Abstract
BACKGROUND
Fibrosis is an important pathological feature of endometriotic lesions of all subtypes. Fibrosis is present in and around endometriotic lesions, and a central role in its development is played by myofibroblasts, which are cells derived mainly after epithelial-to-mesenchymal transition (EMT) and fibroblast-to-myofibroblast transdifferentiation (FMT). Transforming growth factor-β (TGF-β) has a key role in this myofibroblastic differentiation. Myofibroblasts deposit extracellular matrix (ECM) and have contracting abilities, leading to a stiff micro-environment. These aspects are hypothesized to be involved in the origin of endometriosis-associated pain. Additionally, similarities between endometriosis-related fibrosis and other fibrotic diseases, such as systemic sclerosis or lung fibrosis, indicate that targeting fibrosis could be a potential therapeutic strategy for non-hormonal therapy for endometriosis.
OBJECTIVE AND RATIONALE
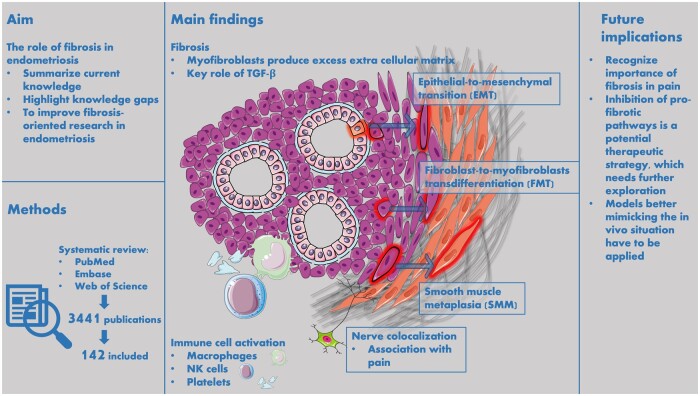
This review aims to summarize the current knowledge and to highlight the knowledge gaps about the role of fibrosis in endometriosis. A comprehensive literature overview about the role of fibrosis in endometriosis can improve the efficiency of fibrosis-oriented research in endometriosis.
SEARCH METHODS
A systematic literature search was performed in three biomedical databases using search terms for ‘endometriosis’, ‘fibrosis’, ‘myofibroblasts’, ‘collagen’, and ‘α-smooth muscle actin’. Original studies were included if they reported about fibrosis and endometriosis. Both preclinical in vitro and animal studies, as well as research concerning human subjects were included.
OUTCOMES
Our search yielded 3441 results, of which 142 studies were included in this review. Most studies scored a high to moderate risk of bias according to the bias assessment tools. The studies were divided in three categories: human observational studies, experimental studies with human-derived material, and animal studies. The observational studies showed details about the histologic appearance of fibrosis in endometriosis and the co-occurrence of nerves and immune cells in lesions. The in vitro studies identified several pro-fibrotic pathways in relation to endometriosis. The animal studies mainly assessed the effect of potential therapeutic strategies to halt or regress fibrosis, for example targeting platelets or mast cells.
WIDER IMPLICATIONS
This review shows the central role of fibrosis and its main cellular driver, the myofibroblast, in endometriosis. Platelets and TGF-β have a pivotal role in pro-fibrotic signaling. The presence of nerves and neuropeptides is closely associated with fibrosis in endometriotic lesions, and is likely a cause of endometriosis-associated pain. The process of fibrotic development after EMT and FMT shares characteristics with other fibrotic diseases, so exploring similarities in endometriosis with known processes in diseases like systemic sclerosis, idiopathic pulmonary fibrosis or liver cirrhosis is relevant and a promising direction to explore new treatment strategies. The close relationship with nerves appears rather unique for endometriosis-related fibrosis and is not observed in other fibrotic diseases.
REGISTRATION NUMBER
N/A.
Keywords: endometriosis, deep endometriosis, fibrosis, myofibroblasts, transforming growth factor-β
Graphical Abstract
Pathways involved in fibrosis development in endometriosis.
Introduction
Endometriosis is the most prevalent benign gynecologic disease, affecting approximately 190 million people worldwide (Zondervan et al., 2020). Persons with endometriosis can experience a variety of symptoms, including dysmenorrhea, chronic pelvic pain and subfertility, resulting in a severe decrease in quality of life (van Aken et al., 2017). Because of the high prevalence of endometriosis and its debilitating effects, endometriosis causes a huge burden, both on an individual as well as at a societal level (Simoens et al., 2012). Nevertheless, many aspects of this disease remain to be investigated.
Endometriosis is characterized by the presence of endometrial-like tissue implants outside the uterine cavity and can be present on the peritoneum, the pelvic organs, in scar tissue after caesarian section and in the thoracic cavity. It is subdivided into three types: peritoneal endometriosis (PER), ovarian endometriotic cysts or endometrioma (OMA), and deep endometriosis (DE). On a histological level, lesions consist of endometrial stromal and epithelial glandular cells and fibrotic deposits (Camboni and Marbaix, 2021). The presence of stromal and epithelial glandular cells in a surgical derived biopsy from visually suspected areas is currently the main criterium of histopathologic diagnosis of endometriosis. However, several leading research groups have recently proposed to highlight fibrosis in the histopathologic definition as well (Guo, 2018; Vigano et al., 2018). Thereby, fibrosis and myofibroblasts, the main extracellular matrix (ECM)-producing cells, are hypothesized to be accountable for endometriosis-related symptoms (Odagiri et al., 2009; Yan et al., 2019b; Garcia Garcia et al., 2023). On the other hand, we know that fibrosis is progressive over time and corresponds with PER lesions changing from red to black to white, which may support the opposite hypothesis that fibrosis is a self-limiting end stage of disease, stopping lesion growth and cyclical bleeding (Matsuzaki et al., 1999; Zhang et al., 2016b). This contrast brings up the question of whether fibrosis is a beneficial or an unfavorable effect.
Under normal circumstances, myofibroblasts fulfill an important role in wound healing (Almadani et al., 2021). By their contraction and production of ECM, myofibroblasts have the ability to congregate wound edges. After tissue homeostasis is reached, myofibroblasts normally go into apoptosis. However, in fibrotic diseases, myofibroblasts persist in an activated state and continue to produce matrix proteins, leading to excess fibrosis (Adler et al., 2020). This can result in pain and, in more severe cases, even lead to a progressive loss of organ function (Hutchenreuther and Leask, 2016). Because these potent characteristics of myofibroblasts and the fibrotic process have been proposed to influence endometriosis progress and its symptoms, it is important to study the myofibroblasts in lesions to gain more knowledge about the exact role of fibrotic processes in the disease.
Fibrosis is defined as the excess deposition of ECM components, mostly collagen, and usually arises during wound healing and inflammation processes (Kuehlmann et al., 2020). Among the cells forming the stromal compartment of endometriosis, myofibroblasts have a pivotal role as they are responsible for this excessive production of ECM leading to fibrosis (Adler et al., 2020). The myofibroblasts mainly derive from fibroblasts by a process called fibroblast-to-myofibroblast transdifferentiation (FMT) (Zhu et al., 2023). Other sources of myofibroblasts are epithelial-to-mesenchymal transition (EMT) and endothelial-to-mesenchymal transition (EndoMT) (Zhang et al., 2016a; Yan et al., 2020a,b). After myofibroblastic differentiation, cells can differentiate further into smooth muscle cells, in a process referred to as smooth-muscle-metaplasia (SMM) (Barcena de Arellano et al., 2011; Ding et al., 2020b). Transforming growth factor-β (TGF-β) is a key stimulating factor in myofibroblastic differentiation (Biernacka et al., 2011). TGF-β signaling is known to be a driver of pathologic fibrosis in several fibrotic diseases like systemic sclerosis, idiopathic pulmonary fibrosis, and liver cirrhosis. The activation of TGF-β signaling can result in the activation of several subsequent pro-fibrotic pathways, among which are Smad, Wingless-related integration (Wnt)/β-catenin, Focal Adhesion Kinase (FAK), and Rho/Rho-associated protein kinase (Rho/ROCK) signaling (Ji et al., 2014; Meng et al., 2016; Zhao et al., 2017; Distler et al., 2019). As pulmonary fibrosis is currently treated with therapeutics interacting in these pathways with some positive effects, parallels between endometriosis and lung fibrosis show potential for investigation (Amati et al., 2023).
In the initial phase of fibrosis, TGF-β and platelet-derived growth factor (PDGF), among other factors, are released due to a continuous or repetitive process of tissue damage and healing, eventually leading to a new fibrotic steady state (Adler et al., 2020). In endometriosis, TGF-β has a pivotal role as a pro-fibrotic signaling factor (Hull et al., 2012; Xiao et al., 2020). It acts as a repetitive signal of tissue injury, potentially triggered by cyclical bleeding as a consequence of the hormonal responsiveness of the endometriotic cells (Laux-Biehlmann et al., 2015). In this process, platelet activation, macrophage infiltration and neuropeptide secretion may be triggered to further stimulate fibrosis (Zhang et al., 2016a; Duan et al., 2018; Liu et al., 2019; 2020). Recently, two reviews focusing on fibrotic pathways in endometriosis have been published (Vigano et al., 2020; Garcia Garcia et al., 2023). Garcia Garcia et al., highlighted important differences in fibrotic processes in ovarian as compared to deep endometriosis. EndoMT contributes more to fibrotic development in ovarian endometriosis, whereas sensory nerves and smooth muscle cells are predominantly involved in deep endometriosis. Vigano et al., provided insight in the cellular processes that are involved in fibrogenesis. Platelets, macrophages, ectopic endometrial cells and sensory nerves produce a variety of cytokines and neuropeptides involved in fibrotic signaling (Vigano et al., 2020).
Closely related to fibrosis is the inflammatory environment of endometriosis. The important role of inflammation in endometriosis is illustrated by a disturbed immune cell composition and an abundance of cytokines in the peritoneal fluid and eutopic endometrium of endometriosis patients (Vallve-Juanico et al., 2019; Abramiuk et al., 2022). Endometriosis can thus be considered both as a fibrotic and as an inflammatory disease (Zhang et al., 2019a; Vigano et al., 2020). However, these two aspects cannot be seen separately based on their inter-connected modifying effects. A yet unanswered question regarding the inflammatory environment is whether endometriotic implants trigger inflammation in their environment, or whether an inflammatory state in the peritoneal cavity and endometrium supports endometriosis development in susceptible individuals (Izumi et al., 2018).
To date, the published reviews have focused on specific aspects of fibrosis in endometriosis, or on fibrosis in specific endometriosis subtypes. They lack a general overview of fibrosis in endometriosis in in vitro, animal and clinical studies. In this systematic review, a comprehensive overview of the current knowledge about fibrosis in endometriosis is presented by congregating various types of research. The aim of this review is to provide a broad basis for researchers exploring fibrosis as a therapeutic target for endometriosis, as resolving fibrosis is a promising strategy for non-hormonal and non-invasive therapeutic options for endometriosis.
Methods
Protocol and registration
This systematic review was reported according to the PRISMA guidelines for systematic reviews (Page et al., 2021). The protocol was registered in the PROSPERO database (registration number: CRD42022295727) in December 2021.
Data source and search strategies
A systematic literature search was performed in September 2023 in the following databases: Pubmed, Embase, and Web of Science. Keywords as well as title or abstract terms for ‘endometriosis’ and ‘ectopic endometrium’, ‘fibrosis’, ‘myofibroblasts’, ‘collagen’, ‘α-smooth muscle actin’ and their synonyms and related terms were combined. The full search strategy is presented in Supplementary File S1. No restrictions based on publication date or language were applied in the initial search. Duplicates were excluded using EndNote 20. A cited-reference search was performed to identify potential additional relevant articles.
Eligibility and study selection
Original studies in English reporting about fibrosis in endometriosis were included in this review. In vitro, animal and human studies were eligible if they reported about the development, presence and/or treatment of fibrosis. Fibrosis as an outcome was defined as the histologic analysis of fibrosis-specific staining or by molecular markers for fibrosis, myofibroblasts or their corresponding genes. These are mainly α-smooth muscle actin (α-SMA, gene symbol: ACTA2) and collagen type I (COL1A1, COL1A2), while including others. Reviews and case reports were excluded, as well as studies exclusively analyzing adenomyosis or eutopic endometrium and studies using immortalized cell lines only.
The selection of studies was performed independently by two authors (GV and MG) using Rayyan. The first round of selection was based on screening of title and abstract. Studies selected by at least one author were included for full text screening. In case of inconsistency between the authors after full text screening, eligibility was discussed between them. If inconsistency persisted, a third author (AN) was consulted. During the selection process the reasons for exclusion were reported.
Data extraction and quality assessment
Data extraction was performed by one author (GV) and systematically checked by a second author (MG). Quality assessment was performed according to validated risk of bias tools: The MINORS tool was used for the observational studies (Slim et al., 2003); a modified version of the ROBINS tool was used for the experimental studies with human-derived material (Sterne et al., 2016; Post et al., 2020); and the SYRCLE tool was used for animal studies (Hooijmans et al., 2014). Quality assessment was performed by one author (GV) and systematically checked by a second author (MG). In case of inconsistency, the risk of bias was discussed between the authors.
Results
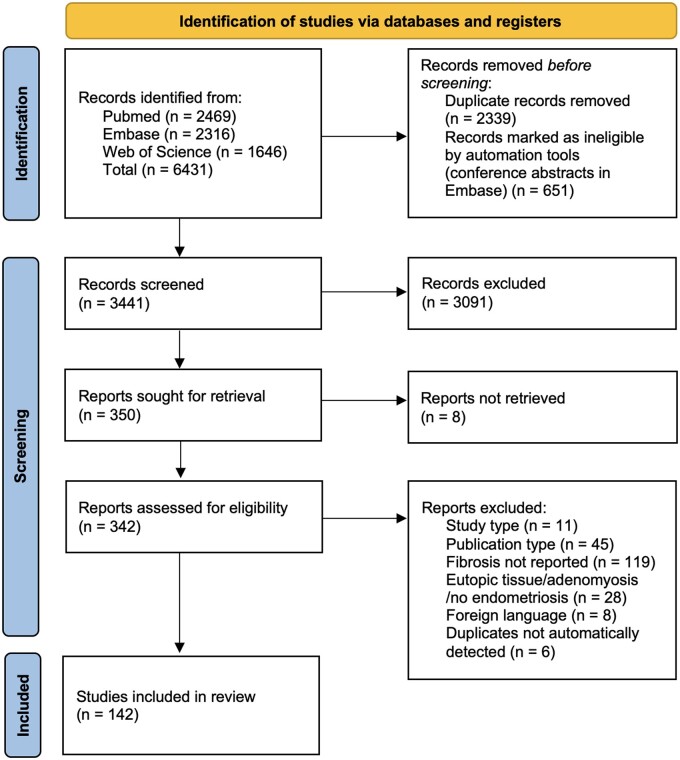
Our search yielded 3441 unique articles. After title and abstract screening 342 articles were included for full text assessment, and 142 articles were ultimately included in our review. The study selection procedure is presented in Fig. 1. There were 11 articles excluded based on their study type being a review or case report; 45 were excluded based on a their publication type (e.g. conference abstracts); 119 studies were excluded based on not reporting fibrosis, meaning that fibrosis was not assessed or not specifically defined; 28 studies were excluded based on not studying ectopic endometriotic tissue; 8 studies in foreign languages were excluded; and six manually detected duplicates were also removed. From the included studies, 44 were human observational studies and 28 were experimental studies using human-derived tissues. The human studies were subdivided per endometriosis subtype. Peritoneal endometriosis was examined in 5 studies, in 33 ovarian endometriosis in 33 and deep endometriosis in 14. In the remaining 20 human studies, more than one endometriosis subtype was examined. In addition to the studies concerning human subjects or tissue, 75 animal studies were included in this review. Some studies were included in more than one group because both human tissue and animal-based experiments were performed. In every following section, studies assessing outcomes at the tissue level are discussed first, and subsequently we zoomed in on cellular and ultimately molecular levels. We started by discussing human observational studies as these most often assessed outcomes at the tissue level.
Figure 1.
PRISMA flow diagram: schematic representation of the study selection process.
Human observational studies
An overview of the 44 human observational studies we included is presented in Table 1. These studies report findings about histological appearance, cellular composition, pro-fibrotic pathways, and clinical parameters.
Table 1.
Observational studies.
| Reference | Sample size | Assay methods | Results | Conclusion | Relevance to fibrosis |
|---|---|---|---|---|---|
|
MULTIPLE ENDOMETRIOSIS SUBTYPES | |||||
| Anaf et al., 2000b; Smooth muscles are frequent components of endometriotic lesions (Anaf et al., 2000b) | 21 PER, 13 OMA, 18 DE, 10 control eutopic, 10 healthy peritoneum | IHC and silver staining | PER 75% SMC covered area; OMA 23%; DE 73–79%, peritoneum 0% | Results support transdifferentiation toward smooth muscle cells | Myofibroblasts transdifferentiate toward smooth muscle-like cells in periphery of lesions |
| Bernacchioni et al., 2021; Sphingosine 1-phosphate (S1P) receptors are dysregulated in endometriosis: possible implications in transforming growth factor β-induced fibrosis (Bernacchioni et al., 2021) | 15 OMA, 30 DE, 30 eutopic control | HE, RT-qPCR, WB | Sphingosine kinase (SK) 1 and S1P receptor expression elevated in OMA and DE compared to eutopic control, SK2 not different. TGF-β, α-SMA and collagen 1A expression elevated in OMA and DE versus eutopic control | The S1P signaling axis may represent a useful biomarker or innovative pharmacologic target for endometriosis | Sphingosine signaling axis expression elevated in line with fibrotic markers in endometriosis |
| Haga et al., 2013; immunohistochemical analysis of thoracic endometriosis (Haga et al., 2013) | 84 thoracic endometriosis, 40 diaphragm controls | HE, IHC | Stroma detected in 100%, glands in 25% and SMCs in 1 of the samples. ER staining was positive in the stromal compartment in 88% and PR in 100% of the samples, CD10 in 88% and α-SMA in 55% | Most thoracic endometriosis biopsies stained positive for CD10, ER and PR, half of them for α-SMA. These markers can be useful for diagnosis | Thoracic endometriosis shares high hormonal receptor expression with other endometriosis subtypes, but showed less α-SMA expression |
| Hao et al., 2022; Activation of α7 nicotinic acetylcholine receptor (α7nAChR) retards the development of endometriosis (Hao et al., 2022) | 17 OMA, 14 DE, 18 healthy endometrium | HE, IHC, Masson stain | α7nAChR staining strongest in epithelial cells in all samples. Expression is decreased in DE and OMA versus healthy endometrium, negative correlated with extent of fibrosis, ASRM score, and dysmenorrhea severity | α7nAChR expression is reduced in endometriosis, especially in DE. Pharmacological activation of α7nAChR decelerates lesional progression and fibrogenesis | α7nAChR is suppressed in endometriosis, contributing to disease and can be a potential anti-fibrotic target |
| Huang et al., 2021; Higher fibrotic content of endometriotic lesions is associated with diminished prostaglandin (PG) E2 signaling (Huang et al., 2021) | 41 OMA, 19 DE | HE, IHC, Masson stain, RT-qPCR | DE lesions contained more fibrosis, less COX-2, EP2, and EP4 than OMA lesion. Prostaglandin signaling markers show negative correlation with fibrosis | PGE2 signaling (COX-2, EP2, EP4 staining) attenuated with progressive fibrosis | PGE2 signaling attenuated with progressive fibrosis |
| Khare et al., 1996; A comparative study of ovarian and pelvic wall-infiltrating endometriosis (Khare et al., 1996) | 10 PER, 10 OMA | IHC, Masson stain | Collagen and SMC-like cells were present in bundles around PER lesions, collagen border in OMA lesions | In PER metaplastic reaction without clear delineation observed, in OMA clear delineation with ovary by collagen bundles | Bundles of collagen and smooth muscle-like cells around PER lesions |
| Konrad et al., 2018; Composition of the stroma in the human endometrium and endometriosis (Konrad et al., 2018) | 17 PER, 26 OMA, 22 DE, 47 healthy endometrium | IHC | PER 8%, OMA 19%, DE 9%, patients eutopic 7%, healthy eutopic 9% α-SMA-positive cells among CD10 positive cells, no significant differences between groups | No differences in α-SMA expression in stromal cells | α-SMA expression in stromal cells similar in various endometriosis subtypes |
| Liu et al., 2018; Histological and immunohistochemical characterization of the similarity and difference between ovarian endometriomas and deep infiltrating endometriosis (Liu et al., 2018) | 25 OMA, 25 DE, 20 healthy endometrium | HE, Masson stain, IHC | OMA and DE expressed markers of EMT, FMT, SMM, and fibrosis, more expression in DE. Platelet aggregation in lesions, probably via enhancer of zeste homolog 2. ER-β increased, PR-β decreased in lesions | OMA and DE both undergo EMT, FMT, SMM, fibrosis; DE more extensively, more fibrosis, less vascularity | DE undergo more extensive fibrotic changes compared to OMA |
| Mechsner et al., 2005; Oxytocin receptor expression in smooth muscle cells of peritoneal endometriotic lesions and ovarian endometriotic cysts (Mechsner et al., 2005) | 120 PER (from 80 patients), 40 OMA, 55 distant peritoneum, 11 healthy peritoneum | IHC | Expression of oxytocin receptor, ER and PR among intrastromal α-SMA-positive cells was high, in fully fibrotic areas receptor expression was low. No correlation found between α-SMA and ASRM score | Hormonal and oxytocin receptors present mostly on intrastromal myofibroblasts, ability to contract | Myofibroblasts lose hormonal receptivity toward periphery of lesions |
| Metzger et al., 1993; Histologic features associated with hormonal responsiveness of ectopic endometrium (Metzger et al., 1993) | 196 endometriosis (subtype undefined) and eutopic endometrium | HE | Hormonal responsiveness was defined as ectopic and eutopic endometrium being in phase. Hormonal responsiveness decreased as fibrosis increased. More fibrosis in lesions with hemorrhage signs. Large cystic glands and endometriomas are less often in phase with eutopic endometrium | Hormonal responsiveness correlates with several histologic features of endometriosis | Hormonal responsiveness decreases as fibrosis is more extensive |
| Nezhat and Kalir, 2002; Comparative immunohistochemical studies of endometriotic lesions and endometriotic cysts (Nezhat and Kalir, 2002) | 30 OMA, 35 not defined endometriosis (no OMA) | IHC | 22/30 cystic lesions stained positive for collagen VI compared to 2/35 of non-cystic lesions. BCL-2 staining in 7/30 cysts and 35/35 non-cystic lesions. No p53 staining in both groups. Matrix metalloproteinase IX mostly positive in non-cystic, semi-positive in cystic lesions | Endometriotic cysts have different protein expression patterns. Collagen VI is overexpressed and BCL-2 shows lower expression in cystic lesions versus non-cystic lesions | Collagen VI more overexpressed in cystic lesions versus other lesions |
| Odagiri et al., 2009; Smooth muscle metaplasia and innervation of endometriotic lesions related to pain (Odagiri et al., 2009) | 3 PER, 12 OMA, 5 DE, 5 eutopic endometrium | Masson stain, IHC | Intense α-SMA staining around stromal region, intense neural cell adhesion molecule staining in lesions, and fibrotic interstitium | Smooth muscle cells and nerve cells are extensively present around lesions and fibrosis, suggesting a relationship between contraction and pain | Clustering of nerve cells and myofibroblasts suggesting a role in pain |
| Shin et al., 2023; Single-cell profiling identifies distinct hormonal, immunologic, and inflammatory signatures of endometriosis-constituting cells (Shin et al., 2023) | 6 OMA, 4 PER, 7 DE | scRNA-seq | 11 cell types were assigned. Macrophage (Mac) subpopulations differed between subtypes, nonclassical monocytes in DIE, resident monocytes in OMA and PER, Mac-2 in PER and DE, Mac-4 (MMP9 expressing) in OMA. 5 fibroblasts subpopulations were identified, Myofibroblast (Mfib)-1, Mfib-2 and Mfib-3 abundance in endometriosis, Mfib-1 (Periostin expressing) and Mfib-2 (mesenchymal marker expressing) main fibroblast in PER and DE, Mfib-3 (Transmembrane 19 and Tenascin-C expressing) in OMA | Subpopulations of cell types varied between endometriosis subtypes, estrogen responsiveness is generally high, and distinct cell subpopulations, among which are myofibroblasts, are found in endometriosis, indicating the heterogeneity of endometriosis | Different subpopulations of myofibroblasts are present in the different subtypes of endometriosis and not present in normal endometrium |
| Yan et al., 2019b; Neuropeptides substance P and calcitonin gene-related peptide accelerate the development and fibrogenesis of endometriosis (Yan et al., 2019b) | 30 OMA, 30 DE, 24 healthy endometrium | Masson stain, IHC, WB, RT-qPCR | DE showed more α-SMA expression and fibrosis than OMA. Fibrosis and α-SMA expression showed positive correlation with nerve fiber density and neuropeptides and their receptors. The severity of dysmenorrhea showed a positive correlation with nerve fiber density | Sensory nerves have an important role in promoting fibrogenesis. Substance P, calcitonin-related peptide, and their receptors stimulate EMT, FMT, and SMM. The anatomical link between DE and multiple nerve plexus could explain higher fibromuscular content in DE | Colocalization of nerves, neuropeptides, and fibrosis implies the contribution of fibrosis to pain in endometriosis |
| Yan et al., 2020b; Platelets induce endothelial-mesenchymal transition and subsequent fibrogenesis in endometriosis (Yan et al., 2020b) | 30 OMA, 30 DE, 30 healthy endometrium | Masson stain, IHC | Highest fibrotic content in DE. Fibroblast-specific protein-1 (FSP-1, as a mesenchymal marker) expression is elevated in endometriotic lesions. Fibrosis showed a positive correlation with FSP-1 and FSP1/CD31 ratio. Co-culture of human umbilical vein endothelial cells (HUVECs) and activated platelets increased and fibrosis markers, TGF-β and Platelet-derived growth factor receptor neutralization abolished effect | Evidence for EndoMT with a critical role of platelet activation | EndoMT supports fibrogenesis with a critical role in platelet activation |
| Yan et al., 2020a; Mesothelial cells participate in endometriosis fibrogenesis through platelet-induced mesothelial-mesenchymal transition (Yan et al., 2020a) | 30 OMA, 30 DE, 30 healthy endometrium | Masson stain, IHC, WB, RT-qPCR | Highest fibrotic content and α-SMA expression in DE. Calretinin (mesothelial marker) showed a positive correlation with fibrosis | Mesothelial cells contribute to fibrosis and lesional progression through platelet-induced mesothelial-mesenchymal transition | Mesothelial origin in endometriotic lesions contribute to development of fibrosis |
| Zheng et al., 2023a; Aberrant expression of histone deacetylase (HDAC) 8 in endometriosis and its potential as therapeutic target (Zheng et al., 2023a) | 38 OMA, 20 DE, 24 healthy endometrium | Masson stain, IHC | HDAC2 staining reduced, HDAC8 elevated in lesions. HDAC1 and HDAC6 elevated in DE, HDAC3 reduced, but in OMA these were similar to control endometrium. Extent of fibrosis highest in DE, also elevated in OMA, fibrosis correlated positively with HDAC1, 6 and 8 and negatively with 2 and 3 | Mainly HDAC8 expression is elevated in endometriosis and correlated with fibrosis, suggesting a role in FMT and SMM, which is supported by an anti-fibrotic effect of HDAC8 inhibition in a mouse experiment | HDAC8 overexpression in endometriosis contributes to fibrogenesis and is a potential therapeutic target |
|
| |||||
|
PERITONEAL ENDOMETRIOSIS | |||||
| Barcena de Arellano et al., 2011; Immunohistochemical characterization of endometriosis-associated smooth muscle cells in human peritoneal lesions (Barcena de Arellano et al., 2011) | 60 PER, 60 distant peritoneal biopsies, 10 healthy peritoneal biopsies | IHC | 25% of the stromal region, 65% of the surrounding tissue, and 31% of the peripheral tissue α-SMA positive. Differentiation grade intrastromal SMC 12–15%; 36–44% surrounding SMC; peripheral SMC 77–80%. No differences in SMC amount based on hormonal medication, cycle, or color of lesion | Increased differentiation toward the periphery, SMC has contractile abilities and may be responsible for pain | Myofibroblasts differentiate toward smooth muscle-like cells in the periphery of lesions |
| Ibrahim et al., 2019; Arrangement of myofibroblastic and smooth muscle-like cells in superficial peritoneal endometriosis and a possible role of transforming growth factor beta 1 (TGF-β1) in myofibroblastic metaplasia (Ibrahim et al., 2019) | 23 PER, 5 distant peritoneum biopsies, 10 healthy peritoneum | IHC | Myofibroblasts are present in all compartments of the lesion, contractile calponin-positive cells mostly in intra-stromal region, and differentiated desmin-positive cells in the periphery. TGF-β receptors are highest in the intra-stromal region | Cell maturity increased toward the periphery of lesions. TGF-β involved in metaplasia | Myofibroblasts differentiate into smooth muscle-like cells in the periphery of lesions |
| Matsuzaki et al., 1999; Fibrogenesis in peritoneal endometriosis (Matsuzaki et al., 1999) | 16 PER, 8 healthy eutopic endometrium | IHC | Different part of the stromal compartment of lesions was stained for collagen between different lesion appearances: 61,5% collagen in black; 33,1% in red; 12,5% eutopic. No differences between cycle stages | Collagen is more present in black versus red lesions | Black lesions contain more collagen than red lesions, phase of the menstrual cycle does not affect collagen content |
| Sohler et al., 2013; Tissue remodeling and non-endometrium-like menstrual cycling are hallmarks of peritoneal endometriosis lesions (Sohler et al., 2013) | 18 PER, 22 distant peritoneum, 17 eutopic endometrium | IHC, RT-qPCR | Fibrosis present in metaplastic lesions, not in hyperplastic lesions; α-SMA mRNA overexpression in lesions compared to eutopic endometrium. Metaplasia based on caldesmon expression. SMC hyperplasia not found in combination with fibrosis. No changes in expression of steroid receptors and tissue remodeling factors trough cycle | Endometriotic lesions do not undergo a menstrual cycle based on microarray analysis | Fibrotic endometriosis lesions do not undergo a regular menstrual cycle |
| Stovall et al., 1992; Immunohistochemical detection of type I, III, and IV collagen in endometriosis implants (Stovall et al., 1992) | 10 PER, 10 eutopic endometrium, 6 healthy eutopic endometrium | IHC | Collagen types were similar between eutopic endometrium and intrastromal area of lesions. Type I collagen was dominant in the fibrotic surrounding of lesions | Various collagen types are present in ectopic and eutopic endometrium. Collagen I dominant in lesion-associated fibrosis | Collagen I is the main ECM component in endometriotic lesion-associated fibrosis |
|
| |||||
|
OVARIAN ENDOMETRIOTIC CYST | |||||
| Cao et al., 2019; Plasma high mobility group box 1 (HMGB1), osteopontin (OPN), and hyaluronic acid (HA) as admissible biomarkers for endometriosis (Cao et al., 2019) | 30 OMA, 20 healthy controls. Lesion biopsies and blood plasma | HE, Masson stain, IHC, ELISA | Moderately fibrotic compared to highly fibrotic lesions: lower expression of OPN, Rage, Interleukin-33, higher HMGB1, Toll-like receptor 4, p-p65, and proliferating cell nuclear antigen. Plasma levels of HMGB1, OPN, and HA in patients elevated compared to controls and showed a positive correlation with the extent of fibrosis | Plasma HMGB1, OPN, and HA are promising biomarkers | Positive correlation between plasma markers and the extent of fibrosis in lesions |
| Ding et al., 2020b; Evidence in support for the progressive nature of ovarian endometriosis (Dinget al., 2020b) | 62 OMA: 30 adolescents (15–19 years), 32 adults (35–39 years) | HE, Masson stain, IHC | In lesions from adults more fibrosis and higher expression of α-SMA. Markers of EMT, FMT, and SMM showed further differentiation. Fibrosis showed a positive correlation with time since ultrasound diagnosis and dysmenorrhea severity | OMA endometriosis lesions in adults are more differentiated in terms of EMT, FMT, and SMM and more fibrotic, which supports the progressive nature of the disease | Fibrotic markers increase in older patients, this supports progressive fibrosis. Fibrosis correlates with dysmenorrhea |
| Guo et al., 2015b; Dating endometriotic ovarian cysts based on the content of cyst fluid and its potential clinical implications (Guo et al., 2015b) | 34 OMA | HE, Masson stain, Picrosirius stain, cyst fluid viscosity measurement | White cyst walls contain 68.2% collagen, mostly type I, more than black cyst walls 54.4%, mostly type I and III. Cyst fluid in white cysts had higher viscosity and iron content | Older endometriotic (white) cysts contain more viscous fluid with higher iron content and more fibrosis: suggesting ReTIAR process resulting in fibrotic lesions resistant to hormonal treatment | Fibrotic area increased in white (older) cyst walls, fibrosis is progressive over time in OMA |
| Kitajima et al., 2011; Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis (Kitajima et al., 2011) | 22 OMA, 11 contralateral ovary biopsies | HE, Masson stain | Follicular density was lower in ovaries with endometriotic cysts. Fibrosis was observed in 80% of ovaries with endometriotic cysts, in 27% without. Fibrosis and presence of cysts independently associated with decreased follicular density | Endometriotic cysts and associated fibrotic tissue may be a cause of reduced ovarian reserve | Fibrosis and endometriotic cysts are independently associated with decreased follicular density |
| Muraoka et al., 2023; Fusobacterium infection facilitates the development of endometriosis through the phenotypic transition of endometrial fibroblasts (Muraoka et al., 2023) | 4 OMA, 4 eutopic endometrium, 4 healthy endometrium | IHC, RT-qPCR, scRNA-seq, FISH | Transgelin (TAGLN) expression was highest in fibroblasts in OMA, also elevated in eutopic endometrium compared to healthy endometrium. Fusobacterium nucleatum was present in 64.3% of eutopic endometrium, 52.4% of ectopic endometrium, 7.1% of healthy endometrium. TGF-β expression was different between F. Nucleatum positive and negative samples | TGF-β promotes myofibroblastic transition, marked by TAGLN expression. TGF-β signaling can be activated by F. nucleatum infection, suggesting a role in pathogenesis. Antibiotic treatment can be a potential therapeutic target | Fusobacterium infection in endometrium triggers myofibroblast activation and thereby attributes to the establishment of endometriotic lesions |
| Nagai et al., 2020; Focal adhesion kinase (FAK)-mediated sequences, including cell adhesion, inflammatory response, and fibrosis as a therapeutic target in endometriosis (Nagai et al., 2020) | 8 OMA, 8 healthy eutopic endometrium | IHC | Focal adhesion kinase (FAK) and monocyte chemoattractant protein-1 expression were upregulated in endometriosis. Co-culture of U937 (macrophage cell line) upregulated TGF-β1 expression | FAK-mediated development of endometriotic lesions is a potential therapeutic target | FAK has a stimulating effect on fibrosis in endometriosis |
| Nie et al., 2022; Identification of lesional attributes of dysmenorrhea severity and the serum antimullerian hormone (AMH) levels in women with ovarian endometriomas (Nie et al., 2022) | 313 OMA | HE, IHC, Masson stain, chemiluminescence for serum hormones | Dysmenorrhea severity correlated negatively with PR-B expression, positively with α-SMA, and lesional fibrosis. AMH levels are not correlated with lesion size, lesional fibrosis, α-SMA, or bilaterality. Adjacent cortical fibrosis varied greatly between patients and correlated negatively with AMH levels | Extent of lesional fibrosis correlated positively with dysmenorrhea severity, not with serum AMH. Ovarian cortical fibrosis correlated negatively with AMH, which argues for early surgical intervention | Lesional fibrosis did not correlate with AMH as a marker for ovarian reserve. Adjacent ovarian cortical fibrosis negatively correlates with AMH. Association between lesional and cortical fibrosis unclear |
| Selcuk et al., 2021; Tumour markers and histopathologic features of ovarian endometriotic cysts (Selcuk et al., 2021) | 97 OMA | HE (thickness fibrosis), blood plasma ELISA | Positive correlation between tumor markers and fibrosis thickness and penetration dept of cyst wall in ovarian tissue. Only CA125 showed a positive correlation with ASRM score | Low levels of tumor markers may permit conservative management, high levels permit surgical intervention based on expected surgical damage on the ovary | Tumor markers are predictive for fibrosis thickness |
| Shi et al., 2017; Transforming growth factor β1 from endometriomas promotes fibrosis in surrounding ovarian tissues via Smad/3 signaling (Shi et al., 2017) | 42 OMA, 29 teratoma controls | HE, Masson stain, IHC, WB, RT-qPCR, FISH | In OMA wall more fibrosis and higher expression of COL1A, α-SMA, TGF-β(R), CTGF, Matrix metalloproteinases, Smad pathway markers | Endometriotic cyst cells produce TGF-β1 leading to fibrosis and adhesions to ovarian tissue via TGF-β1/Smad signaling pathways | Smad pathway is a driver of fibrosis |
| Tsujioka et al., 2009; The efficacy of preoperative hormonal therapy before laparoscopic cystectomy of ovarian endometriomas (Tsujioka et al., 2009) | 96 OMA, 53 untreated, 34 GnRH agonist therapy, 9 danazol | HE, Medical chart review | Hormonal-treated patients had smaller cyst size, no differences in lost primordial follicles, and increased resection time needed. Fibrosis was present in the cyst wall in 45,6% of the untreated group and in all treated patients. More fibrosis between the cyst wall and ovarian tissue | Pre-operative hormonal treatment results in a more favorable pelvic situation. Hormonal treatment did not reduce the loss of primordial follicles. Therapy increases the risk of severe fibrosis and causes difficulties in stripping the cyst wall | Pre-operative hormonal therapy could influence the fibrotic connection between the cyst wall and ovarian tissue |
| Vicino et al., 2009; Fibrotic tissue in the endometrioma capsule: surgical and physiopathologic considerations from histologic findings (Vicino et al., 2009) | 91 OMA | HE (thickness fibrosis), blood plasma ELISA | Negative correlation between fibrotic thickness in cyst wall and CA-125 | High CA-125 correlates with a thinner fibrotic part of the cyst wall and harder stripping removal of the cyst. | CA125 is predictive for thin fibrotic cyst wall |
| Xu et al., 2023; A novel mechanism regulating pyroptosis-induced fibrosis in endometriosis via lnc-MALAT1/miR141-3p/NLRP3 pathway (Xu et al., 2023) | 24 OMA, 24 eutopic endometrium, 26 healthy endometrium | HE, IHC, Masson stain, RT-qPCR, WB | Expression of pyroptosis indicators NLRP3, caspase-1, Gasdermin D and interleukin-1β and fibrotic markers TGF-β1, CTGF, α-SMA, and Fibronectin-1 and extent of fibrosis were increased in OMA versus healthy and patient ectopic endometrium | NLRP3-mediated pyroptosis is upregulated and positively correlated with fibrosis in OMA. Lnc-MALAT1 is increased in endometriosis, which deregulated miR141 expression, leading to increased pyroptosis via NLRP3 expression | NLRP3-mediated pyroptosis via Lnc-MALAT1 regulation promotes fibrosis in endometriosis and is a potential therapeutic target |
| Zhang et al., 2019a; Endometriotic peritoneal fluid promotes myofibroblast differentiation of endometrial mesenchymal stem cells (Zhang et al., 2019a) | 32 OMA, 32 eutopic endometrium, 20 healthy endometrium, Patients and healthy PF | Masson stain, IHC, WB | α-SMA, COL1, CTGF, fibronectin, and the extent of fibrosis increased in OMA compared to eutopic endometrium. Sushi containing domain 2 colocalized with α-SMA in OMA. Patients PF increased fibrotic marker expression in stromal cells | Endometriotic peritoneal fluid promotes myofibroblast differentiation of mesenchymal stem cells | Mesenchymal stem cells are important for fibrosis because they are capable of transdifferentiating to myofibroblasts |
| Zhu et al., 2023; The heterogeneity of fibrogenesis and angiogenesis in endometriosis revealed by single-cell RNA-sequencing (Zhu et al., 2023) | 3 OMA, 3 eutopic endometrium, 3 healthy endometrium | scRNA-seq, HE, IHC, Sirius red stain | Myofibroblasts, pericytes, endothelial cells, and macrophages in abundance in OMA, myofibroblast (MF) C2 dominant MF type (role in ECM organization, TGF-β and Wnt signaling pathway) and derived mainly from FMT | Myofibroblasts, pericytes, and macrophages are potential therapeutic targets | There is an abundance of myofibroblasts in OMA and fibroblasts in eutopic endometrium. Myofibroblasts derived mainly from FMT |
|
DEEP ENDOMETRIOSIS | |||||
| Anaf et al., 2000a; Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules (Anaf et al., 2000a) | 28 DE | HE, Masson stain, IHC | High pain score groups showed more intrafibrotic and intraglandular nerves and peri- and endoneural invasion of endometriosis | There is a close histological relationship between nerves, endometriotic foci, and their fibrotic environment | Colocalization between fibrosis and nerves implies the role of fibrosis in pain |
| Bonte et al., 2002; Histologic appearance of endometriosis infiltrating uterosacral ligaments in women with painful symptoms (Bonte et al., 2002) | 172 DE | HE | Fibrosis was detected in 59.9% of clinical suspect lesions, and smooth muscle hyperplasia in direct contact with glands in 14,5% of lesions. Connective-muscular tissue in close contact with nerve networks. Typical lesions found in 70,8% of clinical suspect lesions | Histologic appearance of suspect lesions is heterogenous. Fibrosis is often present. Smooth muscle hyperplasia is sometimes present, and could be associated with nerve networks | Fibrosis is often present in endometriotic lesions and sometimes associated with nerve networks |
| Ding et al., 2020a; Diagnosing deep endometriosis using transvaginal elastosonography (TVESG) (Ding et al., 2020a) | 34 DE | HE, IHC, MRI, ultrasound, shear-wave elastosonography | DE lesions detected by: pelvic examination 83,3%; ultrasound 66,7%; MRI 83,3%; TVESG 100%. Missed lesions were smaller, higher stiffness. Fibrosis showed a positive correlation with stiffness, α-SMA, and PR, a negative correlation with ER and vascular density | TVESG could help diagnostics by differentiating tissue stiffness between the lesion and surrounding tissue and estimate the developmental stage of the lesion | Stiffness of fibrotic tissue could be used in the diagnostic process |
| Itoga et al., 2003; Fibrosis and smooth muscle metaplasia in rectovaginal endometriosis (Itoga et al., 2003) | 90 DE | HE, Azan, von Gieson, Berlin blue stains, IHC | Fibrosis is present in 89/90 samples. If mild, fibrosis mainly around glands/stroma, if severe, fibrosis incorporated fat and connective tissue around lesions. Lower fibrosis scores in GnRH-treated patients. Smooth muscle metaplasia in 80/90 samples, positive correlation with fibrosis. No differences in GnRH yes or no groups | Smooth-muscle metaplasia is present in fibrotic areas and becomes more severe correlating with increased fibrosis | Fibrosis and smooth-muscle metaplasia are common features of endometriosis. GnRH treatment might prevent fibrogenesis |
| Roman et al., 2009; Histopathological features of endometriotic rectal nodules and the implications for the management by rectal nodule excision (Roman et al., 2009) | 27 DE | HE | 14/27 cases showed infiltration of fibrosis and glands/stroma in same rectal layer. Deeper infiltration of glands/stroma than fibrosis in 24/27. 3/27 deeper fibrosis than glands/stroma | In majority of lesions, gland/stroma infiltration is deeper than fibrosis. Surgical excision of macroscopic fibrosis will leave glands/stroma intact and may continue natural evolution. Could be cause of recurrence | Fibrosis infiltration depth in bowel follows lesion infiltration dept, might indicate fibrogenesis as a reaction to lesion ingrowth |
| Stratopoulou et al., 2021; Identifying common pathogenic features in deep endometriotic nodules and uterine adenomyosis (Stratopoulou et al., 2021) | 13 DE, 14 adenomyosis, 27 eutopic endometrium, 14 healthy endometrium | HE, Picrosirius red stain, IHC | Collagen/stroma rates: Healthy 20% collagen, DE 60%, adenomyosis 65%. DE showed decreased platelet aggregation and increased macrophage infiltration, comparable with adenomyosis | Macrophage accumulation, fibrosis and irregular angiogenesis are common in DE and adenomyosis. DE and adenomyosis show histologic similarities | Fibrosis common in DE, similarities between adenomyosis and DE |
| van Kaam et al., 2008; Fibromuscular differentiation in deeply infiltrating endometriosis is a reaction of resident fibroblasts to the presence of ectopic endometrium (van Kaam et al., 2008) | 20 DE | IHC | Stroma of lesions showed high vimentin and low α-SMA, desmin and SM-MHC expression, fibromuscular tissue around lesion showed strong α-SMA and SM-MHC expression. Smad colocalized with TGF-β receptors in stroma | Fibromuscular differentiation is present in DE lesions and the result of a reaction of the local environment to the presence of ectopic endometrium | More FMT toward periphery of lesions. Smad and TGF-β receptors showed a connection |
| Xie et al., 2013; Potential role of strain elastosonography for detection of the extent of large-scar endometriosis (Xie et al., 2013) | 8 DE | HE, IHC, MRI, ultrasound, strain elastosonography | Elastosonography showed larger extent of lesions (62.4 mm) than MRI (40.9 mm) and conventional US (41.4 mm). All cases showed strong collagen expression | Strain elastosonography could enhance diagnostic accuracy of scar endometriosis | Stiffness of fibrotic tissue could be used in diagnostics |
Studies assessing multiple endometriosis subtypes are only shown in the first section of the table and not in the subsequent following categories to avoid double information. Studies including both observational and intervention approaches are shown in both tables. Information depicted in each table is specific regarding that particular part of the study. The conclusion column shows a conclusion as stated by the authors, so this is including results from both parts of the study. Sample size of number of biopsies is shown, in some cases multiple biopsies from a single patient were included separately. PER, peritoneal endometriosis; OMA, ovarian endometrioma; DE, deep endometriosis; HE, hematoxylin/eosin staining; IHC, immunohistochemistry; IF, immunofluorescence; WB, western blot; RT-qPCR, real-time qualitative polymerase chain reaction; α-SMA, α-smooth muscle actin; TGF-β, transforming growth factor-β; COL, collagen; CTGF or CCN2, connective tissue growth factor; SM-MHC, smooth muscle-myosin heavy chain; EMT, epithelial-to-mesenchymal transition; FMT, fibroblast-to-myofibroblast transdifferentiation; SMM, smooth muscle metaplasia; SMC, smooth muscle cell; ER, estrogen receptor; PR, progesterone receptor; ASRM score, American Society of Reproductive Medicine score.
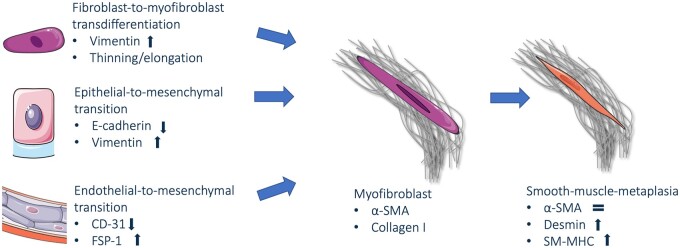
Fibrosis affected tissue and its main cell type myofibroblasts were observed in almost all endometriotic lesions. Myofibroblast associated with endometriosis were found to differentiate from epithelial and endothelial cells toward mesenchymal and ultimately smooth muscle-like cells. This was based on observations of an increased expression of mesenchymal and smooth muscle cell markers like vimentin, desmin, and smooth-muscle myosin heavy chain in all studies assessing these markers as main outcome measures, as schematically presented in Fig. 2 (Anaf et al., 2000b; Itoga et al., 2003; Yan et al., 2020a,b). In general, this stage of differentiation was observed most extensively toward the periphery of lesions (Barcena de Arellano et al., 2011; Ibrahim et al., 2019). This was suggested to be associated with continuation of differentiation and outgrowth over time, since more thorough FMT and more extensive fibrosis is found in lesions in adults compared to adolescents (Guo et al., 2015b; Ding et al., 2020b). Myofibroblasts throughout the central stromal compartment showed less smooth muscle-like characteristics and rather resembled eutopic endometrial stromal cells, suggesting a different stage of myofibroblastic transdifferentiation (Konrad et al., 2018). It was suggested that myofibroblast activation, marked by transgelin expression, is at least in some cases triggered by endometrial infection by Fusobacterium nucleatum, which thereby gives myofibroblasts the ability to initiate endometriosis after retrograde menstruation (Muraoka et al., 2023). The macroscopic aspect and color of peritoneal lesions correlated with the collagen content, which was higher in black compared to red lesions, but was not associated with the amount of SMM (Matsuzaki et al., 1999; Barcena de Arellano et al., 2011). Furthermore, when comparing different endometriosis subtypes, the most extensive fibrosis and myofibroblast transdifferentiation was observed in deep lesions (Liu et al., 2018). In ovarian endometriosis, fibrosis did form a well-organized lining between cysts wall and ovarian tissue, whereas in other subtypes fibrosis was more scattered (Khare et al., 1996). Additionally, more fibrosis was found in the cyst wall of endometrioma in patients treated with hormonal therapy compared to untreated patients (Tsujioka et al., 2009).
Figure 2.
Schematic representation of cellular transitions contributing to myofibroblasts in endometriotic lesions. Fibroblast-to-myofibroblast transdifferentiation (FMT), epithelial-to-mesenchymal transition (EMT), and, to a lesser extent, endothelial-to-mesenchymal transition (EndoMT) are sources of myofibroblasts in endometriosis, marked by expression of α smooth muscle actin (α-SMA). In FMT, expression of vimentin increases, and fibroblasts thin and elongate. In EMT, the expression of E-cadherin decreases, and expression of vimentin increases. In EndoMT, expression of CD-31 decreases, and expression of fibroblast-specific protein (FSP1) increases. Smooth-muscle-metaplasia (SMM) can lead to smooth muscle-like cells in endometriosis, expressing desmin and smooth muscle myosin heavy chain (SM-MHC).
The origin and detailed characterization of cell types present in endometriotic lesions has been studied based on single-cell RNA profiling. Different myofibroblast and macrophage subpopulations were identified across the different subtypes of endometriosis. An abundance of myofibroblasts, marked by Periostin (POSTN), Collagen 6A1 (COL6A1), and Fibronectin (FN1), was found in all subtypes. Myofibroblasts in both PER and DE showed similarities based on their additional specific expression of Secreted frizzled-related protein 1 (SFRP1) and Peptidase domain containing associated with muscle regeneration 1 (PAMR1) or Alpha-2-macroglobulin (A2M) and Collagen 4A1 (COL4A1), whilst the main myofibroblasts in OMA showed additional Transmembrane protein 19 (TMEM19) and Tenascin C (TNC) expression (Shin et al., 2023). Zhu et al., showed the abundance of myofibroblasts and macrophages in OMA. They reported a dominant subpopulation of myofibroblasts characterized by genes involved in TGF-β responsiveness, Wnt signaling, and ECM formation. These myofibroblasts mainly derived from FMT and their proliferative potential was very low.
The process of transdifferentiation was accompanied by a decreased expression of hormone receptors for estrogen and progesterone and markers for prostaglandin signaling (Mechsner et al., 2005; Huang et al., 2021). Collagen types I and IV were found to be the most common type of collagen in endometriosis (Stovall et al., 1992; Nezhat and Kalir, 2002). Besides this, the expression of neural cell adhesion molecule (NCAM) and neuropeptides like substance P (SP) and calcitonin gene-related peptide (CGRP) was positively correlated with increased fibrosis and myofibroblast transdifferentiation, as well as with dysmenorrhea severity, indicating a colocalization between sensory nerves and fibrosis (Anaf et al., 2000a; Bonte et al., 2002; Odagiri et al., 2009; Yan et al., 2019b; Zhang et al., 2019a). These studies hypothesized that due to this colocalization and the correlation between fibrosis and dysmenorrhea severity, fibrosis plays an important role in pain. Compared to healthy endometrium, α7 nicotinic acetylcholine receptor expression was reduced in endometriosis, and correlated negatively with the extent of fibrosis and dysmenorrhea severity (Hao et al., 2022). These findings indicated a role for the cholinergic anti-inflammatory pathway in endometriosis, supported by animal experiments of this group, which are discussed below (Hao et al., 2021).
Based on the observational studies markers of Smad and FAK signaling pathways were upregulated (Shi et al., 2017; Nagai et al., 2020). Upregulation of these signaling pathways was associated with upregulation of TGF-β (van Kaam et al., 2008). Therefore, these pathways were hypothesized to be important pathways in the etiology of fibrosis in endometriosis.
Furthermore, the characteristics of fibrosis show the potential to be used for diagnostic purposes in the future. The potential biomarkers osteopontin, high mobility group box 1 (HMGB1), and hyaluronic acid showed a positive correlation with the extent of fibrosis, in contrast to the association between CA-125 and fibrosis, about which literature reported both a positive and a negative correlation (Vicino et al., 2009; Cao et al., 2019; Selcuk et al., 2021). The stiffness of fibrotic deposits can be detected with elastosonography, which yields a higher diagnostic accuracy than regular ultrasound examination. However, this is only studied for deep endometriosis (Xie et al., 2013; Ding et al., 2020a).
From the perspective of fertility, the extent of fibrosis in ovarian cysts is shown to be independently correlated with a decreased follicular density in the adjacent ovary (Kitajima et al., 2011). On the other hand, Nie et al., did not find a correlation between lesional fibrosis present within the ovarian endometriosis cyst or α-SMA expression in endometrioma and serum levels of anti-mullerian hormone (AMH). They did report a correlation between ovarian fibrosis present in the ovarian cortex, probably triggered by the endometrioma, and serum AMH, however, the interplay between lesional and ovarian fibrosis herein is still unclear (Nie et al., 2022).
Experimental studies with human-derived material
Experimental studies with human-derived material were performed in 28 studies. An overview is presented in Table 2.
Table 2.
Experimental studies with human-derived material.
| Reference | Sample size | Intervention | Assay methods | Results | Conclusion | Relevance to fibrosis |
|---|---|---|---|---|---|---|
|
MULTIPLE ENDOMETRIOSIS SYBTYPES | ||||||
| Matsuzaki and Darcha, 2013; Involvement of the Wnt/B-catenin signaling pathway in cellular and molecular mechanisms of fibrosis in endometriosis (Matsuzaki and Darcha, 2013) | 40 OMA and DE, not specified. 40 eutopic endometrium, 30 healthy endometrium | siRNA knockdown of β-catenin; Tcf/β-catenin antagonists (PKF 115–584, 6.25 µM and CGP049090, 6.25 µM), with or without TGF-β1 (5 ng/ml). Wnt3a stimulation | RT-qPCR | α-SMA and collagen I mRNA expression not altered by β-catenin siRNA in stromal cells. TGF-β1 increased α-SMA and collagen I expression, effect attenuated by β-catenin siRNA, both in ectopic and eutopic cells. α-SMA and collagen I expression decreased by treatment with PKF 115-584 and CGP049090 in ectopic and eutopic stromal cells. Treatment attenuated TGF-β1 dependent increase | Wnt/β-catenin activation may be involved in fibrogenesis in endometriosis | Wnt/β-catenin signaling promotes fibrosis, potential therapeutic target |
| Matsuzaki et al., 2020; Dose-dependent pro- or anti-fibrotic response of endometriotic stromal cells to interleukin (IL)-1β and tumor necrosis factor α (Matsuzaki et al., 2020) | 36 OMA and DE, not specified. 16 eutopic endometrium, 8 healthy endometrium | IL-1β (1–10 pg/ml) or TNF-α (10–1000 pg/ml). With or without TGF-β1 (5 ng/ml) | WB, RT-qPCR, IF | Fibrotic marker expression is higher in ectopic versus eutopic cells. In eutopic cells, no effect of IL-1β or TNF-α. Fibrotic marker expression increased after low-dose IL-1β or TNF-α but decreased after high doses. | Low-grade inflammation stimulates a fibrotic phenotype, whereas high-grade inflammation inactivates a fibrotic phenotype in endometriotic stromal cells | Fibrogenesis reacts differently on high- and low-grade inflammatory stimulus |
| Shao and Wei, 2018; FOXP1 enhances fibrosis via activating Wnt/B-catenin signaling pathway in endometriosis (Shao and Wei, 2018) | 6 OMA and DE, not specified, 6 eutopic endometrium, 6 healthy endometrium | siRNA knockdown of Forkhead box protein 1 (FOXP1); Wnt signaling inhibitor AVX939 | WB, RT-qPCR | Fibrotic markers, β-catenin, and FOXP1 expression are higher in ectopic versus eutopic cells. siRNA knockdown of FOXP1 decreased fibrotic markers expression, β-catenin acetylation, and Wnt signaling. Wnt signaling inhibition attenuated effects of FOXP1 knockdown | FOXP1 is upregulated in endometriotic stromal cells and stimulates fibrosis via Wnt/β-catenin signaling pathways | Wnt/β-catenin signaling has an important role in fibrogenesis |
|
| ||||||
|
OVARIAN ENDOMETRIOTIC CYSTS | ||||||
| Hirakawa et al., 2019; β-catenin signaling inhibitors ICG-001 and C-82 improve fibrosis in preclinical models of endometriosis (Hirakawa et al., 2019) | 11 OMA, 6 healthy endometrium | CREB binding protein (CBP)/β-catenin signaling inhibitors ICG-001 or C-82, concentrations 0–200 µM | WB, RT-qPCR | α-SMA expression higher in ectopic stromal cells than eutopic cells (mRNA and protein). ICG-001 and C-82 treatment downregulated α-SMA mRNA expression but not protein expression, decreased viability and proliferation and increased apoptosis | CBP/β-catenin is an important signaling pathway in endometriosis and a potential therapeutic target | β-catenin signaling has an important role in fibrogenesis |
| Huang et al., 2022b; Tetramethylpyrazine (TMP) retards the progression and fibrogenesis of endometriosis (Huang et al., 2022b) | 5 OMA | Activated platelets; 0, 25, or 100 µM TMP | WB, RT-qPCR | Activated platelets induced FMT-like morphological changes in stromal cells. TMP treatment abolished this effect. TMP dose dependently suppressed of α-SMA, CCN2, and collagen I RNA expression and TGF-β1, α-SMA, p-Smad3, and collagen I protein expression. Treatment attenuated increase of contractility and reduced collagen production | TMP treatment inhibits platelet-induced myofibroblast activation in stromal cells resulting in reduced contractility and collagen production | TMP treatment has anti-fibrotic effect via inhibition of myofibroblast activation induced by platelets |
| Mohankumar et al., 2020; Bis-indole-derived nuclear receptor 4A1 (NR4A1) ligands as inhibitors of endometriosis (Mohankumar et al., 2020) | 2 OMA, experiments in triplicate | DIM-C-pPhOH and DIM-C-pPhOH-3Cl-5-OCH3 in various concentrations. Knockdown with siNR4A1 | WB, RT-qPCR, IF | NR4A1 knockdown decreased expression of BCL-2 family and α-SMA, increased Bax, caspase 3, and induced apoptosis. DIM-C-pPhOH and DIM-C-pPHOH-3-Cl-5-OCH3 decreased expression of fibrotic markers and BCL-2 family, induced apoptosis | NR4A1 is a pro-endometriotic transcription factor and inhibition with Bis-indole-derived antagonist is promising as a new non-hormonal therapy | NR4A1 is a pro-endometriotic factor and inhibition results in decreased expression of fibrotic factors |
| Muraoka et al., 2023; Fusobacterium infection facilitates the development of endometriosis through the phenotypic transition of endometrial fibroblasts (Muraoka et al., 2023) | 4 OMA, 4 eutopic endometrium, 4 healthy endometrium | TAGLN vector, pcDNA3.4-TAGLN, siRNA targeting TAGLN, F. nucleatum co-culture | IHC, RT-qPCR, scRNA-seq, FISH | OMA fibroblast increased expression of transgelin (TAGLN), α-SMA, vimentin. TAGLN siRNA decreased proliferation and contractility, increased by TAGLN stimulation and IL-6. TGF-β upregulated TAGLN expression, abolished by Smad2/3 inhibitor SB431542. F. Nucleatum co-culture with THP1-derived macrophages stimulated M2 differentiation and elevated TAGLN expression | TGF-β promotes myofibroblastic transition, marked by TAGLN expression. TGF-β signaling can be activated by F. nucleatum infection, plays a role in pathogenesis. Antibiotic treatment can be a potential therapeutic target | Fusobacterium infection in endometrium triggers myofibroblast activation and thereby attributes to endometriotic lesion establishment |
| Nagai et al., 2020; Focal adhesion kinase-mediated sequences, including cell adhesion, inflammatory response and fibrosis as a therapeutic target in endometriosis (Nagai et al., 2020) | 1 OMA, 1 eutopic endometrium, 1 healthy endometrium | 5 µM FAK inhibitor, 20 µM MEK inhibitor, 15 µM JNK inhibitor | WB, ELISA | FAK and MCP1 expression was upregulated in endometriosis. TGF-β1 increased α-SMA expression, FAK inhibition attenuated this effect. Co-culture of U937 (macrophage cell line) upregulated TGF-β1 expression, effect attenuated by FAK inhibition | FAK mediated development of endometriotic lesions is a potential therapeutic target | FAK has a stimulating effect in fibrosis in endometriosis |
| Nasu et al., 2010; Heparin is a promising agent for the treatment of endometriosis-associated fibrosis (Nasu et al., 2010) | 9 OMA | Heparin sodium 1–100 µg/ml | WB | Heparin treatment decreased protein expression of α-SMA, RhoA, Rock I and II, and collagen gel contraction | Heparin inhibited Rho/Rock signaling and fibrotic markers, which suggests that the Rho/Rock pathway is the mechanism of action of heparin in influencing myofibroblastic transformation | Heparin has anti-fibrotic properties via inhibition of Rho/Rock signaling |
| Shi et al., 2017; Transforming growth factor β1 from endometriomas promotes fibrosis in surrounding ovarian tissues via Smad2/3 signaling (Shi et al., 2017) | 3 OMA | TGF-β1 (10 ng/ml) | WB, RT-qPCR | Smad signaling pathway markers upregulated directly after TGF β1 stimulation, fibrotic markers increased | Endometriotic cyst cells produce TGF-β1 leading to fibrosis and adhesions to ovarian tissue via TGF-β1/Smad signaling pathways | Smad pathway is a driver of fibrosis |
| Tsuno et al., 2011; Fasudil inhibits the proliferation and contractility and induces cell cycle arrest and apoptosis of human endometriotic stromal cells: a promising agent for the treatment of endometriosis (Tsuno et al., 2011) | 8 OMA | Fasudil (ROCK inhibitor) 100 µM | WB | Fasudil reduced α-SMA, ROCK I and II but not RhoA expression. BCL-2 family expression strongly reduced by fasudil, leading to increased apoptosis. Collagen gel contractility and myofibroblastic differentiation were reduced | Fasudil inhibits cell proliferation, induces cell cycle arrest and apoptosis by down-regulating BCL-2, inhibits collagen contractility and the myofibroblastic transformation, via Rho/ROCK mediated signaling | Fasudil has potential anti-fibrotic properties via ROCK signaling inhibition and apoptosis induction via BCL-2 signaling |
| Tsuno et al., 2009; Decidualization attenuates the contractility of eutopic and ectopic endometrial stromal cells: implications for hormone therapy of endometriosis (Tsuno et al., 2009) | 12 OMA, 8 eutopic endometrium, 9 healthy endometrium | In vitro decidualization by db-cAMP and medroxy-progesteron acetate (MPA) or dienogest | WB, ELISA | RhoA, ROCK I and II, and α-SMA expression and collagen gel contraction reduced after in vitro decidualization by both protocols | Decidualization inhibits the contractility of stromal cells by downregulation of collagen I receptor and Rho-ROCK pathways; inhibits differentiation to myofibroblasts | Contractility and myofibroblastic transformation is attenuated by decidualization, which could be of importance for hormonal interventions |
| Wang et al., 2023; PIM2 promotes the development of ovarian endometriosis by enhancing glycolysis and fibrosis (Wang et al., 2023) | 50 OMA, 50 eutopic endometrium, 50 healthy endometrium | Flag-PIM2, PIM2 inhibitor SMI-4a, PIM2 siRNA PKM2 inhibitor 3K | WB, IHC | PIM2 (proviral insertion in murine lymphomas 2) was upregulated in OMA and positively correlated with HK2, PKM2, SMH (smooth muscle myosin heavy chain), Desmin and α-SMA. Flag-PIM2 increased expression of Desmin, SMH and α-SMA, siRNA knockdown decreased this expression. PKM2 inhibitor abolished stimulating effect | PIM2 promotes glycolysis and fibrogenesis via enhancing PKM2 expression | PIM2 promotes fibrosis in endometriosis via PKM2, SMI-4a is a potential anti-fibrotic target |
| Wu et al., 2018; Exosomal miR-214 from endometrial stromal cells inhibits endometriosis fibrosis (Wu et al., 2018) | 24 OMA, 24 eutopic endometrium, 24 healthy endometrium | miRNA-214 with or without TGF-β stimulation | WB, RT-qPCR, ISH, IF | Expression of α-SMA, CTGF, collagen A1 was increased in OMA. Expression increased in all cells after TGFβ stimulation. Expression in OMA was reduced after miRNA-214 treatment. miRNA-214 attenuated effect of TGF-β stimulation in all cells | miRNA-214 is downregulated in endometriosis, upregulation is a potential therapeutic strategy for endometriosis | The downregulation of miRNA-214 in endometriosis may drive fibrosis via CTGF, upregulation is a potential therapeutic strategy |
| Yan et al., 2019b; Neuropeptides substance P and calcitonin gene-related peptide accelerate the development and fibrogenesis of endometriosis (Yan et al., 2019b) | 8 OMA | Substance P (SP), calcitonin gene-related protein (CGRP), aprepitant, CGRP fragment 8-37 | WB, RT-qPCR, IHC | SP or CGRP treatment induced expression of α-SMA, collagen A1, and markers for myofibroblastic differentiation. Aprepitant and/or CGRP fragment 8-37 (as receptor antagonists) blocked these effects | Sensory nerves have an important role in promoting fibrogenesis. SP, CGRP and their receptors stimulate EMT, FMT, and SMM. Anatomical link between DE and multiple nerve plexus could explain higher fibromuscular content in DE | Colocalization of nerves and fibrosis and fibrosis-stimulating effect of neuropeptides implies contribution of fibrosis to pain in endometriosis |
| Yoshino et al., 2020; Relaxin-2 may suppress endometriosis by reducing fibrosis, scar formation, and inflammation (Yoshino et al., 2020) | 6 OMA | Relaxin-2 100 ng/ml | WB, IHC, RT-qPCR | Relaxin-2 treatment reduced collagen and interleukin-8 expression and collagen gel contraction but did not affect α-SMA and CTGF expression. Protein kinase A inhibition by H89 attenuated effect of relaxin treatment | Relaxin-2 treatment may reduce fibrosis, scar forming, and inflammation in endometriosis | Relaxin-2 reduced formation of collagen but did not affect myofibroblast differentiation, anti-fibrotic properties thereby unclear |
| Yuge et al., 2007; Collagen gel contractility is enhanced in human endometriotic stromal cells: a possible mechanism underlying the pathogenesis of endometriosis-associated fibrosis (Yuge et al., 2007) | 10 OMA, 8 healthy endometrium | Y-27632 (ROCK inhibitor) 0.1–100 µM | WB, ELISA | Expression of RhoA, ROCK I and II, and α-SMA and collagen gel contraction was elevated in OMA cells. Y-27632 reduced fibrotic marker expression and decreased collagen gel contraction | ROCK pathway overexpression and successful ROCK inhibition suggest that ROCK mediated myofibroblastic differentiation is responsible for the collagen contraction in endometriosis | Rho/ROCK inhibition is a potential anti-fibrotic therapeutic strategy |
| Zeng et al., 2018; NR4A1 is involved in fibrogenesis in endometriosis (Zeng et al., 2018) | 23 OMA, 15 healthy endometrium | NR4A1 siRNA knockdown, Csn-β1, TGF-β1, MK2206 | WB, RT-qPCR, IHC | NR4A1 siRNA combined with TGF-β1 increased α-SMA, FN, COL1A1, CTGF expression. Csn-β1 decreased TGF-β1-dependent NR4A1 phosphorylation and decreased α-SMA, FN, COL1A1, and CTGF expression | NR4A1 can regulate fibrogenesis in endometriosis in a TGF-β1 dependent manner | NR4A1 has anti-fibrotic properties, phosphorylated NR4A1 has pro-fibrotic properties, both acting via AKT and TGF- β1 signaling |
| Zhang et al., 2016a; Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation (Zhang et al., 2016a) | 17 OMA | A83-01, co-culture with platelets, with or without activation with thrombin or thrombin alone | WB, IHC, RT-qPCR, ELISA | Expression of markers for EMT, FMT, fibrosis and Smad signaling, and collagen gel contraction increased after co-culture with activated platelets. TGF-β1 inhibition with A83-01 attenuated these effects | Activated platelets promote EMT, FMT, SMM via TGF-β1 and Smad signaling pathway, leading to fibrosis in endometriosis. Platelet-targeted therapy could therefore be a promising therapeutic strategy | Platelets stimulate fibrogenesis via TGF-β |
| Zhang et al., 2021; Downregulation of exosomal miR-214-3p targeting CCN2 contributes to endometriosis fibrosis and the role of exosomes in the horizontal transfer of miR-214-3p (Zhang et al., 2021) | OMA, eutopic endometrium and healthy endometrium stromal cell line | miR-214-3p mimics, miR-214-3p inhibitors | WB, IHC, RT-qPCR | Expression of α-SMA, CCN2, and collagen A1 elevated in OMA, miRNA-214-3p transfection decreased CCN2 expression and fibrotic marker, miRNA-214-3p inhibition increased CCN2 | miRNA-214-3p is downregulated, causing CCN2 increase in endometriosis. miR-214-3p has the potential to stop fibrosis progression via CCN2 signaling. Exosomes are a potential miRNA drug carrier | miRNA-214 downregulation in endometriosis stimulates fibrosis, miRNA therapy inhibits CCN2 to reduce fibrosis. Exosomes have the potential as RNA-based therapy carriers |
| Zhang et al., 2022; Ferroptosis induced by iron overload promotes fibrosis in ovarian endometriosis and is related to subpopulations of endometrial stromal cells (Zhang et al., 2022) | 38 OMA, 38 eutopic endometrium | Ferric ammonium citrate (FAC), erastin; Ferrostatin-1, deferoxamine mesylate | WB, IHC, electron microscopy, iron quantification, HE | Iron deposits and iron ion levels increased in OMA versus eutopic tissue. ROS and markers for ferroptosis 4-NHE, MDA, PTGS2, and NOX1 were increased in OMA. FAC treatment induced ferroptosis and upregulated α-SMA and COL1, effect attenuated by ferroptosis inhibitor ferrostatin-1. Erastin-induced ferroptosis but not fibrotic marker expression | Ferroptosis is induced in endometriosis by increased iron concentration. FAC treatment simulates effects of ferroptosis and caused increased fibrotic marker expression | Iron accumulation in endometriosis can trigger ferroptosis and subsequentially fibrogenesis |
| Zhang et al., 2023b; Flavonoids quercetin and kaempferol are NR4A1 antagonists and suppress endometriosis in female mice (Zhang, Mohankumar, et al., 2023b) | OMA epithelial and stromal cell line | Quercetin 25-100 µM and kaempferol 25-150 µM; NR4A1 siRNA | WB, IF | siNR4A1, quercetin, and kaempferol all inhibited endometriotic, but not normal endometrial cell proliferation. Quercetin and kaempferol suppressed mTOR signaling. siNR4A1, quercetin and kaempferol inhibited the expression of α-SMA, CTGF, COL1A1, and FN in epithelial cells but not stromal cells | NR4A1 has a central role in fibrogenesis and inhibition with quercetin and kaempferol are promising therapeutic targets | NR4A1 has a pro-fibrotic effect, but the discrepancy between the anti-fibrotic effect on epithelial and stromal cells of its inhibitors quercetin and kaempferol needs more research |
|
| ||||||
|
DEEP ENDOMETRIOSIS | ||||||
| González-Foruria et al., 2017; Dysregulation of the ADAM17/Notch signaling pathways in endometriosis: from oxidative stress to fibrosis (González-Foruria et al., 2017) | 8 DE, 8 eutopic endometrium, 8 healthy endometrium, 202 PF | 100 µM DAPT or 2,3 µM FLI-06 (y-secretase inhibitors). H2O2 increasing concentrations 0-40 µM, cell culture supernatants or ADAM17 purified protein 0,01 µg/ml | Picrosirius red stain, WB | Notch cleavage inhibition (DAPT or FLI-6) reduced α-SMA and collagen I levels in DIE, not in control. ADAM17 or supernatant increased cleaved Notch and α-SMA, with or without H2O2 | Increased oxidative stress hyperactivates the ADAM17/Notch signaling pathway and consequently increased expression of fibrotic markers α-SMA and Collagen I | Oxidative stress promotes fibrogenesis and FMT through ADAM17/Notch signaling |
| Leconte et al., 2010; Antiproliferative effects of cannabinoid agonists on deep infiltrating endometriosis (Leconte et al., 2010) | 14 DE, 14 eutopic endometrium, 12 healthy endometrium | WIN 55212-2 0,3 to 40 µM | WB | WIN55212-2 decreased proliferation, ROS production, and (p)AKT, with no effect on (p)ERK and α-SMA | Cannabinoid agonist inhibits Akt signaling and decreases DE stromal cell proliferation and ROS production. No significant effect on α-SMA expression | Cannabinoid agonist treatment does not lead to significantly reduced α-SMA expression |
| Matsuzaki et al., 2023; Interleukin (IL)-10 is not anti-fibrotic but pro-fibrotic in endometriosis: IL-10 treatment of endometriotic stromal cells in vitro promotes myofibroblast proliferation and collagen type I protein expression (Matsuzaki et al., 2023) | 54 DE, 30 healthy endometrium | TGF-β; IL-6, soluble IL-6 receptor (sIL-6R), IL-10 | IF, WB | IL-10 increased col 1 expression, attenuated by STAT3 siRNA. IL-10 increased α-SMA positive cells and collagen contraction, but not col 1 positive cells, attenuated by STAT3 siRNA. Strongest pro-fibrotic effect of IL-10 if administration after TGF-β or IL-6/sIL-6R stimulation, milder effect if administration before | IL-10 is pro-fibrotic via STAT3 activation in endometriosis as it promotes myofibroblast proliferation and collagen expression | IL-10, known as anti-inflammatory cytokine has pro-fibrotic effects in endometriosis, highlighting the complex inflammatory interaction in endometriosis |
| Matsuzaki et al., 2022; Persistent activation of signal transducer and activator of transcription 3 via interleukin (IL)-6 trans-signaling is involved in fibrosis of endometriosis (Matsuzaki et al., 2022) | 36 DE, 24 eutopic endometrium, 32 healthy endometrium | STAT3 siRNA; IL-6, soluble IL-6 receptor (sIL-6R), TGF-β, S3I-201 (STAT3 inhibitor), NF-κB inhibitor BMS-345541 | IF, WB | IL-6 or sIL-6R no effect in healthy, but increased COL1 in endometriotic cells. STAT3 siRNA and S3I-201 decreased COLl1 expression in endometriotic cells. TGF-β and/or IL-6/sIL-6R increased α-SMA positive cells. STAT3 siRNA had no effect, whereas S3I-201 decreased COL1 positive cells, both decreased α-SMA positive cells | Dysregulated STAT3 activation stimulates fibrogenesis via IL-6 and soluble IL-6 receptor signaling in endometriosis | STAT3/IL-6 dysregulation promotes fibrogenesis in endometriosis |
| Matsuzaki et al., 2016; Soft matrices inhibit cell proliferation and inactivate the fibrotic phenotype of deep endometriotic stromal cells in vitro (Matsuzaki et al., 2016) | 40 DE, 40 eutopic endometrium, 23 healthy endometrium | Culture cells on top of 2, 4, 8, 16, 30 kPa stiffness gel, with or without TGF-β 5 ng/mL | IHC, RT-qPCR | In DE cells proliferation, α-SMA and collagen increased on stiffer matrix, strongest with TFG-β, also without. Both patient and healthy eutopic cells showed only α-SMA and collagen expression on high stiffness matrix with TGF-β stimulation | Soft matrix inhibited cell proliferation and decreased fibrotic markers. Stiff matrix increased fibrotic markers. This implies that DE cells react to stiff environment | Stiffness of fibrosis stimulates further fibrotic changes, causing a positive feedback loop |
| Matsuzaki and Darcha, 2014; Antifibrotic properties of epigallocatechin-3-gallate in endometriosis (Matsuzaki and Darcha, 2014) | 45 DE, 10 healthy endometrium | Epigallocatechin-3-gallate (EGCG) and N-acetyl-L-cysteine (NAC), with or without TGF-β 5 ng/ml | WB, RT-qPCR, IF | EGCG treatment decreased fibrotic markers in DE and healthy cells, and attenuated TGF-β-dependent increase of these markers. NAC treatment decreased α-SMA, but did not affect other fibrotic markers in healthy cells, with no effect in ectopic cells. Immunofluorescence showed decrease of α-SMA positive cells after EGCG treatment, no effect of NAC | Epigallocatechin-3-gallate is a potential anti-fibrotic drug candidate | Epigallocatechin-3-gallate as a potential treatment for endometriosis decreases fibrotic markers |
Eutopic endometrium: eutopic endometrium from endometriosis patients as control. Healthy endometrium: eutopic endometrium from non-endometriosis patients as control, in some studies these patients do have other (gynaecologic) diseases, for example, uterine fibroids or mild cervical dysplasia. PER, peritoneal endometriosis; OMA, ovarian endometrioma; DE, deep endometriosis; HE, hematoxylin/eosin staining; IHC, immunohistochemistry; IF, immunofluorescence; WB, western blot; RT-qPCR, real-time qualitative polymerase chain reaction; α-SMA, α-smooth muscle actin; TGF-β, transforming growth factor-β; COL, collagen; CTGF or CCN2, connective tissue growth factor; FN, fibronectin; SM-MHC, smooth muscle-myosin heavy chain; EMT, epithelial-to-mesenchymal transition; FMT, fibroblast-to-myofibroblast transdifferentiation; SMM, smooth muscle metaplasia; SMC, smooth muscle cell; ER, estrogen receptor; PR, progesterone receptor; ASRM score, American Society of Reproductive Medicine score.
Most experimental studies with human-derived material were focused on identification of cellular mechanisms and signaling pathways affecting fibrosis. At the tissue level, a high stiffness of the cellular environment was identified as a promoter of fibrogenesis in endometriosis (Matsuzaki et al., 2016). At the cellular level, an important causative factor is the presence of thrombocytes, as these cells are found to be important in promoting fibrogenesis through secretion of TGF-β (Zhang et al., 2016a). The platelet-inhibiting herbal compound tetramethylpyrazine showed potential to stop pro-fibrotic EMT and FMT and thereby inhibit fibrogenesis (Huang et al., 2022b). A pro-fibrotic effect was also observed from nerves and neural cells, through release of neuropeptides substance P and CGRP (Yan et al., 2019b). The accumulation of iron in OMA can trigger ferroptosis by effects of reactive oxygen species and subsequently cause fibrogenesis (Zhang et al., 2022).
Furthermore, Rho/ROCK, Wnt/β-catenin and Smad are found to significantly affect fibrogenesis. Markers of Rho/ROCK signaling were elevated in endometriosis lesions and inhibition with ROCK antagonists, heparin or fasudil led to a decrease of the fibrotic marker protein expression in vitro (Yuge et al., 2007; Tsuno et al., 2009; Nasu et al., 2010; Tsuno et al., 2011). The pivotal role of Wnt/β-catenin pathway signaling was shown by the inhibitory effect of either β-catenin inhibitors, or forkhead box protein 1 on expression of pro-fibrotic genes (Matsuzaki and Darcha, 2013; Shao and Wei, 2018; Hirakawa et al., 2019). Several interleukins (IL), among which are IL-1, IL-3, IL-6, and IL-10, have also been shown to affect fibrogenesis. IL-1 can have both a pro- or anti-fibrotic effect, depending on the grade of inflammation (Matsuzaki et al., 2020). IL-3, IL-6 and the normally anti-inflammatory IL-10 drive fibrosis via the dysregulated activation of STAT3 (Matsuzaki et al., 2022, 2023).
Besides these pathways, several transcriptional factors also have been identified to play a role in fibrogenesis in endometriosis. Transcription factor NR4A1 regulates BCL-2 expression, which resulted in an anti-apoptotic and pro-fibrotic effect (Zeng et al., 2018; Mohankumar et al., 2020). Flavonoids quercetin and kaempferol are NR4A1 antagonist and showed anti-fibrotic effects in endometriosis (Zhang et al., 2023b). The downregulation of microRNA-214 promoted fibrosis in endometriosis, probably via connective tissue growth factor (CTGF) expression (Wu et al., 2018; Zhang et al., 2021).
Animal studies
We included 75 articles reporting about animal studies. An overview of these studies is presented in Table 3. Most of these studies aimed to test potential anti-fibrotic therapeutics for endometriosis. Nearly all studies were performed in rodents with induced endometriosis. To establish an endometriosis-like model, various methods were applied. Autologous uterine tissue, donor animal uterine tissue or human endometrial tissue was either surgically transplanted into the recipient’s inner abdominal wall or minced and injected either intraperitoneally or subcutaneously. All these methods led to similar ectopic cystic implants consisting of endometrial-like epithelial and stromal cells and fibrosis. The fibrosis was progressive over time and correlated with markers for EMT and FMT, similar to human endometriotic lesions.
Table 3.
Animal studies.
| Reference | Sample size | Intervention | Assay methods | Results | Conclusion | Relevance to fibrosis |
|---|---|---|---|---|---|---|
| Akarca-Dizakar et al., 2022; The therapeutic effects of coenzyme Q10 (CoQ10) on surgically induced endometriosis in Sprague Dawley rats (Akarca-Dizakar et al., 2022) | N = 27 (7, 6, 7, 7); rat. Autologous uterine tissue transplantation | CoQ10 50 or 100 mg/kg, Buserelin (GnRH agonist) | Lesion size, Masson stain, toluidine stain, IHC, ELISA | Significant decrease of lesion size and adhesion scores before and after treatment in low and high dose CoQ10 and Buserelin groups. Mast cell number decreased in all treatment groups and collagen density increased | CoQ10 treatment decreased histopathological effects of endometriosis implants without considerable side effects | Anti-oxidant CoQ10 treatment as well as GnRH agonist treatment decreased lesion size and increased collagen density |
| Arangia et al., 2023; Fisetin, a natural polyphenol, ameliorates endometriosis modulating mast cells derived NLRP-3 inflammasome pathway and oxidative stress (Arangia et al., 2023) | N = 36 exclusive donors (3 * 12); rat. Intraperitoneal injected uterine donor tissue | Fisetin 40 mg/kg | Lesion size, Masson stain, toluidine stain, IHC, WB, ELISA | Lesion size, mast cell infiltration, and activation, the extent of collagen fibers, α-SMA, TGF-β and NLRP-3 inflammasome expression, and markers for oxidative stress were all reduced in fisetin treatment group compared to vehicle controls | Mast cell activation and the NLRP-3 inflammasome pathway and oxidative stress stimulate endometriosis development and fibrosis, fisetin can alter these signaling pathways and thereby inhibit endometriosis formation | Fisetin can reduce fibrosis via inhibiting mast cell activation and oxidative stress pathways |
| Buigues et al., 2018; Evaluation of PAI-1 in endometriosis using a homologous immunocompetent mouse model (Buigues et al., 2018) | N = 56 (2 * 7, donors); mice. subcutaneous transplantation of donor uterine tissue | PAI-039 (PAI-1 inhibitor) 10 mg/kg or control; oral, once daily | Lesion size, Masson stain, IHC | Decreased lesion size in the treatment group. Collagen surrounds lesions in the treatment and control groups, no difference in the stained area. No differences in immune cell infiltration | Plasminogen activator inhibitor-1 overexpression stimulates angiogenic demands of endometriotic lesions. PAI-1 inhibition with PAI-039 decreases lesion size. No clear effect on the amount of collagen | PAI-1 increases endometriotic lesion characteristics but does not influence fibrosis |
| Cao et al., 2019; Plasma high mobility group box 1 (HMGB1), osteopontin (OPN), and hyaluronic acid (HA) as admissible biomarkers for endometriosis (Cao et al., 2019) |
|
None | HE, Masson stain, IHC, ELISA | Lesion size and fibrosis were progressive over time, and hotplate latency decreased. Plasma HMGB1, OPN, and HA correlated with lesional progression and fibrosis | HMGB1, OPN, and HA are potential biomarkers for endometriosis | The correlation between fibrosis and plasma levels of HMGB1, OPN, and HA shows potential as biomarkers for endometriosis |
| Chen et al., 2021; Preoperative and perioperative intervention reduces the risk of recurrence of endometriosis in mice caused by either incomplete excision or spillage and dissemination (Chen et al., 2021) | N = 171 (16, 5 * 10, 4 * 12, donors); mice. Intraperitoneal injection of donor uterine tissue | Ketorolac 7.5 mg/kg before, saline after. Aprepitant 25 mg/kg before and after. Propanolol 10 mg/kg + andrographolide 180 mg/kg before and after | Lesion size, Masson stain, IHC | Spill experiment: E-cadherin, PR-B were elevated in intervention groups, α-SMA, p-p65, VEGF, ADRB2 reduced, fibrosis reduced. Weight correlated pos with α-SMA and fibrosis. Hotplate latency correlated neg with α-SMA and fibrosis. α-SMA | Pre- and perioperative administration of ketorelac, propanolol + andrographolide and aprepitant inhibits outgrowth of endometriotic lesions after spillage or incomplete excision, in combination with reduced fibrogenesis and improved pain behavior in the lesions in a mouse model | Pre- and perioperative interventions could be used to decrease risk of endometriosis recurrence including a inhibiting effect on fibrosis and improved pain behavior |
| Incomplete excision experiment: E-cadherin, PR-B elevated in the intervention group, α-SMA, p-p65, VEGF, ADRB2 reduced in the intervention group. Fibrosis reduced. lesion weight correlated pos with α-SMA and fibrosis. Hotplate latency correlated negatively with α-SMA and fibrosis | ||||||
| Cordaro et al., 2021; Hidrox and endometriosis: biochemical evaluation of oxidative stress and pain (Cordaro et al., 2021) | N = 20 (3 * 5, donors); rats. Intraperitoneal injection of donor uterine tissue | Hidrox (hydroxytyrosol concentrate) 10 mg/kg by gavage daily from day 7 to 14 | Lesion size, Masson stain, IHC, hotplate latency, ROS levels | Reduced lesion size, collagen, α-SMA, Ki67, and BCL-2, increased Bax in Hidrox treatment group. Longer hotplate latency in the treatment group | Hidrox, as a strong anti-inflammatory and anti-oxidant agent, reduces endometriosis lesion size, inflammation, and fibrosis. It restores oxidative balance in hippocampus and relieves endometriosis-associated pain | Hidrox reduced fibrosis in endometriosis via oxidative stress decrease |
| Daftary et al., 2013; A novel role of the Sp/KLF transcription factor KLF11 in arresting the progression of endometriosis (Daftary et al., 2013) | N = 21 (3 * 7); mice. Autologous uterine tissue transplantation | Klf 11 knockout, Klf 9 knockout controls, wild-type controls | Lesion size, Masson stain, RT-qPCR | Klf 11−/− mice had larger lesions, more fibrosis, and higher collagen I expression and deposition versus Klf 9−/− and wild-type controls | Klf 11 seems to have a protective role in the pathologic process of endometriosis | Klf 11 protective role in endometriosis includes fibrosis and other endometriotic lesion characteristics |
| Delaney et al., 2016; KLF10 mediated epigenetic dysregulation of epithelial CD40/CD154 promotes endometriosis (Delaney et al., 2016) | N = 21 (3 * 7); mice. Autologous uterine tissue transplantation | Klf 10 knockout, Klf 11 knockout controls, wild-type controls | Lesion size, Masson stain, RT-qPCR, IHC | Klf10−/− mice had >2-fold increase in lesion size, lesions associated with polymorphonuclear cell infiltrate. Fibrosis score slightly elevated in Klf10−/− mouse versus wild-type, but evident decrease versus Klf11−/− | Klf10 knockout in mice resulted in prominent inflammation and increased lesion size, but only slightly increase in fibrosis | Klf 10 protective role in endometriosis includes inflammation, but not fibrosis |
| Di Paola et al., 2016; Co-micronized palmitoylethanolamide/polydatin (mPEA/PLD) treatment causes endometriotic lesion regression in a rodent model of surgically induced endometriosis (Di Paola et al., 2016) | N = 20 (2 * 10); rat. Autologous uterine tissue transplantation | m(PEA/PLD) 10 mg/kg daily or vehicle control | HE, Masson, Toluidine blue. IHC. WB. Tail-Fick method, Hot plate latency | m(PEA/PLD) treated rats had smaller cysts, less inflammatory cell infiltration and edema, lower pain behavior scores, and larger fibrotic area | m(PEA/PLD) treatment decreased lesion size and increased fibrosis. It reduced inflammatory and angiogenic parameters and pain behavior | Fibrosis increased by m(PEA/PLD) treatment, whereas other lesions characteristically improved |
| Ding et al., 2019; Scutellarin suppresses platelet aggregation and stalls lesional progression in mouse with induced endometriosis (Ding et al., 2019) | N = 27 (3 * 9); mice. Intraperitoneal injection of donor uterine tissue | Scutellarin low dose 7,5 mg/kg, high dose 15 mg/kg every two days IV or vehicle control | HE, Masson, IHC, hotplate latency, peripheral platelet levels | Scutellarin reduced platelet activation, lesion weight, fibrosis, α-SMA, and collagen I expression and increased hotplate latency. Lesion weight correlated positively with fibrosis and α-SMA | Scutellarin is efficacious as a treatment for endometriosis by suppressing platelet aggregation, inhibiting proliferation, angiogenesis, and fibrogenesis, resulting in reduced lesion size and improved pain behavior in mice | Fibrotic markers correlate with lesion weight and platelet-targeted therapy reduced fibrosis |
| Dogan et al., 2023; The effect of rituximab on experimental endometriosis model in rats (Dogan et al., 2023) | N = 24 (12, 11); rat. Autologous uterine tissue transplantation | Rituximab 10 mg/kg | Lesion size, HE, Masson stain, IHC | Implant size was decreased in the rituximab treatment group versus controls, with no difference in HE histology score and fibrosis scores between groups | Rituximab reduced lesion size and therefore is a potential therapeutic agent for endometriosis | Rituximab did not reduce fibrosis and slightly reduced lesion size |
| Dogru et al., 2017; Effect of amygdalin on the treatment and recurrence of endometriosis in an experimental rat study (Dogru et al., 2017) | N = 30 (3 * 10); rat. Autologous uterine tissue transplantation | Amygdalin 5 mg/kg once a week IP, leuprolide 0,0375 mg/kg SC or saline control | Lesion size, Masson stain | Lesion size was smaller in both treatment groups other groups compared to control. No differences in fibrotic area | Amygdalin is superior to leuprolide in reducing endometriosis implant size. No clear effect from any treatment on fibrosis scores | Fibrosis not reduced by amygdalin or leuprolide treatment, lesion size was reduced |
| Duan et al., 2018; The M2a macrophage subset may be critically involved in the fibrogenesis of endometriosis in mice (Duan et al., 2018) | N = 115 (5 * 6, 2 * 6, 4 * 7, donors); mice. Intraperitoneal injection of donor uterine tissue | Macrophage depletion by diphtheria toxin in CD11b-DTR mice. Ex vivo differentiated macrophage IV administration | Lesion size, Masson stain, IHC, hotplate latency | M2 macrophage infiltration and fibrotic markers increased over time. Macrophage depletion reduced fibrosis, lesion weight, and pain behavior. M2a, but not M1 or M2c macrophage suppletion after depletion increased fibrosis | M2a, but not M1 or M2c, macrophages are critically involved in fibrogenesis in endometriosis through promoting EMT, FMT, SMM, and production of pro-fibrotic mediators such as TGF-β1 | M2 macrophages are involved in fibrogenesis |
| Genovese et al., 2022; Molecular and biochemical mechanism of cannabidiol in the management of the inflammatory and oxidative processes associated with endometriosis (Genovese et al., 2022) | N = 30 (3 * 10); rat. Intraperitoneal injection of donor uterine tissue | Cannabidiol (CBD) 10 mg/kg | Lesion size, ultrasound, HE, Masson stain, IHC, WB, open field, hotplate latency, plus maze and acetic-acid induced contractions test | CBD treatment reduced lesion size, markers of oxidative stress, the extent of fibrosis, expression of MMP-9, TGF-β, and nerve growth factor, mast cell infiltration, and pain behavior | Anti-oxidant, anti-fibrotic, and anti-inflammatory activities of cannabidiol can be useful to stop the development of endometriosis | Cannabidiol reduced fibrosis development in endometriosis |
| Grande et al., 2023; Host immunity and KLF11 deficiency together promote fibrosis in a mouse model of endometriosis (Grande et al., 2023) | N = 30 (3 * 10); mice. Autologous or donor uterine tissue transplantation | KLF11−/−, KLF10−/− knockout or wild-type (WT), HATI treatment (auto-transplantation). LacZ-KLF11−/−, Smad3−/+ (donor transplantation) | HE, Masson stain, IHC, RT-qPCR | KLF10−/− and KLF11−/− showed lower adhesion scores than WT after 1, 2, and 3 weeks post-implant, KLF11−/− showed highest scores, in all groups, but most in KLF11−/−, scores progressive over time. HATI treatment reduced scores in KLF11−/− but not affected WT or KLF10−/−. Collagen extent mirrors adhesion scores in all mice. Smad3 controlled LacZ-KLF11−/− donor implants leads to ectopic antigen expression and enhanced fibrosis. Fibrogenesis was attenuated in Smad3 knockout transplants |
|
KLF11 is protective against fibrosis via TGF-β signaling |
| Guo et al., 2015; P-selectin as a potential therapeutic target for endometriosis (Guo et al., 2015a) | N = 64 (6 * 8, 2 * 8); mice. Autologous or donor uterine tissue transplantation | P-selectin knockout in donor or recipients; Platelet transfusion; P-selection-Fc treatment | Lesion size, Masson stain, IHC, hotplate latency | P-selectin knockout (donor and/or recipient) and P-selection-Fc reduced lesion size, platelet aggregation, macrophage infiltration, and fibrotic markers. P-selection-Fc treatment improved hotplate latency | P-selectin knockout or blocking treatment reduced endometriotic implant size and reduced fibrotic markers, TGFB, platelet aggregation, and neovascularization | Platelet activation is a potential fibrosis-based target for endometriosis |
| Guo et al., 2016; Anti-platelet therapy is efficacious in treating endometriosis induced in mouse (Guo et al., 2016) | N = 79 (3 * 10, 4 * 8, donors); mice. Autologous uterine tissue transplantation or intraperitoneal injection of donor uterine tissue | Ozagrel 15 or 30 µg/g daily IP or control; IgG-mediated macrophage and/or platelet depletion | Lesion size, Masson stain, IHC, hotplate latency | Ozagrel reduced lesion size, improved hyperalgesia, and reduced fibrotic markers. Platelet and/or macrophage depletion reduced lesion size, platelet and macrophage infiltration in lesions and fibrotic markers | Anti-platelet interventions reduce lesion size and fibrotic markers and have the potential as a treatment for endometriosis | Platelet activation is a potential fibrosis-based target for endometriosis |
| Guo et al., 2021; NLRP3 inflammasome activation of mast cells by estrogen via nuclear-initiated signaling pathway contributes to the development of endometriosis (Guo et al., 2021) | N = 24 (4 * 8); mice. Intraperitoneal injection of donor uterine tissue | NLRP3 inhibitor CY-09 2,5 mg/kg daily | Lesion size, Masson stain | Il-1β levels in peritoneal fluid were elevated in untreated endometriosis vs healthy controls., but reduced in NLRP3 inhibitor treatment. NLRP3 inhibitor reduced weight, size, and fibrotic area of lesions at 14 and 21 days | Estrogen can promote mast-cell-derived NLRP3 activation and IL-1β secretion, which can lead to development of endometriosis. In vivo mouse experiment showed decreased fibrotic changes after NLRP3 inhibitor treatment | NLRP3 inhibition has the potential as a fibrosis target for endometriosis |
| Hao et al., 2021; Reduced vagal tone in women with endometriosis and auricular vagus nerve stimulation as a potential therapeutic approach (Hao et al., 2021) | N = 90 (3 * 10, 3 * 10, 3 * 10); mice. Intraperitoneal injection of donor uterine tissue | Nervus vagotomy, vagal nerve stimulation (VNS) before or after endometriosis induction | Lesion size, Masson stain, IHC, hotplate latency | Vagotomy increased, VNS (both before and after induction of endometriosis) decreased lesion size, EMT and FMT markers and extent of fibrosis. Hotplate latency decreased after vagotomy and increased after VNS | A sympathetic domination of the autonomic nervous system may have a role in endometriosis. Vagotomy as a model for reduced vagal activity accelerates endometriosis progression and fibrogenesis. Auricular vagal stimulation decelerates endometriosis progression and fibrogenesis | Sympathetic domination of the autonomic nervous system may stimulate endometriosis, also fibrogenesis |
| Hao et al., 2022; Activation of α7 nicotinic acetylcholine receptor retards the development of endometriosis (Hao et al., 2022) | N = 60 (3 * 8, 2 *8 , 20 donors); mice. Intraperitoneal injection of donor uterine tissue | PNU-282987 (α7nAChR agonist), methyllycaconitine citrate (MLA - α7nAChR antagonist) | Lesion size, HE, Masson stain, IHC, hotplate latency | Early PNU treatment reduced lesion size, α-SMA expression, and extent of fibrosis, with no differences between MLA and control group. PNU treatment after the establishment of deep endometriosis reduced lesion size, EMT marker expression and fibrosis, and prolonged latency time compared to controls | Activation of α7nAChR by agonist treatment impeded lesion development, probably via EMT and FMT hindrance. α7nAChR agonist treatment reversed lesion development and fibrosis in a deep endometriosis model | Activation of α7 nicotinic acetylcholine receptor treated endometriosis and fibrosis successfully in mice |
| HayaShi et al., 2020; Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice (HayaShi et al., 2020) | N = 83 (24 recipients, controls, donors); mice. Transplantation of donor uterine tissue in ovarian bursa | None | Lesion size, HE, Masson stain, Berlin blue stain, IHC | Fibrosis was progressive over time, iron accumulation was present in model, accompanied by oxidative stress. Expression of FSH receptor and number of offspring were reduced in model animals | Successful establishment of ovarian endometrioma model. Model accompanies fibrosis and leads to iron-mediated oxidative stress and reduced fertilization | Increased fibrosis was accompanied by iron accumulation and decreased fertility |
| Herington et al., 2013; Dietary fish oil supplementation inhibits the formation of endometriosis-associated adhesions in a chimeric mouse model (Herington et al., 2013) |
|
Dietary fish oil: none, 5% of diet, 10% of diet | Lesion size, HE, Masson stain, IHC, macroscopic adhesion score | Lesion size, adhesion score, extent of fibrosis, and immune cell infiltration were smaller in high and low-dose fish oil suppletion groups | Anti-inflammatory dietary interventions like fish oil supplementation reduce endometriotic implant size, visual adhesions, and collagen accumulation in a xenograft mouse model | Fish oil as anti-inflammatory dietary intervention could prevent fibrogenesis in endometriosis |
| Hirakawa et al., 2019; B-catenin signaling inhibitors ICG-001 and C-82 improve fibrosis in preclinical models of endometriosis (Hirakawa et al., 2019) |
|
ICG-001, 0, 10, 50, or 100 mg/kg IP thrice weekly | Lesion size, Masson stain, Sirius red stain, IHC | Number and weight of lesions, the extent of fibrosis, and α-SMA expression were reduced dose-dependently in treatment groups | CBP/β-catenin signaling pathway is involved in endometriosis. Inhibition with ICG-001 and C-82 inhibits cell proliferation and promotes apoptosis. ICG-001 reduced lesion size and fibrosis in endometriosis mouse model | B-catenin signaling inhibition by ICG-001 is a potential anti-fibrotic intervention in endometriosis |
| Hirakawa et al., 2022; Trophic and immunomodulatory effects of adipose tissue derived stem cells in a preclinical murine model of endometriosis (Hirakawa et al., 2022) | N = 75 (5 * 10, 25); mice. Intraperitoneal injection of donor uterine tissue | Adipose tissue-derived stem cells (ASCs), early (with stemness potential) and late (without) passage; early or late administration | Lesion size, HE, Masson stain, IHC, RT-qPCR | Day 1 and Day 15 administration of early ASCs (EASCs) reduced lesion size and fibrosis thickness, with no differences between control and late ASCs administration. EASCs reduced pro-inflammatory and pro-fibrotic cytokine expression, among which TGF-β1 | Trophic and immunomodulatory properties of ASCs regulate pro-inflammatory and pro-fibrotic cytokines. Regenerative medicine could be an innovative treatment for endometriosis | Early passage ASCs inhibited lesion development and fibrosis via inhibition of TGF-β1 and other pro-fibrotic cytokine expression, regardless of timing before or after lesion establishment |
| Hoorsan et al., 2022; The effectiveness of antioxidant therapy (vitamin C) in an experimentally induced mouse model of ovarian endometriosis (Hoorsan et al., 2022) |
|
Vitamin C 50 mg/kg every 2 days orally | Lesion size, HE, Masson | Lesion size, adhesion score and fibrosis score reduced in treatment group. Follicle number increased in treatment group | Vitamin C treatment reduced endometriosis development and increased fertility parameters of the ovaries | Vitamin C reduced lesion size and fibrosis and increased fertility parameters |
| Huang et al., 2022a; Changing prostaglandin E2 (PGE2) signaling during lesional progression and exacerbation of endometriosis by inhibition of PGE2 receptor EP2 and EP4 (Huang et al., 2022a) |
|
|
Lesion size, HE, Masson, IHC, hotplate latency | Fibrosis increased over time, especially in DE. PGE2 signaling markers COX2, EP2 and EP4 increased in first 2 weeks and decreased later in development, correlated negatively with fibrosis. Hotplate latency of high dose EP2I and EP4I increased, in all EPI treatment groups markers of PGE2 signaling decreased and fibrosis increased. Metformin treatment decreased lesion size and extent of fibrosis and improved hotplate latency | COX-2, EP2 and EP4 expression diminished over time with lesion development. EP2/EP4 inhibitors exacerbated hyperalgesia and increased fibrosis development, metformin reduced fibrosis and hyperalgesia | Prostaglandin signaling diminished as fibrosis progressed, metformin reduced fibrosis development |
| Huang et al., 2022b; Tetramethylpyrazine retards the progression and fibrogenesis of endometriosis (Huang et al., 2022b) |
|
Tetramethylpyrazine (TMP) low (25 mg/kg) or high (100 mg/kg) dose | Lesion size, HE, Masson, IHC, hotplate latency | TMP treatment, in a dose-dependent manner, reduced lesion size, hyperalgesia, extent of fibrosis and lesional platelet aggregation, TGF-β, α-SMA and Col1 IHC expression. Extent of fibrosis correlated positively with lesion size and platelet aggregation and negatively with hotplate latency time | TMP can reduce endometriotic lesion development via platelet aggregation inhibition and inhibition of EMT and FMT | TMP can reduce fibrosis in endometriosis via platelet aggregation inhibition, underlining the importance of platelets in fibrogenesis |
| Hull et al., 2012; Host-derived TGF-β1 deficiency suppresses lesion development in a mouse model of endometriosis (Hull et al., 2012) |
|
TGF-β1−/− knockout | Lesion size, HE, IHC | Lesion weight, macrophage infiltration, α-SMA expression reduced in TGF-β−/− recipient mice | Development of endometriosis depends on the availability of TGF-β1 in the peritoneal environment. Targeting the TGF-β1 pathway could suppress lesion development | TGF-β1 has an essential role in fibrogenesis in endometriosis |
| Hull et al., 2005; Nimesulide, a COX-2 inhibitor, does not reduce lesion size or number in a nude mouse model of endometriosis (Hull et al., 2005) |
|
Nimesulide 25 mg/kg/day SC injection | Lesion size, HE, IHC | Lesion size, lesion number, α-SMA expression, macrophage infiltration, and vWF detected neovascularization did not differ between nimesulide treatment group and control | Nimesulide did not inhibit endometriotic lesion formation in a mouse model. This suggests that COX-2 inhibition is unlikely to influence the establishment or progression of endometriosis | COX-2 inhibition by nimesulide is not effective to reduce endometriosis and the associated myofibroblasts |
| Khan et al., 2018; Epigenetic therapy: novel translational implications for the arrest of environmental dioxin-induced disease in females (Khan et al., 2018) |
|
TCDD (dioxin—toxic environmental contaminant activating CYP enzymes), garcinol (HATI—histone acetyltransferase inhibitor) | Lesion size, HE, Masson stain, IHC, RT-qPCR | TCDD exposure increased lesion size, the extent of fibrosis, and collagen expression. In additional HATI treatment group this effect was attenuated | TCDD exposure increased disease progression and fibrosis via CYP3A4 activation. HATI treatment by garcinol activated KLF11 transcription factor, which diminished the disease progression by TCDD | TCDD, an environmental contaminant is a pro-disease and pro-fibrotic stimulus. This effect can be attenuated by KLF11 activation via HATI treatment |
| Kim et al., 2017; Ginsenoside Rg3 decreased fibrotic and invasive nature of endometriosis by modulating miRNA-27b: in vitro and in vivo studies (Kim et al., 2017) |
|
Rg3E high dose 0.2 mg/g/day, low dose 0.1 mg/g/day | Lesion size, Masson stain, RT-qPCR | Treatment groups showed smaller lesions and lower expression of MMP’s, CTGF, collagen, fibronectin and TGF-β1. Extent of fibrosis decreased dose dependently in treatment groups | miRNA-27b-3p is elevated in endometriosis patients. Rg3E (Korean Red Ginseng extract) alters endometriosis characteristics by reducing miRNA-27b-3p and thereby inhibit invasion and fibrotic characteristics of endometriosis | RgE3 can reduce miRNA-27b-3p and thereby reduce fibrosis |
| Li et al., 2016; Endometriotic mesenchymal stem cells significantly promote fibrogenesis in ovarian endometrioma through the Wnt/B-catenin pathway by paracrine production of TGF-β and Wnt1 (Li et al., 2016) |
|
Intralesional injection of TGF- β1, Wnt1, TGF-β1+Wnt1, Ecto-MSC conditioned medium | Lesion size, HE, Masson stain, IHC | Fibrotic markers increased rapidly from day 14 after endometriosis establishment. Lesion size, extent of fibrosis, and collagen expression were equally increased in all treatment groups | Ecto-MSC conditioned medium promoted proliferation, migration, invasion, and contraction of ecto-ESCs, as characteristics of fibrogenesis. Autocrine production of Wnt1 and TGF-β1 activated Wnt/B-catenin signaling, which stimulates fibrogenesis | Mesenchymal stem cells promoted lesional progression and fibrosis, probably via Wnt1 and TGF-β1 signaling produced by them |
| Liu et al., 2015; Vascular endothelial growth factor receptor-2 inhibitor cediranib causes regression of endometriotic lesions in a rat model (Liu et al., 2015) |
|
Cediranib 4 mg/kg/day | Lesion size, HE, Masson stain, IHC, TUNEL apoptosis assay | Lesion size, microvessel density, and proliferation decreased in the treatment group. The treatment group showed more fibrosis, but equal severe adhesions | Cediranib caused regression of endometriotic implants, associated with decreased angiogenesis. Fibrosis increased, but without severe adhesions | Fibrosis increased by treatment with an angiogenesis inhibitor |
| Liu et al., 2019; Sensory nerve-derived neuropeptides accelerate the development and fibrogenesis of endometriosis (Liu et al., 2019) |
|
|
Lesion size, HE, Masson stain, IHC, hotplate latency | E1: Lesion size, fibrotic markers, proliferation, angiogenesis, and neurokinin receptor 1 (NK1R) expression decreased in chemical denervation groups, most extensively effect by sensory denervation with RTX | Sensory nerves or the NK1R signaling pathway are important in the development of fibrogenesis in endometriosis and may be potential targets for intervention | (Sensory) nerves and the NK1R signaling pathway stimulate fibrogenesis in endometriosis |
| E2: Lesion size, fibrotic markers and NK1R expression decreased, hotplate latency improved in surgical denervation groups, both before and after endometriosis induction | ||||||
| E3: Lesion size, fibrotic markers, EMT, FMT, and SMM markers increased, and hotplate latency impaired in the substance P treatment group, but markers decreased and hotplate latency improved in NK1R antagonist (aprepitant) group | ||||||
| Luo et al., 2020; Sodium tanshinone IIA sulfonate restrains fibrogenesis through induction of senescence in mice with induced deep endometriosis (Luo et al., 2020) |
|
Sodium tanshinone IIA (STS) high or low dose | Lesion size, HE, Masson stain, IHC | Lesion size, the extent of fibrosis, and macrophage (M2) infiltration were reduced, cell senescence markers and apoptosis were increased and hotplate latency improved in the STS treatment group, most extensively in high dose | STS treatment reduced lesion weight and extent of fibrosis in the deep endometriosis model, seemingly through induction of cellular senescence and increased apoptosis and reduced lesional infiltration of M2 macrophages | Sodium tanshinone reduced fibrosis through cellular senescence and apoptosis induction |
| Marcellin et al., 2017; Alteration of Nrf2 and glutamate cysteine ligase expression contribute to lesions growth and fibrogenesis in ectopic endometriosis (Marcellin et al., 2017) |
|
|
Lesion size, HE, Sirius red stain, RT-qPCR | Knockout implants were larger and larger extent of fibrosis. Lower lesion weight and smaller extent of fibrosis in DMF treatment group | Decreased Nrf2 expression is associated with decreased GCL expression and are both present in endometriosis in women. Via oxidative stress this can lead to an increased fibrogenesis as shown in an in vivo experiment by Nrf2 knockout and DMF treatment | Decreased Nrf2 expression can contribute to fibrosis in endometriosis and could be a potential therapeutic target |
| Matsuzaki et al., 2013; Involvement of the Wnt/B-catenin signaling pathway in the cellular and molecular mechanisms of fibrosis in endometriosis (Matsuzaki and Darcha, 2013) |
|
CGP049090, Tcf/β-catenin antagonist, 2 mg/kg/day | Lesion size, Masson stain, Sirius red stain | Fibrosis develops between day 7 and day 14 after model establishment. Smaller extent of fibrosis in CGP049090 treatment groups, both in early treatment start and late treatment start groups | Wnt/β-catenin targeting inhibits fibrogenesis in vitro and in vivo in mouse models, in vivo experiments showed the possibility to reverse established fibrosis | Wnt/β-catenin signaling pathway effective target to prevent and reverse fibrosis |
| Matsuzaki et al., 2014; Antifibrotic properties of epigallocatechin-3-gallate in endometriosis (Matsuzaki and Darcha, 2014) |
|
EGCG (epigallocatechin-3-gallate, intraperitoneal injection 50 mg/kg/day) | Lesion size, Masson stain, Sirius red stain | In treatment group started before fibrosis development extent of fibrosis was comparable with fibrosis directly after lesion establishment. In treatment group started after fibrosis development extent of fibrosis was smaller compared with controls but larger compared with fibrosis directly after lesion establishment | ECGC treatment inhibited TGF-β1-stimulated activation of MAPK and Smad signaling pathways thereby preventing endometriosis development and fibrogenesis in vivo | ECGC treatment inhibited fibrogenesis via MAPK and Smad signaling but did not reverse already established fibrosis |
| Miller et al., 2021; Interleukin-33 activates group 2 innate lymphoid cell expansion and modulates endometriosis (Miller et al., 2021) |
|
IL-33 suppletion, ILC2 antibody depletion by CD90.2 or IL-33 antibody neutralization, wild type or RAG2−/− knockout or RAG2−/− IL2ry−/− knockout mice | HE, Masson stain, IHC | IL-33 treated wild-type mice showed increased extent of fibrosis. IL-33 treated RAG2−/− mice showed increased extent of fibrosis. Both RAG−/− aCD90.2 and RAG2−/− IL-2ry−/− showed no increased extent of fibrosis after IL-33 treatment. IL-33 neutralizing AB treatment decreased extent of fibrosis | IL-33 has an essential role in endometriosis development through ILC2s (group 2 innate lymphoid cells) via stimulating inflammation, immune cell recruitment, lesion proliferation and fibrosis | IL-33 acts via innate lymphoid cells to stimulate endometriosis, various therapeutic strategies inhibiting this pathway inhibited fibrogenesis |
| Mishra et al., 2020; Mouse model for endometriosis is characterized by proliferation and inflammation but not epithelial-to-mesenchymal transition and fibrosis (Mishra et al., 2020) |
|
None | HE, Masson stain, Picrosirius red stain, IHC, RT-qPCR | Adhesion were progressively present. Some collagen IV was detected, collagen I was only detected in muscle layers, not in lesions. No collagen or α-SMA was detected within stromal area | Implantation of autologous uterine fragments leads to the development of ectopic endometrial lesions. The lesions grew progressively and showed inflammatory activity. The lesions did not show EMT or fibrosis | No fibrosis or myofibroblastic differentiation was detected in the used mouse model of endometriosis |
| Mohankumar et al., 2020; Bis-indole-derived nuclear receptor 4A1 (NR4A1, Nur77) ligands as inhibitors of endometriosis (Mohankumar et al., 2020) |
|
C-DIM (methylene substituted diindolylmethane, a NR4A1 antagonist) | Lesion size, HE, IHC, luciferase activity | Lesion size and α-SMA expression were reduced whereas apoptosis markers increased in treatment group in the mouse donor tissue experiment. In the human donor model lesion size was reduced and apoptosis markers were increased in the treatment group, fibrosis was not assessed in this model | NR4A1 is a pro-endometriotic transcription factor and inhibition with Bis-indole-derived antagonist is promising as a new nonhormonal therapy: it reduced growth and α-SMA expression of implants | NR4A1 antagonist therapy is a potential anti-fibrotic target |
| Muraoka et al., 2023; Fusobacterium infection facilitates the development of endometriosis through the phenotypic transition of endometrial fibroblasts (Muraoka et al., 2023) |
|
|
Lesion size, IHC, IF | F. nucleatum also present in endometriosis from infected donors, this group showed larger implants, increased M2 macrophage infiltration, TGF-β and TAGLN expression, not the case for L. iners or E. coli infected donor implants. TAGLN siRNA in recipients reduced stimulating effect of F. nucleatum infection. Antibiotic treatment in donor or recipient mice reduced lesion development as well | F. nucleatum endometrial infection can trigger TAGLN expression via TGF-β signaling, resulting in a fibroblastic phenotype more prone to lead to endometriotic lesion implantation and development. This effect can be reversed by antibiotic elimination of F. nucleatum | F. nucleatum can trigger fibroblast to myofibroblast transdifferentiation marked by TAGLN, resulting in an increased endometriotic implantation rate. This effect can be reversed by antibiotic based elimination of F. nucleatum |
| Nagai et al., 2020; Focal adhesion kinase-mediated sequences, including cell adhesion, inflammatory response, and fibrosis, as a therapeutic target in endometriosis (Nagai et al., 2020) |
|
FAK inhibitor PF573228 | Lesion size, HE, IHC | Lesion size, proliferation score, MCP-1, TGF-β1, and α-SMA expression were decreased in FAK inhibition treatment group. Non-treatment group showed thicker, more fibrotic cyst walls | FAK-inhibition reduced lesion development, TGF-B1 expression, myofibroblastic changes, and macrophage infiltration and thus could be a promising anti-endometriotic treatment pathway | FAK signaling promotes fibrogenesis |
| Nahari and Razi, 2018; Silymarin amplifies apoptosis in ectopic endometrial tissue in rats with endometriosis; implication on growth factor GDNF, ERK1/2, and Bcl-6b expression (Nahari and Razi, 2018) |
|
Silymarin (flavonoid mixture) 50 mg/kg/day | Lesion size, HE, Masson stain, IHC | Lesion size and the number of apoptotic cells were increased and BCL-6b and BCL-2 expression decreased in the treatment group, whereas the extent of fibrosis was increased in the treatment group | SMN acts as an anti-inflammatory and anti-oxidant agent and inhibits endometriosis growth by diminishing GDNF, reducing Bcl-2/6b, but enhances ERk1/2 expression and fibrosis | Silymarin reduced lesion size via inducing apoptosis, but increased fibrosis via Erk signaling |
| Nishimoto-Kakiuchi et al., 2016; Characteristics of histologically confirmed endometriosis in cynomolgus monkeys (Nishimoto-Kakiuchi et al., 2016) |
|
None | HE, IHC, Iron staining | CD10 staining present in lesions. Hemorrhage and inflammation often observed, hemosiderin-laden macrophages and iron deposits present. Α-SMA staining in all cases, surrounding lesions. Nerve fibers present in most cases | Spontaneous endometriosis in Cynomolgus monkeys exhibits characteristics similar to human disease | Myofibroblast differentiation is a characteristic of endometriosis in Cynomolgus monkeys |
| Nishimoto-Kakiuchi et al., 2023; A long acting anti IL-8 antibody improves inflammation and fibrosis in endometriosis (Nishimoto-Kakiuchi et al., 2023) |
|
AMY109 (long-acting anti IL-8 antibody) | Laparoscopy at 3, 6, 9, and 12 months for ASRM and lesion size, HE, Sirius red, IHC | Anti-IL-8 antibody treatment reduced ASRM score, fibrosis, and α-SMA expression in 2 of 4 with spontaneous endometriosis. AMY109 treatment stabilized ASRM size (versus increase in no treatment group), reduced lesion size and fibrosis in the model monkeys | AMY109 anti-IL-8 antibody stopped lesional progression and reduced fibrosis in endometriosis in cynomolgus monkeys, being a potential endometriosis drug | Anti-IL-8 therapy has potential as anti-fibrotic therapy in endometriosis |
| Odagiri et al., 2009; Smooth muscle metaplasia and innervation of interstitium of endometriotic lesions related to pain (Odagiri et al., 2009) |
|
None | HE, IHC | Fibrotic interstitium and intensely α-SMA stained areas present in ectopic lesions, around the formed cysts. NCAM and NGF staining present in ectopic lesions, not in healthy endometrium | Presence of smooth muscle metaplasia and nerve fibers suggests a role of smooth muscle contraction in the endometriosis-associated pain | Histo-anatomical relationship between myofibroblasts and highly innervated interstitium suggests a role in pain |
| Peng et al., 2022; Mechanisms of Thunberg Fritillaria in treating endometriosis based on network pharmacology and the effect of Peiminine on the MEK/ERK pathway (Peng et al., 2022) |
|
Peiminine low dose 2 mg/kg/day, medium dose 4 mg/kg/day, high dose 8 mg/kg/day, dienogest 2 mg/day | HE, Masson stain, IHC, immunofluorescence, WB, RT-qPCR | Extent of fibrosis and expression of vimentin as EMT marker were decreased in treatment groups. Markers for MAPK/ERK signaling were decreased in peiminine groups | Peiminine, as an active component in Thunberg Fritillaria, can downregulate the MAPK/ERK signaling pathway to suppress endometriosis | Peiminine showed anti-fibrotic potential in endometriosis via MAPK/ERK signaling pathway |
| Riccio et al., 2019; B lymphocytes inactivation by ibrutinib limits endometriosis progression in mice (Riccio et al., 2019) |
|
Ibrutinib or anti-CD20 | Lesion size, HE, Sirius red stain, ELISA, RT-qPCR, ultrasonography | Lesion size and expression of COX-2, α-SMA and collagen were decreased in Ibrutinib treatment group, not affected in anti-CD20 treatment group. M1/M2 ratio decreased in spleen, increased in peritoneal cavity after ibrutinib treatment | Bruton’s tyrosine kinase inhibition by Ibrutinib decreased endometriosis progression in mice, by shifting activated B cells to Bregs, while B cell depletion by CD20 antibody treatment had no effect. Peritoneal increase of M1/M2 ratio is a new perspective in treating endometriosis | Endometriosis lesion size and fibrotic markers decreased after Bruton’s tyrosine kinase inhibition, probably because of a decreased B cell activity |
| Shi et al., 2021; WEE1 promotes endometriosis via the Wnt/β-catenin signaling pathway (Shi et al., 2021) |
|
WEE1 inhibitor (AZD1775) with or without estrogen suppletion | HE, Masson stain, RT-qPCR | WEE1 is upregulated by IL-1β. WEE1 upregulation inhibited apoptosis, WEE1 knockdown promoted apoptosis, and attenuated fibrosis. Fibrotic markers were decreased in WEE1 inhibition treatment group. WEE1 and fibrotic marker expression increased in estrogen suppletion group. | WEE1 stimulated ectopic stromal cell migration and fibrosis, via Wnt/β-catenin pathway | WEE1 promotes fibrogenesis via the Wnt/β-catenin pathway. Wnt/β-catenin inhibitor inhibits fibrogenesis |
| Shi et al., 2020; Mechanistic study of vitamin C attenuation of endometriotic fibrosis (Shi et al., 2020) |
|
Vitamin C 500 mg/kg/day IP | Lesion size, HE, Masson stain, RT-qPCR | Lesion size, extent of fibrosis, and expression of collagen I, α-SMA, TGF-β1 and CTGF were decreased in the vitamin C treatment group | Vitamin C treatment decreased endometriosis lesion size and fibrotic marker protein and mRNA expression in a rat model | Vitamin C can reduce fibrosis in endometriosis |
| Siracusa et al., 2021; The methyl ester of 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid reduces endometrial lesions development by modulating the NF-κB and Nrf2 pathways (Siracusa et al., 2021) |
|
CDDO-Me 5 mg/kg/day IP | Lesion size, He, Masson stain, IHC, WB, anti-oxidant activity assay | Lesion size, extent of fibrosis, α-SMA, fibronectin and BCL expression and NF-κB activation were decreased in treatment group | The methyl ester of 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO-Me) reduces endometriotic lesion development by modulating the NF-κB and Nrf2 pathways | CDDO-Me can reduce fibrosis via NF-κB and Nrf2 pathways |
| Taskin et al., 2016; A humanized anti-interleukin (IL)-6 receptor monoclonal antibody, tocilizumab, for the treatment of endometriosis in a rat model (Taskin et al., 2016) |
|
Tocilizumab 8 mg/kg/2 weeks IP | HE, Masson stain, IHC | Lesion size reduced over time in tocilizumab treatment group, but stable in controls. IL-6 expression was comparable between groups. Extent of fibrosis and expression of VEGF was decreased in the treatment group | Tocilizumab (IL-6 receptor mAB) had a regressive effect on endometriosis implants in a rat model | IL-6 inhibition by tocilizumab decreased lesion size and fibrosis |
| Umezawa et al., 2012; Expression profile of extracellular matrix and adhesion molecules in the development of endometriosis in a mouse model (Umezawa et al., 2012) |
|
None | HE, RT-qPCR | Expression of collagens (3a1, 8a1, 1a1), Tnc, Vtn, Lamc1,2 were increased 7 days post-induction vs. sham-operated mice. Lamc2 peaked at 24 h, all other mRNAs at day 7 | RNA expression of integrins, collagens, other ECM proteins peaked at 7 days post-induction. Lamc2 peaked within 24 h, suggesting a role in the initiation of endometriosis | Lamc2 expression could have a role in the initiation of fibrotic development of endometriosis |
| van Kaam et al., 2008; Fibromuscular differentiation in deeply infiltrating endometriosis is a reaction of resident fibroblasts to the presence of ectopic endometrium (van Kaam et al., 2008) |
|
None | HE, IHC | α-SMA highly present 1 week after induction in mouse cells surrounding lesion, not in human stromal cells. Two weeks after induction α-SMA slightly decreased in mouse cells and decline progressively to week 3 and 4. Collagen deposition increased over time | α-SMA expression is induced in the host tissue, suggesting a local reaction to ectopic endometrium leading to FMT rather than FMT of the ectopic cells itself | Myofibroblast differentiation is a reaction of resident fibroblasts rather than endometrial fibroblasts in a mouse model |
| Wang et al., 2023; PIM2 promotes the development of endometriosis by enhancing glycolysis and fibrosis (Wang et al., 2023) |
|
PIM2 knockout donor and/or recipient mice; SMI4a (PIM2 inhibitor) | Lesion size, HE, IHC | Lesions from PIM2 d/d in WT and PIM2 d/d in PIM2 d/d recipients were smaller than WT to WT lesions, SMI4a treatment in WT to WT decreased lesion size. In knockout and treatment groups expression of α-SMA and markers of EMT and FMT was decreased | PIM2 upregulation in endometriosis promotes glycolysis and fibrosis in ectopic lesions, and may be a potential therapeutic target | PIM2 promotes EMT, FMT, and fibrosis in endometriosis, via upregulation of glycolysis |
| Wu et al., 2018; Exosomal miR-214 from endometrial stromal cells inhibits endometriosis fibrosis (Wu et al., 2018) |
|
miRNA-214 loaded exosomes | HE, Masson stain, Sirius red stain, IHC, RT-qPCR | Extent of fibrosis on Masson and Sirius red stain and expression of CTGF and collagen A1 protein and mRNA expression was reduced in exosomal miRNA treatment group | Fibrosis was associated with elevated fibrotic markers CTGF, collagen A1, and α-SMA and a diminished miR-214 expression. miR-214 impacts fibrogenesis via CTGF pathway. miR-214 treatment decreased fibrogenesis in mice and is possible via exosome treatment | miRNA-214 therapy is anti-fibrotic and administration is possible via exosomes |
| Xia et al., 2023; Neferine mediated TGF-β/ERK signaling to inhibit fibrosis in endometriosis (Xia et al., 2023) |
|
Neferine low dose (5 mg/kg/day), medium dose (10 mg/kg/day), high dose (30 mg/kg/day), dienogest (2 mg/day) | Lesion size, HE, Masson stain, IHC, IF, WB | Fibrosis was progressive from day 7 to day 21. Fibrosis decreased in all treatment groups. Fibrotic markers α-SMA, Col-1, CTGF, FN, TGF-β, and p-ERK were increased in all study groups compared to healthy mice but decreased in all treatment groups versus untreated model mice. Largest effect of neferine was observed in high dose group | Inhibition of TGF-β/ERK signaling pathway by neferine can inhibit fibrosis progression in endometriosis | TGF-β/ERK signaling pathway contributes to fibrosis in endometriosis and this pro-fibrotic signaling can be inhibited by neferine |
| Xiao et al., 2020; Platelet and regulatory T cells may induce a type 2 immunity that is conducive to the progression and fibrogenesis of endometriosis (Xiao et al., 2020) |
|
Anti-platelet antibody therapy; regulatory T cell depletion; joint depletion platelets and Tregs | Lesion size, Masson stain, IHC, hotplate latency | Lesion size and extent of fibrosis increased over time in control group. Lesion weight was reduced in intervention group only after 5 weeks. Lesion size, markers for EMT, FMT and extent of fibrosis were reduced in platelet, T regs and joint depletion groups, hotplate latency improved. Type 2 immune reaction cell aggregation, Smad and Akt signaling markers were decreased in intervention groups | Platelets stimulate aggregation of Tregs, Th2 and M2 cells, which facilitated TSLP and GARP expression and TGF-β1 stimulation resulting in fibrogenesis and lesion progression. Platelet and Treg depletion reduced lesions progression by disrupting a type 2 immune reaction. Type 2 immune reaction plays a vital role in fibrogenesis in endometriosis | Fibrogenesis is stimulated via aggregation of regulatory T cells and M2 cells by platelets |
| Xu et al., 2023; A novel pathway regulating pyroptosis-induced fibrosis in endometriosis via Inc-MALAT1/miR-141-3p/NLRP3 pathway (Xu et al., 2023) | N = 24 (3 * 8); mice. Donor uterine tissue transplantation | MCC950 50 mg/kg (NLRP3 inhibitor) | HE, Masson stain, IHC, WB | MCC950 deactivated NLRP3 and decreased pyroptosis markers and IL-1β. Extent of fibrosis, expression of TGF-β1, CTGF, α-SMA, collagen-I and FN-1 were reduced by MCC950 treatment | Lnc-MALAT1 inhibits the inhibitory effect of miR-214-3p on NLRP3 inflammasome, thereby increasing pro-fibrotic signaling | Increased lnc-MALAT1 promotes fibrosis in endometriosis via its inhibiting role on the anti-fibrotic miR-214-3p, via increased NLRP3 inflammasome signaling |
| Yan et al., 2019a; The establishment of a mouse model of deep endometriosis (Yan et al., 2019a) |
|
Substance P (SP), calcitonin gene-related peptide (CGRP), SP+CGRP | Lesion size, Masson stain, IHC, hotplate latency | Lesion size, adhesion scores, extent of fibrosis, α-SMA, and other EMT, FTM, SMM marker expression was increased in treatment groups, especially in combined treatment. FMT progressive over time. Hotplate latency was impaired in treatment groups and showed a positive correlation with EMT, FMT, SMM markers, fibrosis and lesion weight. Correlation stronger with fibrosis than with lesion size | The developed DE model is macroscopically and microscopically similar to human lesions. There is a close correlation between fibrosis and EMT, FMT, and SMM. SP, and CGRP accelerate lesion development through EMT, FMT, and SMM | Substance P and CGRP stimulate endometriotic lesion development and fibrogenesis. Fibrosis important determinant of pain behavior |
| Yin et al., 2020; Enriched environment decelerates the development of endometriosis in mice (Yin et al., 2020) |
|
Enriched environment (EE): larger cages, more social interactions, toys, and physical activity. Either EE before and after induction of in-donors | Lesion size, Masson stain, IHC, hotplate latency | Lesion size, the extent of fibrosis, and α-SMA expression were reduced and hotplate latency improved in the enriched environment before and after lesion establishment group. No significant effects of enriched environment after lesion establishment or for donors. Plasma leptin levels showed a positive correlation with fibrosis and a negative with PPAR-γ expression | Enriched environment decelerates endometriosis development, attenuates hyperalgesia, and reduced fibrogenesis. Likely through increased dopamine receptor D2 and decreased adrenergic receptor B2 | Positive environmental factors can prevent endometriosis development, with no effect after lesion development |
| Yin et al., 2018; Caloric restriction dramatically stalls lesion growth in mice with induced endometriosis (Yin et al., 2018) |
|
Caloric restriction (CR), 30% reduction compared to ad libitum group | Lesion size, Masson stain, IHC, hotplate latency | Lesion size, extent of fibrosis, angiogenesis, proliferation and expression of IGF1, mTOR and pAkt were reduced in caloric restriction before and after lesion induction groups. No differences in hotplate latency were observed | Caloric restriction reduced lesion weight and fibrogenesis, both if started before or after induction of lesions. IHC suggests involvement of PI3K/Akt/mTOR, AMPK, SIRT1, CREB signaling pathways via reduced angiogenesis, proliferation and estrogen production | Caloric restriction may decrease fibrosis in endometriosis |
| Yoshino et al., 2020; Relaxin-2 may suppress endometriosis by reducing fibrosis, scar formation, and inflammation (Yoshino et al., 2020) |
|
Relaxin-2 1 μg/g/day | Lesion size, Masson stain | Lesion size and extent of fibrosis were decreased in RLX-2 treatment group | Relaxin-2 treatment inhibits fibrogenesis and inflammation in endometriosis both in vitro and in a mouse model. Possibly via MAPK pathway | Relaxin-2 effective anti-fibrotic therapy in a mouse model |
| Zeng et al., 2018; NR4A1 is involved in fibrogenesis in ovarian endometriosis (Zeng et al., 2018) |
|
E1: NR4A1−/− knockout; E2: Cytosporone (Csn-B) (NR4A1 agonist) | Lesion size, HE, Masson stain, Sirius red stain, WB, IHC, RT-qPCR | NR4A1 expression decreased and fibrosis increased in NR4A1−/− mice. Csn-B treatment did not affect lesion size. NR4A1 expression increased, p-NR4A1 and extent of fibrosis decreased in Csn-B treatment group | TGF-β1 stimulation phosphorylated NR4A1 through AKT pathway. NR4A1 deficiency promoted fibrosis and Csn-B treatment (a NR4A1 agonist) inhibited this effect and decreased fibrosis in vitro and in a mouse model | NR4A1 has anti-fibrotic properties, phosphorylated NR4A1 has pro-fibrotic properties, both acting via AKT and TGF- β1 signaling |
| Zhang et al., 2016b; Cellular changes consistent with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the progression of experimental endometriosis in baboons (Zhang et al., 2016b) |
|
None | Lesion size, adhesion score, HE, Masson stain, IHC, IF | TGF-β, p-Smad3 and CD42 were progressive over time. Vimentin expression in epithelial cells was absent till 12 months. Different α-SMA expression pattern was observed between intrastromal and surrounding cells, both increased over time. E-cadherin decreased over time. E-cadherin correlated negatively, α-SMA positively with TGF-βand p-Smad3. Fibrosis, desmin and smooth-muscle myosin heavy chain increased progressively from 3 months onward | Repeated tissue injury and repair occurs in endometriotic lesions, leading to EMT, FMT and SMM and ultimately fibrosis | Progressive EMT and FMT are on the basis of over time progressive smooth muscle metaplasia and fibrosis |
| Zhang et al., 2017a; Enhancer of Zeste homolog 2 (EZH2) induces epithelial mesenchymal transition in endometriosis (Zhang et al., 2017a) |
|
3-deazane-planocin A (DZNep, a EZH2 inhibitor) 1 mg/kg or 2.5 mg/kg | Lesion size, IHC, hotplate latency | Lesion size was reduced dose-dependently in DZNep treatment groups. Expression of α-SMA, collagen 1A and markers for EMT were decreased and hotplate latency improved in DZNep treatment groups | EZH2 and associated PRC2 are elevated in endometriosis. EZH2 inhibition suppresses PRC2 expression and EMT activating factors. In vivo EZH2 inhibition with DZNep improved hyperalgesia and reduced EMT and fibrosis in endometriosis mouse model. Platelets can activate EZH2 activity in endometriotic cells | Enhancer of Zeste homolog 2 induces EMT leading to fibrosis, probably after activation by platelets |
| Zhang et al., 2017b; Progressive development of endometriosis and its hindrance by anti-platelet treatment in mice with induced endometriosis (Zhang et al., 2017b) |
|
Tanshinone IIA 12.5 μg/g/2 days | Lesion size, Masson stain, IHC | Lesion size, extent of fibrosis and expression of markers for EMT, FMT and SMM are progressive over time. Hotplate latency impaired over time. In tanshinone IIA treatment group progression was not observed. Lesion development and fibrosis were decreased and hotplate latency improved in treatment group | Endometriosis model in mouse undergo progressive EMT, FMT, SMM, and fibrosis over time. Tanshinone IIA, an anti-platelet drug, inhibits these processes and reduced lesion weight | Anti-platelet therapy by tanshinone IIA is effective to stop endometriosis development and fibrogenesis |
| Zhang et al., 2019b; Activin A promotes myofibroblast differentiation of endometrial mesenchymal stem cells via STAT3-dependent Smad/CTGF pathway (Zhang et al., 2019b) |
|
Activin A, anti-Activin A antibody (AB) | Masson stain, IHC | Extent of fibrosis and expression of α-SMA, collagen I, fibronectin and CTGF were increased in Activin A treatment group versus non-treated control and decreased in the anti-Activin A AB treatment group | Activin A promotes myofibroblast differentiation via STAT3-dependent Smad/CTGF pathway. Activin A inhibition suppresses fibrosis development in mice | Activin A promotes myofibroblast differentiation and fibrosis via Smad signaling, inhibition can be a potential therapeutic target |
| Zhang et al., 2022; Ferroptosis induced by iron overload promotes fibrosis in ovarian endometriosis and is related to subpopulations of endometrial stromal cells (Zhang et al., 2022) | N = 30 (6 * 5); mice. Subcutaneous injection of human endometrial tissue | Control, ferric ammonium citrate (FAC), erastin, FAC + vehicle, FAC+DFO (deferoxamine mesylate, iron chelator), FAC + Fer1 (Ferrostatin-1, ferroptosis inhibitor) | Lesion size, HE, IHC, malondialdehyde assay | FAC treatment enlarged lesion size and enhanced fibrosis, this effect was diminished by Fer-1 and DFO treatment | Mesenchymal stem cell ferroptosis can be induced by endometriosis iron overload, leading to increased fibrosis | Iron accumulation in endometriosis can trigger ferroptosis and subsequentially fibrogenesis |
| Zhang et al., 2023a; Blocking sphingosine 1-phosphate receptor 1 with modulators reduces immune cells infiltration and alleviates endometriosis in mice (Zhang et al., 2023a) |
|
Broad-spectrum S1P modulator FTY720; selective S1P receptor 1 modulator SEW2871 | Lesion size, ultrasonography, HE, Masson stain, IHC, RT-qPCR, flow cytometry | 1 mg/kg FTY720 was identified as adequate dosage. In both treatment groups lesion size and extent of fibrosis, but not α-SMA expression, was decreased. Inflammatory markers IL-1β, TGF-β1, and TNF-α only decreased in SEW2871 group. Immune cell lesional infiltration of CD45+ cells and macrophages, but not CD4+ or CD8+ T cells was decreased in both treatment groups | Both broad and specific S1P receptor modulator decreased endometriosis development via a decreased immune cell infiltration | S1P receptor modulators can reduce immune cell infiltration and fibrosis in endometriosis |
| Zheng et al., 2016; Epigenetic modulation of collagen 1A1: therapeutic implications in fibrosis and endometriosis (Zheng et al., 2016) |
|
|
Lesion size, Masson stain, RT-qPCR |
|
Progressive fibrosis is associated with lesion specific diminished Klf11 expression. Klf11 resulted in histone deacetylation and gene repression of COL1a1. Epigenetic therapy can affect (de)acetylation and thereby is a potential therapeutic strategy | Klf11 repress collagen production and thus fibrogenesis, loss of Klf11 has a pro-fibrotic effect. Epigenetic therapy can be a target for treatment |
| Zheng et al., 2023a; Aberrant expression of histone deacetylase 8 in endometriosis and its potential as a therapeutic target (Zheng et al., 2023a) |
|
PCI- 34051 (HDAC8 inhibitor) | Lesion size, HE, Masson stain, IHC, hotplate latency | Hdac 8 was overexpressed in endometriotic lesions. Hdac 8 inhibition reduced endometriotic lesion development and improved hyperalgesia | Hdac 8 is correlated with lesion development and Hdac 8 inhibition treatment showed therapeutic potential | Hdac 8 inhibition can reduce fibrosis in endometriosis |
| Zheng et al., 2023b; Corroborating evidence for aberrant expression of histone deacetylase 8 in endometriosis (Zheng et al., 2023b) |
|
TM-2-51 (HDAC8 activator); Tubastatin A (HDAC6 inhibitor), PCI- 34051 (HDAC8 inhibitor) | Lesion size, HE, Masson stain, IHC, WB, hotplate latency | Hdac 1, 8, and 6 expression was progressive over time, correlating with fibrosis, but Hdac 2 expression decreased over time. Hdac 8 staining correlated most prominent with lesional fibrosis. Lesional development and fibrosis was increased in Hdac8 activator group and decreased in Hdac inhibitors treatment groups | Hdac 8 expression is progressive during endometriotic lesions development, correlating with fibrosis and Hdac based treatment can be a therapeutic target | Hdac-based interventions showed anti-fibrotic properties in endometriosis |
PER, peritoneal endometriosis; OMA, ovarian endometrioma; DE, deep endometriosis; WT, wild-type; HE, hematoxylin/eosin staining; IHC, immunohistochemistry; IF, immunofluorescence; WB, western blot; RT-qPCR, real-time qualitative polymerase chain reaction; α-SMA, α-smooth muscle actin; TGF-β, transforming growth factor-β; EMT, epithelial-to-mesenchymal transition; FMT, fibroblast-to-myofibroblast transdifferentiation; SMM, smooth muscle metaplasia.
In some studies, the specific aim was to evaluate the development of endometriosis in animal models, rather than to test potential therapeutic agents. FMT, an important process in the development of fibrosis, was shown to be a host reaction to ectopic tissue rather than a reaction of the donor tissue itself (van Kaam et al., 2008). The progressive nature of EMT, FMT, and fibrosis was shown in a baboon endometriosis model, leading to expansion of fibrosis from minor fibrosis at three months to a highly fibrotic lesion at twelve months after endometriosis induction, which strongly arguments for the progressive nature of the disease in human disease as well (Zhang et al., 2016b). In addition to their human observational studies, Muraoka et al. showed that an endometrial infection of donor tissue with F. nucleatum increased lesion size and myofibroblast activation in endometriosis in recipients. This effect was diminished after adequate antibiotic treatment (Muraoka et al., 2023). These findings support the hypothesis that bacterial infection can affect myofibroblastic transdifferentiation and thereby contribute to endometriosis development.
Many animal studies were designed to intervene in the role of different immune cells in endometriotic lesions to achieve an anti-fibrotic effect. Macrophages are known to have a pivotal role in human endometriosis and they are abundantly present in endometriotic implants in animal models as well (Hull et al., 2012; Nishimoto-Kakiuchi et al., 2016; Luo et al., 2020). Particularly, M2-activated macrophages stimulate fibrogenesis, as shown by increased myofibroblast differentiation, fibrosis and pain behaviour after suppletion of ex vivo differentiated macrophages (Duan et al., 2018). Inhibition of mast cell activity, among others with fisetin treatment, also decreased fibrotic development of lesions (Di Paola et al., 2016; Guo et al., 2021; Arangia et al., 2023). Besides this, antibody-based inactivation of B lymphocytes or type 2 innate lymphoid cells led to anti-fibrotic effects in endometriosis models (Riccio et al., 2019; Miller et al., 2021; Dogan et al., 2023).
The immune cell infiltration cascades in endometriosis are partially set in motion because the lesions show a form of tissue injury that normally triggers wound healing mechanisms aiming at resolving the lesion. However, in endometriosis, this wound-healing mechanism fails and triggers repeated platelet aggregation, an important signal for other immune cells to infiltrate the lesions. In this light, many anti-platelet interventions, among which are aggregation inhibitors scutellarin and ozagrel, are shown to reduce macrophage infiltration, lesion growth, and lesional fibrosis in vivo (Guo et al., 2015, 2016; Zhang et al., 2017a; 2017b; Ding et al., 2019; Xiao et al., 2020; Huang et al., 2022b).
Similar to studies in human tissues, sensory nerves and neuropeptides SP and CGRP were identified as pro-fibrotic stimuli (Liu et al., 2019; Yan et al., 2019a). Besides sensory nerves, the autonomous nervous system also influences endometriosis. Sympathetic overstimulation promotes fibrogenesis and activation of nicotinic acetylcholine receptors reducing the development of endometriosis (Hao et al., 2021, 2022). To date, the molecular mechanisms of the interaction between endometriosis and nerves are not well understood yet.
In nearly all included studies, lesion size and the extent of fibrosis are closely correlated. Strikingly, some studies wherein angiogenesis was inhibited reported beneficial effects with lesion size shrinking but an increase in the extent of fibrosis (Liu et al., 2015; Buigues et al., 2018).
Next to cell type-specific interventions, interventions on cellular and molecular pathways were studied. Endometriotic cells may escape apoptosis via overexpression of the anti-apoptotic BCL-2 family proteins and the lack of apoptosis among senescent cells, as illustrated by successful anti-fibrotic interventions targeting these mechanisms (Nahari and Razi, 2018; Luo et al., 2020; Siracusa et al., 2021). The Wnt/β-catenin and Smad signaling pathways were successfully used as anti-fibrotic targets, and may constitute a potential therapeutic approach (Matsuzaki and Darcha, 2013, 2014; Hirakawa et al., 2019; Zhang et al., 2019b; Shi et al., 2021). Mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPK/ERK) signaling is another pathway where interventions caused a reduction in fibrosis (Peng et al., 2022; Xia et al., 2023). Transcription regulating mechanisms were also studied in vivo in mice and indicated that transcription factors Klf10 and Klf11 modulated the fibrotic response in endometriosis (Daftary et al., 2013; Delaney et al., 2016; Zheng et al., 2016; Khan et al., 2018; Grande et al., 2023). MicroRNA miR27b is shown to promote fibrosis, whereas miR214 showed anti-fibrotic effects by interfering in the transcription of NLRP3 (Kim et al., 2017; Wu et al., 2018). The reduced level of miR214 in endometriosis can increase NLRP3 transcription and thereby trigger an increase of IL-1β release and fibrosis (Xu et al., 2023). Histone deacetylase 8 was overexpressed in endometriosis and inhibition showed an anti-fibrotic effect (Zheng et al., 2023a; 2023b).
Risk of bias assessment
The results of the risk of bias assessment are shown in Tables 4, 5, and 6. The risk of bias of the observational studies was assessed with the MINORS tool. Most of them scored as moderate, although few studies were scored according as having a high risk of bias. The experimental studies with human-derived material were assessed using the ROBINS-I tool and most of them were judged to have a moderate to low risk of bias. The animal studies were assessed using the SYRCLE tool. Many animal studies scored a high risk of bias, mainly due to a lack of reporting details about animal facilities.
Table 4.
Bias assessment of observational studies according to MINORS tool.
|
Signaling question numbers correspond with MINORS tool. Score 0: not reported; score 1: reported but inadequate; score 2: reported and adequate. A perfect total score is 16 for non-comparative studies and 24 for comparative studies.
Table 5.
Bias assessment of intervention studies according to ROBINS-I tool.
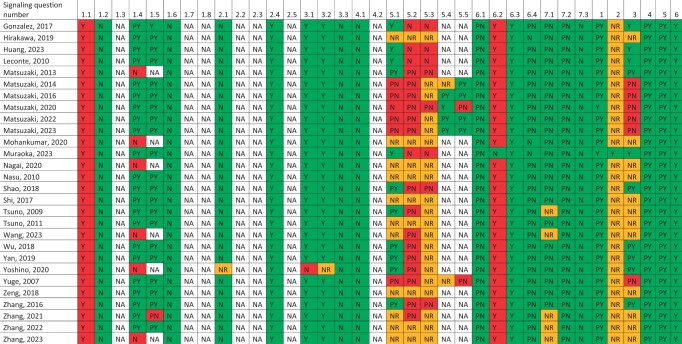
|
Signaling question numbers 1.1-7.3 correspond with ROBINS-I tool, signaling question numbers 1-6 correspond with additional questions for in vitro studies. Scores: Y: yes; PY: probably yes; NR: not reported; PN: probably no; N: no; NA: not applicable.
Table 6.
Bias assessment of animal studies according to SYRCLE tool.
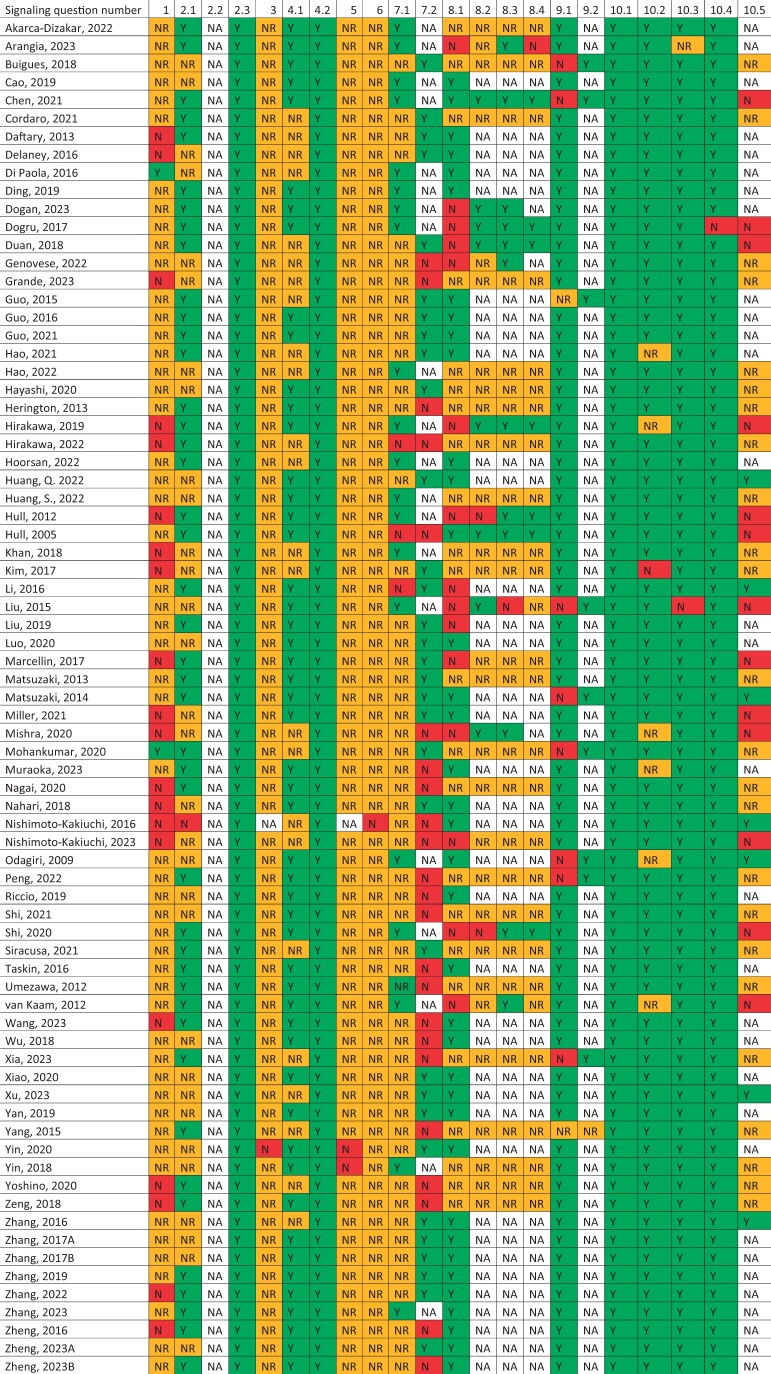
|
Signaling question numbers correspond with SYRCLE tool. Scores: Y: yes; PY: probably yes; NR: not reported; PN: probably no; N: no; NA: not applicable.
Discussion
Interpretation and main findings
The current available knowledge about the role of fibrosis in endometriosis is presented in this systematic review. This includes histologic characterization, molecular processes, clinical parameters, and therapeutic strategies. The main findings of this systematic review are as follows. First, the development of fibrosis in endometriosis is accompanied by the dynamic cellular processes of EMT, FMT, and SMM, resulting in myofibroblasts that enable contraction of the extracellular matrix. Various stages of transdifferentiation can be present within a single lesion. Second, platelet aggregation, probably induced by tissue injury triggers pro-fibrotic signaling and immune cell infiltration. TGF-β is a common activator of pro-fibrotic pathways. Potential therapeutic pathways are often based on preventing platelet activation, or inhibition of Smad and Rho/ROCK. Third, fibrosis, nerves, and neuropeptides are histo-anatomically related, show mutual stimulating effects, and correlate with dysmenorrhea and pain behavior, which suggests the relevance of fibrosis in pain. Finally, numerous therapeutics targeting fibrosis have been tested in vitro and in animal models, but none of them have been tested in the light of endometriosis-related fibrosis in human subjects to date. Nevertheless, the successful regression of endometriosis-related fibrosis in animal studies shows the potential for development of successful therapeutics in humans.
These findings highlight the importance of fibrosis in endometriosis. They also give insight in the etiology of the fibrotic processes. Following this, it is important to note both the similarities and the differences between endometriosis and other fibrotic diseases, including systemic sclerosis and idiopathic pulmonary fibrosis. Among the spectrum of fibrotic diseases, EMT and FMT are common hallmarks. Cellular injury may cause a new fibrotic steady state wherein myofibroblasts are abundantly present due to both cellular differentiation and recruitment (Adler et al., 2020). In endometriosis, the cyclical bleeding of the endometriotic implants could act as a stimulus similar to the continuous injury in other fibrotic diseases. This repetitive signaling can stimulate cellular differentiations EMT and FMT via TGF-β release (Di Gregorio et al., 2020; Wang and Friedman, 2023). Next to activated platelets, M2 differentiated macrophages are an important source of this TGF-β release (Capobianco and Rovere-Querini, 2013; Vigano et al., 2020). Smad and Rho/ROCK pathways are activated via TGF-β in idiopathic pulmonary fibrosis or systemic sclerosis (Knipe et al., 2015; Ye and Hu, 2021; Mendoza and Jimenez, 2022). In idiopathic pulmonary fibrosis, nintedanib and pirfenidone are successful therapeutics which are clinically available. These are identified based on their anti-fibrotic effects in in vitro studies (Lehmann et al., 2018; Amati et al., 2023). Metformin also showed potential in pre-clinical fibrosis research in idiopathic pulmonary fibrosis (Kheirollahi et al., 2019). The repurposing of these compounds for treatment of endometriosis seems promising based on the commonalities in etiology.
On the other hand, the interaction with nerves seems to be rather unique for endometriotic fibrosis as compared to other fibrotic diseases. There is evidence about the involvement of neuropeptides in fibrotic development in other diseases. For example, substance P is described as having a pro-fibrotic effect in several other diseases, like myocardial and idiopathic pulmonary fibrosis, via its receptor neurokinin 1, probably by enhancing TGF-β release and oxidative stress (Peng et al., 2019; Słoniecka and Danielson, 2019). Both pro- and anti-fibrotic effects of CGRP are described in the literature, whereas in endometriosis only pro-fibrotic effects are known (Li et al., 2020; Kayalar and Oztay, 2022). However, the close histo-anatomical relationship between fibrosis and nerves is not described in other fibrotic diseases. Interestingly, endometriosis is unique within the group of fibrotic diseases because in endometriosis pain is a central symptom, which is not the case in most other fibrotic diseases such as, for example, idiopathic pulmonary fibrosis, systemic sclerosis or liver cirrhosis. In most other fibrotic diseases, symptoms occur as a result of organ dysfunction due to tissue stiffness. The pain-related findings in this review also answer the question in our introduction of whether fibrosis is a favorable or unfavorable event in endometriosis. Based on this systematic review, we can consider fibrosis as an unfavorable outcome, as the extent of fibrosis correlated strongly with more severe dysmenorrhea in humans and more pronounced pain behavior in animal studies (Odagiri et al., 2009; Yan et al., 2019b). In contrast, anti-angiogenic therapy leading to an increase of fibrosis at an early stage of lesion development might show beneficial effects because in this case, the early fibrosis formation may hinder further lesion growth and cyclical bleeding of the lesions (Liu et al., 2015).
Strengths and limitations
The systematic methodology of this review has several strengths. First, to our knowledge, this is the first review about fibrosis in endometriosis with a comprehensive systematic approach. The results of both cellular and molecular processes and clinically orientated studies are included in this review. With this approach, we are able to link aetiologic studies to clinically orientated research. In this unique way, all aspects of the relevance of fibrosis in endometriosis have been brought together.
Second, in the systematic risk of bias assessment, we used different validated tools. The most suitable risk of bias tool for the observational studies is the validated MINORS tool and for the animal studies, this is the validated SYRCLE tool (Slim et al., 2003; Hooijmans et al., 2014). For the in vitro experimental studies, the most suitable tool is the ROBINS-I tool (Sterne et al., 2016). We used different tools for different types of studies in order to assess the risk of bias in this comprehensive review in the most reliable way possible. The in vitro design of the experimental studies lack a specific validated assessment tool, so therefore we additionally used the non-validated risk of bias assessment formulated by Post et al. (2020). This systematic assessment for risk of bias helps to value all the evidence presented in this review.
This systematic review has its limitations, too. A significant amount of information presented in this review is based on animal studies. This can be seen as a limitation, as animal models for endometriosis face a number of drawbacks. Most animal models lack a human-like menstrual cycle as well as spontaneous development of endometriosis, which complicates the interpretation of the results of these studies. Therefore, the results of animal studies and potential therapeutics described in this review are not directly useable in current practice in humans.
Next, we excluded some studies not presented in English, as we were unsure if we were able to read and interpret them correctly. From a few articles, we were not able to retrieve a full-text version, so these were also excluded. These studies might have provided us with additional information.
A question not fully answered in this review is what the effect of newly developed anti-fibrotic therapies on already-established endometriosis would be. Most intervention studies are designed in such a way that their therapies are predominantly showing a preventive effect on fibrogenesis. At the moment, efforts to regress fibrosis are underexplored. Some animal experiments try to capture the difference between regression and prevention of fibrosis in their study design by varying the moment of starting their intervention. Some studies were indeed able to show a decrease in fully developed fibrosis. However, this situation is difficult to compare with human subjects but is extremely relevant. Generally, there is a substantial delay in the diagnosis of endometriosis and therefore, future therapies must ideally be able to not only stop fibrosis but also to resolve already established fibrotic tissue.
Future implications
More research is required to bridge the gap between knowledge about the etiology of fibrosis in endometriosis, the current clinical care, and possible therapeutic targets. Based on the pathways identified to be relevant for fibrosis in endometriosis, the similarities between endometriosis and other fibrotic diseases seem very relevant to explore. As research in endometriosis is still in a developing phase relative to its huge societal implications, similarities between broadly extensively studied other diseases including systemic sclerosis and idiopathic pulmonary fibrosis can be extremely useful to explore. Additionally, it might be insightful to investigate which exact mechanisms from which of these diseases align best with pathways of fibrosis in endometriosis.
Another issue highlighted by this review is the lack of studies in human subjects. Already several years ago the relevance of fibrosis in endometriosis was stressed almost simultaneously by two research groups (Guo, 2018; Vigano et al., 2018). Since then, many pre-clinical potential therapeutics have been described, but none of them have been tested in clinical trials regarding their effect on fibrosis in endometriosis. Partially, this may be due to the current gap between endometriosis models and the human in vivo situation. Most pre-clinical work is performed in isolated cells or in rodents, both far from being representative for the clinical situation. An adequate pre-clinical endometriosis model bridging the gap from cells to humans is highly necessary. Fortunately, the number of studies directed at other pre-clinical models for both eutopic and ectopic endometrium is increasing. Such a pre-clinical model should fulfill several requirements (Gołąbek-Grenda and Olejnik 2022). First, it should be as close to the human in vivo situation as possible, thus preferably active immune cells, endometriosis-related hormones and cytokines, and interaction with ECM should be present. Second, the fibrotic environment should be preserved in order to assess the effect of anti-fibrotic agents on fibrosis in endometriosis. Moreover, a suitable model ideally should be reproducible among different research groups and cost-effective. Currently, endometriosis organoids are nearing these fulfillments, recapitulating 3D structure and cell–cell interactions (Boretto et al., 2019; Esfandiari et al., 2021). The recent establishment of a successful eutopic endometrium model will also contribute to developments in endometriosis research (Ahn et al., 2021). With continuing developments in the field of ex vivo tissue culture and organ-on-a-chip systems, useful endometriosis models seem to be reachable in the near future.
Conclusion
In conclusion, this review gives a comprehensive overview of the current evidence about fibrosis with regard to endometriosis. This may help in focusing future research on fibrosis in endometriosis.
Supplementary Material
Contributor Information
Guus Vissers, Department of Obstetrics & Gynaecology, Radboud University Medical Center, Nijmegen, The Netherlands.
Maddalena Giacomozzi, Department of Obstetrics & Gynaecology, Radboud University Medical Center, Nijmegen, The Netherlands.
Wouter Verdurmen, Department of Medical BioSciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Ron Peek, Department of Obstetrics & Gynaecology, Radboud University Medical Center, Nijmegen, The Netherlands.
Annemiek Nap, Department of Obstetrics & Gynaecology, Radboud University Medical Center, Nijmegen, The Netherlands.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ roles
G.V.: conceptualization, methodology, investigation, writing—original draft preparation. M.G.: conceptualization, methodology, investigation, writing—review and editing. W.V.: conceptualization, writing—review and editing. R.P.: conceptualization, writing—review and editing. A.N.: conceptualization, writing—review and editing, supervision.
Funding
No external parties were involved in funding this study.
Conflict of interest
The authors have no conflicts of interest.
References
- Abramiuk M, Grywalska E, Małkowska P, Sierawska O, Hrynkiewicz R, Niedźwiedzka-Rystwej P.. The role of the immune system in the development of endometriosis. Cells 2022;11:2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M, Mayo A, Zhou X, Franklin RA, Meizlish ML, Medzhitov R, Kallenberger SM, Alon U.. Principles of cell circuits for tissue repair and fibrosis. iScience 2020;23:100841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Yoon MJ, Hong SH, Cha H, Lee D, Koo HS, Ko JE, Lee J, Oh S, Jeon NL. et al. Three-dimensional microengineered vascularised endometrium-on-a-chip. Hum Reprod 2021;36:2720–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akarca-Dizakar SÖ, Demirel MA, Coşkun Akçay N, Sipahi M, Karakoç Sökmensüer L, Boyunaga H, Köylü A, Ömeroğlu S.. The therapeutic effects of coenzyme Q10 on surgically induced endometriosis in Sprague Dawley rats. J Obstet Gynaecol 2022;42:3290–3298. [DOI] [PubMed] [Google Scholar]
- Almadani YH, Vorstenbosch J, Davison PG, Am M.. Wound healing: a comprehensive review. Semin Plast Surg 2021;35:141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amati F, Stainer A, Polelli V, Mantero M, Gramegna A, Blasi F, Aliberti S.. Efficacy of pirfenidone and nintedanib in interstitial lung diseases other than idiopathic pulmonary fibrosis: a systematic review. Int J Mol Sci 2023;24:7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaf V, Simon P, El Nakadi I, Fayt I, Buxant F, Simonart T, Peny MO, Noel JC.. Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum Reprod 2000a;15:; 1744–1750. [DOI] [PubMed] [Google Scholar]
- Anaf V, Simon P, Fayt I, Noel J.. Smooth muscles are frequent components of endometriotic lesions. Hum Reprod 2000b;15:767–771. [DOI] [PubMed] [Google Scholar]
- Arangia A, Marino Y, Fusco R, Siracusa R, Cordaro M, D’Amico R, Macrì F, Raffone E, Impellizzeri D, Cuzzocrea S. et al. Fisetin, a natural polyphenol, ameliorates endometriosis modulating mast cells derived NLRP-3 inflammasome pathway and oxidative stress. Int J Mol Sci 2023;24:5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcena de Arellano ML, Gericke J, Reichelt U, Okuducu AF, Ebert AD, Chiantera V, Schneider A, Mechsner S.. Immunohistochemical characterization of endometriosis-associated smooth muscle cells in human peritoneal endometriotic lesions. Hum Reprod 2011;26:2721–2730. [DOI] [PubMed] [Google Scholar]
- Bernacchioni C, Capezzuoli T, Vannuzzi V, Malentacchi F, Castiglione F, Cencetti F, Ceccaroni M, Donati C, Bruni P, Petraglia F.. Sphingosine 1-phosphate receptors are dysregulated in endometriosis: possible implication in transforming growth factor β-induced fibrosis. Fertil Steril 2021;115:501–511. [DOI] [PubMed] [Google Scholar]
- Biernacka A, Dobaczewski M, Frangogiannis NG.. TGF-beta signaling in fibrosis. Growth Factors 2011;29:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonte H, Chapron C, Vieira M, Fauconnier A, Barakat H, Fritel X, Vacher-Lavenu MC, Dubuisson JB.. Histologic appearance of endometriosis infiltrating uterosacral ligaments in women with painful symptoms. J Am Assoc Gynecol Laparosc 2002;9:519–524. [DOI] [PubMed] [Google Scholar]
- Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, Heremans R, Perneel L, Kobayashi H, Van Zundert I. et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol 2019;21:1041–1051. [DOI] [PubMed] [Google Scholar]
- Buigues A, Ferrero H, Martínez J, Pellicer N, Pellicer A, Gómez R.. Evaluation of PAI-1 in endometriosis using a homologous immunocompetent mouse model. Biol Reprod 2018;99:; 326–335. [DOI] [PubMed] [Google Scholar]
- Camboni A, Marbaix E.. Ectopic endometrium: the pathologist's perspective. Int J Mol Sci 2021;22:10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Liu X, Guo S-W.. Plasma high mobility group box 1 (HMGB1), osteopontin (OPN), and hyaluronic acid (HA) as admissible biomarkers for endometriosis. Sci Rep 2019;9:9272–9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capobianco A, Rovere-Querini P.. Endometriosis, a disease of the macrophage. Front Immunol 2013;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu X, Guo S-W.. Preoperative and perioperative intervention reduces the risk of recurrence of endometriosis in mice caused by either incomplete excision or spillage and dissemination. Reprod Biomed Online 2021;43:379–393. [DOI] [PubMed] [Google Scholar]
- Cordaro M, Salinaro AT, Siracusa R, D'Amico R, Impellizzeri D, Scuto M, Ontario ML, Interdonato L, Crea R, Fusco R. et al. Hidrox(R) and endometriosis: biochemical evaluation of oxidative stress and pain. Antioxidants 2021;10:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daftary GS, Zheng Y, Tabbaa ZM, Schoolmeester JK, Gada RP, Grzenda AL, Mathison AJ, Keeney GL, Lomberk GA, Urrutia R.. A novel role of the Sp/KLF transcription factor KLF11 in arresting progression of endometriosis. PLoS One 2013;8:e60165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney AA, Khan Z, Zheng Y, Correa LF, Zanfagnin V, Shenoy CC, Schoolmeester JK, Saadalla AM, El-Nashar S, Famuyide AO. et al. KLF10 mediated epigenetic dysregulation of epithelial CD40/CD154 promotes endometriosis. Biol Reprod 2016;95:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gregorio J, Robuffo I, Spalletta S, Giambuzzi G, De Iuliis V, Toniato E, Martinotti S, Conti P, Flati V.. The epithelial-to-mesenchymal transition as a possible therapeutic target in fibrotic disorders. Front Cell Dev Biol 2020;8:607483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola R, Fusco R, Gugliandolo E, Crupi R, Evangelista M, Granese R, Cuzzocrea S.. Co-micronized palmitoylethanolamide/polydatin treatment causes endometriotic lesion regression in a rodent model of surgically induced endometriosis. Front Pharmacol 2016;7:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Cai X, Zheng H, Guo SW, Liu X.. Scutellarin suppresses platelet aggregation and stalls lesional progression in mouse with induced endometriosis. Reprod Sci 2019;26:; 1417–1428. [DOI] [PubMed] [Google Scholar]
- Ding D, Chen Y, Liu X, Jiang Z, Cai X, Guo S-W.. Diagnosing deep endometriosis using transvaginal elastosonography. Reprod Sci 2020a;27:1411–1422. [DOI] [PubMed] [Google Scholar]
- Ding D, Wang X, Chen Y, Benagiano G, Liu X, Sw G.. Evidence in support for the progressive nature of ovarian endometriomas. J Clin Endocrinol Metab 2020b;105:2189–2202. [DOI] [PubMed] [Google Scholar]
- Distler JHW, Gyorfi AH, Ramanujam M, Whitfield ML, Konigshoff M, Lafyatis R.. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol 2019;15:705–730. [DOI] [PubMed] [Google Scholar]
- Dogan AC, Dogan M, Togrul C, Ozkan NT.. The effects of Rituximab on experimental endometriosis model in rats. J Reprod Immunol 2023;156:103814. [DOI] [PubMed] [Google Scholar]
- Dogru HY, Isguder CK, Ozsoy AZ, Delibas IB, Cakmak B, Arici A.. Effect of amygdalin on the treatment and recurrence of endometriosis in an experimental rat study. Periodicum Biologorum 2017;119:173–180. [Google Scholar]
- Duan J, Liu X, Wang H, Guo SW.. The M2a macrophage subset may be critically involved in the fibrogenesis of endometriosis in mice. Reprod Biomed Online 2018;37:254–268. [DOI] [PubMed] [Google Scholar]
- Esfandiari F, Favaedi R, Heidari-Khoei H, Chitsazian F, Yari S, Piryaei A, Ghafari F, Baharvand H, Shahhoseini M.. Insight into epigenetics of human endometriosis organoids: DNA methylation analysis of HOX genes and their cofactors. Fertil Steril 2021;115:125–137. [DOI] [PubMed] [Google Scholar]
- Garcia Garcia JM, Vannuzzi V, Donati C, Bernacchioni C, Bruni P, Petraglia F.. Endometriosis: cellular and molecular mechanisms leading to fibrosis. Reprod Sci 2023;30:1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T, Cordaro M, Siracusa R, Impellizzeri D, Caudullo S, Raffone E, Macrí F, Interdonato L, Gugliandolo E, Interlandi C et al Molecular and biochemical mechanism of cannabidiol in the management of the inflammatory and oxidative processes associated with endometriosis. Int J Mol Sci 2022;23:5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gołąbek-Grenda A, Olejnik A.. In vitro modeling of endometriosis and endometriotic microenvironment—Challenges and recent advances. Cell Signal 2022;97:110375–110375. [DOI] [PubMed] [Google Scholar]
- González-Foruria I, Santulli P, Chouzenoux S, Carmona F, Chapron C, Batteux F.. Dysregulation of the ADAM17/Notch signalling pathways in endometriosis: from oxidative stress to fibrosis. Mol Hum Reprod 2017;23:488–499. [DOI] [PubMed] [Google Scholar]
- Grande J, Jones TL, Sun Z, Chanana P, Jaiswal I, Leontovich A, Carapanceanu N, Carapanceanu V, Saadalla A, Osman A. et al. Host immunity and KLF 11 deficiency together promote fibrosis in a mouse model of endometriosis. Biochim Biophys Acta Mol Basis Dis 2023;1869:166784. [DOI] [PubMed] [Google Scholar]
- Guo SW. Fibrogenesis resulting from cyclic bleeding: the Holy Grail of the natural history of ectopic endometrium. Hum Reprod 2018;33:353–356. [DOI] [PubMed] [Google Scholar]
- Guo SW, Ding D, Geng JG, Wang L, Liu X.. P-selectin as a potential therapeutic target for endometriosis. Fertil Steril 2015a;103:990–1000.e1008. [DOI] [PubMed] [Google Scholar]
- Guo SW, Ding D, Liu X.. Anti-platelet therapy is efficacious in treating endometriosis induced in mouse. Reprod Biomed Online 2016;33:484–499. [DOI] [PubMed] [Google Scholar]
- Guo SW, Ding D, Shen M, Liu X.. Dating endometriotic ovarian cysts based on the content of cyst fluid and its potential clinical implications. Reprod Sci 2015b;22:873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Xu X, Li T, Yu Q, Wang J, Chen Y, Ding S, Zhu L, Zou G, Zhang X.. NLRP3 inflammasome activation of mast cells by estrogen via the nuclear-initiated signaling pathway contributes to the development of endometriosis. Front Immunol 2021;12:749979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga T, Kumasaka T, Kurihara M, Kataoka H, Miura M.. Immunohistochemical analysis of thoracic endometriosis. Pathol Int 2013;63:429–434. [DOI] [PubMed] [Google Scholar]
- Hao M, Liu X, Guo S-W.. Activation of alpha7 nicotinic acetylcholine receptor retards the development of endometriosis. Reprod Biol Endocrinol 2022;20:85–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao M, Liu X, Rong P, Li S, Guo SW.. Reduced vagal tone in women with endometriosis and auricular vagus nerve stimulation as a potential therapeutic approach. Sci Rep 2021;11:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Nakamura T, Motooka Y, Ito F, Jiang L, Akatsuka S, Iwase A, Kajiyama H, Kikkawa F, Toyokuni S.. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice. Redox Biol 2020;37:101726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herington JL, Glore DR, Lucas JA, Osteen KG, Bruner-Tran KL.. Dietary fish oil supplementation inhibits formation of endometriosis-associated adhesions in a chimeric mouse model. Fertil Steril 2013;99:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa T, Miyabe S, Narahara H, Nasu K, Kouji H, Katoh A, Uemura N.. beta-Catenin signaling inhibitors ICG-001 and C-82 improve fibrosis in preclinical models of endometriosis. Sci Rep 2019;9:20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa T, Yotsumoto F, Shirasu N, Kiyoshima C, Urushiyama D, Yoshikawa K, Miyata K, Kurakazu M, Koga KA, Aoki M. et al. Trophic and immunomodulatory effects of adipose tissue derived stem cells in a preclinical murine model of endometriosis. Sci Rep 2022;12:8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW.. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoorsan H, Simbar M, Tehrani FR, Fathi F, Mosaffa N, Riazi H, Akradi L, Nasseri S, Bazrafkan S.. The effectiveness of antioxidant therapy (vitamin C) in an experimentally induced mouse model of ovarian endometriosis. Womens Health (Lond) 2022;18:17455057221096218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Liu X, Guo S-W.. Higher fibrotic content of endometriotic lesions is associated with diminished prostaglandin E2 signaling. Reprod Med Biol 2021;21:e12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Liu X, Sw G.. Changing prostaglandin E2 (PGE(2)) signaling during lesional progression and exacerbation of endometriosis by inhibition of PGE(2) receptor EP2 and EP4. Reprod Med Biol 2022a;21:e12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Xiao F, Guo SW, Zhang T.. Tetramethylpyrazine retards the progression and fibrogenesis of endometriosis. Reprod Sci 2022b;29:1170–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull ML, Johan MZ, Hodge WL, Robertson SA, Ingman WV.. Host-derived TGFB1 deficiency suppresses lesion development in a mouse model of endometriosis. Am J Pathol 2012;180:880–887. [DOI] [PubMed] [Google Scholar]
- Hull ML, Prentice A, Wang DY, Butt RP, Phillips SC, Smith SK, Charnock-Jones DS.. Nimesulide, a COX-2 inhibitor, does not reduce lesion size or number in a nude mouse model of endometriosis. Hum Reprod 2005;20:350–358. [DOI] [PubMed] [Google Scholar]
- Hutchenreuther J, Leask A.. A tale of two orgins: do myofibroblasts originate from different sources in wound healing and fibrosis? Cell Tissue Res 2016;365:507–509. [DOI] [PubMed] [Google Scholar]
- Ibrahim MG, Sillem M, Plendl J, Taube ET, Schuring A, Gotte M, Chiantera V, Sehouli J, Mechsner S.. Arrangement of myofibroblastic and smooth muscle-like cells in superficial peritoneal endometriosis and a possible role of transforming growth factor beta 1 (TGFbeta1) in myofibroblastic metaplasia. Arch Gynecol Obstet 2019;299:489–499. [DOI] [PubMed] [Google Scholar]
- Itoga T, Matsumoto T, Takeuchi H, Yamasaki S, Sasahara N, Hoshi T, Kinoshita K.. Fibrosis and smooth muscle metaplasia in rectovaginal endometriosis. Pathol Int 2003;53:371–375. [DOI] [PubMed] [Google Scholar]
- Izumi G, Koga K, Takamura M, Makabe T, Satake E, Takeuchi A, Taguchi A, Urata Y, Fujii T, Osuga Y.. Involvement of immune cells in the pathogenesis of endometriosis. J Obstet Gynaecol Res 2018;44:191–198. [DOI] [PubMed] [Google Scholar]
- Ji H, Tang H, Lin H, Mao J, Gao L, Liu J, Wu T.. Rho/Rock cross-talks with transforming growth factor-beta/Smad pathway participates in lung fibroblast-myofibroblast differentiation. Biomed Rep 2014;2:787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayalar O, Oztay F.. CGRP induces myofibroblast differentiation and the production of extracellular matrix in MRC5s via autocrine and paracrine signalings. J Biochem Mol Toxicol 2022;36:e23204. [DOI] [PubMed] [Google Scholar]
- Khan Z, Zheng Y, Jones TL, Delaney AA, Correa LF, Shenoy CC, Khazaie K, Daftary GS.. Epigenetic therapy: novel translational implications for arrest of environmental dioxin-induced disease in females. Endocrinology 2018;159:477–489. [DOI] [PubMed] [Google Scholar]
- Khare VK, Martin DC, Eltorky M.. A comparative study of ovarian and pelvic wall-infiltrating endometriosis. J Am Assoc Gynecol Laparosc 1996;3:235–239. [DOI] [PubMed] [Google Scholar]
- Kheirollahi V, Wasnick RM, Biasin V, Vazquez-Armendariz AI, Chu X, Moiseenko A, Weiss A, Wilhelm J, Zhang JS, Kwapiszewska G et al Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun 2019;10:2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MK, Lee SK, Park JH, Lee JH, Yun BH, Park JH, Seo SK, Cho S, Choi YS.. Ginsenoside Rg3 decreases fibrotic and invasive nature of endometriosis by modulating miRNA-27b: in vitro and in vivo studies. Sci Rep 2017;7:17670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M, Defrère S, Dolmans MM, Colette S, Squifflet J, Van Langendonckt A, Donnez J.. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil Steril 2011;96:685–691. [DOI] [PubMed] [Google Scholar]
- Knipe RS, Tager AM, Liao JK.. The Rho kinases: critical mediators of multiple profibrotic processes and rational targets for new therapies for pulmonary fibrosis. Pharmacol Rev 2015;67:103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad L, Kortum J, Nabham R, Gronbach J, Dietze R, Oehmke F, Berkes E, Tinneberg HR.. Composition of the stroma in the human endometrium and endometriosis. Reprod Sci 2018;25:1106–1115. [DOI] [PubMed] [Google Scholar]
- Kuehlmann B, Bonham CA, Zucal I, Prantl L, Gurtner GC.. Mechanotransduction in wound healing and fibrosis. J Clin Med 2020;9:1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux-Biehlmann A, d'Hooghe T, Zollner TM.. Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends Pharmacol Sci 2015;36:270–276. [DOI] [PubMed] [Google Scholar]
- Leconte M, Nicco C, Ngô C, Arkwright S, Chéreau C, Guibourdenche J, Weill B, Chapron C, Dousset B, Batteux F.. Antiproliferative effects of cannabinoid agonists on deep infiltrating endometriosis. Am J Pathol 2010;177:2963–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Buhl L, Alsafadi HN, Klee S, Hermann S, Mutze K, Ota C, Lindner M, Behr J, Hilgendorff A. et al. Differential effects of Nintedanib and Pirfenidone on lung alveolar epithelial cell function in ex vivo murine and human lung tissue cultures of pulmonary fibrosis. Respir Res 2018;19:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Dai Y, Zhu H, Jiang Y, Zhang S.. Endometriotic mesenchymal stem cells significantly promote fibrogenesis in ovarian endometrioma through the Wnt/β-catenin pathway by paracrine production of TGF-β1 and Wnt1. Hum Reprod 2016;31:1224–1235. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang Z, Li X, Cai J, Li D, Du J, Zhang B, Xiang D, Li N, Li Y.. CGRP derived from cardiac fibroblasts is an endogenous suppressor of cardiac fibrosis. Cardiovasc Res 2020;116:1335–1348. [DOI] [PubMed] [Google Scholar]
- Liu F, Wang L, Zhang XX, Min SY, Liu YX, Zuo Z, Jin ZX, Zl Z.. Vascular endothelial growth factor receptor-2 inhibitor cediranib causes regression of endometriotic lesions in a rat model. Int J Clin Exp Pathol 2015;8:1165–1174. [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yan D, Guo SW.. Sensory nerve-derived neuropeptides accelerate the development and fibrogenesis of endometriosis. Hum Reprod 2019;34:452–468. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Q, Guo SW.. Histological and immunohistochemical characterization of the similarity and difference between ovarian endometriomas and deep infiltrating endometriosis. Reprod Sci 2018;25:329–340. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li M, Wei C, Tang L, Sheng Y, Liu Y, Li D, Ding D, Qiu J, Zhu X.. TSP1-CD47-SIRPalpha signaling facilitates the development of endometriosis by mediating the survival of ectopic endometrium. Am J Reprod Immunol 2020;83:e13236. [DOI] [PubMed] [Google Scholar]
- Luo M, Cai X, Yan D, Liu X, Guo SW.. Sodium tanshinone IIA sulfonate restrains fibrogenesis through induction of senescence in mice with induced deep endometriosis. Reprod Biomed Online 2020;41:373–384. [DOI] [PubMed] [Google Scholar]
- Marcellin L, Santulli P, Chouzenoux S, Cerles O, Nicco C, Dousset B, Pallardy M, Kerdine-Römer S, Just PA, Chapron C. et al. Alteration of Nrf2 and glutamate cysteine ligase expression contribute to lesions growth and fibrogenesis in ectopic endometriosis. Free Radic Biol Med 2017;110:1–10. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Canis M, Darcha C, Dechelotte P, Pouly JL, Bruhat MA.. Fibrogenesis in peritoneal endometriosis. A semi-quantitative analysis of type-I collagen. Gynecol Obstet Invest 1999;47:197–199. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Canis M, Pouly JL, Darcha C.. Soft matrices inhibit cell proliferation and inactivate the fibrotic phenotype of deep endometriotic stromal cells in vitro. Hum Reprod 2016;31:541–553. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Darcha C.. Involvement of the Wnt/β-catenin signaling pathway in the cellular and molecular mechanisms of fibrosis in endometriosis. PLoS One 2013;8:e76808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki S, Darcha C.. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum Reprod 2014;29:1677–1687. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Pouly JL, Canis M.. Dose-dependent pro- or anti-fibrotic responses of endometriotic stromal cells to interleukin-1β and tumor necrosis factor α. Sci Rep 2020;10:9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki S, Pouly JL, Canis M.. Persistent activation of signal transducer and activator of transcription 3 via interleukin-6 trans-signaling is involved in fibrosis of endometriosis. Hum Reprod 2022;37:1489–1504. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Pouly JL, Canis M.. IL-10 is not anti-fibrotic but pro-fibrotic in endometriosis: IL-10 treatment of endometriotic stromal cells in vitro promotes myofibroblast proliferation and collagen type I protein expression. Hum Reprod 2023;38:14–29. [DOI] [PubMed] [Google Scholar]
- Mechsner S, Bartley J, Loddenkemper C, Salomon DS, Starzinski-Powitz A, Ebert AD.. Oxytocin receptor expression in smooth muscle cells of peritoneal endometriotic lesions and ovarian endometriotic cysts. Fertil Steril 2005;83Suppl 1:1220–1231. [DOI] [PubMed] [Google Scholar]
- Mendoza FA, Jimenez SA.. Serine/threonine kinase inhibition as antifibrotic therapy: transforming growth factor-beta and Rho kinase inhibitors. Rheumatology (Oxford) 2022;61:1354–1365. [DOI] [PubMed] [Google Scholar]
- Meng XM, Nikolic-Paterson DJ, Lan HY.. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 2016;12:325–338. [DOI] [PubMed] [Google Scholar]
- Metzger DA, Szpak CA, Haney AF.. Histologic features associated with hormonal responsiveness of ectopic endometrium. Fertil Steril 1993;59:83–88. [PubMed] [Google Scholar]
- Miller JE, Lingegowda H, Symons LK, Bougie O, Young SL, Lessey BA, Koti M, Tayade C.. Interleukin-33 activates group 2 innate lymphoid cell expansion and modulates endometriosis. JCI Insight 2021;6:149699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Galvankar M, Vaidya S, Chaudhari U, Modi D.. Mouse model for endometriosis is characterized by proliferation and inflammation but not epithelial-to-mesenchymal transition and fibrosis. J Biosci 2020;45:105. [PubMed] [Google Scholar]
- Mohankumar K, Li X, Sung N, Cho YJ, Han SJ, Safe S.. Bis-indole-derived nuclear receptor 4A1 (NR4A1, Nur77) ligands as inhibitors of endometriosis. Endocrinology 2020;161:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka A, Suzuki M, Hamaguchi T, Watanabe S, Iijima K, Murofushi Y, Shinjo K, Osuka S, Hariyama Y, Ito M. et al. Fusobacterium infection facilitates the development of endometriosis through the phenotypic transition of endometrial fibroblasts. Sci Transl Med 2023;15:eadd1531. [DOI] [PubMed] [Google Scholar]
- Nagai T, Ishida C, Nakamura T, Iwase A, Mori M, Murase T, Osuka S, Takikawa S, Goto M, Kotani T. et al. Focal adhesion kinase-mediated sequences, including cell adhesion, inflammatory response, and fibrosis, as a therapeutic target in endometriosis. Reprod Sci 2020;27:1400–1410. [DOI] [PubMed] [Google Scholar]
- Nahari E, Razi M.. Silymarin amplifies apoptosis in ectopic endometrial tissue in rats with endometriosis; implication on growth factor GDNF, ERK1/2 and Bcl-6b expression. Acta Histochem 2018;120:757–767. [DOI] [PubMed] [Google Scholar]
- Nasu K, Tsuno A, Hirao M, Kobayashi H, Yuge A, Narahara H.. Heparin is a promising agent for the treatment of endometriosis-associated fibrosis. Fertil Steril 2010;94:46–51. [DOI] [PubMed] [Google Scholar]
- Nezhat FR, Kalir T.. Comparative immunohistochemical studies of endometriosis lesions and endometriotic cysts. Fertil Steril 2002;78:820–824. [DOI] [PubMed] [Google Scholar]
- Nie J, Zhao C, Laganà AS, Liu X, Guo SW.. Identification of lesional attributes of dysmenorrhea severity and the serum antimüllerian hormone levels in women with ovarian endometriomas. Fertil Steril 2022;118:191–202. [DOI] [PubMed] [Google Scholar]
- Nishimoto-Kakiuchi A, Netsu S, Matsuo S, Hayashi S, Ito T, Okabayashi S, Yasmin L, Yuzawa K, Kondoh O, Kato A. et al. Characteristics of histologically confirmed endometriosis in cynomolgus monkeys. Hum Reprod 2016;31:2352–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto-Kakiuchi A, Sato I, Nakano K, Ohmori H, Kayukawa Y, Tanimura H, Yamamoto S, Sakamoto Y, Nakamura G, Maeda A. et al. A long-acting anti-IL-8 antibody improves inflammation and fibrosis in endometriosis. Sci Transl Med 2023;15:eabq5858. [DOI] [PubMed] [Google Scholar]
- Odagiri K, Konno R, Fujiwara H, Netsu S, Yang C, Suzuki M.. Smooth muscle metaplasia and innervation in interstitium of endometriotic lesions related to pain. Fertil Steril 2009;92:1525–1531. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Agogo GO, Guo J, Yan M.. Substance P and fibrotic diseases. Neuropeptides 2019;76:101941. [DOI] [PubMed] [Google Scholar]
- Peng X, Xia Y, Xie J, Liu H, Fan L, Yu C, Ni X.. Mechanism of Thunberg Fritillaria in treating endometriosis based on network pharmacology and the effect of Peiminine on the MEK/ERK pathway. Am J Transl Res 2022;14:6196–6209. [PMC free article] [PubMed] [Google Scholar]
- Post WM, Ruiz-Zapata AM, Grens H, de Vries RBM, Poelmans G, Coenen MJH, Janssen DAW, Heesakkers J, Oosterwijk E, Kluivers KB.. Genetic variants and expression changes in urgency urinary incontinence: A systematic review. Neurourol Urodyn 2020;39:2089–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio LGC, Jeljeli M, Santulli P, Chouzenoux S, Doridot L, Nicco C, Reis FM, Abrão MS, Chapron C, Batteux F.. B lymphocytes inactivation by Ibrutinib limits endometriosis progression in mice. Hum Reprod 2019;34:1225–1234. [DOI] [PubMed] [Google Scholar]
- Roman H, Opris I, Resch B, Tuech JJ, Sabourin JC, Marpeau L.. Histopathologic features of endometriotic rectal nodules and the implications for management by rectal nodule excision. Fertil Steril 2009;92:1250–1252. [DOI] [PubMed] [Google Scholar]
- Selcuk S, Kucukbas M, Koc N, Cam C, Ozkaya E, Eser A, Karateke A.. Tumour markers and histopathologic features of ovarian endometriotic cysts. J Obstet Gynaecol 2021;41:763–768. [DOI] [PubMed] [Google Scholar]
- Shao X, Wei X.. FOXP1 enhances fibrosis via activating Wnt/β-catenin signaling pathway in endometriosis. Am J Transl Res 2018;10:3610–3618. [PMC free article] [PubMed] [Google Scholar]
- Shi L, Xue X, Tian H, Ye H, Wang H, Wang R, Liu Y, Zhang C, Chen Q, Sun L.. WEE1 promotes endometriosis via the Wnt/β-catenin signaling pathway. Reprod Biol Endocrinol 2021;19:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LB, Zhang SY, Shi XJ.. Mechanistic study of vitamin C attenuation of endometriotic fibrosis. Clin Exp Obstet Gynecol 2020;47:383–390. [Google Scholar]
- Shi LB, Zhou F, Zhu HY, Huang D, Jin XY, Li C, Dai Y, Pan YB, Zhang SY.. Transforming growth factor beta1 from endometriomas promotes fibrosis in surrounding ovarian tissues via Smad2/3 signaling. Biol Reprod 2017;97:873–882. [DOI] [PubMed] [Google Scholar]
- Shin S, Chung YJ, Moon SW, Choi EJ, Kim MR, Chung YJ, Lee SH.. Single-cell profiling identifies distinct hormonal, immunologic, and inflammatory signatures of endometriosis-constituting cells. J Pathol 2023;261:323–334. [DOI] [PubMed] [Google Scholar]
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T. et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012;27:1292–1299. [DOI] [PubMed] [Google Scholar]
- Siracusa R, D’Amico R, Cordaro M, Peritore AF, Genovese T, Gugliandolo E, Crupi R, Impellizzeri D, Cuzzocrea S, Fusco R. et al. The methyl ester of 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid reduces endometrial lesions development by modulating the NFkB and Nrf2 pathways. Int J Mol Sci 2021;22:3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J.. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712–716. [DOI] [PubMed] [Google Scholar]
- Słoniecka M, Danielson P.. Substance P induces fibrotic changes through activation of the RhoA/ROCK pathway in an in vitro human corneal fibrosis model. J Mol Med (Berl) 2019;97:1477–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohler F, Sommer A, Wachter DL, Agaimy A, Fischer OM, Renner SP, Burghaus S, Fasching PA, Beckmann MW, Fuhrmann U. et al. Tissue remodeling and nonendometrium-like menstrual cycling are hallmarks of peritoneal endometriosis lesions. Reprod Sci 2013;20:85–102. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stovall DW, Anners JA, Halme J.. Immunohistochemical detection of type I, III, and IV collagen in endometriosis implants. Fertil Steril 1992;57:984–989. [PubMed] [Google Scholar]
- Stratopoulou CA, Camboni A, Donnez J, Dolmans MM.. Identifying common pathogenic features in deep endometriotic nodules and uterine adenomyosis. J Clin Med 2021;10:4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskin MI, Gungor AC, Adali E, Yay A, Onder GO, Inceboz U.. A humanized anti-interleukin 6 receptor monoclonal antibody, tocilizumab, for the treatment of endometriosis in a rat model. Reprod Sci 2016;23:662–669. [DOI] [PubMed] [Google Scholar]
- Tsujioka H, Inoue Y, Emoto M, Sadamori R, Shirota K, Hachisuga T, Kawarabayashi T.. The efficacy of preoperative hormonal therapy before laparoscopic cystectomy of ovarian endometriomas. J Obstet Gynaecol Res 2009;35:782–786. [DOI] [PubMed] [Google Scholar]
- Tsuno A, Nasu K, Kawano Y, Yuge A, Li H, Abe W, Narahara H.. Fasudil inhibits the proliferation and contractility and induces cell cycle arrest and apoptosis of human endometriotic stromal cells: a promising agent for the treatment of endometriosis. J Clin Endocrinol Metab 2011;96:E1944–E1952. [DOI] [PubMed] [Google Scholar]
- Tsuno A, Nasu K, Yuge A, Matsumoto H, Nishida M, Narahara H.. Decidualization attenuates the contractility of eutopic and ectopic endometrial stromal cells: implications for hormone therapy of endometriosis. J Clin Endocrinol Metab 2009;94:2516–2523. [DOI] [PubMed] [Google Scholar]
- Umezawa M, Saito Y, Tanaka-Hattori N, Takeda K, Ihara T, Sugamata M.. Expression profile of extracellular matrix and adhesion molecules in the development of endometriosis in a mouse model. Reprod Sci 2012;19:1365–1372. [DOI] [PubMed] [Google Scholar]
- Vallve-Juanico J, Houshdaran S, Giudice LC.. The endometrial immune environment of women with endometriosis. Hum Reprod Update 2019;25:564–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Aken MAW, Oosterman JM, van Rijn CM, Ferdek MA, Ruigt GSF, Peeters B, Braat DDM, Nap AW.. Pain cognition versus pain intensity in patients with endometriosis: toward personalized treatment. Fertil Steril 2017;108:679–686. [DOI] [PubMed] [Google Scholar]
- van Kaam KJ, Schouten JP, Nap AW, Dunselman GA, Groothuis PG.. Fibromuscular differentiation in deeply infiltrating endometriosis is a reaction of resident fibroblasts to the presence of ectopic endometrium. Hum Reprod 2008;23:2692–2700. [DOI] [PubMed] [Google Scholar]
- Vicino M, Scioscia M, Resta L, Marzullo A, Ceci O, Selvaggi LE.. Fibrotic tissue in the endometrioma capsule: surgical and physiopathologic considerations from histologic findings. Fertil Steril 2009;91:1326–1328. [DOI] [PubMed] [Google Scholar]
- Vigano P, Candiani M, Monno A, Giacomini E, Vercellini P, Somigliana E.. Time to redefine endometriosis including its pro-fibrotic nature. Hum Reprod 2018;33:347–352. [DOI] [PubMed] [Google Scholar]
- Vigano P, Ottolina J, Bartiromo L, Bonavina G, Schimberni M, Villanacci R, Candiani M.. Cellular components contributing to fibrosis in endometriosis: a literature review. J Minim Invasive Gynecol 2020;27:287–295. [DOI] [PubMed] [Google Scholar]
- Wang M, Fan R, Jiang J, Sun F, Sun Y, Wang Q, Jiang A, Yu Z, Yang T.. PIM2 promotes the development of ovarian endometriosis by enhancing glycolysis and fibrosis. Reprod Sci 2023;30:2692–2702. [DOI] [PubMed] [Google Scholar]
- Wang S, Friedman SL.. Found in translation-fibrosis in metabolic dysfunction-associated steatohepatitis (MASH). Sci Transl Med 2023;15:eadi0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Lu P, Mi X, Miao J.. Exosomal miR-214 from endometrial stromal cells inhibits endometriosis fibrosis. Mol Hum Reprod 2018;24:357–365. [DOI] [PubMed] [Google Scholar]
- Xia Y, Guo Y, Zhou J, Fan L, Xie J, Wang Y, Du H, Ni X.. Neferine mediated TGF-beta/ERK signaling to inhibit fibrosis in endometriosis. Am J Transl Res 2023;15:3240–3253. [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Liu X, Guo SW.. Platelets and regulatory T cells may induce a type 2 immunity that is conducive to the progression and fibrogenesis of endometriosis. Front Immunol 2020;11:610963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Zhang X, Zhan J, Ren Y, Wang W.. Potential role of strain elastography for detection of the extent of large-scar endometriosis. J Ultrasound Med 2013;32:1635–1642. [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu H, Xiong W, Peng Y, Li X, Long X, Jin J, Liang J, Weng R, Liu J. et al. A novel mechanism regulating pyroptosis-induced fibrosis in endometriosis via lnc-MALAT1/miR-141-3p/NLRP3 pathwaydagger. Biol Reprod 2023;109:156–171. [DOI] [PubMed] [Google Scholar]
- Yan D, Liu X, Guo SW.. The establishment of a mouse model of deep endometriosis. Hum Reprod 2019a;34:235–247. [DOI] [PubMed] [Google Scholar]
- Yan D, Liu X, Guo SW.. Neuropeptides substance P and calcitonin gene related peptide accelerate the development and fibrogenesis of endometriosis. Sci Rep 2019b;9:2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Liu X, Xu H, Guo SW.. Mesothelial cells participate in endometriosis fibrogenesis through platelet-induced mesothelial-mesenchymal transition. J Clin Endocrinol Metab 2020a;105:4124–4147. [DOI] [PubMed] [Google Scholar]
- Yan D, Liu X, Xu H, Guo SW.. Platelets induce endothelial-mesenchymal transition and subsequent fibrogenesis in endometriosis. Reprod Biomed Online 2020b;41:500–517. [DOI] [PubMed] [Google Scholar]
- Ye Z, Hu Y.. TGF-beta1: gentlemanly orchestrator in idiopathic pulmonary fibrosis (Review). Int J Mol Med 2021;48:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin B, Jiang H, Liu X, Guo SW.. Enriched environment decelerates the development of endometriosis in mouse. Reprod Sci 2020;27:1423–1435. [DOI] [PubMed] [Google Scholar]
- Yin B, Liu X, Guo SW.. Caloric restriction dramatically stalls lesion growth in mice with induced endometriosis. Reprod Sci 2018;25:1024–1036. [DOI] [PubMed] [Google Scholar]
- Yoshino O, Ono Y, Honda M, Hattori K, Sato E, Hiraoka T, Ito M, Kobayashi M, Arai K, Katayama H. et al. Relaxin-2 may suppress endometriosis by reducing fibrosis, scar formation, and inflammation. Biomedicines 2020;8:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuge A, Nasu K, Matsumoto H, Nishida M, Narahara H.. Collagen gel contractility is enhanced in human endometriotic stromal cells: a possible mechanism underlying the pathogenesis of endometriosis-associated fibrosis. Hum Reprod 2007;22:938–944. [DOI] [PubMed] [Google Scholar]
- Zeng X, Yue Z, Gao Y, Jiang G, Zeng F, Shao Y, Huang J, Yin M, Li Y.. NR4A1 is involved in fibrogenesis in ovarian endometriosis. Cell Physiol Biochem 2018;46:1078–1090. [DOI] [PubMed] [Google Scholar]
- Zhang F, Peng M, Zheng X, Wang X, Liu X, Chen C, Lu Y.. Blocking sphingosine 1-phosphate receptor 1 with modulators reduces immune cells infiltration and alleviates endometriosis in mice. Reprod Biomed Online 2023a;47:103304. [DOI] [PubMed] [Google Scholar]
- Zhang L, Mohankumar K, Martin G, Mariyam F, Park Y, Han SJ, Safe S.. Flavonoids quercetin and kaempferol are NR4A1 antagonists and suppress endometriosis in female mice. Endocrinology 2023b;164:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Dong P, Liu X, Sakuragi N, Guo SW.. Enhancer of zeste homolog 2 (EZH2) induces epithelial-mesenchymal transition in endometriosis. Sci Rep 2017a;7:6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Duan J, Liu X, Guo SW.. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol Cell Endocrinol 2016a;428:1–16. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Duan J, Olson M, Fazleabas A, Guo SW.. Cellular changes consistent with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the progression of experimental endometriosis in baboons. Reprod Sci 2016b;23:1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Liu X, Guo SW.. Progressive development of endometriosis and its hindrance by anti-platelet treatment in mice with induced endometriosis. Reprod Biomed Online 2017b;34:124–136. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chang X, Wu D, Deng M, Miao J, Jin Z.. Down-regulation of exosomal miR-214-3p targeting CCN2 contributes to endometriosis fibrosis and the role of exosomes in the horizontal transfer of miR-214-3p. Reprod Sci 2021;28:715–727. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu X, Deng M, Xu C, Zhang Y, Wu D, Tang F, Yang R, Miao J.. Ferroptosis induced by iron overload promotes fibrosis in ovarian endometriosis and is related to subpopulations of endometrial stromal cells. Front Pharmacol 2022;13:930614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Suo L, Chen Y, Zhu L, Wan G, Han X.. Endometriotic peritoneal fluid promotes myofibroblast differentiation of endometrial mesenchymal stem cells. Stem Cells Int 2019a;2019:6183796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wang J, Chen Y, Suo L, Chen H, Zhu L, Wan G, Han X.. Activin a promotes myofibroblast differentiation of endometrial mesenchymal stem cells via STAT3-dependent Smad/CTGF pathway. Cell Commun Signal 2019b;17:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XK, Yu L, Cheng ML, Che P, Lu YY, Zhang Q, Mu M, Li H, Zhu LL, Zhu JJ. et al. Focal adhesion kinase regulates hepatic stellate cell activation and liver fibrosis. Sci Rep 2017;7:4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Liu X, Guo SW.. Aberrant expression of histone deacetylase 8 in endometriosis and its potential as a therapeutic target. Reprod Med Biol 2023a;22:e12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Liu X, Guo SW.. Corroborating evidence for aberrant expression of histone deacetylase 8 in endometriosis. Reprod Med Biol 2023b;22:e12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Khan Z, Zanfagnin V, Correa LF, Delaney AA, Daftary GS.. Epigenetic modulation of collagen 1A1: therapeutic implications in fibrosis and endometriosis. Biol Reprod 2016;94:87. [DOI] [PubMed] [Google Scholar]
- Zhu S, Wang A, Xu W, Hu L, Sun J, Wang X.. The heterogeneity of fibrosis and angiogenesis in endometriosis revealed by single-cell RNA-sequencing. Biochim Biophys Acta Mol Basis Dis 2023;1869:166602. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Missmer SA.. Endometriosis. N Engl J Med 2020;382:1244–1256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.