Abstract
The identification of patients at high risk of Herpes zoster (HZ) requiring a preventive strategy with antiviral prophylaxis and anti-HZ vaccine is a clinically relevant issue in patients with immunological impairment. The absence of trials comparing vaccination to pharmacological prophylaxis or defining their sequential use makes the optimal preventive strategy uncertain. This article presents the results of group discussion among a panel of experts convened ad hoc to review the literature regarding antiviral prophylaxis and vaccine efficacy and safety in populations with malignant and non-malignant hematologic diseases, and in subjects submitted to hematopoietic stem cell transplantation. The expert panel used consensus methodology and proposed solutions for preventive strategies, producing advice for the management of the most relevant unmet clinical needs. This comprehensive overview aims to support the practice of pharmacological and vaccination-based HZ prevention and inform the design and conduct of new studies in the field.
Introduction
Herpes zoster (HZ) is a painful, infectious, cutaneous eruption, usually involving one to three adjacent dermatomes, resulting from reactivation of latent varicella-zoster virus (VZV). Although mortality is low, HZ causes high morbidity, occasionally with life-threatening manifestations (e.g., encephalitis, hepatitis) and a high societal cost mainly related to complications such as postherpetic neuralgia.1 Patients with various hematologic diseases and those undergoing hematopoietic stem cell transplantation (HSCT) are historically considered at high risk of HZ due to their immunological impairment.2
Strategies to prevent symptomatic reactivation of VZV include risk-adapted pharmacological antiviral prophylaxis (AVP) and vaccination. There are currently two different types of HZ vaccines that have been approved for use worldwide and in Europe: a one-dose, live-attenuated vaccine not indicated for subjects with impaired immunity and an adjuvanted recombinant zoster vaccine (aRZV) recommended for immunocompetent adults aged ≥50 years and for immunocompromised adults aged ≥18 years at increased risk of HZ.3-9 The aRZV vaccination schedule consists of two doses of 0.5 mL each: an initial dose followed by a second dose between 2 and 6 months after the first dose. For subjects who are or might become immunodeficient or immunosuppressed due to disease or therapy, and who would benefit from a shorter vaccination schedule, the second dose can be given 1 to 2 months after the initial dose.3
However, the absence of trials comparing vaccination to pharmacological prophylaxis or defining their sequential use makes the overall strategy for HZ prevention in high-risk patients uncertain. Indeed, recommendations issued in 2022 by the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO)10 and by the National Comprehensive Cancer Network (NCCN)11 advise using AVP in certain diseases and conditions and generically recommend vaccination with aRZV in hematologic and HSCT patients without going into details on strategies for concomitant/sequential use of vaccines and AVP (Online Supplementary Table S1).
In this work, a panel of experts constituted ad hoc in view of their expertise in the management of specific hematologic diseases or conditions and of their experience in consensus conference and guidelines projects tried to produce a position paper based on the current literature on vaccine efficacy and safety and AVP in immunocompromised populations with malignant and non-malignant hematologic diseases submitted to chemotherapy and/or immunosuppressive therapy and autologous or allogeneic HSCT. The purpose was to identify, in this setting of patients, critical unmet clinical needs in the prevention of HZ infections and to determine the optimal preventive strategy, by exploiting the consensus methodology approach.
Methods
Two chairmen (CG and GB) appointed an expert panel with nine members, selected as opinion leaders for the following hematologic diseases or conditions: myeloproliferative diseases (GB, MTV, AV), lymphoproliferative diseases (PC, PM, AC), non-oncological hematologic diseases (AMR), stem cell transplants (FC, AMR) and infections in hematology (CG). In addition, a member of the panel with expertise in clinical epidemiology (GB) assured the methodological congruence of the process and an infectious diseases and epidemiology specialist member of the Istituto Superiore di Sanità (the Italian National Institute of Health) (FD) dealt with the legislative and regulatory aspects of vaccination. A consensus-based project applies the methodology of group discussion (questionnaires and nominal group technique). This means that the statements, advice and recommendations generated are not primarily evidence-based (they do not derive from a systematic review and grading of the evidence) because of the limited number of interventional, clinical studies dealing with HZ prevention in hematologic populations.
During an initial meeting, in April 2022, the outline of the project was discussed and the topics forming the structure of the present document were decided. The expert panel agreed on the major relevant issues by generating and rank-ordering key clinical questions, using the criterion of clinical relevance, through a Delphi process.12 In a further phase of the process, the chairmen reviewed evidence about the selected key questions by PubMed searches of English-language literature (from January 2012 to June 2023). Afterwards, panelists drafted statements that addressed one identified relevant issue, while the remaining panelists scored their agreement with those statements and provided suggestions for modifications. The expert panel convened in a consensus meeting during which final proposals and recommendations were prepared. In a round-robin fashion, participants were asked to comment their disagreements with the proposed issues and then to vote for a final statement. For consensus we required 80% votes in favor.
Results
A narrative review of literature published since 2012 on the epidemiology of HZ in hematologic diseases and HSCT, as well as AVP for and vaccination against the infection in the same settings is detailed in Online Supplementary Tables S2 and S3.
The following paragraphs describe a synthesis of the most significant data on the epidemiology and strategies for HZ prevention in various categories of hematologic patients. Proposals and/or recommendations of the expert panel on the prevention of HZ in patients with various hematologic diseases and conditions are summarized in Table 1. All the final proposals and recommendations received more than 80% consensus.
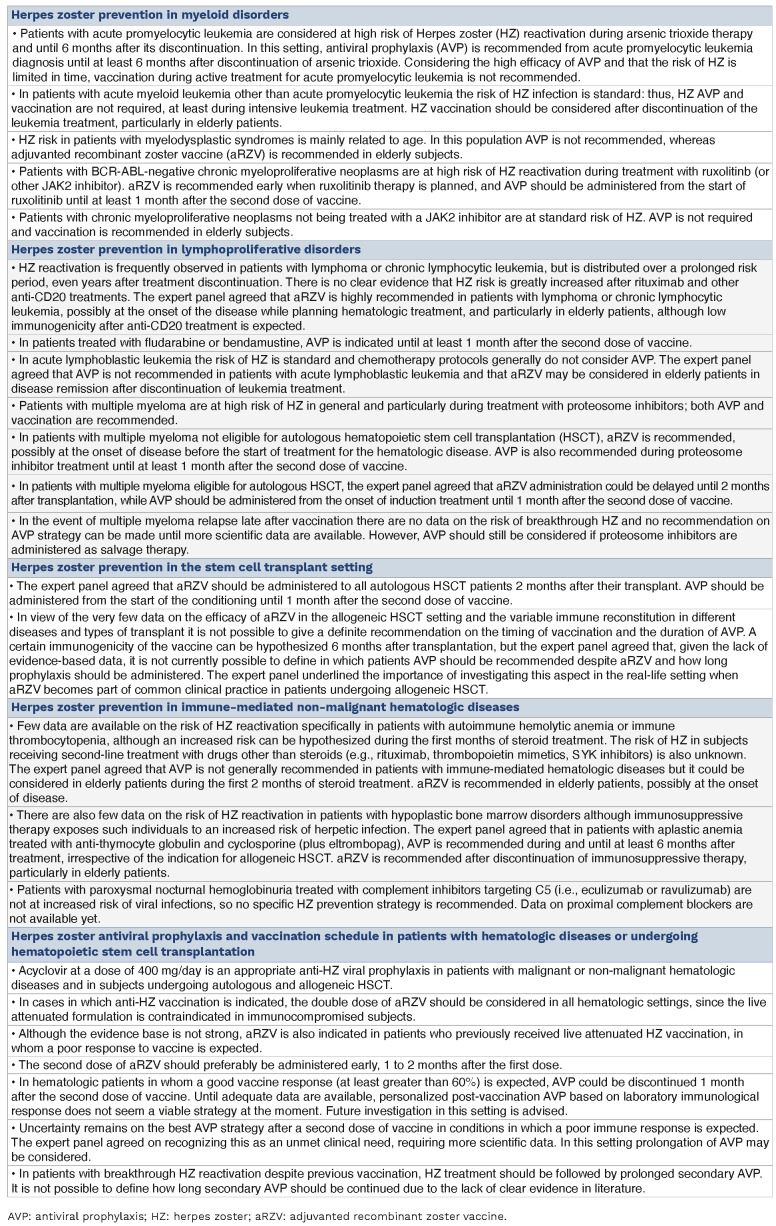
Herpes zoster risk and prevention in myeloid disorders
Data on the incidence of HZ infection specifically in myeloid disorders were derived from three retrospective observational studies on acute promyelocytic leukemia,13-15 two others on chronic myeloproliferative neoplasms and one on myelodysplastic syndromes.16-18
Patients with acute promyelocytic leukemia treated with arsenic trioxide are at high risk of HZ if not receiving AVP; indeed, the incidence ranges from 11.6% to 45.7% within the first 6 months of therapy.13-15 The risk is particularly high in older patients and in those with a previous history of HZ. AVP was shown to significantly reduce the risk of HZ, or delay its onset.14,15
In patients with myeloproliferative neoplasms treatment with ruxolitinib was associated with an increased risk of HZ; this risk ranges from 3.5 to 6.9 cases per 100 patient-years, which is ten times higher than the risk in patients not treated with ruxolitinib.16,17 No information was found on AVP efficacy. Compared to an age-matched immunocompetent population, patients with myelodysplastic syndromes have only a modest increased risk of HZ infection (odds ratio=1.31).18
Table 1.
Proposals and/or recommendations on the prevention of Herpes zoster in patients with hematologic diseases.
With regard to the protective effect of aRZV, Dagnew et al.19 reported 80.4% and 73.7% of humoral and cellular vaccine responses, respectively, in an adult immunocompromised population including patients with acute myeloid leukemia and myelodysplastic syndromes (56 patients in the vaccine group). No patient with a myeloproliferative neoplasm who received aRZV developed HZ infection.
Herpes zoster risk and prevention in lymphoproliferative disorders
Lymphoma and myeloma are both associated with significantly increased odds of HZ, with the magnitude of the increase varying widely.20-33 The association was most significant within 2 years after diagnosis.20
In patients with non-Hodgkin lymphoma (NHL) or Hodgkin lymphoma receiving chemotherapy without AVP the cumulative incidence of herpesvirus infections (93% of which were VZV disease) was 20.2% at 5 years after starting chemotherapy, with a high cumulative dose of corticosteroids and a history of neutropenic fever being independent risk factors for these infections in multivariate analysis.21 AVP was preferentially effective during the first year of treatment since reactivations were reported up to 51.3 months after initial immuno-chemotherapy, particularly in patients treated with rituximab plus bendamustine and in patients receiving rituximab or obinutuzumab maintenance therapy. Rituximab-containing regimens were not associated with a high overall incidence density of HZ, although the addition of rituximab to conventional chemotherapy increased the short-term risk of HZ, with adjusted odd ratios of 1.38 and 1.37 during the 1-year and 2-year follow-up periods, respectively.22
In patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib the incidence of HZ was 5% during a longterm follow-up while 2.9 cases per 1,000 patient-years were documented in those treated with various regimens in the pre-ibrutinib era.24,25
In patients with refractory or relapsed high-grade B-cell lymphoma treated with axicabtagene ciloleucel, a CD19-targeted chimeric antigen receptor (CAR) T-cell therapy, 16% had HZ skin infection.26 The HZ incidence rate was 14.8% for the entire cohort and 25.8% for the cohort of longterm survivors. The median time from CD19-targeted CAR T-cell infusion to the onset of HZ was 15.5 months (range, 8-20 months) and all the HZ infections happened within the first 2 years. Introducing AVP (200 mg oral acyclovir, 3 times a day) effectively reduced the incidence of HZ after CAR T-cell treatment.
An analysis of a UK primary care database of subjects with a variety of solid and hematologic cancers showed that multiple myeloma was associated with the greatest increase in probability of HZ (adjusted odds ratio=4.24) compared to that in the reference group of subjects without a diagnosed malignancy.20 In patients receiving bortezomib without AVP, infection occurred after cycle 1 in 32% of cases, after cycle 2 in 23%, after cycle 3 in 23%, after cycle 4 in 13%, and less than 10% of cases occurred in the following cycles. However, among patients with multiple myeloma who received various schedules of AVP (acyclovir 200 mg/day to 400 mg twice daily or valacyclovir 500 mg/ day) no infections or less than 1% were observed throughout the whole duration of bortezomib treatment and in the follow-up.27-31 Patients in whom AVP was interrupted before the end of cancer treatment had a higher risk of developing HZ infection compared to those who continued AVP (adjusted hazard ratio=3.09).32
The immunogenicity of aRZV vaccination in hematologic malignancies was evaluated in two randomized clinical trials.8,34 The first study8 demonstrated that aRZV elicited robust humoral and cellular responses when administered during and up to 6 months after chemotherapy. Overall, 80.4% of 148 participants with hematologic malignancies other than B-NHL or CLL had a humoral vaccine response at 2 months, compared with 0.8% participants in the placebo group. Conversely, the humoral vaccine response was 45% in B-NHL and 22% in CLL patients. Humoral and cell-mediated immune responses persisted above baseline until month 13 in all subgroups stratified by underlying disease. The frequency of a humoral response to the vaccine was higher among those who were vaccinated after the end of immunosuppressive therapy than among those who were vaccinated while still receiving immunosuppressive treatment. One month after injection of the second dose of the vaccine, the proportion of participants with a cell-mediated immune response to the vaccine was high (83.7%) in all diseases (including B-NHL and CLL) and it was durable since the response was still measurable after 12 months. A post-hoc analysis revealed that the incidence of HZ was 8.5 per 1,000 person-years in the vaccinated group and 66.2 per 1,000 person-years in the placebo group, resulting in 87.2% efficacy against HZ, including patients with B-NHL and CLL.
Zent et al.34 analyzed response to vaccination in patients aged ≥50 years and on treatment with a Bruton tyrosine kinase (BTK) inhibitor because of a diagnosis of CLL or lymphoplasmacytic lymphoma/Waldenström macroglobulinemia. A certain proportion of these patients did have a response to aRZV while on BTK inhibitor therapy. Twenty-four of 32 patients (75%) had a humoral response to vaccination and 21 of these 24 (87.5%) also mounted a T-cell response. Only four (50%) of the eight subjects with no humoral immune response exhibited a T-cell response. A subsequent analysis of the same study evaluated humoral response 24 months (± 3 months) after vaccination.35 Among the patients who had a response at 4 weeks after vaccination, 56.5% had a sustained humoral response 24 months later. The overall humoral response rate for all patients at 24 months compared to pre-vaccination was 41.9%. There was no significant association between prior rituximab therapy and achieving a humoral response. Cellular response was achieved in 81.3% of patients 4 weeks after vaccination and continued to be sustained in 65.4% of patients 24 months later. The overall cellular response rate in all patients at 24 months was 54.8%.
Real-life data on the efficacy and immunogenicity of aRZV in malignant hematologic diseases are summarized in Online Supplementary Table S3. The studies did not provide information on the timing of the vaccination during the course of the disease or on any AVP.
Mutchar et al.36 analyzed the effects of two doses of aRZV given at a 2-month interval to individuals with untreated or BTK inhibitor-treated CLL. At 3 months after vaccination an antibody response was seen in 45% of participants, which was significantly lower than the rate among sex- and age-matched healthy controls (63%; P=0.03). The antibody response did not differ significantly between the CLL patients who were untreated or treated with a BTK inhibitor (51% vs. 36%, respectively; P=0.23). The CD4+ T-cell response to vaccination was significantly lower in study participants than in controls (54% vs. 96%; P<0.001), mainly due to the weaker elicitation among BTK inhibitor-treated patients compared to untreated CLL patients (32% vs. 73%; P=0.008). In two open-label, single-arm clinical trials, Pleyer et al.37,38 investigated the effect of BTK inhibitors on recall immune response to aRZV in CLL patients by comparing the rates in patients who were or were not being treated with BTK inhibition. The response rate to aRZV did not differ significantly between the BTK inhibitor cohort (41.5%) and the treatment-naïve cohort (59.1%). The vaccine antibody response rate in CLL patients was significantly higher in the treatment-naïve cohort (76.8%) than among patients receiving a BTK inhibitor (40%). The cellular response rate was also significantly higher in the treatment-naïve cohort (70%) than in the BTK inhibitor group (41.3%).
In multiple myeloma patients, Sweiss et al.39 documented that the overall rate of seropositivity increased after one (87.9%; P=0.0002) and two (92.6%; P=0.0001) doses of the vaccine. Seroconversion from a baseline negative to positive test was observed in 76.2% and 95.8% patients after one and two doses, respectively.
Herpes zoster risk and prevention in hematopoietic stem cell transplantation
The incidence of HZ ranges from 8% to 25% in autologous HSCT,40,41 and from 13% to 28% in allogeneic HSCT recipients.42,43 AVP is commonly administered to patients after HSCT in order to prevent HZ-associated infections. According to literature data the incidence of HZ reactivation appears similar after the two transplant procedures, however it should be underlined that the above data refer to heterogeneous populations with different transplant risks and the epidemiological data are affected by the use of AVP. In general, the risk is particularly high after allogeneic HSCT and prolonged over time, even for years after the transplant. In 2017 the available literature was systematically reviewed to determine the optimal duration of AVP for prevention of HZ in allogeneic and autologous HSCT recipients.44 Six observational studies were analyzed comprising a total of 3,420 patients. Considering all HSCT recipients, the overall incidence of HZ was 7.8% in the AVP group and 25.6% in the control group, with a pooled relative risk of 0.31. Focusing on the AVP group, the incidence of HZ was 2.1% in the subgroup given prophylaxis for at least 1 year and 15.4% in the subgroup that received prophylaxis for less than 1 year, with a pooled relative risk of 0.23. These data demonstrate that AVP can significantly reduce HZ in HSCT recipients and that prophylaxis should be given for at least 1 year.
More recent literature on anti-HZ prophylaxis has analyzed the effect of transplant type, and the optimal antiviral therapy and dose.45-56 In a study by Kawamura et al.53 in patients who underwent autologous HSCT the first consecutive 30 patients received oral acyclovir at a dose of 1,000 mg/day until engraftment, whereas the following 69 patients were given oral acyclovir at a dose of 200 mg/day. After engraftment, acyclovir was continued at the 200 mg/ day dose at the discretion of the attending physicians in both groups. Patients were next divided into three groups according to the timing at which acyclovir prophylaxis was stopped after the transplant (at engraftment, between engraftment and 1 year after the transplant, and more than 1 year after the transplant). The cumulative incidence of HZ at 1 year was 25.8, 7.7, and 0.0% in the three groups, respectively. No difference was observed in the incidence of HZ according to the AVP dose, suggesting that low-dose acyclovir prophylaxis may be effective at preventing HZ after autologous HSCT.
Zhang et al.49 documented that the duration of AVP and HZ incidence were inversely correlated. Compared with patients who were on AVP for 1-89 days, those whose AVP lasted longer had significantly lower risks of HZ: AVP duration 180-269 days, hazard ratio=0.576 (P=0.019), AVP duration 270-359 days, hazard ratio=0.594 (P=0.023), and AVP for ≥360 days, hazard ratio=0.309 (P<0.001). Abbasov et al.51 reported that no patient given low-dose acyclovir prophylaxis (400 mg/day) developed HZ in the first year after autologous HSCT while 2.8% of patients developed HZ in the second year after discontinuing the AVP.
The impact of longer-term AVP on HZ incidence after cord blood transplantation was studied by Xue et al.52 HZ occurred in 44 recipients (19%) at a median of 23.6 months. The cumulative incidence of HZ by 1 year after cord blood transplantation was 1.8% but increased to 26% by 5 years. A high incidence of HZ after cord blood transplantation, despite AVP for more than 1 year, was found. Based on these findings, the authors suggested a longer duration of prophylaxis for HZ after cord blood transplantation.
A randomized, observer-blind, phase III trial study investigated the role of anti-HZ vaccination in a population of 1,846 adult autologous HSCT recipients7 who were randomized to receive a first dose of either aRZV or placebo 50-70 days after their transplant, followed by a second dose 1-2 months later. Compared to placebo, aRZV was associated with 68.2% efficacy in preventing HZ infection and 89.3% in minimizing the incidence of post-herpetic neuralgia. Analyzing the data from this study, Stadtmauer et al.57 provided an in-depth description of humoral and cell-mediated immune responses by age or underlying disease as well as efficacy of the aRZV by underlying disease. Despite the lower anti-gE antibody concentrations in B-NHL patients, CD4 T-cell frequencies were similar in patients with B-NHL and those with other underlying diseases. Vaccine efficacy against HZ ranged between 42.5% and 82.5% across underlying diseases and was statistically significant in patients with B-NHL and in those with multiple myeloma.
There are two real-life studies on the efficacy of aRZV in the context of allogeneic HSCT.58,59 In a single-center, prospective, observational cohort study, safety and reactogenicity of aRZV, as well as incidence of graft-versus-host disease and confirmed cases of HZ after vaccination were assessed in 158 volunteer allogeneic HSCT recipients.58 The cumulative incidence of graft-versus-host disease in the peri-vaccination period was no different from that in historical controls. There were four cases of HZ in the total vaccinated cohort who received at least one vaccine dose (2.5%) and three cases in the modified total vaccinated cohort who received two vaccine doses (28.3/1,000 person-years). In a study including 79 allogeneic HSCT recipients who were given aRZV, cellular immunity against various VZV antigens was analyzed by interferon-γ ELISpot and patients with or without previous shingles were compared.59 Multivariate analysis showed that previous shingles and sex both had significant effects on VZV immunity, with responses against glycoprotein E being significantly higher in males than in females.
In conclusion, two aRZV doses, administered 50-70 days after transplantation, induced robust and durable humoral and cell-mediated immune responses, irrespective of age and underlying disease, in adults who had undergone auto-logous HSCT. Glycoprotein E-specific CD4 T-cell responses were polyfunctional, and the proportion of polyfunctional CD4 T cells expanded in the 2 years following vaccination. In a post-hoc analysis, it was demonstrated, in each underlying disease, that the efficacy against HZ reflected that observed in the overall population; indeed, there was robust efficacy also in patients with B-NHL, despite their weaker humoral immune response. Data on the efficacy of aRZV in the allogeneic HSCT population are less evident and future clinical trials are needed to elucidate the rate and timing of aRZV immunogenicity in this population.
Herpes zoster risk and prevention in immune-mediated non-malignant hematologic diseases
Few data are available on the risk of HZ reactivation in patients with immune-mediated non-malignant hematologic diseases, such as autoimmune hemolytic anemia and immune thrombocytopenia. Recent studies have shown that the prolonged use of corticosteroids in the geriatric population (>65 years old) can increase the risk of development of HZ (3-fold increase of odds) because of the immunosuppressive effects of these drugs.60 There is a duration-related effect between oral corticosteroid administration and the risk of HZ.
Regarding aplastic anemia, the risk of HZ in subjects not treated with HSCT has been reported generically without specific evidence-based information being provided. Clinical experience indicates that patients with aplastic anemia may develop HZ reactivation while on therapy with anti-thymocyte globulin and cyclosporine, when a global suppression of T-cell function and numbers can occur. Indeed, according to guidelines on the management of aplastic anemia, AVP is not routinely recommended in untreated patients while it is recommended in this population during and after anti-thymocyte globulin therapy.61
Herpes zoster antiviral prophylaxis and vaccination schedules in patients with hematologic diseases or submitted to hematopoietic stem cell transplantation
Secondary objectives of observational studies on AVP have included comparisons of different doses of acyclovir and comparisons of different drugs. Lin et al. reported that the delivery of different dosages and types of anti-HZ drugs resulted in equivalent protective effects.32 Zheng et al. documented that the rate of HZ infection was similar between AVP with intermittent oral famciclovir at a dose of 250 mg twice daily for 9 days, and continuous oral acyclovir (8.4% vs. 7.9%; P=0.835).33 Kawamura et al. found that the number of patients who developed HZ before day 100 after HSCT was not different between a group treated with acyclovir 1,000 mg/day and another treated with acyclovir 200 mg/day.53 Mascarenhas et al. documented that the use of low-dose acyclovir prophylaxis was associated with a low rate of VZV reactivation in the first year after HSCT, with no evidence of clinically significant rebound at 2 years after transplant.55
Two doses of aRZV are necessary regardless of a prior history of shingles or previous receipt of live zoster vaccine. The second dose of aRZV should typically be given 2-6 months after the first. However, for people who are or will be immunodeficient or immunosuppressed and who would benefit from completing the course in a more condensed period of time, the second dose can be administered 1-2 months after the first. If a supplementary dose of aRZV is given sooner than 4 weeks after the first, a second valid dose should be repeated at least 4 weeks after the dose that was given early. The vaccine course should not be resumed if more than 6 months have elapsed since the first dose.
When possible, patients should be vaccinated before becoming immunosuppressed. If vaccination before immunosuppression is not possible, physicians should consider timing vaccination when the immune response is likely to be most robust.
Vaccination deferral in immunodeficient patients should be carefully weighed against the potential decline of response to aRZV, occurring during the most aggressive phases of immunosuppression, which could result in missing a “window of opportunity”.
In theory, laboratory tests to measure vaccine immunoprotection elicited in both B- and T-cell compartments might be exploited to guide post-vaccination AVP; however, at present the few literature data do not enable definition of a post-vaccination AVP strategy based on immunological monitoring.
Conclusions
The availability of a new recombinant vaccine for HZ prevention has provided great impetus to the use of vaccination for immunocompromised patients at high risk of HZ reactivation. However, the lack of randomized trials considerably limits the quality of the evidence that should inform advice and recommendations. The consensus-based statements in this project are provided with the assumption that the experts have an implicit and comprehensive mastery of scientific and practical information to help the most appropriate decisions. Applying these recommendations could not only improve outcomes but also enable data collection to inform future practice. Randomized clinical trials and large, prospective epidemiological surveys are needed to better define some aspects of HZ prevention in hematologic populations with the aim of optimizing the harmonization of the antiviral preventive strategy in terms of duration of AVP after vaccination in specific diseases or conditions and eventual use of AVP late after vaccination, particularly for subjects with chronic hematologic diseases undergoing prolonged treatment. Finally, literature data and the experts’ personal experience highlight the continuous variation in the risk of HZ reactivation related both to the underlying disease or condition and to the different treatments. It is therefore necessary to re-evaluate prevention strategies over time, in step with new therapeutic strategies for individual hematologic diseases.
Supplementary Material
References
- 1.Pan CX, Lee MS, Nambudiri VE. Global herpes zoster incidence, burden of disease, and vaccine availability: a narrative review. Ther Adv Vaccines Immunother. 2022;10:25151355221084535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKay SL, Guo A, Pergam SA, Dooling K. Herpes zoster risk in immunocompromised adults in the United States: a systematic review. Clin Infect Dis. 2020;71(7):e125-e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Medicine Agency. Shingrix: EPAR – Product information. https://www.ema.europa.eu/documents/product-information/shingrix-epar-product-information_en.pdf. Accessed September 2023 [Google Scholar]
- 4.European Medicine Agency. Zostavax: EPAR – Product information. https://www.ema.europa.eu/documents/product-information/zostavax-epar-product-information_en.pdf. Accessed September 2023 [Google Scholar]
- 5.Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. Shingrix [package insert], revised: 07/2021. https://www.fda.gov/media/108597/download. Accessed September 2023 [Google Scholar]
- 7.Bastidas A, de la Serna J, El Idrissi M, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322(2):123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988-1000. [DOI] [PubMed] [Google Scholar]
- 9.Vink P, Mingorance ID, Alonso CM, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer. 2019;125(8):1301-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandherr M, Hentrich M, von Lilienfeld-Toal M, et al. Antiviral prophylaxis in patients with solid tumours and haematological malignancies--update of the Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO). Ann Hematol. 2015;94(9):1441-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2016;14(7):882-913. [DOI] [PubMed] [Google Scholar]
- 12.Delbecq AL, Van de Ven AH, Gustafson DH. Group Techniques for Program Planning. A Guide to Nominal Group and Delphi Processes. Scott, Foresman and Co, Glenview, IL, 1975. [Google Scholar]
- 13.Yamakura M, Tsuda K, Ugai T, et al. High frequency of varicella zoster virus reactivation associated with the use of arsenic trioxide in patients with acute promyelocytic leukemia. Acta Haematol. 2014;131(2):76-77. [DOI] [PubMed] [Google Scholar]
- 14.Freyer CW, Peterson CE, Man Y, Przespolewski A, Baron J, Luger SM. Herpes zoster during arsenic trioxide therapy for acute promyelocytic leukemia. Leuk Lymphoma. 2021;62(3):696-702. [DOI] [PubMed] [Google Scholar]
- 15.Glass JL, Derkach A, Hilden P, et al. Arsenic trioxide therapy predisposes to herpes zoster reactivation despite minimally myelosuppressive therapy. Leuk Res. 2021;106:106569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barraco F, Greil R, Herbrecht R, et al. Real-world non-interventional long-term post-authorisation safety study of ruxolitinib in myelofibrosis. Br J Haematol. 2020;191(5):764-774. [DOI] [PubMed] [Google Scholar]
- 17.Te Linde E, Boots LJE, Daenen LGM, de Witte MA, Bruns AHW. High incidence of herpes zoster in patients using ruxolitinib for myeloproliferative neoplasms: need for prophylaxis. Hemasphere. 2022;6(11):e793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Titmarsh GJ, McMullin MF, McShane CM, Clarke M, Engels EA, Anderson LA. Community-acquired infections and their association with myeloid malignancies. Cancer Epidemiol. 2014;38(1):56-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagnew AF, Vink P, Drame M, Willer DO, Salaun B, Schuind AE. Immune responses to the adjuvanted recombinant zoster vaccine in immunocompromised adults: a comprehensive overview. Hum Vaccin Immunother. 2021;17(11):4132-4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansson E, Forbes HJ, Langan SM, Smeeth L, Bhaskaran K. Herpes zoster risk after 21 specific cancers: population-based case-control study. Br J Cancer. 2017;116(12):1643-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HS, Park JY, Shin SH, et al. Herpesviridae viral infections after chemotherapy without antiviral prophylaxis in patients with malignant lymphoma: incidence and risk factors. Am J Clin Oncol. 2012;35(2):146-150. [DOI] [PubMed] [Google Scholar]
- 22.Cho SF, Wu WH, Yang YH, Liu YC, Hsiao HH, Chang CS. Longitudinal risk of herpes zoster in patients with non-Hodgkin lymphoma receiving chemotherapy: a nationwide population-based study. Sci Rep. 2015;5:14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goenaga Vázquez Y, Cabanillas F, Concepción JR, Díaz Miranda OL. Incidence and risk factors for developing herpes zoster among a cohort of patients diagnosed with lymphoma at a community cancer center. Clin Lymphoma Myeloma Leuk. 2019;19(3):e153-e158. [DOI] [PubMed] [Google Scholar]
- 24.Steingrímsson V, Gíslason GK, Þorsteinsdóttir S, et al. A nationwide study on inpatient opportunistic infections in patients with chronic lymphocytic leukemia in the pre-ibrutinib era. Eur J Haematol. 2021;106(3):346-353. [DOI] [PubMed] [Google Scholar]
- 25.Coutre SE, Byrd JC, Hillmen P, et al. Long-term safety of singleagent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv. 2019;3(12):1799-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Li P, Zhao A, Lei W, Liang A, Qian W. Incidence and prophylaxis of herpes zoster in relapsed or refractory B-cell lymphoma patients after CD19-specific CAR-T cell therapy. Leuk Lymphoma. 2022;63(4):1001-1004. [DOI] [PubMed] [Google Scholar]
- 27.König C, Kleber M, Reinhardt H, Knop S, Wäsch R, Engelhardt M. Incidence, risk factors, and implemented prophylaxis of varicella zoster virus infection, including complicated varicella zoster virus and herpes simplex virus infections, in lenalidomide-treated multiple myeloma patients. Ann Hematol. 2014;93(3):479-484. [DOI] [PubMed] [Google Scholar]
- 28.Minarik J, Pika T, Bacovsky J, Langova K, Scudla V. Low-dose acyclovir prophylaxis for bortezomib-induced herpes zoster in multiple myeloma patients. Br J Haematol. 2012;159(1):111-113. [DOI] [PubMed] [Google Scholar]
- 29.Fukushima T, Sato T, Nakamura T, et al. Daily 500 mg valacyclovir is effective for prevention of varicella zoster virus reactivation in patients with multiple myeloma treated with bortezomib. Anticancer Res. 2012;32(12):5437-5440. [PubMed] [Google Scholar]
- 30.Swaika A, Paulus A, Miller KC, et al. Acyclovir prophylaxis against varicella zoster virus reactivation in multiple myeloma patients treated with bortezomib-based therapies: a retrospective analysis of 100 patients. J Support Oncol. 2012;10(4):155-159. [DOI] [PubMed] [Google Scholar]
- 31.Leng S, Lentzsch S, Shen Y, Tsai WY, Wright JD, Hershman DL, Neugut AI. Use and impact of herpes zoster prophylaxis in myeloma patients treated with proteasome inhibitors. Leuk Lymphoma. 2018;59(10):2465-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin WY, Tsai CK, Yeh CM, et al. Herpes zoster prophylaxis: essential for treating newly diagnosed multiple myeloma patients. Cancer Med. 2023;12(3):3013-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng G, Guan F, Han X, et al. Efficacy of intermittent, oral famciclovir prophylaxis for bortezomib-induced herpes zoster in multiple myeloma patients. Front Oncol. 2022;12:843032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zent CS, Brady MT, Delage C, et al. Short term results of vaccination with adjuvanted recombinant varicella zoster glycoprotein E during initial BTK inhibitor therapy for CLL or lymphoplasmacytic lymphoma. Leukemia. 2021;35(6):1788-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brady MT, Laniewski N, Strawderman M, et al. Long-term results of vaccination with adjuvanted recombinant varicella zoster glycoprotein E during initial Bruton tyrosine kinase inhibitors therapy for chronic lymphocytic leukemia or lymphoplasmacytic lymphoma. Am J Hematol. 2023;98(10):e288-e290. [DOI] [PubMed] [Google Scholar]
- 36.Muchtar E, Koehler AB, Johnson MJ, et al. Humoral and cellular immune responses to recombinant herpes zoster vaccine in patients with chronic lymphocytic leukemia and monoclonal B cell lymphocytosis. Blood. 2021;137(2):185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137(2):185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6(6):1732-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sweiss K, Calip GS, Galivin JP, Rondelli D, Patel P. High rates of varicella zoster virus antibody seroconversion after administration of the adjuvanted, recombinant varicella zoster vaccine in multiple myeloma patients undergoing active treatment. Blood. 2019;134(Suppl 1):3081. [Google Scholar]
- 40.Schuchter LM, Wingard JR, Piantadosi S, Burns WH, Santos GW, Saral R. Herpes zoster infection after autologous bone marrow transplantation. Blood. 1989;74(4):1424-1427. [PubMed] [Google Scholar]
- 41.Barton T, Collis T, Stadtmauer E, Schuster M. Infectious complications the year after autologous bone marrow transplantation or peripheral stem cell transplantation for treatment of breast cancer. Clin Infect Dis. 2001;32(3):391-395. [DOI] [PubMed] [Google Scholar]
- 42.Koc Y, Miller KB, Schenkein DP, et al. Varicella zoster virus infections following allogeneic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Marrow Transplant. 2000;6(1):44-49. [DOI] [PubMed] [Google Scholar]
- 43.Steer CB, Szer J, Sasadeusz J, Matthews JP, Beresford JA, Grigg A. Varicella-zoster infection after allogeneic bone marrow transplantation: incidence, risk factors and prevention with low-dose aciclovir and ganciclovir. Bone Marrow Transplant. 2000;25(6):657-664 [DOI] [PubMed] [Google Scholar]
- 44.Seo HM, Kim YS, Bang CH, et al. Antiviral prophylaxis for preventing herpes zoster in hematopoietic stem cell transplant recipients: a systematic review and metaanalysis. Antiviral Res. 2017;140:106-115. [DOI] [PubMed] [Google Scholar]
- 45.Kamber C, Zimmerli S, Suter-Riniker F, et al. Varicella zoster virus reactivation after autologous SCT is a frequent event and associated with favorable outcome in myeloma patients. Bone Marrow Transplant. 2015;50(4):573-578. [DOI] [PubMed] [Google Scholar]
- 46.Mawatari M, Isoda A, Miyazawa Y, Sawamura M, Matsumoto M. A Japanese single-hospital observational trial with a retrospective case-control analysis of varicella zoster virus reactivation after autologous peripheral blood stem cell transplantation. Transpl Infect Dis. 2015;17(4):544-550. [DOI] [PubMed] [Google Scholar]
- 47.Kawamura K, Hayakawa J, Akahoshi Y, et al. Low-dose acyclovir prophylaxis for the prevention of herpes simplex virus and varicella zoster virus diseases after autologous hematopoietic stem cell transplantation. Int J Hematol. 2015;102(2):230-237. [DOI] [PubMed] [Google Scholar]
- 48.Sahoo F, Hill JA, Xie H, et al. Herpes zoster in autologous hematopoietic cell transplant recipients in the era of acyclovir or valacyclovir prophylaxis and novel treatment and maintenance therapies. Biol Blood Marrow Transplant. 2017;23(3):505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang D, Weiss T, Feng Y, Finelli L. Duration of antiviral prophylaxis and risk of herpes zoster among patients receiving autologous hematopoietic stem cell transplants: a retrospective, observational study. Adv Ther. 2017;34(7):1610-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shinohara A, Osanai S, Izuka Y, et al. Herpes zoster after autologous haematopoietic stem cell transplantation without antiviral prophylaxis. Br J Haematol. 2019;186(6):e195-e197. [DOI] [PubMed] [Google Scholar]
- 51.Abbasov E, Metzner B, Müller TH, et al. Herpes zoster prophylaxis with low-dose acyclovir in patients with malignant lymphoma and multiple myeloma treated with autologous stem cell transplantation. Eur J Haematol. 2022;109(3):298-304. [DOI] [PubMed] [Google Scholar]
- 52.Xue E, Xie H, Leisenring WM, et al. High incidence of herpes zoster after cord blood hematopoietic cell transplant despite longer duration of antiviral prophylaxis. Clin Infect Dis. 2021;72(8):1350-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawamura K, Wada H, Yamasaki R, et al. Low-dose acyclovir prophylaxis for the prevention of herpes simplex virus disease after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2013;15(5):457-465. [DOI] [PubMed] [Google Scholar]
- 54.Blennow O, Fjaertoft G, Winiarski J, Ljungman P, Mattsson J, Remberger M. Varicella-zoster reactivation after allogeneic stem cell transplantation without routine prophylaxis--the incidence remains high. Biol Blood Marrow Transplant. 2014;20(10):1646-1649. [DOI] [PubMed] [Google Scholar]
- 55.Mascarenhas K, Teh JB, Peng K, et al. Efficacy of low-dose zoster prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2020;55(8):1662-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baumrin E, Cheng MP, Kanjilal S, Ho VT, Issa NC, Baden LR. Severe herpes zoster requiring intravenous antiviral treatment in allogeneic hematopoietic cell transplantation recipients on standard acyclovir prophylaxis. Biol Blood Marrow Transplant. 2019;25(8):1642-1647. [DOI] [PubMed] [Google Scholar]
- 57.Stadtmauer EA, Sullivan KM, El Idrissi M, et al. Adjuvanted recombinant zoster vaccine in adult autologous stem cell transplant recipients: polyfunctional immune responses and lessons for clinical practice. Hum Vaccin Immunother. 2021;17(11):4144-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baumrin E, Izaguirre NE, Bausk B, et al. Safety and reactogenicity of the recombinant zoster vaccine after allogeneic hematopoietic cell transplantation. Blood Adv. 2021;5(6):1585-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koldehoff M, Horn PA, Lindemann M. Cellular immune response after vaccination with an adjuvanted, recombinant zoster vaccine in allogeneic hematopoietic stem cell transplant recipients. Vaccines (Basel). 2022;10(5):809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai SW, Lin CL, Liao KF. Case-control study examining the association between herpes zoster and oral corticosteroids use in older adults. Eur Geriatr Med. 2018;9(5):707-712. [DOI] [PubMed] [Google Scholar]
- 61.Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172(2):187-207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.