Abstract
STUDY QUESTION
What is the impact of the EuroNet-PHL-C2 treatment for boys with classical Hodgkin lymphoma (cHL) on semen parameters?
SUMMARY ANSWER
More than half of the patients (52%, n = 16/31) had oligozoospermia or azoospermia at 2 years from cHL diagnosis; particularly boys treated for advanced-stage cHL had low sperm counts and motility.
WHAT IS KNOWN ALREADY
Chemotherapy and radiotherapy to the inguinal region or testes can impair spermatogenesis and result in reduced fertility. The EuroNet-PHL-C2 trial aims to minimize radiotherapy in standard childhood cHL treatment, by intensifying chemotherapy. The present study aims to assess the (gonadotoxic) impact of this treatment protocol on semen parameters and reproductive hormones in boys aged ≤18 years.
STUDY DESIGN, SIZE, DURATION
This international, prospective, multi-centre cohort study was an add-on study to the randomized phase-3 EuroNet-PHL-C2 trial, where the efficacy of standard cHL treatment with OEPA-COPDAC-28 (OEPA: vincristine, etoposide, prednisone, and doxorubicin; COPDAC-28: cyclophosphamide, vincristine, prednisone, and dacarbazine) was compared to intensified OEPA-DECOPDAC-21 chemotherapy (DECOPDAC-21: COPDAC with additional doxorubicin and etoposide and 25% more cyclophosphamide). Patients were recruited between January 2017 and September 2021.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Eligibility criteria included male patients, diagnosed with classical HL before or at the age of 18 years, and treated according to the EuroNet-PHL-C2 protocol in any of the 18 participating sites in the Netherlands, Germany, Belgium, Czech Republic, and Austria. Sperm parameters (sperm concentration, progressive motility, sperm volume, and calculated total motile sperm count) were assessed at diagnosis and 2 years after diagnosis in (post)pubertal boys. Laboratory measurements (serum follicle-stimulating hormone (FSH) and inhibin B) were performed in samples drawn at diagnosis, during treatment (2–3 times), and at 2 years post-diagnosis, and (age-adjusted) analyses were conducted separately for pre-pubertal and (post)pubertal boys. Outcomes were compared between the treatment levels (TL1, TL2, and TL3) and consolidation treatment schemes (COPDAC-28 and DECOPDAC-21).
MAIN RESULTS AND THE ROLE OF CHANCE
In total, 101 boys were included in the present analysis: 73 were (post)pubertal (median age 15.4 years, (IQR 14.4; 16.6), 10 TL1, 29 TL2, 34 TL3, 62% of TL2/3 patients received COPDAC-28) and 28 boys were pre-pubertal (median age 9.6 years (IQR 6.6; 11.4), 4 TL1, 7 TL2, 17 TL3, 38% of TL2/3 patients received COPDAC-28). The study included six boys who had received pelvic radiotherapy; none were irradiated in the inguinal or testicular area. At diagnosis, 48 (post)pubertal boys delivered semen for cryopreservation; 19 (40%) semen samples were oligospermic and 4 (8%) were azoospermic. Low sperm concentration (<15 mil/ml) appeared to be related to the HL disease itself, with a higher prevalence in boys who presented with B symptoms (76% vs 26%, aOR 2.3 (95% CI 1.0; 3.8), P = 0.001) compared to those without such symptoms. At 2 -years post-diagnosis, 31 boys provided semen samples for analysis, of whom 12 (39%) boys had oligozoospermia and 4 (13%) had azoospermia, while 22 boys (71%) had low total motile sperm counts (TMSC) (<20 mil). Specifically, the eight boys in the TL3 group treated with DECOPDAC-21 consolidation had low sperm counts and low progressive motility after 2 years (i.e. median sperm count 1.4 mil/ml (IQR <0.1; 5.3), n = 7 (88%), low sperm concentration, low median progressive motility 16.5% (IQR 0.0; 51.2), respectively). Age-adjusted serum FSH levels were significantly raised and inhibin B levels (and inhibin B:FSH ratios) were decreased during chemotherapy in (post)pubertal boys, with subsequent normalization in 80% (for FSH) and 60% (for inhibin B) of boys after 2 years. Only 4 out of the 14 (post)pubertal boys (29%) with low sperm concentrations after 2 years had elevated FSH (>7.6 IU/l), while 7 (50%) had low inhibin B levels (<100 ng/l). In pre-pubertal boys, reproductive hormones were low overall and remained relatively stable during chemotherapy.
LIMITATIONS, REASONS FOR CAUTION
The present analyses included sperm and laboratory measurements up to 2 years post-diagnosis. Long-term reproductive outcomes and potential recovery of spermatogenesis remain unknown, while recovery was reported up to 5- or even 10-year post-chemotherapy in previous studies.
Boys who were pre-pubertal at diagnosis were still too young and/or physically not able to deliver semen after 2 years and we could not assess a potential difference in gonadotoxicity according to pubertal state at the time of treatment. Overall, the statistical power of the analyses on sperm concentration and quality after 2 years was limited.
WIDER IMPLICATIONS OF THE FINDINGS
Results of the semen analyses conducted among the 31 boys who had provided a semen sample at 2 years post-treatment were generally poor. However, additional long-term and adequately powered data are crucial to assess the potential recovery and clinical impact on fertility. The participating boys will be invited to deliver a semen sample after 5 years. Until these data become available, benefits of intensified chemotherapy in cHL treatment to reduce radiotherapy and lower risk for development of secondary tumours should be carefully weighed against potentially increased risk of other late effects, such as diminished fertility due to the increased chemotherapy burden. Boys with newly diagnosed cHL should be encouraged to deliver sperm for cryopreservation whenever possible. However, patients and clinicians should also realize that the overall state of disease and inflammatory milieu of cHL can negatively affect sperm quality and thereby reduce chance of successful fertility preservation. Furthermore, the measurement of FSH and inhibin B appears to be of low value in predicting low sperm quality at two years from cHL treatment.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by the Dutch charity foundation KiKa (project 257) that funds research on all forms of childhood cancer. C.M.-K., D.K., W.H.W., D.H., MC, A.U., and A.B. were involved in the development of the EuroNet-PHL-C2 regimen. The other authors declare no potential conflict of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: childhood Hodgkin lymphoma, gonadotoxicity, azoospermia, follicle-stimulating hormone, fertility
Introduction
Childhood Hodgkin lymphoma (HL) is a haematological malignancy known for its incidence peak during puberty (Hjalgrim and Jarrett, 2020; Brice et al., 2021). The disease is nowadays highly curable with an overall event-free survival of ∼90% (Mauz-Körholz et al., 2022). Nevertheless, treatment for HL is associated with a risk of late effects including second malignancies and impaired fertility (Schaapveld et al., 2015; Drechsel et al., 2023). Chemotherapy and radiotherapy may damage differentiating spermatogonia and result in loss of sperm cell production in males. Recovery potential depends on the survival of the spermatogonial stem cells within the testis (Weinbauer et al., 2010; Goossens et al., 2020). Infertility becomes definite if the spermatogonial stem cells are fully depleted. Especially alkylating agents and pelvic irradiation have been associated with (definite) gonadal damage, in a dose-dependent manner (Brougham et al., 2003; Romerius et al., 2010; Meistrich, 2013; Drechsel et al., 2023). In addition, the HL disease itself may also affect fertility, as impaired sperm parameters are often observed at time of diagnosis (Drechsel et al., 2023). Although the impact of HL on spermatogenesis is presumed to be temporary, potentially related to a general inflammatory status, the exact underlying mechanisms and duration of these effects currently remain unknown.
Sperm quantity and quality are considered the best indicators of male fertility (Cooper et al., 2010). In addition, in (post)pubertal boys and adult males, measurement of follicle-stimulating hormone (FSH) and inhibin B levels may be useful indirect and interdependent markers of spermatogenesis. FSH stimulates spermatogenesis via the Sertoli cells and inhibin B indirectly plays a role in FSH secretion via feedback mechanisms (Anderson et al., 1997; Meachem et al., 2001; Santi et al., 2020). If spermatogenesis is impaired, FSH levels rise and inhibin B levels diminish (Franchimont et al., 1972; Kelsey et al., 2017). The ratio between inhibin B and FSH may be the most reliable reference to estimate fertility (Andersson et al., 2004; Bordallo et al., 2004; van Casteren et al., 2008). In pre-pubertal boys, however, measurement of endocrine hormones such as FSH is of little value in a setting without a (HCG) stimulation test. Nevertheless, pre-pubertal inhibin B levels are still presumed to reflect the number and integrity of Sertoli cells, potentially offering a prediction of future reproductive ability (Raivio et al., 2000; Andersson and Skakkebaek, 2001; Pierik et al., 2003). Previous studies have observed high incidences of abnormal semen parameters and abnormal reproductive hormones in male childhood HL survivors, yet these studies have often included small patient numbers or studied treatment regimens that are no longer used (Drechsel et al., 2023).
Nowadays, children with classical HL (cHL, ±95% of HL cases) in Europe are treated with vincristine, etoposide, prednisolone, and doxorubicin (OEPA) followed by consolidation treatment with cyclophosphamide, vincristine, prednisone, and dacarbazine (COPDAC) (Mauz-Körholz et al., 2022). The number of administered chemotherapy courses and use of radiotherapy depends on the stage of disease and treatment response. All advanced-stage cHL patients are treated with alkylating agents at a dose equivalent to a CED-score (cyclophosphamide equivalent dose score) ≥4000 mg/m2, which, according to the current PANCARE guideline, is associated with a high risk of infertility in males up to the age of 25 years old (Mulder et al., 2021).
The current EuroNet-PHL-C2 trial aims to reduce the use of radiotherapy in standard cHL treatment in an effort to lower the risk of secondary malignancies, by administering intensified chemotherapy. In the intensified treatment-arm, patients receive DECOPDAC-21 consolidation courses, in which doxorubicin and etoposide are added to COPDAC and the cumulative dose of (gonadotoxic) cyclophosphamide (thus CED-score of treatment) is increased by 25% (European Network-Paediatric Hodgkin Lymphoma Study Group (EuroNet-PHL), 2015). The present add-on study to the EuroNet-PHL-C2 trial was designed to prospectively evaluate the effect of the EuroNet-PHL-C2 protocol for childhood cHL on reproductive markers (i.e. sperm parameters and serum FSH, inhibin B levels and the inhibin B:FSH ratio) in newly diagnosed boys.
Materials and methods
Study design and study population
This international, multi-centre, observational prospective cohort study is an add-on study to the EuroNet-PHL-C2 trial (Clinicaltrials NCT02684708; EudraCT number 2012-004053-88). Inclusion criteria comprised a confirmed diagnosis of cHL in children up to the age of 18 years, scheduled for treatment according to the EuroNet-PHL-C2-protocol. Patients were recruited between January 2017 and September 2021 in 18 participating sites in the Netherlands (n = 5), Belgium (n = 6), Germany (n = 5), Austria (n = 1), and Czech Republic (n = 1). The present analysis included all participating boys, while the data of the participating girls have been described elsewhere (Drechsel et al., 2024).
cHL treatment regimen
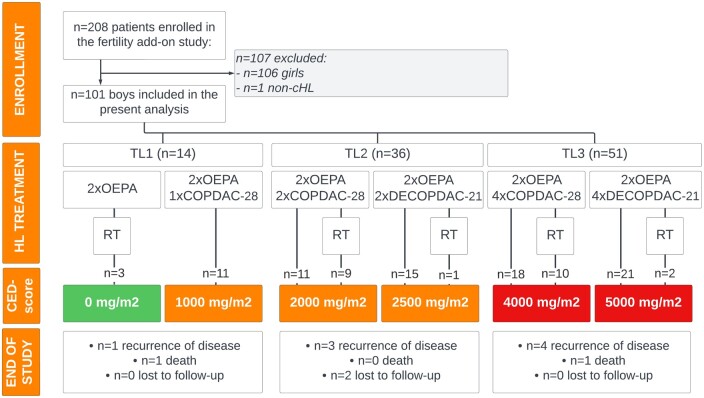
All patients received treatment for cHL according to the EuroNet-PHL-C2 protocol. A schematic description of the protocol is included in Fig. 1. In brief, the intensity of treatment depended on Ann Arbor stage of disease and presence of risk factors (bulky disease with tumour volume ≥200 ml, elevated erythrocyte sedimentation rate (ESR) ≥30 mm/h, E-lesions (extra nodal involvement)) and treatment response determined by PET-CT and MRI. Patients assigned to TL1 (treatment-level 1; early stage cHL) received two induction courses of OEPA followed by one course of COPDAC-28 consolidation treatment (in case of adequate treatment response) or involved-node radiotherapy (20 Gy) (in case of inadequate treatment response). Patients assigned to TL2 and TL3 (intermediate and advanced stages of cHL) received two courses of OEPA and were randomized to receive either standard COPDAC-28 or intensified DECOPDAC-21 consolidation chemotherapy (two courses in case of TL2 and four courses in case of TL3). Patients who declined randomization received standard COPDAC-28 consolidation. TL2 and TL3 patients with adequate response at time of early response assessment (ERA) had no indication for additional radiotherapy. TL2/TL3 patients with inadequate response at ERA underwent a late response assessment after chemotherapy (LRA). The COPDAC-28 treatment-arm received radiotherapy to all initially involved sites (20 Gy) with a boost (10 Gy) to LRA PET-positive sites. The DECOPDAC-21 treatment-arm only received radiotherapy to LRA PET-positive sites (30 Gy). The inclusion of the EuroNet-PHL-C2 trial ended in December 2020, leading to the cessation of randomization between COPDAC-28 and DECOPDAC-21 consolidation. The EuroNet-PHL-C2 protocol was adopted as standard cHL treatment protocol in Europe. Patients included after the end of accrual of the EuroNet-PHL-C2 trial received standard COPDAC-28 consolidation treatment, although in some cases (e.g. advanced-staged patients with inadequate response at ERA, where large radiotherapy fields should be applied), the treating paediatric oncologist could decide to give DECOPDAC-21 consolidation treatment. In the present fertility add-on study, we studied assigned treatment per-protocol.
Figure 1.
Study flow chart. Flow diagram of the fertility add-on study, depicting enrolment, assigned treatment according to the EuroNet-PHL-C2 protocol with corresponding CED-score, and follow-up/end of study for the present study. According to current PANCARE guidelines (Mulder et al., 2021) treatment with a CED-score ≥4000 mg/m2 should be considered high risk of infertility in boys. The depicted CED-score of high-risk treatment is coloured in red, low-risk in orange, and CED-score 0 g/m2 is depicted in green. CED, cyclophosphamide equivalent dose; cHL, classical Hodgkin lymphoma; COPDAC-28, cyclophosphamide, vincristine, prednisone, and dacarbazine; DECOPDAC-21, doxorubicin, etoposide, cyclophosphamide, vincristine, prednisone, and dacarbazine; OEPA, vincristine, etoposide, prednisone, doxorubicin; OPPA, vincristine, procarbazine, prednisone, doxorubicin; TL, treatment level; RT, radiotherapy; n, number.
Data collection and measurements
Data were collected at various time points, including: T0: at diagnosis, T1: after 2× OEPA, T1b: after 1× COPDAC-28 (only for TL1 patients), T2: after 2× COPDAC-28/DECOPDAC-21 (only for TL2/TL3 patients), T3: after 2× COPDAC-28/DECOPDAC-21 (only for TL3 patients), and T4: 2 years post-diagnosis. Study participation and data collection ended prematurely in case of progression of disease, recurrence, death, or loss to follow-up.
The primary outcomes of the present study included quantitative and qualitative sperm parameters from semen samples collected 2 years after diagnosis (in (post)pubertal boys). Secondary outcomes comprised sperm parameters at diagnosis (also compared to baseline semen) and measured serum levels of FSH, inhibin B, and the inhibin B:FSH ratio over time (separately analysed in pre- and (post)pubertal boys).
Semen analysis
All (post)pubertal boys were invited to deliver semen at diagnosis and 2 years post-diagnosis. Boys were instructed to maintain a period of abstinence of minimal 3–5 days prior to sperm collection. Actual duration of abstinence was not recorded in the CRF (case report form). All semen analyses were performed in specialized laboratories on site, in accordance with the WHO-criteria and the Björndahl et al. (2016) checklist (Cooper et al., 2010). Reported results included semen volume (ml), concentration (million/ml), progressive motility (A + B%), and the calculated TMSC (in million, obtained by multiplying sperm concentration × volume × progressive motility divided by 100%). The WHO reference values for human semen characteristics were applied to define abnormal semen parameters (Cooper et al., 2010). A sperm volume of <1.5 ml was defined as hypospermia and a progressive sperm motility <32% was defined as asthenozoospermia. A sperm count ≥15 million/ml was considered normozoospermic (also referred to as ‘normal sperm count’), 5–15 million/ml oligospermic, 0–5 million/ml severe oligospermic, and if there were no spermatozoa in the ejaculate the sample was classified as azoospermic. All azoospermic and (severe) oligospermic sperm counts were labelled as ‘abnormal’. A calculated TMSC <20 million was considered ‘low’.
The collected semen samples at diagnosis were used for cryopreservation after determining semen parameters. Of note, the fertility add-on study was observational, with semen cryopreservation provided and conducted as part of standard care practice. If multiple semen samples were provided in effort to store semen, results of the sample with the highest sperm concentration were used for the study. Attempts and results of TESE (testicular sperm extraction) were separately registered in the CRF. If boys underwent testicular biopsy for the purpose of preserving testicular tissue (potentially as part of another trial, unrelated to the fertility add-on study), the procedure was noted in the CRF, but no additional (tissue) reports were available.
Blood sampling
Upon approval of the patient/parent, blood samples were drawn at each checkup. Samples were stored at the local sites at (minimal) −20°C. All available samples were transported on dry-ice and analysed in the laboratory of Amsterdam UMC, location AMC in May 2023. The lower limit of quantitation (LLOQ) for FSH was 0.1 IU/l with an intra-assay variation of <3.5% and inter-assay variation of <4.8% over the whole concentration range (Alinity I, Abbott Diagnostics). Serum inhibin B levels were analysed using the Gen II Inhibin B Elisa (Beckman Coulter), with an LLOQ 10 ng/l and an inter-assay variations <10% over the whole concentration range.
Patient characteristics and treatment data
Information on age (y), height (m), weight (kg), Tanner stage (G: genital and P: pubic hair) and testicular volume (measured by the attending physician using Prader orchidometer) were obtained during regular checkup visits, using a predesigned CRF. A testicular volume of ≥4 ml (or in case of missing: Tanner-G ≥ 2) was used to define the onset of puberty. Additional details about the cHL staging, administered treatments, and recurrences or secondary malignancies were collected from the central EuroNet-PHL-C2 database (or retrieved from medical files for those patients included after the end of accrual of the EuroNet-PHL-C2 study in December 2020). B symptoms at diagnosis included drenching night sweats, unexplained fever of >38.5°C and/or ≥10% weight loss in the previous 6 months. Active tumour sites within the porta hepatis, splenic hilum, mesenteric, upper/lower para-aortic, iliac, and inguinal areas were referred to as ‘infradiaphragmatic’. The cumulative alkylating agent exposure for each TL and treatment-arm was estimated by calculating the CED-score; see Fig. 1 (Green et al., 2014).
Statistical analysis
Measured continuous values of semen parameters were categorized/dichotomized, using the previously mentioned WHO reference values for human semen characteristics (Cooper et al., 2010).
Continuous and categorical sperm parameters at diagnosis and 2 years post-diagnosis were compared between treatment-arms (TL-stages and COPDAC-28/DECOPDAC-21 consolidation schemes) using chi-square or Fisher’s exact (categorical variables) or Mann–Whitney U-tests (continuous variables). Additionally, we conducted a descriptive comparison of semen concentration before and after treatment in the subset of boys who had provided semen samples at both time points.
Analyses of laboratory measurements were separately conducted in pre- and (post)pubertal boys. Inhibin B:FSH ratios were calculated by dividing serum inhibin B by serum FSH. FSH levels that were reported as ‘<0.1’ were replaced by 0.1 (n = 3) and inhibin B levels ‘<10 µg/l’ were replaced by 10 (n = 2) to enable statistical analysis. For (post)pubertal boys, FSH levels above 7.6 IU/l were considered elevated and inhibin levels below 100 ng/l were considered low (Schoor et al., 2002; Brignardello et al., 2016; Felicetti et al., 2020). For pre-pubertal boys, abnormal reproductive hormone levels were defined as FSH above 5 IU/l and inhibin B below 50 ng/l (Crofton et al., 2002; Kelsey et al., 2016).
Median serum markers and number of patients with elevated FSH or low inhibin B were compared in unadjusted analyses between the treatment arms at the different time points using chi-square/Fisher’s exact test or Mann–Whitney U-test. Furthermore, linear mixed models analyses, adjusted for age at time of sampling, were used to assess laboratory results over time (checkups T0 up to T4). A random intercept at patient level was used to control for the dependency of subsequent observations within the same patient. Potential differences in outcomes between treatment levels (TL1 vs TL2 vs TL3) and treatment-schemes (TL2/3 COPDAC-28 vs TL2/3 DECOPDAC-21) were assessed by adding these variables as covariates to the models.
Differences in laboratory results between (post)pubertal boys with normal or abnormal sperm concentrations were assessed among those who provided both serum and semen samples during either T0 or T4 checkups, using chi-square/Fisher’s exact test or Mann–Whitney U-test. Furthermore, the impact of B symptoms on sperm parameters and laboratory measurements at diagnosis were evaluated by comparing all outcomes between (post)pubertal boys who presented with and without B symptoms in age-adjusted linear (continuous outcomes) and logistic (dichotomous outcomes) regression analyses. Results were respectively presented as adjusted difference (B-coefficient) or odds ratio (OR) with their corresponding 95% CI.
Statistical analyses were performed using IBM SPSS Statistics version 28.0 (IBM Corp., 2021). P-values <0.05 were considered statistically significant.
Ethical approval
This study was conducted according to the Declaration of Helsinki for Medical Research involving Human Subjects. The trial was approved by local ethical committees in each participating country. All patients (≥12 years old) and their parents/guardians (of patients <16 years old) provided written informed consent.
Results
Included boys
101 boys were included in the present analysis; see the study flowchart in Fig. 1. Baseline data are included in Table 1. A total of 73 boys (72%) were (post)pubertal with median age at diagnosis of 15.4 years (IQR 14.4; 16.6). Ten boys (14%) had early stage cHL and were assigned to TL1. The remaining 63 (post)pubertal boys were diagnosed with intermediate (n = 29 TL2, 59% COPDAC-28) or advanced stage (n = 34 TL3, 65% COPDAC-28) of cHL. There were 31 (42.5%) (post)pubertal boys who had B symptoms at the time of cHL diagnosis (n = 22/31 (71%) TL3). Among the 28 pre-pubertal boys (28%), median age at diagnosis was 9.6 years (IQR 6.6; 11.4). Four pre-pubertal boys were assigned to TL1, 7 to TL2 (43% COPDAC-28), and 17 to TL3 (35% COPDAC-28). The CED-score of assigned chemotherapy varied between 0 and 5000 mg/m2 (Fig. 1). In total, 25 (25%) boys received radiotherapy after chemotherapy of whom six were irradiated in the pelvic area (n = 3 TL2-COPDAC-28, n = 3 TL3-COPDAC-28) with target dose 19.8 Gy. However, none of the boys received radiotherapy to the inguinal area, and estimated radiotherapy dose to the testis was less than 0.5–1 Gy for all boys. Median duration of follow-up was 24.0 months (IQR 22.0; 25.0) at time of the analysis. Two (2%) patients died and eight boys (8%) experienced a recurrence of disease during follow-up. One boy was diagnosed with a secondary malignancy (1%) and two (2%) patients were lost to follow-up.
Table 1.
Baseline characteristics of included (post)pubertal and pre-pubertal boys.
| (Post)pubertal boysa | Pre-pubertal boys | |
|---|---|---|
| (n = 73) | (n = 28) | |
| Age at diagnosis (years), median (IQR) | 15.4 [14.4; 16.6] | 9.6 [6.6; 11.4] |
| Ann Arbor stage of disease | ||
| 1 | 1 (1.4%) | 0 (0.0%) |
| 2 | 33 (45.2%) | 7 (25.0%) |
| 3 | 22 (30.1%) | 11 (39.3%) |
| 4 | 17 (23.3%) | 10 (35.7%) |
| B symptomsb | 31 (42.5%) | 12 (42.9%) |
| ESR ≥ 30 mm/h | 44 (61.1%) | 17 (60.7%) |
| Bulky diseasec | 36 (50.7%) | 8 (28.6%) |
| Involved tumour sites in infradiaphragmatic regiond | 23 (31.5%) | 17 (60.7%) |
| Assigned treatment level and consolidation scheme | ||
| TL1 | 10 (13.7%) | 4 (14.3%) |
| TL2, COPDAC-28 | 17 (23.3%) | 3 (10.7%) |
| TL2, DECOPDAC-21 | 12 (16.4%) | 4 (14.3%) |
| TL3, COPDAC-28 | 22 (30.1%) | 6 (21.4%) |
| TL3, DECOPDAC-21 | 12 (16.4%) | 11 (39.3%) |
| Radiotherapy | ||
| Received radiotherapy | 18 (24.7%) | 7 (25.0%) |
| Assigned but not given | 2 (2.7%) | 1 (3.6%) |
| Ended study participation before end of treatment | 1 (1.4%) | 0 (0.0%) |
| Pelvic radiotherapye | 4 (5.5%) | 2 (7.1%) |
| Median follow-up, months (IQR) | 24.0 [23.0; 28.0] | 24.5 [22.0; 26.2] |
| End of study | ||
| Recurrence | 5 (6.8%) | 3 (10.7%) |
| Secondary malignancy | 1 (1.4%) | 0 (0.0%) |
| Death | 2 (2.7%) | 0 (0.0%) |
| Lost to follow-up | 2 (2.7%) | 0 (0.0%) |
Results are presented as median (IQR) or number (%).
Assigned treatment was according to the EuroNet-PHL-C2 protocol. TL1 patients receive 2× OEPA induction followed by either 1× COPDAC-28 or involved node radiotherapy depending on adequate or inadequate treatment-response.TL2/TL3 patients are randomized between the COPDAC-28 and DECOPDAC-21 treatment-arm and receive 2× OEPA induction followed by 2× (TL2) or 4× (TL3) (DE)COPDAC consolidation. Indication for radiotherapy depends on treatment response and treatment-arm.
OEPA, vincristine, etoposide, prednisone, doxorubicin; COPDAC-28, cyclophosphamide, vincristine, prednisone, and dacarbazine; DECOPDAC-21, doxorubicin, etoposide, cyclophosphamide, vincristine, prednisone, and dacarbazine; ESR, erythrocyte sedimentation rate; IQR, interquartile range; TL, treatment level.
(Post)pubertal is defined as testicular volume (measured by Prader orchidometer) ≥4 ml or tanner stage (genital) >1.
B symptoms, i.e. unexplained fever >38.5°C, weight loss of 10% during the past 6 months and drenching night sweats.
Bulky disease is defined as contiguous tumour volume of at least 200 ml.
Including tumour sites in the porta hepatis, splenic hilum, mesenteric, upper para-aortic, lower para-aortic, iliac, and inguinal area.
All patients who received pelvic radiotherapy were within the COPDAC-28 treatment-arm and target dose was 19.8 Gy. None of the boys were irradiated in the inguinal or testicular area, expected radiation directed towards the testes was <0.5–1 Gy.
Semen analysis in (post)pubertal boys
At diagnosis
There were 48 boys (66% of (post)pubertal boys) who delivered semen for cryopreservation before chemotherapy, with a median age of 16.0 years (IQR 15.4; 17.0) at time of semen collection. None of the boys underwent a TESE procedure. The youngest boy who successfully delivered semen was 13.6 years old. Results on semen volume, sperm concentration, progressive motility, and TMSC are reported in Table 2. Overall, 48% of semen samples (n = 23) were abnormal in terms of concentration (<15 mil/ml). More specifically, 4 boys (8%) had azoospermia, 11 (23%) had severe oligozoospermia, and 8 (17%) had oligozoospermia. In total, 22 boys (46%) had asthenozoospermia and 26 (54%) boys had semen volume <1.5 ml. The sperm concentration was statistically significantly lower in the 21 boys who presented with B symptoms compared to those (n = 27) who had no B symptoms at diagnosis (median 7.6 mil/ml (IQR 1.0; 13.6) vs 30.0 mil/ml (IQR 10.9; 68.5), adjusted difference −27.4 (95% CI −52.5; −2.3), P = 0.03, and the OR for having an abnormal sperm concentration was 2.3 (95% CI 1.0; 3.8), P = 0.001), see Supplementary Table S1.
Table 2.
Semen analyses at diagnosis and 2 years post-diagnosis in (post)pubertal boys.
| Semen analysis |
||
|---|---|---|
| At diagnosis | 2 years post-diagnosis | |
| (n = 48) | (n = 31) | |
| Age at time of collection, years (IQR) | 16.0 [15.4; 17.0] | 18.8 [18.0; 19.4] |
| Tanner-G, median (IQR) | V (IV-V) | V (V-V) |
| Mean testicular volume,a median (IQR) | 20.0 [15.0; 22.8] | 20.0 [18.8; 25.0] |
| Semen volume (ml) | 1.4 [0.6; 2.0] | 1.9 [1.3; 3.4] |
| Hypospermia (<1.5 ml) | 26 (54.2%) | 9 (29.0%) |
| Sperm concentration (mil/ml), median (IQR) | 17.0 [2.9; 38.9] | 13.0 [2.2; 35.7] |
| Normozoospermia (≥15 mil/ml) | 25 (52.1%) | 15 (48.4%) |
| Oligozoospermia (5–15 mil/ml) | 8 (16.7%) | 6 (19.4%) |
| Severe oligozoospermia (0–15 mil/ml) | 11 (22.9%) | 6 (19.4%) |
| Azoospernia (0 mil/ml) | 4 (8.3%) | 4 (12.9%) |
| Normal sperm concentration | 25 (52.1%) | 15 (48.4%) |
| Abnormal sperm concentrationb | 23 (47.9%) | 16 (51.6%) |
| Progressive motility a + b (%), median (IQR) | 32.0 [10.8; 49.2] | 48.0 [13.5; 60.9] |
| Asthenozoospermia (<32%) | 22 (45.8%) | 11 (35.5%) |
| Total motile sperm count (TMSC) (mil), median (IQR) | 7.8 [0.3; 28.7] | 12.3 [0.4; 33.9] |
| Low TMSC (<20 mil) | 31 (64.6%) | 22 (71.0%) |
IQR, interquartile range.
Applied references values for semen analysis are according to WHO criteria (Cooper et al., 2010).
Testicular volume defined by Prader orchidometer.
Abnormal sperm concentration includes azoospermia, severe oligozoospermia and oligozoospermia, i.e. <15 mil sperm cells/ml.
None of the boys who were pre-pubertal at diagnosis had delivered sperm at 2 years post-diagnosis.
Of note, five boys (i.e. 1 (post)pubertal and 4 pre-pubertal) underwent testicular biopsy to preserve testicular tissue in a research setting prior to starting cHL treatment.
At 2 years post-diagnosis
At 2 years post-diagnosis, 31 boys collected semen samples for analysis; 16 (52%) had abnormal sperm concentrations, i.e. 4 (13%) had azoospermia, 6 (19%) had severe oligozoospermia, and 6 (19%) had oligozoospermia (see Table 2). The group-median TMSC was low (12.3 million) (IQR 0.4; 33.9) and below reference values in 22 boys (71%). Boys in higher TL-stages had lower sperm concentrations when compared to patients with less advanced cHL (TL3 vs TL2, P = 0.07). All three TL1 staged patients who delivered semen during follow-up had normozoospermia, while 36% (n = 4/11) of the TL2-stage and 71% (n = 12/17) of the TL3-stage boys had abnormal concentrations; see Supplementary Table S2.
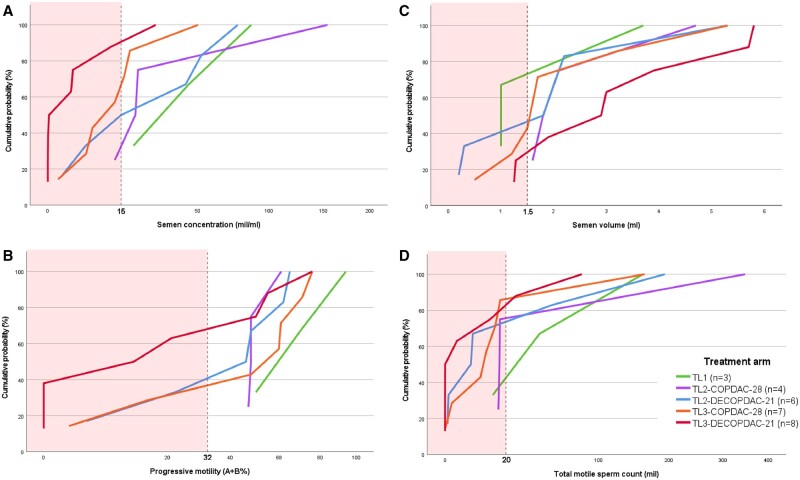
The sperm concentration, progressive motility, volume, and TMSC after 2 years are depicted as cumulative distribution figures per treatment arm in Fig. 2. Median sperm concentration was lowest in the TL3-DECOPDAC-21 arm, when compared to the other TL2/TL3 treatment arms (median sperm concentration 1.4 mil/ml in TL3-DECOPDAC-21 vs 13.0 mil/ml TL3-COPDAC-28, 29.0 mil/ml TL2-DECOPDAC-21 and 20.0 mil/ml TL2-COPDAC-28, P = 0.08 when compared to TL3-COPDAC-28 patients, respectively). Similarly, the median progressive motility was 16.5% in TL3 staged boys treated with DECOPDAC-21, while the median motility ranged between 47.0% and 54.0% in the other TL2/3 treatment arms (P = 0.25 when compared to TL3-COPDAC-28); see Supplementary Table S2. Three out of the six boys who had received pelvic radiotherapy delivered semen after 2 years (n = 1 TL2-COPDAC-28, n = 2 TL3-COPDAC-28); one had a normal sperm concentration after treatment and the other two boys were azoospermic. Of the 31 boys who delivered semen at 2 years post-diagnosis, 28 had also delivered semen at diagnosis. A schematic comparison of their sperm concentration over time, including assigned TL-stage and received consolidation treatment, is included in Supplementary Fig. S1.
Figure 2.
Sperm parameters at 2 years from childhood classical Hodgkin lymphoma diagnosis, according to assigned treatment level and consolidation treatment. Cumulative distribution figures depicting the cumulative proportion of patients with a specific (A) sperm concentration (B) progressive sperm motility (C) semen volume or (D) total motile sperm count (TMSC) at 2 years from Hodgkin lymphoma diagnosis. Vertical dashed lines indicate WHO reference cutoff values to define abnormal sperm parameters, and the red-marked areas highlight the abnormal range (Cooper et al., 2010). TL, treatment level. Assigned treatment was according to the EuroNet-PHL-C2 protocol. TL1 patients received 2× OEPA±1× COPDAC-28 (CED-score = 0–1 g/m2), TL2 patients received 2× OEPA + 2× (DE)COPDAC (CED-score = 2–2.5 g/m2), TL3 patients received 2× OEPA + 4× (DE)COPDAC (CED-score 4–5 g/m2). OEPA, vincristine, etoposide, prednisone, doxorubicin; COPDAC-28, cyclophosphamide, vincristine, prednisone, and dacarbazine; DECOPDAC-21, doxorubicin, etoposide, cyclophosphamide, vincristine, prednisone, and dacarbazine. Additional explanation on how to read a cumulative distribution figure: each of the cumulative distribution figures illustrates the relationship between the respective assessed sperm parameter on the x-axis and the cumulative probability on the y-axis. Each point on the curve represents the cumulative probability of observing a value less than or equal to the corresponding value on the x-axis. For instance, consider figure A (semen concentration): for a sperm concentration of 15 mil/ml on the x-axis, the cumulative probability is 90% for the red line (indicating the TL3-DECOPDAC-21 treatment arm). This implies that ∼90% of the assessed sperm concentrations in this specific subgroup are ≤15 mil/ml.
Serum FSH and inhibin B
(Post)pubertal boys
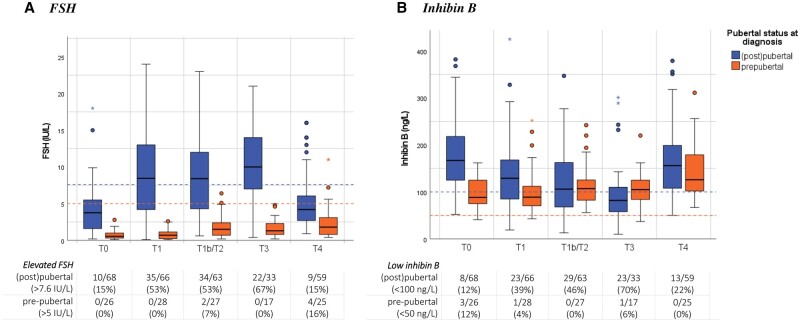
A total of 290 blood samples obtained from boys who were (post)pubertal at time of diagnosis were included in the analysis (n = 68 at diagnosis, n = 163 during treatment, n = 59 at 2 years post-diagnosis). The measured FSH and inhibin B levels are presented as boxplots in Fig. 3. Median FSH was 3.8 IU/l (IQR 1.6; 5.5) and inhibin B 167.0 ng/l (IQR 125.0; 217.0) at diagnosis. Ten boys (15%) had FSH >7.6 IU/l and eight (12%) had low serum inhibin B (<100 ng/l). There were no statistically significant differences in circulating FSH and inhibin B between the boys who presented with B symptoms and those who had no B symptoms at diagnosis (Supplementary Table S1).
Figure 3.
Serum FSH and inhibin B during treatment for childhood Hodgkin lymphoma up to 2 years follow-up in pre- and (post)pubertal boys. (A) FSH, B inhibin. (B) Boxplots depicting the distribution of uncorrected serum FSH or inhibin B from diagnosis up to 2 years post-diagnosis, including the median (centerline), interquartile range (end of the box), and range (end of the whiskers). Separate dots are outliers. Patients who were pre-pubertal at time of diagnosis are shown in orange, and boys who were (post)pubertal at diagnosis (definition testicular volume ≥4 ml or in case of missing Tanner-G ≥2) are shown in blue. The dashed lines in blue highlight applied cut-off scores to define normal (≤7.6 IU/l) or high FSH (>7.6 IU/l), respectively normal (≥100 ng/l) or low inhibin B (<100 ng/l) in (post)pubertal boys. The dashed lines in orange highlight applied cut-off scores to define normal (≤5 IU/l) or high FSH (>5 IU/l), respectively normal- (≥50 ng/l) or low inhibin B (<50 ng/l) in pre-pubertal boys. FSH, follicle-stimulating hormone; N, number. Timing sampling: T0, at diagnosis; T1, after 2× OEPA; T1b, after 1× COPDAC; T2, after 2×(DE)COPDAC; T3, after 4×(DE)COPDAC; T4, 2 years post-diagnosis.
During treatment, serum FSH gradually increased and serum inhibin B gradually decreased, especially among advanced-stage cHL patients (estimated effect after 2× OEPA FSH: 5.2 (95% CI 4.1; 6.4) and inhibin B: −39.8 (95% CI −56.4; −23.2), both P < 0.001, analyses were adjusted for age at time of sampling). Overall, 38 boys (57%) had elevated FSH and 36 (54%) had low inhibin B after completion of therapy; 57% of boys with serum values outside of normal references were TL3 staged patients.
After 2 years, serum FSH and inhibin B levels had often recovered to within normal reference limits (80% FSH recovery, 60% inhibin B recovery), and there were no statistically significant differences in median serum FSH and inhibin B when compared to pre-treatment values. Median FSH was 4.2 IU/l (IQR 2.7; 6.0, 9 boys (15%) high FSH) and inhibin B was 156.0 ng/l (IQR 108.0; 199.0, 13 (22%) boys had low inhibin B). Inhibin B levels and inhibin B:FSH ratio were significantly lower in TL3 patients, when compared to TL2 patients (inhibin B: 121.0 ng/l vs 182.5 ng/l, ratio: 26.0 vs 64.9, P = 0.004 and P = 0.02 according to the linear mixed models, respectively). The linear mixed models did not present any significant differences in reproductive hormones over time when comparing the COPDAC-28/DECOPDAC-21 treatment schemes; see Supplementary Table S3.
Pre-pubertal boys
The total number of included samples obtained from pre-pubertal boys was 123 (n = 26 at diagnosis, n = 72 during treatment, n = 25 at 2 years post-diagnosis). As expected, median serum FSH and inhibin B levels were relatively low at diagnosis (FSH: 0.6 IU/l (IQR 0.3; 1.0) and inhibin B: 88.5 ng/l (IQR 75.2; 123.2)). We observed a small and gradual increase in serum FSH and inhibin B levels during treatment and follow-up (estimated effect at 2 years post-treatment FSH: 1.5 (95% CI 0.7; 2.2) and inhibin B: 43.4 (95% CI 16.4; 58.7), both P < 0.001). After 2 years, median FSH was 1.8 IU/l (IQR 0.8; 3.1) and inhibin B was 126.0 ng/l (IQR 102.0; 179.0).
Serum reproductive markers in boys with normal or abnormal sperm concentration
Table 3 includes an overview of measured reproductive hormones in the (post)pubertal boys who delivered sperm at diagnosis (n = 45 boys of whom both semen and hormone levels were available) and after 2 years (n = 28 boys of whom both semen and hormone levels were available). At diagnosis, there were no statistically significant differences between the reproductive hormones of boys with a normal or abnormal sperm concentration. However, at 2 years from diagnosis, the 14 boys with a sperm concentration below 15 million had higher FSH (5.0 IU/l vs 3.1 IU/l, P = 0.18) and lower inhibin B (102.0 ng/l vs 163.5 ng/l, P = 0.04) levels, resulting in lower inhibin B:FSH ratios (24.7 vs 56.3, P = 0.06) compared to the 14 boys with normal sperm concentration. Nevertheless, 10 out of the 14 boys (71%) with oligozoo/azoospermia still had ‘normal’ FSH <7.6 IU/l, and 7 out of 14 (50%) had inhibin B levels within normal range.
Table 3.
Circulating serum FSH and inhibin B in (post)pubertal boys with a ‘normal’ sperm concentration vs low sperm concentration at diagnosis and 2 years post-diagnosis.
| (Post)pubertal boys | |||||||
|---|---|---|---|---|---|---|---|
| Normal sperm concentration | Abnormal sperm concentration | Normal vs abnormal concentration (P-value) | Normozoospermia | Oligozoospermia | Severe oligozoospermia | Azoospermia | |
| At diagnosis | n = 23 | n = 22 | n = 23 | n = 7 | n = 11 | n = 4 | |
| Serum FSH (IU/l) | 4.8 [3.0; 6.8] | 4.3 [2.7; 5.8] | 0.59 | 4.8 [3.0; 6.8] | 4.3 [2.7; 5.3] | 4.7 [3.4; 6.2] | 3.5 [2.1; 5.2] |
| Elevated FSH (>7.6 IU/l) | 6 (26.1%) | 3 (13.6%) | 0.46 | 6 (26.1%) | 1 (14.3%) | 1 (9.1%) | 1 (25.0%) |
| Serum inhibin B (ng/l) | 169.0 [125.0; 222.5] | 170.0 [130.0; 204.8] | 0.95 | 169.0 [125.0; 222.5] | 165.0 [136.5; 239.0] | 175.0 [125.5; 195.0] | 181.5 [141.0; 208.5] |
| Low inhibin B (<100 ng/l) | 2 (8.7%) | 3 (13.6%) | 0.66 | 2 (66.7%) | 0 (0.0%) | 2 (18.2%) | 1 (25.0%) |
| Inhibin B:FSH ratio | 33.4 [22.6; 70.5] | 41.9 [24.4; 70.4] | 0.91 | 33.4 [22.6; 70.5] | 38.4 [30.6; 86.6] | 37.6 [21.7; 49.1] | 66.6 [38.1; 100.5] |
| After 2 years | n = 14 | n = 14 | n = 14 | n = 5 | n = 6 | n = 3 | |
| Serum FSH (IU/l) | 3.1 [2.7; 4.8] | 5.0 [2.8; 7.7] | 0.18 | 3.1 [2.7; 4.8] | 3.2 [2.3; 5.1] | 5.1 [4.6; 7.1] | 16.0 [8.7; 16.0] |
| Elevated FSH (>7.6 IU/l) | 0 (0.0%) | 4 (28.6%) | 0.10 | 0 (.%) | 0 (.%) | 2 (33.3%) | 2 (66.7%) |
| Serum inhibin B (ng/l) | 163.5 [145.0; 207.2] | 102.0 [74.2; 177.8] | 0.04 | 163.5 [145.0; 207.2] | 147.0 [84.0; 159.0] | 102.0 [77.8; 167.5] | 54.0 [52.5; 186.0] |
| Low inhibin B (<100 ng/l) | 0 (.%) | 7 (50.0%) | 0.006 | 0 (0.0%) | 2 (40.0%) | 3 (50.0%) | 2 (67.0%) |
| Inhibin B:FSH ratio | 56.3 [32.8; 69.4] | 24.7 [11.1; 60.3] | 0.06 | 56.3 [32.8; 69.4] | 28.8 [23.1; 69.1] | 19.6 [11.5; 34.2] | 3.4 [3.3; 124.0] |
Results are presented as median (IQR) and number (percentage). P-values were calculated by Mann–Whitney U-tests (continuous) or chi-square/Fisher’s exact (categorical). Normal sperm concentration and normozoospermia refers to ≥15 mil sperm count; abnormal sperm concentration is defined as sperm count <15 mil/ml; oligozoospermia 5–15 mil/ml, severe oligozoospermia 0–5 mil/ml, and azoospermia 0 sperm cells (Cooper et al., 2010).
FSH, follicle-stimulating hormone; vs, versus.
Discussion
This study prospectively evaluated reproductive markers in boys with newly diagnosed cHL and treated according to the European EuroNet-PHL-C2 treatment protocol. More than half of the (post)pubertal boys (52%) who provided a semen sample had low semen concentrations and even more boys had low TMSC (71%) at 2 years from diagnosis. Serum FSH gradually increased, and inhibin B decreased during chemotherapy with subsequent normalization in most (post)pubertal boys (80% when looking at FSH recovery and 60% when looking at inhibin B recovery), while FSH and inhibin B levels were overall low and remained relatively stable in pre-pubertal boys.
Similar to previous reports, low semen concentration and low motility were prevalent at time of cHL diagnosis (48% and 46%, respectively) (Drechsel et al., 2023). We observed a clear correlation between impaired semen parameters and B symptoms at diagnosis, which are often present in advanced-stage cHL patients as an expression of inflammation. Serum FSH and Inhibin B were far less frequently affected at diagnosis and there was no clear correlation between abnormal semen and abnormal reproductive hormones at diagnosis, as has also been described previously in adult HL patients (Ragni et al., 1985; Rueffer et al., 2001; Sieniawski et al., 2008). These results align with the hypothesis that the impaired health status and inflammatory status at time of cHL diagnosis can temporarily affect the sperm quality, but will probably not cause persisting gonadal damage (Tal et al., 2000; Rueffer et al., 2001; Laddaga et al., 2021).
Moreover, the volume of the collected sperm samples at time of cHL diagnosis was also remarkably low (54% <1.5 ml, n = 26/48). Perhaps, the observed low sperm volume is related to the young age of the included boys, although median testicular volume was 20.0 ml (IQR 15.0; 22.8) among the included boys and many were at a Tanner-G stage V (56%). A previous large nation-wide French cohort study demonstrated that sperm parameters, including ejaculate volume, significantly increased with age at time of cryopreservation (Daudin et al., 2015). Still, the (cancer) disease itself could also have a negative impact on produced sperm volume at time of diagnosis. Three studies among young adolescents (mean age ± 25–26 years) observed significantly lower sperm volumes of samples collected from HL patients, when compared to patients with testicular cancer (Amirjannati et al., 2011; Bizet et al., 2012; Caponecchia et al., 2016). Additionally, the reported median sperm volume was much higher in a large Danish cohort study including healthy males with median age of 19 years, when compared to the observed median sperm volume of collected samples at time of diagnosis in our cohort (2.7 ml (IQR 1.9; 3.7) vs 1.4 ml (IQR 0.6; 2.0), respectively) (Gaml-Sørensen et al., 2024). Clinicians should realize that the presence of HL disease itself might have an adverse effect on sperm parameters and thereby affect the success rate of semen cryopreservation as well as number of stored straws. The storage of testicular tissue might offer a solution for fertility preservation in young, pre-pubertal and/or high-risk boys who are not able to collect semen. In most countries this procedure is currently only offered in research setting and is not yet considered standard of care (Mulder et al., 2021). However, it is active field of research and this may change in the coming years, specifically since in a few countries ethical approval for immature testicular grafting is in place. The impact of disease on (pre-pubertal) testicular tissue is still relatively unknown, yet a recent study observed that the quantity of spermatogonia in pre-pubertal testicular tissue may also be affected by haematological disorders before treatment (Masliukaite et al., 2023).
As expected, abnormal sperm parameters during follow-up appeared to be specifically present in boys assigned to the highest TL-stage with a higher chemotherapy burden, when compared to patients treated for less advanced cHL. Notwithstanding, 36% (n = 4/11) of TL2 patients also had low sperm counts at 2 years from diagnosis, while they received treatment with an estimated low-risk of infertility based on the PANCARE guidelines (i.e. CED-score <4000 mg/mg) (Mulder et al., 2021). It remains unclear whether recovery of spermatogenesis will occur (and to what extent). cHL treatment lasted 3 to 6 months in most boys, hence the semen samples were collected at least 1.5 years after the last administration of chemotherapy. Based on previous reports, recovery of spermatogenesis could occur years after completion of chemotherapy (Howell and Shalet, 2005). Studies in childhood HL patients case-wise reported improved sperm counts even after 10–12 years after treatment, particularly among survivors with a relatively low exposure to alkylating agents (da Cunha et al., 1984; Sy Ortin et al., 1990). At present, we do not have the ability or tools to predict occurrence and timing of (long-term) recovery after cancer treatment. We did observe higher FSH- and lower inhibin B levels in patients with lower sperm concentration in our study, yet still 50% (n = 7/14) of boys with oligozoo/azoospermia had reproductive hormones within normal ranges at 2 years post-diagnosis. FSH and inhibin B levels reflect the early-phases of spermatogenesis, but are not considered predictive of future regeneration of spermatozoa (Khanehzad et al., 2021). Participating boys of the fertility add-on study will be invited to deliver another blood and sperm sample for analysis 5-year post-diagnosis, and these results will be crucial to estimate lasting gonadotoxicity rates.
There is an ongoing discussion whether a pre-pubertal/dormant state of the gonads may act as a protector against gonadotoxicity (Pennisi et al., 1975; Sherins et al., 1978; Bayle-Weisgerber et al., 1984; Hobbie et al., 2005; Zaletel et al., 2010; Brignardello et al., 2016; Drechsel et al., 2023). We observed relatively low FSH and inhibin B levels throughout all assessments among the 28 included boys who were pre-pubertal at time of diagnosis, which is most likely the result of the pre-pubertal state itself, where overall circulating sex steroid levels are generally low (Miles et al., 2015). The observed median inhibin B levels and small increase during the period of 2 years since diagnosis seems to correlate well with the normative model of serum inhibin B published by Kelsey et al. (2016). After 2 years, the boys were still too young and physically too immature (i.e. n = 12 still pre-pubertal, only 1 boy with Tanner 4, all testicular volume ≤12 ml) to deliver sperm for analysis. Therefore, it remains unclear whether cHL treatment affected spermatogonial stem cells and (future) spermatogenesis in these pre-pubertal boys. Larger studies, including long-term data are needed to assess the impact of gonadal development at diagnosis on the gonadotoxicity of treatment.
Potential benefits and risks of intensified chemotherapy to treat cHL should be carefully considered to determine superiority of the two treatment schemes of the EuroNet-PHL-C2 protocol. Any successful reduction in radiotherapy must be weighed against potentially more gonadotoxic chemotherapy, that also contains a higher cumulative dosage of anthracyclines (associated with other late effects such as cardiotoxicity and risk of (female) breast cancer) (Dempke et al., 2023; Wang et al., 2023). Based on the present analysis, advanced-stage cHL patients receiving DECOPDAC-21 could potentially be at a higher risk of impaired spermatogenesis after treatment, when compared to advanced-stage cHL patients receiving the COPDAC-28 consolidation arm of the EuroNet-PHL-C2 protocol. However, considering the limited power due to low numbers and short follow-up of this fertility study, we cannot draw any definite conclusions at the moment.
Strengths and limitations
The fertility add-on study is the first study to evaluate reproductive markers during and after treatment for childhood cHL in a longitudinal design. The results improve knowledge on the acute and late effects of treatment for cHL according to the EuroNet-PHL-C2 protocol in Europe, and the effect of disease itself, on sperm parameters, serum FSH and inhibin B. However, several limitations should be noted. Semen results were based on a single attempt (with some exceptions for cryopreservation at diagnosis) and could not be adjusted for abstinence period or incomplete semen collection. Semen samples were analysed in multiple laboratories, although all adhered to the WHO standard for semen analysis. However, there remains risk of inter-observer variation in the semen analyses, which also extends to the measurement of testicular volume using the Prader Orchidometer. Actually only 50% (n = 28/56) of boys from whom semen collection was expected (based on Tanner stage-G IV or V and/or testicular volume ≥15 ml) delivered semen at 2 years post-diagnosis. The patients could personally benefit from semen collection at diagnosis considering cryopreservation, while at follow-up there were no direct personal benefits, which may have affected participation rate. Moreover, testosterone and luteinizing hormone were not evaluated in the present study. However, the expected added value of measuring these hormones as reproductive markers is fairly limited as, based on previous studies, the hormones usually remain unaffected during chemotherapy and most boys who are treated for cancer during or before puberty actually go through natural pubertal development (Müller, 2002; Stukenborg et al., 2018). The overall sample size and follow-up were limited. Therefore, due to a lack of power, we were unable to perform adjusted subgroup analyses on patients receiving pelvic radiotherapy and pre-pubertal boys at diagnosis.
Clinical implications and future research
The current study demonstrated an adverse effect of cHL treatment according to the EuroNet-PHL-C2 protocol on spermatogenesis in boys. Impaired sperm concentration and reduced progressive sperm motility were commonly observed in samples collected at 2 years from diagnosis. Nevertheless, samples of most boys contained at least some viable sperm cells and the potential for recovery remains uncertain. The measurement of FSH and inhibin B appears to be of low value to predict low sperm quality at 2 years from cHL treatment.
Boys with newly diagnosed cHL should be encouraged to deliver sperm for cryopreservation prior to treatment, whenever possible. The state of disease could negatively affect sperm quality, but boys should not be discouraged if the semen quality is impaired, because oligospermic samples can still be used for future IVF (in vitro fertilization) or ICSI (intracytoplasmic sperm injection) treatment. As long as there are vital sperm cells produced, there is a chance of having a biological child as a male cancer survivor. Although HL primarily occurs during or after puberty, young pre-pubertal boys who are unable to deliver semen for cryopreservation could also be confronted with the disease. According to the current PANCARE guidelines, testicular biopsy for cryopreservation can be offered to patients with substantial risk of infertility based on disease staging and planned treatment, as part of clinical trials or approved protocol (Mulder et al., 2021). Future studies should focus on potential recovery of spermatogenesis on the long term, but also evaluate clinical outcomes, including the desire for children, pregnancy rates (with or without assisted reproductive technology), and utilization of frozen semen samples and testicular tissue. In addition, the impact of new agents to treat HL (such as Brentuximab, Vedotin, Nivolumab, Pembrolizumab) on gonadal function should also be examined.
Summarizing conclusions
The current EuroNet-PHL-C2 protocol for childhood cHL impairs semen quality and quantity. Adverse semen parameters after 2 years were specifically present in boys treated for advanced stage cHL. However, additional long-term and adequately powered data are crucial to assess potential recovery of spermatogenesis and clinical impact on fertility. Serum FSH and inhibin B appear to be temporarily affected by chemotherapy with subsequent normalization after completion of treatment in most post-pubertal boys. In pre-pubertal boys, reproductive hormones were overall low and remained relatively stable during chemotherapy.
Supplementary Material
Acknowledgements
We would like to acknowledge Salena Meivis, Lisanne Raasen, and all other involved research nurses and personnel who assisted in the data collection for this study.
Contributor Information
K C E Drechsel, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Paediatric Haemato-Oncology, Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands; Cancer Center Amsterdam, Treament and quality of life, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
S L Broer, Department of Reproductive Medicine & Gynecology, University Medical Center Utrecht, Utrecht, The Netherlands.
H M K van Breda, Department of Urology, University Medical Centre Utrecht, Utrecht, The Netherlands.
F S Stoutjesdijk, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
E van Dulmen-den Broeder, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
A Beishuizen, Department of Paediatric Haemato-Oncology, Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands; Department of Haematology/Oncology, Erasmus MC—Sophia Children’s Hospital, Rotterdam, The Netherlands.
W H Wallace, Department of Haematology/Oncology, Royal Hospital for Sick Children, Edinburgh, UK.
D Körholz, Department of Pediatric Hematology and Oncology, Universitätsklinikum Giessen und Marburg GmbH, Standort Giessen—Zentrum für Kinderheilkunde und Jugendmedizin, Giessen, Germany.
C Mauz-Körholz, Department of Pediatric Hematology and Oncology, Universitätsklinikum Giessen und Marburg GmbH, Standort Giessen—Zentrum für Kinderheilkunde und Jugendmedizin, Giessen, Germany.
D Hasenclever, Institut für Medizinische Informatik, Statistik und Epidemiologie, Universität Leipzig, Leipzig, Germany.
M Cepelova, Department of Pediatric Hematology and Oncology, University Hospital Motol and 2nd Medical Faculty Charles University, Prague 5, Czech Republic.
A Uyttebroeck, Paediatric Haemato-Oncology, University Hospitals of Leuven, Leuven, Belgium.
L Ronceray, Pediatric Hematology and Oncology, St. Anna Children’s Hospital, Medical University of Vienna, Wien, Austria.
J W R Twisk, Department of Epidemiology and Data Science, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
G J L Kaspers, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Cancer Center Amsterdam, Treament and quality of life, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
M A Veening, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Cancer Center Amsterdam, Treament and quality of life, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Data availability
Individual participant data that underlie this article cannot be shared publicly because of privacy. Pseudonymized data will be shared on reasonable request for an ethically approved study protocol, after compiling a data-sharing agreement.
Authors’ roles
M.A.V., A.B., G.J.L.K., W.H.W., E.v.D.-d.B., D.K., C.M.-K., M.C., A.U., L.R., and F.S.S. were responsible for the trial design and study-setup. D.H. and K.C.E.D. were also involved during the data collection. K.C.E.D. cleaned the data and performed the statistical analysis, supported by J.W.R.T., S.L.B., and M.A.V. K.C.E.D. wrote the first version of the manuscript. All authors were involved in data interpretation, critically reviewed the manuscript, and approved the submitted final version.
Funding
The fertility add-on study was funded by the Dutch Charity Foundation KiKa (Project 257) that funds research on all forms of childhood cancer.
Conflict of interest
C.M.-K., D.K., W.H.W., D.H., M.C., A.U., and A.B. were involved in the development of the EuroNet-PHL-C2 regimen. The other authors declare no potential conflict of interest.
References
- Amirjannati N, Sadeghi M, Hosseini Jadda SH, Ranjbar F, Kamali K, Akhondi MA.. Evaluation of semen quality in patients with malignancies referred for sperm banking before cancer treatment. Andrologia 2011;43:317–320. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Wallace EM, Groome NP, Bellis AJ, Wu FCW.. Physiological relationships between inhibin B, follicle stimulating hormone secretion and spermatogenesis in normal men and response to gonadotrophin suppression by exogenous testosterone. Hum Reprod 1997;12:746–751. [DOI] [PubMed] [Google Scholar]
- Andersson AM, Skakkebaek NE.. Serum inhibin B levels during male childhood and puberty. Mol Cell Endocrinol 2001;180:103–107. [DOI] [PubMed] [Google Scholar]
- Andersson AM, Petersen JH, Jørgensen N, Jensen TK, Skakkebaek NE.. Serum inhibin B and follicle-stimulating hormone levels as tools in the evaluation of infertile men: significance of adequate reference values from proven fertile men. J Clin Endocrinol Metab 2004;89:2873–2879. [DOI] [PubMed] [Google Scholar]
- Bayle-Weisgerber C, Lemercier N, Teillet F, Asselain B, Gout M, Schweisguth O.. Hodgkin’s disease in children. Results of therapy in a mixed group of 178 clinical and pathologically staged patients over 13 years. Cancer 1984;54:215–222. [DOI] [PubMed] [Google Scholar]
- Bizet P, Saias-Magnan J, Jouve E, Grillo JM, Karsenty G, Metzler-Guillemain C, Perrin J.. Sperm cryopreservation before cancer treatment: a 15-year monocentric experience. Reprod Biomed Online 2012;24:321–330. [DOI] [PubMed] [Google Scholar]
- Björndahl L, Barratt CLR, Mortimer D, Jouannet P.. “How to count sperm properly”: checklist for acceptability of studies based on human semen analysis. Hum Reprod 2016;31:227–232. [DOI] [PubMed] [Google Scholar]
- Bordallo MA, Guimarães MM, Pessoa CH, Carriço MK, Dimetz T, Gazolla HM, Dobbin J, Castilho IA.. Decreased serum inhibin B/FSH ratio as a marker of Sertoli cell function in male survivors after chemotherapy in childhood and adolescence. J Pediatr Endocrinol Metab 2004;17:879–887. [DOI] [PubMed] [Google Scholar]
- Brice P, de Kerviler E, Friedberg JW.. Classical Hodgkin lymphoma. Lancet 2021;398:1518–1527. [DOI] [PubMed] [Google Scholar]
- Brignardello E, Felicetti F, Castiglione A, Nervo A, Biasin E, Ciccone G, Fagioli F, Corrias A.. Gonadal status in long-term male survivors of childhood cancer. J Cancer Res Clin Oncol 2016;142:1127–1132. [DOI] [PubMed] [Google Scholar]
- Brougham MFH, Kelnar CJH, Sharpe RM, Wallace WHB.. Male fertility following childhood cancer: current concepts and future therapies. Asian J Androl 2003;5:325–337. [PubMed] [Google Scholar]
- Caponecchia L, Cimino G, Sacchetto R, Fiori C, Sebastianelli A, Salacone P, Marcucci I, Tomassini S, Rago R.. Do malignant diseases affect semen quality? Sperm parameters of men with cancers. Andrologia 2016;48:333–340. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Noonan E, von ES, Auger J, Baker HG, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT. et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231–245. [DOI] [PubMed] [Google Scholar]
- Crofton PM, Evans AE, Groome NP, Taylor MR, Holland CV, Kelnar CJ.. Inhibin B in boys from birth to adulthood: relationship with age, pubertal stage, FSH and testosterone. Clin Endocrinol (Oxf) 2002;56:215–221. [DOI] [PubMed] [Google Scholar]
- da Cunha MF, Meistrich ML, Fuller LM, Cundiff JH, Hagemeister FB, Velasquez WS, McLaughlin P, Riggs SA, Cabanillas FF, Salvador PG.. Recovery of spermatogenesis after treatment for Hodgkin’s disease: limiting dose of MOPP chemotherapy. J Clin Oncol 1984;2:571–577. [DOI] [PubMed] [Google Scholar]
- Daudin M, Rives N, Walschaerts M, Drouineaud V, Szerman E, Koscinski I, Eustache F, Saïas-Magnan J, Papaxanthos-Roche A, Cabry-Goubet R. et al. Sperm cryopreservation in adolescents and young adults with cancer: results of the French National Sperm Banking Network (CECOS). Fertil Steril 2015;103:478–486.e1. [DOI] [PubMed] [Google Scholar]
- Dempke WCM, Zielinski R, Winkler C, Silberman S, Reuther S, Priebe W.. Anthracycline-induced cardiotoxicity—are we about to clear this hurdle? Eur J Cancer 2023;185:94–104. [DOI] [PubMed] [Google Scholar]
- Drechsel K, Pilon M, Stoutjesdijk F, Meivis S, Schoonmade LJ, Wallace W, van Dulmen-den Broeder E, Beishuizen A, Kaspers G, Broer S. et al. Reproductive ability in survivors of childhood, adolescent and young adult Hodgkin lymphoma: a review. Hum Reprod Update 2023;29:486–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel KCE, Broer SL, Stoutjesdijk FS, van Dulmen-den Broeder E, Beishuizen A, Wallace WH, Körholz D, Mauz-Körholz C, Hasenclever D, Cepelova M. et al. The impact of treatment for childhood classical Hodgkin lymphoma according to the EuroNet-PHL-C2 protocol on serum anti-Müllerian hormone. Hum Reprod 2024;39:1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Network-Paediatric Hodgkin Lymphoma Study Group (EuroNet-PHL). Second International Inter-Group Study for Classical Hodgkin Lymphoma in Children and Adolescent: EuroNet-PHL-C2. 2015. https://classic.clinicaltrials.gov/ct2/show/NCT02684708 (26 July 2024, date last accessed). [Google Scholar]
- Felicetti F, Castiglione A, Biasin E, Fortunati N, Dionisi-Vici M, Matarazzo P, Ciccone G, Fagioli F, Brignardello E.. Effects of treatments on gonadal function in long-term survivors of pediatric hematologic malignancies: a cohort study. Pediatr Blood Cancer 2020;67:e28709. [DOI] [PubMed] [Google Scholar]
- Franchimont P, Millet D, Vendrely E, Letawe J, Legros JJ, Netter A.. Relationship between spermatogenesis and serum gonadotropin levels in azoospermia and oligospermia. J Clin Endocrinol Metab 1972;34:1003–1008. [DOI] [PubMed] [Google Scholar]
- Gaml-Sørensen A, Thomsen AH, Tøttenborg SS, Brix N, Hougaard KS, Toft G, Håberg SE, Myrskylä M, Bonde JP, Ramlau-Hansen CH.. Maternal pre-pregnancy BMI and reproductive health in adult sons: a study in the Danish National Birth Cohort. Hum Reprod 2024;39:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, Neglia JP, Sklar CA, Kaste SC, Hudson MM et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2014;61:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens E, Jahnukainen K, Mitchell RT, Van Pelt AMM, Pennings G, Rives N, Poels J, Wyns C, Lane S, Rodriguez-Wallberg KA. et al. Fertility preservation in boys: recent developments and new insights. Hum Reprod Open 2020;2020:hoaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalgrim H, Jarrett RF.. Epidemiology of Hodgkin lymphoma. In: Engert A, Younes A, (eds). Hodgkin Lymphoma: A Comprehensive Overview. Switzerland: Springer International Publishing, 2020, 3–23. https://doi.org/10.1007/978-3-030-32482-7_1. [Google Scholar]
- Hobbie WL, Ginsberg JP, Ogle SK, Carlson CA, Meadows AT.. Fertility in males treated for Hodgkins disease with COPP/ABV hybrid. Pediatr Blood Cancer 2005;44:193–196. [DOI] [PubMed] [Google Scholar]
- Howell SJ, Shalet SM.. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr 2005;(34):12–17. [DOI] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp, 2021. [Google Scholar]
- Kelsey TW, McConville L, Edgar AB, Ungurianu AI, Mitchell RT, Anderson RA, Wallace WHB.. Follicle stimulating hormone is an accurate predictor of azoospermia in childhood cancer survivors. PLoS One 2017;12:e0181377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey TW, Miles A, Mitchell RT, Anderson RA, Wallace WH.. A normative model of serum Inhibin B in young males. PLoS One 2016;11:e0153843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanehzad M, Abbaszadeh R, Holakuyee M, Modarressi MH, Nourashrafeddin SM.. FSH regulates RA signaling to commit spermatogonia into differentiation pathway and meiosis. Reprod Biol Endocrinol 2021;19:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddaga FE, Masciopinto P, Nardelli C, Vacca MP, Masciandaro P, Arcuti E, Cicinelli E, Specchia G, Musto P, Gaudio F.. In male Hodgkin lymphoma patients, impaired fertility may be improved by non-gonadotoxic therapy. Br J Haematol 2021;196:110–115. [DOI] [PubMed] [Google Scholar]
- Masliukaite I, Ntemou E, Feijen EAM, van de Wetering M, Meissner A, Soufan AT, Repping S, Kremer LMC, Jahnukainen K, Goossens E. et al. Childhood cancer and hematological disorders negatively affect spermatogonial quantity at diagnosis: a retrospective study of a male fertility preservation cohort. Hum Reprod 2023;38:359–370. doi: 10.1093/humrep/dead004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauz-Körholz C, Landman-Parker J, Balwierz W, Ammann RA, Anderson RA, Attarbaschi A, Bartelt JM, Beishuizen A, Boudjemaa S, Cepelova M. et al. Response-adapted omission of radiotherapy and comparison of consolidation chemotherapy in children and adolescents with intermediate-stage and advanced-stage classical Hodgkin lymphoma (EuroNet-PHL-C1): a titration study with an open-label, embedded, multinational, non-inferiority, randomised controlled trial. Lancet Oncol 2022;23:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meachem SJ, Nieschlag E, Simoni M.. Inhibin B in male reproduction: pathophysiology and clinical relevance. Eur J Endocrinol 2001;145:561–571. [DOI] [PubMed] [Google Scholar]
- Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril 2013;100:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles AE, Kelsey T, Wallace H.. A normative model of Inhibin B in young males. Maturitas 2015;81:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder RL, Font-Gonzalez A, Green DM, Loeffen EAH, Hudson MM, Loonen J, Yu R, Ginsberg JP, Mitchell RT, Byrne J. et al. ; PanCareLIFE Consortium. Fertility preservation for male patients with childhood, adolescent, and young adult cancer: recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2021;22:e57–e67. [DOI] [PubMed] [Google Scholar]
- Müller J. Disturbance of pubertal development after cancer treatment. Best Pract Res Clin Endocrinol Metab 2002;16:91–103. [DOI] [PubMed] [Google Scholar]
- Ortin TT, Shostak CA, Donaldson SS.. Gonadal status and reproductive function following treatment for Hodgkin’s disease in childhood: the Stanford experience. Int J Radiat Oncol Biol Phys 1990;19:873–880. [DOI] [PubMed] [Google Scholar]
- Pennisi AJ, Grushkin CM, Lieberman E.. Gonadal function in children with nephrosis treated with cyclophosphamide. Am J Dis Child 1975;129:315–318. [DOI] [PubMed] [Google Scholar]
- Pierik FH, Burdorf A, de Jong FH, Weber RF.. Inhibin B: a novel marker of spermatogenesis. Ann Med 2003;35:12–20. [DOI] [PubMed] [Google Scholar]
- Ragni G, Bestetti O, Santoro A, Viviani S, Di Pietro R, De Lauretis L.. Evaluation of semen and pituitary gonadotropin function in men with untreated Hodgkin’s disease. Fertil Steril 1985;43:927–930. [DOI] [PubMed] [Google Scholar]
- Raivio T, Saukkonen S, Jääskeläinen J, Komulainen J, Dunkel L.. Signaling between the pituitary gland and the testes: inverse relationship between serum FSH and inhibin B concentrations in boys in early puberty. Eur J Endocrinol 2000;142:150–156. [DOI] [PubMed] [Google Scholar]
- Romerius P, Ståhl O, Moëll C, Relander T, Cavallin-Ståhl E, Wiebe T, Giwercman YL, Giwercman A, Moe C, Wiebe T. et al. High risk of azoospermia in men treated for childhood cancer. Int J Androl 2010;34:69–76. [DOI] [PubMed] [Google Scholar]
- Rueffer U, Breuer K, Josting A, Lathan B, Sieber M, Manzke O, Grotenhermen F-J, Tesch H, Bredenfeld H, Koch P. et al. Male gonadal dysfunction in patients with Hodgkin’s disease prior to treatment. Ann Oncol 2001;12:1307–1311. [DOI] [PubMed] [Google Scholar]
- Santi D, Crépieux P, Reiter E, Spaggiari G, Brigante G, Casarini L, Rochira V, Simoni M.. Follicle-stimulating hormone (FSH) action on spermatogenesis: a focus on physiological and therapeutic roles. J Clin Med 2020;9:1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaapveld M, Aleman BMP, Eggermond AM, Van Janus CPM, Krol ADG, Maazen RWM, Van Der Roesink J, Raemaekers JMM, Boer JP, De Zijlstra JM. et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med 2015;373:2499–2511. [DOI] [PubMed] [Google Scholar]
- Schoor RA, Elhanbly S, Niederberger CS, Ross LS.. The role of testicular biopsy in the modern management of male infertility. J Urol 2002;167:197–200. [PubMed] [Google Scholar]
- Sherins RJ, Olweny CLM, Ziegler JL.. Gynecomastia and gonadal fysfunction in adolescent boys treated with combination chemotherapy for Hodgkin’s disease. N Engl J Med 1978;299:12–16. [DOI] [PubMed] [Google Scholar]
- Sieniawski M, Reineke T, Josting A, Nogova L, Behringer K, Halbsguth T, Fuchs M, Diehl V, Engert A.. Assessment of male fertility in patients with Hodgkin’s lymphoma treated in the German Hodgkin Study Group (GHSG) clinical trials. Ann Oncol 2008;19:1795–1801. [DOI] [PubMed] [Google Scholar]
- Stukenborg J-B, Jahnukainen K, Hutka M, Mitchell RT.. Cancer treatment in childhood and testicular function: the importance of the somatic environment. Endocr Connect 2018;7:R69–R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal R, Botchan A, Hauser R, Yogev L, Paz G, Yavetz H.. Follow-up of sperm concentration and motility in patients with lymphoma. Hum Reprod 2000;15:1985–1988. [DOI] [PubMed] [Google Scholar]
- van Casteren NJ, Dohle GR Ph D, Romijn JC, van Casteren NJ, Dohle GR, Romijn JC, de Muinck Keizer-Schrama SMPF, Weber RFA, van den Heuvel-Eibrink MM.. Semen cryopreservation in pubertal boys before gonadotoxic treatment and the role of endocrinologic evaluation in predicting sperm yield. Fertil Steril 2008;90:1119–1125. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ronckers CM, Leeuwen FE, van Moskowitz CS, Leisenring W, Armstrong GT, Vathaire F D, Hudson MM, Kuehni CE, Arnold MA. et al. ; International Consortium for Pooled Studies on Subsequent Malignancies after Childhood and Adolescent Cancer. Subsequent female breast cancer risk associated with anthracycline chemotherapy for childhood cancer. Nat Med 2023;29:2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbauer GF, Luetjens CM, Simoni M, Nieschlag E.. Physiology of testicular function. In: Nieschlag E, Behre HM, Nieschlag S (eds). Andrology: Male Reproductive Health and Dysfunction. Berlin Heidelberg: Springer, 2010, 11–59. [Google Scholar]
- Zaletel LZ, Bratanic N, Jereb B.. Gonadal function in patients treated for Hodgkin’s disease in childhood. Radiol Oncol 2010;44:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlie this article cannot be shared publicly because of privacy. Pseudonymized data will be shared on reasonable request for an ethically approved study protocol, after compiling a data-sharing agreement.