Translocation (6;9)(p23;q34.1) is detected in 1% of acute myeloid leukemia (AML) cases and is considered a high-risk subtype by European Leukemia Network (ELN) 2022 criteria.1 Compared to AML overall, t(6;9) AML affects younger adults with a median age of 35-38 years at diagnosis, and is historically associated with poor overall survival (OS) of roughly 14 months.2,3 Most cases (62-88%) harbor internal tandem duplications of fms-like tyrosine kinase 3 (FLT3-ITD).2-6 While preliminary data suggest that FLT3 inhibitors (FLT3i) might provide benefit,7,8 this has not yet been confirmed in larger studies. Although outcomes have improved with allogeneic hematopoietic cell transplantation (alloHCT),6,9,10 survival remains dismal for patients ineligible for transplant.3 This report describes a three-site experience with t(6;9) AML at an academic tertiary care center with a large HCT referral program.
After institutional review board approval, all patients with t(6;9)(p23;q34.1) AML seen across the three Mayo Clinic sites in the US between 2010-2023 were identified via retrospective chart review; this included eight previously-published patients.11 A separate AML cohort excluding t(6;9) was curated pragmatically over a similar time frame. Data from the time of diagnosis or presentation to Mayo Clinic were abstracted. Mutation assessment was performed as described.12 When necessary, FLT3 allelic ratio was estimated from the variant allele fraction (VAF).12,13 Classification and response were assessed by ELN 2022 criteria.1 Measurable residual disease (MRD) was assessed by t(6;9)(p23;q34.1) specific fluorescence in situ hybridization (FISH) of ≥500 nuclei (sensitivity 0.6%) or multiparametric flow cytometry (MFC; sensitivity 0.01%). Categorical variables were compared by Fisher exact or Pearson X2 tests and continuous variables by Mann-Whitney U tests or two-way ANOVA with Tukey correction. Univariate and multivariate analyses utilized Cox proportional hazards models. Survival was assessed via the Kaplan-Meier method with log-rank comparisons. Calculations were performed with BlueSky Statistics (v10.3.1) or GraphPad Prism (v.10.1.2). P<0.05 was considered significant.
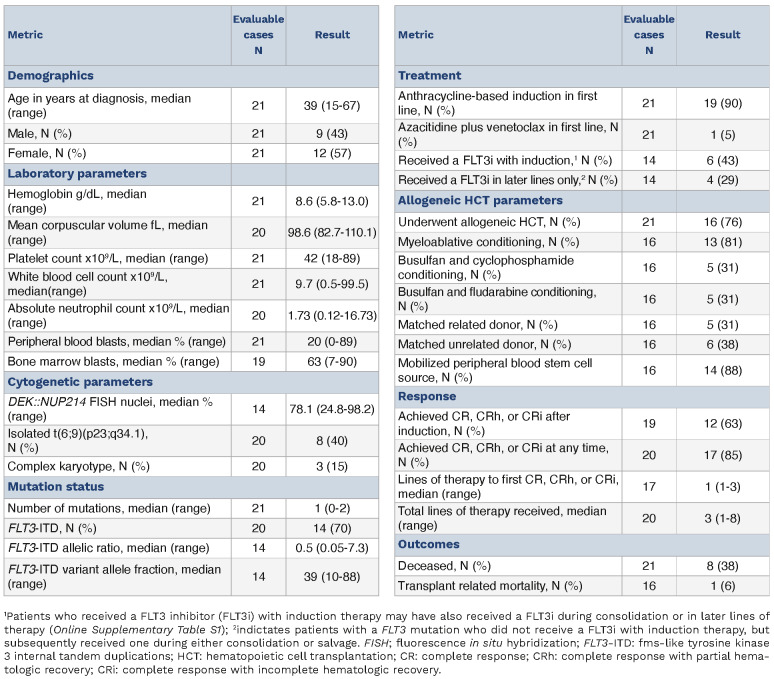
Twenty-one patients (12 females, 57%) with t(6;9) AML were identified with median age 39 years (Table 1). Anemia was common (N=19, 90%) and thrombocytopenia was universal. Peripheral blasts were identified in 19 (90%) patients. Cytogenetic analyses identified isolated t(6;9)(p23;q34.1) in eight (40%) cases, whereas three (15%) exhibited a complex karyotype. Most cases (N=14, 70%) harbored FLT3-ITD mutations (median VAF 39%), which were the sole mutations identified in all 14 cases that harbored them (Online Supplementary Figure S1A, B). No FLT3 tyrosine kinase domain mutations were detected.
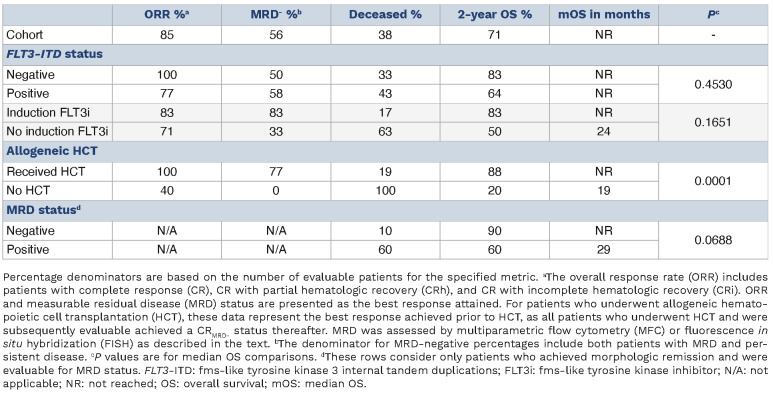
Most (N=19, 90%) received frontline induction therapy with cytarabine plus an anthracycline (Online Supplementary Table S1). Six of 14 (43%) patients harboring FLT3-ITD received a FLT3i during induction. Four (29%) additional patients received a FLT3i with consolidation or salvage therapy. Following induction, 12 of 19 (63%) evaluable patients achieved a compete response (CR) or CR with either partial (CRh) or incomplete (CRi) hematologic recovery. An additional five patients achieved CR/CRh/CRi after salvage or consolidative chemotherapy (Table 1). All FLT3 wild-type (WT) cases eventually achieved a CR/CRh/CRi for an overall response rate (ORR) of 100%. Amongst FLT3-ITD cases, the ORR was 77%. Sixteen patients (76%) proceeded to alloHCT.
The role of MRD assessment in adverse-risk AML is not well defined.14 Because methodology has evolved over time, the present analysis considered MRD based on mean fluorescense intensity (MFC) (N=4) or FISH (N=11) testing within 60 days of induction, prior to transplant, or at post-transplant day +100 (Online Supplementary Figure S1C). After induction, only two of 11 (18%) patients achieving morphologic CR had undetectable MRD. Ten of 11 (91%) evaluable patients were MRD-negative prior to alloHCT. All evaluable patients were MRD-negative at post-transplant day +100. Of the four patients with MRD testing strictly by MFC, all were MRD-negative prior to HCT, and the three evaluable cases remained MRD-negative on day +100 (Online Supplementary Figure S1D). When both MFC and FISH were performed, all results were concordant.
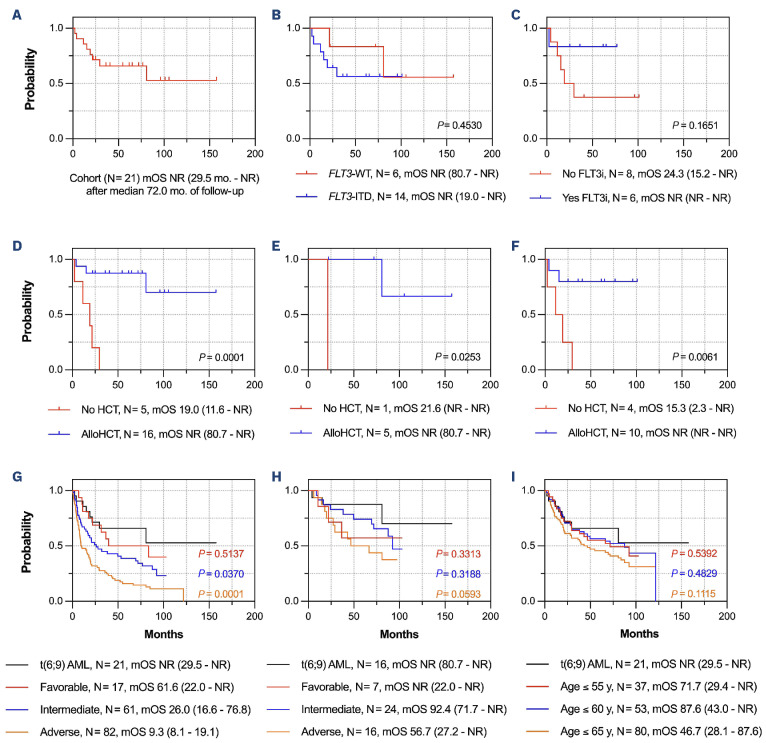
Eight patients (38%) were deceased at last follow-up. With median follow-up of 72 months, the 2-year OS of the cohort was 71% while the median OS (mOS) was not reached (NR; Figure 1A).
The mOS was NR irrespective of FLT3 status (P=0.45; Figure 1B). Amongst FLT3-ITD cases, OS was numerically longer (NR vs. 24 months, P=0.17; Figure 1C) and there were fewer deaths (1 vs. 5) amongst those who received a FLT3i during induction (Table 2). Accordingly, the 2-year OS was numerically higher for those receiving a FLT3i with induction (83% vs. 50%). Although statistically insignificant, these trends again suggest that FLT3i benefit a subgroup of patients.
AlloHCT significantly prolonged survival compared to those who were not transplanted (mOS NR vs. 19 months; P=0.0001; Figure 1D). Two-year survival rates were also superior with alloHCT (88% vs. 20%; Table 2). Moreover, alloHCT was beneficial irrespective of FLT3 status (P<0.03 for both comparisons; Figure 1E, F). There was no difference in survival when patients were stratified by myeloablative versus non-myeloablative conditioning intensity (P=0.55) or conditioning regimen (P=0.40). Transplant-related mortality (TRM) was 6%, as one patient expired on post-HCT day +32 from sinusoidal obstruction syndrome.
Analogous results were obtained when patients with complex cytogenetics (N=3) were excluded, with mOS of 81 months and 2-year OS of 65% for the remaining patients. There was again no difference in mOS when stratified by FLT3 status (P=0.21). AlloHCT similarly improved mOS (NR vs. 19 months; P=0.0014), with 2-year OS of 83% versus 20% for those who did and did not undergo HCT, respectively.
There was no difference in OS based on MRD status after induction (P=0.64) or prior to alloHCT (P=0.75; Online Supplementary Figure S1E, F). When stratified by MRD-negativity at any time prior to HCT (if transplanted), there was a trend toward improved survival compared to MRD-positive patients (mOS NR vs. 29 months; P=0.069; Online Supplementary Figure S1G). Accordingly, the 2-year survival was higher (90% vs. 60%) with fewer deaths (1 vs. 3) amongst MRD-negative patients compared to positive patients (Table 2). However, these analyses are confounded, as all patients who achieved MRD negativity proceeded to alloHCT. Amongst the four patients with MRD testing by MFC, the mOS was NR with 2-year OS of 75% (Online Supplementary Figure S1H).
Survival of the t(6;9) AML cohort was better than anticipated; therefore, these outcomes were compared to those from a separate non-t(6;9) AML cohort (N=160; Online Supplementary Table S2). Patients were classified as ELN favorable (N=17, 11%), intermediate (N=61, 38%), or adverse (N=82, 51%) risk.1 At last follow-up, 47 (29%) patients had undergone alloHCT and 114 (71%) had died. With median follow-up of 87 months, the mOS was 19 months (95% confidence interval [CI]: 15-28) with 2-year OS of 44%. Across the three ELN risk categories, the mOS was 62, 26, and 9 months with 2-year OS of 69%, 53%, and 31%, respectively (Figure 1G). AlloHCT provided a significant survival benefit in the intermediate- and adverse-risk groups (each P<0.0001) but not in the favorable-risk group (P=0.39), as expected for favorable-risk disease.15
Figure 1.
Overall survival in t(6;9)(DEK::NUP214) acute myeloid leukemia and compared to European Leukemia Network risk groups. (A) The median overall survival (mOS) of the entire cohort. (B-F) mOS of fms-like tyrosine kinase 3 (FLT3) wild-type versus FLT3 internal tandem duplications (FLT3-ITD) cases (B); FLT3-ITD-positive cases receiving a FLT3 inhibitor (FLT3i) versus those that did not (C); t(6;9) acute myeloid leukemia (AML) patients undergoing allogeneic hematopoietic cell transplantation (alloHCT) versus those who did not (D); cases with wild-type FLT3 who underwent allogeneic hematopoietic cell transplantation (alloHCT) versus those who did not (E); and those with a FLT3-ITD mutation who underwent alloHCT compared to those who did not (F). (G) Comparison of OS in the t(6;9) AML cohort versus the ELN favorable, intermediate, and adverse-risk groups. (H) Comparison of OS in the t(6;9) AML subset who underwent alloHCT versus the European Leukemia Network (ELN) favorable, intermediate, and adverse-risk groups who also underwent alloHCT. (I) Comparison of OS in the t(6;9) AML cohort versus age-restricted subgroups of the comparison cohort; the median (range) ages of the 3 subgroups are 38 (18-55), 50 (18-60), and 56 (18-65) years (y), respectively. In (G-I), P values depict the comparison between the t(6;9) AML cohort and the color-matched subgroup of the comparison cohort. All survival times are denoted as median (95% confidence interval) in months (mo).
Table 1.
Patient demographics.
Table 2.
Treatment responses and survival outcomes.
Surprisingly, patients with t(6;9) AML fared better than the ELN adverse-risk comparison group (Figure 1G). Rather, the mOS of t(6;9) patients approximated that of the favorable-risk group (NR vs. 62 months; P=0.51) with 2-year OS of 71% versus 69%, respectively. However, patients with t(6;9) AML benefitted from alloHCT whereas those with favorable-risk disease in the comparison cohort did not. Indeed, t(6;9) AML patients who received alloHCT fared similarly to transplanted intermediate-risk patients, with 2-year OS of 88% and 87%, respectively (P=0.32; Figure 1H). Survival was also similar between t(6;9) and non-t(6;9) patients of comparable age (Figure 1I). These data raise the question as to whether t(6;9) AML should be reclassified as intermediate-risk, particularly for those treated with FLT3i and alloHCT. Notably, the 2022 ELN classification schema now categorizes AML with FLT3-ITD (without adverse-risk genetic lesions) in the intermediate-risk group.1
Attempts were made to identify parameters associated with OS. Univariate Cox regression identified MCV (P=0.045) and the peripheral blood blast percentage (P=0.011) as adverse prognostic factors while receipt of alloHCT was beneficial (P=0.0024). Neither FLT3 status, induction FLT3i use, nor best MRD status correlated with OS. In the multivariate model of significant factors, only alloHCT retained significance (hazard ratio=0.11, 95% CI: 0.02-0.68; P=0.017).
Due to the rarity of t(6;9) AML, this study was underpowered to determine whether FLT3i or MRD negativity truly improve survival. Moreover, because this dataset spans an era of evolving MRD assessment standards and few patients had MRD testing by contemporary methods, larger combined analyses are needed to establish the role of frontline FLT3i and MRD monitoring in this setting. Furthermore, the mOS of this cohort (NR) is longer than previously reported (14-27 months),2,3,6 likely signifying transplant referral bias, as alloHCT improves outcomes in t(6;9) AML.6,9-11 Within these limitations, alloHCT significantly improved mOS in this cohort irrespective of F LT 3 or MRD status, and multivariate analysis identified alloHCT as the only prognostic factor.
In conclusion, t(6;9) AML has poor OS in the absence of alloHCT, and all eligible patients should be considered for transplant in first remission irrespective of MRD status. Although a definitive benefit has yet to be proven, FLT3i are an enticing avenue to improve response rates in FLT3-ITD-positive cases. Collectively, these interventions may provide sufficient benefit to reclassify t(6;9) AML as an intermediate-risk disease and need to be validated in larger studies.
Supplementary Material
Funding Statement
Funding: This work was funded in part by the Mayo Foundation for Education and Research.
Data-sharing statement
Original data will be provided to collaborating investigators upon reasonable request to the corresponding authors after requisite institution review board approval.
References
- 1.Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 ELN recommendations from an international expert panel. Blood. 2022;140(12):1345-1377.. [DOI] [PubMed] [Google Scholar]
- 2.Slovak ML, Gundacker H, Bloomfield CD, et al. A retrospective study of 69 patients with t(6;9)(p23;q34) AML emphasizes the need for a prospective, multicenter initiative for rare ‘poor prognosis’ myeloid malignancies. Leukemia. 2006;20(7):1295-1297. [DOI] [PubMed] [Google Scholar]
- 3.Fang H, Yabe M, Zhang X, et al. Myelodysplastic syndrome with t(6;9)(p22;q34.1)/DEK-NUP214 better classified as acute myeloid leukemia? A multicenter study of 107 cases. Mod Pathol. 2021;34(6):1143-1152. [DOI] [PubMed] [Google Scholar]
- 4.Oyarzo MP, Lin P, Glassman A, Bueso-Ramos CE, Luthra R, Medeiros LJ. Acute myeloid leukemia with t(6;9)(p23;q34) is associated with dysplasia and a high frequency of flt3 gene mutations. Am J Clin Pathol. 2004;122(3):348-358. [DOI] [PubMed] [Google Scholar]
- 5.Visconte V, Shetty S, Przychodzen B, et al. Clinicopathologic and molecular characterization of myeloid neoplasms with isolated t(6;9)(p23;q34). Int J Lab Hematol. 2017;39(4):409-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kayser S, Hills RK, Luskin MR, et al. Allogeneic hematopoietic cell transplantation improves outcome of adults with t(6;9) acute myeloid leukemia: results from an international collaborative study. Haematologica. 2020;105(1):161-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong F, Kadia TM, Short NJ, et al. PB1831: utility of FLT3 inhibitors in patients with acute myeloid leukemia (AML) and t(6;9)(p22;q34). Hemasphere. 2022;6:1711-1712. [Google Scholar]
- 8.Day JW, Fox TA, Gupta R, Khwaja A, Wilson AJ, Kottaridis PD. Gilteritinib monotherapy as a transplant bridging option for high risk FLT3-mutated AML with t(6;9)(p23;q34.1);DEK-NUP214 in morphological but not cytogenetic or molecular remission following standard induction chemotherapy. Leuk Res Rep. 2022;17:100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishiyama K, Takami A, Kanda Y, et al. Allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with t(6;9) (p23;q34) dramatically improves the patient prognosis: a matched-pair analysis. Leukemia. 2012;26(3):461-464. [DOI] [PubMed] [Google Scholar]
- 10.Díaz-Beyá M, Labopin M, Maertens J, et al. Allogeneic stem cell transplantation in AML with t(6;9)(p23;q34);DEK-NUP214 shows a favourable outcome when performed in first complete remission. Br J Haematol. 2020;189(5):920-925. [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A, Singh A, Gangat N, et al. Adverse karyotype subcategories in acute myeloid leukemia display significant differences in mutation composition and transplant-augmented survival. Haematologica. 2023;108(1):245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He R, Devine DJ, Tu ZJ, et al. Hybridization capture-based next generation sequencing reliably detects FLT3 mutations and classifies FLT3-internal tandem duplication allelic ratio in acute myeloid leukemia: a comparative study to standard fragment analysis. Mod Pathol. 2020;33(3):334-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tung JK, Suarez CJ, Chiang T, Zehnder JL, Stehr H. Accurate detection and quantification of FLT3 internal tandem duplications in clinical hybrid capture next-generation sequencing data. J Mol Diagn. 2021;23(10):1404-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heuser M, Freeman SD, Ossenkoppele GJ, et al. 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2021;138(26):2753-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data will be provided to collaborating investigators upon reasonable request to the corresponding authors after requisite institution review board approval.