., Troccoli C.I., Ji D., et al. Increased MTH1-specific 8-oxodGTPase activity is a hallmark of cancer in colon, lung and pancreatic tissue. DNA Repair. Nov 2019;83 doi: 10.1016/j.dnarep.2019.102644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patterson J.C., Joughin B.A., Prota A.E., et al. VISAGE reveals a targetable mitotic spindle vulnerability in cancer cells. Cell Syst. Jul 24 2019;9(1):74–92.e8. doi: 10.1016/j.cels.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanjiv K., Calderón-Montaño J.M., Pham T.M., et al. MTH1 inhibitor TH1579 induces oxidative DNA damage and mitotic arrest in acute myeloid leukemia. Cancer Res. Nov 15 2021;81(22):5733–5744. doi: 10.1158/0008-5472.Can-21-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Rosa M., Johnson S.A., Opresko P.L. Roles for the 8-oxoguanine DNA repair system in protecting telomeres from oxidative stress. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.758402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fouquerel E., Lormand J., Bose A., et al. Oxidative guanine base damage regulates human telomerase activity. Nat. Struct. Mol. Biol. Dec 2016;23(12):1092–1100. doi: 10.1038/nsmb.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fouquerel E., Barnes R.P., Uttam S., Watkins S.C., Bruchez M.P., Opresko P.L. Targeted and persistent 8-oxoguanine base damage at telomeres promotes telomere loss and crisis. Mol. Cell. Jul 11 2019;75(1):117–130.e6. doi: 10.1016/j.molcel.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trybek T., Kowalik A., Góźdź S., Kowalska A. Telomeres and telomerase in oncogenesis. Oncol. Lett. Aug 2020;20(2):1015–1027. doi: 10.3892/ol.2020.11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanford S.L., Welfer G.A., Freudenthal B.D., Opresko P.L. Mechanisms of telomerase inhibition by oxidized and therapeutic dNTPs. Nat. Commun. Oct 20 2020;11(1):5288. doi: 10.1038/s41467-020-19115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rai P., Sobol R.W. Mechanisms of MTH1 inhibition-induced DNA strand breaks: the slippery slope from the oxidized nucleotide pool to genotoxic damage. DNA Repair. 2019/05/01/2019;77:18–26. doi: 10.1016/j.dnarep.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Speina E., Arczewska K.D., Gackowski D., et al. Contribution of hMTH1 to the maintenance of 8-oxoguanine levels in lung DNA of non–small-cell lung cancer patients. J. Natl. Cancer Inst.: J. Natl. Cancer Inst. 2005;97(5):384–395. doi: 10.1093/jnci/dji058. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L., Misiara L., Samaranayake G.J., et al. OGG1 co-inhibition antagonizes the tumor-inhibitory effects of targeting MTH1. Redox Biol. Apr 2021;40 doi: 10.1016/j.redox.2020.101848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eshtad S., Mavajian Z., Rudd S.G., et al. hMYH and hMTH1 cooperate for survival in mismatch repair defective T-cell acute lymphoblastic leukemia. Oncogenesis. Dec 5 2016;5(12) doi: 10.1038/oncsis.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Egashira A., Yamauchi K., Yoshiyama K., et al. Mutational specificity of mice defective in the MTH1 and/or the MSH2 genes. DNA Repair. Nov 3 2002;1(11):881–893. doi: 10.1016/s1568-7864(02)00113-1. [DOI] [PubMed] [Google Scholar]
- 67.Pursell Z.F., McDonald J.T., Mathews C.K., Kunkel T.A. Trace amounts of 8-oxo-dGTP in mitochondrial dNTP pools reduce DNA polymerase gamma replication fidelity. Nucleic Acids Res. Apr 2008;36(7):2174–2181. doi: 10.1093/nar/gkn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rotskaya U.N., Rogozin I.B., Vasyunina E.A., Malyarchuk B.A., Nevinsky G.A., Sinitsyna O.I. High frequency of somatic mutations in rat liver mitochondrial DNA. Mutat. Res. Mar 1 2010;685(1–2):97–102. doi: 10.1016/j.mrfmmm.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 69.Sommerville E.W., Dalla Rosa I., Rosenberg M.M., et al. Identification of a novel heterozygous guanosine monophosphate reductase (GMPR) variant in a patient with a late-onset disorder of mitochondrial DNA maintenance. Clin. Genet. Feb 2020;97(2):276–286. doi: 10.1111/cge.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao S., Tahara Y., Kool E.T., Greenberg M.M. Promoter dependent RNA polymerase II bypass of the epimerizable DNA lesion, Fapy•dG and 8-Oxo-2'-deoxyguanosine. Nucleic Acids Res. Jul 22 2024;52(13):7437–7446. doi: 10.1093/nar/gkae529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taiyab A., Choudhury A., Haidar S., et al. Exploring MTH1 inhibitory potential of Thymoquinone and Baicalin for therapeutic targeting of breast cancer. Biomed. Pharmacother. Apr 2024;173 doi: 10.1016/j.biopha.2024.116332. [DOI] [PubMed] [Google Scholar]
- 72.Shahzad N., Fatima M., Mutahir Z., et al. Cytotoxic effect of MTH1 gene silencing in gemcitabine resistant breast cancer cells. Pakistan J. Zool. 2020 doi: 10.17582/journal.pjz/20200406140416. 06/17. [DOI] [Google Scholar]
- 73.Li C., Xue Y., Wu J., et al. MTH1 inhibition synergizes with ROS-inducing agents to trigger cervical cancer cells undergoing parthanatos. Biochim. Biophys. Acta, Mol. Basis Dis. Jun 2024;1870(5) doi: 10.1016/j.bbadis.2024.167190. [DOI] [PubMed] [Google Scholar]
- 74.Shi X.L., Li Y., Zhao L.M., Su L.W., Ding G. Delivery of MTH1 inhibitor (TH287) and MDR1 siRNA via hyaluronic acid-based mesoporous silica nanoparticles for oral cancers treatment. Colloids Surf. B Biointerfaces. Jan 1 2019;173:599–606. doi: 10.1016/j.colsurfb.2018.09.076. [DOI] [PubMed] [Google Scholar]
- 75.Arczewska K.D., Krasuska W., Stachurska A., et al. hMTH1 and GPX1 expression in human thyroid tissue is interrelated to prevent oxidative DNA damage. DNA Repair. Nov 2020;95 doi: 10.1016/j.dnarep.2020.102954. [DOI] [PubMed] [Google Scholar]
- 76.Hansel C., Hlouschek J., Xiang K., et al. Adaptation to chronic-cycling hypoxia renders cancer cells resistant to MTH1-inhibitor treatment which can Be counteracted by glutathione depletion. Cells. Nov 5 2021;10(11) doi: 10.3390/cells10113040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen G., Wang X., He Z., et al. Light-elicited and oxygen-saved iridium nanocapsule for oxidative damage intensified oncotherapy. Molecules. May 28 2023;28(11) doi: 10.3390/molecules28114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Magkouta S.F., Vaitsi P.C., Iliopoulou M.P., et al. MTH1 inhibition alleviates immune suppression and enhances the efficacy of anti-PD-L1 immunotherapy in experimental mesothelioma. Cancers. Oct 12 2023;15(20) doi: 10.3390/cancers15204962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Das I., Tuominen R., Helleday T., Hansson J., Warpman Berglund U., Egyházi Brage S. Coexpression of MTH1 and PMS2 is associated with advanced disease and disease progression after therapy in melanoma. J. Invest. Dermatol. Mar 2022;142(3 Pt A):736–740.e6. doi: 10.1016/j.jid.2021.07.166. [DOI] [PubMed] [Google Scholar]
- 80.Burton D.G., Rai P. MTH1 counteracts oncogenic oxidative stress. Oncoscience. 2015;2(10):785–786. doi: 10.18632/oncoscience.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rai P., Young J.J., Burton D.G., Giribaldi M.G., Onder T.T., Weinberg R.A. Enhanced elimination of oxidized guanine nucleotides inhibits oncogenic RAS-induced DNA damage and premature senescence. Oncogene. Mar 24 2011;30(12):1489–1496. doi: 10.1038/onc.2010.520. [DOI] [PubMed] [Google Scholar]
- 82.Fujishita T., Okamoto T., Akamine T., et al. Association of MTH1 expression with the tumor malignant potential and poor prognosis in patients with resected lung cancer. Lung Cancer. Jul 2017;109:52–57. doi: 10.1016/j.lungcan.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 83.Patel A., Burton D.G., Halvorsen K., et al. MutT Homolog 1 (MTH1) maintains multiple KRAS-driven pro-malignant pathways. Oncogene. May 14 2015;34(20):2586–2596. doi: 10.1038/onc.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li D.N., Yang C.C., Li J., et al. The high expression of MTH1 and NUDT5 promotes tumor metastasis and indicates a poor prognosis in patients with non-small-cell lung cancer. Biochim. Biophys. Acta Mol. Cell Res. Jan 2021;1868(1) doi: 10.1016/j.bbamcr.2020.118895. [DOI] [PubMed] [Google Scholar]
- 85.Zhan D., Zhang X., Li J., Ding X., Cui Y., Jia J. MTH1 inhibitor TH287 suppresses gastric cancer development through the regulation of PI3K/AKT signaling. Cancer Biother. Radiopharm. Apr 2020;35(3):223–232. doi: 10.1089/cbr.2019.3031. [DOI] [PubMed] [Google Scholar]
- 86.Bhavya B., Easwer H.V., Vilanilam G.C., et al. MutT Homolog1 has multifaceted role in glioma and is under the apparent orchestration by Hypoxia Inducible factor 1 alpha. Life Sci. Jan 1 2021;264 doi: 10.1016/j.lfs.2020.118673. [DOI] [PubMed] [Google Scholar]
- 87.Bräutigam L., Pudelko L., Jemth A.S., et al. Hypoxic signaling and the cellular redox tumor environment determine sensitivity to MTH1 inhibition. Cancer Res. Apr 15 2016;76(8):2366–2375. doi: 10.1158/0008-5472.Can-15-2380. [DOI] [PubMed] [Google Scholar]
- 88.Nisimoto Y., Diebold B.A., Cosentino-Gomes D., Lambeth J.D. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. Aug 12 2014;53(31):5111–5120. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takac I., Schröder K., Zhang L., et al. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. Apr 15 2011;286(15):13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Egea J., Fabregat I., Frapart Y.M., et al. European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS) Redox Biol. Oct 2017;13:94–162. doi: 10.1016/j.redox.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ye M., Fang Y., Chen L., et al. Therapeutic targeting nudix hydrolase 1 creates a MYC-driven metabolic vulnerability. Nat. Commun. 2024/03/16 2024;15(1):2377. doi: 10.1038/s41467-024-46572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kumagae Y., Hirahashi M., Takizawa K., et al. Overexpression of MTH1 and OGG1 proteins in ulcerative colitis-associated carcinogenesis. Oncol. Lett. Aug 2018;16(2):1765–1776. doi: 10.3892/ol.2018.8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang X., Song W., Zhou Y., et al. Expression and function of MutT homolog 1 in distinct subtypes of breast cancer. Oncol. Lett. Apr 2017;13(4):2161–2168. doi: 10.3892/ol.2017.5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahmed W., Lingner J. PRDX1 and MTH1 cooperate to prevent ROS-mediated inhibition of telomerase. Genes Dev. May 1 2018;32(9–10):658–669. doi: 10.1101/gad.313460.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X., Zhou Y., Mao F., Lin Y., Shen S., Sun Q. lncRNA AFAP1-AS1 promotes triple negative breast cancer cell proliferation and invasion via targeting miR-145 to regulate MTH1 expression. Sci. Rep. May 6 2020;10(1):7662. doi: 10.1038/s41598-020-64713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma J., Saleem M.H., Ali B., et al. Impact of foliar application of syringic acid on tomato (Solanum lycopersicum L.) under heavy metal stress-insights into nutrient uptake, redox homeostasis, oxidative stress, and antioxidant defense. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.950120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kang N., Ma J., Hu Y., et al. Targeting MutT homolog 1 (MTH1) for breast cancer suppression by using a novel MTH1 inhibitor MA-24 with tumor-selective toxicity. Pharmaceuticals. Feb 23 2024;17(3) doi: 10.3390/ph17030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y., Tang Y., Zhao X-m, et al. A multifunctional non-viral vector for the delivery of MTH1-targeted CRISPR/Cas9 system for non-small cell lung cancer therapy. Acta Biomater. 2022/11/01/2022;153:481–493. doi: 10.1016/j.actbio.2022.09.046. [DOI] [PubMed] [Google Scholar]
- 99.Abbas H.H.K., Alhamoudi K.M.H., Evans M.D., Jones G.D.D., Foster S.S. MTH1 deficiency selectively increases non-cytotoxic oxidative DNA damage in lung cancer cells: more bad news than good? BMC Cancer. 2018/04/16 2018;18(1):423. doi: 10.1186/s12885-018-4332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu J.D., Lin D.W., Page S.T., Lundgren A.D., True L.D., Plymate S.R. Oxidative DNA damage in the prostate may predispose men to a higher risk of prostate cancer. Transl Oncol. Mar 2009;2(1):39–45. doi: 10.1593/tlo.08217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poplawski T., Arabski M., Kozirowska D., et al. DNA damage and repair in gastric cancer--a correlation with the hOGG 1 and RAD51 genes polymorphisms. Mutat. Res. Oct 10 2006;601(1–2):83–91. doi: 10.1016/j.mrfmmm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 102.Zhou W., Ma L., Yang J., et al. Correction: potent and specific MTH1 inhibitors targeting gastric cancer. Cell Death Dis. Oct 16 2020;11(10):868. doi: 10.1038/s41419-020-03010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lamoureux F., Trichet V., Chipoy C., Blanchard F., Gouin F., Redini F. Recent advances in the management of osteosarcoma and forthcoming therapeutic strategies. Expert Rev. Anticancer Ther. Feb 2007;7(2):169–181. doi: 10.1586/14737140.7.2.169. [DOI] [PubMed] [Google Scholar]
- 104.Lee J.A. Osteosarcoma in Korean children and adolescents. Korean. J. Pediatr. Apr 2015;58(4):123–128. doi: 10.3345/kjp.2015.58.4.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moukengue B., Brown H.K., Charrier C., et al. TH1579, MTH1 inhibitor, delays tumour growth and inhibits metastases development in osteosarcoma model. EBioMedicine. Mar 2020;53 doi: 10.1016/j.ebiom.2020.102704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arczewska K.D., Stachurska A., Wojewódzka M., et al. hMTH1 is required for maintaining migration and invasion potential of human thyroid cancer cells. DNA Repair. Sep 2018;69:53–62. doi: 10.1016/j.dnarep.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 107.Qing X., Shao Z., Lv X., et al. Anticancer effect of (S)-crizotinib on osteosarcoma cells by targeting MTH1 and activating reactive oxygen species. Anti Cancer Drugs. Apr 2018;29(4):341–352. doi: 10.1097/cad.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ying H., Dey P., Yao W., et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. Feb 15 2016;30(4):355–385. doi: 10.1101/gad.275776.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Trachootham D., Zhou Y., Zhang H., et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. Sep 2006;10(3):241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 110.Mateo-Victoriano B., Zhang L., Samaranayake G., et al. Abstract 4790: oxidation in the nucleotide pool as a novel targetable vulnerability in pancreatic ductal adenocarcinoma. Cancer Res. 2023;83:4790. doi: 10.1158/1538-7445.AM2023-4790. 4790. [DOI] [Google Scholar]
- 111.Wang J.Y., Jin L., Yan X.G., et al. Reactive oxygen species dictate the apoptotic response of melanoma cells to TH588. J. Invest. Dermatol. Nov 2016;136(11):2277–2286. doi: 10.1016/j.jid.2016.06.625. [DOI] [PubMed] [Google Scholar]
- 112.Cheung E.C., Vousden K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer. May 2022;22(5):280–297. doi: 10.1038/s41568-021-00435-0. [DOI] [PubMed] [Google Scholar]
- 113.Funahashi S., Okazaki Y., Akatsuka S., et al. Mth1 deficiency provides longer survival upon intraperitoneal crocidolite injection in female mice. Free Radic. Res. Mar 2020;54(2–3):195–205. doi: 10.1080/10715762.2020.1743285. [DOI] [PubMed] [Google Scholar]
- 114.Oka S., Leon J., Sakumi K., et al. MTH1 and OGG1 maintain a low level of 8-oxoguanine in Alzheimer's brain, and prevent the progression of Alzheimer's pathogenesis. Sci. Rep. Mar 23 2021;11(1):5819. doi: 10.1038/s41598-021-84640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karch C.M., Goate A.M. Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatr. Jan 1 2015;77(1):43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mizuno Y., Abolhassani N., Mazzei G., et al. Deficiency of MTH1 and/or OGG1 increases the accumulation of 8-oxoguanine in the brain of the App(NL-G-F/NL-G-F) knock-in mouse model of Alzheimer's disease, accompanied by accelerated microgliosis and reduced anxiety-like behavior. Neurosci. Res. Apr 2022;177:118–134. doi: 10.1016/j.neures.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 117.Sheng Z., Oka S., Tsuchimoto D., et al. 8-Oxoguanine causes neurodegeneration during MUTYH-mediated DNA base excision repair. J. Clin. Invest. Dec 2012;122(12):4344–4361. doi: 10.1172/jci65053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamaguchi H., Kajitani K., Dan Y., et al. MTH1, an oxidized purine nucleoside triphosphatase, protects the dopamine neurons from oxidative damage in nucleic acids caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Cell Death Differ. Apr 2006;13(4):551–563. doi: 10.1038/sj.cdd.4401788. [DOI] [PubMed] [Google Scholar]
- 119.Nakabeppu Y., Ohta E., Abolhassani N. MTH1 as a nucleotide pool sanitizing enzyme: friend or foe? Free Radic. Biol. Med. Jun 2017;107:151–158. doi: 10.1016/j.freeradbiomed.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 120.Chen S.H., Oyarzabal E.A., Hong J.S. Critical role of the Mac 1/NOX2 pathway in mediating reactive microgliosis-generated chronic neuroinflammation and progressive neurodegeneration. Curr. Opin. Pharmacol. Feb 2016;26:54–60. doi: 10.1016/j.coph.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shadrina M.I., Slominsky P.A. Genetic architecture of Parkinson's disease. Biochemistry (Mosc.) Mar 2023;88(3):417–433. doi: 10.1134/s0006297923030100. [DOI] [PubMed] [Google Scholar]
- 122.Aslan N.S., Orhan G., Karahalil B. The impacts of prominent gene polymorphisms in DNA repair enzymes on Parkinson's disease. Neurosci. Lett. Sep 14 2020;735 doi: 10.1016/j.neulet.2020.135203. [DOI] [PubMed] [Google Scholar]
- 123.Shimura H., Hattori N., Kang D., Miyako K.-I., Nakabeppu Y., Mizuno Y. Increased 8-oxo-dGTPase in the mitochondria of substantia nigra neurons in Parkinson's disease. Ann. Neurol. 2000;46:920–924. doi: 10.1002/1531-8249(199912)46:63.0.CO;2-R. 01/01. [DOI] [PubMed] [Google Scholar]
- 124.Gmitterová K., Heinemann U., Gawinecka J., et al. 8-OHdG in cerebrospinal fluid as a marker of oxidative stress in various neurodegenerative diseases. Neurodegener. Dis. 2009;6(5–6):263–269. doi: 10.1159/000237221. [DOI] [PubMed] [Google Scholar]
- 125.Hirayama M., Nakamura T., Watanabe H., et al. Urinary 8-hydroxydeoxyguanosine correlate with hallucinations rather than motor symptoms in Parkinson's disease. Parkinsonism Relat. Disorders. Jan 2011;17(1):46–49. doi: 10.1016/j.parkreldis.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 126.Karahalil B., Miser Salihoğlu E., Elkama A., Orhan G., Saygın E., Yardim Akaydin S. Individual susceptibility has a major impact on strong association between oxidative stress, defence systems and Parkinson's disease. Basic Clin. Pharmacol. Toxicol. Jan 2022;130(1):158–170. doi: 10.1111/bcpt.13659. [DOI] [PubMed] [Google Scholar]
- 127.Yoshimura D., Sakumi K., Ohno M., et al. An oxidized purine nucleoside triphosphatase, MTH1, suppresses cell death caused by oxidative stress. J. Biol. Chem. Sep 26 2003;278(39):37965–37973. doi: 10.1074/jbc.M306201200. [DOI] [PubMed] [Google Scholar]
- 128.Amirinejad R., Shirvani-Farsani Z., Naghavi Gargari B., Sahraian M.A., Mohammad Soltani B., Behmanesh M. Vitamin D changes expression of DNA repair genes in the patients with multiple sclerosis. Gene. May 20 2021;781 doi: 10.1016/j.gene.2021.145488. [DOI] [PubMed] [Google Scholar]
- 129.Ljubisavljevic S., Stojanovic I., Basic J., Pavlovic D.A. The validation study of neurofilament heavy chain and 8-hydroxy-2'-deoxyguanosine as plasma biomarkers of clinical/paraclinical activity in first and relapsing-remitting demyelination acute attacks. Neurotox. Res. Oct 2016;30(3):530–538. doi: 10.1007/s12640-016-9639-z. [DOI] [PubMed] [Google Scholar]
- 130.Polachini C.R., Spanevello R.M., Zanini D., et al. Evaluation of delta-aminolevulinic dehydratase activity, oxidative stress biomarkers, and vitamin D levels in patients with multiple sclerosis. Neurotox. Res. Feb 2016;29(2):230–242. doi: 10.1007/s12640-015-9584-2. [DOI] [PubMed] [Google Scholar]
- 131.Kong C.Y., Guo Z., Song P., et al. Underlying the mechanisms of doxorubicin-induced acute cardiotoxicity: oxidative stress and cell death. Int. J. Biol. Sci. 2022;18(2):760–770. doi: 10.7150/ijbs.65258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kibel A., Lukinac A.M., Dambic V., Juric I., Selthofer-Relatic K. Oxidative stress in ischemic heart disease. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/6627144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oldfield C.J., Duhamel T.A., Dhalla N.S. Mechanisms for the transition from physiological to pathological cardiac hypertrophy. Can. J. Physiol. Pharmacol. Feb 2020;98(2):74–84. doi: 10.1139/cjpp-2019-0566. [DOI] [PubMed] [Google Scholar]
- 134.Wang X., Zhang G., Dasgupta S., et al. ATF4 protects the heart from failure by antagonizing oxidative stress. Circ. Res. Jun 24 2022;131(1):91–105. doi: 10.1161/circresaha.122.321050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peng M.L., Fu Y., Wu C.W., Zhang Y., Ren H., Zhou S.S. Signaling pathways related to oxidative stress in diabetic cardiomyopathy. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.907757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Y., Wang X. The role of DNA and RNA guanosine oxidation in cardiovascular diseases. Pharmacol. Res. Jun 2024;204 doi: 10.1016/j.phrs.2024.107187. [DOI] [PubMed] [Google Scholar]
- 137.Di Minno A., Turnu L., Porro B., et al. 8-Hydroxy-2-Deoxyguanosine levels and cardiovascular disease: a systematic review and meta-analysis of the literature. Antioxidants Redox Signal. Apr 1 2016;24(10):548–555. doi: 10.1089/ars.2015.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ohtsubo T., Ohya Y., Nakamura Y., et al. Accumulation of 8-oxo-deoxyguanosine in cardiovascular tissues with the development of hypertension. DNA Repair. Jun 1 2007;6(6):760–769. doi: 10.1016/j.dnarep.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 139.Liu T., Cai J.-P., Zhang L.-Q., et al. The mechanism of RNA oxidation involved in the development of heart failure. Free Radic. Res. 2019/08/03 2019;53(8):910–921. doi: 10.1080/10715762.2019.1646424. [DOI] [PubMed] [Google Scholar]
- 140.Smallwood M.J., Nissim A., Knight A.R., Whiteman M., Haigh R., Winyard P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radical Biology and Medicine. 2018/09/01/. 2018;125:3–14. doi: 10.1016/j.freeradbiomed.2018.05.086. [DOI] [PubMed] [Google Scholar]
- 141.Glennon-Alty L., Hackett A.P., Chapman E.A., Wright H.L. Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radic. Biol. Med. 2018/09/01/2018;125:25–35. doi: 10.1016/j.freeradbiomed.2018.03.049. [DOI] [PubMed] [Google Scholar]
- 142.Ni Q., Zhang P., Li Q., Han Z. Oxidative stress and gut microbiome in inflammatory skin diseases. Review. Front. Cell Dev. Biol. 2022-March-07 2022;10doi doi: 10.3389/fcell.2022.849985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cannavò S.P., Riso G., Casciaro M., Di Salvo E., Gangemi S. Oxidative stress involvement in psoriasis: a systematic review. Free Radic. Res. 2019/08/03 2019;53(8):829–840. doi: 10.1080/10715762.2019.1648800. [DOI] [PubMed] [Google Scholar]
- 144.van der Fits L., Mourits S., Voerman J.S.A., et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 Axis 1. J. Immunol. 2009;182(9):5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 145.Bivik Eding C., Köhler I., Verma D., et al. MTH1 inhibitors for the treatment of psoriasis. J. Invest. Dermatol. 2021/08/01/2021;141(8):2037–2048.e4. doi: 10.1016/j.jid.2021.01.026. [DOI] [PubMed] [Google Scholar]
- 146.Schüler R., Efentakis P., Wild J., et al. T cell-derived IL-17a induces vascular dysfunction via perivascular fibrosis formation and dysregulation of ⋅NO/cGMP signaling. Oxid. Med. Cell. Longev. 2019;2019(1) doi: 10.1155/2019/6721531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alexandrov L.B., Nik-Zainal S., Wedge D.C., et al. Signatures of mutational processes in human cancer. Nature. 2013/08/01 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang L., Leite de Oliveira R., Huijberts S., et al. An acquired vulnerability of drug-resistant melanoma with therapeutic potential. Cell. 2018;173(6):1413–1425.e14. doi: 10.1016/j.cell.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 149.Das I., Gad H., Bräutigam L., et al. AXL and CAV-1 play a role for MTH1 inhibitor TH1579 sensitivity in cutaneous malignant melanoma. Cell Death Differ. 2020/07/01 2020;27(7):2081–2098. doi: 10.1038/s41418-019-0488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bräutigam L., Pudelko L., Jemth A.-S., et al. Hypoxic signaling and the cellular redox tumor environment determine sensitivity to MTH1 inhibition. Cancer Res. 2016;76(8):2366–2375. doi: 10.1158/0008-5472.Can-15-2380. [DOI] [PubMed] [Google Scholar]
- 151.Jin L., Wang J., Liu G., et al. Abstract 2420: Skp2-mediated stabilization of MTH1 promotes survival of melanoma cells upon oxidative stress. Cancer Res. 2018;78(13_Supplement):2420. doi: 10.1158/1538-7445.Am2018-2420. 2420. [DOI] [PubMed] [Google Scholar]
- 152.Cortesi L., Rugo H.S., Jackisch C. An overview of PARP inhibitors for the treatment of breast cancer. Targeted Oncol. May 2021;16(3):255–282. doi: 10.1007/s11523-021-00796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dong L., Wang H., Niu J., et al. Echinacoside induces apoptotic cancer cell death by inhibiting the nucleotide pool sanitizing enzyme MTH1. OncoTargets Ther. 2015;8:3649–3664. doi: 10.2147/ott.S94513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gao Y., Zhu L., Guo J., et al. Farnesyl phenolic enantiomers as natural MTH1 inhibitors from Ganoderma sinense. Oncotarget. Nov 10 2017;8(56):95865–95879. doi: 10.18632/oncotarget.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yokoyama T., Kitakami R., Mizuguchi M. Discovery of a new class of MTH1 inhibitor by X-ray crystallographic screening. Eur. J. Med. Chem. Apr 1 2019;167:153–160. doi: 10.1016/j.ejmech.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 156.Imtiyaz K., Husain Rahmani A., Alsahli M.A., Almatroodi S.A., Rizvi M.M.A. Fisetin induces apoptosis in human skin cancer cells through downregulating MTH1. J. Biomol. Struct. Dyn. 2023/10/13 2023;41(15):7339–7353. doi: 10.1080/07391102.2022.2121323. [DOI] [PubMed] [Google Scholar]
- 157.Wahi D., Soni D., Grover A. A double-edged sword: the anti-cancer effects of emodin by inhibiting the redox-protective protein MTH1 and augmenting ROS in NSCLC. J. Cancer. 2021;12(3):652–681. doi: 10.7150/jca.41160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang M., Zhou S., Chen Q., Wang L., Liang Z., Wang J. Understanding the molecular mechanism for the differential inhibitory activities of compounds against MTH1. Sci. Rep. 2017/01/11 2017;7(1) doi: 10.1038/srep40557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Farand J., Kropf J.E., Blomgren P., et al. Discovery of potent and selective MTH1 inhibitors for oncology: enabling rapid target (In)Validation. ACS Med. Chem. Lett. Mar 12 2020;11(3):358–364. doi: 10.1021/acsmedchemlett.9b00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Saleh A., Gökturk C., Warpman-Berglund U., Helleday T., Granelli I. Development and validation of method for TH588 and TH287, potent MTH1 inhibitors and new anti-cancer agents, for pharmacokinetic studies in mice plasma. J. Pharm. Biomed. Anal. Feb 2015;104:1–11. doi: 10.1016/j.jpba.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 161.Mohammad T., Arif K., Alajmi M.F., et al. Identification of high-affinity inhibitors of pyruvate dehydrogenase kinase-3: towards therapeutic management of cancer. J. Biomol. Struct. Dyn. 2021;39(2):586–594. doi: 10.1080/07391102.2020.1711810. [DOI] [PubMed] [Google Scholar]
- 162.Shen J., Guillén Mancina E., Chen S., et al. Mitotic MTH1 inhibitor TH1579 induces PD-L1 expression and inflammatory response through the cGAS-STING pathway. Oncogenesis. May 25 2024;13(1):17. doi: 10.1038/s41389-024-00518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou S., Wang M., Tong Z., Wang J. The recognition mechanism of crizotinib on MTH1: influence of chirality on the bioactivity. Mol. Phys. 2016;114:1–9. doi: 10.1080/00268976.2016.1145750. 02/12. [DOI] [Google Scholar]
- 164.Musa S., Amara N., Selawi A., et al. Overcoming chemoresistance in cancer: the promise of crizotinib. Cancers. Jul 7 2024;16(13) doi: 10.3390/cancers16132479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sun H., Chen P., Li D., Li Y., Hou T. Directly binding rather than induced-fit dominated binding affinity difference in (S)- and (R)-Crizotinib bound MTH1. J. Chem. Theor. Comput. Feb 9 2016;12(2):851–860. doi: 10.1021/acs.jctc.5b00973. [DOI] [PubMed] [Google Scholar]
- 166.Huber K.V., Salah E., Radic B., et al. Stereospecific targeting of MTH1 by (S)-crizotinib as an anticancer strategy. Nature. Apr 10 2014;508(7495):222–227. doi: 10.1038/nature13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dai X., Guo G., Zou P., et al. (S)-crizotinib induces apoptosis in human non-small cell lung cancer cells by activating ROS independent of MTH1. J. Exp. Clin. Cancer Res. Sep 7 2017;36(1):120. doi: 10.1186/s13046-017-0584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kettle J.G., Alwan H., Bista M., et al. Potent and selective inhibitors of MTH1 probe its role in cancer cell survival. J. Med. Chem. 2016/03/24 2016;59(6):2346–2361. doi: 10.1021/acs.jmedchem.5b01760. [DOI] [PubMed] [Google Scholar]
- 169.Petrocchi A., Leo E., Reyna N.J., et al. Identification of potent and selective MTH1 inhibitors. Bioorg. Med. Chem. Lett. 2016/03/15/2016;26(6):1503–1507. doi: 10.1016/j.bmcl.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 170.Ashrafian S., Farimani M., Sonboli A., Ashrafian H., Kabiri M., Rezadoost H. Simultaneous characterization of nine isolated flavonoids in Iranian Dracocephalum species and in silico study of their inhibitory properties against MTH1 enzyme. South Afr. J. Bot. 2022;146:254–261. doi: 10.1016/j.sajb.2021.10.002. 05/01. [DOI] [Google Scholar]
- 171.Rudling A., Gustafsson R., Almlöf I., et al. Fragment-based discovery and optimization of enzyme inhibitors by docking of commercial chemical space. J. Med. Chem. Oct 12 2017;60(19):8160–8169. doi: 10.1021/acs.jmedchem.7b01006. [DOI] [PubMed] [Google Scholar]
- 172.Kumar A., Kawamura T., Kawatani M., Osada H., Zhang K.Y.J. Identification and structure-activity relationship of purine derivatives as novel MTH1 inhibitors. Chem. Biol. Drug Des. Jun 2017;89(6):862–869. doi: 10.1111/cbdd.12909. [DOI] [PubMed] [Google Scholar]
- 173.Kawamura T., Kawatani M., Muroi M., et al. Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival. Sci. Rep. 2016/05/23 2016;6(1) doi: 10.1038/srep26521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rahm F., Viklund J., Trésaugues L., et al. Creation of a novel class of potent and selective MutT homologue 1 (MTH1) inhibitors using fragment-based screening and structure-based drug design. J. Med. Chem. Mar 22 2018;61(6):2533–2551. doi: 10.1021/acs.jmedchem.7b01884. [DOI] [PubMed] [Google Scholar]
- 175.Yin Y., Sasaki S., Taniguchi Y. Inhibitory effect of 8-halogenated 7-Deaza-2'-deoxyguanosine triphosphates on human 8-Oxo-2'-deoxyguanosine triphosphatase, hMTH1, activities. Chembiochem. Apr 1 2016;17(7):566–569. doi: 10.1002/cbic.201500589. [DOI] [PubMed] [Google Scholar]
- 176.Yin Y., Sasaki S., Taniguchi Y. Effects of 8-halo-7-deaza-2'-deoxyguanosine triphosphate on DNA synthesis by DNA polymerases and cell proliferation. Bioorg. Med. Chem. Aug 15 2016;24(16):3856–3861. doi: 10.1016/j.bmc.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 177.Kasprzak K.S., Bialkowski K. Inhibition of antimutagenic enzymes, 8-oxo-dGTPases, by carcinogenic metals. Recent developments. J. Inorg. Biochem. Apr 2000;79(1–4):231–236. doi: 10.1016/s0162-0134(99)00240-8. [DOI] [PubMed] [Google Scholar]
- 178.Streib M., Kräling K., Richter K., Xie X., Steuber H., Meggers E. An organometallic inhibitor for the human repair enzyme 7,8-dihydro-8-oxoguanosine triphosphatase. Angew Chem. Int. Ed. Engl. Jan 3 2014;53(1):305–309. doi: 10.1002/anie.201307849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Imtiyaz K., Husain Rahmani A., Alsahli M.A., Almatroodi S.A., Rizvi M.M.A. Fisetin induces apoptosis in human skin cancer cells through downregulating MTH1. J. Biomol. Struct. Dyn. Sep-Oct 2023;41(15):7339–7353. doi: 10.1080/07391102.2022.2121323. [DOI] [PubMed] [Google Scholar]
- 180.Rajendraprasad G., Eibes S., Boldú C.G., Barisic M. TH588 and low-dose nocodazole impair chromosome congression by suppressing microtubule turnover within the mitotic spindle. Cancers. Nov 29 2021;13(23) doi: 10.3390/cancers13235995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Warpman Berglund U., Sanjiv K., Gad H., et al. Validation and development of MTH1 inhibitors for treatment of cancer. Ann. Oncol. Dec 2016;27(12):2275–2283. doi: 10.1093/annonc/mdw429. [DOI] [PubMed] [Google Scholar]
- 182.Einarsdottir B.O., Karlsson J., Söderberg E.M.V., et al. Correction: a patient-derived xenograft pre-clinical trial reveals treatment responses and a resistance mechanism to karonudib in metastatic melanoma. Cell Death Dis. Feb 6 2020;11(2):99. doi: 10.1038/s41419-020-2301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Joyce J.A., Pollard J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. Apr 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tajiri T., Maki H., Sekiguchi M. Functional cooperation of MutT, MutM and MutY proteins in preventing mutations caused by spontaneous oxidation of guanine nucleotide in Escherichia coli. Mutat. Res. May 1995;336(3):257–267. doi: 10.1016/0921-8777(94)00062-b. [DOI] [PubMed] [Google Scholar]
- 185.Zheng M., Liu Y., Zhang G., Yang Z., Xu W., Chen Q. The applications and mechanisms of superoxide dismutase in medicine, food, and cosmetics. Antioxidants. Aug 27 2023;12(9) doi: 10.3390/antiox12091675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Glorieux C., Calderon P.B. Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. Sep 26 2017;398(10):1095–1108. doi: 10.1515/hsz-2017-0131. [DOI] [PubMed] [Google Scholar]
- 187.Arai K., Sato Y., Nakajima I., et al. Glutathione peroxidase-like functions of 1,2-diselenane-4,5-diol and its amphiphilic derivatives: switchable catalytic cycles depending on peroxide substrates. Bioorg. Med. Chem. Jan 1 2021;29 doi: 10.1016/j.bmc.2020.115866. [DOI] [PubMed] [Google Scholar]
- 188.Perkins A., Nelson K.J., Parsonage D., Poole L.B., Karplus P.A. Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. Aug 2015;40(8):435–445. doi: 10.1016/j.tibs.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Muri J., Kopf M. The thioredoxin system: balancing redox responses in immune cells and tumors. Eur. J. Immunol. Jan 2023;53(1) doi: 10.1002/eji.202249948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nguyen T., Nioi P., Pickett C.B. The Nrf 2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. May 15 2009;284(20):13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.