Abstract
STUDY QUESTION
What are the current national medically assisted reproduction (MAR) data collection systems across EU Member States, and how can these countries contribute to a unique, cycle-by-cycle registry for the European Monitoring of Medically Assisted Reproduction (EuMAR) project?
SUMMARY ANSWER
The study identified significant variation in MAR data collection practices across Member States, with differences in data types, collection methods, and reporting requirements; the EuMAR project emerges as an opportunity to enhance data standardization and improve MAR data collection in the EU.
WHAT IS KNOWN ALREADY
There is a need for new approaches in MAR data collection that include long-term and cross border follow-up. The EuMAR project intends to establish a unified, cycle-by-cycle registry of data on MAR treatments in EU countries, from which accurate cumulative outcomes can be calculated.
STUDY DESIGN, SIZE, DURATION
This cross-sectional study involved a survey and interviews with stakeholders from 26 EU Member States conducted in 2023 over a period of seven months.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Representatives from national competent authorities and professional associations involved in MAR data collection in EU countries were invited to complete the survey and interviewed to assess current data flows, information requirements, and their interest in the EuMAR project.
MAIN RESULTS AND THE ROLE OF CHANCE
Half of the participating countries reported having a national MAR registry with cycle-by-cycle data (n = 13), while 31% reported having a national registry with aggregated data (n = 8) and 19% reported having no national registry (n = 5). Of the countries with a national cycle-by-cycle registry, eight countries collect identifiable data, five countries collect pseudonymized data, and one country collects fully anonymized data. Informed consent is required in 10 countries. The main advantages that participants expected from a European registry like EuMAR were the possibility of obtaining national statistics in the absence of a national registry and improving the calculation of cumulative outcomes.
LIMITATIONS, REASONS FOR CAUTION
The results of the study are based on self-reported data, which may be subject to bias, however, the validity of the collected information was verified with different means, including follow-up calls for clarifications and sharing final transcript reports. The feasibility of the proposed data flow models will be tested in a pilot study.
WIDER IMPLICATIONS OF THE FINDINGS
Despite the heterogeneity of data collection practices across EU countries, the results show that stakeholders have high expectations of the benefits that the EuMAR registry can bring, namely the improvement of data consistency, cross-border comparability, and cumulative live birth rates, leading to better information for patients, health care providers and policy makers.
STUDY FUNDING/COMPETING INTEREST(S)
The EuMAR project was co-founded by ESHRE and the European Commission (101079865—EuMAR–EU4H-2021-PJ2). No competing interests were declared.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: medically assisted reproduction, data collection, registry, health data registries, European Union, database
Introduction
In a rapidly changing field and an increasingly globalized world, the European Monitoring of Medically Assisted Reproduction (EuMAR) project was launched with the aim of creating a cycle-by-cycle central European registry of data on medically assisted reproduction (MAR) treatments that could provide better insights for professionals, authorities, researchers, and patients. EuMAR is a 3-year project led by ESHRE and co-funded by the European Commission, with three main objectives: to create a data flow system beneficial to all stakeholders; to specify standardized parameters and definitions; and to develop a technical solution for a web-based registry. In line with the objectives of the project, EuMAR is composed of eight Work Packages (WPs), each of which focuses on a specific section, such as the parameters (WP4), the IT solution (WP5), the pilot study (WP6), and the integration into national policies and sustainability (WP3), which is associated with the subject of this paper. Once established, the EuMAR registry aims to improve data transparency and quality assurance and to provide cumulative data per treatment cycle and cross-border follow-up of patients (De Geyter et al., 2023).
The EuMAR project was supported by the European Commission, which in 2022 submitted a proposal for a ‘Regulation on standards of quality and safety for substances of human origin intended for human application (SoHO)’, revising its own collection of data and replacing the Directives 2002/98/EC and 2004/23/EC. In the same year, a proposal for a ‘Regulation on the European Health Data Space (EHDS)’ was also submitted by the European Commission, aiming to ‘facilitate data reuse (secondary use of data) for research, innovation, regulatory and public policy purposes across the European Union (EU)’ (European Parliament, 2024a). It is in this context that EuMAR emerged as an attempt to shape the future of MAR data collection in the EU, adapting to the new European requirements that will eventually come into force in all Member States and interoperating with the resulting data collection ecosystem within the EU.
Currently, data on MAR in Europe are collected by the ESHRE European IVF Monitoring (EIM) Consortium which recorded more than one million MAR cycles in Europe in 2019 alone (Smeenk et al., 2023). From the EIM data collection, which is based on a voluntary submission of data, it is possible to extract the success rates of MAR treatments per embryo transfer. However, due to the aggregated nature of the registry, it is not possible to calculate accurate cumulative live birth rates that would allow for realistic estimates of the overall chance of achieving a live birth over an entire course of treatment. The cumulative approach has been described as the most appropriate way to estimate the chances of treatment success by including the outcomes of all fresh and subsequent frozen-thawed embryo transfers associated to an oocyte retrieval (McLernon et al., 2016). Considering the psychological distress that patients may experience during prolonged MAR treatment and the significant dropout rates during MAR, giving accurate information to patients about their chances of success over subsequent embryo transfers is extremely important (De Neubourg et al., 2016).
The collection of cycle-by-cycle data proposed by EuMAR would not only provide this cumulative perspective but also facilitate a clearer understanding of the current movements of fertility patients and couples across countries and between clinics within the EU (De Geyter et al., 2016).
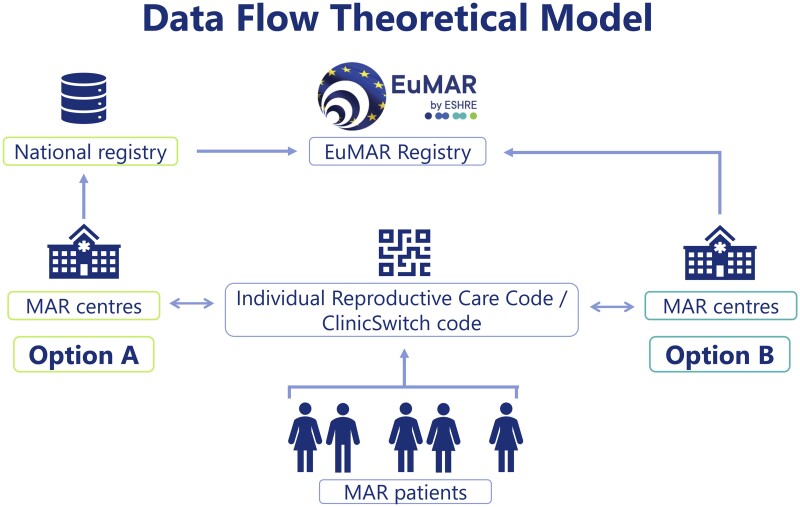
In order to achieve these objectives, the cumulative data and the cross-border follow-up, it is proposed that each patient will be assigned an Individual Reproductive Care Code (IRCC). The IRCC is a unique patient identifier that, after an anonymization process, allows cycle data from an individual patient to be collected without revealing any identifiable information, in full protection of patient data and individual privacy. Should patients move from one clinic to another, a QR code referred to as ClinicSwitch code, can be generated upon their request on the basis of the IRCC, providing a picture of cross-border and cross-institutional trends. In this case, patients’ anonymity is equally fully protected.
There is substantial heterogeneity in current MAR data collection practices in European countries (Calhaz-Jorge et al., 2020), highlighting the importance of creating a data flow model for the EuMAR registry that can be easily adapted to different contexts. A theoretical data flow model was developed during the proposal phase, which includes two different options of data submission: data submission through an existing national cycle-by-cycle registry (option A) and data submission directly from MAR clinics to the EuMAR registry (option B), as shown in Fig. 1.
Figure 1.
EuMAR data flow theoretical model. Countries report cycle-by-cycle data to EuMAR through a national registry (Option A). Alternatively, MAR centres report to the EuMAR registry directly (Option B). In both cases, patients are assigned an Individual Reproductive Care Code (IRCC) and can request a ClinicSwitch code to be presented at a second clinic if they decide to undergo a new treatment cycle elsewhere. EuMAR, European Monitoring of Medically Assisted Reproduction.
In order to determine whether it would be feasible to implement one of the options from the theoretical model in a given country, detailed knowledge of the country’s existing data collection system is required. Thus, a survey was conducted among the institutions responsible for collecting MAR data in EU Member States with the aims to understand the specificities of how data is being collected at a national level and to explore whether there is an interest in contributing cycle-by-cycle data to a European registry. Following the survey, semi-structured interviews were held with the respondents for a more in-depth discussion of the responses. This article summarizes the information gathered through the survey and interviews, thereby providing a detailed overview of the MAR data collection landscape in the EU and the opportunities and barriers to harmonization.
Materials and methods
For the purposes of EuMAR, a national MAR registry was defined as the exercise of collecting data on MAR treatment cycles at national level. This includes any database that records data on MAR activity, regardless of the format used to collect this information or the institution that manages it. Since this definition refers to the recording of treatment cycles, it goes beyond the mandatory data collection under Article 10 of the EU Tissues and Cells Directive, which pertains to information on individual tissues and cells, stating that National Competent Authorities (NCAs) in all EU countries shall receive annual reports of the activities of tissue establishments ‘including the types and quantities of tissues and/or cells procured, tested, preserved, processed, stored and distributed, or otherwise disposed of, and on the origin and destination of the tissues and cells intended for human applications’ (Directive 2004/23/EC).
An online survey with 35 questions (Supplementary File S1) was sent to institutions responsible for collecting MAR data in all 27 EU Member States, namely NCAs and national professional organizations (see Supplementary Table S1). The questionnaire was designed by WP3 members, with input from EuMAR’s Project Steering Committee, with the aims to better understand the data collection systems currently in place in each country and to establish the possible flows of data between countries and EuMAR. Questions were divided into five thematic blocks: (i) general information; (ii) information on MAR data collection; (iii) type of data collected; (iv) legal requirements and data access; and (v) perceptions towards EuMAR. The tool used for this survey was SurveyMonkey and it remained open for responses for four months. The data were collected between May and September 2023.
Before delivery, the survey was validated by two WP3 members who were also representatives of the NCAs in their respective countries (Portugal and Romania). Cognitive interviews were conducted as an established method for identifying issues and correcting survey questions (Beatty and Willis, 2007). In a phone call, draft survey questions were presented and additional verbal information about the survey responses was collected to evaluate the quality of the responses and determine whether the questions led to the information sought. Few edits were needed after the call and only one overlapping suggestion was applied to specify that answers should refer to a single national registry of MAR activity data to avoid confusion, especially for countries with more than one registry (e.g. activity data, vigilance data, and donor registries).
Anticipating potential ambiguous interpretation of responses, and to further explore the topics of the survey, all respondents were contacted to schedule semi-structured interviews. The objectives of the interviews were to: (i) present the project in detail to stakeholders and answer their questions about it; (ii) clarify the survey responses and gather additional information to that collected with the survey, such as whether or not national registries used a unique patient identifier; (iii) define a data flow per country, based on the theoretical model designed; and (iv) explore NCAs and national professional associations’ views on the project and their willingness to participate in the pilot study.
The interviews were carried out by two WP3 ESHRE staff members. A set of pre-defined questions guided the conversation, allowing for flexibility in the order in which they were asked, as well as the extent of probing. There was no imposed number of participants in the phone call, and calls were scheduled for 45–60 min, depending on the availability of attendees. Before the call, a document with their answers to the survey was shared with respondents and after the call, new information was gathered in country profiles, that were shared with the interviewees for revision and approval.
Results
Participants and response rate
In total, 26 out of 27 EU Member States completed the survey; Cyprus was the only country without a response. In 24 countries, the survey was completed by the NCAs, whereas in Germany and Belgium, in the absence of a response from the national authorities, the professional associations in charge of the national MAR registry completed the survey. In Germany, the IVF Registry (Deutsches IVF-Register e. V. (D·I·R)) sent the answers, and in Belgium, the survey was completed by the College of Physicians in Reproductive Medicine, the institution in charge of BELRAP (the Belgian Register for Assisted Procreation). Calls were held with 23 countries. Luxembourg and the Czech Republic did not confirm a date and Romania was not contacted for an additional interview as they already provided all required information in the call to validate the survey.
Types of MAR registries
Most countries (n = 21) reported having a national registry of MAR activity data, whereas only five countries (Estonia, Ireland, Latvia, Luxembourg, and Slovenia) reported not having a MAR activity data collection system.
Sixteen countries indicated having a registry of donors, whereas in 10 countries, there is no donor registry. Ireland and Slovenia both have donor registries, although they do not have MAR activity data registries. In the case of Ireland, there is a compulsory registry of third-party-assisted reproduction procedures, the National Donor-Conceived Person Register, which was created to provide requested information to donor-conceived children about their biological origins.
Mandatory versus voluntary reporting
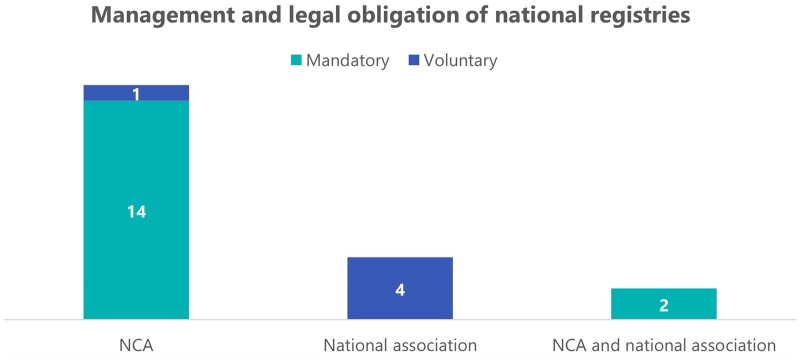
In 16 countries, reporting to a MAR activity data registry is mandatory by law, whereas in Germany, Greece, Poland, Sweden, and The Netherlands, the collection of national MAR data is carried out on a voluntary basis. Of the five countries where the national registry is voluntary, it is only in Greece that the NCA is responsible for the data collection, while in the other four, this work is carried out by a national professional association (Fig. 2). The characteristics of these voluntary registries vary from country to country. In Sweden, the voluntary nature of the data collection does not seem to pose an impediment, as all MAR centres report cycle data to QIVF, the national registry. It is noteworthy that, though it is not mandatory for Swedish centres to send their data to QIVF, this is a pre-requisite to treat publicly funded patients, which could explain the high levels of participation. In Poland and The Netherlands, the lack of a compulsory national registry is compensated for by the national professional associations (PTMRiE in Poland and NVOG in The Netherlands), who independently collect aggregated data every year from MAR centres and send completed forms to EIM. An interesting case is Germany, where the D·I·R is owned by MAR centres themselves, who pay a fee per cycle to send their data to the national registry, as well as an additional fee for quality control checks. Despite the financial costs, Germany reported having a registry with almost 100% coverage of the MAR treatments performed in the country.
Figure 2.
Management and legal obligation of national registries. In light blue are indicated the countries with mandatory national registries (n = 16), out of which 14 are managed by a National Competent Authority (NCA) and 2 by a combination of an NCA and a national association. In dark blue appears the number of voluntary national registries (n = 5), of which four are run by a national association and one is by a NCA.
Mandatory registries are almost in all cases managed exclusively by NCAs, except for in Belgium and Spain, where the national professional association is also involved in the collection of data. Austria reported the peculiarity that their compulsory national registry only collects data from publicly funded MAR cycles, which were indicated to represent about 40–60% of all cycles in Austria. The main purpose of this registry is to determine reimbursements and eligibility requirements for payment of costs, which leads to data of private MAR treatments not being collected in the national registry. Only seven countries reported having 100% of the MAR treatments performed in the country recorded in the national registry (Bulgaria, Italy, Malta, Poland, Portugal, Romania, and Sweden). Eight countries reported recording a range between 81% and 99% of the total of treatments, two countries selected 61% and 80%, two indicated that only 41% and 60% of cycles are registered, and two countries did not provide this information (see Supplementary Table S1). The most common difficulties that NCAs reported in the process of collecting national MAR data are associated with delays in receiving data from MAR centres and insufficient follow-up on deliveries.
Cycle-by-cycle versus aggregated data collection
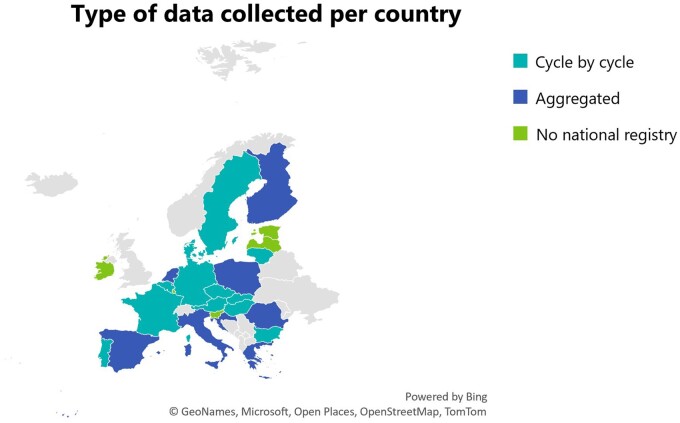
As shown in Fig. 3, most countries with a national activity data registry collect cycle-by-cycle data (n = 13), while eight countries collect aggregated data (Croatia, Finland, Greece, Italy, Poland, Romania, Spain, and The Netherlands).
Figure 3.
Type of data collected per country. Thirteen countries collect cycle-by-cycle data, 8 countries collect aggregated data, and 5 countries reported having no national registry for MAR treatments.
Data protection and consent
Anonymization of data is present in nine national registries, corresponding to the eight countries collecting aggregated data plus Portugal, an exceptional case where cycle-by-cycle data is collected, but data is fully anonymized in the national registry, which has no patient identifier. Instead, ‘treatment codes’ are used, which correspond to every treatment cycle. Data on IVF/ICSI in the Portuguese registry is therefore associated to each oocyte retrieval cycle, including all the embryo transfers related to that ovarian retrieval, but patients starting new IVF/ICSI cycles in the same or a different MAR centre are given a new ‘treatment code’, which means that it is not possible to accurately track the total number of patients or calculate cumulative outcomes over an entire treatment course with several oocyte retrieval cycles. Belgium, France, Germany, and Hungary all have pseudonymized data in their national registries. While Hungary and Belgium use a country-wide unique patient code, France and Germany use patient identifiers that are only unique per MAR centre, which can lead to an overestimation of the number of patients, since individuals will be counted as new patients if they move to a different centre. The remaining eight countries (Czech Republic, Austria, Denmark, Slovakia, Sweden, Bulgaria, Lithuania, and Malta) collect non-anonymized data using a unique patient identifier across the country, which is mainly a national ID or social security number.
Informed consent forms for patients and donors regarding their data being collected are used in ten countries, while 15 countries do not require these (Luxembourg did not provide information on this topic). Seven countries shared additional details on the use of informed consent forms: in Germany and France, these are only used for the collection of certain types of data, such as the national identification number or the date of birth. In Belgium, Hungary, Italy, Portugal, and Romania, where data collection is mandatory, consent forms are being used initially to inform the patients. In Sweden, where the national registry is voluntary, consent forms are not used, but when patients are informed that their data will be registered, they are given the opportunity to opt out. This was reported to be a rare event that happens only with a small minority of patients.
Data use and type of data collected
Most countries indicated using the collected data for monitoring purposes. All countries with a national registry reported having to collect a set of compulsory core parameters.
On the topic of cross-border care, 13 countries responded that they currently do not collect this type of data, but that it would represent an added value to their registries. From the countries with a data collection where no cumulative results can be calculated, i.e. those only collecting aggregated data (n = 8), three selected cumulative data as an indicator of value (Greece, Italy, and Romania). Ten countries also indicated that it would be beneficial to collect additional demographic parameters, such as educational level, income group, ethnicity, or geographic location of patients. These were Belgium, Bulgaria, Denmark, Germany, Greece, Hungary, Italy, Romania, Slovakia, and Sweden.
Possible data flows to EuMAR
A total of 14 countries confirmed preferring Option A of the theoretical data flow model (national registry connected to EuMAR), either because they currently have a cycle-by-cycle data collection (Austria, Belgium, Bulgaria, Denmark, France, Germany, Hungary, Malta, Portugal, and Slovakia Sweden), or because they are working on establishing one (Croatia, Finland, and Italy). Ten countries opted for Option B as the alternative to send data directly to EuMAR, out of which Estonia, Latvia, Slovenia, and The Netherlands reported active efforts to establish a national cycle-by-cycle registry, which they perceived to be the most accurate way to collect MAR data. Spain and Poland mentioned their interest in building cycle-by-cycle national registries but acknowledged not having initiated any formal steps towards establishing one. Lithuania, despite having a cycle-by-cycle registry, opted for clinics reporting directly to EuMAR if many EuMAR parameters were missing in their national registry. The remaining countries without a cycle-by-cycle national registry (Greece, Ireland, and Romania) did not comment on any changes or intentions to change their current systems. No data flow models were established for Czech Republic and Luxembourg, as they did not respond to the requests to have an interview.
Perceptions towards EuMAR
Several countries expressed an initial receptiveness to participate in the pilot study (Belgium, Estonia, France, Germany, Italy, Poland, Portugal, and Slovenia), while others showed a general interest in the project, acknowledging its potential benefits. Perceptions of particular positive impacts of the EuMAR registry in countries depended on the national situation of each country and were generally linked to how EuMAR could fill a gap in their system. For example, by providing a national MAR registry where none exists (Estonia, and Slovenia); or by improving the calculation of cumulative outcomes either where only aggregated data are collected (Italy, and Poland), where no national patient identifiers are used (France, Germany, and Portugal), or where these are only available for a part of the population (e.g. in Belgium, cumulative outcomes are only calculated for residents who have a national security number but cannot be calculated for patients coming from abroad).
In addition to Cyprus who did not respond to the survey, and Czech Republic and Luxembourg who did not interact further after completing the questionnaire, three countries communicated their decision to not continue to actively engage in the EuMAR project. These are Austria, Croatia, and Denmark. For Austria, an incomplete national registry and concerns about patients’ negative perceptions over their health data being shared with an international registry were cited as reasons for not participating in the project. The Croatian NCA was available for future interaction but reported being in the process of completing their national registry. For this reason, they preferred to focus on the new mandatory reporting that will be required by the EU SoHO platform, as envisioned in the SoHO Regulation. During the interviews with Denmark, doubts regarding the use of the IRCC and ClinicSwitch code were raised, which could explain their reluctance to continue engaging.
It became evident throughout the interviews that there were recurrent questions from different stakeholders about the relationship between the EuMAR registry and the EU SoHO platform. While the European Commission proposed mandatory reporting of SoHO entities’ aggregated activity data on donor registration, collection, distribution, import, export, and human application to the EU SoHO platform (European Parliament, 2024b), EuMAR proposes a voluntary, cycle-by-cycle data collection, managed by ESHRE. Each initiative has a different scope and purpose; however, they are both likely to require adaptations at country level. A second recurrent concern observed during the interviews with stakeholders was related to compliance with data sharing and safeguards to the identity of patients. The topic was directly raised by the Austrian NCA, who mentioned this was a delicate subject among patients in Austria, who were expected to be hesitant about the sharing of their data. Similarly, the authority in Malta remarked that the ClinicSwitch code for cross-border care was seen as potentially challenging to implement, as it could be perceived by patients as a risk to their privacy, compounded by the fact that MAR treatments remain a major taboo topic in the country. Other inquiries by stakeholders were related to financial support and the prospective timeline to collect data proposed by EuMAR.
The rest of the countries indicated different levels of interest in the project in general, and towards participating in the pilot study in particular. Eight countries indicated that their current situation did not allow them to actively contribute to a European registry such as EuMAR, in spite of their interest in the project. Specific reasons cited were either: not being allowed to share data outside of their jurisdiction due to their national data protection legislation (Finland) or not knowing if this type of data sharing would be allowed (Bulgaria); changes to their current national registry (Hungary); reduced or limited capacity of staff at the national registry or MAR centres at the time (Slovakia, Ireland, and Luxembourg); and the need to receive more detailed information about the characteristics of the registry before making a decision (Spain, and Latvia). Some of these concerns could be addressed by technical solutions, such as making use of a data federation to avoid data sharing outside of a country’s jurisdiction.
Discussion
This article summarized the information gathered within the EuMAR project from 26 EU Member States on national MAR data collection systems and the interest of countries in participating in a European MAR registry. The findings demonstrate that there is substantial heterogeneity in national data collection systems, ranging from the absence of any registry in some countries to detailed cycle-by-cycle registries with identifiable patient data in others. While the majority of countries showed an interest in the EuMAR project and a general willingness to participate in a European registry, several concerns were identified, notably related to financial costs, timeframe requirements, reluctance to change (e.g. adopting new ways of working), and uncertainties about data sharing based on national legislation. The implementation of the IRCC and ClinicSwitch code, while ensuring the anonymity of patients, was also noted as a point for attention, not only regarding the need for these codes to follow local regulations, but also in relation to patients’ attitudes regarding their own health data and privacy. Different social constructions have an effect on views and concerns over the collection of health data, as shown with the report of the NCAs in Austria and Malta regarding patients’ potential perceptions over their own health data being shared with an international registry. For these reasons, the patient codes to be used in EuMAR have been developed in such a way that patient anonymity is guaranteed at all times.
The percentages of MAR treatments recorded in national registries and the difficulties in collecting MAR data at national level, as reported by different countries, are also worth of consideration. Collecting accurate and complete data on deliveries was identified by many registry managers as a difficult task. In most cases, pregnancy outcome data are collected on a patient-to-patient basis by health professionals at the MAR centre who call or email patients after the expected time of delivery. As, for the most part, the birth will take place in a different location to where the MAR treatment happened, interviewees expressed the benefits that linking MAR registries to national birth registries would bring. This strategy would automate this process and improve the verification of data appropriateness and completeness, on the way to interoperability. While it will not be possible to build such connections during the initial EuMAR project period, the project will explore whether there is a possibility for such an option in the future.
Associating the registration of cycles to reimbursement policies seems to be a successful solution for comprehensive data submission in some countries, such as Sweden and Belgium. Some countries reported difficulties in ensuring that the data received from centres is adequate and complete, and mentioned the need for incentives and sanctions related to data reporting, as well as appropriate methods to facilitate data submission with minimal burden on centres.
The results of the survey and interviews highlighted some of the key contributions of the EuMAR project. Firstly, there were several countries without an existing cycle-by-cycle registry who indicated not having the possibility to build such a registry at national level in the near future. For these countries, the EuMAR project could represent an opportunity to improve the MAR data collection. Secondly, the vast heterogeneity between national data collections highlights the need for data standardization at the European level. Lastly, the EuMAR registry will collect certain data that is currently not often collected but perceived to be of added value by many of the respondents, notably cumulative and cross-border data. Several countries also indicated an interest in collecting more detailed demographic data; however, apart from age, no other demographic parameters will be collected during the EuMAR project, mainly to keep the reporting burden manageable in light of an already extensive list of parameters, and to avoid potential issues with data protection compliance.
It is important to note that the data on the concerns towards EuMAR were collected at a time when many technical aspects of the project were not yet developed. It is possible that the participants’ perceptions on whether it would be possible and of interest to them to contribute to the EuMAR registry will be different when they are fully informed about the technical details of the registry. The timeframe might have also affected stakeholders’ understanding of the EuMAR registry in relation to the EU SoHO platform. While both of these registries will have a different and necessary function, the initiatives were presented at a similar time and both at an early stage of development, which might have inflated perceptions of duplication of efforts and adaptations needed in the reporting for NCAs and MAR centres.
Regarding the data on national MAR registries, a certain degree of subjectivity in the survey responses and information provided during interviews cannot be ruled out, but the overall validity of the data gathered is expected to be very high, since it was collected directly from the persons responsible for the national data collection. In addition, any inconsistencies and unclear answers (e.g. arising from different interpretations of what constitutes a national registry) were clarified in the follow-up calls. Furthermore, validity was ensured by sending final summaries of the information on each country to the participants for review and approval.
The findings presented in this article will feed into the development of the IT solution for the EuMAR registry, as well as the design of the pilot study. While the survey and interviews were conducted with the aim of informing the EuMAR project, the detailed overview of the MAR data collection landscape in the EU can also be of value to inform the scientific community, policymakers and the general public.
Conclusion
The survey and interviews from the MAR authorities in 26 of the 27 Member States provided important insights of the current MAR data collection processes in the EU. It showed heterogeneity, but also the desire to engage and potentially collaborate in a pan-European data collection project that is well managed and collects fully anonymized data. EuMAR is the foundation for the future of EU data collection on MAR treatments following the development of the new European SoHO Regulation and the forthcoming EHDS, which are expected to have an impact on the way MAR data are collected in the EU. The findings presented in this report are currently used for the development of the EuMAR registry and lay the groundwork for its future implementation in different countries. Overall, there has been a positive stakeholder response, which is a promising finding for the next steps of the project and the success of the EuMAR initiative.
Supplementary Material
Acknowledgements
We would like to thank former ESHRE staff members Susanne Hultsch and Kristina Vesela for their contributions to the survey preparation.
Contributor Information
Elena Achótegui Sebastián, ESHRE, Central Office, Strombeek-Bever, Belgium.
Carlos Calhaz-Jorge, Faculty of Medicine, University of Lisbon, Lisbon, Portugal.
Christian De Geyter, Reproductive Medicine and Gynecological Endocrinology (RME), University Hospital, University of Basel, Basel, Switzerland.
Thomas Ebner, Department of Gynecology, Obstetrics and Gynecological Endocrinology, Kepler Universitätsklinikum, Linz, Austria.
Carlos E Plancha, Institute of Histology and Developmental Biology, Faculty of Medicine, University of Lisbon, Lisbon, Portugal.
Veerle Goossens, ESHRE, Central Office, Strombeek-Bever, Belgium.
Anja Pinborg, Fertility Clinic, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
Nikolaos P Polyzos, Dexeus Fertility, Department of Obstetrics, Gynaecology and Reproductive Medicine, Dexeus University Hospital, Barcelona, Spain; Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium.
Laura Rossignoli, ESHRE, Central Office, Strombeek-Bever, Belgium.
Ioana Adina Rugescu, National Transplant Agency Romania, Bucharest, Romania.
Jesper Smeenk, Obstetrics & Gynaecology Department, Elisabeth Twee Steden Hospital, Tilburg, The Netherlands.
Thomas Strowitzki, Department of Gynaecological Endocrinology and Fertility Disorders, Heidelberg University Women’s Hospital, Heidelberg, Germany.
Johanna Tassot, ESHRE, Central Office, Strombeek-Bever, Belgium.
Edgar V Mocanu, Department of Reproductive Medicine, Rotunda Hospital and Royal College of Surgeons in Ireland, Dublin, Ireland.
Nathalie Vermeulen, ESHRE, Central Office, Strombeek-Bever, Belgium.
Christine Wyns, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Brussels, Belgium.
M Cristina Magli, Reproductive Medicine Unit, SISMER, Bologna, Italy.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Authors’ roles
C.M., E.A.S., and J.T prepared the first draft of the article. All authors participated in revising and correcting the text. All authors approved the final article. All authors contributed to the development of the survey.
Funding
The EuMAR project was co-funded by ESHRE and the European Commission (101079865—EuMAR–EU4H-2021-PJ2).
Conflict of interest
A.P. reports grants from Gedeon Richter, Ferring Pharmaceuticals and Merck A/S; consulting fees from IBSA, Ferring, Gedeon Richter, Cryos and Merck A/S; speakers fees from Gedeon Richter, Ferring Pharmaceuticals, Merck A/S and Organon; and travel fees from Gedeon Richter. T.S. reports travel fees from ECDC. The other authors disclosed no conflicts of interest.
References
- Beatty PC, Willis GB.. Research synthesis: the practice of cognitive interviewing. Public Opinion Quart 2007;71:287–311. [Google Scholar]
- Calhaz-Jorge C, De Geyter CH, Kupka MS, Wyns C, Mocanu E, Motrenko T, Scaravelli G, Smeenk J, Vidakovic S, Goossens V.. Survey on ART and IUI: legislation, regulation, funding and registries in European countries: the European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod Open 2020;2020:hoz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geyter C, Calhaz-Jorge C, Goossens V, Magli CM, Smeenk J, Vesela K, Vermeulen N, Wyns C.. EuMAR: a roadmap towards a prospective, cycle-by-cycle registry of medically assisted reproduction in Europe. Hum Reprod Open 2023;2023:hoad011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geyter C, Wyns C, Mocanu E, de Mouzon J, Calhaz-Jorge C.. Data collection systems in ART must follow the pace of change in clinical practice. Hum Reprod 2016;31:2160–2163. [DOI] [PubMed] [Google Scholar]
- De Neubourg D, Bogaerts K, Blockeel C, Coetsier T, Delvigne A, Devreker F, Dubois M, Gillain N, Gordts S, Wyns C.. How do cumulative live birth rates and cumulative multiple live birth rates over complete courses of assisted reproductive technology treatment per woman compare among registries? Hum Reprod 2016;31:93–99. [DOI] [PubMed] [Google Scholar]
- Directive—2004/23/EC—EU Tissue Directive on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells—EUR-Lex, 2004. https://eur-lex.europa.eu/eli/dir/2004/23/oj (1 March 2024, date last accessed).
- McLernon DJ, Maheshwari A, Lee AJ, Bhattacharya S.. Cumulative live birth rates after one or more complete cycles of IVF: A population-based study of linked cycle data from 178,898 women. Hum Reprod 2016;31:572–581. [DOI] [PubMed] [Google Scholar]
- Smeenk J, Wyns C, De Geyter C, Kupka M, Bergh C, Cuevas Saiz I, De Neubourg D, Rezabek K, Tandler-Schneider A, Rugescu I. et al. ; European IVF Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2019: results generated from European registries by ESHRE. Hum Reprod 2023;38:2321–2338.37847771 [Google Scholar]
- European Parliament, Texts Adopted—European Health Data Space—Wednesday, 24 April 2024. 2024a. https://www.europarl.europa.eu/doceo/document/TA-9-2024-0331_EN.html (21 May 2024, date last accessed).
- European Parliament, Texts Adopted—Standards of Quality and Safety for Substances of Human Origin Intended for Human Application—Wednesday, 24 April 2024b. 2024b. Retrieved 21 May 2024. https://www.europarl.europa.eu/doceo/document/TA-9-2024-0353_EN.html (21 May 2024, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.