In the pre-tyrosine kinase inhibitor (TKI) era (i.e., until the early 2000s),1-3 all children and adolescents with Philadelphia positive (Ph+) acute lymphoblastic leukemia (ALL) had an indication for cranial radiotherapy (CRT) or hematopoietic cell transplant (HCT), which usually implied total body irradiation (TBI) in the preparative regimen. The pattern of relapses for patients diagnosed between 1995 and 2005 was well-described in a large international study, which showed that in 610 patients, 3% had an isolated central nervous system (CNS) relapse, 4% a combined bone marrow (BM) and CNS relapse, and 35% an isolated BM relapse.3
In the last two decades, TKI have been added on top of chemotherapy with the use of CRT remaining controversial. In the Children’s Oncology Group (COG) AALL0031 study, all 49 patients received CRT and imatinib as TKI. A total of 11 (22%) patients relapsed, one in CNS only (2%).4,5 In the subsequent COG AALL0622 protocol (opened to recruitment in 2008), dasatinib was given as TKI, and CRT was limited to patients with CNS3 disease. Twenty-two (37%) out of 60 patients relapsed: 4 (7%) with an isolated CNS and 2 (3%) with a combined CNS relapse.6 Thereafter, in the CA180-372/COG AALL1122 study, dasatinib was given as TKI and HCT indications were further reduced, with only 15% of all patients undergoing HCT. CRT was indicated only for patients with CNS3 at diagnosis. Overall, relapses occurred in 38/106 (36%) patients, with isolated CNS relapses in 4 (4%), and combined CNS relapses in 4 (4%).7
In the EsPhALL2004, CRT was planned for all patients not transplanted in first complete remission at a dose of either 12, 18, or 24 Gys depending on age at the time of CRT and/or CNS involvement at diagnosis. Overall, 160 patients were recruited and 81% of them underwent HCT. Relapses occurred in 50 (31%) patients, with isolated CNS relapses in 5 (3%) and combined CNS relapses in 2 (1%).8,9 In the subsequent EsPhALL2010 study (EudraCT 2004-001647-30 and clinicaltrials.gov identifier: 00287105), the fraction of patients who underwent HCT was reduced to 38% (59/155). CRT was prescribed with the same indications as the EsPhALL2004 study. Overall, relapses occurred in 40 (26%) patients, with isolated CNS relapses in 6 (4%) and combined CNS relapses in 11 (7%).10 Of note, the adherence to CRT prescription in the EsPhALL2010 study was low, particularly in countries where contemporary, front-line ALL protocols for Ph- ALL no longer used CRT.
In this paper, we report features and outcomes of patients according to whether they received or not the planned CRT in the EsPhALL2010 study, with a causal approach in order to limit the biases caused by the non-randomized comparison. Results support the concept that CRT has no major role in the treatment of Ph+ ALL.
Overall, 155 patients <18 years of age at diagnosis with Ph+ ALL were enrolled in the EsPhALL2010 study.10 Patients who underwent HCT (N=59) and those who died or relapsed prior to reaching the CRT phase of the protocol (N=15) were excluded from this study. Thus, 81 patients who survived in complete remission for at least seven months from diagnosis are described here.
In the EsPhALL2010 study, CRT was planned at the dose of 12 Gy in patients aged two years or under and in older patients at 18 or 24 Gy if they did not have or had CNS involvement at diagnosis, respectively. Imatinib was added at the dose of 300 mg/m2 in all treatment phases. Details of the EsPhALL2010 treatment protocol have already been reported.10 All patients provided informed consent for participation in the EsPhALL2010 study, which was approved by the ethics committee of each participating institution. The event-free survival (EFS) was defined as the time from diagnosis to first failure, including resistant disease, relapse, death from any cause, or second malignant neoplasm; overall survival (OS) was calculated as the time from diagnosis to death from any cause. Observation periods were censored at the date of last contact if no event was observed. EFS and OS curves were estimated with the Kaplan-Meier method (with Greenwood standard error) and compared with the log-rank test. The cumulative incidence of relapse (CIR) was estimated accounting for competing risks (all other events) and compared with the Gray test. The Cox regression model and the Wald test were used to evaluate the impact of CRT on EFS outcome, adjusted by National Cancer Institute (NCI) criteria.11 In order to take into account imbalances in baseline covariates (related either to the choice to administer CRT or to outcome), the primary analysis adopted a causal approach using the inverse probability of treatment weighting (IPTW). A logistic regression model was fitted to estimate the propensity score, i.e., the probability of treatment assignment conditional on observed baseline covariate (ALL consortia and NCI criteria were regarded as relevant in this setting).
Then, the inverse of this probability was used as a weight in an adjusted Kaplan-Meier estimator and log-rank test.12 Two-sided Fisher exact tests were performed to compare patients who did or did not receive CRT, with respect to baseline characteristics. All analyses were performed using SAS version 9.4.
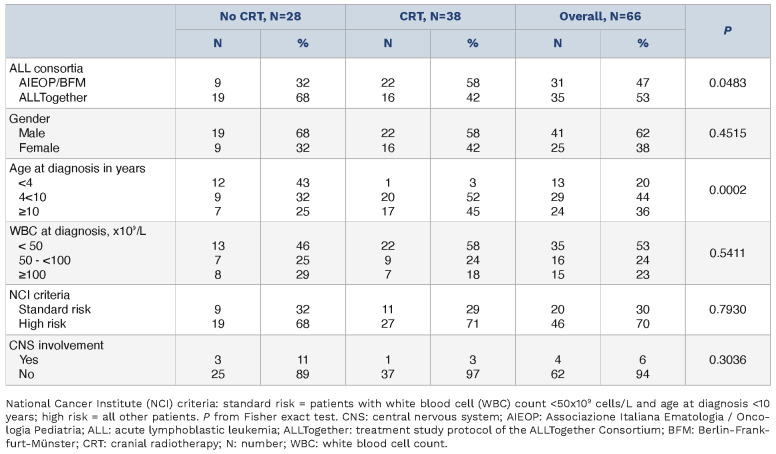
Overall, 81 patients were eligible for CRT. For 15 patients, data on CRT were incomplete and thus 66 were evaluable for outcome according to CRT administration. No difference in characteristics or outcome was observed between these 2 groups (data not shown). A total of 28 (42%) did not receive CRT, and 26 of them received additional intrathecal (IT) therapy. Presenting features of the 2 treatment subgroups are described in Table 1. Patients who did not receive CRT (No-CRT group) were more likely to be treated in countries which later formed the ALLTogether consortium (P=0.0483), where the use of CRT in front-line ALL protocols for non-Ph+ patients was very limited. No-CRT patients were also likely to be younger (P=0.0002). CNS involvement at diagnosis in the 2 treatment groups was documented in 3 out of 28 No-CRT patients and in one out of 38 CRT patients.
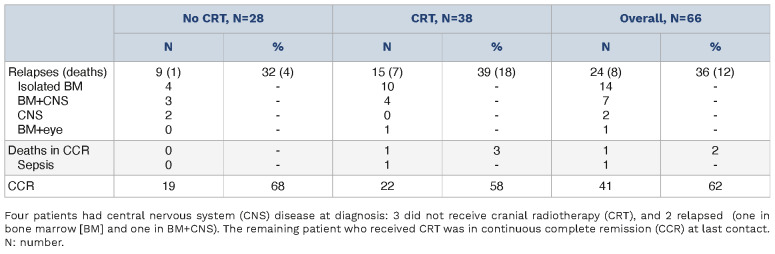
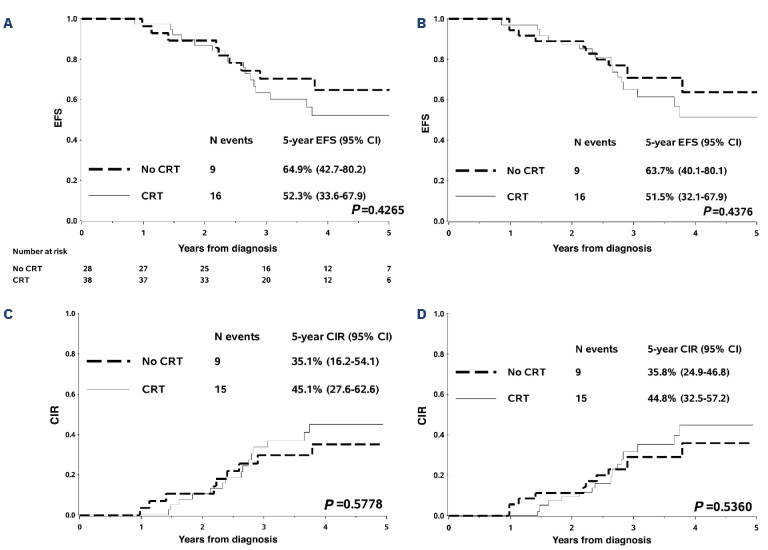
Events are shown in Table 2. Relapses in the No-CRT group occurred in 9 (32%) versus 15 (39%) patients in the CRT group, including 5 and 4 involving CNS, respectively. There was no advantage in terms of EFS and CIR for patients who received CRT: the 5-year EFS (95% CI) was 64.9% (42.7-80.2) versus 52.3% (33.6-67.9) (P=0.4265) and the 5-year CIR (95% CI) was 35.1% (16.2-54.1) versus 45.1% (27.6-62.6) (P=0.5778) in No-CRT versus CRT patients, respectively (Figure 1A, C). The 5-year OS (95% CI) was 96.2% (75.7-99.4) in No-CRT and 71.6% (49.4-85.4) (P=0.0423) in CRT patients (Online Supplementary Figure S1A). At univariate analysis, type of ALL consortium and age did not significantly affect EFS, while NCI criteria did, with NCI standard-risk patients showing a 5-year EFS of 74.8% (44.9-90.0) versus 50.7% (34.5-64.8) in high-risk patients (P=0.0408) (Online Supplementary Figure S1). When analyzed in a multivariable Cox regression model, adjusting by NCI criteria, CRT administration showed no significant impact on outcome: the hazard ratio (HR) of any event for CRT versus No-CRT was 1.44 (95% CI: 0.64-3.27, P=0.3821), while the HR for NCI high-risk versus standard-risk was 2.96 (95% CI: 1.01-8.64, P=0.0477). The description of impact of CRT on EFS, within NCI subgroups, confirms the overall finding of no difference according to treatment (Online Supplementary Figure S2). The 5-year weighted EFS (95% CI) and CIR (95% CI), based on the IPTW approach, were consistent with unadjusted estimates: 63.7% (40.1-80.1) versus 51.5% (32.1-67.9) (P=0.4376) and 35.8% (24.9-46.8) versus 44.8% (32.5-57.2) (P=0.5360) in No-CRT versus CRT patients, respectively (Figure 1B, D). The weighted 5-year OS (95% CI) was 96.9% (73.4-99.7) versus 75.5% (53.0-88.3), although this difference was not significant (P=0.0854) (Online Supplementary Figure S1B). Almost half of the patients who had an indication for CRT did not receive it. The outcome of patients who did or did not receive CRT was similar. Isolated CNS relapses occurred in only 2 out of 66 patients, both in the No-CRT group, which, however, did not contribute to a higher CIR at any sites, including isolated BM relapses. CNS3 disease at diagnosis occurred only in 4 patients and thus no conclusions can be drawn on this issue.
Table 1.
Baseline characteristics of 66 patients on chemotherapy at the end of Delayed Intensification I by cranial radiotherapy administration.
Table 2.
Outcome of 66 patients on chemotherapy at the end of Delayed Intensification I, by cranial radiotherapy administration.
Figure 1.
Kaplan-Meier estimates and cumulative incidence of relapse by cranial radiotherapy administration. Standard Kaplan-Meier estimates of event-free survival (EFS) (A), weighted Kaplan-Meier estimates of event-free survival (B), cumulative incidence of relapse (C), and weighted cumulative incidence of relapse (D) by cranial radiotherapy (CRT) administration. The initial plateau in the curves reflects the fact that all 66 patients included in the analysis were still in complete remission and on protocol chemotherapy at the planned time of CRT administration (i.e., the end of Delayed Intensification I, about seven months after diagnosis). The weighted estimates were obtained applying a causal approach based on the inverse probability of treatment weighting. CI: Confidence Interval; CIR: cumulative incidence of relapse; N: number.
The limitation of this study is in its retrospective and observational nature, as the definition of the 2 groups of patients receiving or not receiving CRT did not rely on randomization, but on lack of adherence to protocol. This complicated the assessment of treatment effect on outcome, as apparent differences could be due to systematic differences in baseline covariates. The IPTW method was applied to address this issue and led to weighted Kaplan-Meier estimates which confirmed no evidence of benefit for CRT in this context.
In summary, our data support the concept that CRT has no major role in the treatment of Ph+ ALL, which is in keeping with the evidence provided by the Ponte di Legno group on non-Ph+ ALL,13 although the possibility that there is a potential benefit for patients with CNS disease at diagnosis cannot be excluded. The administration of IT therapy and high-dose chemotherapy to replace CRT spares patients from neuro-cognitive late effects (particularly severe in younger patients) and second malignancies.14,15 The omission of CRT may be even more attractive in protocols which adopt dasatinib as TKI and may have additional relevance for patients who need a TBI-conditioning regimen for HCT in second-line treatment.
Supplementary Material
Acknowledgments
We thank Thai Hoa Tran for the productive discussions during the development of the joint EsPhALL/COG protocol for the treatment of ABL-class ALL (AALL2131/EsPhALL2022), which stimulated this work.
Funding Statement
Funding: The work was partially supported by the grant PRIN 2022SYXEHJ (principal investigator MGV). Projet Hospitalier de Recherche Clinique-Cancer and Novartis France (to VG).
Data-sharing statement
Questions regarding data sharing should be addressed to the corresponding author.
References
- 1.Schrappe M, Arico M, Harbott J, et al. Philadelphia chromosome-positive (Ph+) childhood acute lymphoblastic leukemia: good initial steroid response allows early prediction of a favorable treatment outcome. Blood. 1998;92(8):2730-2741. [PubMed] [Google Scholar]
- 2.Arico M, Valsecchi MG, Camitta B, et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med. 2000;342(14):998-1006. [DOI] [PubMed] [Google Scholar]
- 3.Arico M, Schrappe M, Hunger SP, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosomepositive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol. 2010;28(31):4755-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27(31):5175-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz KR, Carroll A, Heerema NA, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia. 2014;28(7):1467-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slayton WB, Schultz KR, Kairalla JA, et al. Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with Philadelphia chromosome-positive acute lymphoblastic leukemia: results of Children’s Oncology Group Trial AALL0622. J Clin Oncol. 2018;36(22):2306-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunger SP, Tran TH, Saha V, et al. Dasatinib with intensive chemotherapy in de novo paediatric Philadelphia chromosomepositive acute lymphoblastic leukaemia (CA180-372/COG AALL1122): a single-arm, multicentre, phase 2 trial. Lancet Haematol. 2023;10(7):e510-e520. [DOI] [PubMed] [Google Scholar]
- 8.Biondi A, Schrappe M, De Lorenzo P, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012;13(9):936-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biondi A, Cario G, De Lorenzo P, et al. Long-term follow up of pediatric Philadelphia positive acute lymphoblastic leukemia treated with the EsPhALL2004 study: high white blood cell count at diagnosis is the strongest prognostic factor. Haematologica. 2019;104(1):e13-e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biondi A, Gandemer V, De Lorenzo P, et al. Imatinib treatment of paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (EsPhALL2010): a prospective, intergroup, open-label, single-arm clinical trial. Lancet Haematol. 2018;5(12):e641-e652. [DOI] [PubMed] [Google Scholar]
- 11.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14(1):18-24. [DOI] [PubMed] [Google Scholar]
- 12.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24(20):3089-3110. [DOI] [PubMed] [Google Scholar]
- 13.Vora A, Andreano A, Pui CH, et al. Influence of cranial radiotherapy on outcome in children with acute lymphoblastic leukemia treated with contemporary therapy. J Clin Oncol. 2016;34(9):919-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halsey C, Buck G, Richards S, Vargha-Khadem F, Hill F, Gibson B. The impact of therapy for childhood acute lymphoblastic leukaemia on intelligence quotients; results of the risk-stratified randomized central nervous system treatment trial MRC UKALL XI. J Hematol Oncol. 2011;4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loning L, Zimmermann M, Reiter A, et al. Secondary neoplasms subsequent to Berlin-Frankfurt-Munster therapy of acute lymphoblastic leukemia in childhood: significantly lower risk without cranial radiotherapy. Blood. 2000;95(9):2770-2775. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Questions regarding data sharing should be addressed to the corresponding author.