Abstract
STUDY QUESTION
Is there an association between premature ovarian insufficiency (POI) and severe autoimmune diseases before and after POI diagnosis?
SUMMARY ANSWER
Women with POI had at least one hospital-treated autoimmune disorder preceding POI diagnosis 2.6 times more often compared with matched female controls, and a 2- to 3-fold risk for these diseases for several years after POI diagnosis.
WHAT IS KNOWN ALREADY
It has been suggested that autoimmunity is an important factor in the pathogenesis of POI. Estimations of the prevalence of POI cases with autoimmune origin have ranged from 4% to 50%.
STUDY DESIGN, SIZE, DURATION
This population-based registry study included 3972 women diagnosed with spontaneous POI between 1988 and 2017 and 15 708 female population controls and used both case–control and cohort analysis. Autoimmune disease diagnoses were evaluated from childhood until the end of the year 2017.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Women with POI were identified from the reimbursement registry of the Finnish Social Insurance Institution by their right to hormone replacement therapy (HRT). Four female population controls matched by age and municipality of residence were searched for each POI case to form a reference cohort. Women with a history of cancer or bilateral oophorectomy were excluded. Severe autoimmune disorder diagnoses for the years 1970–2017 were identified from the Hospital Discharge Registry. Odds ratios (ORs) with 95% confidence intervals (CI) were calculated using binary logistic regression for cases of having any, or one or more, specific autoimmune diseases preceding the index date (the date when reimbursement for HRT was granted for the POI) among women with POI as compared to controls. Standardized incidence ratios (SIR) with 95% CIs for getting diagnosed with an autoimmune disease after the index date in 3-year follow-up periods among women with POI (who did not have these diseases prior to the index date) were also calculated. The expected numbers of autoimmune disease cases were based on the incidence of first-onset severe autoimmune disease among the controls.
MAIN RESULTS AND THE ROLE OF CHANCE
The prevalence of having at least one severe autoimmune disease in women with POI was 5.6% (n = 233), with an OR of 2.6 (95% CI 2.2, 3.1) when compared to population controls. Women with POI had an increased prevalence of several specific autoimmune diseases prior to the index date compared to controls: polyglandular autoimmune diseases (OR 25.8, 95% CI 9.0, 74.1), Addison’s disease (OR 22.9, 95% CI 7.9, 66.1), vasculitis (OR 10.2, 95% 4.3, 24.5), systemic lupus erythematosus (OR 6.3 95% CI 4.2, 20.3), rheumatoid arthritis (OR 2.3, 95% CI 1.7, 3.2), sarcoidosis (OR 2.3, 95% CI 1.2, 4.5), inflammatory bowel diseases (OR 2.2, 95% CI 1.5, 3.3), and hyperthyroidism (OR 1.9, 95% CI 1.2, 3.1); whereas the prevalence of diabetes type 1 and ankylosing spondylitis did not differ between the women with POI and the reference cohort. The SIRs for being diagnosed for the first time with a severe autoimmune disease after POI diagnosis was 2.8 (95% CI 2.3, 3.4), during the first three years after POI diagnosis, decreasing gradually to 1.3 (1.1, 1.6) after 12 years.
LIMITATIONS, REASONS FOR CAUTION
This study only included autoimmune disorders diagnosed in specialized health care; hence, the overall prevalence of autoimmune disorders in women with POI may be higher.
WIDER IMPLICATIONS OF THE FINDINGS
Severe autoimmune diseases have a strong association with POI, suggesting that immunological mechanisms play a pivotal role in POI. Future studies should focus on specific autoimmune mechanisms behind POI, from both preventive and curative perspectives.
STUDY FUNDING/COMPETING INTEREST(S)
This work was financially supported by Oulu University Hospital. S.M.S. received grants from the Finnish Menopause Society, the Finnish Medical Foundation, and the Juho Vainio Foundation. H.S. received grants from the Finnish Menopause Society, the Oulu Medical Research Foundation, the Finnish Research Foundation of Gynecology and Obstetrics, UniOGS graduate school, The Finnish Medical Society Duodecim, Orion Research Foundation, and the University of Oulu Scholarship Fund. M.-M.O. received a grant from the Sakari Alhopuro Foundation and the Finnish Diabetes Research Foundation. None of the funders had any involvement in the study design or its execution or reporting. The authors do not have any competing interests to report.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: premature ovarian insufficiency, autoimmune disease, menopause, etiology, comorbidity
Introduction
Spontaneous premature ovarian insufficiency (POI), defined as cessation of ovarian function before the age of 40 years, affects 1% of women and has various negative health- and quality-of-life-related effects (Sullivan et al., 2016). In addition to chromosomal disorders (Silvén et al., 2023), previous cancers and bilateral oophorectomies, autoimmune mechanisms are a well-known etiologic factor of POI, and it has been estimated that 4–55% of POI cases are of autoimmune origin (Szeliga et al., 2021; Chen et al., 2022). Autoimmune diseases are characterized by autoreactive T-cells and organ-specific or non-organ-specific autoantibodies (Silva et al., 2014). Ovarian autoimmune processes leading to POI can manifest in isolation. However, they are often associated with other autoimmune conditions, of which thyroid and adrenal autoimmune disorders are among the most common (Szeliga et al., 2021). Women with POI have an increased prevalence of autoimmune antibodies, and thyroid antibody positivity associates with an increased risk of developing POI (Hsieh and Ho, 2021; Beitl et al., 2024).
Various autoimmune conditions have been associated with lower ovarian reserve, as determined by anti-Müllerian hormone (AMH) levels. The association of low AMH levels in reproductive-aged women has been reported at least in cases of thyroid autoimmunity, diabetes mellitus type 1 (DMI), rheumatoid arthritis, systemic lupus erythematosus, and ankylosing spondylitis (Luo et al., 2020; Yalçın Bahat et al., 2021; Hasegawa et al., 2022; Yang et al., 2022; Zhang et al., 2022). One abstract reported that women with certain autoimmune disorders are at a 2- to 5-fold risk of POI (McLaren et al., 2011).
Several previous studies have estimated the prevalence of autoimmune disorders in study populations from dozens to hundreds of POI patients (Kim et al., 1997; Bachelot et al., 2009; Bakalov et al., 2012; Ayesha et al., 2016; Szlendak-Sauer et al., 2016; Jiao et al., 2017; Grossmann et al., 2020). Many severe autoimmune disorders, however, are very rare, with a population prevalence of less than 1/1000 (Bakalov et al., 2012). Some autoimmune disorders have a typical onset at a young age, while others may manifest at middle age or later (Cooper and Stroehla, 2003). Hence, studies with large study populations and long follow-up periods are needed to investigate the association between severe autoimmune diseases and POI.
We hypothesized that women diagnosed with POI have an increased prevalence of severe autoimmune diseases (diseases that are diagnosed and treated in specialized health care) prior to POI diagnosis and that the incidence of these diseases remains elevated after POI diagnosis as compared to the general female population. As both severe autoimmune diseases and POI of autoimmune origin reflect general susceptibility to autoimmune conditions, women who get diagnosed with POI during their lifetime are likely to be prone to developing autoimmune diseases both before and after being diagnosed with POI. We aimed to investigate this hypothesis using nationwide registry data to retrieve population-based information on these conditions in women with POI.
Materials and methods
In Finland, comprehensive health data are stored in several registries using personal identity codes (PICs) given to all citizens and immigrants living permanently in Finland. The uniqueness of each PIC makes it possible to combine data from different registries. From the medicine reimbursement registry maintained by the Social Insurance Institution of Finland (SII), we identified women who had been granted the right to full reimbursement for hormone replacement therapy (HRT) because of POI diagnosis under the age of 40 years, between the years 1988 and 2017 (cases). In Finland, the diagnosis of POI is based on the criteria defined by the European Society for Human Reproduction and Embryology (ESHRE) guidelines for POI (Webber et al., 2016). The criteria to receive the right of reimbursement has changed slightly over the years but has always aligned with ESHRE guidelines. All women fulfilling the criteria are entitled to full reimbursement for HRT until the average age of natural menopause (50 years). When a woman is diagnosed with POI, the treating physician writes a certificate describing in detail how the diagnostic criteria are fulfilled, regardless of whether the patient starts HRT at the time of diagnosis. The certificate is then sent to SII, where another medical specialist verifies that the criteria for POI are fulfilled, after which the right for reimbursement is granted.
For each case with POI, the Digital and Population Data Services Agency (DVV) randomly selected four female population controls from the Finnish Population Information System, matched by month and year of birth and municipality of residence constituting a reference cohort. The same control women constituted the reference cohort for the cohort analysis on risk of autoimmune diseases after POI diagnosis. The controls also had to be alive and living in Finland on the date that HRT reimbursement was granted for the respective POI case (the index date).
The diagnoses of severe autoimmune diseases (diseases that are diagnosed and treated in specialized health care) were collected from the Hospital Discharge Register (HDR), where the diagnoses made among specialized health care are stored with International Classification of Diseases (ICD) codes. HDR data were available from 1970 to 2017. We identified ICD-8 (1970–1986), ICD-9 (1987–1995), and ICD-10 (1996–2017) codes as these coding systems have been used during the lifetimes of the women in our study population. The codes we used in this study are listed in Supplementary Table S1. Autoimmune diseases that are mainly diagnosed and treated in primary health care, such as hypothyroidism and celiac disease, were excluded from this study. As many autoimmune disorders are rare, we combined similar diseases into larger entities for the analyses.
As the aim was to investigate the association between spontaneous POI and autoimmune disorders, women with bilateral oophorectomy (n = 748) or a history of cancer before the index date (n = 1195) were identified by the HDR and the Finnish Cancer Registry and excluded from the analyses. We also excluded individuals who had undergone gender reassignment (n = 235).
Statistical methods
A binary regression model was used to calculate odds ratios (ORs) with 95% confidence intervals (CI) for women with POI, (i) with at least one severe autoimmune disease and (ii) with a specific autoimmune disease prior to the index date, compared to reference cohort.
The standardized incidence ratios (SIR) of being diagnosed with a severe autoimmune disease after the index date with 95% CIs were calculated for women with POI compared to reference cohort, and stratified into 3-year follow-up periods starting from the date of POI diagnosis. Women who had a severe autoimmune disease before the POI diagnosis were excluded. For calculating the SIRs, the end of the follow-up was the first date of a severe autoimmune diagnosis, the end of follow-up (31 December 2017), the date of migration, or the date of death, whichever came first. The SIR was defined as the ratio of the observed number of autoimmune diseases among women with POI to the expected number of autoimmune diseases calculated by multiplying the number of person years of the women with POI with the incidence of autoimmune cases in the reference cohort, stratified by calendar year and by time since the index date.
Study approvals and data processing
This study was approved by the Finnish Institute for Health and Welfare (THL/1973/5.05.00/2019), the Social Insurance Institution (135/522/2018), and the Digital and Population Data Services Agency (VRK 4304-2019-2). Approval from the ethics committee was not required due to the study’s registry-based nature and no registered person was contacted. Data processing was done using RStudio (Rstudio Inc.) and SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA). The statistical analyses were performed with IBM SPSS Statistics 28.0 (IBM Corp., Armonk, NY, USA). Anonymization was done before the analyses, and the researchers could not access identifiable personal data. The data were analyzed and reported following the STROBE statement.
Results
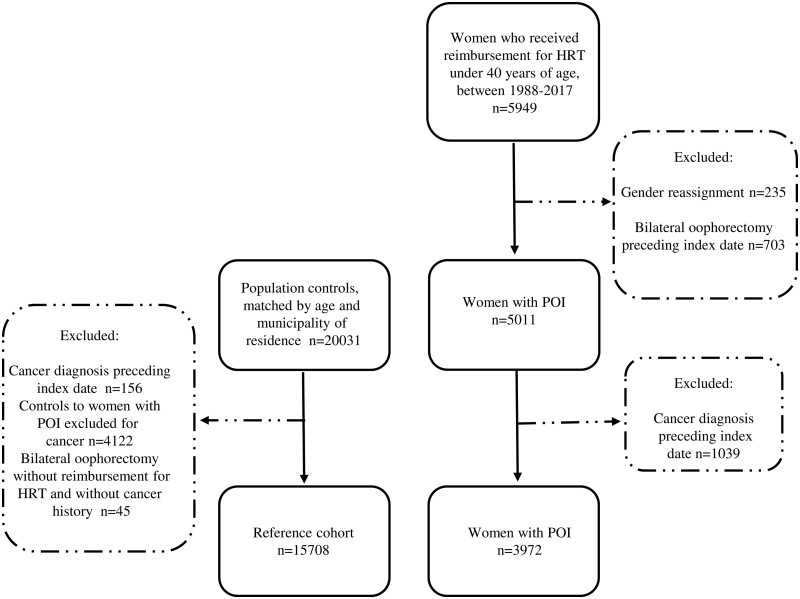
The flow chart of the study population, of which the ORs of having an autoimmune disease prior to the index date were calculated, is shown in Fig. 1. The final study population included 3972 women with POI and 15 708 female controls without POI (reference cohort). The median age at POI diagnosis in the whole study population was 35.9 years (IQR 7.9 years).
Figure 1.
Flow chart of the study population. POI, premature ovarian insufficiency; HRT, hormone replacement therapy; index date, the date when replacement for HRT was granted for the respective POI case.
Prevalence of autoimmune disorders
Among women with POI, 5.6% (n = 223) were diagnosed with at least one autoimmune disorder prior to the index date and 12.7% (n = 503) were diagnosed with at least one autoimmune disorder after the index date, by the end of the follow-up period. The numbers of severe autoimmune diseases among women with POI and in the reference cohort prior to the index date and during the whole study period are shown in Table 1. The prevalences of severe autoimmune disease diagnoses among women with POI and in the reference cohort preceding the index date and during the whole study period are presented in Table 2.
Table 1.
Total number of severe autoimmune diseases in women with premature ovarian insufficiency (POI) (n = 3972) and in the reference cohort (N = 15 708), prior to the index datea and during the whole study period.b
| Prior the index datea |
During the whole study periodb |
|||
|---|---|---|---|---|
| Number of autoimmune diseases | Women with POI n (%) | Reference cohort n (%) | Women with POI n (%) | Reference cohort n (%) |
| 0 | 3749 (94.4) | 15 352 (97.7) | 3469 (87.3) | 14 624 (93.1) |
| 1 | 194 (4.9) | 338 (2.2) | 409 (10.3) | 999 (6.4) |
| 2 | 27 (0.7) | 17 (0.1) | 77 (1.9) | 77 (0.5) |
| ≥3 | <5 (<0.1) | <5 (<0.03) | 17 (0.4) | 8 (0.1) |
The date when reimbursement for hormone replacement therapy (HRT) was granted for the case with POI.
By the end of the year 2017, the date of migration, or the date of death.
Table 2.
Prevalence of autoimmune diseases in women with premature ovarian insufficiency (POI) (n = 3972) and reference cohort (n = 15 708), prior to and after POI diagnosis.a
| Autoimmune disease | Prior to POI diagnosis of the index case |
After the POI diagnosisb |
||
|---|---|---|---|---|
| POI n (%) | Reference cohort n (%) | POI n (%) | Reference cohort n (%) | |
| Endocrine diseases | ||||
| Hyperthyroidism | 26 (0.7) | 54 (0.3) | 58 (1.5) | 199 (1.3) |
| Polyglandular autoimmune disorders (APECED, Schmidt syndrome, unspecified) | 26 (0.7) | <5 (<0.03) | 12(0.3) | <5 (<0.03) |
| Diabetes type I | 21 (0.5) | 73 (0.5) | 54 (1.4) | 83 (0.5) |
| Addison’s disease | 23 (0.6) | <5 (<0.03) | 21 (0.5) | 8 (0.05) |
| Inflammatory diseases | ||||
| Rheumatoid arthritis | 56 (1.4) | 96 (0.6) | 82 (2.1) | 207 (1.3) |
| Ankylosing spondylitis | 9 (0.2) | 33 (0.2) | 22 (0.6) | 45 (0.3) |
| Inflammatory bowel diseases (Crohn’s disease, colitis ulcerosa) | 41 (1.0) | 70 (0.5) | 61 (1.5) | 159 (1.0) |
| Sarcoidosis | 14 (0.4) | 24 (0.2) | 25 (0.6) | 62 (0.4) |
| Vasculitis | 18 (0.5) | 7 (0.04) | 19 (0.5) | 16 (0.01) |
| Systemic lupus erythematosus | 21 (0.5) | 12 (0.1) | 10 (0.3) | 21 (0.1) |
Prior to or after the index date, i.e. the date when reimbursement for hormone replacement therapy (HRT) was granted for the woman with POI.
Until the end of the year 2017, the date of migration, or the date of death.
The odds of women with POI being diagnosed with a severe autoimmune disease prior to the index date
The OR for having at least one severe autoimmune disorder prior to the index date was 2.6 (95% CI 2.2, 3.1) in women with POI compared to controls. At the time of the index date, the prevalence of each severe autoimmune diagnosis that was included in the study, except ankylosing spondylitis and DMI, was increased among women who were diagnosed with POI (Table 3). Moreover, the prevalence of a polyglandular autoimmune disorder and Addison’s disease preceding the index date were remarkably high in women who were diagnosed with POI compared to reference cohort (OR 25.8, 95% 9.0, 74.1 and OR 22.9, 95% CI 7.9, 66.1, respectively).
Table 3.
Odds ratios (ORs) with 95% CIs for women with premature ovarian insufficiency (POI, n = 3972) having an autoimmune disease prior to POI diagnosis,a as compared to reference cohort (n = 15 708).
| Autoimmune diseases | OR | 95% CI |
|---|---|---|
| Endocrine diseases | ||
| Hyperthyroidism | 1.9 | 1.2, 3.1 |
| Polyglandular autoimmune diseases (APECED, Schmidt syndrome, unspecified) | 25.8 | 9.0, 74.1 |
| Diabetes type I | 1.1 | 0.7, 1.9 |
| Addison’s disease | 22.9 | 7.9, 66.1 |
| Inflammatory diseases | ||
| Rheumatoid arthritis | 2.3 | 1.7, 3.2 |
| Ankylosing spondylitis | 1.1 | 0.5, 2.3 |
| Inflammatory bowel diseases (Crohn’s disease and colitis ulcerosa) | 2.2 | 1.5, 3.3 |
| Sarcoidosis | 2.3 | 1.2, 4.5 |
| Vasculitis | 10.2 | 4.3, 24.5 |
| Systemic lupus erythematosus | 6.3 | 4.2, 20.3 |
Prior to the index date, i.e. the date when reimbursement for hormone replacement therapy (HRT) was granted for the case with POI.
SIRs after being diagnosed with POI
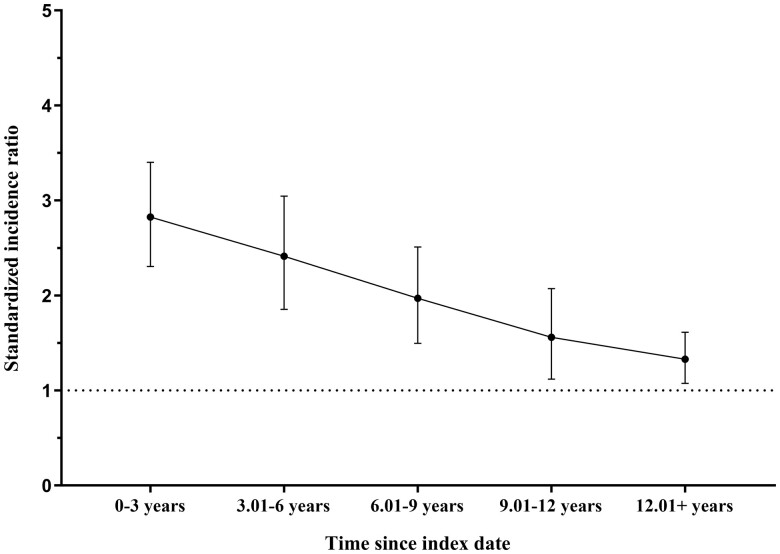
The SIRs in 3-year follow-up periods for severe autoimmune disease diagnosis for women with POI who had none of these diseases preceding the index date are shown in Fig. 2. The SIR decreased from 2.8 (95% CI 2.3, 3.4) during the first three years after POI diagnosis to 1.3 (1.1, 1.6) in the follow-up of ≥12 years after the POI diagnosis.
Figure 2.
Standardized incidence ratios, with 95% confidence interval (CI) bars, for being diagnosed with autoimmune disease after the index date among POI women, compared to the cohort of the control women, by 3-year follow-up periods since the index date. Women with autoimmune diseases prior to the index date were excluded.
Discussion
The results of this large nationwide registry-based study suggest that there is a strong association between POI and severe autoimmune diseases, as the prevalence of severe autoimmune diseases preceding POI diagnosis was over 2-fold greater in women with POI compared to the reference cohort, while the incidence of severe autoimmune diseases after POI diagnosis was 2- to 3-fold greater for several years. The results strengthen the hypothesis that autoimmune processes play a pivotal role in the pathogenesis of POI. As both severe autoimmune diseases and POI of autoimmune origin reflect general susceptibility, it is reasonable to expect that severe autoimmune diseases may manifest either before or after POI. The prevalence of autoimmune diseases increased during the study period, not only among the women with POI but also among their matched population controls, which is in line with reports of the increasing prevalence of autoimmune diseases worldwide (Miller, 2023). There is also evidence that cyclic ovarian production of female sex steroids has a pivotal role in maintaining normal immune system function, and hence it has been suggested that early cessation of ovarian function may also expose women to some autoimmune diseases such as rheumatoid arthritis (Bove, 2013).
For decades, it has been suggested that autoimmune mechanisms play an important role in the pathogenesis of POI (Irvine et al., 1968). However, the estimated proportion of autoimmune POI cases has been highly variable, with a wide range of 4–50% (Szeliga et al., 2021; Chen et al., 2022). Jiao et al. (2017) reported that 6.0% of 632 POI patients with secondary amenorrhea, with a mean age of 29.3 years, had been diagnosed with at least one autoimmune disease (Jiao et al., 2017). In the study population of Bachelot et al. (2009), with 357 women with POI with a mean age of 26.5 years, 10% were diagnosed with an autoimmune disorder. Also, a higher prevalence has been reported, as Szlendak-Sauer et al. (2016) reported that 34% of 98 women with POI, aged 18–39 years, fulfilled the criteria of autoimmune polyglandular syndrome, namely autoimmune thyroid disorder and some other non-ovarian autoimmune disorder (Szlendak-Sauer et al., 2016). The aforementioned studies reported the prevalence of autoimmune disorders among women with POI, and the study by Szlendak-Sauer et al. (2016) also reported the prevalence of autoimmune polyglandular syndrome among 75 healthy, non-matched controls, but to the best of our knowledge there are no previous studies that had compared the odds for autoimmune diseases among women with POI to matched female population controls; thus, our study significantly adds to the existing literature. A study by Sardu et al. (2012) reported that 9.6% of the 14 167 women aged 15–89 years in their population-based sample were diagnosed with at least one autoimmune disorder (Sardu et al., 2012). This prevalence is higher than in our control female population, as this study also included less severe disorders, such as autoimmune thyroiditis and celiac disease.
To the best of our knowledge, no previous studies have investigated the incidence of autoimmune diseases after being diagnosed with POI. We suggest that the high incidence of first-onset severe autoimmune diseases during the first years after being diagnosed with POI, as shown in this study, further confirms that the activation of autoimmune mechanisms at the time POI develops among many patients. However, the tendency to develop autoimmune diseases among POI patients seems to be long-term, considering the high prevalence of severe autoimmune diseases preceding POI and the increased incidence even more than a decade after being diagnosed with POI.
Compared to earlier studies investigating the prevalence of autoimmune disorders among women with POI, the most notable strength of this study is the high number of women with POI in the study population. Because of this high number, we were able to investigate the prevalence and incidence of rare autoimmune diseases among women with POI. Many previous studies are based on study populations of a few dozen to a few hundred POI patients. Moreover, the study period of this study was as long as 30 years, while previous studies have been mainly cross-sectional and investigated the prevalence of autoimmune disorders close to POI diagnosis. To the best of our knowledge, no previous studies have investigated the incidence of autoimmune diseases after POI diagnosis or compared the incidence of autoimmune diseases between women with POI and female population controls. Hence, our study makes a novel contribution to the field of etiology and comorbidity of POI.
Finnish registry data, on which the diagnoses of POI and autoimmune disorders were based, have been described as highly reliable in evaluations of the quality of the data (Heino et al., 2018; Helle et al., 2022). The POI diagnoses in our study were based on a full reimbursement right for HRT, and the process to apply for this reimbursement included independent evaluations from two physicians. We believe that this two-step process identifies POI patients more reliably than searching with the diagnostic codes for POI from the HDR. Only spontaneous POI cases were included, as we could reliably exclude the POI cases caused by bilateral oophorectomy, as well as the cases of receiving a cancer diagnosis preceding POI diagnosis. In addition, in Finland, the autoimmune disorders included in this study are diagnosed and treated among specialized health care, except on rare occasions. Hence, their diagnoses are systematically recorded in the mandatory HDR.
The study also has some limitations. Some disorders of autoimmune origin, such as celiac disease and hypothyroidism, can be diagnosed and treated solely in primary health care. This is why these diagnoses are under-reported in the HDR. Hence, this study focused only on more severe autoimmune diseases routinely treated in specialized health care. As we aimed to investigate the risk of severe autoimmune diseases among women with spontaneous POI, we excluded the women with bilateral oophorectomy. It is known that also ovary-sparing hysterectomy at an early age associates with impaired ovarian reserve (Singha et al., 2016) and may expose to earlier menopause, which was not taken into account in this study. It is likely that POI is underdiagnosed among hysterectomized women. However, we believe that hysterectomized, undiagnosed POI cases are a small minority and excluding them would not make a difference to the results. Furthermore, due to registry-based study setting, we had no access to some confounders that are associated with the risk for autoimmune disease and POI, like body mass index and smoking (Harpsøe et al., 2014; Khan and Wang, 2019; Shelling and Ahmed Nasef, 2023). It was either not possible to interview or examine all participants to screen them for symptoms and signs of autoimmune diseases. However, the severe autoimmune diseases included in this study manifest with significant symptoms, and hence we presume that undiagnosed cases are rare.
Our study provides novel information at the population-based level about the association of severe autoimmune disorders and POI. Women diagnosed with POI have a more than 2-fold prevalence of severe autoimmune diseases, and an increased incidence of these diseases for even more than a decade after being diagnosed with POI, compared with female population controls. The study results strengthen the hypothesis that autoimmune mechanisms play an important role in the pathogenesis of POI. Future studies should focus on the immunological mechanism of POI from preventative and curative perspectives.
Supplementary Material
Contributor Information
S M Savukoski, Department of Obstetrics and Gynecology, Oulu University Hospital, Wellbeing Services County of North Ostrobothnia, Pohde, Oulu, Finland; Research Unit of Clinical Medicine, University of Oulu, Oulu, Finland; Medical Research Center, University of Oulu and Oulu University Hospital, Wellbeing Services County of North Ostrobothnia, Oulu, Finland.
H Silvén, Department of Obstetrics and Gynecology, Oulu University Hospital, Wellbeing Services County of North Ostrobothnia, Pohde, Oulu, Finland; Research Unit of Clinical Medicine, University of Oulu, Oulu, Finland; Medical Research Center, University of Oulu and Oulu University Hospital, Wellbeing Services County of North Ostrobothnia, Oulu, Finland.
P Pesonen, Infrastructure for Population Studies, Faculty of Medicine, University of Oulu, Oulu, Finland.
E Pukkala, Health Sciences Unit, Faculty of Social Sciences, Tampere University, Tampere, Finland; Finnish Cancer Registry, Institute for Statistical and Epidemiological Cancer Research, Helsinki, Finland.
M Gissler, Information Department, THL Finnish Institute for Health and Welfare, Helsinki, Finland; Academic Primary Health Care Centre, Region Stockholm, Stockholm, Sweden; Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden.
E Suvanto, Department of Obstetrics and Gynecology, Oulu University Hospital, Wellbeing Services County of North Ostrobothnia, Pohde, Oulu, Finland; Research Unit of Clinical Medicine, University of Oulu, Oulu, Finland; Medical Research Center, University of Oulu and Oulu University Hospital, Wellbeing Services County of North Ostrobothnia, Oulu, Finland.
M -M Ollila, Department of Obstetrics and Gynecology, Oulu University Hospital, Wellbeing Services County of North Ostrobothnia, Pohde, Oulu, Finland; Research Unit of Clinical Medicine, University of Oulu, Oulu, Finland; Medical Research Center, University of Oulu and Oulu University Hospital, Wellbeing Services County of North Ostrobothnia, Oulu, Finland; Department of Internal Medicine, Oulu University Hospital, Wellbeing Services County of North Ostrobothnia, Pohde, Oulu, Finland.
M Niinimäki, Department of Obstetrics and Gynecology, Oulu University Hospital, Wellbeing Services County of North Ostrobothnia, Pohde, Oulu, Finland; Research Unit of Clinical Medicine, University of Oulu, Oulu, Finland; Medical Research Center, University of Oulu and Oulu University Hospital, Wellbeing Services County of North Ostrobothnia, Oulu, Finland.
Data availability
The data underlying this article were provided by Findata under license. Data will be shared on request to Findata.
Authors’ roles
All authors participated in planning the study design. S.M.S. and P.P. performed the data analyses, while the drafting of the article was accomplished by S.M.S., H.S., M.-M.O., and M.N. All authors were involved in critical discussion and comments on the manuscript and accepted the final version of this paper.
Funding
Oulu University Hospital; Finnish Menopause Society (S.M.S.); Finnish Medical Foundation (S.M.S.); Juho Vainio Foundation (S.M.S.); Finnish Menopause Society (H.S.); Oulu Medical Research Foundation (H.S.); Finnish Research Foundation of Gynecology and Obstetrics, UniOGS Graduate School (H.S.); Finnish Medical Society Duodecim (H.S.), Orion Research Foundation (H.S.); University of Oulu Scholarship Fund (H.S.); Sakari Alhopuro Foundation (M.-M.O.); Finnish Diabetes Research Foundation (M.-M.O.).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ayesha, Jha V, Goswami D.. Premature ovarian failure: an association with autoimmune diseases. J Clin Diagn Res 2016;10:QC10–QC12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelot A, Rouxel A, Massin N, Dulon J, Courtillot C, Matuchansky C, Badachi Y, Fortin A, Paniel B, Lecuru F, et al. ; POF-GIS Study Group. Phenotyping and genetic studies of 357 consecutive patients presenting with premature ovarian failure. Eur J Endocrinol 2009;161:179–187. [DOI] [PubMed] [Google Scholar]
- Bakalov VK, Gutin L, Cheng CM, Zhou J, Sheth P, Shah K, Arepalli S, Vanderhoof V, Nelson LM, Bondy CA.. Autoimmune disorders in women with turner syndrome and women with karyotypically normal primary ovarian insufficiency. J Autoimmun 2012;38:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitl K, Ott J, Rosta K, Holzer I, Foessleitner P, Steininger J, Panay N.. Premature ovarian insufficiency and autoimmune profiles: a prospective case-control study. Climacteric 2024;27:187–192. [DOI] [PubMed] [Google Scholar]
- Bove R. Autoimmune diseases and reproductive aging. Clin Immunol 2013;149:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wu S, Wang M, Zhang H, Cui M.. A review of autoimmunity and immune profiles in patients with primary ovarian insufficiency. Medicine (Baltimore) 2022;101:e32500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, Stroehla BC.. The epidemiology of autoimmune diseases. Autoimmun Rev 2003;2:119–125. [DOI] [PubMed] [Google Scholar]
- Grossmann B, Saur S, Rall K, Pecher A-C, Hübner S, Henes J, Henes M.. Prevalence of autoimmune disease in women with premature ovarian failure. Eur J Contracept Reprod Health Care 2020;25:72–75. [DOI] [PubMed] [Google Scholar]
- Harpsøe MC, Basit S, Andersson M, Nielsen NM, Frisch M, Wohlfahrt J, Nohr EA, Linneberg A, Jess T.. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol 2014;43:843–855. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Kitahara Y, Osuka S, Tsukui Y, Kobayashi M, Iwase A.. Effect of hypothyroidism and thyroid autoimmunity on the ovarian reserve: a systematic review and meta-analysis. Reprod Med Biol 2022;21:e12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino A, Niinimäki M, Mentula M, Gissler M.. How reliable are health registers? Registration of induced abortions and sterilizations in Finland. Inform Health Soc Care 2018;43:310–319. [DOI] [PubMed] [Google Scholar]
- Helle N, Niinimäki M, Linnakaari R, But A, Gissler M, Heikinheimo O, Mentula M.. National register data are of value in studies on miscarriage – validation of the healthcare register data in Finland. Acta Obstet Gynecol Scand 2022;101:1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh Y-T, Ho JYP.. Thyroid autoimmunity is associated with higher risk of premature ovarian insufficiency-a nationwide Health Insurance Research Database study. Hum Reprod 2021;36:1621–1629. [DOI] [PubMed] [Google Scholar]
- Irvine WJ, Chan MM, Scarth L, Kolb FO, Hartog M, Bayliss RI, Drury MI.. Immunological aspects of premature ovarian failure associated with idiopathic Addison’s disease. Lancet 1968;2:883–887. [DOI] [PubMed] [Google Scholar]
- Jiao X, Zhang H, Ke H, Zhang J, Cheng L, Liu Y, Qin Y, Chen Z-J.. Premature ovarian insufficiency: phenotypic characterization within different etiologies. J Clin Endocrinol Metab 2017;102:2281–2290. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wang H.. Environmental exposures and autoimmune diseases: contribution of gut microbiome. Front Immunol 2019;10:3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TJ, Anasti JN, Flack MR, Kimzey LM, Defensor RA, Nelson LM.. Routine endocrine screening for patients with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol 1997;89:777–779. [DOI] [PubMed] [Google Scholar]
- Luo W, Mao P, Zhang L, Chen X, Yang Z.. Assessment of ovarian reserve by serum anti-Müllerian hormone in patients with systemic lupus erythematosus: a meta-analysis. Ann Palliat Med 2020;9:207–215. [DOI] [PubMed] [Google Scholar]
- McLaren JF, Haynes K, Barnhart KT, Sammel MD, Strom BL.. Early menopause in women with chronic inflammatory disease: a population-based cohort study. Fertil Steril 2011;96:S3–S4. [Google Scholar]
- Miller FW. The increasing prevalence of autoimmunity and autoimmune diseases: an urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr Opin Immunol 2023;80:102266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C, Cocco E, Mereu A, Massa R, Cuccu A, Marrosu MG, Contu P.. Population based study of 12 autoimmune diseases in Sardinia, Italy: prevalence and comorbidity. PLoS One 2012;7:e32487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelling AN, Ahmed Nasef N.. The role of lifestyle and dietary factors in the development of premature ovarian insufficiency. Antioxidants(Basel) 2023;8:1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CA, Yamakami LYS, Aikawa NE, Araujo DB, Carvalho JF, Bonfá E.. Autoimmune primary ovarian insufficiency. Autoimmun Rev 2014;13:427–430. [DOI] [PubMed] [Google Scholar]
- Silvén H, Savukoski SM, Pesonen P, Pukkala E, Ojaniemi M, Gissler M, Suvanto E, Niinimäki M.. Association of genetic disorders and congenital malformations with premature ovarian insufficiency: a nationwide register-based study. Hum Reprod 2023;38:1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singha A, Saha S, Bhattacharjee R, Mondal S, Choudhuri S, Biswas D, Das SK, Ghosh S, Mukhopadhyay S, Chowdhury S.. Deterioraron of ovarian function after total abdominal hysterectomy with preservaron of ovaries. Endocr Pract 2016;22:1387–1392. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Sarrel PM, Nelson LM.. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril 2016;106:1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeliga A, Calik-Ksepka A, Maciejewska-Jeske M, Grymowicz M, Smolarczyk K, Kostrzak A, Smolarczyk R, Rudnicka E, Meczekalski B.. Autoimmune diseases in patients with premature ovarian insufficiency—our current state of knowledge. Int J Mol Sci 2021;22:2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szlendak-Sauer K, Jakubik D, Kunicki M, Skórska J, Smolarczyk R.. Autoimmune polyglandular syndrome type 3 (APS-3) among patients with premature ovarian insufficiency (POI). Eur J Obstet Gynecol Reprod Biol 2016;203:61–65. [DOI] [PubMed] [Google Scholar]
- Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, Keizer-Schrama S, Hogervorst E, Janse F, et al. ; European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 2016;31:926–937. [DOI] [PubMed] [Google Scholar]
- Yalçın Bahat P, Kadiroğulları P, Topbas Selcuki NF, Yücel B, Çakmak K, Üreyen Özdemir E.. Ovarian reserve in patients with ankylosing spondylitis. Arch Gynecol Obstet 2021;303:189–193. [DOI] [PubMed] [Google Scholar]
- Yang W, Lin C, Zhang M, Lv F, Zhu X, Han X, Cai X, Ji L.. Assessment of ovarian reserve in patients with type 1 diabetes: a systematic review and meta-analysis. Endocrine 2022;77:205–212. [DOI] [PubMed] [Google Scholar]
- Zhang X-H, Zhang Y-A, Chen X, Qiao P-Y, Zhang L-Y.. Assessment of the ovarian reserve by serum anti-Müllerian hormone in rheumatoid arthritis patients: a systematic review and meta-analysis. Int Arch Allergy Immunol 2022;183:462–469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by Findata under license. Data will be shared on request to Findata.