Abstract
The recent progress with ruxolitinib treatment might improve quality of life as well as overall survival in patients with primary myelofibrosis. Therefore, the optimal timing of allogeneic hematopoietic cell transplantation (HCT) in the ruxolitinib era remains to be elucidated. We constructed a Markov model to simulate the 5-year clinical course of transplant candidates with primary myelofibrosis and compared outcomes between those who underwent immediate HCT and those whose HCT was delayed until after ruxolitinib failure. Since older age was associated with an increased risk of mortality, we analyzed patients aged <60 and ≥60 years separately in subgroup analyses. Life expectancy was consistently longer in the groups undergoing delayed HCT after ruxolitinib failure regardless of the patients’ age. Regarding quality-adjusted life years, a baseline analysis showed that immediate HCT was inferior to delayed HCT after ruxolitinib failure (2.19 vs. 2.26). In patients aged <60 years, immediate HCT was equivalent to delayed HCT after ruxolitinib failure (2.31 vs. 2.31). On the other hand, in patients aged ≥60 years, immediate HCT was inferior to delayed HCT after ruxolitinib failure (1.98 vs. 2.21). A one-way sensitivity analysis showed that the utility of being alive without chronic graft-versus-host disease after immediate HCT was the most influential parameter for quality-adjusted life years, and that a value higher than 0.836 could reverse the superiority of delayed HCT after ruxolitinib failure. As a result, delayed HCT after ruxolitinib failure is expected to be superior to immediate HCT, especially in patients aged ≥60 years, and is also a promising strategy even in those aged <60 years.
Introduction
Primary myelofibrosis (PMF) is characterized by stem cell-derived clonal myeloproliferation based on constitutive JAKSTAT signaling,1 and patients with PMF often suffer from anemia, hepatosplenomegaly, and constitutional symptoms, which are associated with impaired quality of life (QoL).2 The only curative treatment for PMF is allogeneic hematopoietic cell transplantation (HCT), but transplant-related mortality after HCT remains a major problem.3,4 In addition, chronic graft-versus-host disease (GvHD) after HCT can impair QoL considerably.5 Ruxolitinib is a selective inhibitor of JAK1/2 and has the potential to reduce disease-related symptoms and improve QoL in PMF.6 Moreover, the decrease in the size of the spleen with ruxolitinib was reported to correlate with longer overall survival.7 Thus, progress in treatment with ruxolitinib might affect the decision regarding the timing of HCT for transplant candidates.8 Immediate HCT at the time of diagnosis is currently recommended for patients with International Prognostic Scoring System (IPSS) intermediate-2 and high-risk disease in the recommendations from European LeukemiaNet.9-11 However, pretransplant ruxolitinib treatment may be able to delay HCT until disease progression, while unfavorable outcomes after HCT have also been reported after disease progression with ruxolitinib.12 Therefore, the optimal timing of allogeneic HCT for PMF in the ruxolitinib era remains to be elucidated.
Ideally, a randomized prospective trial is recommended to compare the outcomes of these clinical strategies. However, this seems impractical. A decision analysis is an alternative statistical technique that makes it possible to evaluate clinical decisions under uncertain conditions. QoL can also be considered in the analytic method as quality-adjusted life years (QALY). Cipkar et al. previously performed a decision analysis to determine the optimal timing of HCT for PMF, in which the effect of ruxolitinib on survival was not considered.13 Using a Markov model, we performed a decision analysis for transplant candidates with PMF to determine the optimal strategy between immediate HCT after diagnosis and delayed HCT after ruxolitinib failure.14
Methods
Patients
Based on the recommendations from European LeukemiaNet, we considered adult patients with PMF who were categorized into the IPSS intermediate-2-risk and high-risk groups and had an HLA-matched donor as candidates for this analysis, since upfront HCT was recommended as an initial therapy in these patients.10,11,15 We assumed that all patients in our model had splenomegaly at the time of diagnosis. A large cohort and long-term observation are required for the Markov model to define appropriate transition probabilities and utilities that change with time. Thus, we referred to the report by Hernández-Boluda et al.,16 describing a retrospective study including 2,916 patients, to estimate the clinical course of immediate HCT. Regarding the clinical course of patients undergoing delayed HCT after the failure of ruxolitinib treatment, we mainly referred to the report by Harrison et al.,17 which was a long-term comparison in the Controlled Myelofibrosis Study with Oral JAK Inhibitor Treatment (COMFORT)-II trial including 219 patients.
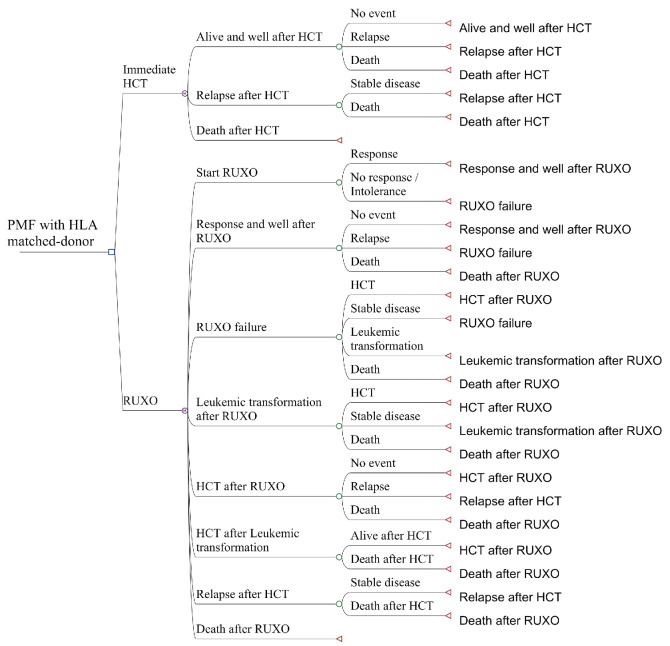
Figure 1.
The decision tree. The leftmost line shows the targeted patients: subjects with primary myelofibrosis (PMF) with an HLA-matched donor. The square is a decision node. The second line shows the two strategies that were compared: “Immediate hematopoietic cell transplantation” (HCT) and “Ruxolitinib treatment” (RUXO). At the decision node, we could decide to proceed to either of these. A Markov node (circled M) leads to a chance node, which is followed by two to four possible states.
Model structure
We constructed a decision tree and compared QALY of two strategies: “immediate HCT” and “delayed HCT after ruxolitinib failure”. Based on previously published reports, a response to ruxolitinib was defined as at least a 35% reduction in spleen volume.18,19 Ruxolitinib failure was defined as the lack of a reduction in the spleen volume of >35% from baseline or an increase in spleen volume after a >35% reduction had been achieved. In the group that underwent “delayed HCT after ruxolitinib failure”, patients received ruxolitinib as an initial therapy, and proceeded to HCT after failure of the drug.
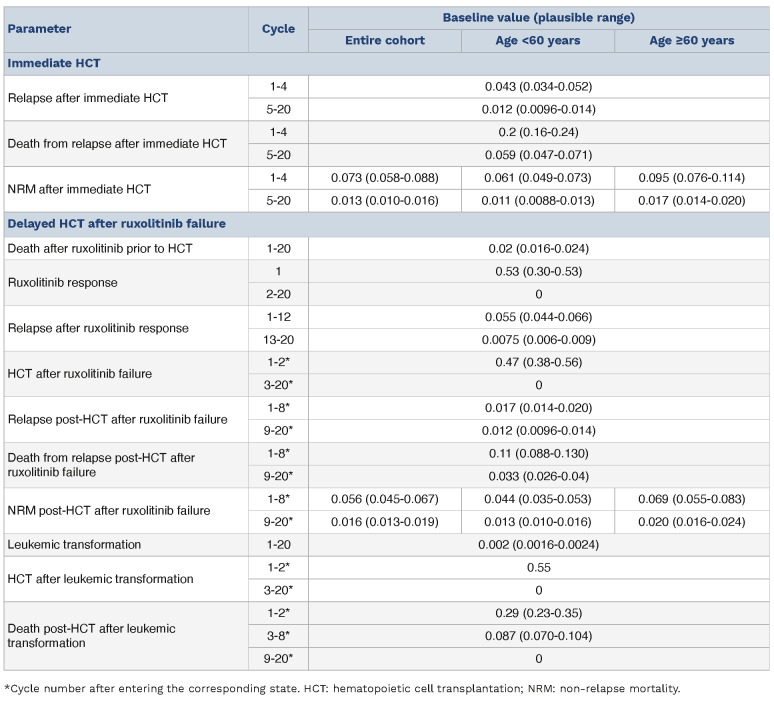
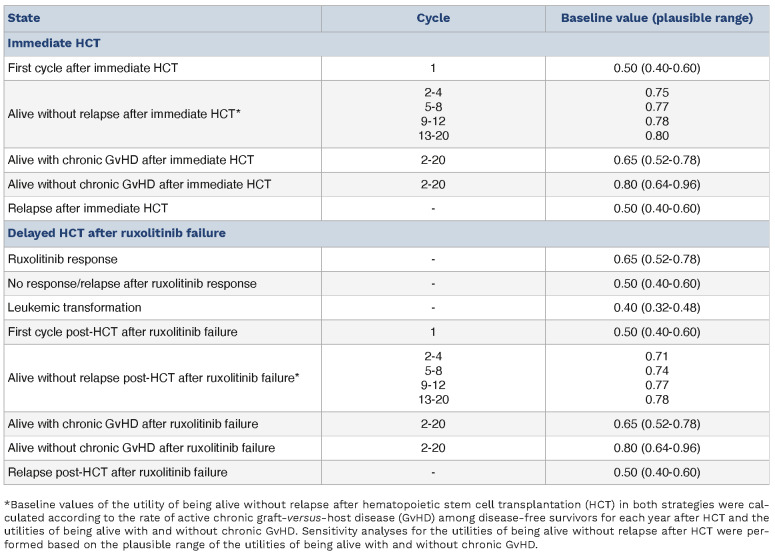
In the decision tree (Figure 1), the square on the left is a decision node, and a choice can be made between “Immediate HCT” or “RUXO”. Each decision is followed by a Markov node, which branches into several Markov health states including an only-absorbing state of “Death”. Each branch proceeds to a chance node, and each node has two to four possible states with specific transition probabilities (Table 1). In the subgroup analyses, we analyzed patients aged <60 and ≥60 years old separately because age ≥60 years was reported to be associated with an increased risk of non-relapse mortality.16 The cycle length was 3 months, and the analysis was performed for 20 cycles, or 5 years. QALY were calculated by multiplying QoL score for the health states (utilities) by the number of years for which a patient was expected to live in the health state. QALY for each decision were calculated based on the probability weighting of QALY obtained by the Markov processes with an annual discount rate of 3%, which is a standard method based on the concept that the value of future life-years is less than the current value (Table 2).20,21 For calculating utilities, we referred to the reports by Kurosawa et al., Shanavas et al., Harrison et al., and Mesa et al.6,12,18,22 Details of transition probabilities and utilities are provided in the Online Supplementary Methods.23
Table 1.
Summary of transition probabilities.
This study was approved by the Institutional Review Board of Jichi Medical University Saitama Medical Center.
Results
Baseline analysis
The 5-year overall survival probability calculated in this model was 47.1% for patients who underwent immediate HCT and 60.1% for those whose HCT was delayed after ruxolitinib failure after the clinical decisions, which seemed compatible with the original overall survival (Figure 2).16,17 QALY were 2.19 and 2.26 after the decisions for immediate HCT and delayed HCT after ruxolitinib failure, while the expected life-years without adjusting for QoL were 3.22 and 3.96 years, respectively.
Sensitivity analyses
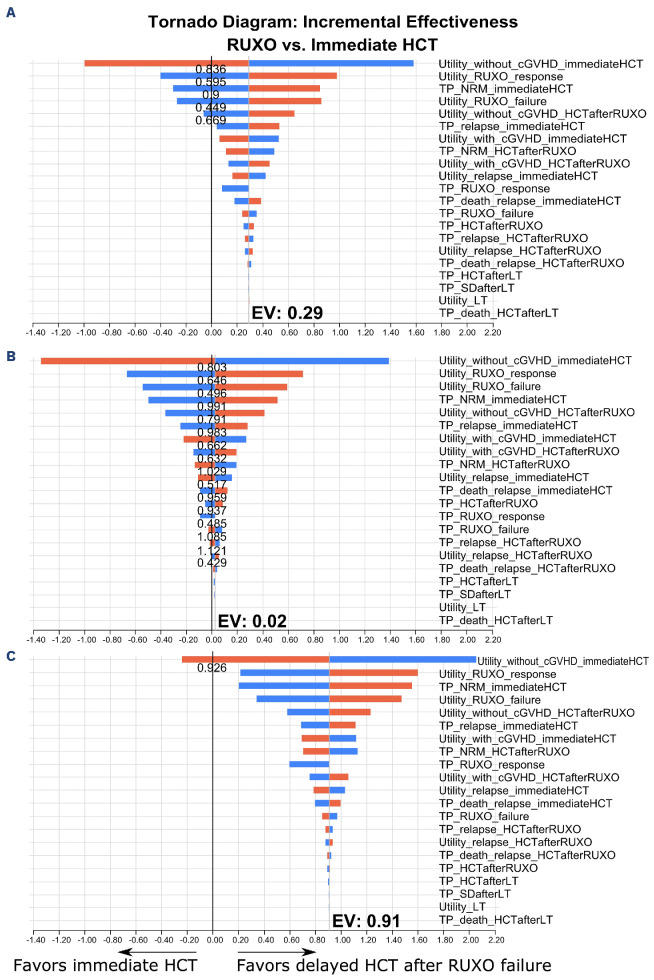
In one-way sensitivity analyses, the utility of being alive without chronic GvHD after immediate HCT was the most influential parameter for QALY, followed by the utility of a ruxolitinib response, a transition probability for non-relapse mortality after immediate HCT, the utility of ruxolitinib failure, and the utility of being alive without chronic GvHD following HCT after ruxolitinib failure (Figure 3A). The superiority of delayed HCT after ruxolitinib failure was reversed if the utility of being alive without chronic GvHD after immediate HCT was higher than 0.84 or the transition probability for non-relapse mortality after immediate HCT was 0.9 times lower than the original value. When the utilities of a ruxolitinib response and failure were lower than 0.595 or 0.449, and the utility of being alive without chronic GvHD post-HCT after ruxolitinib failure was lower than 0.669, the superiority of delayed HCT after ruxolitinib failure was also reversed. Next, we performed probabilistic sensitivity analyses using a Monte Carlo simulation. Out of 1,000 simulations, delayed HCT after ruxolitinib failure was superior to immediate HCT in 95.8% (Online Supplementary Figure S1A).
Table 2.
Summary of utilities.
Subgroup analyses according to age
In patients aged <60 years, immediate HCT was equivalent to delayed HCT after ruxolitinib failure, with QALY of 2.31 and 2.31, repectively. On the other hand, in patients aged ≥60 years, immediate HCT was inferior to delayed HCT after ruxolitinib failure, with QALY of 1.98 and 2.21, respectively. There were fewer expected life-years in the strategy of immediate HCT compared with delayed HCT after ruxolitinib failure in patients aged <60 years (3.39 vs. 4.04 years) and in those aged ≥60 years (2.93 vs. 3.88 years, respectively). Similar to the results in the entire cohort, one-way sensitivity analyses showed that the impact of the utility of being alive without chronic GvHD after immediate HCT was most influential in both age groups. In the group aged <60 years, the superiority of delayed HCT after ruxolitinib failure was reversed when the utility of being alive with and without chronic GvHD after immediate HCT was higher than 0.662 and 0.803, that of a ruxolitinib response and failure was lower than 0.646 or 0.496, that of being alive with and without chronic GvHD following HCT after ruxolitinib failure was lower than 0.632 and 0.791, and that of relapse after immediate HCT and post-HCT after ruxolitinib failure was higher than 0.517 and 0.429, respectively (Figure 3B). Moreover, immediate HCT was superior to delayed HCT after ruxolitinib failure when the transition probability for non-relapse mortality after immediate HCT and following HCT after ruxolitinib failure was 0.991 times lower and 1.029 times higher that of relapse after immediate HCT, and following HCT after ruxolitinib failure was 0.983 times lower and 1.121 times higher that of death due to relapse after immediate HCT was 0.959 times lower, that of HCT after ruxolitinib failure was 0.937 times lower than the original values, and that of a ruxolitinib response and failure was lower than 0.485 or 1.085 times higher. Instead, in the group aged ≥60 years, the superiority of delayed HCT after ruxolitinib was reversed only when the utility of being alive without chronic GvHD after immediate HCT was higher than 0.926 (Figure 3C).
Probabilistic sensitivity analyses showed that QALY were better after the decision for delayed HCT after ruxolitinib failure in 54.9% of the patients in the group aged <60 years and in 100.0% of those in the group aged ≥60 years (Online Supplementary Figure S1B, C).
Discussion
Using a Markov model, we performed the current decision analysis to compare the use of immediate HCT after diagnosis and delayed HCT after ruxolitinib failure for transplant candidates with PMF who had an HLA-matched donor. To the best of our knowledge, this is the first study to evaluate the optimal timing of HCT for PMF in the ruxolitinib era. We found that these strategies had similar QALY in the group of patients aged <60 years, while delayed HCT after ruxolitinib failure led to superior QALY in the group aged ≥60 years. There were more expected life-years in patients undergoing delayed HCT after ruxolitinib failure compared with patients undergoing immediate HCT in both age groups. Thus, immediate HCT might contribute to improved QoL at the cost of a risk of transplant-related mortality, while delayed HCT after ruxolitinib failure is expected to result in longer survival with relatively impaired QoL.
Figure 2.
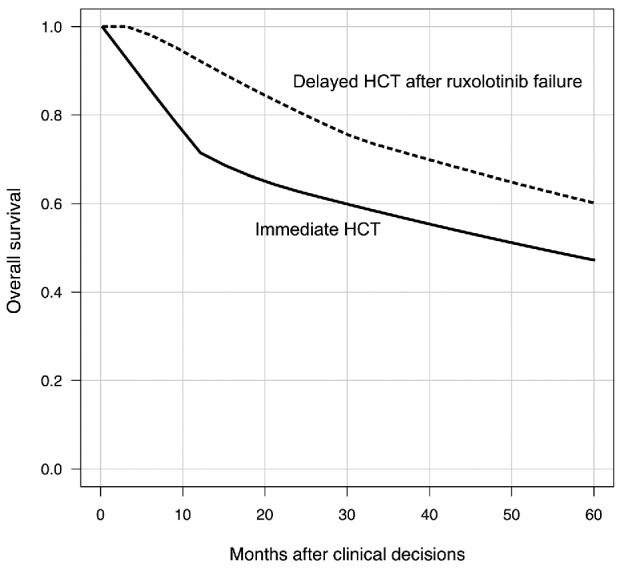
Estimated overall survival after the decisions. HCT: hematopoietic stem cell transplantation.
In one-way sensitivity analyses, the utility of being alive without chronic GvHD after immediate HCT strongly influenced the results. Chronic GvHD also strongly affects QoL after HCT,24 and the incidence of chronic GvHD decreases over time. Thus, QoL might tend to gradually recover after HCT, and we determined the utilities of being alive with and without chronic GvHD after HCT based on the data regarding current GvHD-free, relapse-free survival.25 Although these studies were not aimed only at PMF, QoL and the incidence of chronic GvHD did not seem to be affected by the type of disease.26 Nevertheless, in the current study, QALY after the decision of delayed HCT after ruxolitinib failure were superior to those after immediate HCT. The utility of a ruxolitinib response also strongly affected QALY. Ruxolitinib reduces spleen volume and myelofibrosis-associated symptoms such as fatigue, insomnia, and appetite loss.19 Therefore, patients who achieve marked reductions in these symptoms are likely to benefit from the strategy of delayed HCT after ruxolitinib failure.
Figure 3.
Tornado diagrams of one-way sensitivity analyses for transition probabilities and utilities. (A-C) Tornado diagrams of one-way sensitivity analyses for transition probabilities and utilities are shown for the entire cohort (A), patients aged <60 years (B) and patients aged ≥60 years (C). Plausible ranges are shown with respect to the original values. The x-axis shows the incremental quality-adjusted life years of “Delayed hematopoietic cell transplantation (HCT) after ruxolitinib failure” against “Immediate HCT”. The red and blue bars indicate increases and decreases in transition probabilities and utilities from the baseline values. The width of each bar reflects the impact of the parameter. RUXO: ruxolitinib; cGVHD: chronic graft-versus-host disease; TP: transition probabilities; NRM: non-relapse mortality; LT: leukemic transformation; SD: stable disease; EV: expected value.
As shown in the subgroup analyses, the utility of being alive without chronic GvHD after immediate HCT strongly influenced the results in both age groups. In the group aged ≥60 years, no other parameters could reverse the superiority of delayed HCT after ruxolitinib failure, and delayed HCT after ruxolitinib failure might be a promising strategy. On the other hand, in patients aged <60 years, immediate HCT seemed to give results equivalent to those with delayed HCT after ruxolitinib failure, and the superiority of delayed HCT after ruxolitinib failure could be reversed depending on several parameters. Therefore, both strategies seem to be worth considering in patients aged <60 years.
Most previous studies that have compared HCT treatment and non-HCT treatment showed that HCT could achieve long-term survival at the cost of impaired QoL because of chronic GvHD.21,22 On the other hand, QoL in patients with PMF-associated symptoms was inferior to that in those with chronic GvHD in studies on PMF.6,18 Thus, HCT for PMF might be expected to improve QoL from baseline along with the risk of transplant-related mortality. As a result, for transplant candidates with PMF, we might need to select between immediate HCT to improve QoL and delayed HCT after ruxolitinib failure to prolong survival duration. The prognosis of patients treated with ruxolitinib in the current study seemed to be better than the prognoses in reports based on real-world data.27,28 Transition probabilities in delayed HCT after ruxolitinib failure were determined based on data from several clinical trials,7,17 whereas those in immediate HCT were based on a database study.16 Baseline conditions in cohorts registered in clinical trials tend to be better than those in the real world. Thus, the prognosis of delayed HCT after ruxolitinib failure might be overestimated in the current study, while we also considered real-world data about ruxolitinib failure in sensitivity analyses. Further studies based on real-world data are warranted to compare immediate HCT and delayed HCT after ruxolitinib failure. In addition, overall survival after immediate HCT might be slightly lower than that in the previous report.16 It might be because the exact rate of death due to relapse was not available, and we calculated transition probabilities for death due to relapse based on the estimated rate. However, considering that we assumed patients with higher disease risk in our model, the setting seemed to be appropriate. Moreover, our results seemed to be inconsistent with the previous study by Kröger et al., in which the outcomes after HCT were equivalent between no or lost response to pre-HCT ruxolitinib and those without ruxolitinib treatment prior to HCT29 The discrepancy could be explained by the difference in analyzed timepoints. The previous study compared their survival from the day of HCT, while we compared the survival time from the decision of strategies at diagnosis. When the time to HCT from diagnosis was taken into consideration, our study demonstrated that delayed HCT after ruxolitinib failure would be superior to immediate HCT.
Our study had some limitations. First, transition probabilities in immediate HCT were derived from a single, retrospective study.16 In that study, the graft source in most cases was peripheral blood, and more than half of patients underwent HCT from unrelated donors. Different background data could change the results of the current study. Since the incidence of chronic GvHD especially affected QoL, the graft source and donor type should be considered in individual estimations. Indeed, a lower risk of GvHD was reported in patients undergoing bone marrow transplantation from haploidentical donors with post-transplant cyclophosphamide compared with peripheral blood stem cell transplantation from HLA-matched unrelated donors.30 Post-transplant cyclophosphamide should be incorporated and evaluated in our model in the future. Moreover, we determined QoL after HCT based on chronic GvHD status, while GvHD alone is not the whole story of QoL after HCT. Second, in the current model, a response to ruxolitinib was assumed based on a decrease in spleen volume. Thus, it can be difficult to assess the response to ruxolitinib in patients who do not have splenomegaly. The clinical decision regarding HCT in the real world might be based on transfusion dependency, adverse cytogenetics, and high-risk mutations in addition to a ruxolitinib response.9,31,32 Third, some transition probabilities and utilities were not available from previous published data, and were estimated by combining several reports. When making decisions based on the current model, these limitations should be considered.
In conclusion, for transplant candidates with PMF and an HLA-matched donor, immediate HCT and delayed HCT after ruxolitinib failure showed comparable QALY in patients aged <60 years. On the other hand, in the group of patients aged ≥60 years, delayed HCT after ruxolitinib failure is expected to be superior to immediate HCT. Consideration of the risk of chronic GvHD might help when making individual decisions.
Supplementary Material
Funding Statement
Funding: There was no funding relevant to this study.
Data-sharing statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Tefferi A. Primary myelofibrosis: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(5):801-821. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie E, Al-Janadi A, Kessler C, et al. Patient-reported outcomes of patients with myelofibrosis or essential thrombocythemia enrolled in the MOST study. Leuk Lymphoma. 2022;63(13):3138-3153. [DOI] [PubMed] [Google Scholar]
- 3.McLornan DP, Hernandez-Boluda JC, Czerw T, et al. Allogeneic haematopoietic cell transplantation for myelofibrosis: proposed definitions and management strategies for graft failure, poor graft function and relapse: best practice recommendations of the EBMT Chronic Malignancies Working Party. Leukemia. 2021;35(9):2445-2459. [DOI] [PubMed] [Google Scholar]
- 4.Bewersdorf JP, Sheth AH, Vetsa S, et al. Outcomes of allogeneic hematopoietic cell transplantation in patients with myelofibrosis-a systematic review and meta-analysis. Transplant Cell Ther. 2021;27(10):873.e1-873.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khera N, Storer B, Flowers ME, et al. Nonmalignant late effects and compromised functional status in survivors of hematopoietic cell transplantation. J Clin Oncol. 2012;30(1):71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison CN, Mesa RA, Kiladjian JJ, et al. Health-related quality of life and symptoms in patients with myelofibrosis treated with ruxolitinib versus best available therapy. Br J Haematol. 2013;162(2):229-239. [DOI] [PubMed] [Google Scholar]
- 7.Vannucchi AM, Kantarjian HM, Kiladjian JJ, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015;100(9):1139-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta V, Hari P, Hoffman R. Allogeneic hematopoietic cell transplantation for myelofibrosis in the era of JAK inhibitors. Blood. 2012;120(7):1367-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895-2901. [DOI] [PubMed] [Google Scholar]
- 12.Shanavas M, Popat U, Michaelis LC, et al. Outcomes of allogeneic hematopoietic cell transplantation in patients with myelofibrosis with prior exposure to Janus kinase 1/2 inhibitors. Biol Blood Marrow Transplant. 2016;22(3):432-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cipkar C, Kumar S, Thavorn K, Kekre N. Optimal timing of allogeneic stem cell transplantation for primary myelofibrosis. Transplant Cell Ther. 2022;28(4):189-194. [DOI] [PubMed] [Google Scholar]
- 14.Naimark D, Krahn MD, Naglie G, Redelmeier DA, Detsky AS. Primer on medical decision analysis: part 5--working with Markov processes. Med Decis Making. 1997;17(2):152-159. [DOI] [PubMed] [Google Scholar]
- 15.Kröger N, Giorgino T, Scott BL, et al. Impact of allogeneic stem cell transplantation on survival of patients less than 65 years of age with primary myelofibrosis. Blood. 2015;125(21):3347-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernández-Boluda JC, Pereira A, Kröger N, et al. Determinants of survival in myelofibrosis patients undergoing allogeneic hematopoietic cell transplantation. Leukemia. 2021;35(1):215-224. [DOI] [PubMed] [Google Scholar]
- 17.Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30(8):1701-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesa RA, Gotlib J, Gupta V, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebocontrolled trial. J Clin Oncol. 2013;31(10):1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787-798. [DOI] [PubMed] [Google Scholar]
- 20.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104(2):579-585. [DOI] [PubMed] [Google Scholar]
- 21.Koreth J, Pidala J, Perez WS, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol. 2013;31(21):2662-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurosawa S, Yamaguchi T, Mori T, et al. Patient-reported quality of life after allogeneic hematopoietic cell transplantation or chemotherapy for acute leukemia. Bone Marrow Transplant. 2015;50(9):1241-1249. [DOI] [PubMed] [Google Scholar]
- 23.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pidala J, Kurland B, Chai X, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117(17):4651-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon SR, Sizemore C, Zhang X, et al. Current graft-versus-host disease-free, relapse-free survival: a dynamic endpoint to better define efficacy after allogenic transplant. Biol Blood Marrow Transplant. 2017;23(7):1208-1214. [DOI] [PubMed] [Google Scholar]
- 26.Palmer J, Kosiorek HE, Wolschke C, et al. Assessment of quality of life following allogeneic stem cell transplant for myelofibrosis. Biol Blood Marrow Transplant. 2019;25(11):2267-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coltro G, Sant’Antonio E, Palumbo GA, et al. Assessment of the efficacy and tolerability of ruxolitinib for the treatment of myelofibrosis patients in a real-life setting: an Italian MYNERVA project. Cancer Med. 2023;12(7):8166-8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verstovsek S, Parasuraman S, Yu J, et al. Real-world survival of US patients with intermediate- to high-risk myelofibrosis: impact of ruxolitinib approval. Ann Hematol. 2022;101(1):131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kröger N, Sbianchi G, Sirait T, et al. Impact of prior JAK-inhibitor therapy with ruxolitinib on outcome after allogeneic hematopoietic stem cell transplantation for myelofibrosis: a study of the CMWP of EBMT. Leukemia. 2021;35(12):3551-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagler A, Labopin M, Dholaria B, et al. Comparison of haploidentical bone marrow versus matched unrelated donor peripheral blood stem cell transplantation with posttransplant cyclophosphamide in patients with acute leukemia. Clin Cancer Res. 2021;27(3):843-851. [DOI] [PubMed] [Google Scholar]
- 31.Tefferi A, Guglielmelli P, Nicolosi M, et al. GIPSS: genetically inspired prognostic scoring system for primary myelofibrosis. Leukemia. 2018;32(7):1631-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: mutation-enhanced international prognostic score system for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36(4):310-318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.