Abstract
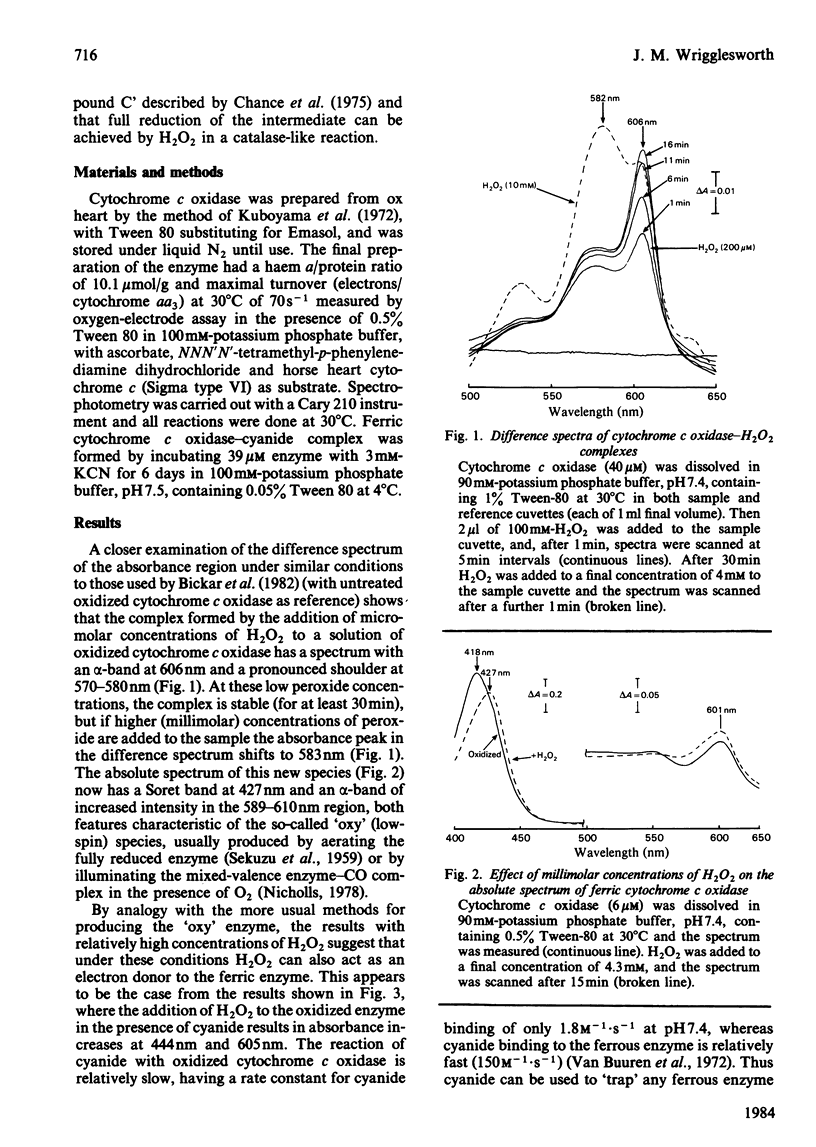
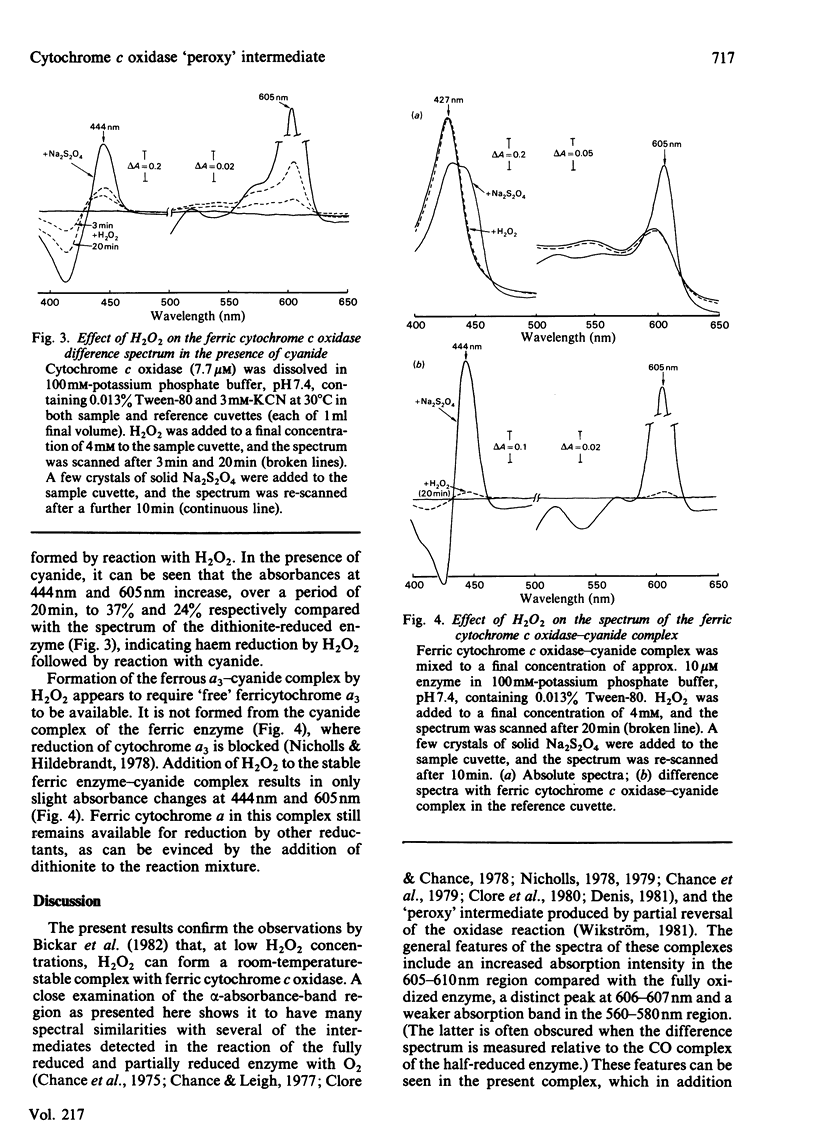
In the presence of micromolar concentrations of H2O2, ferric cytochrome c oxidase forms a stable complex characterized by an increased absorption intensity at 606-607 nm with a weaker absorption band in the 560-580 nm region. Higher (millimolar) concentrations of H2O2 result in an enzyme exhibiting a Soret band at 427 nm and an alpha-band of increased intensity in the 589-610 nm region. Addition of H2O2 to ferric cytochrome c oxidase in the presence of cyanide results in absorbance increases at 444nm and 605nm. These changes are not seen if H2O2 is added to the cyanide complex of the ferric enzyme. The results support the idea that direct reaction of H2O2 with ferric cytochrome a 3 produces a 'peroxy' intermediate that is susceptible to further reduction by H2O2 at higher peroxide concentrations. Electron flow through cytochrome a is not involved, and the final product of the reaction is the so-called 'pulsed' or 'oxygenated' ferric form of the enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini E., Brunori M., Colosimo A., Greenwood C., Wilson M. T. Oxygen "pulsed" cytochrome c oxidase: functional properties and catalytic relevance. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3128–3132. doi: 10.1073/pnas.74.8.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernofsky C., Wanda S. Y. Reduction of cytochrome c by hydrogen peroxide and its inhibition by superoxide dismutase. Biochem Biophys Res Commun. 1983 Feb 28;111(1):231–238. doi: 10.1016/s0006-291x(83)80141-7. [DOI] [PubMed] [Google Scholar]
- Bickar D., Bonaventura J., Bonaventura C. Cytochrome c oxidase binding of hydrogen peroxide. Biochemistry. 1982 May 25;21(11):2661–2666. doi: 10.1021/bi00540a013. [DOI] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr Oxygen intermediates and mixed valence states of cytochrome oxidase: infrared absorption difference spectra of compounds A, B, and C of cytochrome oxidase and oxygen. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4777–4780. doi: 10.1073/pnas.74.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Compound C2, a product of the reaction of oxygen and the mixed-valence state of cytochrome oxidase. Optical evidence for a type-I copper. Biochem J. 1979 Mar 1;177(3):931–941. doi: 10.1042/bj1770931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- Clore G. M., Andréasson L. E., Karlsson B., Aasa R., Malmström B. G. Characterization of the intermediates in the reaction of mixed-valence state soluble cytochrome oxidase with oxygen at low temperatures by optical and electron-paramagnetic-resonance spectroscopy. Biochem J. 1980 Jan 1;185(1):155–167. doi: 10.1042/bj1850155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G. M., Chance E. M. The mechanism of reaction of fully reduced membrane-bound cytochrome oxidase with oxygen at 176K. Biochem J. 1978 Sep 1;173(3):799–810. doi: 10.1042/bj1730799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Resolution of two compound C-type intermediates in the reaction with oxygen of mixed-valence state membrane-bound cytochrome oxidase. Biochim Biophys Acta. 1981 Jan 14;634(1):30–40. doi: 10.1016/0005-2728(81)90125-0. [DOI] [PubMed] [Google Scholar]
- Denis M. The involvement of the fully oxidized state in cytochrome oxidase reaction with oxygen studied with the 655 and nm band as a probe. FEBS Lett. 1977 Dec 15;84(2):296–298. doi: 10.1016/0014-5793(77)80710-2. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F. Cytochrome c oxidase: a synopsis. Arch Biochem Biophys. 1978 May;188(1):1–14. doi: 10.1016/0003-9861(78)90348-x. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. THE REACTION OF CYTOCHROME OXIDASE WITH CYTOCHROME C. J Biol Chem. 1965 Feb;240:888–894. [PubMed] [Google Scholar]
- Greenwood C., Wilson M. T., Brunori M. Studies on partially reduced mammalian cytochrome oxidase. Reactions with carbon monoxide and oxygen. Biochem J. 1974 Feb;137(2):205–215. doi: 10.1042/bj1370205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblatt J. A., Kells D. I., Williams G. R. The conformational states of oxidized and oxygenated beef-heart cytochrome c oxidase. Can J Biochem. 1975 Apr;53(4):461–466. doi: 10.1139/o75-063. [DOI] [PubMed] [Google Scholar]
- Krasna A. I. Nonparticipation of hydrogen peroxide in the cytochrome oxidase reaction. Arch Biochem Biophys. 1965 Oct;112(1):209–211. doi: 10.1016/0003-9861(65)90033-0. [DOI] [PubMed] [Google Scholar]
- Kuboyama M., Yong F. C., King T. E. Studies on cytochrome oxidase. 8. Preparation and some properties of cardiac cytochrome oxidase. J Biol Chem. 1972 Oct 25;247(20):6375–6383. [PubMed] [Google Scholar]
- Nicholls P. A new carbon monoxide-induced complex of cytochrome c oxidase. Biochem J. 1978 Dec 1;175(3):1147–1150. doi: 10.1042/bj1751147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls P., Chanady G. A. Interactions of cytochrome aa3 with oxygen and carbon monoxide. The role of the 607 nm complex. Biochim Biophys Acta. 1981 Feb 12;634(2):256–265. doi: 10.1016/0005-2728(81)90144-4. [DOI] [PubMed] [Google Scholar]
- Nicholls P. Effects of inhibitory ligands on the aerobic carbon monoxide complex of cytochrome c oxidase. Biochem J. 1979 Dec 1;183(3):519–529. doi: 10.1042/bj1830519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls P., Hildebrandt V. Binding of ligands and spectral shifts in cytochrome c oxidase. Biochem J. 1978 Jul 1;173(1):65–72. doi: 10.1042/bj1730065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORII Y., OKUNUKI K. STUDIES ON CYTOCHROME A. X. EFFECT OF HYDROGEN PEROXIDE ON ABSORPTION SPECTRA OF CYTOCHROME A. J Biochem. 1963 Sep;54:207–213. doi: 10.1093/oxfordjournals.jbchem.a127773. [DOI] [PubMed] [Google Scholar]
- Orii Y. The cytochrome c peroxidase activity of cytochrome oxidase. J Biol Chem. 1982 Aug 25;257(16):9246–9248. [PubMed] [Google Scholar]
- Reed C. A., Landrum J. T. Structural models for the heme a3/copper active site of cytochrome c oxidase. FEBS Lett. 1979 Oct 15;106(2):265–267. doi: 10.1016/0014-5793(79)80510-4. [DOI] [PubMed] [Google Scholar]
- Wikström M. Energy-dependent reversal of the cytochrome oxidase reaction. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4051–4054. doi: 10.1073/pnas.78.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. T., Peterson J., Antonini E., Brunori M., Colosimo A., Wyman J. A plausible two-state model for cytochrome c oxidase. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7115–7118. doi: 10.1073/pnas.78.11.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren K. J., Nicholis P., van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . VI. Reaction of cyanide with oxidized and reduced enzyme. Biochim Biophys Acta. 1972 Feb 28;256(2):258–276. doi: 10.1016/0005-2728(72)90057-6. [DOI] [PubMed] [Google Scholar]


