Abstract
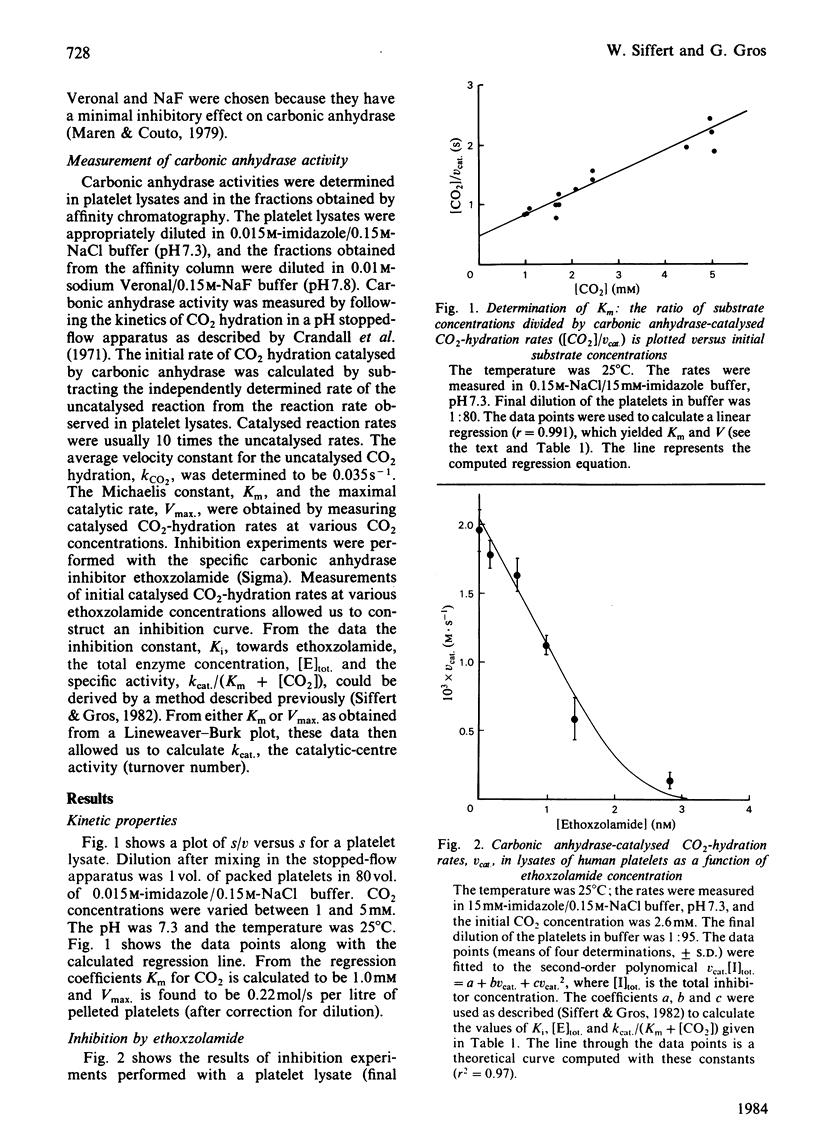
The carbonic anhydrase activity of human platelets was investigated by measuring the kinetics of CO2 hydration in supernatants of platelet lysates by using a pH stopped-flow apparatus. An average carbonic anhydrase concentration of 2.1 microM was determined for pellets of human platelets. Analysis of the kinetic properties of this carbonic anhydrase yielded a Km value of 1.0 mM, a catalytic-centre activity kcat. of 130000 s-1 and an inhibition constant Ki towards ethoxzolamide of 0.3 nM. From these values, CO2 hydration inside platelets is estimated to be accelerated by a factor of 2500. When platelet lysates were subjected to affinity chromatography, only the high-activity carbonic anhydrase II could be eluted from the affinity column, whereas the carbonic anhydrase isoenzyme I, which is known to occur in high concentrations in human erythrocytes, appeared to be absent.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akkerman J. W., Holmsen H., Loughnane M. Simultaneous measurement of aggregation, secretion, oxygen uptake, proton production, and intracellular metabolites in the same platelet suspension. Anal Biochem. 1979 Sep 1;97(2):387–393. doi: 10.1016/0003-2697(79)90090-3. [DOI] [PubMed] [Google Scholar]
- Carter N., Shiels A., Tashian R. Carbonic anhydrase III isoenzyme from human and bovine muscle [proceedings]. Biochem Soc Trans. 1978;6(3):552–553. doi: 10.1042/bst0060552. [DOI] [PubMed] [Google Scholar]
- Crandall E. D., Klocke R. A., Forster R. E. Hydroxyl ion movements across the human erythrocyte membrane. Measurement of rapid pH changes in red cell suspensions. J Gen Physiol. 1971 Jun;57(6):664–683. doi: 10.1085/jgp.57.6.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes L. B., Trelstad R. L. Cell polarity in precartilage mouse limb mesenchyme cells. Dev Biol. 1980 Aug;78(2):511–520. doi: 10.1016/0012-1606(80)90350-4. [DOI] [PubMed] [Google Scholar]
- Horne W. C., Norman N. E., Schwartz D. B., Simons E. R. Changes in cytoplasmic pH and in membrane potential in thrombin-stimulated human platelets. Eur J Biochem. 1981 Nov;120(2):295–302. doi: 10.1111/j.1432-1033.1981.tb05703.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A., Salganicoff L. The internal pH of isolated serotonin containing granules of pig platelets. J Biol Chem. 1978 Oct 10;253(19):7061–7068. [PubMed] [Google Scholar]
- Khalifah R. G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem. 1971 Apr 25;246(8):2561–2573. [PubMed] [Google Scholar]
- Koester M. K., Register A. M., Noltmann E. A. Basic muscle protein, a third genetic locus isoenzyme of carbonic anhydrase? Biochem Biophys Res Commun. 1977 May 9;76(1):196–204. doi: 10.1016/0006-291x(77)91686-2. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Couto E. O. The nature of anion inhibition of human red cell carbonic anhydrases. Arch Biochem Biophys. 1979 Sep;196(2):501–510. doi: 10.1016/0003-9861(79)90302-3. [DOI] [PubMed] [Google Scholar]
- Osborne W. R., Tashian R. E. An improved method for the purification of carbonic anhydrase isozymes by affinity chromatography. Anal Biochem. 1975 Mar;64(1):297–303. doi: 10.1016/0003-2697(75)90434-0. [DOI] [PubMed] [Google Scholar]
- Siffert W., Gros G. Carbonic anhydrase C in white-skeletal-muscle tissue. Biochem J. 1982 Sep 1;205(3):559–566. doi: 10.1042/bj2050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H., Jonsson B. H., Lindskog S. The catalytic mechanism of carbonic anhydrase. Hydrogen-isotope effects on the kinetic parameters of the human C isoenzyme. Eur J Biochem. 1975 Nov 1;59(1):253–259. doi: 10.1111/j.1432-1033.1975.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Wistrand P. J. The importance of carbonic anhydrase B and C for the unloading of CO2 by the human erythrocyte. Acta Physiol Scand. 1981 Dec;113(4):417–426. doi: 10.1111/j.1748-1716.1981.tb06918.x. [DOI] [PubMed] [Google Scholar]


