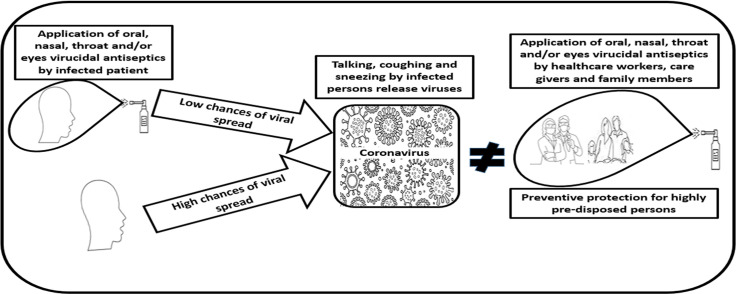
Highlights
-
•
COVID-19 has killed millions of people all over the globe.
-
•
Vaccines and drugs are beneficial but have not stopped the spread of SARS-CoV-2.
-
•
A third force to help in the prevention of coronavirus diseases is essential.
-
•
Some antiseptic oral and nasal wash/spray are effective in reducing viral load.
-
•
This will help reduce/halt the spread of SARS-CoV-2 and other respiratory diseases.
Keywords: COVID-19, Povidone iodine, SARS CoV-2, Coronavirus, Mouthwash/rinse, Healthcare workers
Abstract
Notably, severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and coronavirus disease 2019 (COVID-19) have all had significant negative impact on global health and economy. COVID-19 alone, has resulted to millions of deaths with new cases and mortality still being reported in its various waves. The development and use of vaccines have not stopped the transmission of SARS coronavirus 2 (SARS-CoV-2), the etiological agent of COVID-19, even among vaccinated individuals. The use of vaccines and curative drugs should be supplemented with adoption of simple hygiene preventive measures in the fight against the spread of the virus, especially for healthcare workers. Several virucidal topical antiseptics, such as povidone-iodine (PVP-I), citrox, cyclodextrins among others, have been demonstrated to be efficacious in the inactivation of SARS-CoV-2 and other coronaviruses in both in vitro and in vivo studies. The strategic application of these virucidal formulations could provide the additional impetus needed to effectively control the spread of the virus. We have here presented a simple dimension towards curtailing the dissemination of COVID-19, and other coronaviruses, through the application of effective oral, nasal and eye antiseptics among patients and medical personnel. We have further discussed the mechanism of action of some of these commonly available virucidal solutions while also highlighting some essential controversies in their use.
Graphical abstract
1. Introduction
The coronaviruses are the etiological agents responsible for deadly diseases in several birds and mammals (Fehr and Perlman, 2015; Alluwaimi et al., 2020; Wang et al., 2020). In humans, these viruses are usually disseminated through airborne droplets and fluid from infected persons (Wang et al., 2020; Jayaweera et al., 2020; Shereen et al., 2020). The virus got its first detailed description in the 1960s and its name was derived from the distinctive corona (i.e. crown) shape of the projecting glycosylated club-shaped spikes from the surrounding envelope of the particle (Li, 2016; Nova, 2021). Coronaviruses belong to the Orthocoronavirinae subfamily, in Coronaviridae family, Nidovirales order and realm Riboviria (Alluwaimi et al., 2020; Wang et al., 2020; Nova, 2021).
Four out of the 7 coronaviruses which are known to infect humans, do spread with seasonal regularity, resulting to symptoms similar to mild cold with flu-like discomforts. Since the onset of the 21st century, 3 of these coronaviruses have been established to cross species barrier leading to very serious pneumonia in infected persons (Carod-Artal, 2020; Dhama et al., 2020; Walls et al., 2020). The notable strains are SARS-CoV-2, the etiological agent of COVID-19, MERS-CoV and SARS-CoV which cause the Middle East respiratory syndrome (MERS) and the severe acute respiratory syndrome (SARS), respectively (Dhama et al., 2020; Swelum et al., 2020). The reported causes of death are complex, though it is mainly due to heightened immune response which leads to damages in several organ systems all over the body (Walls et al., 2020).
SARS was originally identified as a distinct strain of the coronavirus in 2003. Although the origin of the virus is not quite clear, the first reported human incident was however in 2002 in China's Guangdong province. The virus eventually became a pandemic, leading to 8098 infections in 26 countries in over five continents with 774 deaths (Walls et al., 2020; Cherry and Krogstad, 2004; Muller and McGeer, 2007). MERS was identified in 2012 among persons showing symptoms of fever, cough, shortness of breath and occasional gastrointestinal problems like diarrhoea in Saudi Arabia. Official animal hosts for the virus have not been confirmed yet, but evidence suggests dromedary camels as the potential reservoir. MERS-CoV disseminated to about 27 countries, infecting over 2494 people with 858 reported deaths (Zumla et al., 2015; de Wit et al., 2016; Chafekar and Fielding, 2018).
The new coronavirus, SARS-CoV-2, is responsible for the deadly COVID-19 disease. This virus was previously unknown before its discovery in December 2019 in the city of Wuhan in Hubei province of China. The virus was isolated and sequenced by January 2020 (Zhu et al., 2019; Safari et al., 2020). The virus was declared an epidemic of public health emergency, and as a pandemic on 30th of January and 11th of March 2020, respectively, by the World Health Organization (Cucinotta and Vanelli, 2020; WHO 2021). According to the WHO, as at June 2, 2024, there had been 775,583,309 confirmed cases of COVID-19, out of which 7,050,691 deaths had been reported to WHO (World Health Organization Data 2024). Morbidity and mortality attributable to SARS-CoV-2 has exceeded that reported for both MERS-CoV and SARS CoV.
In the current therapeutic approach for handling coronavirus respiratory diseases, most of the recent updates have been on the use of vaccines, drugs, antibodies, immune-suppressants, angiotensin-converting enzyme (ACE) inhibitors and blockers (South et al., 2020; Chung et al., 2021; Ita, 2021; Majumder and Minko, 2021; Tarighi et al., 2021). Among all these, the use of vaccination is the most widely adopted. It is generally agreed that vaccines and vaccination hold the best means of ending the COVID-19 pandemic (Keegan et al., 2021; Viana et al., 2021; Nnaemeka et al., 2023). Nevertheless, vaccination has a number of limitations, prominent among them being vaccine escape variants of the virus that can be transmitted to both vaccinated and non-vaccinated individuals (Riemersma et al., 2022; Singanayaman et al., 2022). Hence, there is the need to consider other means of controlling the viral spread to compliment the use of vaccination. There has been very little reference on the use of mouthwash or gargles, throat, nose and eye sprays in the prevention of infection establishment or in the hindrance of progression of the disease, particularly since its first port of call before entering the lungs or digestive tract is the throat.
When infectious diseases spread through aerosol or by direct contact, respiratory pathogens attach to and then colonize the mucus of the oropharynx, which leads to the development of upper respiratory tract infections (URTI) (Murphy et al., 2009; Bosch et al., 2013; Rosas-Salazar et al., 2021). Considering the infection complexity and/or disease progression, it is pertinent to contemplate “how a prophylactic mouth/throat rinse and nose/eye spray with virucidal activities may hinder the progression of infection and establishment of disease” (Chopra et al., 2021; Pattanshetty et al., 2021; Gandhi et al., 2021). Both oral and oropharyngeal cavities are habitats for a wide variety of microbes with pathogenic potentials. This therefore should necessitate evaluation of antiseptic compounds with broad spectrum antimicrobial activity for inclusion in oral/nasal formulations to ensure oropharyngeal coverage (Chopra et al., 2021; Kanagalingam et al., 2015; Vieira Colombo et al., 2016; Kumpitsch et al., 2019).
Reports have shown that the viral load in human's oropharyngeal mucosa is high in both symptomatic and asymptomatic patients during SARS-CoV-2 infection (Zou et al., 2020). There is, therefore, the need to minimize this viral load in the oropharynx adopting adequate prophylactic measures (Chopra et al., 2021; Gandhi et al., 2021). For this purpose, considering the use of prophylactic mouthwash with anti-viral activity to remove/lower SARS-CoV-2 loads in the oropharyngeal mucosa is vital. Since SARS-CoV-2 is quite susceptible to oxidation (Suhail et al., 2020; Forcados et al., 2021; Tu et al., 2021), the use of 0.5% H2O2 or 0.2% povidone-iodine (PVP-I) as mouthwash/gargle may help minimize the risk of transmission of SARS-CoV-2 (Pattanshetty et al., 2021).
2. Applications of mouthwash, throat, nose and eye sprays in pandemics involving respiratory diseases
2.1. Povidone-Iodine (PVP-I)
PVP-I is made-up of iodine and polyvinylpyrrolidone, a water-soluble polymer, which is also known as polyvidone or povidone. It displays antimicrobial activity as it dissociates to release iodine. It is this iodine that penetrates the target microbe, oxidizing nucleic acids and disrupting proteins by attacking the sulfuryl and disulphide bonds of the protein (Zhao et al., 2016; Kurakula and Rao, 2020; Carrouel et al., 2021; Ghafoor et al., 2021). Thus, PVP-I kills microorganisms through the perturbation metabolic pathways and disorganization of microbial cell membrane (Carrouel et al., 2021). There are two potent antiseptic components of PVP-I, viz: hypoiodous acid (HIO) and molecular iodine (I2). Both deliver the potent free iodine which can oxidize the cell membrane and amino acids of pathogens. Through cellular receptors oxidation, PVP-I inhibits attachment of viral pathogens to these receptors (Kanagalingam et al., 2015; Sriwilaijaroen et al., 2009).
For over six decades, PVP-I has been used as an effective topical antiseptic agent. It is attributed to having the broadest antimicrobial spectrum when compared to some other antiseptics such as polyhexanide, chlorhexidine, hexetidine and octenidine (Selvaggi et al., 2003; Lachapelle et al., 2013). It has activity against bacteria and bacterial spores, protozoa, fungi and some viruses (Lachapelle et al., 2013). The persistent effect of PVP-I was demonstrated in a research that used 1% PVP-I as a preprocedural antimicrobial agent in persons with different levels of oral hygiene. Reduction in microbial load was reported to be maintained for minimum of four hours (Domingo et al., 1996).
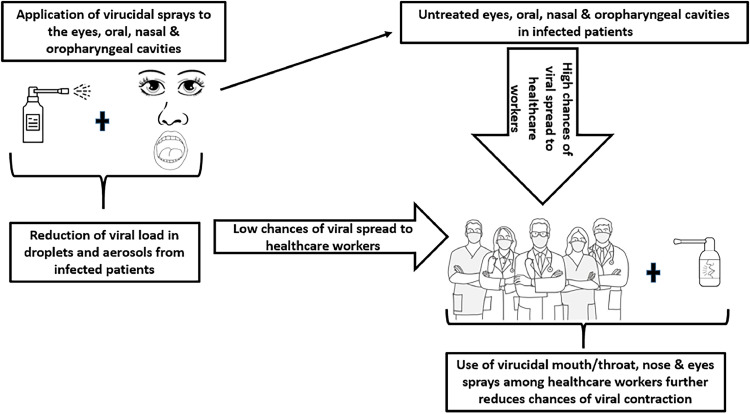
Several works have shown the efficacy of using different PVP-I antiseptics in deactivating SARS-CoV-2, SARS-CoV and MERS-CoV in in vitro assays both singly and in combination with other antiseptics (Table 1). The action of PVP-I oral/nasal rinses and sprays on SARS-CoV-2 could largely be due to the susceptibility of the virus to oxidation (Pattanshetty et al., 2021; Forcados et al., 2021; Tu et al., 2021). The application of these PVP-I products as topical oral/nasal antiseptic solutions has been reported to be safe (Chopra et al., 2021; Frank et al., 2020; Arefin, 2021). Therefore, in cases of COVID-19 outbreak, applications of PVP-I products have been proposed for use in mouthwash/gargle, nasal spray and in skin cleanser for both patients and healthcare workers (HCWs), as well as in disinfectants used for intubation, bronchoscopy and endoscopy instruments [(Arefin, 2021; Malaysian Health Technology Assessment Section (MaHTAS) 2020; Naqvi et al., 2020)]. The use of oral and intra-nasal PVP-I applications by infected persons and their attendant HCWs during this COVID-19 pandemic has been advocated to help reduce the viral load and transmission through aerosols and droplets (Cascella et al., 2022) (Fig. 1).
Table 1.
The efficacy of topical antiseptics against coronaviruses.
| Virucidal agent/Active ingredients | Tested concentrations | Corona Virus tested | Treatment time | Effect/Activity | Reference |
|---|---|---|---|---|---|
| PVP-I products such as: Isodine Nodo Fresh, Isodine Palm, Isodine Gargle, Isodine and Isodine Scrub1 | 0.23, 0.25, 0.47,1 and 1%1 | SARS-CoV | 2 mins | Infectivity of virus was reduced below detectable level from 1.17 × 106 TCID50/mL by all the PVP-I products | (Kariwa et al., 2006) |
| PVP-I mouthwash/gargle | 0.23% | SARS-CoV and MERS-CoV | 15 s | Viruses were inactivated [titre value reduction of ≥ 4 log10 (99.99%) compared with control] in clean and dirty conditions | (Eggers et al., 2018) |
| PVP-I products: PVP-I mouthwash/gargle, skin cleanser and surgical scrub1 | 1, 4 and 7.5%1 | MERS-CoV | 15 s | Reduction of MERS-CoV titre value by ≥ 4 log10 (99.99%) in both clean and dirty conditions by all the PVP-I products | (Eggers et al., 2015a) |
| PVP-I products: throat spray, gargle/mouth wash, skin cleanser and antiseptic solution1 | 0.45, 1, 7.5 and 10% | SARS-CoV-2 | 30 s | Reduction of SARS-CoV-2 titre value by ≥ 4 log10 (99.99%), compared with control, by all the PVP-I products. Furthermore, 1:2 dilution of the mouth wash also lowered SARS-CoV-2 titre value by ≥ 4 log10 | (Anderson et al., 2020) |
| PVP-I oral rinses and hydrogen peroxide | PVP-I: 1, 2.5 and 3%; H2O2: 3 and 6% | SARS-CoV-2 | 15 and 30 s | SARS-CoV-2 was completely inactivated (i.e. < 0.67 log10 CCID50/0.1 mL) by all the tested concentrations of PVP-I compared to control. While H2O2 was only minimally effective against SARS-CoV-2 (i.e. ≤ 3.67 and ≤ 3.33 log10 CCID50/0.1 mL for 3 %, then ≤ 4.0 and ≤ 2.5 log10 CCID50/0.1 mL for 6%) after 15 s and 30 s, respectively | (Bidra et al., 2020) |
| PVP-I nasal antiseptics | 0.5, 1.25, and 2.5 % | SARS-CoV-2 | 15 and 30 s | SARS-CoV-2 was completely inactivated by all the tested concentrations of PVP-I (by ˃ 3 log10 of 50 % cell culture infectious dose) within 15 s | (Frank et al., 2020) |
| PVP-I mouthwash and gargle | 0.5 and 1% | SARS-CoV-2 | 15, 30 and 60 s | Greater than 5 log10 reduction of SARS-CoV-2 titre value by 1 % PVP-1 at ≥15 s treatment exposure under dirty and clean conditions. While the 0.5% PVP-I gave a ˃ 5 log10 and ˃ 5 log10 reductions by 15 and 30 s of treatment, respectively, in clean and dirty conditions, all compared to control | (Hassandarvish et al., 2020) |
| PVP-I nasal and oral rinse antiseptics | 0.25 – 2.5% | SARS-CoV-2 | 60 s | The PVP-I products tested reduced SARS-CoV-2 to ≤ 1 log10 CCID50/0.1 mL from a viral load of 5.3 log10 CCID50/0.1 mL | (Pelletier et al., 2021) |
| Oral rinses with these active ingredients: PVP-I, H2O2, chlorhexidinebis, octenidine dihydrochloride, dequalinium chloride/benzalkonium chloride, ethanol/essential oils and polyaminopropyl biguanide | – | SARS-CoV-2 | 30 s | PVP-I, dequalinium chloride and ethanol/essential oils significantly reduced the infectivity of the tested 3 strains of SARS-COV-2 (≥ 3.11 log10) | (Meister et al., 2020) |
| Stabilized hypochlorous acid; chlorhexidine (CHX) gluconate (both alcohol based and free); PVP-1; dipotassium oxalate; and H2O21 | 0.01–0.02; 0.2; 0.58; 1.4; and 1.5%1 | SARS-CoV-2 | 1 min | Reduction of viral titre value by ≥ 4.1 to ≥5.5 log10 after treatment with all the mouthwashes except for the 0.2 CHX gluconate and 1.5 H2O2 that did not give activity against SARS-CoV-2. | (Davies et al., 2021) |
Products and their concentrations in respective order of listing.
Fig. 1.
Application of virucidal antiseptics reduces the spread/dissemination of respiratory viral diseases like COVID-19 to healthcare workers.
Considering the reported in vitro effectiveness of PVP-I antiseptics, gargling with PVP-I mouthwash/rinse may be quite an effective preventive measure against the dissemination of respiratory viruses (Chopra et al., 2021; Eggers et al., 2018; Ahmad, 2021). Furthermore, since the reported average time of viral shedding in COVID-19 patients was 20 days, and 37 days at the most in China (Zou et al., 2020), an effective gargle/mouthwash and nasal spray, especially one containing PVP-I, would be very useful in reducing transmission of the disease to medical personnel and care givers in isolation centers. It may also prevent patients who have recovered from the disease from infecting their loved ones through viral shedding (Chopra et al., 2021; Arefin, 2021). It could also prevent those who are vaccinated from spreading the virus and from being re-infected, since it is well established that infection and spread of the virus also occur in vaccinated persons (Keegan et al., 2021; Bergwerk et al., 2021; Franco-Paredes, 2022). The benefits of using PVP-I gargle was reported in Japanese clinical respiratory guidelines (Sriwilaijaroen et al., 2009; Japan Ministry of Health, Labour and Welfare (JMHLW) 2007; Eggers, 2019), which recommends the gargling with PVP-I solution as a potent antiseptic agent against influenza pandemic and in the prevention of nosocomial pneumonia (Japan Ministry of Health, Labour and Welfare (JMHLW) 2007; Kariwa et al., 2004).
During lacrimal surgeries, the surgeon usually comes into contact with ocular surface, nasal tissues and tears. Hence, among ophthalmology healthcare personnel, the risk of transmitting SARS-CoV-2 may be higher in lacrimal surgeons (Ali et al., 2020). PVP-I may be used in all the frequently contacted areas in specific concentrations proven to be safe in the respective anatomical regions (Ali et al., 2020). The viral load on ocular surface could be adequately managed with PVP-I eye drops (1%) (Grzybowski et al., 2018; Koerner et al., 2018), while 0.4% reconstituted PVP-I solution could be used for both lacrimal and nasal drainage tissues, and 1% PVP-I gargles applied in the oral mucosa (Pattanshetty et al., 2021). In vitro studies have demonstrated that about 5 - 10% PVP-I could be ciliotoxic to epithelium of the respiratory system while 10% could lead to iodine toxicity (Kim et al., 2015). On the other hand, the use of 0.5% PVP-I nose drops and mouthwash/rinse have been confirmed to be safe, without allergic reactions, in both patients and HCWs (Lachapelle et al., 2013; Khan et al., 2020; Kirk-Bayley et al., 2020). However, PVP-I usage is contraindicated in pregnant women, and in patients having iodine allergy as well as in those with thyroid diseases, or persons undertaking treatments with radioactive iodine (Gray et al., 2013).
The dental authorities of some countries have recommended application of an effective antiviral mouth rinse/wash during oral treatment to help protect both personnel and patients. However, there is currently no such recommendations from the WHO or most national Ministries of Health to use mouth rinses/wash by COVID-19 patients or as a preventive and protective measure to the general population (Alharbi et al., 2020; Ather et al., 2020). Considering that mouth rinses and nasal sprays may act as agents that could help in the reduction of viral load of SARS-CoV-2 in both asymptomatic and symptomatic patients, the adoption of these antiseptics in the crusade against COVID-19 pandemic is quite an attractive concept (Carrouel et al., 2021) and ought to be evaluated for inclusion in health policy. Mouth rinses/washes which contain PVP-I or cetylpyridinium chloride (CPC) can decrease SARS-CoV-2 oral load and the consequent risk of transmitting the virus through generated droplets and aerosols in normal life and dental procedures, respectively (Castro-Ruiz and Vergara-Buenaventura, 2020; Dev Kumar et al., 2020; Herrera et al., 2020). Therefore, usage of preprocedural mouth rinses (PPMRs) was recommended to prevent nosocomial SARS-CoV-2 transmission (Peng et al., 2020).
SARS-CoV-2 is found in secretions of both oropharynx and nasopharynx. Saliva is a crucial means of the viral transmission. There is usually a high load of SARS-CoV-2 in the saliva, especially in the early disease stage. The virus is detected in over 90% saliva samples obtained from patients with COVID-19. The number of viral infective copies/mL in these samples could amount to 1.2 × 108 (To et al., 2020; Yoon et al., 2020). When an individual coughs, sneezes, converses or breathes, such a one generates saliva droplets harbouring microorganisms. A cough or five minutes of conversation could produce about three thousand saliva droplets while a sneeze could produce about forty thousand droplets which can be transmitted several meters in the air (Baghizadeh, 2020). Over 60 μm of saliva droplets could lead to the spread of SARS-CoV-2 when there are close contacts among people (Carrouel et al., 2021). Droplets with SARS-CoV-2 could penetrate susceptible hosts through the eyes and/or mouth, or could be inhaled straight to the lungs through the nose. Thus, the infected host can become sick with COVID-19 disease (Carrouel et al., 2021; Dhand and Li, 2020; Lelieveld et al., 2020). However, the application of PVP-I mouth rinses reduces viral load in the saliva/salivary glands which could consequently help in the prevention of disease spread by decreasing the chances of contracting the virus.
The activated spike protein on SARS-CoV-2 virus, binds to its receptor, ACE2, making the virus to act like a pathogen (Duan et al., 2020; Hoffmann et al., 2020; Shang et al., 2020). ACE2 and the proprotein convertase furin, transmembrane serine protease 2 (TMPRSS2), are involved in the penetration of the virus into host cells and have high expression in the salivary glands (Hoffmann et al., 2020; Zupin et al., 2021). At the eye level, ACE2 and TMPRSS2 are both expressed on humans’ ocular surfaces (Carrouel et al., 2021). Since ACE2 is expressed on the eye surface cells which produce TMPRSS2 also, this helps SARS-CoV-2 enter host cells (Ni et al., 2020; Salamanna et al., 2020; Zhou et al., 2020), which is why people are advised to avoid auto-infection through rubbing their eyes with unclean hands (Coroneoand Collignon, 2021; Dawood, 2021). Considering the fact that the virus can establish itself on ocular surfaces, it can therefore be transmitted via the tear duct which connects the eyes with the nasal cavity and thereafter infects the respiratory cells (Sun et al., 2020; Agrawal et al., 2021; Liu et al., 2021).
Conjunctivitis commonly known as pinkeye can be a symptom of COVID-19 and it is recommended that people with symptoms, characterized by redness, tearing, itchiness, gritty sensation and discharge in the eyes, should get tested for COVID-19 (Scalinci and Trovato Battagliola, 2020; Al-Namaeh, 2021; Ozturker, 2021). There have been reports of SARS-CoV-2 detection in the conjunctival and tears swab samples collected from COVID-19 patients (Kaya et al., 2020; Arora et al., 2021). Eye protection goggles or glasses are highly recommended for HCWs in order to avoid infection through droplet transmission (Chu et al., 2020; Byambasuren et al., 2021). As earlier stated, viral load on ocular surfaces can be effectively reduced with 1% PVP-I eye drops (Grzybowski et al., 2018; Koerner et al., 2018).
The few available in vivo clinical studies which utilized PVP-I treatments on COVID-19 patients have indicated the possibility of the antiseptic reducing SARS-CoV-2 viral load in the saliva/mouths of people and sustaining such reduced viral load for some hours (Table 2). Application of PVP-I products is therefore a prospective option in the reduction of COVID-19 transmission risk and a wonderful additional preventive/protective measure for HCWs (Chopra et al., 2021; Arefin, 2021; Cascella et al., 2022).
Table 2.
In vivo clinical studies of some topical antiseptics on SARS-CoV-2.
| Virucidal Agent & concentration | Treatment time | Number of patients | Ages of patients (yr) | Kind of samples | Method of viral detection | Effect/Activity | Reference |
|---|---|---|---|---|---|---|---|
| 0.12% Chlorhexidine mouthwash | 30 s | 02 | 46 and 65 | Nasopharyngeal, oropharyngeal, sputum & urine | rRT-PCR | Saliva viral load decreased transiently for about 2 h after usage of mouthwash. However, there was an eventual increase in viral load by 2 to 4 h after mouthwash | (Yoon et al., 2020) |
| PVP-I (0.5%), cetylpyridinium chloride (CPC) (0.075%) & chlorhexidine gluconate (CHX) (0.2%) | 30 s | 16 | 35.7 ± 8.5 – 43.6 ± 8.61 | Saliva | RT-PCR | No significant difference in the cycle threshold (Ct) values of patients in the tested groups and water control at all the tested times. There was however a significant increase of Ct value fold changes of CPC (at 5 min & 6 h) and PVP-I (at 6 h) tested groups compared to the control group fold change. Therefore, PVP-I & CPC had a sustained reducing effect on SARS-CoV-2 load in the saliva of tested persons | (Seneviratne et al., 2021) |
| 1% PVP-I | 1 min | 04 | 43, 54, 73 and 74 | Nasopharyngeal & saliva | rRT-PCR | Significant drop in SARS-CoV-2 load in two patients after administration of PVP-I which was sustained for at least 3 h | (Martínez Lamas et al., 2020) |
| β-cyclodextrin-citrox mouthwash (CDCM) | 1 min | 176 | 18 - 85 | Saliva | RT-PCR | CDCM gave significantly (p = 0.036) better effect than the placebo by 4 h after the first dose resulting in 12.58% viral median percentage decrease; the small median value for CDCM was further sustained by the second dose. There was also greater median decrease percentage in the viral load for CDCM group when compared to the placebo by day 7 | (Carrouel et al., 2021) |
| 0.12% chlorhexidine gluconate (CHX) oral rinse & oropharyngeal spray | 30 s | 294 COVID-19 patients + 15 healthcare workers | 23 – 89 (median age: 62) | Oropharyngeal swab | rRT-PCR | SARS-CoV-2 was eliminated in the oropharynx of 61.1% patients who applied only CHX oral rinse as against 5.5% of control patients who did not. While there was 86% eradication of SARS-CoV-2 in patients who used both CHX oropharyngeal spray and oral rinse as against 6.3% control group who did not after 4 days of treatment. Furthermore, none of the healthcare personnel that used CHX developed COVID-19 as against 50% rate among their colleagues in other hospitals | (Huang and Huang, 2021) |
| 0.2% CHX & 1% PVP-I gargle/mouth-wash | 30 s | 61 | 17 – 85 (45.3 ± 16.7)1 | Saliva | rRT-PCR | There was significant difference in the delta Ct obtained from the control group (with distilled water) and that of the two treated groups – CHX (P = 0.0024) and PVP-I (P = 0.012). However, there was no significance between CHX and PVP-I treated groups. There was also significant difference (P < 0.0001) in delta Ct of the treated groups before and after treatments while such was not witnessed in the control group | (Elzein et al., 2021) |
| PVP-I (2%), H2O2 (1%), cetylpyridinium chloride (0.07%) & CHX (0.12%) | 1 min | 84 | Saliva | rRT-PCR | There was no statistical difference in the SARS-CoV-2 load after use of all the tested mouthwashes | (Ferrer et al., 2021) | |
| 1% H2O2 mouthrinse | 30 s | 12 | 22 – 81 (median age: 55) | Oropharyngeal | RT-PCR | SARS-CoV-2 load was not reduced by 1% H2O2 in tested patients | (Gottsauner et al., 2020) |
Mean age ± SD.
2.1.1. Emphasis on oral hygiene in a respiratory pandemic era
Respiratory and oral tract pathogens could cause serious threat to humans’ health (Jain et al., 2001; Grief, 2013). The widespread of nosocomial infections greatly add to the increase of morbidity and mortality among patients, especially the vulnerable ones (Koch et al., 2015; Haque et al., 2018). The practice of appropriate hygiene is usually recommended among individuals and HCWs in an emerging infectious disease outbreak to help curtail the dissemination of such disease by interrupting its transmission. The observance of routine adequate oral hygiene can further increase the positive outcome of general hygiene practice, especially against respiratory pathogens (Chopra et al., 2021; Arefin, 2021; Cascella et al., 2022; Kirk-Bayley et al., 2020; Yimenu et al., 2020). In this regard, the application of PVP-I mouthwash/gargle presents a useful protection measure against the spread of respiratory and oral tract infections (Chopra et al., 2021; Ahmad, 2021).
Daily gargling was recommended by the Ministry of Health, Labour and Welfare in Japan as a protective and/or preventive hygiene protocol to limit spread of URTIs (Japan Ministry of Health, Labour and Welfare (JMHLW) 2007), and this was greatly promoted during the 2009 H1N1 swine flu outbreak (Kramar and Eggers, 2020). This practice has been confirmed by several studies investigating the functions of oral gargling in some healthy individuals and people suffering from persistent URTIs (Nagatake et al., 2002; Satomura et al., 2005; Kitamura et al., 2007). Since clinical studies that applied PVP-I to decrease respiratory infections in different settings have validated its efficacy, its use in the current pandemic should hence be considered (Martínez Lamas et al., 2020; Elzein et al., 2021).
Oral hygiene with PVP-I could be of particular importance in specific groups of patients such as the immunocompromised who are at great risk of extended viral shedding that could facilitate the potential of antiviral drug resistance, nosocomial transmission and evolution of new variants (Eggers et al., 2018; Corey et al., 2021). There is currently a risk of prolonged viral shedding in COVID-19 patients even after recovery therefore the prospect of reducing this viral shedding by applying oral gargling with 1% PVP-1 should be explored (Chopra et al., 2021; Arefin, 2021). With its established safety profile, PVP-I might just be the added lifesaver and lifeguard, in addition to vaccination, to protect HCWs as they take care of COVID-19 patients who have active diseases or asymptomatic patients in isolation. Several in vitro and in vivo studies have confirmed the potency of PVP-I against coronaviruses, including SARS-CoV-2 (Table 1, Table 2).
2.2. Citrox
Citrox is a derivative of citrus fruits. It constituents include natural soluble bioflavonoids and hydroxylated phenolic compounds which are obtained from plants (Carrouel et al., 2021). Flavonoids are an essential class of natural products. These include several subgroups, such as flavonols, flavones, isoflavones and chalcones (Panche et al., 2016). Bioflavonoids that are produced from plants have been demonstrated to have antimicrobial activities against viruses, bacteria and fungi (Middleton et al., 2000; Nair et al., 2006; Russo et al., 2020). Flavonoids usually display a broad spectrum of activities because of their antioxidant properties as well as their potentials to modulate several cell receptors and/or enzymes (Panche et al., 2016). These activities include anti-inflammatory (Spagnuolo et al., 2018), anti-allergic (Park et al., 2020), antiangiogenic (Khater et al., 2020), cytostatic (Go et al., 2018), analgesic (Nesterova et al., 2017), apoptotic (Tavsan and Kayali, 2019), hepatoprotective (Ma et al., 2020), among others.
A number of flavonoids have been demonstrated to elicit efficacious activities against several human viruses. For example, two flavonoids (orbifolin and pedalitin) from Pterogyne nitens were shown to prevent the entrance of hepatitis C virus into the tested host cells at non-cytotoxic concentrations (Shimizu et al., 2017). Moreover, some other flavonoids, such as baicalin, herbacetin, isobavachalcone, helichrysetin, quercetin 3-β-d-glucoside, amentoflavone, etc., inhibited the activity of MERS-CoV protease (MERS-CoV/SARS-CoV-2 3CLpro), which performs vital roles in the replication of the MERS virus (Ryu et al., 2010; Jo et al., 2019), and that of SARS-CoV-2 3-chymotrypsin-like cysteine protease.
Since chymotrypsin-like and papain‐like proteases are essential coronaviral encoded proteins responsible for both replications of the virus and inhibition of host immune response, targeting these proteases are therefore quite attractive measures in the treatment and prevention of coronaviral diseases (Zhang and Liu, 2020). Interestingly, some flavonoids have already been demonstrated to have inhibitory effects against these proteases (Park et al., 2020; Ryu et al., 2010; Jo et al., 2019; Mouffouk et al., 2021). Some other flavonoids, such as hesperidin, isorhamnetin, rutin etc., were reported to show potential good activity against SARS-CoV-2 through binding to the ACE2 and/or TMPRSS2 thereby preventing their expression and interaction with SARS-CoV-2 (Mouffouk et al., 2021; Hu et al., 2020; Muchtaridi et al., 2020; Cheng et al., 2021; Zhan et al., 2021). Furthermore, since SARS-CoV-2 is susceptible to oxidative reaction, it is therefore recommended to apply a mouth rinse/gargle with oxidizing agents like citrox to decrease the oral salivary microbiota which include potential SARS-CoV-2 carriage (Carrouel et al., 2021; Carrouel et al., 2020).
2.3. Cyclodextrins (CDs)
Cyclodextrins (CDs) are natural glucose derivatives which possess rigid cyclic structure that contain α (1–4)–linked gluco‑pyranoside units (Jambhekar and Breen, 2016). α-, β- and γ-CDs contain 6-, 7- and 8-units of glucopyranoside, respectively. CDs are applied in the improvement of bioavailability and/or in water-solubility of medicines (Saokham et al., 2018). They can also be applied in preventing or reducing gastrointestinal and ocular irritations, decreasing or eliminating disagreeable smells or tastes, preventing interactions among drugs or drug additives in formulations. The advantages of CDs usage include: better biocompatibility compared to most oxides in oral products (i.e., gold or silver); ease of use; lack resistance reactions, and are non-toxic (Adeoye and Cabral-Marques, 2017; Muankaew and Loftsson, 2018; Menezes et al., 2019). CDs applications are becoming very valuable in the prevention of viral infections of the mucosal membranes in the throat, mouth and nose (Garrido et al., 2020).
Sulfonated CDs have been reported to show antiviral activities against HIV, albeit, their activity was discovered to be virus specific and virustatic (Jones et al., 2020). The structure of CDs may be modified and applied in infection control or as virucidal agents (Braga, 2019). For example, methylated β-CD can inhibit some viruses, including coronavirus by sequestering the viral cholesterol or by removing it from the cell membranes of the host thereby deforming the viral envelope (Danthi and Chow, 2004; Pratelli and Colao, 2015; Palacios-Rápalo et al., 2021). Hydroxypropyl-β-CD has been applied as a vaccine adjuvant to provide immunity in the cynomolgus monkey model against H1N1 influenza virus (Carrouel et al., 2020).
The modified cyclodextrin (CD) with mercaptoundecane sulfonic acids has been reported to have promising antiviral activities. By inhibiting the outer membrane of a virus, this modified CD molecule can destroy virions simply by contact instead of inhibiting viral growth. And this antiviral mechanism appears to be similar technique used against all other viruses (Carrouel et al., 2020; Jones et al., 2020). These modified CDs are biocompatible with broad-spectrum activities even at micro-molar concentrations against a lot of viruses including respiratory syncytial virus (RSV), Zika virus, herpes simplex virus (HSV) and dengue virus in vitro assays. They are also effective against clinical and laboratory strains of HSV-2 and RSV in ex vivo vaginal and respiratory tissue culture models, respectively (Jones et al., 2020).
Application of CDs to mucous membrane of the oropharynx can help prevent infection and viral spread in the mouth, nose and throat. This can be potentially exploited in the reduction of SARS-CoV-2 load that may be transmitted via aerosols and droplets especially in this COVID-19 pandemic era. This will greatly reduce virus transmission and the chances of infecting HCWs and care givers by infected patients (Carrouel et al., 2020).
The combination of β-CD and citrox in one mouthwash solution has been demonstrated to produce efficacious effect against SARS-CoV-2. β-CD and citrox mouthwash (CDCM) were demonstrated to significantly reduced SARS-CoV-2 and sustained the low viral load by the seventh day (Carrouel et al., 2021; Carrouel et al., 2020) (Table 2). Therefore, this combination may really be beneficial in COVID-19 control and prevention.
2.4. Chlorhexidine and hydrogen peroxide
Chlorhexidine (CHX) is a known cationic bisbiguanide which is applied as a broad-spectrum antiseptic in general medical practice (Gilbert and Moore, 2005; Thangavelu et al., 2020). CHX has antiviral activities that are particularly potent against enveloped viruses (Huang and Huang, 2021; Elzein et al., 2021; Montefiori et al., 1990).
There seems to be a controversy in the recommendation/adoption of CHX as adequate mouthwash antiseptic against SARS-CoV-2 as some researchers have reported it to be ineffective against SARS-CoV-2 in both in vitro and in vivo clinical studies (Meister et al., 2020; Davies et al., 2021; Ferrer et al., 2021) (Table 1, Table 2). However, some other studies have demonstrated it to show adequate in vivo activities against SARS-CoV-2 (Yoon et al., 2020; Huang and Huang, 2021; Elzein et al., 2021) (Table 2). Further studies are therefore needed to authenticate both the in vitro and in vivo claims on CHX activities.
Hydrogen peroxide is a colourless chemical compound which is broadly used for its antimicrobial properties. The efficacy of H2O2 has been shown on some human viruses, of which influenza viruses and coronaviruses were the most sensitive (Dev Kumar et al., 2020; Gottsauner et al., 2020; Dembinski et al., 2014) (Table 1). H2O2 liberates oxygen-free radicals from the envelope of these viruses thereby disrupting the lipid membrane (Peng et al., 2020; O'Donnell et al., 2020). An important advantage of using H2O2 is that it is safe on the mucosal membranes whether used as mouth washes/rinse or as nasal sprays, even when applied at 3% concentration for 6 months (Caruso et al., 2020).
Furthermore, the American Dental Association recommended the use of 1.5% H2O2 (or 0.2% PVP-1) as a preprocedural mouth rinse (PPMR) (American Dental Association (ADA) 2022), because of its efficacy against microbes. However, H2O2, just like CHX has got a number of controversial reports that cast doubts on its efficacy against SARS-CoV-2 in both in vitro (Meister et al., 2020; Davies et al., 2021) and in vivo (Ferrer et al., 2021) studies (Table 1, Table 2). This therefore may affect its adoption as a preferential antiseptic oral/nasal rinse in this COVID-19 pandemic.
3. Challenges associated with the use of mouth and throat washes
Perhaps, one of the biggest challenges of prolonged usage of some mouth/throat washes is the possibility of them affecting the oral/pharyngeal microbiota which might lead to dysbiosis (Carvalho et al., 2024; Brookes et al., 2023). The category of beneficial microorganisms disrupted ushers in the real consequent long-term effect of using such oral antiseptics. For example, the reduction/elimination of essential nitrate-reducing bacteria, such as some species of Veillonella, Provotella, Haemophilus, Rothia, Actinomyces and/or Neisseria from the oral cavity by mouth/throat washes, could lead to high blood pressure, obesity among other negative effects (Brookes et al., 2023; Bescos et al., 2020; Liu et al., 2023). This is because these nitrate-reducing bacteria are supposed to reduce the salivary nitrates in ingested food or from endogenous sources to give nitrite and nitric oxide which is essential in the modulation of cardiovascular vessels (Brookes et al., 2023; Bescos et al., 2020; Sitanaya et al., 2023). However, it is very important to state that several researches have either supported or negated this claim of dysbiosis which therefore requires further studies to substantiate (Brookes et al., 2023; Bescos et al., 2020; Fan et al., 2018; Brookes et al., 2021; Mitsui and Harasawa, 2017; Ren et al., 2023; Plummer et al., 2022). Ultimately, just like most other medications, the type and usage of mouth/throat washes should be based on medical prescriptions to avoid any possible side effects (Radzki et al., 2022).
Other challenges that might arise from the use of some types of antiseptic mouth and throat washes such as CHX, PVP-1, etc., include: malabsorption and increased risk of systemic diseases, change in saliva lactate and pH, diabetes mellitus, oral cancer and Alzheimer's disease (Carvalho et al., 2024; Bescos et al., 2020; Sitanaya et al., 2023; Brookes et al., 2021; Alrashdan et al., 2023). However, most of these side effects lack adequate scientific evidence at the moment.
4. Conclusion
So much emphases have been placed on vaccination and hand hygiene in this COVID-19 pandemic era. How about oral/nasal hygiene? The roles of oropharyngeal and nasopharyngeal mucosae in respiratory viral dissemination are critical in the control and management of the COVID-19 pandemic. Therefore, adopting and/or emphasising effective oral and other vital mucosal hygiene, through the use of antiseptics, might be an effective additional measure to reduce SARS-CoV-2 spread, especially among COVID-19 patients and their care givers. Reducing and/or eliminating these mucosal viral loads by the use of antiseptics practically decrease the chances of viral spread through aerosols and droplets which are essential channels of COVID-19 transmission.
CRediT authorship contribution statement
Kizito I. Okeke: Conceptualization, Writing – original draft. Chukwuemeka Samson Ahamefule: Writing – review & editing. Obianuju O. Nnabuife: Writing – review & editing. Ibuchukwu N. Orabueze: Writing – review & editing. Christian U. Iroegbu: Writing – review & editing. Kingsley A. Egbe: Writing – review & editing. Anthony C. Ike: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgement
This work was not supported by any research grant.
Data availability
No data was used for the research described in the article.
References
- Adeoye O., Cabral-Marques H. Cyclodextrin nanosystems in oral drug delivery: a mini review. Int. J. Pharm. 2017;531(2):521–531. doi: 10.1016/j.ijpharm.2017.04.050. [DOI] [PubMed] [Google Scholar]
- Agrawal R., Ding J., Wei X. Conjunctival findings in patients with coronavirus disease 2019. JAMa Ophthalmol. 2021;139(2):253. doi: 10.1001/jamaophthalmol.2020.5810. [DOI] [PubMed] [Google Scholar]
- Ahmad L. Impact of gargling on respiratory infections. All. Life. 2021;14(1):147–158. doi: 10.1080/26895293.2021.1893834. [DOI] [Google Scholar]
- Al-Namaeh M. COVID-19 and conjunctivitis: a meta-analysis. Ther. Adv. Ophthalmol. 2021;13 doi: 10.1177/25158414211003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi A., Alharbi S., Alqaidi S. Guidelines for dental care provision during the COVID-19 pandemic. Saudi. Dent. J. 2020;32(4):181–186. doi: 10.1016/j.sdentj.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.J., Hegde R., Nair A.G., Bajaj M.S., Betharia S.M., Bhattacharjee K., Chhabra A.K., Das J.K., Dudeja G., Grover A.K., Honavar S.G., Kim U., Mahesh L., Mukherjee B., Sethi A., Sharma M., Singh U. All india ophthalmological society - oculoplastics association of india consensus statement on preferred practices in oculoplasty and lacrimal surgery during the COVID-19 pandemic. Indian J. Ophthalmol. 2020;68(6):974–980. doi: 10.4103/ijo.IJO_1415_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alluwaimi A.M., Alshubaith I.H., Al-Ali A.M., Abohelaika S. The coronaviruses of animals and birds: their zoonosis, vaccines, and models for SARS-CoV and SARS-CoV2. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.582287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrashdan M.S., Leao J.C., Doble A., McCullough M., Porter S. The effects of antimicrobial mouthwashes on systemic disease: what is the evidence? Int. Dent. J. 2023;73(2):S82–S88. doi: 10.1016/j.identj.2023.08.012. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Dental Association (ADA) (2022) American Dental Association Interim Guidance for Minimizing Risk of COVID-19 Transmission. Updated April 1, 2020. http://success.ada.org/∼/media/CPS/Files/COVID/ADA_COVID_Rapid_Advice_Treat_Pts.pdf; Accessed January 27, 2022.
- Anderson D.E., Sivalingam V., Kang A.E.Z., Ananthanarayanan A., Arumugam H., Jenkins T.M., Hadjiat Y., Eggers M. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect. Dis. Ther. 2020;9(3):669–675. doi: 10.1007/s40121-020-00316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arefin M.K. Povidone iodine (pvp-i) oro-nasal spray: an effective shield for covid-19 protection for health care worker (HCW), for all. Indian J. Otolaryngol. Head. Neck. Surg. 2021;2021:1–6. doi: 10.1007/s12070-021-02525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora R., Goel R., Kumar S., Chhabra M., Saxena S., Manchanda V., Pumma P. Evaluation of SARS-CoV-2 in tears of patients with moderate to severe COVID-19. Ophthalmology. 2021;128(4):494–503. doi: 10.1016/j.ophtha.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ather A., Patel B., Ruparel N.B., Diogenes A., Hargreaves K.M. Coronavirus disease 19 (COVID-19): implications for clinical dental care. J. Endod. 2020;46(5):584–595. doi: 10.1016/j.joen.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghizadeh F.M. Oral saliva and COVID-19. Oral Oncol. 2020;108 doi: 10.1016/j.oraloncology.2020.104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Levin E.G., Rubin C., Indenbaum V., et al. COVID-19 breakthrough infections in vaccinated Health Care Workers. N. Engl. J. Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bescos R., Ashworth A., Cutler C., Brookes Z.L., Belfield L., Rodiles A., Casas-Agustench P., Farnham G., Liddle L., Burleigh M., White D., Easton C., Hickson M. (2020) Effects of chlorhexidine mouthwash on the oral microbiome. 10:5254. 10.1038/s41598-020-61912-4. [DOI] [PMC free article] [PubMed]
- Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Comparison of in vitro inactivation of SARS COV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses. J. Prosthodont. 2020;29(7):599–603. doi: 10.1111/jopr.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch A.A., Biesbroek G., Trzcinski K., Sanders E.A., Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS. Pathog. 2013;9(1) doi: 10.1371/journal.ppat.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga S.S. Cyclodextrins: emerging medicines of the new millennium. Biomolecules. 2019;9(12):801. doi: 10.3390/biom9120801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes Z.L.S., Belfield L.A., Ashworth A., Casas-Agustench P., Raja M., Pollard A.J., Bescos R. Effects of chlorhexidine mouthwash on the oral microbiome. J. Dent. 2021;113 doi: 10.1016/j.jdent.2021.103768. [DOI] [PubMed] [Google Scholar]
- Brookes Z., Teoh L., Cieplik F., Kumar P. Mouthwash effects on the oral microbiome: are they good, bad, or balanced? Int. Dent. J. 2023;73(2):S74–S81. doi: 10.1016/j.identj.2023.08.010. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byambasuren O., Beller E., Clark J., Collignon P., Glasziou P. The effect of eye protection on SARS-CoV-2 transmission: a systematic review. Antimicrob. Resist. Infect. Control. 2021;10(1):156. doi: 10.1186/s13756-021-01025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carod-Artal F.J. Neurological complications of coronavirus and COVID-19. Rev Neurolog. 2020;70(9):311–322. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- Carrouel F., Conte M.P., Fisher J., Gonçalves L.S., Dussart C., Llodra J.C., Bourgeois D. COVID-19: a recommendation to examine the effect of mouthrinses with β-cyclodextrin combined with citrox in preventing infection and progression. J. Clin. Med. 2020;9(4):1126. doi: 10.3390/jcm9041126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrouel F., Gonçalves L.S., Conte M.P., Campus G., Fisher J., Fraticelli L., Gadea-Deschamps E., Ottolenghi L., Bourgeois D. Antiviral activity of reagents in mouth rinses against SARS-CoV-2. J. Dent Res. 2021;100(2):124–132. doi: 10.1177/0022034520967933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso A.A., Del Prete A., Lazzarino A.I., Capaldi R., Grumetto L. Might hydrogen peroxide reduce the hospitalization rate and complications of SARS-CoV-2 infection? Infect. Control Hosp. Epidemiol. 2020;41(11):1360–1361. doi: 10.1017/ice.2020.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho L.R.R.A., Boeder A.M., Shimari M., Kleschyov A.L., Esberg A., Johansson I., Weitzberg E., Lundberg J.O., Carlstrom M. Antibacterial mouthwash alters gut microbiome, reducing nutrient absorption and fat accumulation in Western diet-fed mice. Sci. Rep. 2024;14(1):4025. doi: 10.1038/s41598-024-54068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL): 2022. Features, evaluation, and treatment of coronavirus (COVID-19) 2022. [PubMed] [Google Scholar]
- Castro-Ruiz C., Vergara-Buenaventura A. Povidone-iodine solution: a potential antiseptic to minimize the risk of COVID-19? A narrative review. J. Int. Soc. Prev. Community Dent. 2020;10(6):681–685. doi: 10.4103/jispcd.JISPCD_304_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafekar A., Fielding B.C. MERS-CoV: understanding the latest human coronavirus threat. Viruses. 2018;10(2):93. doi: 10.3390/v10020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F.J., Huynh T.K., Yang C.S., Hu D.W., Shen Y.C., Tu C.Y., Wu Y.C., Tang C.H., Huang W.C., Chen Y., Ho C.Y. Hesperidin is a potential inhibitor against SARS-CoV-2 infection. Nutrients. 2021;13(8):2800. doi: 10.3390/nu13082800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J.D., Krogstad P. SARS: the first pandemic of the 21st century. Paediatr Res. 2004;56(1):1–5. doi: 10.1203/01.PDR.0000129184.87042.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra A., Sivaraman K., Radhakrishnan R., Balakrishnan D., Narayana A. Can povidone iodine gargle/mouthrinse inactivate SARS-CoV-2 and decrease the risk of nosocomial and community transmission during the COVID-19 pandemic? An evidence-based update. Jpn. Dent. Sci. Rev. 2021;57:39–45. doi: 10.1016/j.jdsr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J., COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J.Y., Thone M.N., Kwon Y.J. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L., Beyrer C., Cohen M.S., Michael N.L., Bedford T., Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N. Engl. J. Med. 2021;385:562–566. doi: 10.1056/NEJMs62104756. Pmid:34347959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coroneo M.T., Collignon P.J. SARS-CoV-2: eye protection might be the missing key. Lancet Microbe. 2021;2(5):e173–e174. doi: 10.1016/S2666-5247(21)00040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danthi P., Chow M. Cholesterol removal by methyl-beta-cyclodextrin inhibits poliovirus entry. J. Virol. 2004;78(1):33–41. doi: 10.1128/jvi.78.1.33-41.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K., Buczkowski H., Welch S.R., Green N., Mawer D., Woodford N., Roberts A.D.G., Nixon P.J., Seymour D.W., Killip M.J. Effective in vitro inactivation of SARS-CoV-2 by commercially available mouthwashes. J. Gen. Virol. 2021;102(4) doi: 10.1099/jgv.0.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood A.A. Transmission of SARS CoV-2 virus through the ocular mucosa worth taking precautions. Vacunas. 2021;22(1):56–57. doi: 10.1016/j.vacun.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembinski J.L., Hungnes O., Hauge A.G., Kristoffersen A.C., Haneberg B., Mjaaland S. Hydrogen peroxide inactivation of influenza virus preserves antigenic structure and immunogenicity. J. Virol. Methods. 2014;207:232–237. doi: 10.1016/j.jviromet.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Dev Kumar G., Mishra A., Dunn L., Townsend A., Oguadinma I.C., Bright K.R., Gerba C.P. Biocides and novel antimicrobial agents for the mitigation of coronaviruses. Front. Microbiol. 2020;11:1351. doi: 10.3389/fmicb.2020.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., Patel S.K., Sharun K., Pathak M., Tiwari R., Yatoo M.I., Malik Y.S., Sah R., Rabaan A.A., Panwar P.K., Singh K.P., Michalak I., Chaicumpa W., Martinez-Pulgarin D.F., Bonilla-Aldana D.K., Rodriguez-Morales A.J. SARS-CoV-2 jumping the species barrier: zoonotic lessons from SARS, MERS and recent advances to combat this pandemic virus. Travel. Med. Infect. Dis. 2020;37 doi: 10.1016/j.tmaid.2020.101830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhand R., Li J. Coughs and sneezes: their role in transmission of respiratory viral infections, including SARS-CoV-2. Am J Resp Crit Care Med. 2020;202(5):651–659. doi: 10.1164/rccm.202004-1263PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo M.A., Farrales M.S., Loya R.M., Pura M.A., Uy H. The effect of 1% povidone iodine as a pre-procedural mouthrinse in 20 patients with varying degrees of oral hygiene. J. Philipp. Dent. Assoc. 1996;48(2):31–38. [PubMed] [Google Scholar]
- Duan L., Zheng Q., Zhang H., Niu Y., Lou Y., Wang H. The SARS-CoV-2 spike glycoprotein biosynthesis, structure, function, and antigenicity: implications for the design of spike-based vaccine immunogens. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.576622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers M., Eickmann M., Zorn J. Rapid and effective virucidal activity of povidone-iodine products against Middle East respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus Ankara (MVA) Infect. Dis. Ther. 2015;4(4):491–501. doi: 10.1007/s40121-015-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers M., Koburger-Janssen T., Eickmann M., Zorn J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect. Dis. Ther. 2018;7(2):249–259. doi: 10.1007/s40121-018-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers M. Infectious disease management and control with povidone iodine. Infect. Dis. Ther. 2019;8(4):581–593. doi: 10.1007/s40121-019-00260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzein R., Abdel-Sater F., Fakhreddine S., Hanna P.A., Feghali R., Hamad H., Ayoub F. In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial. J Evid-Based Dent Pract. 2021;21(3) doi: 10.1016/j.jebdp.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Peters B.A., Min D., Ahn J., Hayes R.B. Comparison of the oral microbiome in mouthwash and whole saliva samples. PLoS. One. 2018;13(4) doi: 10.1371/journal.pone.0194729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Maier H, Bickerton E, Britton P, editors. Coronaviruses: an overview of their replication and pathogenesisCoronaviruses. Methods Mol. Biol. 2015 doi: 10.1007/978-1-4939-2438-7_1. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer M.D., ÁS Barrueco, Martinez-Beneyto Y., Mateos-Moreno M.V., Ausina-Márquez V., García-Vázquez E., Puche-Torres M., Giner M.J.F., González A.C., Coello J.M.S., Rueda I.A., Aubá J.M.V., Español C.C., Velasco A.L., Abad D.S., García-Esteban S., Artacho A., López-Labrador X., Mira A. Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2. Sci. Rep. 2021;11(1):24392. doi: 10.1038/s41598-021-03461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcados G.E., Muhammad A., Oladipo O.O., Makama S., Meseko C.A. Metabolic implications of oxidative stress and inflammatory process in SARS-CoV-2 pathogenesis: therapeutic potential of natural antioxidants. Front. Cell Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.654813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Paredes C. Transmissibility of SARS-CoV-2 among fully vaccinated individuals. Lancet Infect. Dis. 2022;22(1):16. doi: 10.1016/S1473-3099(21)00768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S., Capriotti J., Brown S.M., Tessema B. Povidone-iodine use in sinonasal and oral cavities: a review of safety in the COVID-19 era. Ear Nose Throat J. 2020;99(9):586–593. doi: 10.1177/0145561320932318. [DOI] [PubMed] [Google Scholar]
- Gandhi G., Thimmappa L., Upadhya N., Carnelio S. Could mouth rinses be an adjuvant in the treatment of SARS-CoV-2 patients? An appraisal with a systematic review. Int. J. Dent. Hyg. 2021;20(1):136–144. doi: 10.1111/idh.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido P.F., Calvelo M., Blanco-González A., Veleiro U., Suárez F., Conde D., Cabezón A., Piñeiro Á., Garcia-Fandino R. The lord of the nanorings: cyclodextrins and the battle against SARS-CoV-2. Int. J. Pharm. 2020;588 doi: 10.1016/j.ijpharm.2020.119689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafoor D., Khan Z., Khan A., Ualiyeva D., Zaman N. Excessive use of disinfectants against COVID-19 posing a potential threat to living beings. Curr. Res. Toxicol. 2021;2:159–168. doi: 10.1016/j.crtox.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P., Moore L.E. Cationic antiseptics: diversity of action under a common epithet. J App Microbiol. 2005;99(4):703–715. doi: 10.1111/j.1365-2672.2005.02664.x. [DOI] [PubMed] [Google Scholar]
- Go Y.H., Lee H.J., Kong H.J., Jeong H.C., Lee D.Y., Hong S.K., Sung S.H., Kwon O.S., Cha H.J. Screening of cytotoxic or cytostatic flavonoids with quantitative fluorescent ubiquitination-based cell cycle indicator-based cell cycle assay. R. Soc. Open. Sci. 2018;5(12) doi: 10.1098/rsos.181303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsauner M.J., Michaelides I., Schmidt B., Scholz K.J., Buchalla W., Widbiller M., Hitzenbichler F., Ettl T., Reichert T.E., Bohr C., Vielsmeier V., Cieplik F. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin. Oral Investig. 2020;24(10):3707–3713. doi: 10.1007/s00784-020-03549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P.E., Katelaris C.H., Lipson D. Recurrent anaphylaxis caused by topical povidone-iodine (Betadine) J. Paediatr. Child Health. 2013;49(6):506–507. doi: 10.1111/jpc.12232. [DOI] [PubMed] [Google Scholar]
- Grief S.N. Upper respiratory infections. Prim. Care. 2013;40(3):757–770. doi: 10.1016/j.pop.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzybowski A., Kanclerz P., Myers W.G. The use of povidone-iodine in ophthalmology. Curr Opinion Ophthalmol. 2018;29(1):19–32. doi: 10.1097/ICU.0000000000000437. [DOI] [PubMed] [Google Scholar]
- Haque M., Sartelli M., McKimm J., Abu Bakar M. Health care-associated infections - An overview. Infect. Drug Resist. 2018;11:2321–2333. doi: 10.2147/IDR.S177247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassandarvish P., Tiong V., Sazaly A.B., Mohamed N.A., Arumugam H., Ananthanarayanan A., Qasuri M., Hadjiat Y. Povidone iodine gargle and mouthwash. Brit Dent J. 2020;228(12):900. doi: 10.1038/s41415-020-1794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera D., Serrano J., Roldán S., Sanz M. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin. Oral Investig. 2020;24(8):2925–2930. doi: 10.1007/s00784-020-03413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Cai X., Song X., Li C., Zhao J., Luo W., Zhang Q., Ekumi I.O., He Z. Possible SARS-coronavirus 2 inhibitor revealed by simulated molecular docking to viral main protease and host toll-like receptor. Future Virol. 2020 doi: 10.2217/fvl-2020-0099. [DOI] [Google Scholar]
- Huang Y.H., Huang J.T. Use of chlorhexidine to eradicate oropharyngeal SARS-CoV-2 in COVID-19 patients. J. Med. Virol. 2021;93(7):4370–4373. doi: 10.1002/jmv.26954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ita K. Coronavirus disease (COVID-19): current status and prospects for drug and vaccine development. Arch. Med. Res. 2021;52(1):15–24. doi: 10.1016/j.arcmed.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N., Lodha R., Kabra S.K. Upper respiratory tract infections. Indian J Paediatr. 2001;68(12):1135–1138. doi: 10.1007/BF02722930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambhekar S.S., Breen P. Cyclodextrins in pharmaceutical formulations I: structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today. 2016;21(2):356–362. doi: 10.1016/j.drudis.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Japan Ministry of Health, Labour and Welfare (JMHLW) 2007. Pandemic Influenza Preparedness Action Plan of the Japanese government.http://www.mhlw.go.jp/english/topics/influenza/dl/pandemic02.pdf Accessed January 12, 2022. [Google Scholar]
- Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S., Kim H., Kim S., Shin D.H., Kim M.S. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem. Biol. Drug Des. 2019;94(6):2023–2030. doi: 10.1111/cbdd.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.T., Cagno V., Janeček M., Ortiz D., Gasilova N., Piret J., Gasbarri M., Constant D.A., Han Y., Vuković L., Král P., Kaiser L., Huang S., Constant S., Kirkegaard K., Boivin G., Stellacci F., Tapparel C. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 2020;6(5):eaax9318. doi: 10.1126/sciadv.aax9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagalingam J., Feliciano R., Hah J.H., Labib H., Le T.A., Lin J.C. Practical use of povidone-iodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infections. Int. J. Clin. Pract. 2015;69(11):1247–1256. doi: 10.1111/ijcp.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariwa H., Fujii N., Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions, and chemical reagents. Jap J Vet Res. 2004;52(3):105–112. [PubMed] [Google Scholar]
- Kariwa H., Fujii N., Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212(S1):S119–S123. doi: 10.1159/000089211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya H., Çalışkan A., Okul M., Sarı T., Akbudak İ.H. Detection of SARS-CoV-2 in the tears and conjunctival secretions of coronavirus disease 2019 patients. J. Infect. Dev. Ctries. 2020;14(9):977–981. doi: 10.3855/jidc.13224. [DOI] [PubMed] [Google Scholar]
- Keegan L.T., Truelove S., Lessler J. Analysis of vaccine effectiveness against COVID-19 and emergence of Delta and other variants of concern in Utah. JAMa Netw. Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.40906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.M., Parab S.R., Paranjape M. Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in Covid 19 pandemic. Am. J. Otolaryngol. 2020;41(5) doi: 10.1016/j.amjoto.2020.102618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khater M., Greco F., Osborn H.M.I. Antiangiogenic activity of flavonoids: a systematic review and meta-analysis. Molecules. 2020;25(20):4712. doi: 10.3390/molecules25204712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Rimmer J., Mrad N., Ahmadzada S., Harvey R.J. Betadine has a ciliotoxic effect on ciliated human respiratory cells. J. Laryngol. Otol. 2015;129(S1):S45–S50. doi: 10.1017/S0022215114002746. [DOI] [PubMed] [Google Scholar]
- Kirk-Bayley J., Sunkaraneni V., Challacombe S. The use of povidone iodine nasal spray and mouthwash during the current COVID-19 pandemic may reduce cross infection and protect healthcare workers. SSRN Electr J. 2020 doi: 10.2139/ssrn.3563092. [DOI] [Google Scholar]
- Kitamura T., Satomura K., Kawamura T., Yamada S., Takashima K., Suganuma N., Namai H., Komura Y., Great Cold Investigators-I Can we prevent influenza-like illnesses by gargling? Intern. Med. 2007;46(18):1623–1624. doi: 10.2169/internalmedicine.46.0104. [DOI] [PubMed] [Google Scholar]
- Koch A.M., Nilsen R.M., Eriksen H.M., Cox R.J., Harthug S. Mortality related to hospital-associated infections in a tertiary hospital; repeated cross-sectional studies between 2004 and 2011. Antimicrob. Resist. Infect. Control. 2015;4:57. doi: 10.1186/s13756-015-0097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner J.C., George M.J., Meyer D.R., Rosco M.G., Habib M.M. Povidone-iodine concentration and dosing in cataract surgery. Surv. Ophthalmol. 2018;63(6):862–868. doi: 10.1016/j.survophthal.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Kramar A., Eggers M. Prevention of respiratory viral infections by virucidal mucosal antisepsis among medical staff and the community. Hyg. Med. 2020;45(9):1–9. [Google Scholar]
- Kumpitsch C., Koskinen K., Schöpf V., Moissl-Eichinger C. The microbiome of the upper respiratory tract in health and disease. BMC. Biol. 2019;17(1):87. doi: 10.1186/s12915-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurakula M., Rao G.S.N.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): as excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020;60 doi: 10.1016/j.jddst.2020.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachapelle J.M., Castel O., Casado A.F., Leroy B., Micali G., Tennstedt D., Lambert J. Antiseptics in the era of bacterial resistance: a focus on povidone iodine. Clin. Pract. 2013;10(5):579–592. doi: 10.2217/cpr.13.50. [DOI] [Google Scholar]
- Lelieveld J., Helleis F., Borrmann S., Cheng Y., Drewnick F., Haug G., Klimach T., Sciare J., Su H., Pöschl U. Model calculations of aerosol transmission and infection risk of COVID-19 in indoor environments. Int. J. Environ. Res. Public Health. 2020;17(21):8114. doi: 10.3390/ijerph17218114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev. Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Xiao Q., Sun C.B. Conjunctival findings in patients with coronavirus disease 2019. JAMa Ophthalmol. 2021;139(2):253–254. doi: 10.1001/jamaophthalmol.2020.5813. [DOI] [PubMed] [Google Scholar]
- Liu T., Chen Y.C., Jeng S.L., Chang J.J., Wang J.Y., Lin C.H., Tsai P.F., Ko N.Y., Ko W.C., Wang J.L. Short-term effects of chlorhexidine mouthwash and listerine on oral microbiome in hospitalized patients. Front. Cell Infect. Microbiol. 2023;13 doi: 10.3389/fcimb.2023.1056534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Wei R., Sang Z., Dong J. Structural characterization, neuroprotective and hepatoprotective activities of flavonoids from the bulbs of Heleocharis dulcis. Bioorg. Chem. 2020;96:03630. doi: 10.1016/j.bioorg.2020.103630. [DOI] [PubMed] [Google Scholar]
- Majumder J., Minko T. Recent developments on therapeutic and diagnostic approaches for COVID-19. Am Assoc Pharm Sci J. 2021;23(1):14. doi: 10.1208/s12248-020-00532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaysian Health Technology Assessment Section (MaHTAS) Based on Available Evidence up to 3 July 2020. Medical Development Division, Ministry of Health; Malaysia: 2020. Povidone-iodine for disinfection of SARS-CoV-2; p. 2020. [Google Scholar]
- Martínez Lamas L., Diz Dios P., Pérez Rodríguez M.T., Del Campo Pérez V., Cabrera Alvargonzalez J.J., López Domínguez A.M., Fernandez Feijoo J., Diniz Freitas M., Limeres Posse J. Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests. Oral Dis. 2020;2020 doi: 10.1111/odi.13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister T.L., Brüggemann Y., Todt D., Conzelmann C., Müller J.A., Groß R., Münch J., Krawczyk A., Steinmann J., Steinmann J., Pfaender S., Steinmann E. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J. Infect. Dis. 2020;222(8):1289–1292. doi: 10.1093/infdis/jiaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes P.D.P., Andrade T.A., Frank L.A., de Souza E.P.B.S.S., Trindade G.D.G.G., Trindade I.A.S., Serafini M.R., Guterres S.S., Araújo A.A.S. Advances of nanosystems containing cyclodextrins and their applications in pharmaceuticals. Int. J. Pharm. 2019;559:312–328. doi: 10.1016/j.ijpharm.2019.01.041. [DOI] [PubMed] [Google Scholar]
- Middleton E., Kandaswami C., Theoharides T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52(4):673–751. [PubMed] [Google Scholar]
- Mitsui T., Harasawa R. The effects of essential oil, povidone-iodine, and chlorhexidine mouthwash on salivary nitrate/nitrite and nitrate-reducing bacteria. J. Oral Sci. 2017;59(4):597–601. doi: 10.2334/josnusd.16-0593. [DOI] [PubMed] [Google Scholar]
- Montefiori D.C., WE-Jr Robinson, Modliszewski A., Mitchell W.M. Effective inactivation of human immunodeficiency virus with chlorhexidine antiseptics containing detergents and alcohol. J. Hosp. Infect. 1990;15(3):279–282. doi: 10.1016/0195-6701(90)90036-n. [DOI] [PubMed] [Google Scholar]
- Mouffouk C., Mouffouk S., Mouffouk S., Hambaba L., Haba H. Flavonols as potential antiviral drugs targeting SARS-CoV-2 proteases (3CLpro and PLpro), spike protein, RNA-dependent RNA polymerase (RdRp) and angiotensin-converting enzyme II receptor (ACE2) Eur. J. Pharmacol. 2021;891 doi: 10.1016/j.ejphar.2020.173759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muankaew C., Loftsson T. Cyclodextrin-based formulations: a non-invasive platform for targeted drug delivery. Basic Clin. Pharmacol. Toxicol. 2018;122(1):46–55. doi: 10.1111/bcpt.12917. [DOI] [PubMed] [Google Scholar]
- Muchtaridi M., Fauzi M., Khairul Ikram N.K., Mohd Gazzali A., Wahab H.A. Natural flavonoids as potential angiotensin-converting enzyme 2 inhibitors for anti-SARS-CoV-2. Molecules. 2020;25(17):3980. doi: 10.3390/molecules25173980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M.P., McGeer A. Severe acute respiratory syndrome (SARS) coronavirus. Semin Resp Crit Care Med. 2007;28(2):201–212. doi: 10.1055/s-2007-976492. [DOI] [PubMed] [Google Scholar]
- Murphy T.F., Bakaletz L.O., Smeesters P.R. Microbial interactions in the respiratory tract. Pediatr. Infect. Dis. J. 2009;28(S10):S121–S126. doi: 10.1097/INF.0b013e3181b6d7ec. [DOI] [PubMed] [Google Scholar]
- Nagatake T., Ahmed K., Oishi K. Prevention of respiratory infections by povidone-iodine gargle. Dermatology. 2002;204(S1):S32–S36. doi: 10.1159/000057722. [DOI] [PubMed] [Google Scholar]
- Nair M.P., Mahajan S., Reynolds J.L., Aalinkeel R., Nair H., Schwartz S.A., Kandaswami C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin Vaccin Immunol. 2006;13(3):319–328. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi S.H.S., Citardi M.J., Cattano D., Ostrosky-Zeichner L., Knackstedt M.I., Karni R.J. Povidone-iodine solution as SARS-CoV-2 prophylaxis for procedures of the upper aerodigestive tract a theoretical framework. J Otolaryngol - Head Neck Surg. 2020;49(1):77. doi: 10.1186/s40463-020-00474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterova Y.V., Povet'eva T.N., Zibareva L.N., Suslov N.I., Zueva E.P., Aksinenko S.G., Afanas'eva O.G., Krylova S.G., Amosova E.N., Rybalkina O.Y., Lopatina K.A. Anti-inflammatory and analgesic activities of the complex of flavonoids from Lychnis chalcedonica L. Bull. Exp. Biol. Med. 2017;163(2):222–225. doi: 10.1007/s10517-017-3771-5. [DOI] [PubMed] [Google Scholar]
- Ni W., Yang X., Yang D., Bao J., Li R., Xiao Y., Hou C., Wang H., Liu J., Yang D., Xu Y., Cao Z., Gao Z. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit care (Lond Engl) 2020;24(1):422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnaemeka V.C., Okafor N.A., Orababar O.Q., Anikwe R., Onwe R.O., Uzochukwu N.P., Tsiterimam T.S., Nwokoye N.N., Ike A.C. COVID-19 vaccine acceptance in Nigeria: a rapid systematic review and meta-analysis. medRxiv. 2023 doi: 10.1101/2023.02.16.23286008. [Preprint] [DOI] [Google Scholar]
- Nova N. Cross-species transmission of coronaviruses in humans and domestic mammals, what are the ecological mechanisms driving transmission, spillover, and disease emergence? Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.717941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell V.B., Thomas D., Stanton R., Maillard J.Y., Murphy R.C., Jones S.A., Humphreys I., Wakelam M.J.O., Fegan C., Wise M.P., Bosch A., Sattar S.A. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function. 2020;1(1):zqaa002. doi: 10.1093/function/zqaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturker Z.K. Conjunctivitis as sole symptom of COVID-19: a case report and review of literature. Eur. J. Ophthalmol. 2021;31(NP2):NP161–NP166. doi: 10.1177/1120672120946287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Rápalo S.N., De Jesús-González L.A., Cordero-Rivera C.D., Farfan-Morales C.N., Osuna-Ramos J.F., Martínez-Mier G., Quistián-Galván J., Muñoz-Pérez A., Bernal-Dolores V., Del Ángel R.M., Reyes-Ruiz J.M. Cholesterol-rich lipid rafts as platforms for SARS-CoV-2 entry. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.796855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.H., Min S.Y., Yu H.W., Kim K., Kim S., Lee H.J., Kim J.H., Park Y.J. Effects of apigenin on RBL-2H3, RAW264.7, and HaCaT cells: anti-Allergic, anti-inflammatory, and skin-protective activities. Int. J. Mol. Sci. 2020;21(13):4620. doi: 10.3390/ijms21134620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanshetty S., Narayana A., Radhakrishnan R. Povidone-iodine gargle as a prophylactic intervention to interrupt the transmission of SARS-CoV-2. Oral Dis. 2021;27(S3):S752–S753. doi: 10.1111/odi.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J.S., Tessema B., Frank S., Westover J.B., Brown S.M., Capriotti J.A. Efficacy of povidone-iodine nasal and oral antiseptic preparations against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) Ear Nose Throat J. 2021;100(S2):S192–S196. doi: 10.1177/0145561320957237. [DOI] [PubMed] [Google Scholar]
- Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020;12(1):9. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer E.L., Maddaford K., Murray G.L., Fairley C.K., Pasricha S., Mu A., Bradshaw C.S., Williamson D.A., Chow E.P.F. The impact of mouthwash on the oropharyngeal microbiota of men who have sex with men: a substudy of the OMEGA trial. Microbiol. Spectr. 2022;10(1) doi: 10.1128/spectrum.01757-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Colao V. Role of the lipid rafts in the life cycle of canine coronavirus. J. Gen. Virol. 2015;96(Pt2):331–337. doi: 10.1099/vir.0.070870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzki D., Wilhelm-Weglarz M., Pruska K., Kusiak A., Ordyniec-Kwa´snica I.A. Fresh look at mouthwashes —What is inside and what is it for? Int. J. Environ. Res. Public Health. 2022;19 doi: 10.3390/ijerph19073926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Zhang Y., Xiang Y., Hu T., Cheng R., Cai H. The efficacy of mouthwashes on oral microorganisms and gingivitis in patients undergoing orthodontic treatment: a systematic review and meta-analysis. BMC. Oral Health. 2023;23(1):204. doi: 10.1186/s12903-023-02920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemersma K.K., Haddock L.A., III, Wilson N.A., Minor N., Eickhoff J., Grogan B.E., Kita-Yarbro A., Halfmann P.J., Segaloff H.E., Kocharian A., et al. Shedding of infectious SARS-CoV-2 despite vaccination. PLoS. Pathog. 2022;18(9) doi: 10.1371/journal.ppat.1010876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Salazar C., Kimura K.S., Shilts M.H., Strickland B.A., Freeman M.H., Wessinger B.C., Gupta V., Brown H.M., Rajagopala S.V., Turner J.H., Das S.R. SARS-CoV-2 infection and viral load are associated with the upper respiratory tract microbiome. J. Allergy Clin. Immunol. 2021;147(4):1226–1233. doi: 10.1016/j.jaci.2021.02.001. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M., Moccia S., Spagnuolo C., Tedesco I., Russo G.L. Roles of flavonoids against coronavirus infection. Chem-Biolog Interact. 2020;328 doi: 10.1016/j.cbi.2020.109211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y.B., Jeong H.J., Kim J.H., Kim Y.M., Park J.Y., Kim D., Nguyen T.T., Park S.J., Chang J.S., Park K.H., Rho M.C., Lee W.S. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CL(pro) inhibition. Bioorg. Med. Chem. 2010;18(22):7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safari I., InanlooRahatloo K., Elahi E. Evolution of SARS-CoV-2 genome from December 2019 to late March 2020: emerged haplotypes and informative Tag nucleotide variations. J. Med. Virol. 2020;93(4):2010–2020. doi: 10.1002/jmv.26553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamanna F., Maglio M., Landini M.P., Fini M. Body localization of ACE-2: on the trail of the keyhole of SARS-CoV-2. Front. Med. 2020;7 doi: 10.3389/fmed.2020.594495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saokham P., Muankaew C., Jansook P., Loftsson T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules. 2018;23(5):1161. doi: 10.3390/molecules23051161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satomura K., Kitamura T., Kawamura T., Shimbo T., Watanabe M., Kamei M., Takano Y., Tamakoshi A., Great Cold Investigators-I Prevention of upper respiratory tract infections by gargling: a randomized trial. Am. J. Prev. Med. 2005;29(4):302–307. doi: 10.1016/j.amepre.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Scalinci S.Z., Trovato Battagliola E. Conjunctivitis can be the only presenting sign and symptom of COVID-19. IDCases. 2020;20:e00774. doi: 10.1016/j.idcr.2020.e00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaggi G., Monstrey S., Van Landuyt K., Hamdi M., Blondeel P. The role of iodine in antisepsis and wound management: a reappraisal. Acta Chir. Belg. 2003;103(3):241–247. doi: 10.1080/00015458.2003.11679417. [DOI] [PubMed] [Google Scholar]
- Seneviratne C.J., Balan P., Ko K.K.K., Udawatte N.S., Lai D., Ng D.H.L., Venkatachalam I., Lim K.S., Ling M.L., Oon L., Goh B.T., Sim X.Y.J. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection. 2021;49(2):305–311. doi: 10.1007/s15010-020-01563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc Nat Acad Sci USA. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu J.F., Lima C.S., Pereira C.M., Bittar C., Batista M.N., Nazaré A.C., Polaquini C.R., Zothner C., Harris M., Rahal P., Regasini L.O., Jardim A.C.G. Flavonoids from Pterogyne nitens Inhibit hepatitis C virus entry. Sci. Rep. 2017;7(1):16127. doi: 10.1038/s41598-017-16336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singanayaman A., Hakki S., Dunning J., Madon K.J., Crone M.A., Koychera A., Derqui-Fernandez N., Barnett J.L., Whitefield M.G., Varro R., et al. Community transmission and viral load kinetics of the individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022;22(2):183–195. doi: 10.1016/S1473-3099(21)006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitanaya R., Lesmana H., Irayani S., Hamid E.M., Achmad H., Gobel F.A., Adawiyah E. Effect of using povidone iodine and herbal propolis antibacterial mouth on blood pressure. J. Popul. Ther. Clin. Pharmacol. 2023;30(8):e477–e484. doi: 10.47750/jptcp.2023.30.08.051. 26. [DOI] [Google Scholar]
- South A.M., Brady T.M., Flynn J.T. ACE2 (Angiotensin-Converting Enzyme 2), COVID-19, and ACE inhibitor and Ang II (Angiotensin II) receptor blocker use during the pandemic: the pediatric perspective. Hypertension. 2020;76(1):16–22. doi: 10.1161/HYPERTENSIONAHA.120.15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnuolo C., Moccia S., Russo G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018;153:105–115. doi: 10.1016/j.ejmech.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Sriwilaijaroen N., Wilairat P., Hiramatsu H., Takahashi T., Suzuki T., Ito M., Ito Y., Tashiro M., Suzuki Y. Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: its effects on hemagglutination and sialidase activities. Virol. J. 2009;6:124. doi: 10.1186/1743-422X-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhail S., Zajac J., Fossum C., Lowater H., McCracken C., Severson N., Laatsch B., Narkiewicz-Jodko A., Johnson B., Liebau J., Bhattacharyya S., Hati S. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review. Protein J. 2020;39(6):644–656. doi: 10.1007/s10930-020-09935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.B., Wang Y.Y., Liu G.H., Liu Z. (2020) Role of the eye in transmitting human coronavirus: what we know and what we do not know. Front Public Health 8:155. 10.3389/fpubh.2020.00155. [DOI] [PMC free article] [PubMed]
- Swelum A.A., Shafi M.E., Albaqami N.M., El-Saadony M.T., Elsify A., Abdo M., Taha A.E., Abdel-Moneim A.-M.E., Al-Gabri N.A., Almaiman A.A., Al-wajeeh A.S., Tufarelli V., Staffa V.N., Abd El-Hack M.E. COVID-19 in human, animal, and environment: a review. Front. Vet. Sci. 2020;7:578. doi: 10.3389/fvets.2020.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarighi P., Eftekhari S., Chizari M., Sabernavaei M., Jafari D., Mirzabeigi P. A review of potential suggested drugs for coronavirus disease (COVID-19) treatment. Eur. J. Pharmacol. 2021;895 doi: 10.1016/j.ejphar.2021.173890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavsan Z., Kayali H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. PharmacOther. 2019;116 doi: 10.1016/j.biopha.2019.109004. [DOI] [PubMed] [Google Scholar]
- Thangavelu A., Kaspar S.S., Kathirvelu R.P., Srinivasan B., Srinivasan S., Sundram R. Chlorhexidine: an elixir for periodontics. J. Pharm. Bioallied. Sci. 2020;12(S1):S57–S59. doi: 10.4103/jpbs.JPBS_162_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K., Tsang O.T., Yip C.C., Chan K.H., Wu T.C., Chan J.M., Leung W.S., Chik T.S., Choi C.Y., Kandamby D.H., Lung D.C., Tam A.R., Poon R.W., Fung A.Y., Hung I.F., Cheng V.C., Chan J.F., Yuen K.Y. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020;71(15):841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y., Tang W., Yu L., Liu Z., Liu Y., Xia H., Zhang H., Chen S., Wu J., Cui X., Zhang J., Wang F., Hu Y., Deng D. Inactivating SARS-CoV-2 by electrochemical oxidation. Sci Bull. 2021;66(7):720–726. doi: 10.1016/j.scib.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana J., van Dorp C.H., Nunes A., Gomes M.C., van Boren M., Kretzschmar M.E., Veldhoen M., Rozhnova G. Controlling the pandemic during the SARS-CoV-2 vaccination rollout. Nat Comm. 2021;12:3674. doi: 10.1038/s41467-021-23938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira Colombo A.P., Magalhães C.B., Hartenbach F.A., Martins do Souto R., Maciel da Silva-Boghossian C. Periodontal-disease-associated biofilm: a reservoir for pathogens of medical importance. Microb. Pathog. 2016;94:27–34. doi: 10.1016/j.micpath.2015.09.009. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization; 2021. Coronavirus Disease (COVID 19) Pandemic. Update June 24, 2021. Accessed January 27, 2022. [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Grunewald M., Perlman S. Coronaviruses: an updated overview of their replication and pathogenesis. Maier H, Bickerton E, editors. Coronaviruses: an updated overview of their replication and pathogenesisCoronaviruses. Methods Mol. Biol.. 2020 doi: 10.1007/978-1-0716-0900-2_1. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Data (2024) WHO COVID-19 dashboard. https://data.who.int/dashboards/covid19/ Accessed June 17, 2024.
- Yimenu D.K., Adelo E.S., Siraj E.A., Kassie T.A., Hammeso W.W., Demeke C.A., Emiru Y.K. Health professionals oral health knowledge and practice: unleashing the hidden challenges. J. Multidiscip. Healthc. 2020;13:459–469. doi: 10.2147/JMDH.S254964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.G., Yoon J., Song J.Y., Yoon S.Y., Lim C.S., Seong H., Noh J.Y., Cheong H.J., Kim W.J. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J. Korean Med. Sci. 2020;35(20):e195. doi: 10.3346/jkms.2020.35.e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y., Ta W., Tang W., Hua R., Wang J., Wang C., Lu W. Potential antiviral activity of isorhamnetin against SARS-CoV-2 spike pseudotyped virus in vitro. Drug Dev. Res. 2021;82(8):1124–1130. doi: 10.1002/ddr.21815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Hu X., Li Z., Wang F., Xia Y., Hou S., Zhong H., Zhang F., Gu N. Use of polyvinylpyrrolidone-iodine solution for sterilisation and preservation improves mechanical properties and osteogenesis of allografts. Sci. Rep. 2016;6:38669. doi: 10.1038/srep38669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Xu Z., Castiglione G.M., Soiberman U.S., Eberhart C.G., Duh E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surface. 2020;18(4):537–544. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F. Tan W & China Novel Coronavirus Investigating and Research Team (2020) A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. Lancet. 2015;386(9997):995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupin L., Pascolo L., Crovella S. Is FURIN gene expression in salivary glands related to SARS-CoV-2 infectivity through saliva? J. Clin. Pathol. 2021;74(4):209–211. doi: 10.1136/jclinpath-2020-206788. [DOI] [PubMed] [Google Scholar]
- de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.