Abstract
Cadmium (Cd) contamination poses significant risks to agricultural productivity and human health, particularly through its accumulation in staple crops such as bread wheat (Triticum aestivum L.). This study evaluated Cd accumulation and tolerance among six bread wheat cultivars exposed to six Cd concentrations (0, 2.5, 5, 10, 15, 20, and 25 mg kg−1 soil). Phenotypic assessments and quantitative real-time PCR (qRT-PCR) were conducted to analyze the expression patterns of TaNRAMP and TaZIP genes in various tissues and developmental stages of wheat, which play crucial roles in Cd uptake and transport. Results demonstrated significant variability in Cd accumulation. The Barat cultivar exhibited the lowest accumulation in grain (ranging from 0.21 to 8.8 mg kg−1) and the highest tolerance. In contrast, Kavir and Pishtaz displayed elevated Cd levels in both grain and straw, while Parsi accumulated more Cd in straw at lower concentrations (56.9 mg kg−1 in Cd concentration of 10 mg kg−1 soil). The gene expression analysis revealed that most cultivars showed increased expression of TaNRAMP genes, particularly TaNRAMP2 in Cd concentration of 10 mg kg−1 soil, which facilitates Cd uptake from the soil, and TaZIP genes, such as TaZIP4 and TaZIP7, involved in transporting Cd within the plant. Notably, the expression of TaZIP1 was significantly lower in cultivars with high Cd accumulation, suggesting a potential regulatory mechanism for Cd tolerance. Furthermore, cultivars exhibiting higher Cd levels correlated with increased expression of stress-responsive genes, indicating a broader response to Cd stress. These findings highlight Barat’s potential for bread-making applications due to its low Cd accumulation, while Morvarid and Pishtaz which show reduced Cd content in the straw even under high Cd exposure are better suited for animal feed. This research underscores the genetic variability of wheat cultivars in response to Cd stress and provides essential insights into the molecular mechanisms underlying Cd accumulation, offering valuable information for breeding programs aimed at developing Cd-tolerant varieties to ensure food security in contaminated regions.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78425-z.
Keywords: Bread wheat, Cultivars, Soil, Cadmium, Gene expression
Subject terms: Plant sciences, Plant stress responses, Abiotic
Introduction
Wheat, a major cereal crop for billions of people, belongs to the Poaceae family, comprising various species, with T. aestivum L. (bread wheat) and Triticum turgidum var. durum (pasta wheat) being the most widely cultivated and recognized1. Bread wheat is an essential agricultural product, with approximately 35% of the global food supply relying on it. It serves as a significant source of carbohydrates, proteins, minerals, and vitamins for human consumption2–4. Furthermore, its versatility in food processing has resulted in the production of an array of products such as bread, pasta, and cereals, establishing wheat as a dietary staple for numerous populations5.
The quality and safety of wheat crops are subject to a multitude of influencing factors, among which the presence of heavy metals in the soil emerges as a critical concern. Heavy metals, including Cd, lead, and arsenic, have the potential to accumulate in wheat plants when present in excessive concentrations within the soil6. These metals are absorbed by the plant’s root system and may subsequently translocate to various plant tissues, including the grain, which constitutes a primary component of the human diet. Accumulation of heavy metals in wheat grains raises significant health concerns, as heavy metals are known to exhibit toxicity, posing risks to human health through the consumption of contaminated grains7–10. Therefore, the execution of efficient agronomic practices, including the adoption of wheat cultivars with low accumulation rates11,12, play a crucial role in safeguarding the nutritional quality and safety of wheat-based products in the food supply chain.
As a toxic and unnecessary element in humans and animals, Cd may become a serious menace through transfer from plants and food chain13. The accumulation of Cd in plants, including wheat and rice, involves intricate processes. This heavy metal, resembling essential nutrients like calcium (Ca) and iron (Fe), enter plant roots through transporters and channels. Once inside the roots, Cd can follow either apoplastic or symplastic pathways, moving between cell walls and intercellular spaces or within the plant’s symplastic continuum. Cd accumulation in plants is influenced by a combination of various factors. Notably, even the application of exogenous components can have a profound impact on Cd accumulation in leaves. Soil properties, including pH, organic matter content, and salinity affect Cd availability to plants14–18. Molecular mechanisms within plants, such as metal transporters14,18 and metal-chelating compounds19,20, regulate the uptake and sequestration of Cd. Additionally, plant genotype plays a crucial role, with different species and cultivars exhibiting varying levels of metal tolerance and accumulation11,12.
The evaluation of wheat varieties for their propensity to accumulate Cd has been a focal point of scientific research. Several studies have revealed substantial variations in Cd uptake and accumulation across different wheat cultivars. Xiao et al. investigated the influence of different wheat genotypes on Cd accumulation and found significant variations in Cd tolerance and accumulation traits21. In the exploration of arsenic (As), Cd, and Pb accumulation within diverse wheat varieties, the findings elucidated a discernible trend in metal concentrations, with a descending order observed in the distribution across root, leaf, stem, and seed tissues. Notably, a statistically significant variation was discerned in the bioaccumulation factors of the aforementioned metals across distinct wheat varieties. Cd, in particular, exhibited a higher propensity for accumulation compared to both As and Pb. The established hierarchy of heavy metal accumulation in wheat grain, as documented, follows the sequence: “Zn < Pb < Cr < Cu < As < Hg < Cd”22–24. A study conducted by Kubo et al.25 revealed significant variations in grain Cd concentration among 237 wheat genotypes under Cd stress. Response of wheat genotypes to heavy metal stress has been documented in previous reports. This diversity in response can be attributed to the inherent ability of certain genotypes to exhibit resistance to the stress imposed by heavy metals26–29.
Regardless of the intrinsic mechanism of heavy metal absorption related to the type of genotype, soil properties can be another critical factor in metal accumulation in plants. According to the report of Tang et al., the concentration of Cd mainly depends on the two factors of genotypic difference and soil type and a significant correlation has been observed between these two factors30. Elgharbawy et al. showed that Zn, Pb, and Cd posed negative impacts on the seed germination, as well as root and shoot growth of various wheat genotypes. Moreover, the study highlighted the distinct responses exhibited by different wheat genotypes in response to heavy metal stress. They suggested that the genotypes demonstrating tolerance at the early stages of growth have the potential to be used as donor parents to produce cultivars tolerant to heavy metal stress27.
Plants under the stress of Cd, employ various mechanisms to detoxify, remove, and accumulate Cd in certain parts. One of these mechanisms involves the production of metal transporters that regulate Cd uptake and distribution in plant. For example, Cd transport can be regulated by some transporters such as ZIP, low-affinity calcium transporters (LCT), and NRAMP. Additionally, specific cation channels and Cd chelates facilitate the entry of Cd into root cells15. The ZIP family of transporters, including OsZIP6 and OsZIP7, are involved in Cd transport in rice plants. OsZIP6 is located on the plasma membrane in root and shoot tissues, facilitating the Cd transport from the root to the shoot. OsZIP7, located in the roots, is responsible for Cd loading into the xylem31,32. A previous study has indicated that OsZIP1 is able to act as a metal exporter in rice when Zn, Cu and Cd concentrations are excessive in the environment33. The NRAMP family comprises a group of membrane proteins that are involved in the transport of heavy metals within plants. Specifically, OsNRAMP1 and OsNRAMP5 transport Cd across the plasma membrane14, AtNRAMP6 serves as an intracellular Cd transporter, and TpNRAMP3 is a transporter responsible for Cd accumulation34. Cation Diffusion Facilitating Proteins (CDF) are a group of important metal transporters that play a crucial role in facilitating the flow of metals from the cytoplasm to either the extracellular space or intracellular compartments in plants. These proteins are commonly referred to as metal-tolerant proteins (MTP). An illustrative example of an MTP is MTP1 in rice (OsMTP1) which specifically functions as a transporter for Cd35. Heavy metal transporter (HMA3) in rice has been found to be closely associated with major QTLs related to Cd accumulation in grain36. This finding highlights the potential of HMA3 as a valuable marker in marker association selection programs. In a study investigating the response of eight high-yielding wheat cultivars to Pb and Cd stress, it was observed that the expression of TaHMA2 and TaABCC2/3/4 (transporter and metal detoxifier) significantly increased in the cultivar that exhibit resistance to Cd and Pb stress37. OsHMA3 is a mediator for the flow of Cd to the root vacuoles, thereby regulating its transfer from the roots to the stems in rice.
Despite extensive research on heavy metal accumulation in crops, a specific gap exists in understanding the differential responses of various Iranian wheat cultivars to Cd stress, particularly regarding their underlying molecular mechanisms. This study aims to address this gap by assessing six important bread wheat cultivars for their Cd accumulation ability and tolerance, contributing to food safety and environmental remediation by mitigating the introduction of toxic metals into the food chain. The selection of these cultivars was made with careful consideration of several factors: they represent a diverse range of genetic backgrounds, essential for understanding variations in heavy metal accumulation and tolerance. Additionally, these cultivars are well-adapted to the varied environmental conditions in Iran, demonstrating resilience across different regions. Their strong agronomic traits and yield potential ensure practical relevance, while historical performance data on heavy metal accumulation and tolerance informed their selection. We subjected these cultivars to different levels of Cd stress and evaluated their responses through comprehensive analyses of Cd accumulation, phenotypic traits, and gene expression related to key metal transporters, specifically TaNRAMP and TaZIP genes in various tissues and wheat developmental stages. Key findings from this research include significant variability in Cd accumulation among the cultivars, with the Barat cultivar exhibiting the lowest Cd levels and highest tolerance, while the Kavir and Pishtaz cultivars showed higher accumulation. Gene expression analysis indicated that TaNRAMP genes showed increased expression in cultivars with higher Cd accumulation, while TaZIP1 genes were significantly upregulated in cultivars with lower Cd accumulation. This highlights the potential for breeding programs to focus on the Barat cultivar for sustainable wheat production in contaminated soils.
Results
Measurement of cd accumulation
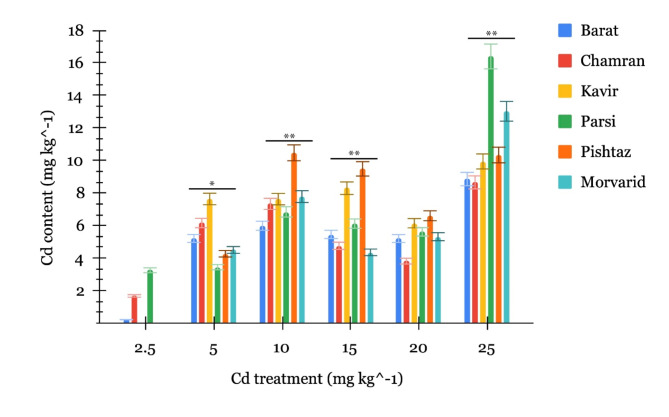
The investigation of Cd accumulation in grains of six wheat cultivars (Barat, Chamran, Kavir, Parsi, Pishtaz, and Morvarid) elucidated distinct trends and cultivar-specific responses across varying Cd concentrations (Fig. 1).
Fig. 1.
Accumulation of Cd in the grain of six wheat cultivars at the stage of grain maturity under different Cd treatment (mg kg−1). The bars represent means ± SE. The asterisks “*” and “**” represent significant effects among cultivars in each Cd level based on the one-way ANOVA at p < 0.05 and p < 0.01 levels, respectively.
At a concentration of 2.5 mg Cd kg−1 soil, the Barat cultivar showed the lowest Cd accumulation in grains at 0.21 mg kg−1, while Chamran and Parsi had moderate accumulations of 1.67 and 3.25 mg kg−1, respectively. Kavir’s grain Cd content increased from non-detectable levels at 2.5 mg kg-1 soil to 7.63 mg kg−1 at 5 mg kg−1 soil, indicating its capacity to accumulate Cd at higher concentrations. Both Barat and Chamran also had considerable increases in grain Cd accumulation, reflecting their sensitivity to elevated Cd levels. In contrast, Parsi showed a non-significant increase at 5 mg kg−1 soil, suggesting a possible saturation or regulatory mechanism. Pishtaz and Morvarid, which had no measurable Cd at 2.5 mg kg−1 soil, exhibited 4.26 and 4.49 mg kg−1, respectively, at 5 mg kg−1 soil, indicating responsiveness to increased Cd content.
Under the 10 mg Cd kg−1 soil treatment, most cultivars showed increased grain Cd accumulations ranging from 5.98 to 10.46 mg kg−1. At this level, Barat had the lowest and Pishtaz the highest Cd accumulations. At 15 mg Cd kg−1 soil, all cultivars except Kavir showed decreased Cd accumulation compared to the 10 mg kg−1 treatment. At 20 mg Cd kg−1 soil, Chamran and Kavir had substantial decreases in grain Cd accumulation, Pishtaz and Morvarid maintained moderate levels, while Barat and Parsi showed no significant change. At the highest concentration (25 mg Cd kg−1 soil), all cultivars had increased grain Cd accumulations compared to the 20 mg kg−1 treatment, with Parsi exhibiting the most substantial increase, indicating its capacity to accumulate Cd at elevated concentrations.
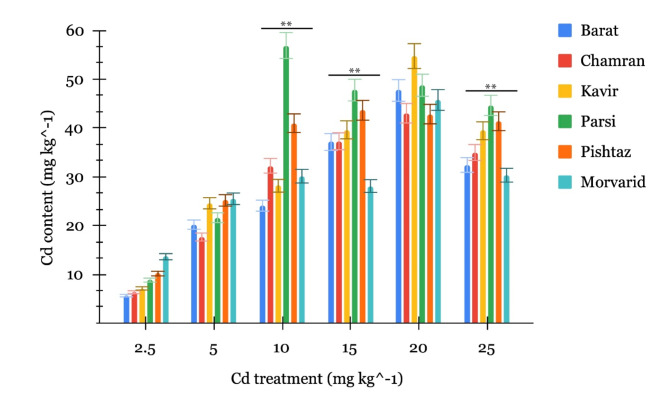
The investigation into Cd accumulation in straw tissue also revealed diverse responses among cultivars, as depicted in Fig. 2.
Fig. 2.
Accumulation of Cd in the straw of six wheat cultivars at the stage of grain maturity under different Cd treatment (mg kg−1). The bars represent means ± SE. The asterisks “*” and “**” represent significant levels among cultivars in each Cd level based on theone-way ANOVA at p < 0.05 and p < 0.01, respectively.
At the 2.5 mg Cd kg−1 soil treatment, Morvarid exhibited the highest accumulation (13.61 mg Cd kg−1), surpassing all other cultivars, while Barat displayed the lowest accumulation. Moving to 5 mg Cd kg−1 soil treatment, the Cd accumulation patterns in the six wheat cultivars continued to exhibit distinct variations compared to the 2.5 mg Cd kg−1 treatment. Pishtaz and Morvarid demonstrated the most significant increases in Cd accumulation, indicating their heightened sensitivity to moderate Cd concentrations. Morvarid, which had the highest accumulation at 2.5 mg Cd kg−1, showed a substantial further increase in straw Cd accumulation to 25.48 mg Cd kg−1 under the 5 mg Cd kg−1 soil concentration. Conversely, Chamran displayed the lowest increase in straw Cd accumulation among the cultivars under this treatment. At 10 mg Cd kg−1 soil concentration, the Cd accumulation patterns in the six wheat cultivars continued to undergo significant changes, revealing remarkable differences compared to the 2.5 and 5 mg Cd kg−1 soil treatments. Parsi exhibited a substantial increase in straw Cd content, with a peak accumulation of 56.89 mg Cd kg−1, emphasizing its heightened susceptibility to higher Cd concentrations. Pishtaz, which displayed one of the highest accumulations at 2.5 mg Cd kg−1, also maintained a considerable Cd uptake at the higher soil Cd concentration of 10 mg kg−1. In addition, Chamran showed a notable rise in its Cd accumulations compared to the levels observed at the lower soil Cd concentrations, indicating a discernible variation in its sensitivity to elevated Cd concentrations. Conversely, Barat revealed the least significant increase in Cd accumulation when compared to other cultivars. This suggests that, while Barat remained responsive to heightened Cd concentrations, its capacity for increased Cd uptake was relatively limited compared to other cultivars exposed to the same soil Cd treatment. At 15 mg Cd kg−1 soil, Kavir and Barat demonstrated the highest increment in straw Cd accumulation, respectively, indicating their sensitivity to elevated Cd levels in the soil. In contrast, Morvarid and Parsi exhibited a pronounced decrease in Cd accumulation compared to the 10 mg Cd kg−1 treatment, suggesting the possibility of saturation or the involvement of regulatory mechanisms. At the soil treatment of 20 mg Cd kg−1, Kavir demonstrated a notable rise in straw Cd content, reaching 54.73 mg Cd kg−1, indicating a heightened sensitivity to elevated Cd concentrations. In contrast, Pishtaz exhibited a slight decline in straw Cd accumulation compared to the 15 mg Cd kg−1 soil treatment, suggesting potential influence of saturation or regulatory mechanisms. At the highest soil Cd concentration of 25 mg Cd kg−1, a notable trend emerged as all six wheat cultivars. Unlike the accumulation patterns observed in the grains, the cultivars exhibited a decrease in Cd accumulation in their straw compared to the preceding 20 mg Cd kg−1 soil treatment. Morvarid and Barat, in particular, demonstrated the most significant decreases in Cd accumulation, suggesting a potential saturation effect or regulatory mechanisms limiting further Cd uptake.
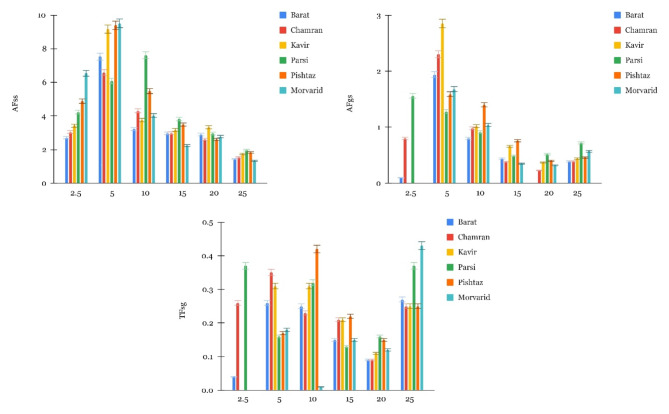
Accumulation factor (AF) and translocation factor (TF) results
The AFss (Cd in straw/soil) of Cd in straw relative to soil were analyzed across six wheat cultivars subjected to varying Cd treatment levels (Fig. 3). Overall, notable variations for AFss were observed among the wheat cultivars across treatment levels. At 2.5 mg Cd kg−1 soil treatment, the cultivars Morvarid and Pishtaz exhibited relatively higher AFss values compared to other cultivars. Conversely, Barat demonstrated relatively lower value at this soil Cd concentration level, suggesting low potential for Cd accumulation in the straw of this cultivar under this treatment condition. However, at the 5 mg Cd kg−1 treatment, the Barat and Kavir exhibited an intensified AFss response to the increased soil Cd level in comparison to the 2.5 mg Cd kg−1 soil treatment. At the 10 mg Cd kg−1 soil treatment, Parsi demonstrated the highest AFss, suggesting a substantial uptake of Cd into the straw, whereas Morvarid exhibited the lowest AFss value, indicating reduced Cd accumulation in its straw. At higher Cd concentrations (15, 20, and 25 mg Cd kg−1), AFss values decreased across all cultivars, indicating reduced Cd accumulation in straw, though the differences between cultivars were not statistically significan. In addition to AFss, Cd accumulation in grains relative to soil (AFgs) was also calculated (Fig. 3). At higher soil Cd concentrations, AFgs values were lower across all cultivars, similar to the trend in AFss. Notably, at 5 mg Cd kg−1, Barat, Chamran, and Kavir showed higher AFgs values compared to other cultivars, indicating a relatively greater Cd accumulation in their grains at this treatment level.The translocation of Cd from straw to grain (TFsg) was also examined across cultivars (Fig. 3). A decreasing trend in TFsg values was observed at 15 and 20 mg Cd kg−1 treatments, indicating reduced Cd movement from straw to grain under higher Cd exposure. However, at 2.5 mg Cd kg−1, TFsg values increased for all cultivars, suggesting a shift in Cd translocation compared to higher Cd treatments. This variability underscores the complex relationship between Cd exposure levels and cultivar-specific Cd translocation patterns.
Fig. 3.
Cd accumulation factor (AF) and translocation factor (TF) in straw and grain of six wheat cultivars under different Cd treatment levels (mg kg−1).
Phenotypical traits assay
In this study, we examined the impact of varying Cd concentrations on several growth and developmental parameters across six wheat cultivars. We recorded plant height, spike length, flag leaf length, peduncle length, grain weight, number of seeds, shoot dry weight, days to flowering, and days to harvest ripe for each cultivar under different soil Cd concentrations. Significant differences in responses to Cd-induced stress were observed among the cultivars. The Barat cultivar showed a notable increase in plant height at 5 mg Cd kg−1 soil, indicating potential tolerance to moderate Cd concentrations, while Pishtaz exhibited reduced plant height, indicating heightened sensitivity. Spike length and flag leaf length responses were also cultivar-specific; Chamran had increased spike length at 10 mg Cd kg−1 soil, and Parsi showed reduced flag leaf length at 20 and 25 mg Cd kg−1 soil. Grain weight and seed production varied across cultivars, highlighting the complex interaction between genetic factors and Cd stress.
Shoot dry weight responses varied significantly among cultivars. Kavir showed increased shoot dry weight at 2.5 and 10 mg Cd kg−1 soil, while Pishtaz declined at 20 mg Cd kg−1 soil. Days to flowering and days to harvest ripe were influenced by Cd contents, with distinct cultivar-specific adjustments. Overall, Barat and Chamran exhibited the maximum plant height, while Parsi and Kavir displayed the minimum, indicating reduced growth under Cd stress. Morvarid had the highest grain weight and the number of grains, along with maximum days to flowering, highlighting its extended reproductive period. Morvarid also displayed significant variability across multiple traits, suggesting a dynamic and versatile response to Cd stress, underscoring its potential resilience and adaptability under diverse environmental conditions.
The correlation matrix (Table S1) showed that Grain Cd content exhibited a strong positive correlation (0.68) with straw Cd content. Cd contents were positively correlated with spike length and shoot dry. However, both grain and straw Cd content showed weak associations with days to flowering and days to harvest ripe, suggesting that the timing of flowering and harvest had minimal effects on Cd content. Similarly, straw Cd content also has a weak negative correlation (-0.032) with days to flowering, indicating that variations in flowering time have little impact on Cd accumulation in straw. For days to harvest ripe, the correlation with grain Cd content is weakly positive (0.055), and with straw Cd content, it is slightly stronger but still weakly positive (0.150). These results imply that while the timing of harvest may have a marginal influence on Cd levels, it is not a major determinant compared to other factors. Overall, these weak correlations suggest that Cd accumulation in wheat is not strongly influenced by the timing of flowering or harvest, highlighting that other physiological or environmental factors likely play a more significant role in Cd uptake and storage. Indeed, days to flowering and days to harvest ripe had weak negative correlations with most of the traits. Spike length and shoot dry weight had strong positive correlations with Cd content. Spike length demonstrated strong positive correlation with most of the traits except days to flowering and days to ripening. Number of grains in spike was also positively correlated with most of the traits.
The results of the Cadmium Tolerance Index (CTI) indicated significant variability in tolerance among the wheat cultivars across different cadmium concentrations (Table S2). At lower Cd levels (2.5 mg/kg), all cultivars exhibited relatively high CTI percentages for most traits, with Barat demonstrating the highest overall tolerance, especially in grain weight and spike length. However, as cadmium concentration increased, the CTI values decreased for all cultivars. Chamran and Parsi showed the most substantial declines in CTI across all traits, indicating higher sensitivity to cadmium stress. At 25 mg/kg, most cultivars had notably low CTI percentages, particularly in traits such as shoot dry weight and grain weight. These findings highlight the differential responses of the cultivars to cadmium exposure, with Barat emerging as a more resilient option for breeding programs focused on improving cadmium tolerance in wheat.
Quantitative analysis of gene expression
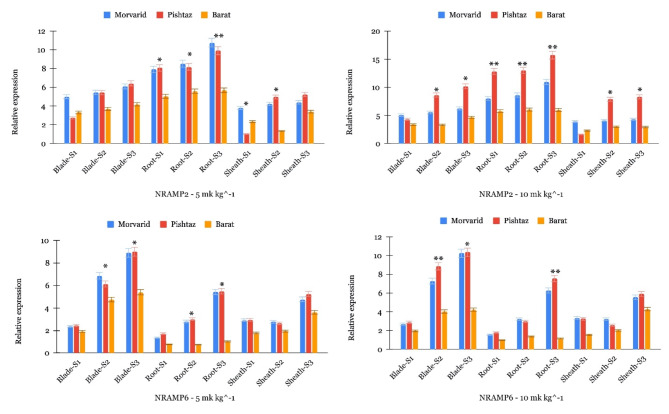
Although multiple gene families are known to be involved in Cd uptake and translocation, including NRAMP, ZIP, MTP, HMA, YSL, and LCT, many of these genes have already been extensively investigated in various plant species, including wheat. To provide novelty and contribute new insights to the field, we chose to focus on genes that have been either specifically assayed in wheat or have been studied in a limited context. Hence, we selected genes including TaNRAMP2, TaNRAMP6, TaZIP1, TaZIP3, TaZIP5, TaZIP7, and TaZIP9 for assay in this experiment, most of which have not been directly evaluated in wheat by qRT-PCR. The gene expression assay revealed notable discrepancies in the expression levels of TaNRAMP2 and TaNRAMP6 among the various wheat cultivars across different tissue types and sampling stages. To summarize, the analysis of TaNRAMP2 gene expression indicated that it tends to have higher levels of expression in roots compared to blades and sheaths, regardless of the Cd concentrations and wheat cultivars being studied (Fig. 4).
Fig. 4.
Expression profiles of TaNRAMP genes across different tissues during three developmental stages. The y-axis indicates the expression level of TaNRAMP genes, while the x-axis represents three tissues: blade, root, and sheath at three developmental stages: tillering (S1), booting (S2), and flowering (S3) for Morvarid, Barat, and Pishtaz cultivars. Asterisks indicate statistical significance levels among cultivars within each tissue/stage, where “*” denotes significance at p < 0.05 and “**” denotes significance at p < 0.01 based on one-way ANOVA.
When comparing the levels of TaNRAMP2 expression among the three cultivars, Morvarid and Pishtaz consistently demonstrated higher expression levels in most tissue types and sampling stages compared to Barat. Upon increasing the Cd concentration to 10 mg kg−1, the expression levels of TaNRAMP2 were further enhanced across all tissue types and sampling stages for all cultivars. The lower expression of the TaNRAMP2 gene in the Barat variety aligns with its tendency to accumulate lower Cd in its tissues compared to other wheat cultivars like Morvarid and Pishtaz. This correlation implies a potential association between gene expression levels and the ability of wheat plants to uptake or accumulate Cd. The reduced expression of NRAMP2 in Barat indicates a strong and effective mechanism to prevent Cd uptake in comparison to Morvarid and Pishtaz. The assessment of TaNRAMP6 gene expression demonstrated divergent patterns when compared to TaNRAMP2, as TaNRAMP6 displayed higher expression levels in leaves and lower levels in roots across different wheat cultivars and sampling stages. Notably, similar to TaNRAMP2, Morvarid and Pishtaz consistently showed higher TaNRAMP6 expression levels than Barat in most tissue types and stages, suggesting their heightened sensitivity to Cd stress. Upon exposure to 10 mg kg−1Cd, TaNRAMP6 expression levels were generally increased across all tissues and stages, indicating an intensified response to higher Cd concentrations. Entering the higher stages and the growth of wheat, the expression of TaNRAMP6 gene has also increased, but this rhythm was not significant in the TaNRAMP2 gene.
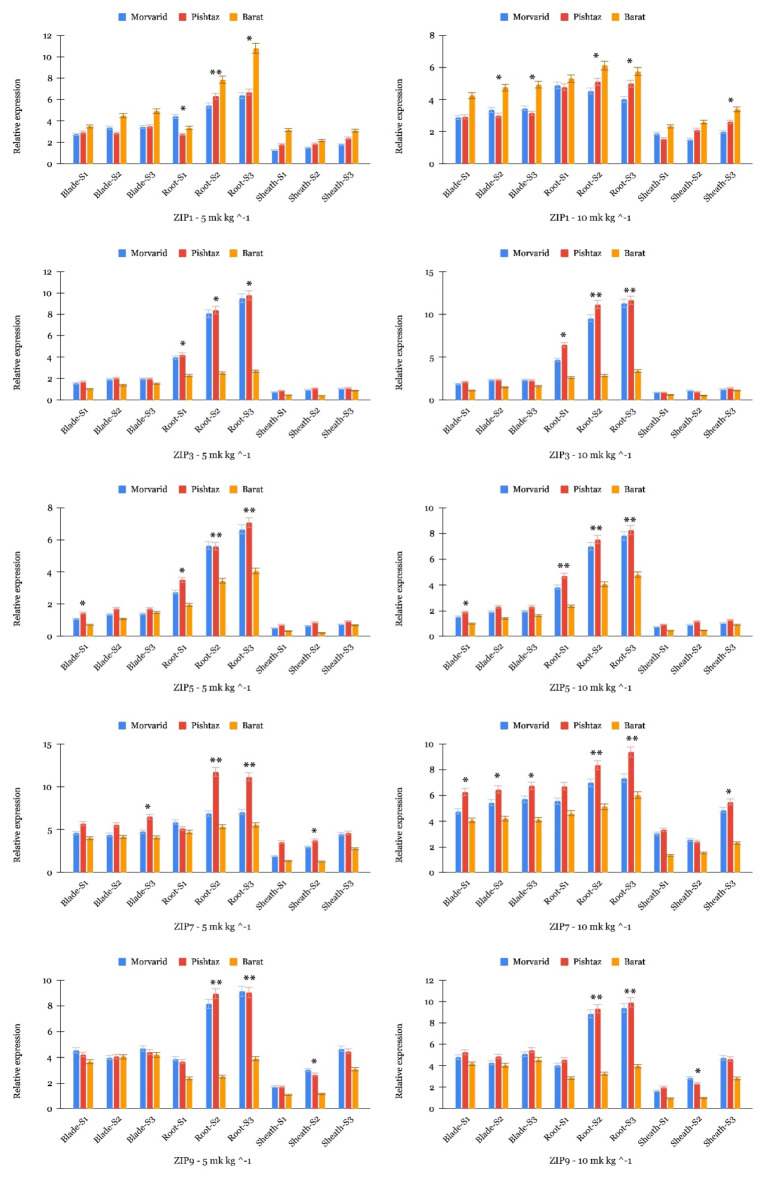
Among the analyzed ZIP genes (Fig. 5), TaZIP1, TaZIP3, TaZIP5, TaZIP7, and TaZIP9 all exhibited higher expression levels in root tissues across the three wheat cultivars, regardless of the concentration of Cd. This indicates a crucial role for these genes in the uptake and transportation of Cd within wheat roots. However, TaZIP1, TaZIP7, and TaZIP9 showed higher expression levels in blade and sheath tissues compared to TaZIP3 and TaZIP5. This variation in expression suggests that ZIP3 and ZIP5 may be involved in the transport of Cd within shoot tissues, potentially contributing to the sequestration or redistribution of Cd within the plant. Additionally, when comparing the expression levels among the wheat cultivars, Morvarid and Pishtaz consistently exhibited higher expression levels of ZIP genes, except for TaZIP1, compared to Barat, suggesting a genetic predisposition for increased sensitivity or response to Cd stress. Nevertheless, in certain instances, such as TaZIP5 and TaZIP7, Pishtaz displayed higher expression levels than Morvarid in most tissues. Alongside the identified expression patterns of TaZIP5 and TaZIP7, it is notable that TaZIP1 consistently displayed elevated expression levels in Barat compared to both Morvarid and Pishtaz across diverse tissues and Cd concentrations. This observation suggests a potential involvement of TaZIP1 in the Cd uptake mechanism by indicating higher expression, particularly in Morvarid and Pishtaz, contributing to their heightened sensitivity to Cd stress. Furthermore, exposure to higher Cd concentrations (10 mg kg−1) generally led to increased expression levels of ZIP genes across all tissue types and sampling stages.
Fig. 5.
Expression profiles of TaZIP genes across different tissues during three developmental stages. The y-axis indicates the expression level of TaZIP genes, while the x-axis represents three tissues: blade, root, and sheath at three developmental stages: tillering (S1), booting (S2), and flowering (S3) for Morvarid, Barat, and Pishtaz cultivars. Asterisks indicate statistical significance levels among cultivars within each tissue/stage, where “*” denotes significance at p < 0.05 and “**” denotes significance at p < 0.01 based on one-way ANOVA.
In addition to our initial analysis, we further examined the gene expression data using a three-factor ANOVA to assess the effects of genotype, Cd level, and tissue type on gene expression at each developmental stage. The results of this analysis are presented in Table S3, and Fig. S2–S12, which indicates significant effects for each gene across all stages at the 0.001 level. These findings highlight the strong influence of the three factors on gene expression and provide a more comprehensive understanding of the interactions between genotype, Cd treatment, and tissue type in our study.
Discussion
As one of the most toxic heavy metals, Cd accumulates in soil primarily through industrial activities and wastewater irrigation, contaminating agricultural lands38,39. Its uptake by bread wheat (T. aestivum L.), a staple crop for a large portion of the global population, raises serious concerns about food safety and public health. Cd accumulation in wheat grains can lead to severe health issues upon consumption. Therefore, the identification and selection of low-Cd accumulating wheat cultivars, understanding the mechanisms underlying Cd stress in wheat, and developing strategies to mitigate Cd accumulation in grains are essential for ensuring food security and human health. Hence this study aimed to evaluate the Cd accumulation and tolerance level in some bread wheat cultivars, analyzing the phenotypic traits, and investigating the gene expression patterns associated with Cd stress response in these wheat cultivars.
The assessment of Cd accumulation across six wheat cultivars revealed distinctive patterns and cultivar-specific responses to varying soil Cd concentrations. This observation aligns with previous studies indicating genetic variability in Cd accumulation among wheat cultivars40–46. Cd accumulation patterns differed between grain and straw. At 2.5 and 5 mg Cd kg−1 soil, there were no significant differences in Cd accumulation between cultivars. However, at 10 mg Cd kg−1 soil, Barat had the lowest Cd levels in both grain and straw, while Parsi had the highest straw Cd accumulation despite lower levels in grain. This pattern persisted at 15 mg Cd kg−1 soil, with Parsi showing high straw Cd but low grain Cd levels, indicating a cultivar-specific response to Cd stress. Kavir and Pishtaz exhibited higher grain Cd levels, while other cultivars did not show significant differences. Barat and Parsi are promising for bread-making, especially in environments with Cd concentrations up to 10 mg Cd kg−1 soil. For animal feed production, where straw Cd levels are critical, cultivars like Kavir and Pishtaz, which show low to moderate Cd in straw despite higher grain levels, are preferable. At 20 and 25 mg Cd kg−1 soil, Cd levels in grain initially decreased but then increased, while straw Cd levels increased up to 20 mg Cd kg−1 soil before decreasing at 25 mg Cd kg−1 soil. This fluctuation highlights the complex, tissue-specific responses to Cd exposure, likely due to differences in Cd transport and storage mechanisms. These findings emphasize the role of genetic background in shaping wheat cultivars’ responses to Cd, suggesting varied susceptibility and tolerance levels among cultivars. This observation is consistent with previous research, such as the study conducted by Kubo et al., which demonstrated cultivar-specific responses to Cd stress in various Japanese wheat cultivars25. Similarly, the research by Liu et al. elucidated distinct Cd accumulation patterns among different wheat cultivars, highlighting the role of genetic factors in determining Cd tolerance40. Furthermore, the findings of our study are consistent with the work of Qiao et al., who reported differential Cd accumulation in wheat cultivars with diverse genetic backgrounds, emphasizing the importance of genetic variability in Cd response mechanisms47,48. The phenotypic assessment also revealed diverse responses of wheat cultivars to Cd-induced stress, highlighting the complex interplay between genetic factors and environmental conditions. For instance, Barat exhibited significant variations in plant height, spike length, and other traits, indicating its potential tolerance or adaptability to moderate Cd concentrations as it has been indicated in other species like maize48,49. This adaptability may be associated with enhanced root architecture48, allowing for improved nutrient and water uptake, which could mitigate the detrimental effects of Cd. In contrast, Pishtaz displayed heightened sensitivity, as reflected in reduced plant height and other growth parameters under similar Cd treatments. This reduced vigor may hinder Pishtaz’s ability to cope with Cd exposure, making it less suitable for cultivation in contaminated soils. The observed variability in phenotypic traits underscores the importance of selecting cultivars with desirable agronomic traits and Cd tolerance for sustainable wheat production in Cd-contaminated soils. Certain cultivars that exhibit resilience in the face of Cd-induced stress, like Barat, could be further studied and utilized in breeding programs aimed at developing Cd-tolerant wheat varieties, ensuring food security in Cd-contaminated regions. Ultimately, integrating both phenotypic assessments and genetic insights will be crucial for enhancing the breeding strategies focused on improving Cd tolerance in wheat. The gene expression analysis elucidated the molecular mechanisms underlying Cd stress response in these wheat cultivars. Previous research suggests that NRAMP family genes play a significant role in metal transport in plants, including Cd uptake and translocation50–53. NRAMP254–56 and NRAMP652,57 expressions showed a certain effect, with their expression increasing with higher Cd levels. Our results indicated that TaNRAMP2 and TaNRAMP6 expressions exhibit a certain concentration-dependent effect, with their expression levels increasing with higher Cd concentrations. The variation in expression levels of these genes among the cultivars suggests cultivar-specific responses to Cd stress. Pishtaz and Morvarid exhibited relatively higher expression levels of TaNRAMP2 and TaNRAMP6 compared to Barat. This higher expression of these genes in these cultivars suggests a potential mechanism for increased Cd uptake and accumulation in this cultivar under Cd stress conditions. Conversely, Barat displayed lower expression levels for both genes, which possibly contributes to reduced Cd accumulation in these cultivars. Previous research, particularly in model plants like rice, has demonstrated that higher expression levels of NRAMP255,58–60 and NRAMP658,61 are associated with increased Cd accumulation.
The tissue-specific expression of TaNRAMP2 and TaNRAMP6 plays a crucial role in mediating Cd uptake and accumulation in plants, including wheat cultivars such as Pishtaz, Morvarid, and Barat. Previous studies have revealed that NRAMPs expression is not uniform across different tissues but rather exhibits specific spatial distribution patterns within the plant. In particular, TaNRAMP2 expression tends to be higher in roots compared to other tissues, reflecting its primary role in metal ion uptake from the soil. This elevated expression in roots facilitates the transport of Cd ions across the plasma membrane and their subsequent translocation to the aerial parts of the plant56,59,60. Additionally, TaNRAMP2 expression has been detected in other tissues, including stems and leaves, albeit at lower levels compared to roots. This broader expression pattern suggests that TaNRAMP2 may also contribute to Cd transport within the plant’s vascular system, facilitating the movement of Cd ions from roots to shoots. According to the results of the tissue-expression of TaNRAMP6, although it was found to be upregulated in the roots, indicating its involvement in Cd uptake from the soil, intriguingly, higher expression levels were observed in the blade or leaf tissues. Indeed, expression of TaNRAMP2 and TaNRAMP6 in the blade and sheath tissues of Morvarid and Pishtaz is substantiated by their higher Cd accumulation in both grain and straw compared to Barat. This correlation underscores the role of NRAMP genes in facilitating Cd uptake and accumulation in wheat cultivars, particularly in tissues such as blades and sheaths, contributing to the observed differences in Cd levels between cultivars. This finding suggests a higher potential role especially for TaNRAMP6 beyond Cd uptake, possibly in Cd translocation or sequestration within the aerial parts of the plant. Previous research has provided insights into the tissue-specific expression patterns of some NRAMP genes in wheat, such as NRAMP3. For instance, in a study investigating the expression pattern of TpNRAMP3 across different tissues and growth stages of wheat, notable variations were observed. At the jointing stage, TpNRAMP3 exhibited its highest expression levels in leaf blades, followed by roots and new leaves, while basal stems and leaf sheaths showed the lowest expression levels. During the booting stage, TpNRAMP3 expression peaked in old leaf blades, followed by old leaf sheaths and flag leaf blades, with subsequent expression detected in roots, flag leaf sheaths, and immature spikes, which exhibited comparatively lower expression34. These findings underscore the dynamic regulation of NRAMP gene expression across different tissues and growth stages of wheat, shedding light on their potential roles in nutrient uptake, transport, and distribution within the plant.
In addition to evaluating the expression of NRAMP genes, we also investigated the role of key ZIP genes in response to Cd stress. ZIP transporters play a crucial role in metal uptake, distribution, and detoxification in plants61,62. Our study focused on assessing the expression patterns of select ZIP genes, such as TaZIP1, in various tissues of wheat cultivars under Cd stress conditions. TaZIP1 displayed differential expression levels, particularly in roots compared to basales and sheaths. Notably, we observed higher expression of TaZIP1 in cultivars with lower Cd accumulation (Barat) not only in root but also in aerial organs, suggesting a potential role in limiting Cd uptake and translocation to grain wheat. In previous research, the function of TaZIP1 in the detoxification of Cd has been extensively evaluated, particularly in model plants like rice33,63. Overexpression or knockdown experiments of ZIP1 in rice have demonstrated its significant impact on Cd stress responses, with transgenic lines exhibiting altered Cd accumulation and tolerance33. These findings provide valuable insights into the molecular mechanisms by which ZIP transporters regulate Cd detoxification pathways in plants and underscore the importance of understanding ZIP-mediated Cd transport for enhancing Cd tolerance in crops like wheat. In further investigation, TaZIP3, TaZIP5, TaZIP7, and TaZIP9 displayed higher expression levels in root tissues across wheat cultivars, suggesting their involvement in Cd uptake and transportation within the roots. However, unlike TaZIP3 and TaZIP5, TaZIP7 and TaZIP9 exhibited higher expression levels in blade tissues. This differential expression pattern suggests that TaZIP7 and TaZIP9 may play a role in Cd transport within above-ground tissues, potentially contributing to Cd sequestration or redistribution within the plant. This finding aligns with prior research, which indicates that TaZIP7 facilitates the uptake of Cd into the xylem of rice roots and collaborates with OsZIP3 and OsHMA to facilitate intervascular transport in nodules64–66. Additionally, our findings revealed that the expression of these four ZIP genes was lower in Barat (except TaZIP1), a wheat cultivar known for its low Cd accumulation, compared to other cultivars. While there is limited research on the role of these genes in wheat under Cd stress, our findings align with previous studies indicating the involvement of certain ZIP genes in Cd uptake across plant species. For instance, investigations in rice have highlighted the cooperative function of OsZIP5 and OsZIP9 in Zn/Cd uptake, wherein knockout mutants exhibited compromised Zn/Cd absorption and subsequent growth impairment67. Similarly, a genome-wide examination of ZIP genes in Arabidopsis and rice revealed distinct expression patterns in response to Cd stress, with some ZIP genes conferring increased Cd accumulation and sensitivity when expressed in yeast cells68.
Its notable that the interaction and compensation of other metal transporter genes in the NRAMP and ZIP families beyond those studied are crucial in understanding the complex mechanisms of Cd stress response in plants. For instance, AtNRAMP1-6 except AtNRAMP4, have been shown to be involved in Mn and Cd transport in Arabidopsis69. Likewise, OsNRAMP5 is involved in transport of Cd and Mn in rice70. Similarly, OsZIP5 and OsZIP9 have been implicated in Cd and Zn transport in rice67. This results indicates how the compensatory mechanisms of metal transporter genes in the NRAMP and ZIP families are essential to Cd stress response in plants.
Conclusion
This study aimed to identify bread wheat cultivars with low Cd accumulation traits to ensure food safety and support environmental remediation. We evaluated six cultivars under Cd stress through accumulation, phenotypic, and gene expression analyses, revealing that Barat and Parsi are promising for bread-making due to their resistance to Cd accumulation, particularly at higher concentrations, while Pishtaz demonstrated sensitivity to elevated Cd levels but showed some resistance at lower concentrations (less than 5 mg kg−1). Morvarid and Chamran consistently limited Cd accumulation at increased soil concentrations, suggesting their suitability for animal feed production in contaminated soils, pending further evaluation. Additionally, Cd accumulation in grains correlated positively with straw Cd content, emphasizing the need to monitor both tissues for assessing overall Cd uptake. Significant differences in phenotypic traits were observed, with Barat and Chamran exhibiting superior growth parameters under Cd stress. Our gene expression analysis highlighted distinct regulatory patterns, as TaNRAMP genes showed higher expression levels in roots, particularly in Barat, which exhibited lower expression, indicating an effective mechanism to limit Cd uptake. Conversely, TaZIP genes, especially TaZIP1, TaZIP3, TaZIP5, TaZIP7, and TaZIP9, were elevated in root tissues across all cultivars, underscoring their crucial roles in Cd uptake and potential transport to aerial organs, with higher expression of TaZIP1 in Barat suggesting its involvement in limiting Cd uptake. Overall, the identification of cultivars like Barat, Morvarid, and Chamran provides valuable insights for future wheat breeding efforts aimed at enhancing Cd tolerance and ensuring food safety, with recommendations for incorporating these cultivars into breeding programs focused on developing Cd-resistant varieties and conducting further gene expression studies in field conditions to explore the underlying genetic mechanisms of Cd tolerance.
Material and methods
Plant material and experimental design
In this investigation, a factorial experiment was conducted utilizing six distinct wheat cultivars (Fig. S1) originated from Iran: Barat (Pedigree - SLVS*2/PASTOR), Chamran (Pedigree −50y//Attila/Bacanora), Kavir (Pedigree - Jit716/Kal//V534/3/Stm), Parsi (Pedigree - NaN), Pishtaz (Pedigree - Alvd//Aldan/Ias58), and Morvarid (Pedigree - Milan/Sha7). The selection of the six wheat cultivars for assessment in this study was undertaken with accuracy, taking into account several compelling reasons. Firstly, the cultivars were chosen to represent a diversity of genetic backgrounds, recognizing the crucial role of genetic variability in influencing a plant’s ability to accumulate and tolerate heavy metals. Secondly, the selected cultivars were specifically chosen for their demonstrated adaptability to the diverse environmental conditions present in Iran, reflecting their resilience and suitability for cultivation across different regions. Moreover, the selection of the cultivars was based on their agronomic traits and yield potential. The inclusion of high-yielding cultivars ensures that the study remains relevant to practical agricultural applications, promoting the integration of sustainable practices without compromising crop productivity. Lastly, historical data regarding the performance of these cultivars in terms of heavy metal accumulation or tolerance was taken into account.
The experimental arrangement to assess the effects of Cd levels on the cultivars was factorial based on a completely randomized design with three replications. The Cd concentrations (first factor) ranged from 0 as the control, to 2.5, 5, 10, 15, 20, and 25 mg kg−1 soil) applied on five cultivars (Second factor). The heavy metal concentrations selected for this experiment were determined based on standards established by the Iranian Department of Environment, ensuring alignment with the regulatory compliance. This approach facilitates meaningful comparisons, supports risk assessment, and contributes to public health considerations, enhancing the scientific rigor and applicability of our study in assessing wheat cultivars for their response to Cd accumulation. We also paid attention to previous studies to select the optimal Cd concentration for our experiments71–75.
The soil used in this study was sampled from research farm land in Isfahan University of Technology located in Iran (32°32′ N, 51°23′ E). The soil properties, including texture, field capacity (FC), and concentrations of Cd, were precisely measured, and the results are presented in Table 1.
Table 1.
Some physical and chemical properties of the soil used in this study.
| Silt (%) | Clay (%) | Sand (%) | FC1 (%) | PH | Cd contents (mg kg−1) |
|---|---|---|---|---|---|
| 43 | 39.2 | 17.8 | 27 | 7 | ND |
ND not detected.
1Field Capacity.
Greenhouse culture
Three-kilogram soil samples were transferred into pots with a diameter of 20 cm and a height of 18 cm. Soil was spiked adding a distilled water solution of Cd(NO3)2, mixing thoroughly and allowing to age 30 days up to field capacity. Ca(NO3)2 was also used in the controls to compensate the effect of nitrate in the treated pots. Subsequently, 500 mL volumes from each solution, corresponding to 80% of the soil sample’s water holding capacity (WHC), were uniformly added to the soil samples. This process resulted in the establishment of seven concentration levels: 0, 2.5, 5, 10, 15, 20, and 25 Cd mg kg−1 soil.
To emulate natural conditions, the treated soils underwent multiple wetting and drying cycles during a month. Finally, the soil within each pot was thoroughly mixed prior to seed planting. Ten grains of each cultivar were sowed in 21 pots for 7 levels of Cd treatment in 3 replications, 5 plants in each pot were maintained. They were kept in the controlled conditions of the greenhouse. Throughout the plant growth phase, soil moisture levels were carefully maintained at the field capacity and at the stage of grain maturity, wheat plants were harvested.
Phenotypic traits assessment
The examination of phenotypic traits assumes significance in light of the observable impact of Cd stress on the morphological and phenological characteristics of plants. In this study, a comprehensive analysis was undertaken to quantify alterations in various traits, encompassing plant height, spike length, and root length at the end of the pollination stage. Besides, root and shoot dry weights were measured after harvesting through placing vegetative organs in an oven at 75 °C for 24 h. Additionally, the assessment extended to yield-related parameters, specifically the quantity of grains produced. Moreover, phenological traits were scrutinized, encompassing the duration of time required for tillering, the emergence of the longest stem (extension), flowering initiation, and the period until the attainment of harvestable ripeness. To comprehensively evaluate the tolerance of different wheat cultivars to Cd stress, CTI was calculated for several phenotypic traits. The CTI was calculated for each cultivar and trait using the following formula: CTI (%) = (Trait value at Cd concentration / Trait value at control samples) × 100.
Cd concentration measurements
To determine the concentration of Cd in grains and straw, which were harvested at the stage of grain maturity, samples from all cultivars were assessed under uniform storage conditions, duration, and moisture levels. To manage variability and outliers, five uniform plants were selected from each pot as a replicate for analysis. For each replicate, a 0.2 g sample of oven-dried and pulverized tissue, composed of these selected plants, was digested with nitric acid to determine the Cd concentration in grains and straw” To measure the bioavailable Cd concentration in the soil samples, a mixture of DTPA (diethylenetriaminepentaacetic acid), CaCl2 (calcium chloride), and TEA (triethanolamine) was used as the extracting solution76. In this method, 50 milliliters of the extractant was added to a 12.5-g of soil (< 2 mm), followed by agitation for 2 hours. After centrifugation (6,000 rpm, 10 min), the supernatant was filtered using Whatman paper. Subsequently, the concentrations of Cd in the resulting plant and soil extracts were determined using a Rayleigh, WFX-210 flame atomic absorption spectrometer.
AF and TF
The statistical analysis involved the calculation of AF and TF to evaluate the plant’s efficiency in accumulating and translocating Cd within grains and straw46. The AF is determined by the ratio of Cd concentration in the plant’s tissues (grains or straw) to the Cd concentration in the soil, reflecting the plant’s capacity to accumulate this heavy metal. On the other hand, the TF represents the ratio of Cd in the above-ground plant parts (grains or straw) to that in the straws, indicating the translocation efficiency of Cd from roots to shoots.
AFss = [Cd] in straws/[Cd] in soil.
AFgs = [Cd] in grains/[Cd] in soil.
TFsg = [Cd] in grains/[Cd] in straws.
Furthermore, variations in Cd concentrations were precisely analyzed among different wheat cultivars. The significant differences between the Cd treatments from the same cultivar were analyzed using one-way ANOVA via Duncan’s multiple range test.
Gene expression assay
Based on preliminary experiments, three wheat cultivars were selected for gene expression analysis due to their demonstrated highest diversity in response to Cd stress, covering the spectrum of sensitivity to Cd: Morvarid (sensitive), Pishtaz (semi-sensitive), and Barat (resistant). This selection ensured a comprehensive understanding of Cd uptake and translocation mechanisms by capturing a wide range of genetic responses. The experimental setup involved cultivating seeds in soil treated with two Cd concentrations, 5 mg kg−1 and 10 mg kg−1, chosen because they induced significant differences among the cultivars. Subsequently, samples of the blade, sheath, and root were collected at three crucial stages of development: tillering (Zadok’s growth stages 20–29), booting (Zadok’s growth stage 45), and flowering (Zadok’s growth stage 58). Then, RNA was extracted in triplicate utilizing the DENAzist column RNA isolation kit, in accordance with the manufacturer’s instructions. The concentration and purity of the isolated RNA were evaluated through the utilization of a NanoDrop Spectrophotometer (NanoDrop Technologies) and agarose gel electrophoresis to guarantee the quality of the RNA samples. After verifying the integrity of the RNA, quantification of the RNA samples was carried out, leading to the commencement of cDNA library construction. For the cDNA library preparation, aimed at reverse transcription quantitative polymerase chain reaction (RT-qPCR), the initial step involved treating the isolated RNA samples with DNase I enzyme to eliminate potential genomic DNA contamination. Specifically, 2 µg of total RNA underwent incubation with 1 U of DNase I (Thermo Fisher Scientific) at 37°C for 30 min, followed by heat inactivation at 75 °C for 10 min. Subsequently, the DNase I-treated RNA was subjected to reverse transcription using Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT, Thermo Fisher Scientific). This process entailed incubating a reaction mixture comprising 1 µg of DNase I-treated RNA, 200 U M-MLV RT, 500 µM dNTPs, 5 µM random hexamer primers, 10 mM DTT, and 20 U RNase inhibitor at 37 °C for 1 h. Following the incubation period, the enzyme was inactivated at 70 °C for 15 min. The resulting cDNA served as a reliable template for subsequent quantitative PCR analysis, ensuring accurate assessment of gene expression levels. The qRT-PCR experiments were conducted using the StepOne Real-Time PCR system, with a final reaction volume of 15 µL. This reaction volume comprised 7.5 µL of SYBR Green Master Mix (BioFACT, Korea), 2 µL of diluted cDNA, and 0.5 µL of each primer designed utilizing the Primer3 tool (10 pM, Table 2), with the remaining volume filled with PCR-grade water. The qRT-PCR protocol consisted of an initial denaturation step at 95 °C for 5 min, followed by 40 cycles comprising denaturation at 95 °C for 10 s, annealing at the primer-specific temperature for 20 s, extension at 72 °C for 20 s, and a final melting curve analysis. The statistical analysis of gene expression data was performed employing the 2−∆∆Ct method77,78 with the Actin II (ACT2) gene serving as the internal reference (housekeeping) gene for normalization purposes79. In the gene expression analysis, a one-way ANOVA followed by Tukey’s post hoc test was conducted to assess the differences in gene expression among cultivars at each developmental stage and Cd concentration. This approach allowed us to focus on cultivar-specific comparisons, which were of primary interest for this study, while considering the effects of developmental stages and Cd concentrations, as reflected in the plots. Significant differences were identified at p < 0.05 and p < 0.01, indicated by single (*) and double asterisks (**), respectively. Furthermore, we conducted a three-factor ANOVA analysis using the ‘rtPCR’ package80 in R to evaluate the combination effects of genotype, Cd level, and tissue type, and developmental stage on gene expression. This analysis was performed for each stage across all genes separately.
Table 2.
Characteristics of primers are used in qRT-PCR.
| Gene name | Primer sequence | Amplicon Length (bp) |
|---|---|---|
| TaNRAMP2 | F: CCTTCGACTGCTTCATCTTTCT | 182 |
| R: GATGGTCTTTGAGCTCAGCTTT | ||
| TaNRAMP6 | F: ACCGCACAATCTCTTCCTACAT | 218 |
| R: AAGTGAGACTGCTGCATGTGTT | ||
| TaZIP1 | F: GCTTCCACTCCATCTTCGAG | 200 |
| R: ACCCCAACCGGGCTTGAC | ||
| TaZIP3 | F: CCATCCACCATCAAGCCTCT | 200 |
| R: GCTGAGTTGAAGACTCCCTC | ||
| TaZIP5 | F: GTTGACGTTCCACCAGCTCT | 160 |
| R: CGTCGTACACCGAGGATATC | ||
| TaZIP7 | F: TCTCATTCCACCAGTTCTTCGA | 250 |
| R: TCGGCTGCGATTAGATCCAC | ||
| TaZIP9 | F: ACGAGAACAGCCCCAACAC | 180 |
| R: AAGCAGCAAGGAGAGGTTTAC |
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
ZA, FB, MM, and FN: conceptualization, investigation (responsible for most experimental work), formal analysis, validation (qRT-PCR), visualization, and writing review & editing. AS: conceptualization, formal analysis (responsible for all bioinformatic analysis), validation, visualization, supervision, and writing - original draft as well as review & editing. RM, BEST, MSh, and MMM: conceptualization, investigation; formal analysis, writing-review and editing. All authors contributed to the article and approved the submitted version.
Funding
No funding was received for conducting this study.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fatemeh Beigi, Mahdiye Mortazavi and Farzaneh Najafi contributed equally to this work.
References
- 1.Salamini, F., Ozkan, H., Brandolini, A., Schäfer-Pregl, R. & Martin, W. Genetics and geography of wild cereal domestication in the near east. Nat. Rev. Genet.3, 429–441 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Jones, J., Peña, R., Korczak, R., Braun, H. J. & Carbohydrates grains, and wheat in nutrition and health: An overview part I. Role of carbohydrates in Health. Cereal Foods World60, 224–233 (2015). [Google Scholar]
- 3.Shewry, P. R. & Hey, S. J. The contribution of wheat to human diet and health. Food Energy Secur.4, 178–202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Khaishany, M. et al. Genetic variation of wheat for salt tolerance based on physiological and agronomic traits. Int. J. Agric. Biol.20, 2853–2861 (2018). [Google Scholar]
- 5.Delcour, J. & Hoseney, R. Principles of cereal science and technology. AACC Int. 229–289 (2010).
- 6.Alloway, B. J. In Sources of Heavy Metals and Metalloids in Soils BT - Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability 11–50 (ed Alloway, B. J.) (Springer Netherlands, 2013). 10.1007/978-94-007-4470-7_2
- 7.Shi, G. L. et al. Accumulation and distribution of arsenic and cadmium in winter wheat (Triticum aestivum L.) at different developmental stages. Sci. Total Environ.667, 532–539 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Pirhadi, M., Shariatifar, N., Bahmani & Manouchehri, A. Heavy metals in wheat grain and its impact on human health: A mini-review. J. Chem. Health Risks3, 421–426. 10.22034/jchr.2021.1924307.1269 (2021).
- 9.Wang, C. C., Tian, W., Xiang, P., Xu, W. & Guan, D. X. Mechanism of heavy metal uptake and transport in soil-rice/wheat system and regulation measures for safe production. Zhongguo Huanjing Kexue/China Environ. Sci.10.19674/j.cnki.issn1000-6923.20210929.003 (2021). [Google Scholar]
- 10.Zhuang, Z. et al. Source-specific risk assessment for cadmium in wheat and maize: towards an enrichment model for China. J. Environ. Sci. (China). 125, 723–734 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Li, S. et al. Selection of low-cadmium and high-micronutrient wheat cultivars and exploration of the relationship between agronomic traits and grain cadmium. Environ. Sci. Pollut Res.29, 42884–42898 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Bai, L. et al. Stability and adaptability of wheat cultivars with low cadmium accumulation based on farmland trials. Eur. J. Agron.144, 126764 (2023). [Google Scholar]
- 13.Bosch, A. C., O’Neill, B., Sigge, G. O., Kerwath, S. E. & Hoffman, L. C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric.96, 32–48 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Uraguchi, S. & Fujiwara, T. Cadmium transport and tolerance in rice: Perspectives for reducing grain cadmium accumulation. Rice (N Y). 5, 5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abedi, T. & Mojiri, A. Cadmium uptake by wheat (Triticum aestivum L.): an overview. Plants 9 (2020). [DOI] [PMC free article] [PubMed]
- 16.Kaur, R. et al. Heavy metal stress in rice: Uptake, transport, signaling, and tolerance mechanisms. Physiol. Plant.173, 430–448 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Verbruggen, N., Hermans, C. & Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New. Phytol. 181, 759–776 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Ai, H., Wu, D., Li, C. & Hou, M. Advances in molecular mechanisms underlying cadmium uptake and translocation in rice. Front. Plant. Sci.13, 1003953 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cobbett, C. S. Phytochelatins and their roles in heavy metal detoxification. Plant. Physiol.123, 825–832 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sebastian, A. & Prasad, M. N. V. Cadmium minimization in rice. A review. Agron. Sustain. Dev.34, 155–173 (2014). [Google Scholar]
- 21.Xiao, Y. et al. Differences in cadmium uptake and accumulation in seedlings of wheat varieties with low- and high-grain cadmium accumulation under different drought stresses. Plants 12 (2023). [DOI] [PMC free article] [PubMed]
- 22.Wang, C. C. et al. Heavy metal(loid)s in agriculture soils, rice, and wheat across China: Status assessment and spatiotemporal analysis. Sci. Total Environ.882, 163361 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Guo, G., Lei, M., Wang, Y., Song, B. & Yang, J. Accumulation of As, Cd, and Pb in sixteen wheat cultivars grown in contaminated soils and associated health risk assessment. Int. J. Environ. Res. Public Health. 10.3390/ijerph15112601 (2018). [DOI] [PMC free article] [PubMed]
- 24.Yang, W. et al. Heavy metals and associated health risk of wheat grain in a traditional cultivation area of Baoji, Shaanxi, China. Environ. Monit. Assess.191 (2019). [DOI] [PubMed]
- 25.Kubo, K. et al. Cadmium concentration in grains of Japanese wheat cultivars: Genotypic difference and relationship with agronomic characteristics. Plant. Prod. Sci.11, 243–249 (2008). [Google Scholar]
- 26.Mourad, A. M. I., Amin, A. E. E. A. Z. & Dawood, M. F. A. Genetic variation in kernel traits under lead and tin stresses in spring wheat diverse collection. Environ. Exp. Bot.192, 104646 (2021). [Google Scholar]
- 27.Elgharbawy, S., Abdelhamid, M., Mansour, E. & Salem, A. Rapid screening wheat genotypes for tolerance to heavy metals. 10.1007/978-3-030-64323-2_6 (2021).
- 28.Bhatti, K. H. et al. Effect of heavy metal lead (PB) stress of different concentration on wheat (Triticum aestivum L). Middle East. J. Sci. Res.14, 148–154 (2013). [Google Scholar]
- 29.Awaad Auda, H. & Moustafa, E. Identification of genetic variation among Bread Wheat genotypes for lead tolerance using Morpho – physiological and molecular markers. J. Am. Sci.6 (2010).
- 30.Tang, L. et al. Characterization of fava bean (Vicia faba L.) genotypes for phytoremediation of cadmium and lead co-contaminated soils coupled with agro-production. Ecotoxicol. Environ. Saf.171, 190–198 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Kuruvilla, P. G. K., Mathew, M. & S. & Functional characterization of a transition metal ion transporter, OsZIP6 from rice (Oryza sativa L). Plant. Physiol. Biochem.97, 165–174 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Zhang, J. et al. Research advances in cadmium uptake, transport and resistance in rice (Oryza sativa L). Cells11 (2022). [DOI] [PMC free article] [PubMed]
- 33.Liu, X. S. et al. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant. Biol.19, 283 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng, F. et al. Cloning and characterization of TpNRAMP3, a metal transporter from Polish wheat (Triticum polonicum L). Front. Plant. Sci.9, 1354 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das, N., Bhattacharya, S. & Maiti, M. K. Enhanced cadmium accumulation and tolerance in transgenic tobacco overexpressing rice metal tolerance protein gene OsMTP1 is promising for phytoremediation. Plant. Physiol. Biochem.105, 297–309 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Li, S. et al. Variation in the tonoplast cadmium transporter heavy metal ATPase 3 (HMA3) homolog gene in Aegilops tauschii. PLoS ONE18, e0279707 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shafiq, S. et al. Lead, Cadmium and Zinc Phytotoxicity alter DNA methylation levels to Confer heavy metal tolerance in wheat. Int. J. Mol. Sci.20 (2019). [DOI] [PMC free article] [PubMed]
- 38.Sizmur, T. & Richardson, J. Earthworms accelerate the biogeochemical cycling of potentially toxic elements: Results of a meta-analysis. Soil. Biol. Biochem.148, 107865 (2020). [Google Scholar]
- 39.Bamagoos, A. A., Alharby, H. F. & Abbas, G. Differential uptake and translocation of cadmium and lead by quinoa: A multivariate comparison of physiological and oxidative stress responses. Toxics10 (2022). [DOI] [PMC free article] [PubMed]
- 40.Liu, N. et al. Screening stably low cadmium and moderately high micronutrients wheat cultivars under three different agricultural environments of China. Chemosphere241, 125065 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Zhang, D. et al. Root characteristics critical for cadmium tolerance and reduced accumulation in wheat (Triticum aestivum L). J. Environ. Manage.305, 114365 (2022). [DOI] [PubMed] [Google Scholar]
- 42.He, C. et al. Evaluation of three wheat (Triticum aestivum L.) cultivars as sensitive cd biomarkers during the seedling stage. PeerJ8, e8478 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halim, M., Rahman, M. M., Mondal, D., Mallavarapu, M. & Naidu, R. Bioaccumulation and tolerance indices of cadmium in wheat plants grown in cadmium-spiked soil: Health risk assessment. Front. Environ. Sci.9 (2021).
- 44.Gao, X., Mohr, R. M., McLaren, D. L. & Grant, C. A. Grain cadmium and zinc concentrations in wheat as affected by genotypic variation and potassium chloride fertilization. Food Crop Res.122, 95–103 (2011). [Google Scholar]
- 45.Eđed Rebekić, A. & Lončarić, Z. Genotypic difference in cadmium effect on agronomic traits and grain zinc and iron concentration in winter wheat. Emirates J. Food Agric.28, 1 (2016). [Google Scholar]
- 46.Lu, M. et al. Identification of wheat (Triticum aestivum L.) genotypes for food safety on two different cadmium contaminated soils. Environ. Sci. Pollut Res. Int.27, 7943–7956 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Qiao, L. et al. Novel quantitative trait loci for grain cadmium content identified in hard white spring wheat. Front. Plant. Sci.12, 756741 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.An, T. et al. Variability in cadmium stress tolerance among four maize genotypes: Impacts on plant physiology, root morphology, and chloroplast microstructure. Plant. Physiol. Biochem.205, 108135 (2023). [DOI] [PubMed] [Google Scholar]
- 49.Deng, S. et al. Effects of Cd stress on morphological and physiological characteristics of maize seedlings. Agronomy. 10.3390/agronomy14020379 (2024).
- 50.Ehrnstorfer, I. A., Geertsma, E. R., Pardon, E., Steyaert, J. & Dutzler, R. Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport. Nat. Struct. Mol. Biol.21, 990–996 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Milner, M. J. et al. Root and shoot transcriptome analysis of two ecotypes of Noccaea caerulescens uncovers the role of NcNramp1 in cd hyperaccumulation. Plant. J.78, 398–410 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Pottier, M. et al. Identification of mutations allowing natural resistance associated macrophage proteins (NRAMP) to discriminate against cadmium. Plant. J.83, 625–637 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Leonhardt, T., Sácký, J. & Kotrba, P. Functional analysis RaZIP1 transporter of the ZIP family from the ectomycorrhizal Zn-accumulating Russula Atropurpurea. Biometals Int. J. Rle Met. Ions Biol. Biochem. Med.31, 255–266 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Zhao, J. et al. Genome-wide association study and candidate gene analysis of rice cadmium accumulation in grain in a diverse rice collection. Rice (N Y)11, 61 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang, J. D., Xie, Y., Zhang, H., Zhang, S. & Zhao, F. J. The vacuolar transporter OsNRAMP2 mediates Fe remobilization during germination and affects cd distribution to rice grain. Plant. Soil476, 79–95 (2022). [Google Scholar]
- 56.Yang, W. et al. OsNRAMP2 facilitates cd efflux from vacuoles and contributes to the difference in grain cd accumulation between japonica and indica rice. Crop J.11, 417–426 (2023). [Google Scholar]
- 57.Cailliatte, R., Lapeyre, B., Briat, J. F., Mari, S. & Curie, C. The NRAMP6 metal transporter contributes to cadmium toxicity. Biochem. J.422, 217–228 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Hu, X., Li, T., Xu, W. & Chai, Y. Distribution of cadmium in subcellular fraction and expression difference of its transport genes among three cultivars of pepper. Ecotoxicol. Environ. Saf.216, 112182 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Wang, D. et al. Overexpression of Leymus chinensis vacuole transporter NRAMP2 in rice increases Mn and cd accumulation. Plant. Stress. 11, 100344 (2024). [Google Scholar]
- 60.Zhang, Y. et al. The expression of the StNRAMP2 gene determined the accumulation of cadmium in different tissues of potato. Int. J. Mol. Sci.24 (2023). [DOI] [PMC free article] [PubMed]
- 61.Shi, X., Sun, H., Chen, Y., Pan, H. & Wang, S. Transcriptome sequencing and expression analysis of cadmium (cd) transport and detoxification related genes in cd-accumulating salix integra. Front. Plant. Sci.7, 1577 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li, S. et al. Genome-wide identification of wheat ZIP gene family and functional characterization of the TaZIP13-B in plants. Front. Plant. Sci.12, 748146 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feng, S. J. et al. Variation of DNA methylation patterns associated with gene expression in rice (Oryza sativa) exposed to cadmium. Plant. Cell. Environ.39, 2629–2649 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Yamaji, N., Xia, J., Mitani-Ueno, N. & Yokosho, K. Feng Ma, J. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant. Physiol.162, 927–939 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sasaki, A., Yamaji, N., Mitani-Ueno, N., Kashino, M. & Ma, J. F. A node-localized transporter OsZIP3 is responsible for the preferential distribution of Zn to developing tissues in rice. Plant. J.84, 374–384 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Tian, S. et al. Co-expression of multiple heavy metal transporters changes the translocation, accumulation, and potential oxidative stress of cd and zn in rice (Oryza sativa). J. Hazard. Mater.380, 120853 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Tan, L. et al. ZINC TRANSPORTER5 and ZINC TRANSPORTER9 function synergistically in Zinc/Cadmium uptake. Plant. Physiol.183, 1235–1249 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng, X., Chen, L. & Li, X. Arabidopsis and rice showed a distinct pattern in ZIPs genes expression profile in response to cd stress. Bot. Stud.59, 22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao, H. et al. NRAMP2, a trans-golgi network-localized manganese transporter, is required for Arabidopsis root growth under manganese deficiency. New. Phytol. 217, 179–193 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Ishimaru, Y. et al. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci. Rep.2, 286 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qing, M., Lin, L., Sun, J. & Ming’an, L. Effects of Cadmium stress on antioxidant enzymes activities and soluble protein content in Cyphomandra betacea Seedlings. IOP Conf. Ser. Earth Environ. Sci.199, 32025 (2018). [Google Scholar]
- 72.Shen, Q., Yang, Q., Ren, B. & Zhang, M. Adsorption and desorption characteristics of cd, Cu and Pb in different soil aggregates through soil profile under single and ternary systems. Pedosphere. 10.1016/j.pedsph.2024.02.004 (2024). [Google Scholar]
- 73.Soni, S., Jha, A. B., Dubey, R. S. & Sharma, P. Mitigating cadmium accumulation and toxicity in plants: The promising role of nanoparticles. Sci. Total Environ.912, 168826 (2024). [DOI] [PubMed] [Google Scholar]
- 74.Song, Y. et al. Impact of industrial pollution of cadmium on traditional crop planting areas and land management: A case study in Northwest China. Land. 10.3390/land10121364 (2021).
- 75.Wang, X., Song, Y., Ma, Y., Zhuo, R. & Jin, L. Screening of cd tolerant genotypes and isolation of metallothionein genes in alfalfa (Medicago sativa L). Environ. Pollut. 159, 3627–3633 (2011). [DOI] [PubMed] [Google Scholar]
- 76.Lindsay, W. L. & Norvell, W. A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil. Sci. Soc. Am. J.42, 421–428 (1978). [Google Scholar]
- 77.Rao, X., Huang, X., Zhou, Z. & Lin, X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath.3, 71–85 (2013). [PMC free article] [PubMed] [Google Scholar]
- 78.Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods. 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Tenea, G. N., Bota, P., Cordeiro Raposo, A. & Maquet, A. Reference genes for gene expression studies in wheat flag leaves grown under different farming conditions. BMC Res. Notes4, 373 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mirzaghaderi, G. rtpcr: A Package for Statistical Analysis of Real-Tme PCR Data in R. 10.21203/rs.3.rs-4193266/v1 (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.