Abstract
Belantamab mafodotin (belantamab) is a first-in-class anti-B-cell maturation antigen (BCMA) antibody-drug conjugate approved for the treatment of triple-class refractory multiple myeloma. It provides a unique therapeutic option for patients ineligible for chimeric antigen receptor (CAR) T and bispecific antibody therapy, and/or patients progressing on anti-CD38 treatment where CAR T and bispecifics might be kept in reserve. Wider use of the drug can be challenged by its distinct ocular side effect profile, including corneal microcysts and keratopathy. While dose reduction has been the most effective way to reduce these toxicities, the underlying mechanism of this BCMA off-target effect remains to be characterized. In this study, we provide the first evidence for soluble BCMA (sBCMA) in lacrimal fluid and report on its correlation with tumor burden in myeloma patients. We confirm that corneal cells do not express BCMA, and show that sBCMA-belantamab complexes may rather be internalized by corneal epithelial cells through receptor-ligand independent pinocytosis. Using an hTcEpi corneal cell-line model, we show that the pinocytosis inhibitor EIPA significantly reduces belantamab-specific cell killing. As a proof of concept, we provide detailed patient profiles demonstrating that, after belantamab-induced cell killing, sBCMA is released into circulation, followed by a delayed increase of sBCMA in the tear fluid and subsequent onset of keratopathy. Based on the proposed mechanism, pinocytosis-induced keratopathy can be prevented by lowering the entry of sBCMA into the lacrimal fluid. Future therapeutic concepts may therefore consist of belantamab-free debulking therapy prior to belantamab consolidation and/or concomitant use of γ-secretase inhibition as currently evaluated for belantamab and nirogacestat in ongoing studies.
Introduction
B-cell maturation antigen (BCMA, also referred to as TNFRSF17 or CD269), a cell surface receptor that is almost exclusively expressed on the surface of plasmablasts and differentiated plasma cells,1 has recently emerged as a promising therapeutic target in multiple myeloma (MM). The approvals of anti-BCMA directed CAR T-cell therapies and bispecific antibodies (bsAb) are practice-changing milestones in the treatment of MM.2-6 However, severe side effects, including infections, hematotoxicity and neurological disorders, along with availability and financial limitations, render a relevant number of patients ineligible to these therapies.7-9 Recently emerged characterization of T-cell subsets in large CAR T and bsAb clinical trials additionally indicates that T-cell redirecting therapies are less effective in heavily pretreated patients with exhausted or contracted effector cell phenotypes.10-12 This observation underscores the persistent clinical need for BCMA-targeting therapies whose mechanism of action is agnostic of T-cell functionality. Belantamab mafodotin (belantamab) is an afucosylated, humanized anti-BCMA mAb conjugated to the microtubule toxin monomethyl auristatin F (MMAF) by a protease-resistant maleimidocaproyl linker.13 It depicts the first-in-class antibody-drug conjugate (ADC) that gained accelerated Food and Drug Administration (FDA) approval for the treatment of patients with triple-class-refractory MM and ≥4 prior lines of therapy.14-17 This label however was withdrawn upon FDA request as belantamab did not meet its primary endpoint of progression-free survival (PFS) benefit in the confirmatory phase III DREAMM-3 trial for RRMM which tested single-agent belantamab over pomalidomide-dexamethasone (Pd).18 Despite this setback and given the unmet need for effective backbone agents in patients progressing on first-line anti-CD38 treatment, belantamab remained under investigation in multiple latestage clinical trials to explore its efficacy and safety in combination with other agents.19-21 An interim analysis from the head-to-head DREAMM-7 trial recently revealed a substantial and highly significant PFS benefit for belantamab-bortezomib-dexamethasone (BVd) over Vd combined with the CD38 monoclonal antibody daratumumab (DVd), together with a concomitant survival benefit suggested on preliminary analysis.22
These observations have stimulated continued efforts to establish the value of belantamab within the treatment landscape of RRMM. Wider use of the drug remains impeded because of its distinct ocular side effect profile, ranging from dry eye or blurred vision to corneal events such as corneal microcyst-like epithelial changes (MEC) and subsequent keratopathy, albeit proving reversible and manageable with dose reduction and changed schedule. At the recommended dose level of 2.5 mg/kg, such ocular events may occur in up to 71% of patients.23 Whilst to date, dose reductions are the only way to efficiently reduce these ocular toxicities, the underlying mechanism of this BCMA off-target effect remains to be explored. It has been hypothesized that belantamab is being transported to the eye via the vessels of the corneal limbus.24 Here, similarly to other ADC, MMAF or the entire belantamab molecule could be internalized into corneal progenitor cells via non-specific pinocytosis and be subsequently pushed into the corneal center during tissue differentiation.25 It may thus be speculated that pharmacologic inhibition of pinocytosis may depict a promising strategy to reduce belantamab-mediated cytotoxicity in corneal progenitor cells. The cornea itself does not express BCMA, however it is known that the extracellular domain of the BCMA glycoprotein may be shed from the cell membrane via γ-secretases to circulate in the blood as soluble BCMA (sBCMA).26 In this study, we hypothesized that sBCMA acts as a carrier which may shuttle belantamab to the vessels of the corneal limbus where both cross the tissue barrier to enter the lacrimal fluid and spread across the corneal surface. Our study provides the first evidence for the presence of sBCMA in the tears of MM patients and demonstrates strong binding affinity between sBCMA and belantamab while circulating throughout the bloodstream. Mechanistically, we describe the uptake of sBCMA-bound belantamab by corneal epithelial cells via pinocytosis and show how pinocytosis inhibition can be used to design more effective mitigation strategies for keratopathy in belantamab-exposed MM patients.
Methods
Measurement of soluble B-cell maturation antigen
Serum was collected after 1 hour of blood draw (5-10 mL) via centrifugation at 2,000 g for 10 minutes and was diluted at 1:500 or 1:1000 in dilution buffer (Sample Diluent NS, Abcam, Cambridge, UK) of the commercially available human BCMA ELISA kit (ab263875, Abcam). Tear fluid was collected from patients’ conjunctival fornices using polyvinyl sponges followed by high-speed centrifugation (15,000 g for 15 minutes). BCMA quantification via enzyme-linked immunosorbant assay was performed following the manufacturer’s instructions.
Response assessment
Response assessment was performed in accordance with the International Myeloma Working Group (IMWG) response criteria. Ocular adverse events (AE) were rated according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. All procedures were performed in accordance with the Declaration of Helsinki and national ethical standards. Informed consent was obtained from all patients on our study (ethics vote #08/21).
Eye examination of patients
All patients received ophthalmological assessment at baseline, after every three treatment cycles, and upon clinical decision during treatment. According to the keratopathy and visual acuity (KVA) scale,27 patients were graded regarding the severity of corneal changes and the decline of best-corrected visual acuity from baseline. Corneal adverse events were mitigated as recommended by withholding treatment until improvement and resuming treatment at the next level of dose reduction.
Cell culture
The human telomerase-immortalized corneal epithelial cell line hTcEpi was purchased from Evercyte (Vienna, Austria). Cells were expanded into working banks and were reinstated freshly from the working bank after every 3-4 passages. Keratinocyte growth medium-2 (KGM-2) BulletKit (cat# CC-3107) was purchased from Lonza (Basel, Switzerland) and was used for routine cell culture. Cells were grown at 37°C supplemented with 5% CO2.
Drug treatment
hTcEpi cells were seeded into 96-well plates (20,000 cells per well), incubated for 2 hours before treatment with the respective compounds and subjected to Alamarblue measurements after 72 hours of drug treatment. Treatment with the pinocytosis inhibitor 5-(N-ethyl-N-isopropyl) amiloride (EIPA, 20 µM) was performed for 2 hours. EIPA was removed before adding belantamab at a concentration of 1,315 nM (200 µg/mL). For sBCMA co-cultures, sBCMA was added at a 2:1 ratio based on our binding experiments, i.e., at a concentration of 2,630 nM. Drug solutions for each experiment were freshly prepared from concentrated stocks (solvents for stock solutions: phosphate-buffered saline for BCMA, dimethyl sulfoxide for EIPA).
Surface plasmon resonance spectroscopy
Interactions between belantamab and sBCMA were measured by surface plasmon resonance spectroscopy (SPR) using a Biacore™ X100 SPR system (Cytiva, Marlborough, MA). Measurements were performed at 25°C using HBS150T (10 mM HEPES pH 7.4; 150 mM NaCl; 3.4 mM EDTA; 0.005% [v/v] Tween 20) as running buffer. The flow rate for the acquisition of interaction data was set to 25 µL/minute (min) in all experiments. Association was measured for 60 seconds, then dissociation was initiated by perfusing the running buffer, and the dissociation phase was monitored for 300 seconds. For immobilization of sBCMA on the sensor surface, the carboxylate groups of a CM5 sensor chip (Cytiva) were activated first by perfusing a 1:1 mixture of N-hydroxy-succinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) according to the manufacturer’s instructions. Next, sBCMA (100 µg/mL in 10 mM sodium acetate, pH 4.0) was injected onto the activated sensor for 360 seconds at a flow rate of 25 µL/min to cause sBCMA coating to a density of 2.500 resonance units (RU). Unreacted activated carboxylate groups were quenched by injecting 1 M ethanolamine (pH 8.5) for 300 seconds. Finally, lanes were probed with 10, 5, 2.5 and 1.25 nM belantamab. A flow channel carrying no sBMCA as ligand was used as control to monitor interaction specificity. Interaction of the analyte belantamab with its control surface was subtracted from the raw data to allow for the removal of unspecific binding and bulk effects. Regeneration of the chip surface was performed by injection of two 60-second pulses of 100 mM glycine (pH 2.5) at a flow rate of 100 µL/min. Interaction data were analyzed using the Biacore X100 Evaluation Software 2.0.2 (Cytiva), applying a bivalent interaction model and using local fitting for the rate constants. Association rate constant kon values and dissociation rate constant koff values were obtained by fitting data of individual experiments. Equilibrium binding dissociation constants (KD) were deduced using the equation KD=koff/kon. All SPR measurements were performed in at least three independent experiments.
Gene expression analysis
Total RNA from the MM cell lines and hTcEpi cells was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany). cDNA was synthesized using SuperScript VILO cDNA-Synthesis Kit (ThermoFisher Scientific, Waltham, MA). Gene expression analysis was performed using TaqMan Real-Time-PCR-Assays (ThermoFisher Scientific) using GUS4B or GAPDH as housekeeping genes, As a reference, TNFRSF17 gene expression was determined using publicly available libraries of MM cell lines and ocular cell subsets.28,29 Published data of normalized transcripts per million (nTPM) of human cancer cell lines and ocular cell subsets was summarized as heatmaps using Prism (version 10.1, GraphPad Inc, Boston, MA).
Statistics
All data were plotted as mean with median and range using violin plots where applicable. Statistical difference between two and three groups was determined by unpaired t test (Mann-Whitney) and one-way ANOVA, respectively.
Results
Soluble B-cell maturation antigen in lacrimal fluid correlates with serum soluble B-cell maturation antigen and remission state
Our first goal was to provide proof-of-principle that sBCMA can be detected in the serum of MM patients with active disease. We investigated a cohort of 16 individuals, including seven healthy volunteers and nine MM patients, the latter in part with controlled versus active disease, defined as IMWG remission state of stable disease or better (≥SD, N=5) versus progression (N=4).
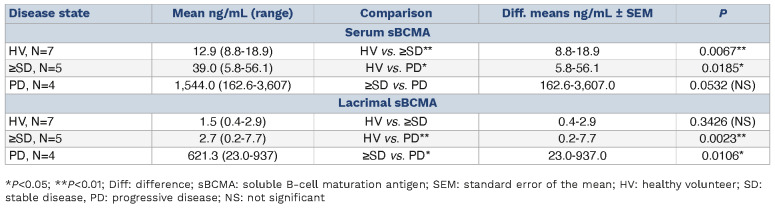
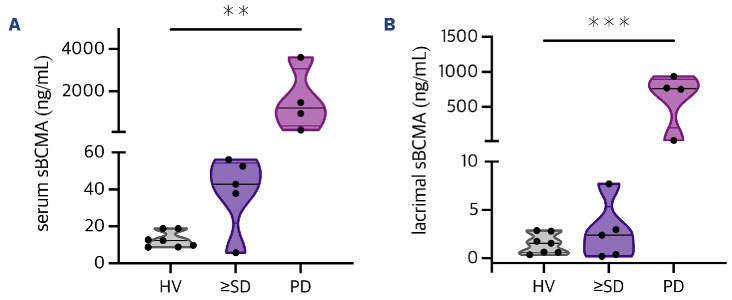
In this cohort, serum sBCMA was lowest in healthy individuals with a mean of 12.9 ng/mL (range, 8.8-18.9 ng/ mL) (Table 1; Figure 1A). In comparison, patients with ≥SD (mean 39.0 ng/mL; range, 5.8-56.1 ng/mL; P=0.0067) and progressive disease (mean 1,544 ng/mL; range, 162.6-3,607 ng/mL; P=0.0185) had significantly higher sBCMA levels in the serum (Table 1). This confirms prior reports on serum sBCMA serving as a proxy of tumor burden in MM.30,31 Based on this preliminary observation and given the distinct ocular side effect profile of BCMA-directed antibody drug conjugates in MM, we next hypothesized that sBCMA may also be present in the tear fluid of MM patients with active disease. We found levels of lacrimal sBCMA to be lowest in healthy individuals (mean 1.5 ng/mL; range, 0.4-2.9 ng/ mL) (Figure 1B). Interestingly and in contrast to our prior analysis for serum sBCMA, the levels of lacrimal sBCMA remained similarly low also in patients with controlled disease (mean 2.7 ng/mL; range, 0.2-7.7 ng/mL; P=0.3426). In contrast, sBCMA was substantially increased in the lacrimal fluid of MM patients with active disease measured at a mean of 621.3 ng/mL (range, 23.0-937 ng/mL), which proved to be significantly elevated if compared to HV (P=0.0023) and ≥SD patients (P=0.0106). This indicates that sBCMA in lacrimal fluid may serve as a measure of tumor burden in MM patients. In contrast to serum sBCMA, lacrimal sBCMA showed diverging kinetics as levels in the tear fluid of patients with clinically active MM were significantly higher as compared to those with controlled disease.
B-cell maturation antigen-coding TNFRSF17 is not expressed by corneal epithelial cells
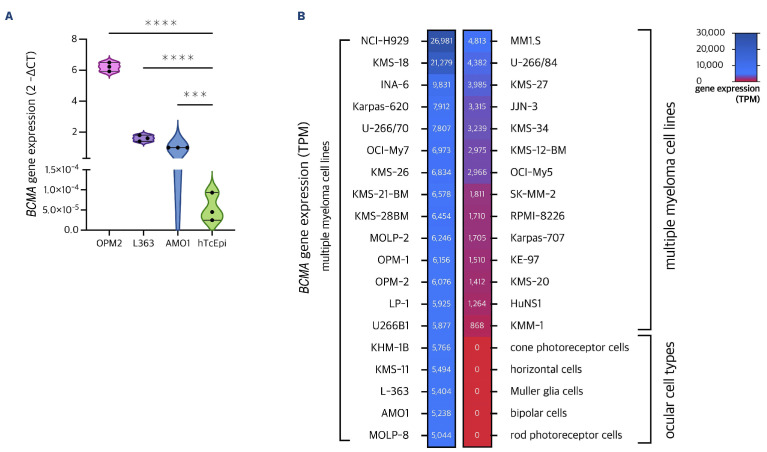
In order to measure TNFRSF17 expression in corneal epithelial cells, we utilized qPCR and investigated hTcEpi cells along with three MM cell lines serving as positive controls. Our analysis revealed that there was no expression of TNFRSF17 in hTcEpi cells (2-ΔCT=0.000054), which proved significantly lower than in the AMO1 (P=0.0003), L363 (P<0.0001) and OPM2 (P<0.0001) cell lines (Figure 2A). In order to further validate our results, we examined TNFRSF17 expression in various ocular cell types and MM cell lines as available through publicly accessible datasets.28,29 No expression of TNFRSF17 (nTPM=0) was seen in any type of ocular tissue as revealed by single-cell transcriptomic data. This compared to a consistently stable expression of TNFRSF17 in MM cell lines, and confirmed our in vitro observation on TNFRSF17 expression being absent on human corneal epithelial cells (Figure 2B).
Table 1.
Comparison of soluble B-cell maturation antigen levels in serum and lacrimal fluid across disease states.
Belantamab and microtubule toxin monomethyl auristatin F show dose-dependent killing in hTcEpi
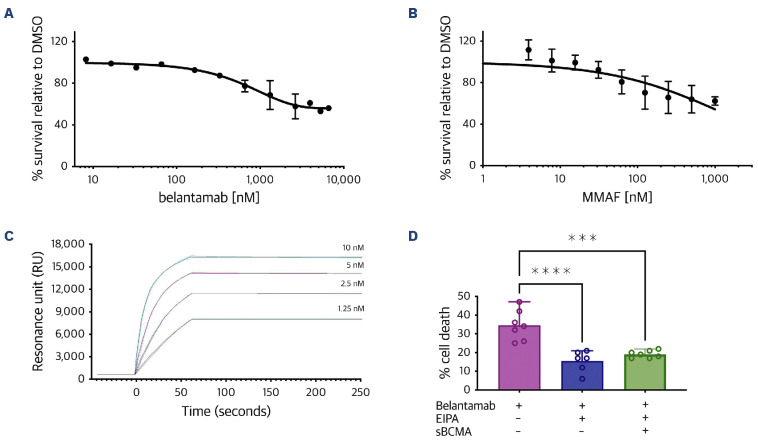
Sensitivity of corneal epithelial cells to belantamab was measured in hTcEpi with serial dilutions of belantamab ranging from 10-10,000 nM in vitro. Our results demonstrated a dose-dependent killing of hTcEpi cells after 72 hours of incubation with belantamab as measured via Alamarblue assay (Figure 3A) despite the absence of the target antigen BCMA on the corneal cell surface. In addition, we treated the cells with various concentrations of MMAF ranging between 1-10,000 nM and observed dose-dependent sensitivity to MMAF similar to belantamab treatment (Figure 3B). These observations suggest that corneal epithelial cells are sensitive to toxic effects of MMAF either in its free form or if conjugated to an antibody as with belantamab. Of note, despite using a very high concentration for both drugs, the maximum killing efficacy observed was ~50% compared to solvent control (dimethyl sulfoxide [DMSO]) implying that MMAF or ADC do not have targeted killing properties, but act by a non-specific mode of transportation, i.e., pinocytosis.
Figure 1.
Soluble B-cell maturation antigen distribution in serum and lacrimal fluid of multiple myeloma patients and healthy volunteers. Violin plots were used to show the levels of soluble B-cell maturation antigen (sBCMA) (ng/mL) in serum versus lacrimal fluid. (A) Three-group comparison revealed a significant increase (one-way ANOVA, P=0.0091) of serum sBCMA levels for patients at the time of progressive disease (PD, N=4, mean 1,544 ng/mL; range, 162.6-3,607 ng/mL) if compared to healthy volunteers (HV, N=7, mean 12.9 ng/mL; range, 8.8-18.9 ng/mL) and patients with a remission state of stable disease (SD) or better (N=5, mean 39.0 ng/mL; range, 5.8-56.1 ng/mL). (B) For lacrimal fluid, this increase in sBCMA levels for PD patients (mean 621.3 ng/mL; range, 23.0-937 ng/mL) versus patients with ≥SD (mean 2.7 ng/mL; range, 0.2-7.7 ng/mL) versus HV (mean 1.5 ng/mL; range, 0.4-2.9 ng/mL) was even more pronounced (one-way ANOVA, P=0.0004). **P<0.005; ***P<0.0005.
Figure 2.
BCMA gene expression across tissues and cell lines. (A) Violin plots were used to show the levels of BCMA gene expression (ng/mL) quantified by quantitative polymerase chain reaction (qPCR) using Taqman real-time PCR in 3 multiple myeloma (MM) cell lines (OPM2, L363, AM01) and human telomerase-immortalized corneal epithelial (hTcEpi) cells. Expression is given as fold gene expression normalized to AM01 using the b-b Ct method. BCMA gene expression is highest in OPM2, followed by L363 and AM01. AM01 (P=0.0003), L363 (P<0.0001) and OPM2 (P<0.0001) show significantly higher BCMA gene expression as compared to hTcEpi for which BCMA gene expression remains absent. (B) Heatmap summarizing TNFRSF17 expression in a larger set of MM cell lines and distinct tissues from the ocular system. Expression data was retrieved from publicly available datasets (humanproteinatlas.org) and is given as normalized transcripts per million (TPM). ***P<0.001; ****P<0.0001.
Figure 3.
Mechanisms of belantamab toxicity in human telomerase-immortalized corneal epithelial cells. Dose-titration curves for human telomerase-immortalized corneal epithelial (hTcEpi) cells treated with increasing concentrations of belantamab (A) and MMAF (B) for 72 hours as quantified by Alamarblue assay. (C) Measurements of dissociation constants (KD) for the interaction between belantamab and soluble B-cell maturation antigen (sBCMA) by surface plasmon resonance spectroscopy (SPR). Experimental curves are given in black, fitted curves in other colors. (D) Bar chart illustrating belantamab-specific cell killing in hTcEpi in the presence versus absence of the pinocytosis inhibitor EIPA (20 µM). hTcEpi cells were treated for 2 hours with EIPA followed by 3 days treatment with belantamab (1,315 nM [200 µg/mL]) with or without sBCMA (2,630 nM). All samples were quantified by Alamarblue assay. DMSO: dimethyl sulfoxide. ****P<0.0001; ***P=0.0002.
Belantamab shows strong affinity binding to soluble B-cell maturation antigen
In order to investigate the binding strength of belantamab to sBCMA, we performed surface plasmon resonance (SPR) analysis between belantamab and sBCMA. SPR analysis revealed a high affinity binding of belantamab to sBCMA molecules, with KD1 17.4±4.2 nM and KD2 5.6±1.4 nM. In the course of data evaluation, the best fit of the experimental curves was achieved by applying a bivalent binding model instead of a simple Langmuir-type 1:1 interaction model. This is consistent with the interpretation of a high-affinity, bivalent binding (1:2) mode (Figure 3C) implying that one belantamab antibody molecule has a strong binding affinity to two neighboring immobilized sBCMA molecules on the sensor chip surface. This strong interaction may explain the mode of transportation of sBCMA-bound belantamab from peripheral blood into lacrimal fluid and hence in close proximity of the cornea.
Inhibition of pinocytosis reduces cell killing in hTcEpi
Given the observation that we do not achieve full eradication of hTcEpi cells despite using high concentrations of MMAF and belantamab and in light of reports that corneal epithelial cells possess the ability of pinocytosis,32 we treated hTcEpi cells with the pinocytosis inhibitor EIPA followed by belantamab treatment to assess the intake of belantamab via pinocytosis. Strikingly, pretreatment with EIPA significantly reduced belantamab-specific killing from 34.5% to 15.5% (P<0.0001) (Figure 3D). Pretreatment with EIPA was also tested in the presence of sBCMA-bound belantamab, and for this condition led to a comparable reduction of specific killing down to 19.1% (P=0.0002). These results confirmed that hTcEpi cells have the ability to absorb belantamab and sBCMA-bound belantamab via pinocytosis, which may explain the mechanism of corneal toxicity in patients treated with belantamab.
High soluble B-cell maturation antigen in lacrimal fluid predisposes to keratopathy
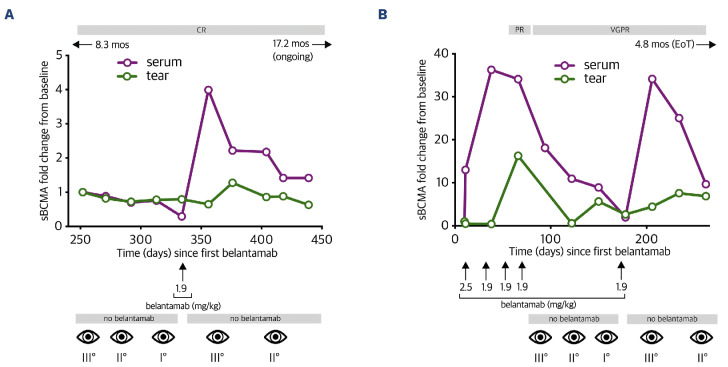
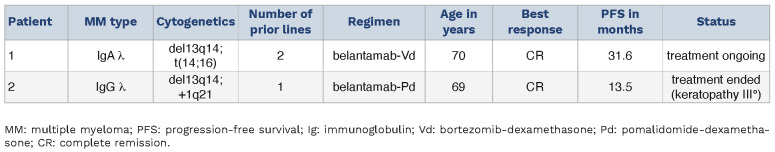
Next, we correlated sBCMA levels in serum and lacrimal fluid with the occurrence of keratopathy in two long-term responders to belantamab. Patient 1 was a 70-year-old male who underwent treatment with belantamab-bortezomib-dexamethasone (belantamab-Vd) and had already achieved complete remission to belantamab at the start of our analysis (Figure 4A). Initial assessment confirmed constantly low levels for sBCMA in both the serum and lacrimal fluid, thus matching to the depth of remission in this patient. At this time, belantamab treatment had been paused for 3 months due to keratopathy grade 3 according to CTCAE criteria, but got redosed upon amelioration of keratopathy at day 82. Following belantamab re-exposure, sBCMA in the serum immediately surged to 4-fold of the baseline value whereas lacrimal sBCMA showed only a modest 1.3-fold incline, along with re-exacerbation of keratopathy which resulted in another dosing break for belantamab (Figure 4A). Despite this intermittent dosing scheme and fluctuating keratopathy, the patient continues to pursue belantamab-Vd treatment and remains in complete remission at a PFS of 31.6 months (Table 2). Patient 2 was a 69-year-old female treated with belantamab-pomalidomide-dexamethasone (belantamab-Pd). The patient was belantamab-naïve at the start of investigation (Figure 4B). Upon first exposure, we observed an immediate 30-fold increase in serum sBCMA. At a latency of 28 days, lacrimal sBCMA levels had equally surged by 37-fold to baseline. After four monthly belantamab infusions, the patient had achieved a very good partial response (VGPR), but treatment had to be stopped due to the onset of grade 3 keratopathy. The patient was re-exposed to belantamab after 48 days. This resulted in another 34-fold surge of serum sBCMA whereas lacrimal sBCMA increased more modestly by 4.4-fold, yet sufficiently for re-exacerbation of grade 3 keratopathy which ultimately led to side-effect-related termination of belantamab-Pd at a PFS of 13.5 months despite excellent response in this patient (Table 2). These data demonstrate that detectability of sBCMA in lacrimal fluid is a phenomenon that precedes keratopathy in belantamab-exposed MM patients. While sBCMA may generally serve as a proxy of tumor burden and effective tumor lysis, serum levels seem a more sensitive marker that allows disease monitoring after a longer treatment break even in patients with complete remission, whereas lacrimal sBCMA shows relevant deflection mostly in patients with previously untreated disease or in patients with suboptimal depth of response. Validation of exact thresholds of lacrimal sBCMA that trigger keratopathy in patients was beyond the scope of this study and remains to be determined in future prospective trials.
Figure 4.
Kinetics of soluble B-cell maturation antigen increase in serum and lacrimal fluid. Serial profile of serum and lacrimal soluble B-cell maturation antigen (sBCMA) levels along with CTCAE levels for keratopathy in response to belantamab exposure at multiple time points in 2 patients (Pt). Pt1 (A) with a progression-free survival (PFS) of 31.6 months and Pt2 (B) with a PFS of 13.5 months (mos). X-axis indicates days since first belantamab treatment. Numbers next to eye symbol depict keratopathy grade according to CTCAE criteria. CR: complete remission; PR: partial response; VGPR: very good partial response; EoT: end of treatment; mos: months; ↑: belantamab treatment with respective belantamab dosing (mg/kg); ←/→: indicating duration of belantamab treatment (in months) prior/after investigational period.
Discussion
In this study, we report on the use of sBCMA as a proxy of tumor burden in MM and while the role of serum sBCMA as a biomarker in MM is nowadays widely accepted,33,34 our data provides first evidence for the detectability of sBCMA in the lacrimal fluid of MM patients.
Table 2.
Patient characteristics.
This observation seems critical for patients undergoing treatment with the BCMA-directed antibody drug conjugate belantamab as it may help to develop improved mitigation strategies to prevent belantamab-induced keratopathy, a major side effect impeding wider use of this highly effective drug in the clinic. At the same time, the exact mechanism by which belantamab exerts its ocular damage needs further elucidation to optimize mitigation.
It has been reported that belantamab can be detected in the tears of rabbits after single or repeated belantamab infusions,35 yet attempts to reproduce these measurements in humans have remained unsuccessful. Our data indicates that belantamab shows striking binding affinity to sBCMA, and once bound, may be carried along with sBCMA throughout the bloodstream. We show that sBCMA is detectable in the lacrimal fluid of MM patients. In contrast, sBCMA levels in the serum are generally higher and elevated also in patients with less tumor burden. This may indicate that sBCMA detectability in the tear fluid of MM patients reflects an overflow phenomenon once the amount of circulating sBCMA has reached a critical threshold. As we confirm that corneal cells do not express TNFRSF17,28,29 it may be assumed that the belantamab-binding sBCMA complex enters the lacrimal compartment through the vasculature of the limbus and is absorbed by cells from the corneal basal epithelial layer by a receptor-ligand independent internalization process commonly known as pinocytosis (Figure 5).36,37 Pinocytosis is driven by actin polymerization which allows for membrane protrusions to create an enclosed cavity that is next internalized as vesicle.38 In order to assess the role of pinocytosis for the pathogenesis of belantamab-induced keratopathy, we investigated the ability of EIPA, a preclinical inhibitor of the sodium/ hydrogen exchange machinery which blocks pinosome formation without affecting other endocytic pathways,39,40 to prevent belantamab-induced cytotoxicity in human corneal epithelial cells. Strikingly, EIPA significantly reduced belantamab-specific cell killing in the hTcEpi corneal cell line model. Interestingly, EIPA also protected hTcEpi cells from sBCMA-bound belantamab, confirming pinocytosis as a critical mode of action in the context of belantamab-induced keratopathy.
Due to its interference with ion transport and intracellular pH levels, EIPA cannot be administered as a clinical-grade therapeutic. At the same time, repurposing FDA-approved agents has emerged as a potential avenue to identify clinically available inhibitors of pinocytosis, with imipramine, a tricyclic antidepressant acting by presynaptic reuptake inhibition, depicting the most promising candidate in this regard.41
The DREAMM-1 and DREAMM-2 trial protocols investigated several mitigation strategies to reduce belantamab-induced keratopathy.14,16 The use of cooling eye masks, vasoconstrictors or corticosteroids have been disappointing in regard to keratopathy-protective effects,42 whereas the use of rigid gas-permeable corneal contact lenses can lead to major visual improvement to enable therapy continuation.43
Current strategies concentrate on dose reductions and extended treatment-free intervals, along with the use of preservative-free artificial tears. Based on the proposed mechanism, pinocytosis-induced keratopathy can be diminished by pre-emptive strategies that lower the entry of sBCMA into the lacrimal fluid. This may be achieved by lowering the tumor mass using belantamab-free debulking therapy prior to belantamab treatment or the concomitant use of y-secretase inhibition that can successfully stabilize the target antigen expression on MM cells.44 The combination of belantamab and the y-secretase inhibitor nirogacestat is currently being examined in the phase I/II DREAMM-5 trial (clinicaltrials gov. Identifier: NCT04126200) for relapsed/ refractory MM patients with ≥3 prior lines of therapy. In the first interim safety analysis, severe ocular toxicity (CTCAE ≥grade 3) was noted in ten of 34 (29%) of patients treated with belantamab plus nirogacestat as compared to 22 of 37 (59%) patients with belantamab monotherapy.45 While this data remains preliminary and additional studies are needed, these early results suggest that this mitigation strategy may provide an effective and safe way to reduce belantamab-induced keratopathy while maintaining anti-tumor efficacy.
In summary, we are first to describe the presence of sBCMA in the lacrimal fluid of MM patients and present pinocytosis as the underlying mechanism of keratopathy induction.
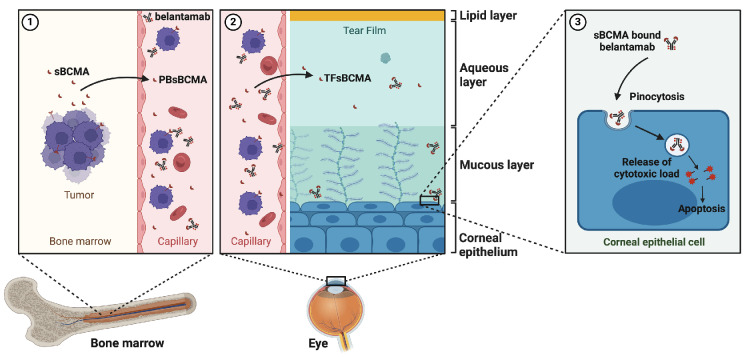
Figure 5.
Proposed mechanism of belantamab-induced keratopathy. (1) Upon high tumor burden or sudden tumor lysis, soluble B-cell maturation antigen (sBCMA) is shed from the surface of bone marrow-residing multiple myeloma (MM) cells to circulate into the bloodstream. (2) Serum sBCMA crosses the endothelial barrier at the corneal limbus of the eye and enters the lacrimal fluid; the amount of sBCMA in the tear fluid may serve as proxy for tumor load in the body. (3) In its bound form to sBCMA, belantamab is carried to the lacrimal fluid and absorbed by corneal epithelial cells via fluid endocytosis (pinocytosis). Inside the cell, belantamab is subsequently released via hydroxylation leading to apoptosis of the corneal epithelium. PB: peripheral blood; TF: tear film. (Created with Biorender.com).
Thus, inhibition of pinocytosis, along with lowering sBCMA levels in belantamab-treated patients are promising strategies to mitigate side effects of this highly effective agent in the clinic.
Funding Statement
Funding: JMW is supported by the Germany Ministry of Education and Research (BMBF) and Interdisziplinäres Zentrum für Klinische Forschung (IZKF) Würzburg. KMK is supported by Stifterverband. LR and KMK are supported by Mildred-Scheel-Nachwuchszentrum (MSNZ) Würzburg
Data-sharing statement
All data is available on request.
References
- 1.Laâbi Y, Gras MP, Carbonnel F, et al. A new gene, BCM, on chromosome 16 is fused to the interleukin 2 gene by a t(4;16) (q26;p13) translocation in a malignant T cell lymphoma. EMBO J. 1992;11(11):3897-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munshi NC, Anderson LD, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705-716. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Otero P, Ailawadhi S, Arnulf B, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med. 2023;388(11):1002-1014. [DOI] [PubMed] [Google Scholar]
- 4.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314-324. [DOI] [PubMed] [Google Scholar]
- 5.Martin T, Usmani SZ, Berdeja JG, et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. 2023;41(6):1265-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Usmani SZ, Garfall AL, van de Donk NWCJ, et al. Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): a multicentre, open-label, single-arm, phase 1 study. Lancet. 2021;398(10301):665-674. [DOI] [PubMed] [Google Scholar]
- 7.Rejeski K, Perez A, Sesques P, et al. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/ refractory large B-cell lymphoma. Blood. 2021;138(24):2499-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Oekelen O, Aleman A, Upadhyaya B, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat Med. 2021;27(12):2099-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Y, Martin TG, Usmani SZ, et al. CARTITUDE-1 final results: phase 1b/2 study of ciltacabtagene autoleucel in heavily pretreated patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2023;41(16_suppl):8009. [Google Scholar]
- 10.Dhodapkar KM, Cohen AD, Kaushal A, et al. Changes in bone marrow tumor and immune cells correlate with durability of remissions following BCMA CAR T therapy in myeloma. Blood Cancer Discov. 2022;3(6):490-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garfall AL, Dancy EK, Cohen AD, et al. T-cell phenotypes associated with effective CAR T-cell therapy in postinduction vs relapsed multiple myeloma. Blood Adv. 2019;3(19):2812-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firestone RS, McAvoy D, Shekarkhand T, et al. CD8 effector T cells enhance response in BCMA-exposed and -naïve multiple myeloma. Blood Adv. 2024;8(7):1600-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tai Y-T, Mayes PA, Acharya C, et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood. 2014;123(20):3128-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trudel S, Lendvai N, Popat R, et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol. 2018;19(12):1641-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trudel S, Lendvai N, Popat R, et al. Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019;9(4):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21(2):207-221. [DOI] [PubMed] [Google Scholar]
- 17.Richardson PG, Lee HC, Abdallah A-O, et al. Single-agent belantamab mafodotin for relapsed/refractory multiple myeloma: analysis of the lyophilised presentation cohort from the pivotal DREAMM-2 study. Blood Cancer J. 2020;10(10):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimopoulos MA, Hungria VTM, Radinoff A, et al. Efficacy and safety of single-agent belantamab mafodotin versus pomalidomide plus low-dose dexamethasone in patients with relapsed or refractory multiple myeloma (DREAMM-3): a phase 3, open-label, randomised study. Lancet Haematol. 2023;10(10):e801-e812. [DOI] [PubMed] [Google Scholar]
- 19.Nooka AK, Weisel K, van de Donk NW, et al. Belantamab mafodotin in combination with novel agents in relapsed/ refractory multiple myeloma: DREAMM-5 study design. Future Oncol. 2021;17(16):1987-2003. [DOI] [PubMed] [Google Scholar]
- 20.Trudel S, McCurdy A, Louzada ML, et al. Belantamab mafodotin, pomalidomide and dexamethasone in refractory multiple myeloma: a phase 1/2 trial. Nat Med. 2024;30(2):543-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trudel S, Davis R, Lewis NM, et al. DREAMM-8: a phase III study of the efficacy and safety of belantamab mafodotin with pomalidomide and dexamethasone (B-Pd) vs pomalidomide plus bortezomib and dexamethasone (PVd) in patients with relapsed/refractory multiple myeloma (RRMM). Blood. 2020;136(Suppl 1):4.32614961 [Google Scholar]
- 22.Press release: GSK announces positive results from DREAMM-7 head-to-head phase III trial for Blenrep in relapsed/refractory multiple myeloma. https://www.gsk.com/en-gb/media/press-releases/gsk-announces-positive-results-from-dreamm-7-head-to-head-phase-iii-trial-for-blenrep. Accessed January 11, 2024. [Google Scholar]
- 23.Nooka AK, Cohen AD, Lee HC, et al. Single-agent belantamab mafodotin in patients with relapsed/refractory multiple myeloma: final analysis of the DREAMM-2 trial. Cancer. 2023;129(23):3746-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marquant K, Quinquenel A, Arndt C, Denoyer A. Corneal in vivo confocal microscopy to detect belantamab mafodotin-induced ocular toxicity early and adjust the dose accordingly: a case report. J Hematol Oncol. 2021;14(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonial S, Nooka AK, Thulasi P, et al. Management of belantamab mafodotin-associated corneal events in patients with relapsed or refractory multiple myeloma (RRMM). Blood Cancer J. 2021;11(5):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurent SA, Hoffmann FS, Kuhn P-H, et al. γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun. 2015;6:7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahab A, Rafae A, Mushtaq K, et al. Ocular toxicity of belantamab mafodotin, an oncological perspective of management in relapsed and refractory multiple myeloma. Front Oncol. 2021;11:678634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Human Protein Atlas. https://www.proteinatlas.org/humanproteome/cell+line. Accessed January 11, 2024. [Google Scholar]
- 29.Karlsson M, Zhang C, Méar L, et al. A single-cell type transcriptomics map of human tissues. Sci Adv. 2021;7(31):eabh2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez E, Li M, Kitto A, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol. 2012;158(6):727-738. [DOI] [PubMed] [Google Scholar]
- 31.Visram A, Soof C, Rajkumar SV, et al. Serum BCMA levels predict outcomes in MGUS and smoldering myeloma patients. Blood Cancer J. 2021;11(6):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng H, Park JK, Lavker RM. Autophagy and macropinocytosis: keeping an eye on the corneal/limbal epithelia. Invest Ophthalmol Vis Sci. 2017;58(1):416-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alomari M, Kunacheewa C, Manasanch EE. The role of soluble B cell maturation antigen as a biomarker in multiple myeloma. Leuk Lymphoma. 2023;64(2):261-272. [DOI] [PubMed] [Google Scholar]
- 34.Shen Y, Liu J, Wang B, et al. Serum soluble BCMA can be used to monitor relapse of multiple myeloma patients after chimeric antigen receptor T-cell immunotherapy. Curr Res Transl Med. 2023;71(2):103378. [DOI] [PubMed] [Google Scholar]
- 35.Farooq AV, Degli Esposti S, Popat R, et al. Corneal epithelial findings in patients with multiple myeloma treated with antibody-drug conjugate belantamab mafodotin in the pivotal, randomized, DREAMM-2 study. Ophthalmol Ther. 2020;9(4):889-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao H, Atkinson J, Gulesserian S, et al. Modulation of macropinocytosis-mediated internalization decreases ocular toxicity of antibody-drug conjugates. Cancer Res. 2018;78(8):2115-2126. [DOI] [PubMed] [Google Scholar]
- 37.Mahalingaiah PK, Ciurlionis R, Durbin KR, et al. Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacol Ther. 2019;200:110-125. [DOI] [PubMed] [Google Scholar]
- 38.Bernitt E, Döbereiner H-G, Gov NS, Yochelis A. Fronts and waves of actin polymerization in a bistability-based mechanism of circular dorsal ruffles. Nat Commun. 2017;8:15863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Commisso C, Davidson SM, Soydaner-Azeloglu RG, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497(7451):633-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivanov AI. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol Biol. 2008;440:15-33. [DOI] [PubMed] [Google Scholar]
- 41.Lin H-P, Singla B, Ghoshal P, et al. Identification of novel macropinocytosis inhibitors using a rational screen of Food and Drug Administration-approved drugs. Br J Pharmacol. 2018;175(18):3640-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popat R, Warcel D, O’Nions J, et al. Characterization of response and corneal events with extended follow-up after belantamab mafodotin (GSK2857916) monotherapy for patients with relapsed multiple myeloma: a case series from the first-time-in-human clinical trial. Haematologica. 2020;105(5):e261-e263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keye P, Engelhardt M, Wäsch R, Böhringer D, Reinhard T. Management of belantamab mafodotin-associated keratopathy with rigid gas-permeable corneal contact lenses. Cornea. 2023;42(6):744-746. [DOI] [PubMed] [Google Scholar]
- 44.Pont MJ, Hill T, Cole GO, et al. γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood. 2019;134(19):1585-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callander N, Richardson P, Hus M, et al. P913: Low-dose belantamab mafodotin (belamaf) in combination with nirogacestat vs. belamaf monotherapy in patients with relapsed/refractory multiple myeloma (RRMM): phase 1/2 DREAMM-5 platform sub-study 3. Hemasphere. 2023;7(Suppl):e9722122. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is available on request.