Abstract
Background and Aims
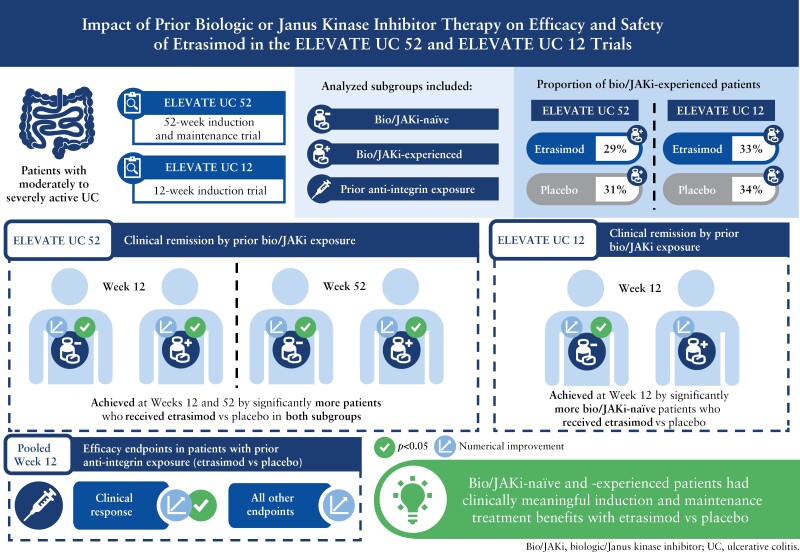
Etrasimod is an oral, once daily, selective, sphingosine 1-phosphate [S1P]1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis [UC]. This subgroup analysis evaluated the efficacy and safety of etrasimod 2 mg once daily vs placebo by prior biologic/Janus kinase inhibitor [bio/JAKi] exposure in ELEVATE UC 52 and ELEVATE UC 12.
Methods
Pre-defined efficacy endpoints were assessed at Weeks 12 and 52 in ELEVATE UC 52 and Week 12 in ELEVATE UC 12 in bio/JAKi-naïve and -experienced patients, and at Week 12 [pooled] based on prior advanced therapy exposure mechanism.
Results
In the ELEVATE UC 52 and ELEVATE UC 12 analysis populations, 80/274 [29.2%] and 74/222 [33.3%] patients receiving etrasimod and 42/135 [31.1%] and 38/112 [33.9%] patients receiving placebo, respectively, were bio/JAKi-experienced. In both bio/JAKi-naïve and -experienced patients, a significantly greater proportion receiving etrasimod vs placebo achieved clinical remission [p < 0.05] in ELEVATE UC 52 at Weeks 12 [naïve: 30.9% vs 9.7%; experienced: 17.5% vs 2.4%] and 52 [naïve: 36.6% vs 7.5%; experienced: 21.3% vs 4.8%]; in ELEVATE UC 12, this was observed only for bio/JAKi-naïve patients [naïve: 27.7% vs 16.2%, p = 0.033; experienced: 18.9% vs 13.2%, p = 0.349]. Similar patterns were observed for most efficacy endpoints. Among patients with prior anti-integrin exposure [N = 90], a significantly greater proportion achieved clinical response [54.1% vs 27.6%, p = 0.030], but not clinical remission [9.8% vs 3.4%, p = 0.248], with etrasimod vs placebo.
Conclusions
Bio/JAKi-naïve and -experienced patients had clinically meaningful induction and maintenance treatment benefits with etrasimod vs placebo.
ClinicalTrials.gov
Keywords: Ulcerative colitis, etrasimod, advanced therapies
Graphical Abstract
Graphical Abstract.
1. Introduction
Ulcerative colitis [UC] is a chronic, relapsing, inflammatory disease of the colon characterised by symptoms of rectal bleeding, diarrhoea, urgency, and tenesmus, which are associated with decreased quality of life.1,2 The goal of UC therapy is to achieve and maintain long-term clinical and endoscopic remission.3 Treatment of UC follows a stepwise approach, with medication selection being guided by the severity and extent of disease; in this approach, patients with mildly to moderately active UC are initially treated with 5-aminosalicylates [5-ASA] and/or corticosteroids [CS].1,2,4,5 For patients with moderately to severely active UC, treatment is stepped up to advanced therapies including parenterally administered biologic therapies, such as tumour necrosis factor [TNF] antagonists, anti-integrins, and interleukin inhibitors, or targeted synthetic small molecules, such as sphingosine 1-phosphate [S1P] receptor modulators and Janus kinase inhibitors [JAKi].1,4–10 Patients who do not respond or have lost response to one or more advanced UC therapies are less likely to respond to the subsequent advanced UC therapy.11,12 Therefore, understanding the efficacy and safety of advanced therapy as well as the characteristics of patients, both naïve and with prior exposure to advanced UC therapies, is of increasing importance.11,13
Etrasimod is an oral, once daily [QD], selective S1P1,4,5 receptor modulator for the treatment of moderately to severely active UC. The efficacy and safety of etrasimod 2 mg QD in patients with moderately to severely active UC were demonstrated in a phase 3 induction and maintenance study [ELEVATE UC 52; NCT03945188] and a phase 3 induction study [ELEVATE UC 12; NCT03996369].14 In ELEVATE UC 52 and ELEVATE UC 12, a significantly greater proportion of patients who received etrasimod achieved the primary efficacy endpoint of clinical remission vs placebo and all key secondary efficacy endpoints.14 Patients were eligible for inclusion if they had prior exposure to up to two biologics or one biologic and one JAKi. Patients with prior exposure to biologics and/or JAKi [bio/JAKi] made up 30% and 33% of patients in ELEVATE UC 52 and ELEVATE UC 12, respectively. In the overall population, induction and maintenance treatment with etrasimod was well tolerated.14
This analysis evaluated the efficacy and safety of etrasimod vs placebo in adults with UC, enrolled in ELEVATE UC 52 and ELEVATE UC 12, who were naïve to or had prior exposure to one or more advanced [bio/JAKi] UC therapies, including subgroups of patients who had prior exposure to an anti-integrin therapy or prior exposure to bio/JAKi therapy excluding anti-integrins.
2. Materials and Methods
2.1. Patients and study design
Efficacy and safety data from patients who participated in the phase 3 ELEVATE UC 52 and ELEVATE UC 12 clinical trials were included in this analysis. Full study design details have been described elsewhere.14 Briefly, ELEVATE UC 52 comprised a 12-week induction period followed by a 40-week maintenance period with a treat-through design. ELEVATE UC 12 comprised a 12-week induction period. Eligible patients were aged 16–80 years, with moderately to severely active UC (a modified Mayo score [MMS] of 4–9, with a centrally read endoscopic subscore [ES] ≥ 2 and rectal bleeding subscore [RBS] ≥ 1) and a documented history of inadequate response, loss of response, or intolerance to at least one approved UC therapy. This included prior exposure to up to two biologic therapies, or prior exposure to one biologic therapy and one JAKi. Patients were randomised 2:1 at baseline to etrasimod 2 mg QD or placebo QD, and were stratified according to prior exposure to bio/JAKi therapy, baseline CS use, and baseline disease activity [MMS of 4–6 or 7–9]. Background concomitant treatment with 5-ASA and/or CS was permitted provided patients were on a stable dose ≥ 2 weeks immediately prior to randomisation for 5-ASA and 4 weeks immediately prior to the screening endoscopy assessment for CS use. The washout period prior to randomisation was > 8 weeks for prior treatment with a biologic therapy and > 5 half-lives for prior treatment with a small molecule therapy. Investigators were directed to taper CS in ELEVATE UC 52 after the Week 12 assessment.
In this analysis, patients were analysed in nine non-mutually exclusive subgroups: bio/JAKi-naïve, bio/JAKi-experienced, prior exposure to one bio/JAKi, prior exposure to ≥ 2 bio/JAKi, any prior exposure to JAKi, prior exposure to JAKi only, any prior exposure to anti-integrin therapy, prior exposure to anti-integrin therapy only, and prior exposure to bio/JAKi therapy that was not an anti-integrin. The first four subgroups were pre-specified analyses in which efficacy endpoints are reported using data from the primary analysis population of patients with a baseline MMS of 5–9, consistent with the primary efficacy analysis.14 Data for the total population of patients with a baseline MMS of 4–9 are reported in the Supplementary Material. The latter two subgroups were post hoc analyses performed in a pooled population of patients with a baseline MMS of 4–9 from both ELEVATE UC 52 and ELEVATE UC 12, to maximise the number of patients available for analysis. The any prior exposure to JAKi subgroup included patients who had prior exposure to JAKi therapy, with or without prior exposure to biologics. The prior exposure to JAKi only subgroup included patients who had prior exposure to JAKi therapy but not biologics. The prior anti-integrin therapy only subgroup included patients who had prior exposure to exactly one anti-integrin therapy but not any other bio/JAKi therapies. The JAKi class included tofacitinib, upadacitinib, filgotinib, and other investigative therapies. The anti-integrin class included vedolizumab, etrolizumab, and other investigative anti-integrin therapies.
2.2. Efficacy and safety evaluations
Full details of the efficacy and safety evaluations performed in the overall ELEVATE UC clinical programme have been reported previously.14 In the primary analysis of ELEVATE UC 52 and ELEVATE UC 12, the primary efficacy endpoints were the achievement of clinical remission (stool frequency subscore [SFS] = 0 [or = 1 with a ≥ 1-point decrease from baseline] RBS = 0, and ES ≤ 1 [excluding friability]) assessed at Weeks 12 and 52 in ELEVATE UC 52 and at Week 12 in ELEVATE UC 12. Key secondary efficacy endpoints included the achievement of endoscopic improvement [ES ≤ 1], symptomatic remission (SFS = 0 [or = 1 with a ≥ 1-point decrease from baseline] and an RBS = 0), and endoscopic improvement–histological remission [EIHR; ES ≤ 1 with histological remission measured by a Geboes Index score < 2.0]. Clinical response [≥ 2-point and ≥ 30% decrease from baseline in MMS, and a ≥ 1-point decrease from baseline in RBS or an absolute RBS ≤ 1] was included as a pre-specified endpoint. The above efficacy endpoints were assessed at Weeks 12 [both trials] and 52 [ELEVATE UC 52 only]. CS-free clinical remission [remission at Week 52 with no CS use for at least the past 12 study weeks immediately before Week 52] and sustained clinical remission [clinical remission at both Weeks 12 and 52] were assessed at Week 52 of ELEVATE UC 52 only. Due to small sample sizes in the subgroups of prior exposure to anti-integrin therapy and prior exposure to bio/JAKi therapy that was not an anti-integrin, these analyses are reported up to Week 12. Additionally, mean changes from baseline in SFS, RBS, MMS, and composite RBS + SFS were assessed at Week 12 for patients with prior exposure to anti-integrin therapy only.
Additionally, changes from baseline over time per study visit were analysed for symptomatic response and symptomatic remission separately from Weeks 2 to 52 for ELEVATE UC 52 and from Weeks 2 to 12 in ELEVATE UC 12 among the bio/JAKi-naïve and -experienced patients. Symptomatic response was defined as a decrease from baseline ≥ 30% in composite RBS and SFS.
Safety was assessed in patients who received etrasimod or placebo up to Week 52 in ELEVATE UC 52 and up to Week 12 in ELEVATE UC 12 trials. Pooled data from ELEVATE UC 52 and ELEVATE UC 12 for treatment-emergent adverse events [TEAEs] recorded throughout the trials up to 30 days after the last administration of study treatment are reported for bio/JAKi-naïve and -experienced patients in this analysis. Serious adverse events [SAEs] were defined as any events that resulted in death, were life-threatening, required patient hospitalisation or prolongation of existing hospitalisation, resulted in a persistent or significant disability or incapacity, resulted in a congenital anomaly or birth defect, or were deemed medically significant.
Additionally, the ELEVATE UC clinical programme identified adverse events of special interest [AESI] deemed mechanistically associated with S1P modulation. Evaluation of potential AESIs involved the review of a subset of reported TEAEs designated Targeted Medical Events and relevant laboratory parameters. These events underwent medical review to determine which qualified as AESIs. Categories of AESI included cardiovascular events, macular oedema, pulmonary disorders, infections [such as herpes zoster], liver injury, posterior reversible encephalopathy syndrome, and malignancies.
2.3. Statistical analyses
Efficacy data were evaluated as part of an a priori analysis in the statistical plans for ELEVATE UC 52 and ELEVATE UC 12. Analyses were based on treatment assigned in the full analysis set or full analysis set with a baseline MMS of 5–9. Safety analyses were based on treatment received in the safety set. Both the full analysis and safety sets included all randomised patients who received ≥ 1 dose of study treatment in the ELEVATE UC clinical programme.
Week 12 efficacy data were analysed separately for the individual clinical trials, ELEVATE UC 52 and ELEVATE UC 12, unless otherwise specified. Week 52 efficacy data are from ELEVATE UC 52 only. For analyses of all binary efficacy endpoints at a visit, treatment comparisons (treatment differences and 95% confidence intervals [CIs]) and two-sided p-values were obtained using the Cochran–Mantel–Haenszel [CMH] method assuming common proportion difference within each subgroup, adjusting to prior bio/JAKi therapy exposure [if applicable], baseline CS use, and baseline disease activity [MMS of 4–6 or 7–9]. These adjustments were also applied [if applicable] to the pooled analyses. Missing response information was considered as nonresponse. There are no hypothesis tests performed in this post hoc analysis; p-values are reported for descriptive purposes, without adjustment for multiple comparisons.
Additional interaction analyses were performed to assess potential differences in treatment effects [etrasimod vs placebo] between bio/JAKi-naïve and -experienced patients for the primary and key secondary binary efficacy endpoints at Week 12 and Week 52. Three different testing approaches were used to evaluate if observed differences in treatment effect were supported by data across different modelling assumptions. The first method generated adjusted treatment difference using the adjusted CMH method described above. The second method evaluated the absolute difference in treatment effects using the unadjusted crude estimates of proportion differences. Both methods assumed asymptotic normal distribution of the absolute difference. The third method used logistic regression to model endpoints on the fixed effects of treatment, prior bio/JAKi exposure, and treatment by prior bio/JAKi subgroup interaction, adjusted for the same stratification factors as the CMH method. In this method, the interaction effect was assessed using the Wald test statistics of the logistic regression. Two-sided p-values were generated for all three methods; a p value < 0.05 was considered suggestive that an interaction was present. Due to the exploratory nature of these analyses, p-values were not adjusted for multiple comparisons.
For the safety data, numbers, percentages, and incidence rates [per 100 patient-years] of patients with TEAEs, SAEs, and AESI, were evaluated.
2.4. Ethics approval
All studies were registered with ClinicalTrials.gov and were conducted in compliance with the Declaration of Helsinki and were approved by the Institutional Review Boards at each investigational centre participating in the studies. All patients provided written informed consent.
3. Results
3.1. Patient disposition and baseline characteristics
The demographics and clinical characteristics stratified by prior bio/JAKi exposure [naïve, experienced, 1, 2] for patients with a baseline MMS of 4–9 are summarised in Table 1 and separately for patients with a baseline MMS of 5–9 in Supplementary Table S1. In the ELEVATE UC 52 and ELEVATE UC 12 primary analysis populations [baseline MMS of 5–9], 80/274 [29.2%] and 74/222 [33.3%] patients who received etrasimod and 42/135 [31.1%] and 38/112 [33.9%] patients who received placebo, respectively, were bio/JAKi-experienced.
Table 1.
Baseline demographics and clinical characteristics of patients in ELEVATE UC 52 and ELEVATE UC 12, stratified by prior bio/JAKi exposure [baseline MMS 4–9].
| ELEVATE UC 52 | ELEVATE UC 12 | |||
|---|---|---|---|---|
| Placebo QD [N = 144] |
Etrasimod 2 mg QD [N = 289] |
Placebo QD [N = 116] |
Etrasimod 2 mg QD [N = 238] |
|
| N1 | ||||
| Naïve | 99 | 205 | 77 | 159 |
| Experienced | 45 | 84 | 39 | 79 |
| 1 | 25 | 44 | 20 | 36 |
| ≥ 2a | 20 | 40 | 19 | 43 |
| Age, mean [SD], years | ||||
| Naïve | 38.6 [14.6] | 39.7 [13.7] | 42.0 [14.1] | 40.9 [13.9] |
| Experienced | 39.8 [12.8] | 44.8 [14.1] | 37.3 [11.1] | 39.3 [12.5] |
| 1 | 38.3 [12.9] | 45.6 [14.9] | 39.7 [12.3] | 41.0 [14.0] |
| ≥ 2 | 41.6 [12.8] | 43.8 [13.3] | 34.8 [9.3] | 37.8 [11.2] |
| Female, n [%] | ||||
| Naïve | 43 [43.4] | 102 [49.8] | 32 [41.6] | 69 [43.4] |
| Experienced | 13 [28.9] | 35 [41.7] | 11 [28.2] | 34 [43.0] |
| 1 | 5 [20.0] | 18 [40.9] | 4 [20.0] | 16 [44.4] |
| ≥ 2 | 8 [40.0] | 17 [42.5] | 7 [36.8] | 18 [41.9] |
| Extent of UC, n [%]b | ||||
| Pancolitis | ||||
| Naïve | 26 [26.3] | 59 [29.1]a | 23 [29.9] | 45 [28.3] |
| Experienced | 21 [47.7]a | 34 [40.5] | 18 [46.2] | 32 [40.5] |
| 1 | 10 [40.0]a | 13 [29.5] | 8 [40.0] | 19 [52.8] |
| ≥ 2 | 11 [55.0] | 21 [52.5] | 10 [52.6] | 13 [30.2] |
| Left-sided colitis | ||||
| Naïve | 68 [68.7] | 128 [63.1]a | 43 [55.8] | 105 [66.0] |
| Experienced | 22 [50.0]a | 44 [52.4] | 20 [51.3] | 41 [51.9] |
| 1 | 13 [54.2]a | 27 [61.4] | 12 [60.0] | 16 [44.4] |
| ≥ 2 | 9 [45.0] | 17 [42.5] | 8 [42.1] | 25 [58.1] |
| Proctitis [central read] | ||||
| Naïve | 8 [8.1] | 21 [10.2] | 12 [15.6] | 10 [6.3] |
| Experienced | 1 [2.2] | 6 [7.1] | 1 [2.6] | 5 [6.3] |
| 1 | 1 [4.0] | 4 [9.1] | 0 | 1 [2.8] |
| ≥ 2 | 0 | 2 [5.0] | 1 [5.3] | 4 [9.3] |
| Duration of UC, mean [SD], years | ||||
| Naïve | 4.7 [4.6] | 5.5 [6.3] | 6.6 [6.7] | 6.5 [6.6] |
| Experienced | 8.5 [6.5] | 12.2 [9.6] | 9.8 [8.1] | 8.8 [6.5] |
| 1 | 8.3 [7.3] | 12.9 [10.3] | 8.2 [6.3] | 8.2 [6.5] |
| ≥ 2 | 8.8 [5.3] | 11.5 [8.9] | 11.4 [9.6] | 9.3 [6.4] |
| Baseline MMS, mean [SD] | ||||
| Naïve | 6.6 [1.2] | 6.7 [1.2] | 6.5 [1.2] | 6.5 [1.2] |
| Experienced | 6.8 [1.1] | 6.8 [1.2] | 6.9 [1.2] | 6.7 [1.2] |
| 1 | 6.8 [1.0] | 6.8 [1.1] | 7.2 [1.1] | 6.7 [1.2] |
| ≥ 2 | 6.8 [1.3] | 6.8 [1.3] | 6.6 [1.2] | 6.6 [1.2] |
| Baseline MMS 5–9, n [%] | ||||
| Naïve | 93 [93.9] | 194 [94.6] | 74 [96.1] | 148 [93.1] |
| Experienced | 42 [93.3] | 80 [95.2] | 38 [97.4] | 74 [93.7] |
| 1 | 24 [96.0] | 41 [93.2] | 20 [100.0] | 34 [94.4] |
| ≥ 2 | 18 [90.0] | 39 [97.5] | 18 [94.7] | 40 [93.0] |
| Baseline MMS 7–9, n [%] | ||||
| Naïve | 55 [55.6] | 120 [58.5] | 40 [51.9] | 82 [51.6] |
| Experienced | 32 [71.1] | 56 [66.7] | 23 [59.0] | 47 [59.5] |
| 1 | 18 [72.0] | 32 [72.7] | 15 [75.0] | 22 [61.1] |
| ≥ 2 | 14 [70.0] | 24 [60.0] | 8 [42.1] | 25 [58.1] |
| Baseline CS use, n [%] | ||||
| Naïve | 28 [28.3] | 65 [31.7] | 19 [24.7] | 37 [23.3] |
| Experienced | 14 [31.1] | 28 [33.3] | 15 [38.5] | 28 [35.4] |
| 1 | 6 [24.0] | 14 [31.8] | 5 [25.0] | 10 [27.8] |
| ≥ 2 | 8 [40.0] | 14 [35.0] | 10 [52.6] | 18 [41.9] |
| Any prior anti-integrin exposure, n [%] | ||||
| Experienced | 19 [42.2] | 28 [33.3] | 10 [25.6] | 33 [41.8] |
| Prior anti-integrin exposure only, n [%]c | ||||
| Experienced | 6 [13.3] | 9 [10.7] | 1 [2.6] | 8 [10.1] |
| Any prior JAKi exposure, n [%] | ||||
| Experienced | 9 [20.0] | 20 [23.8] | 9 [23.1] | 15 [19.0] |
bio/JAKi, biologic/Janus kinase inhibitor; CS, corticosteroid; MMS, modified Mayo score; N, number of patients by treatment group in the full analysis set; N1, number of patients by treatment and prior bio/JAKi exposure subgroups; n, number of patients with each specific outcome; SD, standard deviation; UC, ulcerative colitis; QD, once daily.
aProtocol deviation of > 2 prior bio/JAKi therapies was evident for 12 patients who received etrasimod and three patients who received placebo in ELEVATE UC 52, and four patients each in the etrasimod and placebo treatment groups in ELEVATE UC 12.
bIn ELEVATE UC 52, two bio/JAKi-naïve patients who received etrasimod and one patient with prior exposure to one bio/JAKi who received placebo had missing Extent of Disease data at baseline. Percentages were calculated as a proportion of patients with observed Extent of Disease at baseline.
cIncluded patients who had prior exposure to only one prior anti-integrin and no prior exposure to other bio/JAKi; the majority of patients who received only anti-integrin therapy received only one therapy.
In the pooled population of patients with a baseline MMS of 4–9 from both trials, 61/527 [11.6%] and 29/260 [11.2%] patients who received etrasimod and placebo, respectively, had prior exposure to anti-integrin therapy. Of these patients, 36 [59.0%] and 20 [69.0%] had prior failure of TNF antagonist therapy. Among bio/JAKi-naïve patients in this pooled population [baseline MMS of 4–9], 72/364 [19.8%] patients who received etrasimod and 40/176 [22.7%] patients who received placebo had prior failure of oral 5-ASA treatment only. In bio/JAKi-experienced patients in the pooled population, prior failure of only oral 5-ASA occurred in 1/163 [0.6%] patient who received etrasimod and 1/84 [1.2%] patient who received placebo; prior failure of TNF antagonist therapy occurred in 101/163 [62.0%] and 54/84 [64.3%] patients, respectively; prior failure of TNF antagonist therapy or anti-integrin therapy occurred in 110/163 [67.5%] and 59/84 [70.2%] patients, respectively.
Generally, across the etrasimod and placebo treatment groups, greater proportions of bio/JAKi-experienced patients had pancolitis, a baseline MMS of 7–9 and a longer mean duration of UC compared with bio/JAKi-naïve patients [Table 1].
There were noticeable similarities and differences in demographics and baseline characteristics between patients with exposure to one vs ≥ 2 bio/JAKi [Table 1].
3.2. Efficacy
3.2.1. Efficacy endpoints by prior bio/JAKi exposure
In ELEVATE UC 52, among both bio/JAKi-naïve and -experienced patients, significantly greater proportions of patients who received etrasimod achieved clinical remission, clinical response, and endoscopic improvement at Weeks 12 and 52, and CS-free clinical remission at Week 52 vs placebo [Figure 1]. Significantly greater proportions of bio/JAKi-naïve patients who received etrasimod met the endpoints of symptomatic remission, EIHR, and sustained clinical remission at Weeks 12 and/or 52 vs placebo. Among bio/JAKi-experienced patients, the difference vs placebo was not significant [p > 0.05] for symptomatic remission and sustained clinical remission [Table 2].
Figure 1.
[A] Clinical remission,a [B] clinical response,b [C] endoscopic improvement,c and [D] CS-free clinical remissiond in ELEVATE UC 52 and ELEVATE UC 12, stratified by prior bio/JAKi exposure [baseline MMS 5–9]. Consistent with the statistical analysis plan and primary efficacy analyses, subgroup efficacy analyses were performed in patients with a baseline MMS of 5–9. Treatment comparisons [treatment differences and 95% CIs] and two-sided p-values were obtained using the CMH method assuming common proportion difference within each subgroup, adjusting to prior bio/JAKi therapy exposure [if applicable], baseline CS use, and baseline disease activity [MMS of 4–6 or 7–9]. Patients missing an assessment at the specified analysis visit were considered nonresponders; p < 0.05 are highlighted in bold. aClinical remission was defined as an SFS = 0 [or = 1 with a ≥ 1-point decrease from baseline], RBS = 0, and ES ≤ 1 [excluding friability]. bClinical response was defined as a ≥ 2-point and ≥ 30% decrease from baseline in MMS and a ≥ 1-point decrease from baseline in RBS or an absolute RBS ≤ 1. cEndoscopic improvement was defined as an ES ≤ 1. dCS-free clinical remission was defined as remission at Week 52 and had not been receiving CS for ≥ 12 study weeks before Week 52. Δ, adjusted percentage difference for etrasimod minus placebo; bio/JAKi, biologic/Janus kinase inhibitor; CI, confidence interval; CMH, Cochran–Mantel–Haenszel; CS, corticosteroid; ES, endoscopic subscore; MMS, modified Mayo score; RBS, rectal bleeding subscore; SFS, stool frequency subscore; QD, once daily; UC, ulcerative colitis.
Table 2.
Selected efficacy endpoints in ELEVATE UC 52 and ELEVATE UC 12, stratified by prior bio/JAKi exposure [baseline MMS 5–9].
| Endpoint, n [%] by prior bio/JAKi exposure | ELEVATE UC 52 | ELEVATE UC 12 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 12 | Week 52 | Week 12 | |||||||
| Placebo QD [N = 135] |
Etrasimod 2 mg QD [N = 274] |
Percentage difference [95% CI] p value |
Placebo QD [N = 135] |
Etrasimod 2 mg QD [N = 274] |
Percentage difference [95% CI] p value |
Placebo QD [N = 112] |
Etrasimod 2 mg QD [N = 222] |
Percentage difference [95% CI] p value |
|
| N1 | |||||||||
| Naïve | 93 | 194 | N/A | 93 | 194 | N/A | 74 | 148 | N/A |
| Experienced | 42 | 80 | N/A | 42 | 80 | N/A | 38 | 74 | N/A |
| Symptomatic remissiona | |||||||||
| Naïve | 22 [23.7] | 101 [52.1] | 28.8 [17.59, 40.06] <0.001 |
19 [20.4] | 97 [50.0] | 29.0 [18.03, 39.91] <0.001 |
23 [31.1] | 73 [49.3] | 18.6 [5.21, 32.01] 0.006 |
| Experienced | 7 [16.7] | 25 [31.3] | 13.3 [− 1.52, 28.17] 0.078 |
6 [14.3] | 22 [27.5] | 11.2 [− 3.16, 25.63] 0.126 |
10 [26.3] | 31 [41.9] | 17.2 [− 0.30, 34.76] 0.054 |
| EIHRb | |||||||||
| Naïve | 6 [6.5] | 47 [24.2] | 17.6 [9.50, 25.73] <0.001 |
10 [10.8] | 55 [28.4] | 18.7 [10.04, 27.30] <0.001 |
8 [10.8] | 28 [18.9] | 8.5 [− 0.74, 17.72] 0.071 |
| Experienced | 0 | 11 [13.8] | 13.7 [5.90, 21.55] <0.001 |
1 [2.4] | 18 [22.5] | 19.1 [8.66, 29.51] <0.001 |
2 [5.3] | 8 [10.8] | 6.1 [− 3.91, 16.07] 0.233 |
| Sustained clinical remissionc | |||||||||
| Naïve | N/A | N/A | N/A | 2 [2.2] | 41 [21.1] | 19.3 [12.69, 25.92] <0.001 |
N/A | N/A | N/A |
| Experienced | N/A | N/A | N/A | 1 [2.4] | 8 [10.0] | 7.5 [− 1.21, 16.13] 0.092 |
N/A | N/A | N/A |
Consistent with the statistical analysis plan and primary efficacy analyses, subgroup efficacy analyses were performed in patients with a baseline MMS of 5–9. Treatment comparisons [treatment differences and 95% CIs] and two-sided p-values were obtained using the CMH method assuming common proportion differences within each subgroup, adjusting to prior bio/JAKi therapy exposure [if applicable], baseline CS use, and baseline disease activity [MMS of 4–6 or 7–9]. Patients missing an assessment at the specified analysis visit were considered nonresponders; p < 0.05 are highlighted in bold.
Bio/JAKi, biologic/Janus kinase inhibitor; CI, confidence interval; CMH; Cochran–Mantel–Haenszel; CS, corticosteroid; EIHR, endoscopic improvement–histological remission; ES, endoscopic subscore; MMS, modified Mayo score; N, number of patients by treatment group in the full analysis set; N1, number of patients by treatment and prior bio/JAKi exposure subgroups; n, number of patients with each specific outcome; N/A, not applicable; RBS, rectal bleeding subscore; SFS, stool frequency subscore; SD, standard deviation; QD, once daily; UC, ulcerative colitis.
aSymptomatic remission was defined as an SFS = 0 [or = 1 with a ≥ 1-point decrease from baseline] and an RBS = 0.
bEIHR was defined as an ES ≤ 1 [excluding friability] with histological remission as measured by a Geboes Index score < 2.0.
cSustained clinical remission was defined as clinical remission at both Weeks 12 and 52.
In ELEVATE UC 12, significantly greater proportions of bio/JAKi-naïve patients who received etrasimod achieved clinical remission, clinical response, endoscopic improvement, and symptomatic remission at Week 12 vs placebo, and significantly greater proportions of bio/JAKi-experienced patients achieved clinical response at Week 12 vs placebo [Figure 1 and Table 2].
In the comparison of patients with prior exposure to one and ≥ 2 bio/JAKi, significantly greater proportions of patients with prior exposure to one bio/JAKi who received etrasimod achieved clinical remission, clinical response, endoscopic improvement, and CS-free clinical remission vs placebo at Weeks 12 and/or 52 in ELEVATE UC 52 [Supplementary Figure S1]; however, numerical improvements vs placebo were not significant among patients with prior exposure to ≥ 2 bio/JAKi. Other efficacy endpoints in these subgroups showed similar trends [Supplementary Table S2]. In ELEVATE UC 12, only patients with prior exposure to one bio/JAKi who received etrasimod had significant improvements in clinical remission vs placebo at Week 12 [Supplementary Figure S1] and in symptomatic remission at Week 12 [Supplementary Table S2]. In both studies, the treatment differences in patients who received etrasimod vs placebo who achieved clinical remission were numerically similar among those who were bio/JAKi-naïve and those with prior exposure to one bio/JAKi, and numerically higher in both these groups than among patients with prior exposure to ≥ 2 bio/JAKi [Figure 1 and Supplementary Figure S1].
At Week 12, clinical remission was achieved by a significantly greater proportion of patients who received etrasimod vs placebo in the ELEVATE UC 12 prior exposure to JAKi only subgroup; a numerically greater proportion of patients achieved clinical remission in the ELEVATE UC 12 any prior exposure to JAKi subgroup and in both subgroups in ELEVATE UC 52 [Supplementary Figure S2]. In both subgroups in ELEVATE UC 52 and the prior exposure to JAKi only subgroup in ELEVATE UC 12, a numerically greater proportion of patients who received etrasimod vs placebo achieved endoscopic improvement at Week 12; results for clinical remission, endoscopic improvement, and CS-free clinical remission at Week 52 were inconsistent [Supplementary Figure S2].
Efficacy results among patients with baseline MMS of 4–9 were generally consistent with those reported among patients with baseline MMS of 5–9, with significant differences for all endpoints vs placebo in bio/JAKi-naïve patients at Weeks 12 and/or 52, and a less consistent treatment effect in the bio/JAKi-experienced subgroup [Supplementary Figure S3 and Supplementary Table S3].
3.2.2. Efficacy endpoints by prior anti-integrin exposure
Among patients with any prior anti-integrin exposure pooled from ELEVATE UC 52 and ELEVATE UC 12, a significantly greater proportion of those who received etrasimod [n = 61] vs placebo [n = 29] achieved clinical response at Week 12, and a numerically greater proportion achieved all efficacy endpoints [Figure 2]. These treatment differences were generally consistent with the improvements observed among patients with prior exposure to bio/JAKi therapies that were not anti-integrin therapy, with the exception of clinical remission and EIHR [Figure 2]. Although limited by the small sample size, mean changes from baseline in SFS, RBS, MMS, and composite RBS + SFS for Week 12 data pooled from ELEVATE UC 52 and ELEVATE UC 12 were generally similar between patients who received etrasimod vs placebo who had prior exposure to anti-integrin therapy only [Supplementary Figure S4].
Figure 2.
Efficacy endpoints at Week 12 in ELEVATE UC 52 and ELEVATE UC 12 [pooled] in patients with prior exposure to anti-integrin therapy and prior exposure to bio/JAKi therapy excluding anti-integrins [baseline MMS 4–9]. This is a subgroup analysis of the pooled full analysis set of patients with a baseline MMS score of 4–9 in ELEVATE UC 52 and ELEVATE UC 12. Treatment comparisons [treatment differences and 95% CIs] and two-sided p-values were obtained using the CMH method assuming common proportion difference with the subgroup, adjusting to baseline CS use, baseline disease activity [MMS of 4–6 or 7–9], and study stratification [ELEVATE UC 52 or ELEVATE UC 12]. Patients missing an assessment at the specified analysis visit were considered nonresponders; p < 0.05 are highlighted in bold. The anti-integrin class included vedolizumab, etrolizumab, and other investigative anti-integrin therapies. Patients with prior anti-integrin exposure may also have had prior exposure to other bio/JAKi therapies. aClinical remission was defined as an SFS = 0 [or = 1 with a ≥ 1-point decrease from baseline], RBS = 0, and ES ≤ 1 [excluding friability]. bClinical response was defined as a ≥ 2-point and ≥ 30% decrease from baseline in MMS, and a ≥ 1-point decrease from baseline in RBS or an absolute RBS ≤ 1. cEndoscopic improvement was defined as an ES ≤ 1. dEIHR was defined as an ES ≤ 1 with histological remission as measured by a Geboes Index score < 2.0. eSymptomatic remission was defined as an SFS = 0 [or = 1 with a ≥ 1-point decrease from baseline] and an RBS = 0. fSymptomatic response was defined as ≥ 30% decrease from baseline in composite RB and SF subscores. bio/JAKi, biologic/Janus kinase inhibitor; CI, confidence interval; CMH; Cochran–Mantel–Haenszel; CS, corticosteroid; EIHR, endoscopic improvement–histological remission; ES, endoscopic subscore; MMS, modified Mayo score; QD, once daily; RBS, rectal bleeding subscore; SFS, stool frequency subscore; SD, standard deviation; UC, ulcerative colitis.
3.2.3. Interaction analyses
At Week 52 in ELEVATE UC 52, all three interaction methods show that an absolute difference in treatment effects between bio/JAKi-naïve and -experienced patients may exist for the sustained clinical remission endpoint [p < 0.05; Supplementary Table S4]. For symptomatic remission at Week 52 in ELEVATE UC 52, absolute difference in treatment effects was only demonstrated by the logistic regression method, although p-values were trending towards this for the other two methods [p < 0.1]. Other endpoints that trended towards the 5% threshold were clinical remission and CS-free clinical remission at Week 52 in ELEVATE UC 52 and symptomatic remission at Week 12 in ELEVATE UC 52. None of the endpoints in ELEVATE UC 12 met the 5% threshold [Supplementary Table S4].
3.2.4. Symptomatic response and remission over time
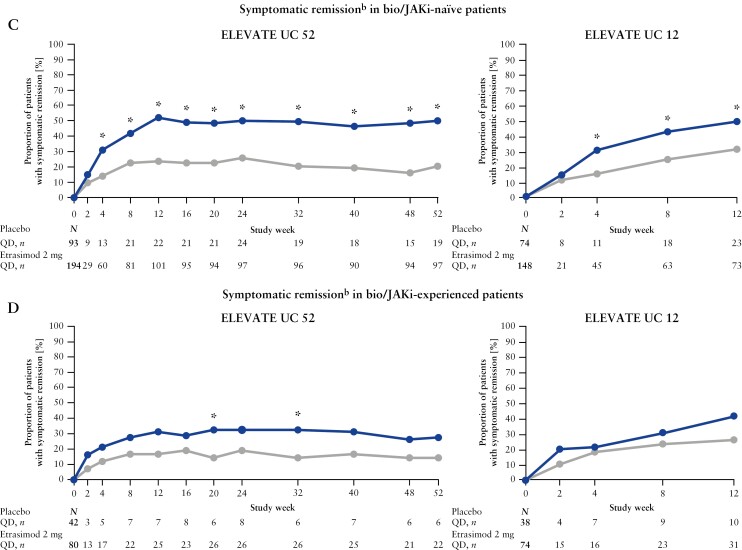
A greater proportion of bio/JAKi-naïve patients who received etrasimod vs placebo achieved significantly improved symptomatic response from Week 4 in ELEVATE UC 52 [63.9% vs 43.0%, p < 0.001] and from Week 2 in ELEVATE UC 12 [40.5% vs 24.3%, p < 0.01; Figure 3A]. In bio/JAKi-experienced patients, a greater proportion of those who received etrasimod vs placebo achieved significantly improved symptomatic response from Week 24 in ELEVATE UC 52 [43.8% vs 23.8%, p = 0.04] and at Week 12 in ELEVATE UC 12 [60.8% vs 42.1%, p = 0.04; Figure 3B].
Figure 3.
[A and B] Symptomatic responsea and [C and D] symptomatic remissionb over time in ELEVATE UC 52 and ELEVATE UC 12, stratified by prior bio/JAKi exposure [baseline MMS 5–9]; *p < 0.05. Consistent with the statistical analysis plan and the primary efficacy analyses, subgroup efficacy analyses were performed in patients with a baseline MMS of 5–9. Treatment comparisons [treatment difference and 95% CIs] and two-sided p-values were obtained using the CMH method assuming common proportion difference within each subgroup, adjusting to prior bio/JAKi therapy [if applicable], baseline CS use, and baseline disease activity [MMS of 4–6 or 7–9]. Patients missing an assessment at the specified analysis visit were considered nonresponders; p < 0.05 are highlighted in bold. aSymptomatic response was defined as a decrease from baseline ≥ 30% in composite RBS and SFS. bSymptomatic remission was defined as SFS = 0 [or = 1 with a ≥ 1-point decrease from baseline] and an RBS = 0. bio/JAKi, biologic/Janus kinase inhibitor; CI, confidence interval; CMH; Cochran–Mantel–Haenszel; CS, corticosteroid; MMS, modified Mayo score; N, total number of patients; n, number of patients with evaluable data within each category; RBS, rectal bleeding subscore; SFS, stool frequency subscore; QD, once daily; UC, ulcerative colitis.
A greater proportion of bio/JAKi-naïve patients who received etrasimod vs placebo achieved significantly improved symptomatic remission from Week 4 in both ELEVATE UC 52 [30.9% vs 14.0%, p < 0.001] and ELEVATE UC 12 [30.4% vs 14.9%, p < 0.001; Figure 3C]. In bio/JAKi-experienced patients, a greater proportion of those who received etrasimod vs placebo achieved significantly improved symptomatic remission from Week 20 in ELEVATE UC 52 [32.5 vs 14.3, p = 0.03]; no significant improvement was observed in ELEVATE UC 12 [Figure 3D].
3.3. Safety
Safety data for the pooled population of patients in ELEVATE UC 52 and ELEVATE UC 12 stratified by prior bio/JAKi exposure are summarised in Table 3. Among patients who received etrasimod, TEAEs were reported in 218/364 [59.9%] bio/JAKi-naïve patients and 100/163 [61.3%] bio/JAKi-experienced patients. Among those who received placebo, TEAEs were reported in 86/176 [48.9%] bio/JAKi-naïve patients and 49/84 [58.3%] bio/JAKi-experienced patients. The most common TEAEs by preferred term reported in both bio/JAKi-naïve and -experienced patients who received etrasimod were ‘Headache’ and ‘Colitis ulcerative’ [including UC worsening/flares].
Table 3.
Summary of safety in ELEVATE UC 52 and ELEVATE UC 12, stratified by prior bio/JAKi exposure [baseline MMS 4–9; pooled].
| Patients, n [%] [IR, per 100 PY] | ELEVATE UC 52 + ELEVATE UC 12 | |||
|---|---|---|---|---|
| Bio/JAKi-naïve | Bio/JAKi-experienced | |||
| Placebo QD [N = 176] |
Etrasimod 2 mg QD [N = 364] |
Placebo QD [N = 84] |
Etrasimod 2 mg QD [N = 163] |
|
| Any TEAEs | 86 [48.9] | 218 [59.9] | 49 [58.3] | 100 [61.3] |
| Any SAEs | 9 [5.1] [12.31] | 16 [4.4] [8.23] | 2 [2.4] [6.58] | 10 [6.1] [13.36] |
| Any AEs leading to discontinuation | 3 [1.7] [3.97] | 17 [4.7] [8.56] | 5 [6.0] [16.56] | 8 [4.9] [10.53] |
| AEs leading to death | 0 | 0 | 0 | 0 |
| Most common TEAEsa | ||||
| Headache | 6 [3.4] [8.12] | 23 [6.3] [12.26] | 3 [3.6] [9.98] | 12 [7.4] [16.56] |
| Colitis ulcerativeb | 9 [5.1] [12.12] | 18 [4.9] [9.15] | 5 [6.0] [16.95] | 13 [8.0] [17.48] |
| Pyrexia | 3 [1.7] [4.00] | 16 [4.4] [8.24] | 6 [7.1] [20.28] | 6 [3.7] [7.97] |
| Nausea | 2 [1.1] [2.67] | 15 [4.1] [7.70] | 2 [2.4] [6.61] | 4 [2.5] [5.32] |
| Dizziness | 0 | 12 [3.3] [6.19] | 1 [1.2] [3.27] | 6 [3.7] [8.07] |
| Alanine aminotransferase increased | 1 [0.6] [1.32] | 10 [2.7] [5.09] | 1 [1.2] [3.28] | 1 [0.6] [1.30] |
| Arthralgia | 5 [2.8] [6.79] | 9 [2.5] [4.58] | 1 [1.2] [3.31] | 8 [4.9] [10.74] |
| Infections [all] | 32 [18.2] [51.43] | 63 [17.3] [36.08] | 14 [16.7] [52.27] | 36 [22.1] [54.40] |
| Serious infections | 4 [2.3] [5.42] | 3 [0.8] [1.51] | 1 [1.2] [3.28] | 0 |
| Herpes zoster | 1 [0.6] [1.32] | 1 [0.3] [0.50] | 1 [1.2] [3.28] | 1 [0.6] [1.30] |
| Opportunistic infectionsc | 0 | 1 [0.3] [0.50] | 1 [1.2] [3.28] | 0 |
| AESId | ||||
| Bradycardiae | 0 | 2 [0.5] [1.00] | 0 | 0 |
| Sinus bradycardiae | 0 | 2 [0.5] [1.00] | 0 | 0 |
| AV block, 1st degree | 0 | 2 [0.5] [1.01] | 0 | 0 |
| AV block, 2nd degree [Mobitz I] | 0 | 1 [0.3] [0.50] | 0 | 0 |
| Hypertensionf | 2 [1.1] [2.65] | 9 [2.5] [4.56] | 0 | 4 [2.5] [5.33] |
| Macular oedema | 0 | 0 | 0 | 1 [0.6] [1.30] |
For AEs with 0 patients with events, percentage values, and IRs are also 0 and so are not displayed.
AE, adverse event; AESI, adverse events of special interest; AV, atrioventricular; bio/JAKi, biologic/Janus kinase inhibitor; IR, incidence rate; MedDRA, Medical Dictionary for Regulatory Activities; N, total number of patients; n, number of patients with evaluable data within each category; PY, patient-years; QD, once daily; SAE, serious adverse event; TEAE, treatment-emergent adverse event; UC, ulcerative colitis.
aThe most common TEAEs included above are the five most frequently occurring TEAEs among patients who received etrasimod, per subgroup. Common TEAEs were defined as those reported > 1% patients [etrasimod treatment group] and with a higher IR in the etrasimod treatment group than in the placebo group, in the overall population.
b‘Colitis ulcerative’ includes UC worsening/UC flares.
cBased on MedDRA Standardised Query for Opportunistic infections [narrow definition], MedDRA version 24.1. Included ‘Cytomegalovirus infection’ for the etrasimod group and ‘Tuberculosis’ for the placebo group.
dAESIs are sponsor-designated events of interest, defined as a subset of TEAEs that met the criteria of the sponsor’s medical review.
eFive additional cases of ‘Bradycardia’/‘Sinus bradycardia’ were reported by study investigators; however, these did not meet the AESI review criteria.
fIncluded ‘Hypertension’, ‘Essential hypertension’, ‘Hypertensive crisis’, and ‘Blood pressure increased’.
SAEs were reported in 16/364 [4.4%] and 9/176 [5.1%] bio/JAKi-naïve patients who received etrasimod and placebo, respectively. Corresponding values for bio/JAKi-experienced patients were 10/163 [6.1%] and 2/84 [2.4%], respectively. In the placebo treatment group, numerically lower proportions of bio/JAKi-naïve patients reported adverse events leading to discontinuation vs bio/JAKi-experienced patients [Table 3]. No deaths occurred in any subgroup. The proportions of patients with infections [including herpes zoster] were similar among bio/JAKi-naïve and -experienced patients, regardless of treatment group. AESIs are reported in Table 3.
4. Discussion
Our study showed that both bio/JAKi-naïve and -experienced patients had significantly and clinically meaningful treatment effects with etrasimod relative to placebo in both the induction and maintenance periods, with a similar safety profile seen across these subgroups. These findings demonstrate the robustness of the treatment benefits observed in the primary analysis of ELEVATE UC 52 and ELEVATE UC 12.
In ELEVATE UC 52, bio/JAKi-naïve patients who received etrasimod vs placebo achieved significant improvements in all induction and maintenance efficacy endpoints, while bio/JAKi-experienced patients achieved significant improvements in most endpoints, except for symptomatic remission and sustained clinical remission. In ELEVATE UC 12, bio/JAKi-naïve patients who received etrasimod vs placebo demonstrated significant improvements in all efficacy endpoints except for EIHR; in bio/JAKi-experienced patients, numerical improvements were observed for all efficacy endpoints and statistically significant results for clinical response. The proportion of patients in the etrasimod treatment group achieving each endpoint was consistent across trials for most efficacy endpoints. The proportion of patients who received placebo in ELEVATE UC 12 who achieved efficacy endpoints was generally higher than observed in ELEVATE UC 52, resulting in smaller differences between treatment groups. The results in the primary analysis population [baseline MMS of 5–9] were consistent with the total population of patients with baseline MMS of 4–9.
Patients with prior exposure to one bio/JAKi therapy who received etrasimod achieved significant improvements vs placebo in some, but not all, efficacy endpoints at Weeks 12 and 52. Less consistent treatment benefits were observed for patients with prior exposure to ≥ 2 bio/JAKi therapies. Further prospective studies are required in larger cohorts of patients with prior exposure to advanced therapy to more precisely define the benefits of long-term therapy with etrasimod in this subgroup of patients.
A unique aspect of the study was an opportunity to evaluate the efficacy of etrasimod in patients with prior exposure to the anti-integrin therapy vedolizumab in UC. Vedolizumab is often the first-line biologic therapy given to patients with UC, where it has demonstrated superior efficacy relative to adalimumab.15,16 Notably, 11% of patients enrolled in the ELEVATE programme had prior exposure to anti-integrin therapy. Although the mechanisms by which vedolizumab and S1P receptor modulators exert a therapeutic effect are presumed different, they are both believed to block recruitment of circulating lymphocytes into the gastrointestinal tract.17–19 Accordingly, patients with UC with prior exposure to vedolizumab might have a diminished response to treatment with S1P receptor modulators. Despite this, recent findings for ozanimod, another S1P receptor modulator, showed greater efficacy relative to placebo in patients with prior vedolizumab exposure.20 In the present analysis, the pooled subset of patients who received etrasimod with prior exposure to anti-integrin therapy, although limited in interpretation due to sample size, showed numerical improvements relative to placebo in all efficacy endpoints. These improvements were of a similar magnitude to those observed in the bio/JAKi-experienced excluding anti-integrins population. Similarly, among patients with any prior exposure to JAKi and those with prior exposure to JAKi only, numerically greater proportions of patients who received etrasimod vs placebo achieved some induction efficacy endpoints.
Symptomatic improvement is a key early target in the management of UC.21 In both the ELEVATE UC 52 and ELEVATE UC 12 clinical trials, bio/JAKi-naïve patients who received etrasimod demonstrated improvements in symptomatic response and remission vs placebo as early as Weeks 2 and 4, with improvements in symptomatic remission and response observed later for bio/JAKi-experienced patients. These findings suggest that treatment with etrasimod early in the disease course is likely to be associated with better patient outcomes.
The ELEVATE UC clinical programme is the first trial of an S1P receptor modulator with a treat-through design to evaluate efficacy by prior bio/JAKi exposure.14 Several studies have evaluated clinical remission in the induction period in patients treated with advanced UC therapies, such as vedolizumab, tofacitinib, mirikizumab, and ustekinumab, with or without prior exposure to biologics. However, most of these studies re-randomised either all patients or responders at the end of the induction period.11,13,22–25 Across all advanced UC therapies in which efficacy by prior biologic exposure has been investigated, all demonstrated numerically greater rates of clinical remission vs placebo in biologic-experienced patients at the end of induction, although greater improvements were generally observed in biologic-naïve vs -experienced patients.11,13,22–26 Here, in keeping with those findings, both bio/JAKi-naïve and -experienced patients who received etrasimod demonstrated greater efficacy in most endpoints vs placebo, including clinical remission.
Previously presented safety data for etrasimod showed a favourable safety profile14; analyses of the safety data by prior bio/JAKi exposure showed similar results for both bio/JAKi-naïve and -experienced patients. The proportions of patients with SAEs were consistent between the bio/JAKi-naïve and -experienced subgroups who received etrasimod. The proportions of patients with infections, including serious infections, were similar across subgroups. S1P receptor modulators may be associated with increased risk of bradyarrhythmia, atrioventricular conduction delays, and macular oedema23,27; however, respectively, these events occurred in < 2% of bio/JAKi-naïve and -experienced patients included in this analysis and the proportions of patients with these adverse events were consistent with those reported in the ELEVATE UC clinical programme population, regardless of prior bio/JAKi exposure.
This study is limited by the fact that ELEVATE UC 52 and ELEVATE UC 12 were not powered to demonstrate statistical differences among subgroups of patients with prior bio/JAKi exposure. Whereas the data were evaluated as part of an a priori analysis in the statistical analysis plans for ELEVATE UC 52 and ELEVATE UC 12, the analyses were not controlled for multiplicity. Analyses in the any prior exposure to anti-integrin, prior exposure to bio/JAKi excluding anti-integrins, any prior exposure to JAKi, prior exposure to JAKi only, and prior exposure to anti-integrin only subgroups were all post hoc and were performed in a smaller subset of patients. The relatively small subgroup sample sizes limit the interpretation of differences between these subgroups for efficacy and safety data. However, for the TEAEs and the assessment of AESI data presented, the findings were consistent overall with what has been previously observed in patients with UC treated with etrasimod.14 Finally, the analysis population comprised patients enrolled based on the inclusion and exclusion criteria for ELEVATE UC 52 and ELEVATE UC 12 and was not enriched for the enrolment of heavily pretreated patients with UC.
5. Conclusion
In conclusion, etrasimod was superior to placebo as an induction and maintenance therapy in both bio/JAKi-naïve and -experienced patients in ELEVATE UC 52, although the treatment effect was more consistent among bio/JAKi-naïve vs -experienced patients. Safety findings were similar to those reported previously for etrasimod. Ultimately, the findings from this subgroup analysis provide useful insights to help guide the clinical use of etrasimod in patients according to their prior exposure to advanced UC therapies.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
Acknowledgements
The authors would like to thank the patients, investigators, and study teams who were involved in the etrasimod UC programme. Christopher J Rabbat [an employee of Pfizer Inc at the time of the analysis] contributed to the design of the study and the data interpretation. Medical writing support, under the direction of the authors, was provided by Niall Tyrer, MBiolSci, CMC Connect, a division of IPG Health Medical Communications and Chimwemwe Chibambo, MBChB, CMC Connect, a division of IPG Health Medical Communications, and was funded by Pfizer, New York, NY, USA, in accordance with Good Publication Practice [GPP 2022] guidelines [Ann Intern Med 2022;175:1298–304].
Contributor Information
Séverine Vermeire, Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium.
Bruce E Sands, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Laurent Peyrin-Biroulet, Department of Gastroenterology, Nancy University Hospital, F-54500 Vandœuvre-lès-Nancy, France; INSERM, NGERE, University of Lorraine, F-54000 Vandœuvre-lès-Nancy, France; INFINY Institute, Nancy University Hospital, F-54500 Vandœuvre-lès-Nancy, France; FHU-CURE, Nancy University Hospital, F-54500 Vandœuvre-lès-Nancy, France; Groupe Hospitalier privé Ambroise Paré-Hartmann, Paris IBD Center, F-92200 Neuilly sur Seine, France; Division of Gastroenterology and Hepatology, McGill University Health Centre, Montreal, QC, Canada.
Geert R D’Haens, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Amsterdam, The Netherlands.
Julian Panés, Formerly Department of Gastroenterology, Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Spain.
Andres J Yarur, Division of Gastroenterology and Hepatology, Inflammatory Bowel Disease Center, Cedars Sinai Medical Center, Los Angeles, CA, USA.
Douglas C Wolf, Atlanta Gastroenterology Associates, Atlanta, GA, USA.
Timothy Ritter, Department of Research, GI Alliance, Southlake, TX, USA.
Stefan Schreiber, Department of Internal Medicine, University Hospital Schleswig-Holstein, Kiel University, Kiel, Germany.
John C Woolcott, Pfizer Inc, Collegeville, PA, USA.
Irene Modesto, Pfizer Inc, New York, NY, USA.
Michael Keating, Pfizer Inc, New York, NY, USA.
Kevin Shan, Pfizer Inc, New York, NY, USA.
Joseph Wu, Pfizer Inc, Cambridge, MA, USA.
Michael V Chiorean, Inflammatory Bowel Disease Center, Swedish Medical Center, Seattle, WA, USA.
Filip Baert, Department of Gastroenterology, AZ Delta, Roeselare, Belgium.
Marla C Dubinsky, Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Martina Goetsch, Pfizer AG, Zürich, Switzerland.
Silvio Danese, Division of Gastroenterology and Digestive Endoscopy, IRCCS San Raffaele Hospital and Vita-Salute San Raffaele University, Milan, Italy.
Brian G Feagan, Division of Gastroenterology, Department of Medicine, Western University, London, ON, Canada; Alimentiv Inc, London, ON, Canada.
Funding
This study was sponsored by Pfizer.
Conflict of Interest
SV received lecture/speaker fees from AbbVie, Dr. Falk Pharma, Ferring, Galapagos, Hospira, Janssen, MSD, Takeda, and Tillotts; consultancy/advisory fees from AbbVie, AbolerIS Pharma, Alimentiv, Arena, AstraZeneca, Avaxia, BMS, Boehringer Ingelheim, Celgene, CVasThera, Dr. Falk Pharma, Eli Lilly, Ferring, Galapagos, Genentech/Roche, Gilead Sciences, Hospira, IMIDomics, Janssen, Johnson and Johnson, Materia Prima, MiroBio, Morphic, MRM Health, MSD, Mundipharma, Pfizer Inc, ProDigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Surrozen, Takeda, Theravance Biopharma, Tillotts, and Zealand Pharma; grants/research support from AbbVie, Pfizer Inc, Galapagos, Janssen, and Takeda. BES: received consulting fees from AbbVie, Abivax, Alimentiv, Amgen, Arena, Artugen Therapeutics, AstraZeneca, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Calibr, Celgene, Celltrion, ClostraBio, Equillium, Enthera, Evommune, Fresenius Kabi, Galapagos, Genentech [Roche], Gilead Sciences, GlaxoSmithKline, Gossamer Bio, InDex Pharmaceuticals, Innovation Pharmaceuticals, Inotrem, Janssen, Kaleido, Kallyope, Lilly, Merck, Morphic, MRM Health, Pfizer Inc, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Sun Pharma, Surrozen, Takeda, Target RWE, Teva, Theravance Biopharma, TLL Pharmaceutical, Ventyx Biosciences; speaking fees from Abivax, Bristol Myers Squibb, Janssen, Lilly, Pfizer, Takeda; research grants from Bristol Myers Squibb, Janssen, Pfizer, Takeda, Theravance Biopharma; other support from Bristol Myers Squibb, Janssen, Lilly, Pfizer, Takeda; shareholder/stock options for Ventyx Biosciences. LP-B received fees from Adacyte, AbbVie, Abivax, Alimentiv, Alma Bio Therapeutics, Amgen, Applied Molecular Transport, Arena, Biogen, BMS, Celltrion, Connect Biopharma, Cytoki Pharma, Enthera, Ferring, Fresenius Kabi, Galapagos, Genentech, Gilead Sciences, Gossamer Bio, GSK, HAC Pharma, IAG Image Analysis, InDex Pharmaceuticals, Inotrem, Janssen, Lilly, Medac, Mopac, Morphic, MSD, Norgine, Nordic Pharma, Novartis, OM Pharma, ONO Pharmaceutical Co, Ose Immunotherapeutics, Pandion Therapeutics, Par’Immune, Pfizer Inc, Prometheus, Protagonist Therapeutics, Roche, Roivant Sciences, Samsung, Sandoz, Sanofi, Takeda, Theravance Biopharma, Thermo Fisher, TiGenix, Tillotts, Viatris, VectivBio, Ventyx, Vifor, and Ysopia. GRD’H served as an adviser for AbbVie, AstraZeneca, Alimentiv, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Cosmo, Eli Lilly, Galapagos, GSK, Johnson and Johnson, Takeda, Pfizer Inc, Polpharma, Prometheus Biosciences, Tillotts, and Ventyx; received speaker fees from AbbVie, Boehringer Ingelheim, Celltrion, Eli Lilly, Johnson and Johnson, Takeda, Pfizer Inc, and Tillotts. JP received personal fees from AbbVie, Arena, Athos, Atomwise, Boehringer Ingelheim, Celgene, Celsius, Celltrion, Ferring, Galapagos, Genentech/Roche, GSK, Janssen, Mirum, Morphic, Pandion Therapeutics, Pfizer Inc, Progenity, Prometheus, Protagonist Therapeutics, Revolo Biotherapeutics, Sanofi, Takeda, Theravance Biopharma, and Wassermann; grant support from AbbVie and Pfizer Inc. AJY received consultancy fees from Pfizer Inc, Arena, Takeda, and Bristol Myers Squibb; lecture and speaking fees from Bristol Myers Squibb. DCW received research grant support from AbbVie, Arena, Bristol Myers Squibb, Janssen, Pfizer Inc, Takeda, and Ventyx; lecture fees from AbbVie, Bristol Myers Squibb, Janssen, Pfizer Inc, and Takeda; consultancy fees from AbbVie, Arena, Bristol Myers Squibb, Janssen, Pfizer Inc, and Takeda. TR served on speaker panels for Takeda, Janssen, Pfizer Inc, Bristol Myers Squibb, AbbVie, and Lilly; served on a data adjudication committee for Ferring/Rebiotix; served on advisory boards for AbbVie, Ardelyx, Arena, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Ferring, Genentech/Roche, Gilead Sciences, and Intercept Pharma; shareholder for Iterative Scopes. SS received lecture/speaker fees from AbbVie, Arena, Biogen, Bristol Myers Squibb, Celgene, Celltrion, Dr. Falk Pharma, Fresenius Kabi, Janssen, MSD, Pfizer Inc, and Takeda; consultancy/advisory fees from AbbVie, Arena, Biogen, Bristol Myers Squibb, Celgene, Celltrion, Dr. Falk Pharma, Fresenius Kabi, Gilead Sciences, I-Mab, Janssen, MSD, Mylan, Pfizer Inc, Protagonist Therapeutics, Provention Bio, Takeda, and Theravance Biopharma. JW, IM, MK, KS, JW, and MG are employees and shareholders of Pfizer Inc. MVC received grant support from Pfizer Inc, Janssen, Novartis, and BMS; consulting fees from AbbVie, Arena, BMS, Medtronic, Pfizer Inc, Prometheus, Lilly, Janssen, and Takeda; speaker fees from AbbVie, Janssen, Medtronic, Pfizer Inc, BMS, Takeda, and Fresenius Kabi. FB received financial support for research/grants from AbbVie, Amgen, Janssen, and Takeda; received lecture fees and served on a speaker’s bureau for AbbVie, Arena, Celltrion, Ferring, Galapagos, Janssen, Merck Sharp & Dohme, Pfizer Inc, and Takeda; consultancy fees from AbbVie, Amgen, Arena, Celgene, Celltrion, Ferring, Fresenius Kabi, Janssen, Merck Sharp & Dohme, Pfizer Inc, and Sandoz. MCD received consulting fees from AbbVie, Abivax, Arena, AstraZeneca, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Genentech, Gilead Sciences, Janssen, Pfizer Inc, Prometheus Laboratories, Prometheus Biosciences, Takeda, and UCB; grant/research support from Janssen; shareholder/royalties and directorship/ownership interest in Trellus Health. SD received lecture/speaker fees from AbbVie, Amgen, Ferring, Gilead Sciences, Janssen, Mylan, Pfizer Inc, and Takeda; consulting/advisory fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Gilead Sciences, Hospira, Janssen, Johnson and Johnson, MSD, Mundipharma, Pfizer Inc, Roche, Sandoz, Takeda, TiGenix, UCB, and Vifor; holds directorship/ownership in Gastroenterology and Endoscopy. BGF serves as Senior Scientific Director for Alimentiv Inc, which provides central reading services; he is not a company employee and has no equity stake in the organisation which is owned by a medical trust; received speakers fees from AbbVie, Janssen, and Takeda; served as a consultant/advisory board member for AbbVie, AbolerIS Pharma, Agomab Therapeutics, AllianThera Biopharma, Amgen, AnaptysBio, Applied Molecular Transport, Arena, Avoro Capital Advisors, Atomwise, BIOJAMP, Biora Therapeutics, Boehringer Ingelheim, Boxer, Celsius Therapeutics, Celgene/BMS, Connect Biopharma, Cytoki, Disc Medicine, Duality, EcoR1, Eli Lilly, Equillium, Ermium, First Wave BioPharma, FirstWord Group, Galapagos, Galen/Atlantica, Genentech/Roche, Gilead Sciences, Gossamer Bio, GSK, Hinge Bio, HotSpot Therapeutics, InDex Pharmaceuticals, Imhotex, Immunic Therapeutics, JAK Academy, Janssen, Japan Tobacco Inc., Kaleido Biosciences, Landos Biopharma, Leadiant Biosciences, L.E.K. Consulting, Lenczner Slaght, LifeSci Capital, Lument AB, Millennium, MiroBio, Morgan Lewis, Morphic, Mylan, OM Pharma, Origo Biopharma, Orphagen, Pandion Therapeutics, Pendopharm, Pfizer Inc, Prometheus Therapeutics and Diagnostics, PlayToKnow AG, Progenity, Protagonist Therapeutics, PTM Therapeutics, Q32 Bio, Rebiotix, Redx, Roche, Sandoz, Sanofi, Seres Therapeutics, Silverback Therapeutics, Surrozen, Takeda, Teva, Thelium Therapeutics, TiGenix, Tillotts, Ventyx, VHSquared, Viatris, Ysios, Ysopia, and Zealand Pharma; shareholder for Gossamer Pharma.
Author Contributions
JW, IM, MK, KS, JW, and MG contributed to the conceptualisation and methodology of the study. JW and KS contributed to the formal analysis and validation of the data. All authors contributed to the writing, reviewing, and editing of the manuscript.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel J-F.. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Magro F, Gionchetti P, Eliakim R, et al.; European Crohn’s and Colitis Organisation [ECCO]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11:649–70. [DOI] [PubMed] [Google Scholar]
- 3. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease [STRIDE]: determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 4. Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis 2022;16:2–17. [DOI] [PubMed] [Google Scholar]
- 5. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD.. ACG Clinical Guideline: ulcerative colitis in adults. Am J Gastroenterol 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 6. Harbord M, Eliakim R, Bettenworth D, et al.; European Crohn’s and Colitis Organisation [ECCO]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017;11:769–84. [DOI] [PubMed] [Google Scholar]
- 7. Singh S, Allegretti JR, Siddique SM, Terdiman JP.. AGA technical review on the management of moderate to severe ulcerative colitis. Gastroenterology 2020;158:1465–96.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferretti F, Cannatelli R, Monico MC, Maconi G, Ardizzone S.. An update on current pharmacotherapeutic options for the treatment of ulcerative colitis. J Clin Med 2022;11:2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. European Medicines Agency. Zeposia, Summary of Product Characteristics. 2020. https://www.ema.europa.eu/documents/product-information/zeposia-epar-product-information_en.pdf Accessed 2 November 2023. [Google Scholar]
- 10. European Medicines Agency. Xeljanz, Summary of Product Characteristics. 2017. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf Accessed 2 November 2023. [Google Scholar]
- 11. Feagan BG, Rubin DT, Danese S, et al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol 2017;15:229–39.e5. [DOI] [PubMed] [Google Scholar]
- 12. Vavricka SR, Greuter T, Cohen BL, et al. Corticosteroid-free efficacy and safety outcomes in patients receiving tofacitinib in the OCTAVE Sustain maintenance study. Therap Adv Gastroenterol 2022;15:17562848221090834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sandborn WJ, Peyrin-Biroulet L, Sharara AI, et al. Efficacy and safety of tofacitinib in ulcerative colitis based on prior tumor necrosis factor inhibitor failure status. Clin Gastroenterol Hepatol 2022;20:591–601.e8. [DOI] [PubMed] [Google Scholar]
- 14. Sandborn WJ, Vermeire S, Peyrin-Biroulet L, et al. Etrasimod as induction and maintenance therapy for ulcerative colitis [ELEVATE]: two randomised, double-blind, placebo-controlled, phase 3 studies. Lancet 2023;401:1159–71. [DOI] [PubMed] [Google Scholar]
- 15. Sands BE, Peyrin-Biroulet L, LoftusDanese EVS, et al.; VARSITY Study Group. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med 2019;381:1215–26. [DOI] [PubMed] [Google Scholar]
- 16. Moens A, Verstockt B, Alsoud D, Sabino J, Ferrante M, Vermeire S.. Translating results from VARSITY to real world: adalimumab vs vedolizumab as first-line biological in moderate to severe IBD. Inflamm Bowel Dis 2022;28:1135–42. [DOI] [PubMed] [Google Scholar]
- 17. Argollo M, Furfaro F, Gilardi D, et al. Modulation of sphingosine-1-phosphate in ulcerative colitis. Expert Opin Biol Ther 2020;20:413–20. [DOI] [PubMed] [Google Scholar]
- 18. Peyrin-Biroulet L, Christopher R, Behan D, Lassen C.. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun Rev 2017;16:495–503. [DOI] [PubMed] [Google Scholar]
- 19. Wyant T, Fedyk E, Abhyankar B.. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis 2016;10:1437–44. [DOI] [PubMed] [Google Scholar]
- 20. Sands BE, Anjali J, Harris A, et al. Efficacy of ozanimod in patients with ulcerative colitis who were previously exposed to vedolizumab: True North post hoc analysis. Am J Gastroenterol 2022;117:S4–S5. [Google Scholar]
- 21. Turner D, Ricciuto A, Lewis A, et al.; International Organization for the Study of IBD. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE] Initiative of the International Organization for the Study of IBD [IOIBD]: determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160:1570–83. [DOI] [PubMed] [Google Scholar]
- 22. D’Haens G, Dubinsky M, Kobayashi T, et al.; LUCENT Study Group. Mirikizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2023;388:2444–55. [DOI] [PubMed] [Google Scholar]
- 23. Sandborn WJ, Feagan BG, D’Haens G, et al.; True North Study Group. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2021;385:1280–91. [DOI] [PubMed] [Google Scholar]
- 24. Sands BE, Sandborn WJ, Panaccione R, et al.; UNIFI Study Group. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–14. [DOI] [PubMed] [Google Scholar]
- 25. Vermeire S, Danese S, Hébuterne X, et al. OP04 Efficacy of upadacitinib in ulcerative colitis: a phase 3 post hoc analysis of biologic- and anti-TNF-inadequate response patients. J Crohns Colitis 2023;17:i8–9. [Google Scholar]
- 26. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257–65.e1. [DOI] [PubMed] [Google Scholar]
- 27. US Food and Drug Administration. ZEPOSIA® [Ozanimod]: Highlights of Prescribing Information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209899s000lbl.pdf Accessed 31 October 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.