Abstract
Campylobacter jejuni (C. jejuni) is a leading foodborne illness causing bacteria, and poultry is a major reservoir of this pathogen. With the recent increase in broiler production under the “no antibiotics ever” (NAE) system, this study aimed to assess the prevalence, antibiotic resistance, and virulence characteristics of C. jejuni isolated from NAE raised broilers. A total of 270 cloacal swabs were collected from the live-hang areas of 3 commercial processing plants over 9 wk. Each processing plant was visited 3 times at a 1-wk interval, and 30 samples were collected per visit. Among the total 270 cloacal swab samples, C. jejuni was isolated from 44 (16.3%) samples . Of these isolates, 65.9% possessed toxin-producing genes cdtA, cdtB, and cdtC, and invasion gene ciaB. The prevalence of antibioitc resistance genes aph (3′)-IIIa, erm(B) were 59.1%, and 50%, respectively. Nine (20.45%) C. jejuni isolates were identified as multidrug resistant (MDR), and 18 (40.9%) isolates showed resistance to at least 1 tested antibiotic. The highest resistance was observed against tetracycline (29.5%), followed by nalidixic acid (25%), whereas 22.7% of isolates were resistant to 2 clinically important antibiotics, azithromycin and ciprofloxacin. These results suggest that there ishigh prevalence level of multi-drug resistant C. jejuni with toxin producing virulence genes in the NAE-raised broilers sampled in this study, indicating the potential for serious human illnesses if transmitted through the food chain.
Key words: NAE raised broiler,Campylobacter jejuni; genotypic and phenotypic antibiotic resistance; virulence gene; food safety
INTRODUCTION
Campylobacteriosis, primarily caused by Campylobacter jejuni (C. jejuni), is the leading global foodborne illness (Kaakoush et al., 2015; Skarp et al., 2016; Collins, 2022). Symptoms of Campylobacter infection to humans include acute gastroenteritis, diarrhea, vomiting, and abdominal cramps (Allos, 2001). Sometimes, Campylobacter infection can lead to severe extra gastrointestinal diseases such as Miller Fisher Syndrome (MFS), Guillain-Barre syndrome (GBS) (Ford et al., 2014), and reactive arthritis (Pope et al., 2007). In the United States, the incidence rate of campylobacteriosis was reported as 19.5 cases per 100,000 people, resulting in an estimated 1.5 million illnesses annually (Tack et al., 2020). The economic impact of these human infections was estimated to be around $6.9 billion annually (Scharff 2020; Tack et al., 2020). Additionally, attribution studies have identified that consumption of the contaminated chicken and turkey accounts 71.3% of nondairy human Campylobacter infection (IFSAC, 2021). In 2019, it was estimated that there were 458,400 antibiotic-resistant human Campylobacter infections, and 70 deaths occurred in the United States (CDC 2019; Francois Watkins et al., 2021). The incidence of drug-resistant Campylobacter outbreaks has increased by 44% from 2013 to 2019 (310,000 cases in 2013 to 448,400 cases in 2019) (CDC 2019), with drug-resistant Campylobacter infection doubling in the last 2 decades (NARMS 2015). Typically, fluoroquinolones (such as ciprofloxacin) and macrolides (such as azithromycin) are prescribed for the treatment of Campylobacter infection (Allos, 2001; Shane et al., 2017). However, the sensitivity of Campylobacter to fluoroquinolones and macrolides has decreased by approximately 30% (CDC 2019). Infection with antimicrobial resistant bacterial pathogens significantly increases the infection's severity and mortality risk (Cecchini et al., 2015; WHO, 2017; Dadgostar, 2019).
Poultry provides the optimum condition for the proliferation of C. jejuni and serves as a reservoir host (Beery et al., 1988; Horrocks et al., 2009). Approximately 56.5% of human campylobacteriosis is connected to consuming and handling contaminated poultry (Wilson et al., 2008; EFSA, 2021). In the past, subtherapeutic levels of antibiotics (such as Bacitracin, Tetracycline, Virginiamycin) have been consistently used as feed additives in poultry to promote growth and reduce the occurrence of enteric diseases (Diarra et al., 2007). However, due to growing concerns about antibiotic resistance in both food animals and humans, the use of medically important antibiotics in food animals has been prohibited in the United States (FDA, 2012; FDA, 2013; Page et al., 2021), and all antibiotics use in food animals has been banned in the EU (ESVAC, 2018). This change in antibiotic usage policy has led to a shift in the poultry production system, with a move from the antibiotic used to reduced or no antibiotics ever (NAE) program (Singer et al., 2020). Approximately 50 to 60% of broilers produced in the United States are raised under the NAE system (Poultry Health, 2020). The reduction or elimination of antibiotic usage in broiler feed may have impacted the prevalence and characteristics of Campylobacter in the broilers. Therefore, it is crucial to assess the prevalence and characteristics of C jejuni in NAE-raised broilers. There is limited information available on the prevalence and characteristics of C. jejuni in NAE-raised broilers. The present study aims to investigate the prevalence of C. jejuni in NAE-raised chicken in 2 states (Mississippi and Alabama) of United States and to determine the phenotypic and genotypic antimicrobial resistance and virulence characteristics of the isolated C jejuni strains.
MATERIALS AND METHODS
Sample Collection
The samples were collected between July 2020 to September 2020. All procedures used were approved by the Institutional Animal Care and Use Committee (IACUC) of Mississippi State University with protocol numbers 19-330. A total of 270 cloacal swab samples were collected from live hang areas of 3 commercial processing plants in Mississippi and Alabama that only process NAE-raised broilers. Each processing plant was visited 3 times at 1 wk intervals andfrom each visit, 30 cloacal swab samples were collected. The swab samples were collected using CultureSwab Cary-Blair Agar Transportation System (BD BBL, Berkshire, England, UK), transported in a cooler with ice to the Mississippi State University laboratory, and processed within 4 h.
Isolation of C. Jejuni
C. jejuni isolation was performed based on the Microbiology Laboratory Guidebook (MLG) 41.04 method with some modifications as described by Moran et al. (2011). Briefly, the cloacal swab was pre-enriched using a mixture of 5 mL of 2 × Blood Free- Bolton Broth (BF-BB) supplemented with Oxoid Bolton Broth Selective supplements consisting of vancomycin, cefoperazone, trimethoprim, and amphotericin B (Oxoid, ThermoFisher Scientific, Waltham, MA, USA) and 5 mL of BPW, making total volume 10 mL. The broth mixture was then incubated at 42°C for 48 h under microaerobic conditions (85% nitrogen, 10% carbon dioxide, and 5% oxygen) using Mart anaerobic jars with an Anoxomat II System (Mart Microbiology B. V., Lichtenvoorde, Oost Gelre, Netherlands). The loopfull (∼10 μl) enriched samples were cultured onto Campylobacter selective agar plates 3 times to obtain the pure single isolate. Each time a single colony was obtained from the media plate and streaked into another plate. The Campylobacter selective agar contained Campylobacter agar base (Oxoid, ThermoFisher Scientific, Waltham, MA), selective supplements (Oxoid, ThermoFisher Scientific, Waltham, MA), and 5% laked horse blood (Remel Laked Horse Blood, ThermoFisher Scientific, Waltham, MA). Cultured bacterial plates were incubated under microaerophilic conditions at 42°C for 48 h. After incubation, colony morphology was observed, and typical C. jejuni colonies (small to medium, grayish in coloration with an irregular or round edge and mucoid appearance) were picked for subsequent culture and storage.
Molecular Identification of C. Jejuni
A previously isolated bacterial colony was cultured in the 5 mL 1 × BF-BB supplemented with Oxoid Bolton Broth Selective supplements (Oxoid, ThermoFisher Scientific, Waltham, MA). The bacterial cell pellet was collected via centrifuge of the broth at 13,000 rpm (Eppendorf 5415 Centrifuge, Eppendorf, NY) for 3 min. The genomic DNA was extracted using QIAamp Fast DNA Stool Mini Kit (Qiagen, Germany) following manufacturer protocol. Following the DNA extraction, the quality of the DNA was checked via agarose gel electrophoresis and quantified via NanoDrop (Thermo Scientific, Wilmington, MA). Two C. jejuni specific gene hippo [hip400F 5′ GAAGAGGGTTTGGGTGGTG, hip1134R 5′ AGCTAGCTTCGCATAATAACTTG] and Cj-CdtC [Cj-CdtCF 5′ TTTAGCCTTTGCAACTCCTA, Cj-CdtCR 5′ AAGGGGTAGCAGCTGTTAA] genes were amplified via PCR. PCR mixture (10 µl) containing 5 µl of 2× GoTaq Green Master Mix (Promega, Madison, WI), 0.25 µl of each forward and reverse primer (10 µM), 1 µl DNA (20 ng/µl), and 3.5 µl of sterile molecular H2O. An Eppendorf Master cycler (Eppendorf, Westbury, NY) thermocycler was used under the following conditions: 2 min at 95°C; and 35 cycles of 30 s denaturation at 95°C, 30 s annealing at 60°C, and 60 s for the extension at 72°C and final extension of 5 min at 72°C (Linton et al., 2000; On and Jordan, 2003; Asakura et al., 2008). The amplified PCR product was visualized via gel electrophoresis using 2% agarose gel, and the gel image was visualized using a Kodak Gel Logic 200 Imaging System (Eastman Kodak Co., Rochester, NY).
Detection of Virulence Gene
The prevalence of thirteen virulence genes responsible for adhesion (cadF, jlpA, pebA, porA, and pldA), invasion (ciaB), toxin production (cdtA, cdtB, and cdtC), flagella gene (flaAB, flgB, and flhB) and type secretion system IV (virB9) were tested. Information about the primer is listed in Table 1. The virulence gene was detected following the procedure and primer described by Poudel et al. (2022).
Table 1.
List of primers used and thermocycler setting1 used for the amplification of the virulence genes.
| Set | Target genes | Accession No. | Primer name | Sequence (5′–3′) | Length (nt) | Tm (°C) | Amplicon Size(bp) | References |
|---|---|---|---|---|---|---|---|---|
| A2 | ciaB | NP_282066 | ciaB.F335 | GGTCTAACTTCATCAACCCTTTGC | 24 | 62.9 | 658 | Poudel et al. (2022) |
| ciaB.R992 | CTCATGCGGTGGCATTAGAATG | 22 | 62.7 | |||||
| cadF | NP_282616 | cadF.F20 | GCATCCACTCTTCTATTATCCGC | 23 | 62.8 | 543 | Poudel et al. (2022) | |
| cadF.R562 | ATTCCGTCTTAGTGATTCTTTGGC | 24 | 61.2 | |||||
| cdtA | NP_281292 | cdtA.F3 | ATCGTACCTCTCCTTGGCG | 19 | 62.3 | 440 | Poudel et al. (2022) | |
| cdtAR442 | CGGAGCAGCTTTAACGGTTTG | 21 | 62.6 | |||||
| cdtC | NP_281290 | cdtC.F260 | GCTCCAAAGGTTCCATCTTCTAAG | 24 | 62.9 | 263 | Poudel et al. (2022) | |
| cdtC.R522 | GCAACTCCTACTGGAGATTTGAAAG | 25 | 62.9 | |||||
| cdtB | NP_281291 | cdtB.F152 | GCTTGAGTTGCGCTAGTTGG | 20 | 62.4 | 180 | Poudel et al. (2022) | |
| cdtB.R331 | TGGAGGAACAGATGTAGGAGC | 21 | 62.6 | |||||
| B2 | virB9 | YP_980061 | virB9.F429 | AAGAACACGCTTTGCAATGGC | 21 | 60.6 | 535 | Poudel et al. (2022) |
| virB9.R964 | CGATGATCCTAGTCTCTACTGGAC | 24 | 64.6 | |||||
| pebA | NP_282073 | pebA.F40 | GCTCTAGGTGCTTGTGTTGC | 20 | 62.4 | 436 | Poudel et al. (2022) | |
| pebA.R476 | GTAGTTGCAGCTTGAGCCAC | 20 | 62.4 | |||||
| porA | NP_282406 | porA.F740 | TCAACTGGACACTTGAAGGTGC | 22 | 62.7 | 342 | Poudel et al. (2022) | |
| porA.R1082 | CCACCATATACGAAGTCAGCACC | 23 | 64.8 | |||||
| flhB | NP_281526 | flhB.F531 | GGTTGCACAGCTTACTTGGC | 20 | 62.4 | 257 | Poudel et al. (2022) | |
| flhB.R788 | ACATCCGCACCTGCAACATC | 20 | 62.4 | |||||
| jlpA | NP_282133 | jlpA.F998 | GCACACAGGGAATCGACAGC | 20 | 64.5 | 119 | Poudel et al. (2022) | |
| jlpA.R1116 | AAATGACGCTCCGCCCATTAAC | 22 | 62.7 | |||||
| C3 | flaAB | NP_282485 (flaA) NP_282484 (flaB) | flaA.R1094 | CAGTTGGAACAGGACTTGGAG | 21 | 62.6 | ∼1500 | Poudel et al. (2022) |
| flaB.R253 | GCTCATCCATAGCCTTATCAGCAG | 24 | 64.6 | |||||
| pldA | Part of NC_002163 | pldA.F422 | GCCTATACTCAAACTTCTTGGTGG | 24 | 60.6 | 499 | Poudel et al. (2022) | |
| pldA.R940 | AGTCTATAAGGCTTTCTCCATAGCC | 25 | 62.9 | |||||
| flgB | NP_281712 | flgB.F25 | GAACTGGTCACTGGTGCTTTAGC | 23 | 64.6 | 224 | Poudel et al. (2022) |
PCR thermocycler condition was initial denaturation at 95 °C for 3 min followed by 35 cycles of 95° C for 30 s, 60° C for 30 s, 72° C for 60 s, and a final extension step of 72° C for 5 min.
Virulence genes run as a pentaplex PCR.
Virulence gene run individually for PCR.
Motility Assay
The C. jejuni motility assay was conducted in 0.4% semi-solid Muller-Hinton Agar Plates following the procedure of Pascoe et al. (2019) with slight modifications. Briefly, the C. jejuni colonies were picked from the sub-cultured agar plates and suspended in Muller-Hinton Broth (MHB) (Oxoid, ThermoFisher Scientific, Waltham, MA). The MHB was standardized with 0.5 McFarland Standard solution using spectrophotometry (Thermo Scientific Sensitire Nephelometer, ThermoFisher Scientific, Waltham, MA). The standardized 1 mL solution was then transferred to 5 mL of MHB, and 2 µl diluted MHB was pipetted into the center of 60 mm semi-solid Muller-Hinton Agar plates in duplicates. The cultured agar plates were incubated at 42 °C for 24 h under microaerophilic conditions. Following the incubation, the halo diameter of the bacterial motility was measured using a vernier caliper. Based on the diameter of the spread, bacteria were categorized into 3 categories nonmotile, motile, and hypermotile (Pascoe et al., 2019). Bacteria that did not spread across the agar plate were categorized as nonmotile. If a bacterial spread diameter was >0.5 cm, then categorized as motile, whereas a diameter >1.5 cm was categorized as hypermotile.
Detection of Antibiotics Resistance Genes
Prevalence of 6 antibiotics resistance genes (ARG) was tested tet(O), aph(2′)-Ig, aph(3″)-IIIa, blaOXA-61, blaOXA-184, and ermB. The tested ARGs are responsible for the resistance to tetracycline [tet(O)], aminoglycosides [aph(2′)-Ig, aph(3″)-IIIa], β-lactam (blaOXA-61, blaOXA-184), and erythromycin (ermB). The PCR was conducted following the primer and thermocycler conditions as previously described by Poudel et al. (2022) (Table 2).
Table 2.
List of primers used and thermocycler setting1 used for the amplification of the antimicrobial resistance genes.
| Set | Target genes | Accession No. | Orientation | Sequence (5′–3′) | Length (nt) | Tm (°C) | Amplicon Size (bp) | Reference |
|---|---|---|---|---|---|---|---|---|
| A2 | aph (3′)-IIIa | NG_047420 | Forward | TGCACTTTGAACGGCATGATG | 21 | 56.5 | 432 | Poudel et al. (2022) |
| Reverse | TGTCATACCACTTGTCCGCC | 20 | 57.3 | |||||
| aph (2″)-Ig | NG_047407 | Forward | GATTTACCTGCCTTGATTCCGG | 22 | 56.0 | 523 | Poudel et al. (2022) | |
| Reverse | TTCGCCGAAATCTTTCCCA | 19 | 54.6 | |||||
| blaOXA-184 | NG_049485 | Forward | GCTCTCAAGTGCCTGCTTTT | 20 | 56.0 | 317 | Poudel et al. (2022) | |
| Reverse | AAATCCAACAATCCAAGCCAAA | 22 | 53.6 | |||||
| blaOXA-61 | NG_049801 | Forward | CTTTCTCTCCCGCTTCCACT | 20 | 56.8 | 203 | Poudel et al. (2022) | |
| Reverse | ACCAATTCTTCTTGCCACTTCTTT | 24 | 55.3 | |||||
| tet(O) | NG_048260 | Forward | AATATTCAGAGAAAAGGCGGCG | 22 | 55.7 | 686 | Poudel et al. (2022) | |
| Reverse | GCAGCCATAAAGAACCCCCT | 20 | 57.6 | |||||
| B3 | ermB | KC575115 | Forward | GGGCATTTAACGACGAAACTGG | 22 | 62.7 | 421 | Cheng et al. (2020) |
PCR thermocycler condition was initial denaturation at 95 °C for 3 min followed by 35 cycles of 95° C for 30 s, 55° C for 30 s, 72° C for 60 s, and a final extension step of 72° C for 5 min.
Antibiotics resistance genes run as a pentaplex PCR.
Antibiotics resistance gene run individually for PCR.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing (AST) was conducted following the broth micro dilution method using the Sensitire Campylobacter CAMPY2 plate (ThermoFisher Scientific, Waltham, MA) following manufacturer protocol. Briefly, the previously isolated and identified C. jejuni were subcultured in Campylobacter agar base for 48 h at 42 °C under microaerophilic conditions. The colonies were picked up from the agar plate and suspended in cation adjusted MHB (Thermo Scientific, Waltham, MA). The MHB was standardized with 0.5 McFarland Standard solution using spectrophotometry (Thermo Scientific Sensitire Nephelometer, CatLog: V301). A 100 µl of standardized MHB solution was then transferred to 11 mL MHB with lysed horse blood (Thermo Scientific, Waltham, MA). Subsequently, 50 µl broth solution was dispensed into CAMPY2 microtiter plates (Trek Diagnostics System, Thermo Scientific, Waltham, MA), which contains known concentrations ranging between 0.12 µg/mL to 128 µg/mL of 8 antibiotics (azithromycin, ciprofloxacin, clindamycin, erythromycin, florfenicol, gentamicin, tetracycline, and nalidixic acid). The microtiter plates were incubated at 37°C for 48 h under microaerophilic conditions. After incubation, the micro titer plate was visualized using the Vision System (Thermo Scientific, Waltham, MA), and the results were read manually. C. jejuni ATCC 33560 strain was used as the positive control.
Statistical Analysis
The MIC breaking point from Central for Disease Control and Prevention (https://www.cdc.gov/narms/antibiotics-tested.html) was utilized to interpret phenotypic antibiotic susceptibility test results; the breaking point value is listed in Table 3. For the concordance analysis and Cohen's kappa test (κ) value between the genotypic and phenotypic antibiotic resistance was only calculated for antibiotic tetracycline and tet(O) using methods described by Mackinnon, (2000). Even though 8 other antibiotics were tested we were unable to do concordance analysis due to the presence of the ≥1 cells having value zero resulting error, during calculation, which might be due to the lower positive sample. Correlation between the flagellar genes (flgB, flhB, flaAB) and phenotypic motility (combined hypermotile and motile) of C. jejuni was assessed using logistic regression utilizing SAS 9.4 (SAS, NC) (SAS Institute, 2013). The level of significance was determined at P ≤ 0.05. The descriptive calculation and graph were created using GraphPad Prism 9.1.2 (GraphPad Software, San Diego, CA).
Table 3.
Cut-off values used to determine the antibiotic resistance of Campylobacter along with the information about the concentration of the disc used.
| CLSI1 Class | Antimicrobial agents | Antimicrobial concentration range (µg/mL) | MIC2 interpretive criteria (µg/mL) |
||
|---|---|---|---|---|---|
| Susceptible | Resistance | Reference source | |||
| Macrolides | AZI | 0.015-64 | ≤0.25 | ≥0.5 | NARMS3 |
| ERY | 0.03-64 | ≤4 | ≥8 | NARMS | |
| Lincosamides | CLI | 0.03-16 | ≤0.5 | ≥1 | NARMS |
| Aminoglycosides | GEN | 0.25-32 | ≤2 | ≥4 | NARMS |
| Quinolones | CIP | 0.015-64 | ≤0.5 | ≥1 | NARMS |
| NAL | 4-64 | ≤16 | ≥32 | NARMS | |
| Tetracyclines | TET | 0.06-64 | ≤1 | ≥2 | NARMS |
| Phenicol | FLO | 0.03-64 | ≤4 | ≥8 | NARMS |
Abbreviations: AZI, azithromycin; CIP, ciprofloxacin; CM, clindamycin; ERY, erythromycin; FLO, florfenicol; GEN, gentamycin; NAL, nalidixic acid; TET, tetracycline.
Clinical and Laboratory Standards Institute.
Minimum Inhibatory Concentration.
National Antimicrobial Resistance Monitoring System.
RESULTS
C. jejuni Prevalence, Virulence, and Motility
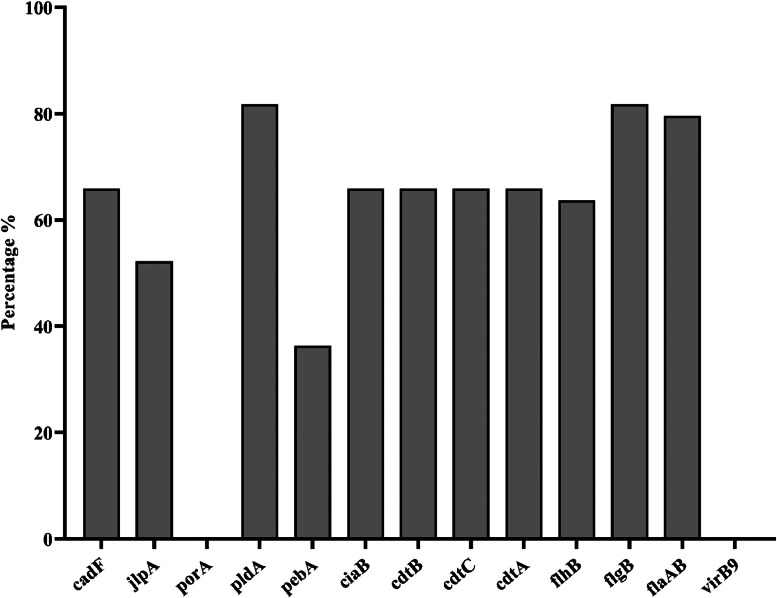
The overall prevalence of C. jejuni was found to be 16.3% (44/270) (Table 4). There was variation in prevalence of C. jejuni between the sampling point and between the processing plant. Among the 3 processing plant visit, plant 3 had higher prevalence compared to plant 1 and 2. Of the 44 C. jejuni positive samples, the adhesion gene pldA and flagellar gene flgB were present in 81.8% of the isolates, followed by another flagellar gene flaAB in 79.55% of the isolates. The Campylobacter adherence factor gene cadF, toxin gene cluster cdtA, cdtB, and cdtC, and invasion gene ciaB were present in 65.9% of the isolates. However, the genes porA and type IV secretion gene virB9 were absent in all isolates (Figure 1).
Table 4.
Isolation statistics of chicken C. jejuni strains.
| Processing Plant | Location | Sampling Time | No of swab | No. of PCR positive (%) | No. of PCR positive (%) |
|---|---|---|---|---|---|
| Plant 1 | Mississippi | 2020 July | 30 | 4 (13.3%) | 14 (15.5%) |
| Mississippi | 2020 July | 30 | 7 (23.3%) | ||
| Mississippi | 2020 August | 30 | 3 (10.0%) | ||
| Plant 2 | Alabama | 2020 August | 30 | 1 (3.3%) | 7 (7.7%) |
| Alabama | 2020 August | 30 | 4 (13.3%) | ||
| Alabama | 2020 August | 30 | 2 (6.7%) | ||
| Plant 3 | Mississippi | 2020 September | 30 | 2 (6.7%) | 23 (25.5%) |
| Mississippi | 2020 September | 30 | 0 (0%) | ||
| Mississippi | 2020 September | 30 | 21 (70.0%) | ||
| 270 | 44 (16.3%) |
Figure 1.
Percentage of virulence genes in C. jejuni isolates from NAE-raised broilers (n = 44). The bar plots display the presence of virulence genes in C. jejuni isolates obtained from broilers. These results provide insights into the genetic profiles of C. jejuni strains and their potential virulence factors in broiler.
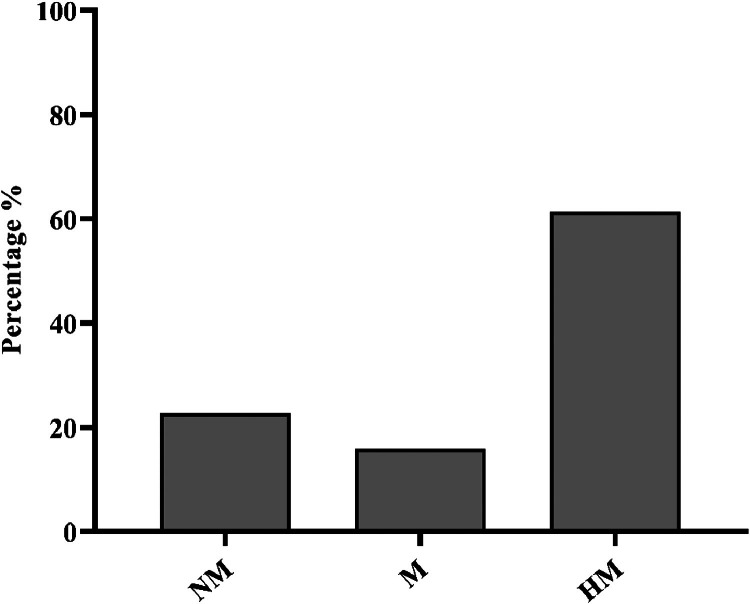
The swarming motility of the isolated C. jejuni was determined in the semisolid agar. Out of the 44 C. jejuni isolates, 61.36% (27/44) were hypermotile, 15.9% (7/44) were motile, and 22.7% (10/44) were nonmotile (Figure 2). The phenotypic motility (combined hypermotile and motile) of C. jejuni was highly correlated with all 3 tested flagellar genes (flgB, flhB, flaAB), with respective P-values of P < 0.001, 0.0059, and <0.001.
Figure 2.
Motility profiles of C. jejuni isolates from NAE-raised broiler cloacal swabs (n = 44). The bar plot shows the distribution of motility profiles among C. jejuni isolates obtained from broiler cloacal swabs. The categories include nonmotile (NM), motile (M), and hypermotile (HM) isolates.
C. Jejuni Antibiotics Resistance and Antibiotics Susceptibility
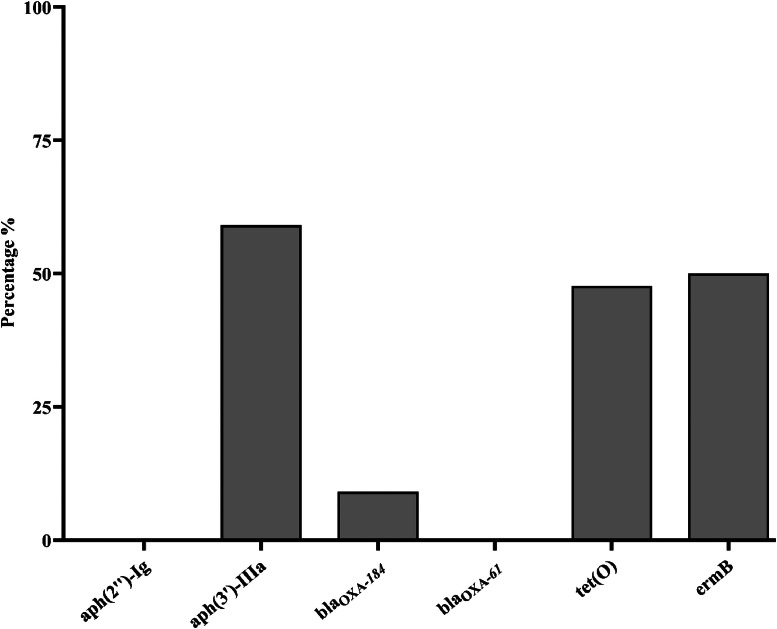
Six antibiotic resistance genes (aph(3′)-IIIa, aph(2″)-Ig, blaOXA-61, blaOXA-184, tet(O), and ermB) associated with resistance against 4 antibiotic classes (aminoglycoside, β-lactamase, tetracycline, and macrolide) were tested. The most prevalent resistance gene was aph(3′)-IIIa conferring resistance to aminoglycoside detected in 26 (59.9%) isolates. The erythromycin resistance gene ermB was detected in 22 (50%) isolates. The tetracycline resistance gene tet(O) was present in 21 (47.7%) isolates. The β-lactamase resistance gene blaOXA-184 was detected in 4 (9.09%) isolates, whereas blaOXA-61 was absent in all isolates (Figure 3).
Figure 3.
Percentage of antimicrobial resistance gene presence in C. jejuni isolates from NAE-raised broilers (n = 44). The bar plots illustrate the percentage of antimicrobial resistance gene presence in C. jejuni isolates obtained from the cloacal swab of NAE-raised broilers. Six antibiotic resistance genes [aph(3′)-IIIa, aph(2″)-Ig, blaOXA-61, blaOXA-184, tet(O), and ermB] associated with resistance against 5 antibiotic classes (aminoglycoside, β-lactamase, tetracycline, fluoroquinolones, macrolide) were tested.
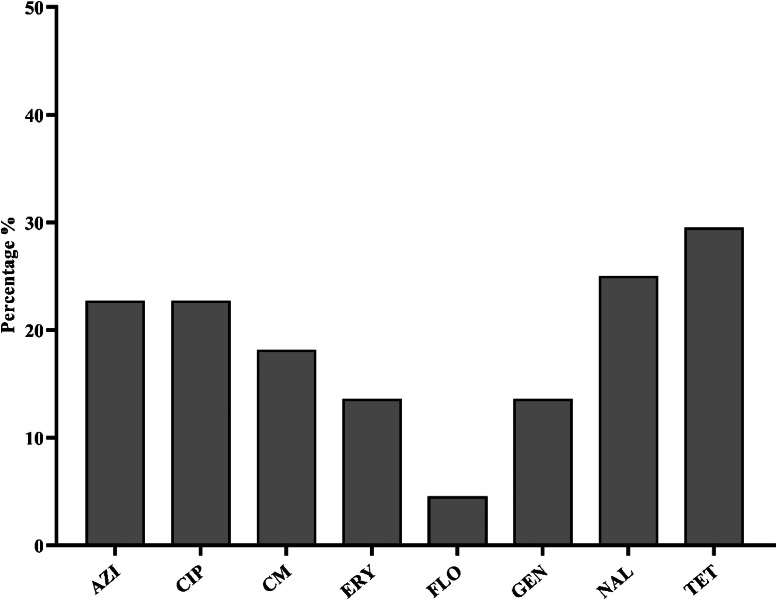
The 44 C. jejuni isolates were tested for susceptibility to 8 antibiotics from 6 different antimicrobial classes, and the results are summarized in Figure 4. The highest resistance was observed against tetracycline, with 13 (29.5%) isolates showing resistance, followed by nalidixic acid with 11 (25%) isolates, azithromycin and ciprofloxacin with 10 (22.7%) isolates each, clindamycin with 8 (18.1%) isolates, erythromycin and gentamicin with 6 (13.6%) isolates each. The lowest resistance was observed against florfenicol, with 2 (4.5%) isolates (Figure 4). Among the tested antibiotics, 9 (20.45%) C. jejuni isolates exhibited multidrug resistance (MDR), and 26 (59.09%) isolates were susceptible to all tested antibiotics. One isolate (2.27%) showed resistance to all 6-antimicrobial classes, followed by 3 (6.81%) isolates resistant to 5 antimicrobial classes (Table 5).
Figure 4.
Phenotypic antibiotics resistance profile of C. jejuni isolated from NAE-raised broiler cloacal swabs (n = 44). The bar plots show the percentage distribution of antibiotic resistance among C. jejuni isolates derived from broiler cloacal swabs. The bar represents the percentage to specific antibiotics, including AZI: azithromycin, CIP: ciprofloxacin, CM: clindamycin, ERY: erythromycin, FLO: florfenicol, GEN: gentamycin, NAL: nalidixic acid, TET: tetracycline.
Table 5.
Antimicrobial resistance patterns of the tested C. jejuni isolates against 9 antimicrobial agents of 6 antimicrobial class.
| Resistance pattern | Antimicrobial class | No of resistance isolates (%) |
|---|---|---|
| GEN, AZI, ERY, FLO, CIP, NAL, TET, CM, | Aminoglycoside, Macrolide, Phenicol, Quinolones, Tetracycline, Lincosamides | 1 (2.27%) |
| GEN, AZI, ERY, FLO, CIP, NAL, TET, | Aminoglycoside, Macrolide, Phenicol, Quinolones, Tetracycline | 1 (2.27%) |
| GEN, AZI, ERY, CIP, NAL, TET, CM, | Aminoglycoside, Macrolide, Quinolones, Tetracycline, Lincosamides | 1 (2.27%) |
| GEN, AZI, CIP, NAL, TET, CM, | Aminoglycoside, Macrolide, Quinolones, Tetracycline, Lincosamides | 1 (2.27%) |
| AZI, ERY, CIP, NAL, TET, CM, | Macrolide, Quinolones, Tetracycline, Lincosamides | 2 (4.54%) |
| GEN, AZI, CIP, NAL, CM, | Aminoglycoside, Macrolide, Quinolones, Lincosamides | 1 (2.27%) |
| GEN, AZI, CIP, NAL, | Aminoglycoside, Macrolide, Quinolones | 1 (2.27%) |
| AZI, ERY, TET, CM, | Macrolide, Tetracycline, Lincosamides | 1 (2.27%) |
| CIP, NAL, TET | Quinolones, Tetracycline | 2 (4.54%) |
| AZI, CM | Lincosamides Macrolide | 1 (2.27%) |
| TET | Tetracycline | 4 (9.09%) |
| NAL | Quinolones | 1 (2.27%) |
| CM | Lincosamides | 1 (2.27%) |
| None | None | 26 (59.09%) |
Abbreviations: AZI, azithromycin; CIP, ciprofloxacin; CM, clindamycin; ERY, erythromycin; FLO, florfenicol; GEN, gentamycin; NAL, nalidixic acid; TET, tetracycline.
The concordance analysis of the tet(O) and tetracycline antibiotics shows moderate agreement between the antibiotic's resistance and presence of the gene in the genome, as we observed the Cohen's kappa test (κ) value 0.68 with the specificity 87% and 48% sensitivity.
DISCUSSION
In the present study, we aimed to evaluate the prevalence, virulence genes, ARGs, and phenotypic antimicrobial susceptibility (AST) of C. jejuni isolates obtained from NAE-raised broilers. Our study revealed a prevalence of 16.3% for C. jejuni in NAE-raised broilers, which aligns with our previous investigation reporting a 15.7% prevalence of C. jejuni in environmental samples including broiler feces, cloacal swab, and litter samples from NAE broiler farms (Poudel et al., 2022). Current C. jejuni prevalence in NAE-raised birds was similar to (15 % -19.7 %) the previous studies, which studied the prevalence of C. jejuni in conventionally raised birds (Rosenquist et al., 2013; Gaucher et al., 2015; Golden and Mishra, 2020). Additionally, a meta-analysis predicts the prevalence of Campylobacter spp in conventionally raised birds to be 15.8% (Golden and Mishra, 2020). These findings indicate that the prevalence of C. jejuni has not increased in NAE-raised birds compared to conventionally raised birds. Although it is generally expected that the removal of antibiotics from the bird's diet could potentially increase enteric pathogen along with the C. jejuni colonization compared to a conventional system (Cox, 2005), our finding suggests that the prevalence of C. jejuni has not increased in NAE-raised broilers compared to conventionally raised birds. Potentially, the enhanced biosecurity and adoption of modern housing facilities in poultry farms might have played a significant role in maintaing similar levels of contamination. These modern houses can reduce the contact between commercial boilers with rodents, flies, cockroaches, beetles, and wild birds that may serve as a potential reservoir for bacteria transmission.
C. jejuni has many virulent factors, i.e., flagellar, adherence, toxins, invasion, and secretion. The virulence genes are the key to the pathogenesis of the bacteria. The current study tested 13 virulence genes associated with adhesion (cadF, jlpA, pebA, porA, pldA), toxin production (cdtA, cdtB, cdtC), invasion (ciaB), secretion (virB9), and motility (flaAB, flgB, and flhB). The cytolethal distending toxins (CDT) are a major heat-labile exotoxin produced by Gram-negative bacteria, which play a crucial role in the pathogenesis of Campylobacter (Jain et al., 2008). These toxins could interact with eukaryotic cells, leading to cell cycle arrest at the G2/M stage and ultimately resulting in cell death (Gargi et al., 2012; Bezine et al., 2014). In the present study, 65.9% of the C. jejuni isolates possessed all 3 cytotoxin subunit genes (cdtA, cdtB, and cdtC). The prevalence of cdt complex in our study was lower compared to previous studies, where the cdtA, cdtB, and cdtC were detected at frequencies 90-100% in C. jejuni isolated from human and poultry feces (Datta et al., 2003; Rozynek et al., 2005; Wieczorek et al., 2018). Similar to the cdt genes, the observed frequency of flagellar genes flgB (81.8%), flaAB (79.5%) had lower prevalence compared to C. jejuni isolated from the human, poultry feces, and poultry meat, where they detected approx. 100% occurrence (Datta et al., 2003; Quetz et al., 2012; Wieczorek et al., 2018; Melo et al., 2019; Sierra-Arguello et al., 2021). Additionally, in our study, 77.26% (combined hypermotile and motile) of isolated C. jejuni were motile, and there was a high correlation between the flagellar gene and the motility of C. jejuni. The primary role of flagella of the bacteria is to provide chemotactic motility and help them reach the favorable niche, adhesion, colonization, and invasion (Ottemann and Miller, 1997; Josenhans and Suerbaum, 2002; Lux and Shi, 2004; Haiko and Westerlund-Wikström, 2013; Chaban et al., 2018). Therefore, it has been postulated that the higher motility of Campylobacter means higher the risk of invading host-cell and higher the pathogenesis of bacteria (Hazell et al., 1986; Lertsethtakarn et al., 2011; Baldvinsson et al., 2014). Additionally, a positive correlation was observed between the motility and invasiveness of C. jejuni isolated from the poultry samples in human intestinal epithelial cells (Corcionivoschi et al., 2015). These findings suggest that the majority of C. jejuni isolated from NAE broilers sampled in this study possess greater colonizing and invasion potential but possess lower capabilities to produce cytolethal-distending toxins compared to human and other poultry meat C. jejuni isolates.
Although the removal of the antibiotics can negatively impact the growth performance of birds, to address the increased problem of antibiotic resistance, the usage of antibiotics in poultry production has been reduced, and a “no antibiotic ever” program has been introduced (Gaucher et al., 2015; Cowieson and Kluenter, 2019; EFSA, 2019). When the birds are constantly exposed to antibiotics, bacteria present in poultry can develop resistance against treated antibiotics, and these antibiotic-resistance bacteria can spread to humans through the food chain (Dibner and Richards, 2005; Diarra et al., 2007; Diarra and Malouin, 2014). Antimicrobial resistance (AMR) is a major public health concern throughout the world, and antibiotic resistant Campylobacter spp. has been categorized as a serious public health threat (CDC, 2019). In this study, we analyzed the presence of AMR and AST on C. jejuni isolated from the cloacal swab of an NAE-raised broiler. In our study, 20.45% C. jejuni isolates were multidrug resistance (MDR), and isolates possess ARGs and show resistance against tetracycline, nalidixic acid ciprofloxacin, azithromycin, clindamycin, gentamicin and erythromycin at varying intensity. The European Union and the United States have banned fluoroquinolones since 2001 in poultry and have progressively removed the usage of other antibiotics from poultry diet since 2006 in the EU and 2013 in the United States (FDA, 2012; FDA, 2013; ESVAC, 2018; Page et al., 2021). The impact of these regulatory changes on antibiotic usage has been significant. In chickens, the usage of medically important antibiotics has decreased by 69% in total from 2016 to 2021 (FDA, 2021). Furthermore, aminoglycosides, macrolides, sulfonamides, and tetracyclines have seen reductions of 19%, 89%, 66%, and 59%, respectively, in chicken production (FDA, 2021). The prevalence of multidrug-resistant (MDR) C. jejuni reported in our study was higher compared to <3% reported by (Bailey et al., (2019), who collected samples in midwest USA. However, the prevalence of MDR obtained in this study was slightly lower then MDR reported (30%) by Hull et al., (2021), who collected samples from chicken in North Carolina. Additionally, the resistance of C. jejuni against the tested antibiotics falls within a similar range or even higher compared to previous studies in which C. jejuni was isolated from either conventionally raised birds or undefined poultry sources (Cody et al., 2012; Obeng et al., 2012; Giacomelli et al., 2014; Sifré et al., 2015; Sierra-Arguello et al., 2015; Narvaez-Bravo et al., 2017; Premarathne et al., 2017; Szczepanska et al., 2017; Khan et al., 2018; Schiaffino et al., 2019; NARMS, 2019; Varga et al., 2019; Rivera-Mendoza et al., 2020; Tang et al., 2020; Cobo-Díaz et al., 2021; Uddin et al., 2021; Liao et al., 2021). These results indicate that despite removing antibiotics from broiler production, antibiotic resistance to C. jejuni still prevails in broiler production system. The higher frequencies of resistance observed may be attributed to the previous availability of these antibiotics as growth promoters in poultry (Miranda et al., 2008; Apata, 2009; Elviss et al., 2009; Giacomelli et al., 2014; Diarra and Malouin, 2014; Ljubojević et al., 2017; Miranda et al., 2018) and C. jejuni was still maintaining it in their genome, as Erythromycin resistance gene was found to be stable in C. jejuni even after 33 passage in nonantibiotics medium (Caldwell et al., 2008).
Although in this study we did not test the presence of all the genes associated with resistance to the tested phenotypic antibiotics susceptibility, but among the tested there was higher ARGs compared to the showed phenotypic resistance. Aminoglycoside phosphotransferases (aph) is mainly coded by aph(3′)-IIIa and aph(2″)-If, which confers resistance against amikacin, gentamicin, and kanamycin (Fong and Berghuis, 2009; Yao et al., 2017). In our study aminoglycoside resistance genes aph(3′)-IIIa was present in 59.9% of isolates; however, only 13.63% isolates were resistance to Gentamicin. Similarly, a total of 50% C. jejuni isolated in this study possess ermB gene whereas only 13.6% isolates were resistance against erythromycin phenotypically. The potential reasons for the higher ARGs compared to the phenotypic resistant might associated with unexpression resistance genes due to potential mutation in the genes and other factors associated with the gene expression like temperature and growth medium (Ocejo et al., 2021).
In this study, 29.5% isolates were resistant to tetracycline and 47.7% isolates possess tetracycline resistance gene tet(O). The results obtained for tet(O) gene was close to the results obtained by Cobo-Díaz et al. (2021), who observed 36.4% isolates positive for tet(O) gene while analyzing 39,789 C. jejuni genome sequence available in the NCBI database. Furthermore, Abdi-Hachesoo et al. (2014) observed 51.8% tet(O) gene in C. jejuni isolated from poultry carcass. However, Wozniak-Biel et al. (2018) observed higher frequency (70-100%) of tet(O) gene in the broiler and turkey samples. The observation of a higher frequency of tetracycline resistance might be due to its availability as growth promoters in poultry (Ljubojević et al., 2017) and tetracycline accounts for 71% of total antibiotics used in USA up to 2015 . However, the use of tetracycline as a growth promoter was restricted in the USA from 1 January 2017, and its use was confined to therapeutic purposes only (US FDA, 209 Guideline). In our study, we observed moderate concordance (κ = 0.68) between genotypic and phenotypic resistance of tetracycline, which was lower compared to other studies that utilizes WGS to identify concordance (Marotta et al., 2019; Whitehouse et al., 2018; Painset et al., 2020). The lower concordance might be due to the robust ness of WGS compared to PCR for the accurate identification of target genes (Moran et al., 2011).
CONCLUSION
The present study showed that the prevalence of C. jejuni has not increased in NAE-raised broilers. However, C. jejuni isolated from cloacal swabs of NAE-raised broilers possessed a higher resistance to clinically important antibiotics (tetracycline, ciprofloxacin, and azithromycin), which are commonly used during human clinical cases of campylobacteriosis. Additionally, majority C. jejuni isolated in this study were hypermotile and possessed virulence genes that can play a crucial role in the pathogenesis of campylobacteriosis in human. The observed resistance to clinically important antibiotics and the presence of virulence genes indicates that the C. jejuni from NAE-raised broilers still pose a public health threat even though there was a shift in the poultry production system from the conventional to NAE.
DISCLOSURES
The authors have declared no conflict of interest.
ACKNOWLEDGMENTS
This publication is a contribution of the Mississippi Agricultural and Forestry Experiment Station, under US Department of Agriculture, Hatch project accession number MIS-322430/NE2442. This material is based upon work that is supported by the US Poultry and Egg Association, Award No. #724.
REFERENCES
- Abdi-Hachesoo B., Khoshbakht R., Sharifiyazdi H., Tabatabaei M., Hosseinzadeh S., Asasi K. Tetracycline Resistance genes in campylobacter jejuni and c. coli isolated from poultry carcasses. Jundishapur J. Microbiol. 2014;7:e12129. doi: 10.5812/jjm.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allos B.M. Campylobacter jejuni infections: Update on emerging issues and trends. Clin. Infect. Dis. 2001;32:1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- Apata D. Antibiotic resistance in poultry. Int J. Polt. Sci. 2009;8:404–408. [Google Scholar]
- Asakura M., Samosornsuk W., Hinenoya A., Misawa N., Nishimura K., Matsuhisa A., Yamasaki S. Development of a cytolethal distending toxin (cdt) gene-based species-specific multiplex PCR assay for the detection and identification of Campylobacter jejuni, Campylobacter coli and Campylobacter fetus. FEMS Immunol. Med. Microbiol. 2008;52:260–266. doi: 10.1111/j.1574-695X.2007.00369.x. [DOI] [PubMed] [Google Scholar]
- Bailey M.A., Taylor R.M., Brar J.S., Corkran S.C., Velásquez C., Novoa Rama E., Oliver H.F., Singh M. Prevalence and antimicrobial resistance of Campylobacterfrom antibiotic-free broilers during organic and conventional processing. Poult. Sci. 2019;98:1447–1454. doi: 10.3382/ps/pey486. [DOI] [PubMed] [Google Scholar]
- Baldvinsson S.B., Sørensen M.C.H., Vegge C.S., Clokie M.R., Brøndsted L. Campylobacter jejuni motility is required for infection of the flagellotropic bacteriophage F341. Appl Environ. Microbiol. 2014;80:7096–7106. doi: 10.1128/AEM.02057-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery J., Hugdahl M., Doyle M. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 1988;54:2365–2370. doi: 10.1128/aem.54.10.2365-2370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezine E., Vignard J., Mirey G. The cytolethal distending toxin effects on mammalian cells: A DNA damage perspective. Cell. 2014;3:592–615. doi: 10.3390/cells3020592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D.B., Wang Y., Lin J. Development, stability, and molecular mechanisms of macrolide resistance in Campylobacter jejuni. Antimicrobial. Agent. Chemother. 2008;52:3947–3954. doi: 10.1128/AAC.00450-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; Atlanta, GA: 2019. Antibiotic resistance threats in the United States; pp. 1–113.https://www.cdc.gov/drugresistance/biggest_threats.html Accessed Oct. 2024. [Google Scholar]
- Cecchini M., Langer J., Slawomirski L. Organization for Economic Co-operation and Development; Paris: 2015. Antimicrobial resistance in G7 countries and beyond: economic issues, policies and option for action. [Google Scholar]
- Chaban B., Coleman I., Beeby M. Evolution of higher torque in Campylobacter-Type bacterial flagellar motors. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-017-18115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Zhang W., Lu Q., Wen G., Zhao Z., Luo Q., Shao H., Zhang T. Point deletion or insertion in cmeR-box, A2075G substitution in 23s rRNA, and presence of erm(B) are key factors of erythromycin resistance in Campylobacter jejuni and Campylobacter coli isolated from central China. Front. Microb. 2020;11:203. doi: 10.3389/fmicb.2020.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobo-Díaz J.F., González del Río P., Álvarez-Ordóñez A. Whole resistome analysis in Campylobacter jejuni and C. coli genomes available in public repositories. Front. Microbiol. 2021;12:1–16. doi: 10.3389/fmicb.2021.662144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody A.J., McCarthy N.M., Wimalarathna H.L., Colles F.M., Clark L., Bowler I.C.J.W., Maiden M.C.J., Dingle K.E. A longitudinal 6-year study of the molecular epidemiology of clinical Campylobacter isolates in Oxfordshire, United Kingdom. J. Clin. Microbiol. 2012;50:3193–3201. doi: 10.1128/JCM.01086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J.P. Preliminary incidence and trends of infections caused by pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 US Sites, 2016–2021. MMWR. Morb Mortal Wkly Rep. 2022;71:1260–1264. doi: 10.15585/mmwr.mm7140a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcionivoschi N., Gundogdu O., Moran L., Kelly C., Scates P., Stef L., Cean A., Wren B., Dorrell N., Madden R.H. Virulence characteristics of hcp+ Campylobacter jejuni and Campylobacter coli isolates from retail chicken. Gut. Pathog. 2015;7:1–11. doi: 10.1186/s13099-015-0067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowieson A., Kluenter A. Contribution of exogenous enzymes to potentiate the removal of antibiotic growth promoters in poultry production. Anim. Feed Sci. Technol. 2019;250:81–92. [Google Scholar]
- Cox L.A. Potential human health benefits of antibiotics used in food animals: A case study of virginiamycin. Environm. Int. 2005;31:549–563. doi: 10.1016/j.envint.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Dadgostar P. Antimicrobial resistance: implications and costs. Infect. Drug Resist. 2019;12:3903–3910. doi: 10.2147/IDR.S234610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S., Niwa H., Itoh K. Prevalence of 11 pathogenic genes of Campylobacter jejuni by PCR in strains isolated from humans, poultry meat and broiler and bovine faeces. J. Med. Microbiol. 2003;52:345–348. doi: 10.1099/jmm.0.05056-0. [DOI] [PubMed] [Google Scholar]
- Diarra M.S., Malouin F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Microbiol. 2014;5:282. doi: 10.3389/fmicb.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra M.S., Silversides F.G., Diarrassouba F., Pritchard J., Masson L., Brousseau R., Bonnet C., Delaquis P., Bach S., Skura B.J., Topp E. Impact of feed supplementation with antimicrobial agents on growth performance of broiler chickens, Clostridium perfringens and Enterococcus counts, and antibiotic resistance phenotypes and distribution of antimicrobial resistance determinants in Escherichia coli isolates. Appl. Environ. Microbiol. 2007;73:6566–6576. doi: 10.1128/AEM.01086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Elviss N.C., Williams L.K., Jørgensen F., Chisholm S.A., Lawson A.J., Swift C., Owen R.J., Griggs D.J., Johnson M.M., Humphrey T.J., Piddock L.J.V. Amoxicillin therapy of poultry flocks: effect upon the selection of amoxicillin-resistant commensal Campylobacter spp. J. Antimicrob. Chemother. 2009;64:702–711. doi: 10.1093/jac/dkp277. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority and European Center for Disease Prevention and Control The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019;17 doi: 10.2903/j.efsa.2019.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority, and European Centre for Disease Prevention and Control The European Union One Health 2019 Zoonoses Report. EFSA J. 2021;19 doi: 10.2903/j.efsa.2021.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency (ESVAC) European Medicines Agency; Amsterdam, Netherlands: 2018. Sales of veterinary antimicrobial agents in 31 European countries in 2018: Trends from 2010 to 2018 (Tenth ESVAC Report, Ema/184855/2017)https://www.who.int/foodsafety/publications/antimicrobials- Accessed Oct. 2024. [Google Scholar]
- FDA . U.S. Food and Drug Administration; Silver Spring, MD: 2012. Guidance for Industry #209: The judicious use of medically important antimicrobial drugs in food-producing animals. [Google Scholar]
- FDA . U.S. Food and Drug Administration; Silver Spring, MD: 2013. Guidance for Industry# 213: new animal drugs and new animal drug combination products administered in or on medicated feed or drinking water of food-producing animals: recommendations for drug sponsors for voluntarily aligning product use conditions with GFI# 209. [Google Scholar]
- FDA, 2021. 2020 Summary report on Antimicrobials sold or distributed for use in food-producing animals.
- Fong D.H., Berghuis A.M. Structural basis of aph(3′)-IIIa-mediated resistance to n1-substituted aminoglycoside antibiotics. Antimicrob. Agents Chemother. 2009;53:3049–3055. doi: 10.1128/AAC.00062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L., Kirk M., Glass K., Hall G. Sequelae of foodborne illness caused by 5 pathogens, Australia, circa 2010. Emerg. Infect Dis. 2014;20:1865–1871. doi: 10.3201/eid2011.131316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois Watkins L.K., Laughlin M.E., Joseph L.A., Chen J.C., Nichols M., Basler C., Breazu R., Bennett C., Koski L., Montgomery M.P., Hughes M.J., Robertson S., Lane C.G., Singh A.J., Stanek D., Salehi E., Brandt E., McGillivary G., Mowery J., DeMent J., Aubert R.D., Geissler A.L., de Fijter S., Williams I.T., Friedman C.R. Ongoing outbreak of extensively drug-resistant Campylobacter jejuni infections associated with us pet store puppies, 2016-2020. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.25203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargi A., Reno M., Blanke S.R. Bacterial toxin modulation of the eukaryotic cell cycle: Are all cytolethal distending toxins created equally. Front. Cell. Infect. Microbiol. 2012;2:124. doi: 10.3389/fcimb.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher M., Quessy S., Letellier A., Arsenault J., Boulianne M. Impact of a drug-free program on broiler chicken growth performances, gut health, Clostridium perfringens and Campylobacter jejuni occurrences at the farm level. Polt. Sci. 2015;94:1791–1801. doi: 10.3382/ps/pev142. [DOI] [PubMed] [Google Scholar]
- Giacomelli M., Salata C., Martini M., Montesissa C., Piccirillo A. Antimicrobial resistance of Campylobacter jejuni and Campylobacter coli from poultry in Italy. Microb. Drug. Resist. 2014;20:181–188. doi: 10.1089/mdr.2013.0110. [DOI] [PubMed] [Google Scholar]
- Golden C.E., Mishra A. Prevalence of Salmonella and Campylobacter spp. In alternative and conventionally produced chicken in the United States: A systematic review and meta-analysis. J. Food Prot. 2020;83:1181–1197. doi: 10.4315/JFP-19-538. [DOI] [PubMed] [Google Scholar]
- Haiko J., Westerlund-Wikström B. The role of the bacterial flagellum in adhesion and virulence. Biology. 2013;2:1242–1267. doi: 10.3390/biology2041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell S.L., Lee A., Brady L., Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J. Infect. Dis. 1986;153:658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- Horrocks S., Anderson R., Nisbet D., Ricke S. Incidence and ecology of Campylobacter jejuni and C. coli in animals. Anaerobe. 2009;15:18–25. doi: 10.1016/j.anaerobe.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Hull D.M., Harrell E., van Vliet A.H.M., Correa M., Thakur S. Antimicrobial resistance and interspecies gene transfer in Campylobacter coli and Campylobacter jejuni isolated from food animals, poultry processing, and retail meat in North Carolina, 2018–2019. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Food Safety Analysis Collaboration (IFSAC), 2021. Foodborne illness source attribution estimates for 2019 for Salmonella, Escherichia coli O157, Listeria monocytogens, and Campylobacter using multi-year outbreak surveillance data, United States.
- Jain D., Prasad K.N., Sinha S., Husain N. Differences in virulence attributes between cytolethal distending toxin positive and negative Campylobacter jejuni strains. J. Med. Microbiol. 2008;57:267–272. doi: 10.1099/jmm.0.47317-0. [DOI] [PubMed] [Google Scholar]
- Josenhans C., Suerbaum S. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 2002;291:605–614. doi: 10.1078/1438-4221-00173. [DOI] [PubMed] [Google Scholar]
- Kaakoush N.O., Castano-Rodriguez N., Mitchell H.M., Man S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan J.A., Rathore R.S., Abulreesh H.H., Qais F.A., Ahmad I. Prevalence and antibiotic resistance profiles of Campylobacter jejuni isolated from poultry meat and related samples at retail shops in Northern India. Foodborne Pathog. Dis. 2018;15:218–225. doi: 10.1089/fpd.2017.2344. [DOI] [PubMed] [Google Scholar]
- Lertsethtakarn P., Ottemann K.M., Hendrixson D.R. Motility and chemotaxis in Campylobacter and Helicobacter. Annu. Rev. Microbiol. 2011;65:389–410. doi: 10.1146/annurev-micro-090110-102908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y.S., Chen B.H., Teng R.H., Wang Y.W., Chang J.H., Liang S.Y., Tsao C.S., Hong Y.P., Sung H.Y., Chiou C.S. Antimicrobial resistance in Campylobacter coli and Campylobacter jejuni from human campylobacteriosis in Taiwan, 2016 to 2019. Antimicrob. Agents Chemother. 2021;66 doi: 10.1128/AAC.01736-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton D., Gilbert M., Hitchen P.G., Dell A., Morris H.R., Wakarchuk W.W., Gregson N.A., Wren B.W. Phase variation of a β-1,3 galactosyltransferase involved in generation of the ganglioside GM1-like lipo-oligosaccharide of Campylobacter jejuni. Mol. Microbiol. 2000;37:501–514. doi: 10.1046/j.1365-2958.2000.02020.x. [DOI] [PubMed] [Google Scholar]
- Ljubojević D., Pelić M., Radosavljević V., Ćirković M. Food safety hazards related to fish produced in aquaponics. European Aquaculture Society; Dubrovnik, Croatia: 2017. pp. 676–677. [Google Scholar]
- Lux R., Shi W. Chemotaxis-guided movements in bacteria. Crit. Rev. Oral Biol. Med. 2004;15:207–220. doi: 10.1177/154411130401500404. [DOI] [PubMed] [Google Scholar]
- Mackinnon A. A spreadsheet for the calculation of comprehensive statistics for the assessment of diagnostic tests and inter-rater agreement. Comput. Biol. Med. 2000;30:127–134. doi: 10.1016/s0010-4825(00)00006-8. [DOI] [PubMed] [Google Scholar]
- Marotta F., Garofolo G., Di Marcantonio L., Di Serafino G., Neri D., Romantini R., Sacchini L., Alessiani A., Di Donato G., Nuvoloni R. Antimicrobial resistance genotypes and phenotypes of Campylobacter jejuni isolated in Italy from humans, birds from wild and urban habitats, and poultry. PloS One. 2019;14 doi: 10.1371/journal.pone.0223804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo R.T., Grazziotin A.L., Júnior E.C.V., Prado R.R., Mendonça E.P., Monteiro G.P., Peres P.A.B.M., Rossi D.A. Evolution of Campylobacter jejuni of poultry origin in Brazil. Food Microbiol. 2019;82:489–496. doi: 10.1016/j.fm.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Miranda J., Vázquez B., Fente C., Barros-Velázquez J., Cepeda A., Franco C. Evolution of resistance in poultry intestinal Escherichia coli during three commonly used antimicrobial therapeutic treatments in poultry. Polt. Sci. 2008;87:1643–1648. doi: 10.3382/ps.2007-00485. [DOI] [PubMed] [Google Scholar]
- Miranda C.D., Godoy F.A., Lee M.R. Current status of the use of antibiotics and the antimicrobial resistance in the Chilean salmon farms. Front. Microbiol. 2018;9:1284. doi: 10.3389/fmicb.2018.01284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran L., Kelly C., Cormican M., McGettrick S., Madden R. Restoring the selectivity of Bolton broth during enrichment for Campylobacter spp. from raw chicken. Lett. Appl. Microbiol. 2011;52:614–618. doi: 10.1111/j.1472-765X.2011.03046.x. [DOI] [PubMed] [Google Scholar]
- National Antimicrobial Resistance Monitoring System (NARMS). (2015). NARMS 2015 human isolates surveillance report. Centers for Disease Control and Prevention. Accessed June 2022. https://www.cdc.gov/narms/reports/annual-human-isolates-report-2015.html.
- National Antimicrobial Resistance Monitoring System (NARMS). (2019). NARMS Update: Integrated report summary 2016-2017. U.S. Food and Drug Administration. Accessed June 2022. https://www.fda.gov/animal-veterinary/national-antimicrobial-resistance-monitoring-system/2019-narms-update-integrated-report-summary.
- Narvaez-Bravo C., Taboada E.N., Mutschall S.K., Aslam M. Epidemiology of antimicrobial resistant Campylobacter spp. isolated from retail meats in Canada. Int. J. Microbiol. 2017;253:43–47. doi: 10.1016/j.ijfoodmicro.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Obeng A.S., Rickard H., Sexton M., Pang Y., Peng H., Barton M. Antimicrobial susceptibilities and resistance genes in Campylobacter strains isolated from poultry and pigs in Australia. J. Appl. Micrbiol. 2012;113:294–307. doi: 10.1111/j.1365-2672.2012.05354.x. [DOI] [PubMed] [Google Scholar]
- Ocejo M., Oporto B., Lavín J.L., Hurtado A. Whole genome-based characterisation of antimicrobial resistance and genetic diversity in Campylobacter jejuni and Campylobacter coli from ruminants. Sci. Rep. 2021;11:8998. doi: 10.1038/s41598-021-88318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- On S.L.W., Jordan P.J. Evaluation of 11 PCR assays for species-level identification of Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 2003;41:330–336. doi: 10.1128/JCM.41.1.330-336.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottemann K.M., Miller J.F. Roles for motility in bacterial-host interactions. Mol. Microbiol. 1997;24:1109–1117. doi: 10.1046/j.1365-2958.1997.4281787.x. [DOI] [PubMed] [Google Scholar]
- Page, E.T., Short, G., Sneeringer, S., & Bowman, M. (2021). The marker for chicken raised without antibiotics 2012-17. United States Department of Agriculture, Economic Research Service. Accessed Oct. 2021. https://www.ers.usda.gov/publications/pub-details/?pubid=102186.
- Painset A., Day M., Doumith M., Rigby J., Jenkins C., Grant K., Dallman T.J., Godbole G., Swift C. Comparison of phenotypic and WGS-derived antimicrobial resistance profiles of Campylobacter jejuni and Campylobacter coli isolated from cases of diarrhoeal disease in England and Wales, 2015–16. J. Antimicrob. Chemother. 2020;75:883–889. doi: 10.1093/jac/dkz539. [DOI] [PubMed] [Google Scholar]
- Pascoe B., Williams L.K., Calland J.K., Meric G., Hitchings M.D., Dyer M., Ryder J., Shaw S., Lopes B.S., Chintoan-Uta C. Domestication of Campylobacter jejuni NCTC 11168. Microb. Genom. 2019;5 doi: 10.1099/mgen.0.000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope J.E., Krizova A., Garg A.X., Thiessen-Philbrook H., Ouimet J.M. Campylobacter reactive arthritis: a systematic review. Semin. Arthritis Rheum. 2007;37:48–55. doi: 10.1016/j.semarthrit.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel S., Li T., Chen S., Zhang X., Cheng W.-H., Sukumaran A.T., Kiess A.S., Zhang L. Prevalence, antimicrobial resistance, and molecular characterization of Campylobacter isolated from broilers and broiler meat raised without antibiotics. Microbiol. Spectr. 2022;10 doi: 10.1128/spectrum.00251-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poultry Health Today. 2020, Nearly 60% of the US broilers now raised without antibiotics, but that number may have peaked. Accessed May 2020. https://poultryhealthtoday.com/nearly-60-of-us-broilers-now-raised-without-antibiotics-but-that-number-may-have-peaked/
- Premarathne J.M.K.J.K., Anuar A.S., Thung T.Y., Satharasinghe D.A., Jambari N.N., Abdul-Mutalib N.A., Yew Huat J.T., Basri D.F., Rukayadi Y., Nakaguchi Y., Nishibuchi M., Radu S. Prevalence and antibiotic resistance against tetracycline in Campylobacter jejuni and C. coli in cattle and beef meat from Selangor, Malaysia. Front. Microbiol. 2017;8:2254. doi: 10.3389/fmicb.2017.02254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quetz d.J.S., Lima I.F.N., Havt A., Prata M.M.G., Cavalcante P.A., Medeiros P.H.Q.S., Cid D.A.C., Moraes M.L., Rey L.C., Soares A.M., Mota R.M.S., Weigl B.H., Guerrant R.L., Lima A.A.M. Campylobacter jejuni infection and virulence-associated genes in children with moderate to severe diarrhoea admitted to emergency rooms in northeastern Brazil. J. Med. Microbiol. 2012;61:507–513. doi: 10.1099/jmm.0.040600-0. [DOI] [PubMed] [Google Scholar]
- Rivera-Mendoza D., Martínez-Flores I., Santamaría R.I., Lozano L., Bustamante V.H., Pérez-Morales D. Genomic analysis reveals the genetic determinants associated with antibiotic resistance in the zoonotic pathogen Campylobacter spp. distributed globally. Front. Microbiol. 2020;11:2231. doi: 10.3389/fmicb.2020.513070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist H., Boysen L., Krogh A.L., Jensen A.N., Nauta M. Campylobacter contamination and the relative risk of illness from organic broiler meat in comparison with conventional broiler meat. Int. J. Food Microbiol. 2013;162:226–230. doi: 10.1016/j.ijfoodmicro.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Rozynek E., Dzierzanowska-Fangrat K., Jozwiak P., Popowski J., Korsak D., Dzierzanowska D. Prevalence of potential virulence markers in Polish Campylobacter jejuni and Campylobacter coli isolates obtained from hospitalized children and from chicken carcasses. J. Med. Microbiol. 2005;54:615–619. doi: 10.1099/jmm.0.45988-0. [DOI] [PubMed] [Google Scholar]
- SAS Institute . SAS Inst. Inc.; Cary, NC: 2013. SAS Institute SAS Proprietary Software Release 9.4. (2013) [Google Scholar]
- Scharff R.L. Food attribution and economic cost estimates for meat- and poultry-related illnesses. J. Food Prot. 2020;83:959–967. doi: 10.4315/JFP-19-548. [DOI] [PubMed] [Google Scholar]
- Schiaffino F., Colston J.M., Paredes-Olortegui M., Francois R., Pisanic N., Burga R., Penataro-Yori P., Kosek M.N. Antibiotic resistance of Campylobacter species in a pediatric cohort study. Antimicrob. Agents Chemother. 2019;63:e01911–e01918. doi: 10.1128/AAC.01911-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane A.L., Mody R.K., Crump J.A., Tarr P.I., Steiner T.S., Kotloff K., Langley J.M., Wanke C., Warren C.A., Cheng A.C., Cantey J., Pickering L.K. 2017 Infectious diseases society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin. Infect. Dis. 2017;65:e45–e80. doi: 10.1093/cid/cix669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Arguello Y.M., Morgan R.B., Perdoncini G., Lima L.M., Gomes M.J.P., do Nascimento V.P. Resistance to β-lactam and tetracycline in Campylobacter spp.isolated from broiler slaughterhouses in southern Brazil. Pesquisa Vet. Brasileira. 2015;35:637–642. [Google Scholar]
- Sierra-Arguello Y.M., Perdoncini G., Rodrigues L.B., Ruschel dos Santos L., Apellanis Borges K., Quedi Furian T., Pippi Salle C.T., de Souza Moraes H.L., Pereira Gomes M.J., Pinheiro do Nascimento V. Identification of pathogenic genes in Campylobacter jejuni isolated from broiler carcasses and broiler slaughterhouses. Sci. Rep. 2021;11:1–8. doi: 10.1038/s41598-021-84149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifré E., Salha B.A., Ducournau A., Floch P., Chardon H., Mégraud F., Lehours P. EUCAST recommendations for antimicrobial susceptibility testing applied to the three main Campylobacter species isolated in humans. J. Microbiol Methods. 2015;119:206–213. doi: 10.1016/j.mimet.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Singer R.S., Porter L.J., Schrag N.F.D., Davies P.R., Apley M.D., Bjork K. Estimates of on-farm antimicrobial usage in broiler chicken production in the United States, 2013-2017. Zoon. Public Health. 2020;1:22–35. doi: 10.1111/zph.12764. [DOI] [PubMed] [Google Scholar]
- Skarp C., Hänninen M.-L., Rautelin H. Campylobacteriosis: The role of poultry meat. Clin. Microbiol. Infect. 2016;22:103–109. doi: 10.1016/j.cmi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Szczepanska B., Andrzejewska M., Spica D., Klawe J.J. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from children and environmental sources in urban and suburban areas. BMC Microbiol. 2017;17:80. doi: 10.1186/s12866-017-0991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack D.M., Ray L., Griffin P.M., Cieslak P.R., Dunn J., Rissman T., Jervis R., Lathrop S., Muse A., Duwell M. Preliminary incidence and trends of infections with pathogens transmitted commonly through food-Foodborne Diseases Active Surveillance Network, 10 US Sites, 2016-2019. Morb. Mortal. Wkly Rep. 2020;69:509. doi: 10.15585/mmwr.mm6917a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M., Zhou Q., Zhang X., Zhou S., Zhang J., Tang X., Lu J., Gao Y. Antibiotic resistance profiles and molecular mechanisms of Campylobacter from chicken and pig in China. Front Microbiol. 2020;11:2722. doi: 10.3389/fmicb.2020.592496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M., Neogi S.B., Islam S.S., Ferdous J., Khan M., Rahman S., Yamasaki S., Kabir S. Occurrence and multidrug resistance of Campylobacter spp. at duck farms and associated environmental and anthropogenic risk factors in Bangladesh. BMC Infct. Dis. 2021;21:1–17. doi: 10.1186/s12879-021-06834-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga C., Guerin M.T., Brash M.L., Slavic D., Boerlin P., Susta L. Antimicrobial resistance in Campylobacter jejuni and Campylobacter coli isolated from small poultry flocks in Ontario, Canada: A two-year surveillance study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse C.A., Young S., Li C., Hsu C.-H., Martin G., Zhao S. Use of whole-genome sequencing for Campylobacter surveillance from NARMS retail poultry in the United States in 2015. Food Microbiol. 2018;73:122–128. doi: 10.1016/j.fm.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Wieczorek K., Wolkowicz T., Osek J. Antimicrobial resistance and virulence-associated traits of Campylobacter jejuni isolated from poultry food chain and humans with diarrhea. Front. Microbiol. 2018;9:1508. doi: 10.3389/fmicb.2018.01508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D.J., Gabriel E., Leatherbarrow A.J.H., Cheesbrough J., Gee S., Bolton E., Fox A., Fearnhead P., Hart C.A., Diggle P.J. Tracing the source of Campylobacteriosis. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2017). Campylobacter. WHO Fact Sheet. Accessed May 2020. https://www.who.int/news-room/fact-sheets/detail/campylobacter.
- Wozniak-Biel A., Bugla-Płoskońska G., Kielsznia A., Korzekwa K., Tobiasz A., Korzeniowska-Kowal A., Wieliczko A. High prevalence of resistance to fluoroquinolones and tetracycline Campylobacter Spp. isolated from poultry in Poland. Microb. Drug Resist. 2018;24:314–322. doi: 10.1089/mdr.2016.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Liu D., Wang Y., Zhang Q., Shen Z. High prevalence and predominance of the aph (2 ″)-If gene conferring aminoglycoside resistance in Campylobacter. Antimicrobial. Agents Chemother. 2017;61:e00112–e00117. doi: 10.1128/AAC.00112-17. [DOI] [PMC free article] [PubMed] [Google Scholar]